Abstract
AIM: In the present study, antibody and peripheral blood mononuclear cells (PBMC) proliferative responses against hepatitis C virus (HCV) antigens were evaluated in HCV chronically infected patients.
METHODS: Paired serum and PBMC samples were taken six months apart from 34 individuals, either treated or not, and tested by enzyme-linked immunosorbent assay (ELISA) and carboxyfluorescein succinimidyl ester staining.
RESULTS: Over 70% of the patients showed specific IgG and IgM against capsid, E1 and NS3, while HVR-1 was recognized by half of the patients. An increase in the levels of the anti-capsid IgM (P = 0.027) and IgG (P = 0.0006) was observed in six-month samples, compared to baseline. Similarly, a significantly higher percent of patients had detectable IgA reactivity to capsid (P = 0.017) and NS3 (P = 0.005) after six months, compared to baseline. Particularly, IgA against structural antigens positively correlated with hepatic damage (P = 0.036). IgG subclasses evaluation against capsid and NS3 revealed a positive recognition mediated by IgG1 in more than 80% of the individuals. On the contrary, less than 30% of the patients showed a positive proliferative response either of CD4+ or CD8+ T cells, being the capsid poorly recognized.
CONCLUSION: These results confirm that while the cellular immune response is narrow and weak, a broad and vigorous humoral response occurs in HCV chronic infection. The observed correlation between IgA and hepatic damage may have diagnostic significance, although it warrants further confirmation.
Keywords: Hepatitis C, Antibody response, Lymphoproliferation, Core, Envelope
INTRODUCTION
Hepatitis C virus (HCV) constitutes a major health problem, since it is infecting an estimated 170 million people worldwide. Probably, the most characteristic feature of this virus is its propensity to cause chronic infection, which is established in the majority of cases and has become a leading indication to liver transplantation in Western countries[1]. The burden of HCV infection is even more dramatic due to the absence of preventive or therapeutic vaccines. Additionally, the best available treatments, based on pegylated interferon plus ribavirin, are generally effective only in 50% of cases[2]. Thus, the development of new treatments and prophylactic interventions is currently a priority.
Despite almost two decades of intense research, since its description by Choo and coworkers[3], correlates of protection have not been entirely established for HCV infection. The specific humoral response to acute infection is considered of relatively low titer and delayed in time from the moment of infection[4,5]. Antibodies directed to the capsid protein are the first ones to be detected[4], and have been determined to be principally of IgG and, secondly, of IgM classes in chronic infection[6]. Particularly, the significance of IgM anti-HCV capsid in chronic infection has been studied[7,8]. Results indicate that IgM anti-HCV capsid occurrence is directly related to viremia levels[8] and these antibodies have been found to decrease or disappear in patients in disease remission and increase when the disease reactivates after therapy[6]. Another feature reported as characteristic of HCV-specific antibody response is its restriction, except for the capsid, to the IgG1 isotype, the rest of the subclasses being poorly prevalent[4].
Evidence for a significant role of antibody responses in viral clearance seems conflicting, since it has been observed that subjects with antibody deficiencies may spontaneously clear HCV infection[9]. Several epitopes of the envelope glycoproteins have been identified as targets of neutralizing antibodies[10]. However, in the majority of the patients, chronic infection is established in spite of their presence[11], probably due to their absence or low titers in early phases of the infection[12].
On the other hand, the importance of a sustained, multispecific CD4+, as well as CD8+, T cell responses, targeting numerous epitopes early in infection, has been highlighted[13-16]. In persistently infected patients, CD4+ and CD8+ T cells are found at low frequencies in peripheral blood, but seem to be compartmentalized in the liver[17]. It has been demonstrated that, in most subjects, a detectable cell-mediated immune response is generated at the onset of acute infection, but this response progressively disappears in those where HCV infection becomes persistent[18]. Impaired production of interferon-gamma (IFN-γ) and interleukin- (IL-) 2, as well as incapability to proliferate in vitro, have been demonstrated for CD4+ T cells of chronically infected patients[18-20]. Similarly, CD8+ T cells of persistently infected subjects fail to produce IFN-γ and tumor necrosis factor-alpha (TNF-α) in functional assays[21,22].
In this work, we aimed at evaluating HCV-specific immune response in chronically infected patients, treated or untreated, using paired blood samples taken 6 months apart. IgG, IgM and IgA levels, as well as IgG1-4 subclasses and peripheral blood mononuclear cells proliferative responses against HCV core, envelope and NS3 antigens were measured. Additionally, we studied the relationship between immune parameters and patients’ demographic characteristics.
MATERIALS AND METHODS
Study population
The study cohort included 34 patients with chronic HCV genotype 1 infection. The enrolment of patients was conducted at the National Institute of Gastroenterology (Havana, Cuba). Written informed consent was obtained from every patient prior to start of the study. All procedures were conducted in accordance with the national ethics guidelines and the Declaration of Helsinki, as revised in 1996. A patient with chronic HCV infection was defined as an individual with detectable HCV RNA and sustained liver injury for more than 6 mo, as monitored by liver function tests [alanine aminotransferase (ALT)/aspartate aminotransferase (AST)] and/or liver biopsy, scored according to the Ishak system. Patients were either treatment naïve or had been treated with the interferon-α (IFN-α) and ribavirin combination during the study period, except one subject who had received IFN-α monotherapy. Blood samples were taken at baseline (T = 0) and 6 mo later (T = 6). Demographic data of patients involved in the study are shown in Table 1.
Table 1.
