Abstract
Pernicious anemia (PA) is a macrocytic anemia that is caused by vitamin B12 deficiency, as a result of intrinsic factor deficiency. PA is associated with atrophic body gastritis (ABG), whose diagnosis is based on histological confirmation of gastric body atrophy. Serological markers that suggest oxyntic mucosa damage are increased fasting gastrin and decreased pepsinogen I. Without performing Schilling’s test, intrinsic factor deficiency may not be proven, and intrinsic factor and parietal cell antibodies are useful surrogate markers of PA, with 73% sensitivity and 100% specificity. PA is mainly considered a disease of the elderly, but younger patients represent about 15% of patients. PA patients may seek medical advice due to symptoms related to anemia, such as weakness and asthenia. Less commonly, the disease is suspected to be caused by dyspepsia. PA is frequently associated with autoimmune thyroid disease (40%) and other autoimmune disorders, such as diabetes mellitus (10%), as part of the autoimmune polyendocrine syndrome. PA is the end-stage of ABG. Long-standing Helicobacter pylori infection probably plays a role in many patients with PA, in whom the active infectious process has been gradually replaced by an autoimmune disease that terminates in a burned-out infection and the irreversible destruction of the gastric body mucosa. Human leucocyte antigen-DR genotypes suggest a role for genetic susceptibility in PA. PA patients should be managed by cobalamin replacement treatment and monitoring for onset of iron deficiency. Moreover, they should be advised about possible gastrointestinal long-term consequences, such as gastric cancer and carcinoids.
Keywords: Pernicious anemia, Autoimmune diseases, Atrophic gastritis, Intrinsic factor, Autoantibodies, Parietal cells, Vitamin B12 deficiency, Helicobacter pylori
INTRODUCTION
Pernicious anemia (PA) (also known as Biermer’s disease[1] and Addisonian anemia[2]) is a macrocytic anemia due to vitamin B12 (cobalamin) deficiency, which, in turn, is the result of deficiency of intrinsic factor, a protein that binds avidly to dietary vitamin B12 and promotes its transport to the terminal ileum for absorption[3]. The deficiency of intrinsic factor is a consequence of the presence of atrophic body gastritis (ABG), which results in the destruction of the oxyntic mucosa, and thus, the loss of parietal cells, which normally produce chlorhydric acid as well as intrinsic factor[4]. The term PA is sometimes used as synonym for cobalamin deficiency or for macrocytic anemia, but to avoid ambiguity, PA should be reserved for conditions that result from impaired secretion of intrinsic factor and atrophy of oxyntic mucosa[5]. However, differential diagnosis may sometimes be challenging due to the limit of available diagnostic tools.
PA is considered an autoimmune disorder due to the frequent presence of gastric autoantibodies directed against intrinsic factor, as well as against parietal cells. PA is often considered a synonym of autoimmune gastritis, because PA is thought to be the end stage of an autoimmune process that results in severe damage of the oxyntic gastric mucosa[6]. Recent experimental and clinical data strongly suggest an involvement of long-standing Helicobacter pylori (H pylori) infection in the pathogenesis of ABG and PA, but it is still under debate whether PA may be included among the long-term consequences of H pylori gastritis[7].
The present review focuses on novel aspects regarding the pathogenesis, clinical presentation, and diagnosis of PA, as well as the management of PA patients from a gastroenterological point of view.
PA: AN AUTOIMMUNE DISORDER OR AN INFECTIOUS DISEASE?
PA is the end-stage of ABG and is generally considered an autoimmune disease. The autoimmune origin of PA is based on the presence of parietal cell and/or intrinsic factor autoantibodies, and the frequent association with other autoimmune disorders, such as autoimmune thyroid disease (ATD), type 1 diabetes, and vitiligo[6,8].
ABG associated with PA is often called autoimmune gastritis or type A gastritis, which is defined as a type of chronic atrophic gastritis restricted to the body mucosa, characterized by a severe, diffuse atrophy of the oxyntic glands and hypochlorhydria, and a normal antral mucosa[4]. Another classical histological feature of ABG is the absence of H pylori on gastric mucosal biopsies[4]. It is now accepted that long-standing H pylori infection is able to induce atrophy of the gastric mucosa, and H pylori is considered the main causative agent of multifocal atrophic gastritis, in which the antrum is almost invariably involved[9]. Thus, ABG is generally considered a separate entity from H pylori-related atrophic gastritis, mainly because the prevalence of H pylori infection in patients with severe ABG and PA has been found to be low[10,11]. However, in the past few years, the question has been raised whether H pylori may be implicated in the pathogenesis of ABG, and, as a basic mechanism for the induction of gastric autoimmunity by H pylori infection, molecular mimicry has been proposed[7,12,13]. Molecular mimicry is defined as the possibility that sequence similarities between foreign and self-peptides are sufficient to result in the cross-activation of autoreactive T or B cells by pathogen-derived peptides. It is a phenomenon associated with some pathogens in which the antigens that evoke an immune response have enough similarity to the body’s own proteins to cause an autoimmune reaction, such as in rheumatoid arthritis, mediated by cross-reactive T cells and/or circulating antibodies. In fact, gastric H+/K+-ATPase has been recognized as the major autoantigen in experimental and human ABG[14-16], and autoreactive gastric CD4+ T cells that recognize H+/K+-ATPase and H pylori antigens have been recently described in ABG[17,18]. Thus, PA and ABG seem to be an example of pathogen-induced, organ-specific autoimmunity, in which genetic susceptibility plays an important role in relation to the loss of immunological tolerance[18]. In fact, the immunological basis of molecular mimicry lies in the recognition by T-cell antigen receptors of antigenic peptides bound to human leucocyte antigen (HLA) molecules on the surface of antigen-presenting cells, and inappropriate activation of T cells may occur as a result of the upregulation of HLA molecules in genetically susceptible individuals[19]. A specific HLA-DR pattern was suggested in PA patients several years ago[20], and more recently, blocking experiments with anti-DR and anti-DQ antibodies have shown that DR antigen probably represents the HLA restriction element in ABG[17]. By using a DNA-based, sequence-specific oligonucleotide technology, we observed in our series of PA patients that the genotypes HLA-DRB1*03 and DRB1*04, which are known to be associated with other autoimmune disease (such as type 1 diabetes and ATD)[21], were significantly associated with PA, compared to a control group (unpublished data), which supports the idea that genetic susceptibility for autoimmunity may play a role in PA.
Table 1 shows the literature regarding H pylori infection and related gastric histological features in some PA patients[10,22-24]. The presence of H pylori infection was diagnosed by histology in up to 30% (median 11%), but by serology (IgG) in up to 51% (median 20.5%) of PA patients. It is well known that the diagnosis of H pylori infection may be difficult in patients with ABG. H pylori may disappear over time due to the hostile gastric microenvironment, and past infection may be demonstrated by serological positivity to H pylori in a large majority of patients with ABG or PA[10,25-27]. A recent study has reported that seropositivity against H pylori antigens may be demonstrated in a very high percentage of patients with ABG by using ad hoc immunoblotting[28]: in this study 47.8% of ABG patients had PA and all but two of them presented with seropositivity against H pylori antigens, including CagA and VacA. As far as regards histological features of the gastric body, in the vast majority of PA patients (> 70%) this disorder is associated with severe body atrophy and the presence of intestinal metaplasia. From data reported in Table 1, another interesting observation emerges: irrespective of the presence of H pylori infection, in about half of PA patients, the gastric antrum is involved, and about one-third have antral atrophic gastritis, whose presence is strongly related to H pylori infection[9]. This observation challenges the widely accepted notion that PA occurs exclusively in association with the classical histological feature of corpus-restricted atrophic gastritis. All these data taken together support the idea that long-standing H pylori infection probably plays an important role in many genetically susceptible PA patients. In these patients, the active infectious process has been gradually replaced by an autoimmune process directed by autoreactive gastric CD4+ T cells that recognize H+/K+-ATPase and H pylori antigens, which ends in a burned-out infection and the irreversible destruction of the gastric body mucosa. The failure to demonstrate H pylori infection in some of these individuals does not necessarily argue against the role of the bacterium in these patients, but more likely indicates that a point of no return may be reached beyond which the autoimmune process may no longer require the continued presence of the inducing pathogen[29].
Table 1.