Demographic characteristics of patients with chronic hepatitis C
| Characteristic | Value |
| Age (yr) | |
| Mean ± SD | 48 ± 11 |
| Median (Interquartile range) | 49 (39-54) |
| Race (n/%) | |
| Caucasian | 31/91.2 |
| Black | 2/5.8 |
| Mixed | 1/2.9 |
| Body Mass Index (kg/m2) | |
| Mean ± SD | 26.4 ± 4.5 |
| Median (Interquartile range) | 26.4 (23.3-28.9) |
| Gender (n/%) | |
| Female | 22/64.7 |
| Male | 12/35.3 |
| Possible source of infection (n/%) | |
| Transfusion/surgery | 27/79.4 |
| Unknown | 7/20.6 |
| Treatment (n/%) | |
| IFN-α + ribavirin | 23/67.6 |
| IFN-α | 1/2.9 |
| Untreated | 10/29.4 |
| Hepatic damage1 (n/%) | |
| Undetermined | 9/26.5 |
| Mild | 15/44.1 |
| Moderate | 7/20.6 |
| Severe | 3/8.8 |
| Alcohol consumption (n/%) | |
| Yes | 4/11.7 |
| No | 30/88.2 |
Necro-inflammatory activity.
Antigens
The recombinant proteins Co.120[23], E1.340[24] and NS3[25] are expressed in modified Escherichia coli and purified to 90%, except E1.340 which is purified to 85%. E2.680 recombinant protein is expressed in modified Picchia pastoris yeast and purified to 85%[26]. The HVR-1 peptide comprises amino acids 384-414 (TGTYVTGGTAARGVSQFTGLFTSGPSQKIQL) of the E2 protein[27]. All the recombinant proteins and the HVR-1 synthetic peptide correspond to a genotype 1b strain. Peptide pools individually comprising the whole sequence of the capsid, E1 and E2 proteins of HCV-1a strain were also used for Peripheral blood mononuclear cells (PBMC) proliferation assays. These peptides were 18 amino acids in length, overlapping adjacent peptides by 10 amino acids. Peptide pools were kindly donated by Dr Naglaa Shoukry (Centre de Recherche du CHUM, Montreal, Canada).
Evaluation of antibody response against HCV antigens
To detect human antibodies to HCV structural antigens, 96-well microtiter plates (Costar, Cambridge, MA, USA) were coated with 100 μL of Co.120 (10 μg/mL), E1.340 (10 μg/mL), HVR-1 synthetic peptide (2 μg/mL) or NS3 (5 μg/mL) diluted in coating buffer (50 mmol/L carbonate buffer, pH 9.6) followed by 16-h incubation at 4°C. The wells were washed four times with 0.1% Tween 20 in phosphate buffered saline (0.14 mol/L NaCl, 0.003 mol/L KCl, 0.01 mol/L Na2HPO4, 0.001 mol/L KH2PO4, pH 7.5) (PBST) and blocked with 200 μL of PBST containing 2% skim milk (Oxoid Ltd, England) and 5% goat normal serum (blocking solution) for 1 h at 25°C. After four washes with PBST, each well received 100 μL of a 1:10 dilution of human sera in blocking solution and the plates were incubated at 37°C for 1 h. Sera were diluted 1:80 in blocking solution for the evaluation of the specific response against E1.340. The plates were washed four times with PBST. Then, 100 μL of horseradish peroxidase-conjugated goat anti-human IgM, IgA or IgG secondary antibodies (Sigma, St Louis, USA), 1:10 000, 1:25 000 and 1:30 000 diluted, respectively, in PBST plus 2% skim milk, were added and the plates were incubated at 37°C for 1 h, followed by four washes with PBST. IgG subclasses were evaluated with the secondary biotinylated antibodies against human IgG1, IgG2, IgG3 and IgG4 (Sigma-Aldrich, St Louis, USA) respectively diluted 1:24 000, 1:5000, 1:5000 and 1:1000 in blocking solution. After four washes with PBST, an additional 1 h incubation step at 37°C with extravidin-peroxidase conjugate (Sigma, St Louis, USA), 1:1000 diluted in PBST plus 2% skim milk, was carried out followed by four washes with PBST. In every case, positive reactions were visualized with o-phenylenediamine (Sigma-Aldrich, St Louis, USA) 0.05% in substrate buffer (0.1 mol/L citric acid, 0.2 mol/L NaH2PO4, pH 5.0) with 0.015% H2O2 (Merck, Germany) as substrate. Reactions were stopped with 50 μL of 2.5 mol/L H2SO4. Measurement of absorbance (A) at 492 nm was made in a SensIdent Scan reader plate (Merck, Darmstadt, Germany). At least two human sera, anti-HCV negative by UMELISA (Center for Immunoassay, Cuba), were used as negative controls in each experiment. Anti-HCV positive human sera (as tested by UMELISA, Center for Immunoassay, Cuba), having a known antibody titre of at least 1:150 against the corresponding antigen, served as positive controls. The cut-off value to consider a positive antibody (Ab) response was established as twice the mean A492nm of the negative control sera.
PBMC preparation
Blood anticoagulated with acid citrate dextrose (1:9) was processed within 2 h after sample collection. PBMC from HCV patients and a healthy individual were isolated using Ficoll-Paque PLUS density gradients (Amersham, Oslo, Norway), and adjusted to 5-10 × 106 cells/mL in freezing medium consisting of nine parts of foetal bovine serum (FBS; Hyclone) and one part of DMSO (Sigma, Deisenhofen, Germany). PBMC were stored for 16 h in 1°C freezing containers (Nalgene Nunc International, Rochester, New York, USA) at 80°C and then transferred into liquid nitrogen until use.
Evaluation of CD4+ and CD8+ T cell proliferative response against HCV antigens
T cell proliferation assays were used to analyze HCV specific T cell responses against proteins Co.120, E1.340, E2.680 and NS3 or peptide pools covering core, E1 and E2 HCV proteins, depending on patients’ PBMC availability. Cryopreserved PBMC were thawed quickly in a 37°C water bath, and washed twice with R10 medium. After a 16-h resting period at 37°C and 50 mL/L of CO2, cells were washed twice with PBS, adjusted to 20 × 106 cells/mL and labeled with 4 μmol/L of carboxyfluorescein succinimidyl ester (CFSE) for 8 min at room temperature in the dark. The reaction was stopped by adding 1 volume of human AB serum (Sigma-Aldrich, St. Louis, USA). Next, PBMC were washed twice with PBS and once with RPMI 1640 medium (Sigma-Aldrich, St. Louis, USA). Cells were finally adjusted to 2 × 106 cells/mL and stimulated or not with the peptide pools (1 μg/mL) and proteins (2 μg/mL, except for NS3, of which a concentration of 5 μg/mL was used) for 6 days at 37°C and 50 mL/L of CO2. Cells incubated with media alone were considered as negative control. Concanavalin A (ConA, Sigma-Aldrich, St. Louis, USA, 5 μg/mL) was used as positive control. Cells were harvested, stained with surface antibodies and analyzed by flow cytometry. Anti-CD4 allophycocyanin (APC) (clone # 11 830), anti-CD8 phycoerythrin (PE) (clone # 37 006) and anti-CD8 APC (clone # 37 006) monoclonal antibodies, 4 μg/mL, 2.5 μg/mL and 5 μg/mL respectively, were from R&D Systems (R&D Systems, Minneapolis, USA). The stimulation index (SI) was calculated by dividing the proliferative frequency (%) in the presence of antigen by the proliferative frequency (%) without antigen. The stimulation index was considered positive if ≥ 2.5 after peptide stimulation and ≥ 3 after protein stimulation.
Statistical analysis
GraphPad Prism version 4.00 statistical software (GraphPad Software, San Diego, CA) was generally used to carry out statistical analysis. Unpaired t test (for data sets with a Gaussian distribution and equal variances) and Mann Whitney test (for data sets with non-Gaussian distribution or different variances) were used to compare the magnitude of a given response between the two evaluated time points. For comparison of the number of positive samples at the two evaluated moments, Fisher’s exact test was used. Correlations between variables were analyzed by Spearman’s rank correlation coefficient, using SPSS 11.5.1 Software for Windows. Significant differences were considered when P < 0.05.
RESULTS
Study subjects
The present study was designed to evaluate the specific immune response against HCV in genotype 1 chronically infected patients, in a 6-mo follow-up period. Of the 34 patients initially enrolled in the study, only 31 could be contacted for a second blood extraction 6 mo later. In this cohort, 73.5% of the patients were over 40 years of age, 67.6% had been treated with the standard combined therapy with IFN-α and ribavirin and only 11.7% of the individuals reported to consume alcohol. Over 73% of the patients had undergone a liver biopsy and of them, 64.7% showed a mild to moderate necro-inflammatory activity, as measured by Ishak scores. Table 1 summarizes the principal demographic characteristics.
Humoral immune response to HCV antigens
The reactivity to HCV antigens was assessed in serum samples from 34 patients chronically infected with HCV. All the patients displayed a positive antibody response against several of the evaluated antigens. At baseline, IgG and IgM reactivities were present in more than 70% of the individuals against Co.120, E1.340 and NS3 proteins (Table 2). Regarding the reactivity towards HVR-1, IgG could only be detected in half of the individuals; still, it was dominant over the rest of the evaluated classes. IgA was generally less frequently detected than IgG and IgM against all antigens.
Table 2.
Reactivity of the main antibody classes against core, E1, HVR-1 and NS3 antigens in sera taken at baseline
| Isotypes |
Percentage of patients with a positive response against the indicated antigen |
|||
| Core | E1 | HVR-1 | NS3 | |
| IgG | 91.1 | 70.8 | 51.5 | 88.2 |
| IgM | 76.4 | 78.7 | 5.8 | 79.4 |
| IgA | 52.9 | 39.3 | 12.1 | 50 |
The assessment of the reactivity of the IgG subclasses, at baseline, against the highly conserved capsid and NS3 antigens, revealed that their recognition was mediated by IgG1 in more than 80% of the patients (Table 3). At the same time, in 78.7% of the individuals a positive IgG4 response could be detected against NS3, while it was detectable only in nearly half of the samples against Co.120. IgG2 and IgG3 were detected in a small percent of the tested samples against both antigens (Table 3).
Table 3.
Reactivity of IgG subclasses against core and NS3 antigens in sera taken at baseline
| IgG Subclasses |
Percentage of patients with a positive response against the indicated antigen |
|
| Core | NS3 | |
| IgG1 | 85.2 | 82.3 |
| IgG2 | 17.6 | 39.3 |
| IgG3 | 11.7 | 12.1 |
| IgG4 | 48.4 | 78.7 |
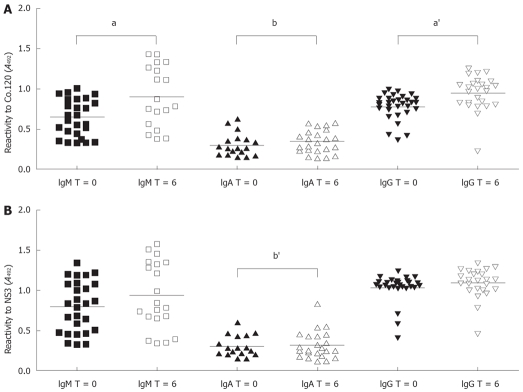
The reactivity of the main classes IgM, IgA and IgG against Co.120 and NS3 was not only assessed at baseline (T = 0), but also 6 months later (T = 6) (Figure 1). The comparison of these two time points revealed that there was a significantly higher percentage of individuals with a positive IgA response against both antigens at T = 6 (Co.120 52.9% vs 88.4%, P = 0.017; NS3 50.5% vs 88.4%, P = 0.005). Interestingly, none of the patients lost the specific IgA reactivity from baseline to the end of the study: instead, the observed increase was totally due to de novo responses at T = 6. Regarding the magnitude of the response, a statistically significant difference was observed in the IgG and IgM classes against Co.120 (Figure 1A). The mean reactivity was higher at T = 6 when compared to baseline (IgM 0.6419 vs 0.9099, P = 0.027; IgG 0.7802 vs 0.9532, P = 0.0006).
Figure 1.