H pylori infection and related gastric histological features in a series of PA patients n (%)
| First author/publication year[Ref.] | No. of patients | Mean age (yr) | F:M ratio | Geographical origin | Severe body atrophy | Body intestinal metaplasia | Antral inflammation | Antral atrophic gastritis | Positive H pylori histology | Positive H pylori serology |
| Fong TL/1991[22] | 28 | 59 | 1.2:0.8 | USA1 | ND | 18 (64) | 14 (50) | 2 (7) | 3 (11) | 2 (7) |
| Haruma K/1995[23] | 24 | 65 | 0.9:1.1 | Japan | 24 (100) | 18 (75) | 22 (92) | 17 (71) | 0 (0) | 0 (0) |
| Sari R/2000[24] | 30 | 60 | 0.9:1.1 | Turkey | 15 (50) | 13 (43) | 14 (47) | 8 (27) | 12 (30) | ND |
| Annibale B/2000[10] | 81 | 62 | 0.9:1.1 | Italy | 56 (69) | 70 (86) | 43 (53) | 27 (30) | 8 (10) | 41 (51) |
| Annibale B/2009[unpublished data] | 177 | 60 | 1:1 | Italy | 124 (70) | 161 (91) | 81 (46) | 40 (23) | 19 (11) | 61 (34) |
Hispanic, n = 20; white, n = 3; black, n = 5. ND: Not done.
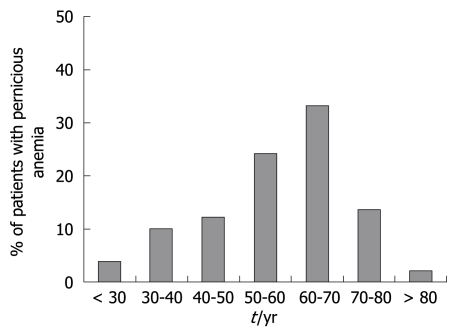
PA is frequently described as a disease of adults > 60 years of age[8,30,31]. Among our unpublished series of 177 PA patients, about one half were < 60 years of age; in particular, 4% of patients were < 30 years and 10% were 30-40 years of age (Figure 1). Table 1 shows that the mean age of PA patients in published studies ranges from 59 to 62 years. These data challenge the common notion that PA is an exclusive disease of the elderly, and suggest that, in clinical practice, PA is probably under-diagnosed in elderly and younger patients[32]. Stratification by age cohorts (< 20 years to > 60 years) of ABG patients identified by hypergastrinemia and positive parietal cell antibodies has shown a regular and progressive increase in mean corpuscular volume and levels of ferritin and gastrin, and a decrease in vitamin B12 levels. However, the prevalence of H pylori infection has decreased from > 80% at age < 20 years to 12.5% at > 60 years[32]. This reminds us that: (1) iron deficiency is a complication of achlorhydria and may precede the development of PA[4]; (2) ABG patients frequently present with iron deficiency anemia[33-35]; and (3) iron deficiency may be present concomitantly with PA[36]. These findings further support the idea that PA seems to be a long-duration disease that is related to H pylori, gastric achlorhydria and atrophy, which begins many years before the establishment of clinical vitamin B12 deficiency.
Figure 1.
Age cohorts of a series of patients with PA (n = 177) consecutively diagnosed between 1992 and 2005 at an academic gastroenterology unit.
CLINICAL PRESENTATION OF PA
The clinical presentation of PA is often insidious for various reasons. The onset and progression of PA are very slow. As a consequence, patients often are not aware of their symptoms related to anemia, because over time they have become used to them. In many such cases, the underlying disease may not be suspected until a complete red blood count has been performed. However, patients with PA may seek medical advice due to non-specific symptoms related to the presence of anemia per se, such as weakness, asthenia, decreased mental concentration, headache, and especially, in elderly patients, cardiological symptoms such as palpitations and chest pain[3,6]. Less frequently, patients with PA may present only with neurological symptoms, such as paresthesia, unsteady gait, clumsiness, and in some cases, spasticity. Indeed, vitamin B12 deficiency may cause peripheral neuropathy and lesions in the posterior and lateral columns of the spinal cord (subacute combined degeneration) and in the cerebrum, and these lesions progress from demyelinization to axonal degeneration and eventual neuronal death. It is particularly important to recognize these symptoms early, because the neurological lesions may not be reversed after replacement therapy with vitamin B12[3,5]. Finally, the onset of PA may be observed in patients undergoing medical treatment for other autoimmune conditions frequently associated with PA, such as ATD, type 1 diabetes, and vitiligo, as part of the autoimmune polyendocrine syndromes[37].