Comparison of antibody reactivity to HCV recombinant Co.120 (A) and NS3 (B) proteins in serum samples from baseline (T = 0) and 6 mo (T = 6) of follow-up. Symbols represent individual values. Only samples showing a positive reactivity are displayed. The horizontal lines represent mean values. Letters over brackets indicate statistical significance (a denotes differences in response magnitude, aP = 0.027, a'P = 0.0006; b denotes differences in the number of positive samples, bP = 0.017, b'P = 0.005).
Correlation analyses between demographic variables and humoral responses revealed that alcohol consumption was negatively correlated with the responses of the main classes IgM (P = 0.01), IgA (P = 0.019) and IgG (P = 0.028), as well as IgG4 (P = 0.00000182) (Table 4). On the other hand, positive IgG4 positively correlated with the fact of being treated (P = 0.014) and the grade of hepatic damage (P = 0.047). Additionally, the hepatic damage, expressed as necro-inflammatory activity, also correlated with IgA (P = 0.036), while fibrosis did not (P = 0.487).
Table 4.
Correlation between demographic variables and aspects of humoral response
| Alcohol consumption | Specific treatment | Hepatic damage1 | |
| IgM2 | R = -0.53b | R = 0.313 | R = 0.107 |
| P = 0.01 | P = 0.071 | P = 0.609 | |
| IgA | R = -0.402a | R = 0.238 | R = 0.422a |
| P = 0.019 | P = 0.176 | P = 0.036 | |
| IgG3 | R = -0.383a | R = 0.257 | R = 0.100 |
| P = 0.028 | P = 0.149 | P = 0.635 | |
| IgG4 | R = -0.717b | R = 0.418a | R = 0.401a |
| P = 0.000 | P = 0.014 | P = 0.047 |
Necro-inflammatory activity;
IgM positive response to HCV structural antigens;
IgG positive response to HCV HVR-1 peptide; R = Spearman’s correlation coefficient;
Significant correlation at 0.05 level;
Significant correlation at 0.01 level.
T cell proliferative response to HCV antigens
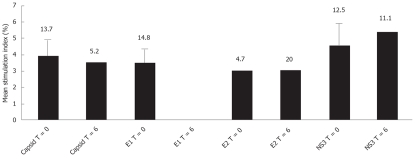
PBMC were analyzed in a proliferation assay for their capacity to expand in response to stimulation with HCV antigens. Samples from both T = 0 and T = 6 were evaluated. Depending on patients PBMC availability, the response to the structural antigens was evaluated by stimulation with the corresponding recombinant protein and a peptide pool. Cells were surface-stained with anti-CD4 to evaluate whether the CD4+ cell subpopulation proliferated when stimulated with specific HCV antigens. Particularly, a specific proliferative response was neither detected against the capsid and NS3 proteins at T = 0 nor to E2 at T = 6 (data not shown). Only 10% of the evaluated samples showed a positive response to the capsid at the end of the study, being this antigen the less frequently recognized. The highest percent of positive samples (25%) was detected towards E2 at baseline, followed by NS3 at T = 6 (20%), while E1 protein was always recognized by 14% of the individuals. None of the patients showed a positive proliferative response against more than one antigen.
On the other hand, the analysis of CD8+ cells allowed the identification of specific responses to all the tested antigens, except to E1 at T = 6 (Figure 2). However, these responses were present in less than 15% of the patients against each antigen. Two patients showed a positive proliferative response simultaneously against the capsid and E1 proteins at T = 0, while another individual recognized E1 at baseline and also E2, 6 months later. CD8+ cells of only one individual proliferated against all tested antigens (capsid, envelope and NS3 proteins) at T = 0; unfortunately, this patient could not be contacted for a second blood sample, and therefore, the persistence of this peculiar response could not be assessed. Taking into account the results of both CD4+ and CD8+ cell proliferation, we did not detect any patient in which the two time-point samples were consistently positive throughout the study.
Figure 2.
Comparison of CD8+ T cell proliferative response to HCV structural and non-structural antigens in baseline and six months follow up samples. Bars represent mean stimulation index of positive samples only. Error bars represent standard deviation of the mean. Numbers above bars indicate percent of positive samples.
DISCUSSION
In this study, we assessed HCV-specific immune responses in a group of HCV chronically infected patients at two different time-points six months apart. The evaluation of the antibody response against viral antigens revealed that all the patients displayed a positive antibody response against several of the evaluated antigens. Specifically, in the majority of patients the reactivity was dominated by IgG and IgM, both in terms of number of positive patients and the magnitude of the response. Particularly, the presence of IgM anti-HCV-capsid antibodies have been regarded as a negative prognostic marker of response to treatment[28] and a factor associated to recurrence of hepatitis and its severity in HCV-infected liver transplant recipients[29]. Therefore, their presence in our cohort of chronic patients reinforces the notion of their inefficacy in this phase of the infection. Our results are in agreement with previous works reporting elevated prevalence of both immunoglobulin classes in chronically infected patients[7,30]. These studies only refer to the specific response to the capsid protein; our results extend this notion to other antigens such as E1 and NS3.
Among the IgG subtypes, IgG1 has been, by far, the most frequently found against all tested viral antigens[5,30]. Moreover, it has been reported that the antibody response to most HCV antigens is highly restricted to this subclass[31], the rest of the subclasses being rarely detected. This response restriction to IgG1 has lead studies aiming to find relations of antibody production to long-term outcome after therapy. In fact, it has been observed that IgG1 specific to an N-terminal epitope of the capsid protein decrease in complete responders, while remain unchanged in non-responders. Therefore, these antibodies have been proposed as markers of the efficacy of IFN-α therapy[30]. Our results showed a high prevalence of IgG1 against the capsid, and also against NS3, HCV’s most conserved antigens. Nevertheless, 78.7% of the patients also showed a positive IgG4 response against NS3. It is of general knowledge that in chronic viral infections in humans, viral proteins generally elicit the IgG1 and IgG3 subtypes and to a lesser extent, IgG2 and IgG4. IgG4 has been frequently found dominating in responses to prolonged antigenic stimulation[32] and has been identified as a major component of circulating immune-complexes in chronic hepatitis B virus-infected individuals[33]. Given that antibodies of IgG4 subclass do not activate the complement system through the classic pathway and have a low affinity to Fc-γ receptors, their presence is considered a factor that may contribute to chronicity.
The assessment of correlations between immunological and demographic variables in our study revealed that IgM to structural antigens, IgG to the HVR-1, IgA and IgG4 responses negatively correlated with alcohol consumption, indicating that this habit may dampen the potentiality for generating a diverse immune response. In contrast, the fact of having been treated with the standard therapy positively correlated with the presence of IgG4 responses. Regarding the involvement of IgG4, it has been observed that the nonselective modulatory effect of IFN-α treatment may contribute to widen the diversity of specific IgG subclasses profiles in hepatitis B virus infection, contributing to the high participation of IgG4[34]. To our knowledge, this effect of antiviral therapy has not been assessed specifically in HCV infection, but seems a plausible hypothesis supporting our findings.
Another positive correlation that could be detected was that between hepatic damage, expressed as necro-inflammatory activity, and IgA response. It has been observed that TGF-β1, which is produced by hepatic stellate cells and Kupffer’s cells, induces the isotype switching to IgA in B lymphocytes proliferating in vitro[35]. This cytokine is a prominent profibrogenic factor during inflammation, tissue regeneration and fibrogenesis[36] and in line with this, it has been demonstrated that HCV patients have elevated levels of circulating TGF-β1 versus controls[37]. To our knowledge, whether there is a direct relation between serum IgA and TGF-β1 circulating levels in HCV chronic patients has not been explored so far. Nevertheless, our results warrant further studies, although they do not point out to a direct correlation with fibrosis, but rather with the necro-inflammatory activity. On the other hand, the liver plays an important role in IgA clearance, and the loss of hepatic function due to chronic inflammation and damage may reduce normal IgA catabolism, and contribute to its accumulation in serum[38]. Therefore, the observed correlation might be probably indicating that IgA increase is a consequence, rather than a cause, of hepatic damage.
Additionally, IgG4 responses positively correlated with hepatic damage. As previously discussed, IgG4 has not been usually found as a dominant component of HCV specific immune response. Therefore, the implications of the presence of this subclass have not been explored so far in patients chronically infected with HCV. Nevertheless, a series of inflammatory and autoimmune diseases, in which IgG4 have a strong participation, have been described: these are known as IgG4-related sclerosing diseases[39]. In general, these disorders are characterized by high serum IgG4 levels, which are closely associated with disease activity, in a context of chronic lymphocyte infiltration and fibrosis of the affected organs[39]. Although the role of IgG4 in these disorders remains obscure, it has been suggested that the formation and accumulation of immune complexes and the activation of the alternative complement pathway contribute to disease activity[39]. Further studies with larger cohort of patients are needed to definitively discern the real role of IgG4 in chronic hepatitis C as well as in other chronic inflammatory disorders.
We also evaluated the proliferative response of PBMC of chronic patients against stimulation with HCV antigens, in paired samples taken six months apart. Only a small percent of samples showed a positive response against each of the tested antigens. Moreover, the great majority of the patients displayed a detectable proliferation only to a single antigen and this response was never constant in the two evaluated time points. These results are in agreement with previous works reporting instability, low frequencies and a small number of targeted epitopes by both CD4+ and CD8+ T cells in peripheral blood of chronic patients[14,16,40]. The characteristic asymptomatic course of this disease hinders the accumulation of immunological data regarding the very early phase of the infection; therefore, it has been difficult, so far, to discriminate between primary T cell failure and early T cell exhaustion or deletion, once chronic infection has already been established. In fact, both phenomena seem to operate in different patients and equally lead to persistence[41]. Many mechanisms are postulated to be involved in T cell failure, namely impaired antigen presentation[42,43], reduced cytokine secretion by antigen presenting cells[44], immunomodulatory effects exerted by different viral factors[45,46] and increased levels of CD4 + CD25high Treg cells[47].
In summary these results confirm that in patients chronically infected with HCV, either naïve or non-responders to the standard therapy with IFN-α plus ribavirin, cellular proliferative responses are rarely detected, usually weak, not sustained and narrowly directed. On the other hand, the humoral response is characterized by a broad representation of antibody classes and subclasses, some of which have not been demonstrated to contribute to viral clearance, but rather to persistence. Particularly, the association of specific IgA response to necro-inflammatory activity paves the way to further studies to confirm its utility as an easy-to-measure marker of increased histological activity.
COMMENTS
Background
Correlates of protection against hepatitis C virus (HCV) are extensively pursued in nowadays research. Early, vigorous and sustained peripheral blood mononuclear cells (PBMC) proliferative responses specific to HCV have been regarded as pivotal for viral clearance. On the other hand, antibody responses’ contribution is still controversial and the significance of specific antibody classes during chronic infection has been investigated.
Research frontiers
So far, in HCV infection, the most extensively studied antibodies are those directed to the capsid protein. Several data indicate that IgM anti-HCV capsid occurrence is directly related to viremia levels. Additionally, HCV-specific antibody response is regarded as restricted to the IgG1 isotype, except for the capsid. The rest of the classes and IgG subclasses have been found very rarely represented, and therefore their significance in acute and chronic HCV is unclear.
Innovations and breakthroughs
Correlation analysis between demographic variables and humoral response confirmed the negative influence of alcohol consumption on the immune response, particularly on responses of the main immunoglobulin classes. On the other hand, IgG4, an IgG subclass characteristic of chronic antigenic stimulation, positively correlated with the grade of necro-inflammatory activity and the fact of being treated with the standard therapy; the latter already demonstrated for hepatitis B virus (HBV), but not for HCV. Additionally, for the first time a positive correlation between necro-inflammatory activity with HCV-specific IgA was found.