Although the primary cause of PA is ABG, rarely the disease may result from gastrointestinal tract symptoms. The reason for the apparent paradox may lie in the fact that ABG is associated with hypochlorhydria, and symptoms of the upper gastrointestinal tract are often related to the presence of chlorhydric acid. However, hypochlorhydria itself may cause impaired gastric emptying, which eventually leads to dyspeptic symptoms such as epigastric discomfort, postprandial bloating and fullness, and early satiety[38]. In our experience, awareness and concern about upper gastrointestinal or neurological symptoms often are not sufficient to seek medical advice, since patients over time become used to these slowly and insidiously presenting complaints. Only 3% of PA patients presented directly to our gastroenterology unit for long-standing dyspepsia, and only 3% were referred from a neurologist. At the time of diagnosis of PA, dyspeptic symptoms were complained of by 28% of patients, and neurological symptoms were present in 19% (unpublished data).
An increased association of PA with other autoimmune diseases, such as type 1 diabetes (3%-4%)[39], vitiligo (2%-8%)[3], and in particular, ATD (3%-32%)[40] has been reported. Among our unpublished series of 177 PA patients, 41% had associated ATD and 10% presented with vitiligo or alopecia, which indicates that a subgroup of PA patients can be considered as having a type II autoimmune polyendocrine syndrome. In a recent study, we have observed that ABG and ATD occur in a closely linked fashion, with ATD being present in about 40% of ABG patients[41]. These data suggest that, in patients with autoimmune disorders, in particular ATD, a possible association with PA should be suspected and excluded. The diagnosis of concomitant autoimmune thyroiditis and PA may have an important clinical implication, in particular, in those patients who require replacement therapy with thyroxine. Recently, it has been reported that patients with impaired acid secretion may present with thyroxine malabsorption that requires an increased dose of the drug[42], and in patients with PA, associated hypochlorhydria is always present, due to the loss of oxyntic mucosa[4].
Useful information about which patients may have PA can be derived also from epidemiological data. According to the older literature, PA is thought to be particularly common among individuals of Scandinavian, English or Irish ancestry, whereas it appears to be much less common in Caucasians of Italian or Greek origin[43]. However, more recently, the disease have been reported in black and Latin American subjects[30,44], and as shown in Table 1, series of PA patients have been diagnosed in the United States, Turkey and Italy, and even in Japan. The reason for the different distribution of PA among different ethnical groups is not known yet, but probably lies in their different genetic background, and in different awareness and diagnostic accuracy for this often overseen disorder. In the so-called high-risk groups, about nine new cases are detected per 100 000 population per year, and about 0.13% of the population is affected[31]. A more recent population survey has reported that 1.9% of persons aged > 60 years have undiagnosed PA[30].
A female preponderance ranging from 1.7 to 2.0:1 has been reported in white subjects[3]. This sex distribution has been confirmed in the more recent population survey of persons > 60 years old that was conducted in California, in which the prevalence of PA was 2.7% in women and 1.4% in men[30]. However, data reported in Table 1 concerning United States, Japanese, Turkish and Italian PA patients seem not to confirm the female preponderance described in older studies.
DIAGNOSIS OF PA
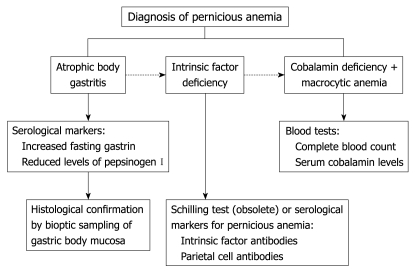
PA is defined as the presence of a hemoglobin concentration < 13 g/dL for men and < 12 g/dL for women[45], mean corpuscular volume ≥ 100 fL[5], low levels of cobalamin (vitamin B12)[5], together with the concomitant presence of ABG and intrinsic factor deficiency (Figure 2). By definition, PA is associated with ABG, and strict diagnostic criteria for ABG are based on the histological confirmation of gastric body mucosal atrophy and enterochromaffin-like (ECL) cell hyperplasia, associated with hypochlorhydria to pentagastrin stimulation[4]. Increased levels of fasting gastrin and decreased levels of pepsinogen I are well accepted serological markers[46,47], which suggest the presence of oxyntic mucosa damage, which should be confirmed, however, by appropriate histological sampling of gastric body mucosa to diagnose ABG definitively.