Applications
Particularly, the association of specific IgA response to necro-inflammatory activity paves they way to further studies to confirm its utility as a non invasive, easy-to-measure marker of increased histological activity in chronic HCV infection.
Peer review
An interesting study assessing the humoral response in chronic HCV patients. The association of elevated IgG and globulins with fibrosis have been described as referenced. It is an interesting observation to find IgA specific to HCV antigens.
Acknowledgments
We thank the patients for their willingness to participate into this study. We also thank Dr. Raffick-Pierre Sékaly and Dr. Naglaa Shoukry for training in determination of cellular immune response and Carmen Valenzuela and Dr. Julio C Alvarez for assisting in study design. We are grateful to Jeny Marante, Acacia Trujillo, Elena Ferrer and Aina Méndez for excellent technical assistance, MSc Hugo Nodarse and Dr. Luis Rivera for invaluable help in patient enrollment and Dr. Enrique Arús for assisting in the general coordination of the study. Dr. Santiago Dueñas-Carrera holds a Grant from PanAmerican Health Organization.
Footnotes
Supported by Grant from Pan American Health Organization, held by Dr. Santiago Dueñas-Carrera
Peer reviewers: Dr. Vincent Lai, Derby NHS Foundation Trust, Utooxeter Road, Derby, DE22 3NE, United Kingdom; Bo-Jian Zheng, MD, PhD, Department of Microbiology, the University of Hong Kong, University Pathology Building, Queen Mary Hospital, Pokfulam Road, Hong Kong, China
S- Editor Xiao LL L- Editor Negro F E- Editor Zheng XM
References
- 1.Hoofnagle JH. Course and outcome of hepatitis C. Hepatology. 2002;36:S21–S29. doi: 10.1053/jhep.2002.36227. [DOI] [PubMed] [Google Scholar]
- 2.Yuan HJ, Lee WM. Nonresponse to treatment for hepatitis C: current management strategies. Drugs. 2008;68:27–42. doi: 10.2165/00003495-200868010-00003. [DOI] [PubMed] [Google Scholar]
- 3.Choo QL, Kuo G, Weiner AJ, Overby LR, Bradley DW, Houghton M. Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome. Science. 1989;244:359–362. doi: 10.1126/science.2523562. [DOI] [PubMed] [Google Scholar]
- 4.Chen M, Sällberg M, Sönnerborg A, Weiland O, Mattsson L, Jin L, Birkett A, Peterson D, Milich DR. Limited humoral immunity in hepatitis C virus infection. Gastroenterology. 1999;116:135–143. doi: 10.1016/s0016-5085(99)70237-4. [DOI] [PubMed] [Google Scholar]
- 5.Netski DM, Mosbruger T, Depla E, Maertens G, Ray SC, Hamilton RG, Roundtree S, Thomas DL, McKeating J, Cox A. Humoral immune response in acute hepatitis C virus infection. Clin Infect Dis. 2005;41:667–675. doi: 10.1086/432478. [DOI] [PubMed] [Google Scholar]
- 6.Nagayama R, Miyake K, Tsuda F, Okamoto H. IgM antibody to a hepatitis C virus core peptide (CP14) for monitoring activity of liver disease in patients with acute or chronic hepatitis C. J Med Virol. 1994;42:311–317. doi: 10.1002/jmv.1890420320. [DOI] [PubMed] [Google Scholar]
- 7.Quiroga JA, van Binsbergen J, Wang CY, Pardo M, Navas S, Trines C, Herrero M, Carreño V. Immunoglobulin M antibody to hepatitis C virus core antigen: correlations with viral replication, histological activity, and liver disease outcome. Hepatology. 1995;22:1635–1640. [PubMed] [Google Scholar]
- 8.Yuki N, Hayashi N, Ohkawa K, Hagiwara H, Oshita M, Katayama K, Sasaki Y, Kasahara A, Fusamoto H, Kamada T. The significance of immunoglobulin M antibody response to hepatitis C virus core protein in patients with chronic hepatitis C. Hepatology. 1995;22:402–406. [PubMed] [Google Scholar]
- 9.Chapel HM, Christie JM, Peach V, Chapman RW. Five-year follow-up of patients with primary antibody deficiencies following an outbreak of acute hepatitis C. Clin Immunol. 2001;99:320–324. doi: 10.1006/clim.2001.5036. [DOI] [PubMed] [Google Scholar]
- 10.Zeisel MB, Fafi-Kremer S, Fofana I, Barth H, Stoll-Keller F, Doffoel M, Baumert TF. Neutralizing antibodies in hepatitis C virus infection. World J Gastroenterol. 2007;13:4824–4830. doi: 10.3748/wjg.v13.i36.4824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meunier JC, Engle RE, Faulk K, Zhao M, Bartosch B, Alter H, Emerson SU, Cosset FL, Purcell RH, Bukh J. Evidence for cross-genotype neutralization of hepatitis C virus pseudo-particles and enhancement of infectivity by apolipoprotein C1. Proc Natl Acad Sci USA. 2005;102:4560–4565. doi: 10.1073/pnas.0501275102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pestka JM, Zeisel MB, Bläser E, Schürmann P, Bartosch B, Cosset FL, Patel AH, Meisel H, Baumert J, Viazov S, et al. Rapid induction of virus-neutralizing antibodies and viral clearance in a single-source outbreak of hepatitis C. Proc Natl Acad Sci USA. 2007;104:6025–6030. doi: 10.1073/pnas.0607026104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grüner NH, Gerlach TJ, Jung MC, Diepolder HM, Schirren CA, Schraut WW, Hoffmann R, Zachoval R, Santantonio T, Cucchiarini M, et al. Association of hepatitis C virus-specific CD8+ T cells with viral clearance in acute hepatitis C. J Infect Dis. 2000;181:1528–1536. doi: 10.1086/315450. [DOI] [PubMed] [Google Scholar]
- 14.Day CL, Lauer GM, Robbins GK, McGovern B, Wurcel AG, Gandhi RT, Chung RT, Walker BD. Broad specificity of virus-specific CD4+ T-helper-cell responses in resolved hepatitis C virus infection. J Virol. 2002;76:12584–12595. doi: 10.1128/JVI.76.24.12584-12595.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thimme R, Bukh J, Spangenberg HC, Wieland S, Pemberton J, Steiger C, Govindarajan S, Purcell RH, Chisari FV. Viral and immunological determinants of hepatitis C virus clearance, persistence, and disease. Proc Natl Acad Sci USA. 2002;99:15661–15668. doi: 10.1073/pnas.202608299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cox AL, Mosbruger T, Lauer GM, Pardoll D, Thomas DL, Ray SC. Comprehensive analyses of CD8+ T cell responses during longitudinal study of acute human hepatitis C. Hepatology. 2005;42:104–112. doi: 10.1002/hep.20749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grabowska AM, Lechner F, Klenerman P, Tighe PJ, Ryder S, Ball JK, Thomson BJ, Irving WL, Robins RA. Direct ex vivo comparison of the breadth and specificity of the T cells in the liver and peripheral blood of patients with chronic HCV infection. Eur J Immunol. 2001;31:2388–2394. doi: 10.1002/1521-4141(200108)31:8<2388::aid-immu2388>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 18.Folgori A, Spada E, Pezzanera M, Ruggeri L, Mele A, Garbuglia AR, Perrone MP, Del Porto P, Piccolella E, Cortese R, et al. Early impairment of hepatitis C virus specific T cell proliferation during acute infection leads to failure of viral clearance. Gut. 2006;55:1012–1019. doi: 10.1136/gut.2005.080077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ulsenheimer A, Gerlach JT, Gruener NH, Jung MC, Schirren CA, Schraut W, Zachoval R, Pape GR, Diepolder HM. Detection of functionally altered hepatitis C virus-specific CD4 T cells in acute and chronic hepatitis C. Hepatology. 2003;37:1189–1198. doi: 10.1053/jhep.2003.50194. [DOI] [PubMed] [Google Scholar]
- 20.Semmo N, Day CL, Ward SM, Lucas M, Harcourt G, Loughry A, Klenerman P. Preferential loss of IL-2-secreting CD4+ T helper cells in chronic HCV infection. Hepatology. 2005;41:1019–1028. doi: 10.1002/hep.20669. [DOI] [PubMed] [Google Scholar]
- 21.Wedemeyer H, He XS, Nascimbeni M, Davis AR, Greenberg HB, Hoofnagle JH, Liang TJ, Alter H, Rehermann B. Impaired effector function of hepatitis C virus-specific CD8+ T cells in chronic hepatitis C virus infection. J Immunol. 2002;169:3447–3458. doi: 10.4049/jimmunol.169.6.3447. [DOI] [PubMed] [Google Scholar]
- 22.Spangenberg HC, Viazov S, Kersting N, Neumann-Haefelin C, McKinney D, Roggendorf M, von Weizsäcker F, Blum HE, Thimme R. Intrahepatic CD8+ T-cell failure during chronic hepatitis C virus infection. Hepatology. 2005;42:828–837. doi: 10.1002/hep.20856. [DOI] [PubMed] [Google Scholar]
- 23.Dueñas-Carrera S, Morales J, Acosta-Rivero N, Lorenzo LJ, García C, Ramos T, Guerra I, Peña M. Variable level expression of hepatitis C virus core protein in a prokaryotic system. Analysis of the humoral response in rabbit. Biotecnología Aplicada. 1999;16:226–231. [Google Scholar]
- 24.Lorenzo LJ, García O, Acosta-Rivero N, Dueñas-Carrera S, Martínez G, Alvarez-Obregón J, Pichardo D, Ramos A, Guerra I, Morales J. Expression and immunological evaluation of the Escherichia coli-derived hepatitis C virus envelope E1 protein. Biotechnol Appl Biochem. 2000;32(Pt 2):137–143. doi: 10.1042/ba20000040. [DOI] [PubMed] [Google Scholar]
- 25.Pentón N, Musacchio A, Rivera JM, Roca J, Ponce M, Rodríguez D, Caballero A, Tallo YI, Narciandi RE. Antigenicity of a recombinant NS3 protein representative of ATPase/helicase domain from hepatitis C virus. Clin Biochem. 2003;36:41–49. doi: 10.1016/s0009-9120(02)00365-x. [DOI] [PubMed] [Google Scholar]
- 26.Martinez-Donato G, Acosta-Rivero N, Morales-Grillo J, Musacchio A, Vina A, Alvarez C, Figueroa N, Guerra I, Garcia J, Varas L, et al. Expression and processing of hepatitis C virus structural proteins in Pichia pastoris yeast. Biochem Biophys Res Commun. 2006;342:625–631. doi: 10.1016/j.bbrc.2006.01.157. [DOI] [PubMed] [Google Scholar]
- 27.Dueñas-Carrera S, Viña A, Garay HE, Reyes O, Alvarez-Lajonchere L, Guerra I, González LJ, Morales J. Immunological evaluation of Escherichia coli-derived hepatitis C virus second envelope protein (E2) variants. J Pept Res. 2001;58:221–228. doi: 10.1034/j.1399-3011.2001.00795.x. [DOI] [PubMed] [Google Scholar]
- 28.Papatheodoridis GV, Delladetsima JK, Katsoulidou A, Sypsa V, Albrecht M, Michel G, Hatzakis A, Tassopoulos NC. Significance of IgM anti-HCV core level in chronic hepatitis C. J Hepatol. 1997;27:36–41. doi: 10.1016/s0168-8278(97)80277-2. [DOI] [PubMed] [Google Scholar]
- 29.Bizollon T, Ahmed SN, Guichard S, Chevallier P, Adham M, Ducerf C, Baulieux J, Trepo C. Anti-hepatitis C virus core IgM antibodies correlate with hepatitis C recurrence and its severity in liver transplant patients. Gut. 2000;47:698–702. doi: 10.1136/gut.47.5.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hirayama M, Maruyama T, Mitsui H, Maekawa H, Yamada H, Hashimoto N, Koike K, Kimura S, Yasuda K, Iino S, et al. IgG1 anti-P2 as a marker of response to interferon in patients with chronic hepatitis C. Clin Exp Immunol. 2001;126:92–100. doi: 10.1046/j.1365-2249.2001.01648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen M, Sällberg M, Sönnerborg A, Weiland O, Mattsson L, Jin L, Birkett A, Peterson D, Milich DR. Limited humoral immunity in hepatitis C virus infection. Gastroenterology. 1999;116:135–143. doi: 10.1016/s0016-5085(99)70237-4. [DOI] [PubMed] [Google Scholar]
- 32.Aalberse RC, van der Gaag R, van Leeuwen J. Serologic aspects of IgG4 antibodies. I. Prolonged immunization results in an IgG4-restricted response. J Immunol. 1983;130:722–726. [PubMed] [Google Scholar]
- 33.Rath S, Devey ME. IgG subclass composition of antibodies to HBsAg in circulating immune complexes from patients with hepatitis B virus infections. Clin Exp Immunol. 1988;72:164–167. [PMC free article] [PubMed] [Google Scholar]
- 34.Gregorek H, Madaliński K, Woynarowski M, Mikolajewicz J, Syczewska M, Socha J. IgG subclass distribution of hepatitis B surface antigen antibodies induced in children with chronic hepatitis B infection after interferon-alpha therapy. J Infect Dis. 2000;181:2059–2062. doi: 10.1086/315515. [DOI] [PubMed] [Google Scholar]
- 35.Zan H, Cerutti A, Dramitinos P, Schaffer A, Casali P. CD40 engagement triggers switching to IgA1 and IgA2 in human B cells through induction of endogenous TGF-beta: evidence for TGF-beta but not IL-10-dependent direct S mu-->S alpha and sequential S mu-->S gamma, S gamma-->S alpha DNA recombination. J Immunol. 1998;161:5217–5225. [PMC free article] [PubMed] [Google Scholar]
- 36.Schuppan D, Krebs A, Bauer M, Hahn EG. Hepatitis C and liver fibrosis. Cell Death Differ. 2003;10 Suppl 1:S59–S67. doi: 10.1038/sj.cdd.4401163. [DOI] [PubMed] [Google Scholar]
- 37.Peterson MC. Elevated circulating transforming growth factor beta-1 may explain poorer renal survival in type II diabetics with chronic hepatitis C. Med Sci Monit. 2007;13:RA81–RA85. [PubMed] [Google Scholar]
- 38.Woof JM, Kerr MA. The function of immunoglobulin A in immunity. J Pathol. 2006;208:270–282. doi: 10.1002/path.1877. [DOI] [PubMed] [Google Scholar]
- 39.Nakanuma Y, Zen Y. Pathology and immunopathology of immunoglobulin G4-related sclerosing cholangitis: The latest addition to the sclerosing cholangitis family. Hepatol Res. 2007;37 Suppl 3:S478–S486. doi: 10.1111/j.1872-034X.2007.00243.x. [DOI] [PubMed] [Google Scholar]
- 40.Lauer GM, Barnes E, Lucas M, Timm J, Ouchi K, Kim AY, Day CL, Robbins GK, Casson DR, Reiser M, et al. High resolution analysis of cellular immune responses in resolved and persistent hepatitis C virus infection. Gastroenterology. 2004;127:924–936. doi: 10.1053/j.gastro.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 41.Thimme R, Oldach D, Chang KM, Steiger C, Ray SC, Chisari FV. Determinants of viral clearance and persistence during acute hepatitis C virus infection. J Exp Med. 2001;194:1395–1406. doi: 10.1084/jem.194.10.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bain C, Inchauspé G. [Dendritic cells and hepatitis C virus] Pathol Biol (Paris) 2001;49:464–465. doi: 10.1016/s0369-8114(01)00166-3. [DOI] [PubMed] [Google Scholar]
- 43.Lee CH, Choi YH, Yang SH, Lee CW, Ha SJ, Sung YC. Hepatitis C virus core protein inhibits interleukin 12 and nitric oxide production from activated macrophages. Virology. 2001;279:271–279. doi: 10.1006/viro.2000.0694. [DOI] [PubMed] [Google Scholar]
- 44.Anthony DD, Yonkers NL, Post AB, Asaad R, Heinzel FP, Lederman MM, Lehmann PV, Valdez H. Selective impairments in dendritic cell-associated function distinguish hepatitis C virus and HIV infection. J Immunol. 2004;172:4907–4916. doi: 10.4049/jimmunol.172.8.4907. [DOI] [PubMed] [Google Scholar]
- 45.Zhu N, Ware CF, Lai MM. Hepatitis C virus core protein enhances FADD-mediated apoptosis and suppresses TRADD signaling of tumor necrosis factor receptor. Virology. 2001;283:178–187. doi: 10.1006/viro.2001.0896. [DOI] [PubMed] [Google Scholar]
- 46.Accapezzato D, Francavilla V, Rawson P, Cerino A, Cividini A, Mondelli MU, Barnaba V. Subversion of effector CD8+ T cell differentiation in acute hepatitis C virus infection: the role of the virus. Eur J Immunol. 2004;34:438–446. doi: 10.1002/eji.200324540. [DOI] [PubMed] [Google Scholar]
- 47.Smyk-Pearson S, Golden-Mason L, Klarquist J, Burton JR Jr, Tester IA, Wang CC, Culbertson N, Vandenbark AA, Rosen HR. Functional suppression by FoxP3+CD4+CD25(high) regulatory T cells during acute hepatitis C virus infection. J Infect Dis. 2008;197:46–57. doi: 10.1086/523651. [DOI] [PubMed] [Google Scholar]