Figure 2.
Diagnostic flow-chart for PA.
As far as regards gastric mucosa histology, classical findings associated with PA are the presence of corpus-restricted atrophy with a spared antrum, as well as the presence of hyperplasia of ECL cells[4,6]. As shown in Table 1, in about 50% of PA patients, antral mucosa is not spared, and in about 27% of PA patients, a concomitant antral atrophic gastritis may be observed. These data strongly suggest that an extension of gastritis to the gastric antrum does not necessarily exclude the diagnosis of PA and the presence of gastric autoimmunity. The determination of ECL cell hyperplasia is helpful in the histological diagnosis of ABG, because the presence of this histological change may be considered an indirect confirmation of the presence of hypochlorhydria. This leads to hypergastrinemia, which in turn, is a trophic factor for ECL cells that leads to their hyperplasia, and eventually, to the development of gastric carcinoids[48].
Intrinsic factor deficiency can be proven by the now obsolete Schilling test. To confirm that the cobalamin deficiency is the result of intestinal malabsorption due to intrinsic factor deficiency, urinary excretion of orally administered vitamin B12 is low, and is increased by administration of vitamin B12 and intrinsic factor. Unfortunately, the availability of this test is vanishing due to problems related to its radioactive reagents. Therefore, in clinical practice, the presence of intrinsic factor deficiency may not be proven, and increasing reliance is placed on the detection of intrinsic factor antibodies for the diagnosis of PA, which are viewed as useful markers of this disease[49]. Earlier studies have reported positivity for intrinsic factor antibodies in 40%-60% of patients with PA[50,51], which rises to 60%-80% with increasing duration of disease[52].
Recently, we reassessed the diagnostic performance of intrinsic factor and parietal cell antibodies in PA patients by using a novel ELISA[48], which yielded for intrinsic factor antibodies, a sensitivity and specificity of 37% and 100%, respectively, and for parietal cell antibodies, a sensitivity and specificity of 81.5% and 90.3%, respectively. The combined assessment of both autoantibodies significantly increased their diagnostic performance, which yielded 73% sensitivity for PA, while maintaining 100% specificity. Thus, our data show that, by combining the assessment of intrinsic factor and parietal cell autoantibodies, the diagnostic performance of these surrogate markers for PA may be notably improved. Beyond being a specific hallmark of PA, the positivity for intrinsic factor and parietal cell antibodies may be interpreted as an expression of oxyntic mucosal damage, because a positive correlation between the increasing histological score of body mucosa atrophy and the titer of both antibodies can be observed[27,35].
Accurate differential diagnosis of other causes of cobalamin deficiency is mandatory. As shown in Table 2, cobalamin deficiency may result from other causes of impaired absorption in the stomach or intestine, or by decreased intake due to vegetarianism. Among cases of mal-digestion, there are very rare cases related to severe pancreatic insufficiency, but more interesting is the recent evidence of mal-digestion of dietary cobalamin in patients with corpus-predominant H pylori gastritis, which leads to impaired acid secretion and consequent increased intragastric pH[53,54]. In fact, dietary cobalamin is bound to salivary proteins, which needs to be cleaved in the presence of chlorhydric acid before it can be bound to intrinsic factor and be absorbed in the terminal ileum[4]. In these cases of mal-digestion of dietary cobalamin, the Schilling test would be normal, which indicates that cobalamin deficiency is not due to intrinsic factor deficiency. Without performing a Schilling test, it may be challenging to discriminate between the presence of PA and mal-digestion of dietary cobalamin. However, from a practical point of view, the clinical management of these two groups of patients is similar. As observed[54,55], when atrophy of the gastric body mucosa is mild and active H pylori infection is present, in patients with mal-digestion of dietary cobalamin, a reversal of body mucosal atrophy, anemia and cobalamin deficiency following eradication treatment may be achieved. An accurate differential diagnosis should be carried out also for macrocytic anemia, which may underlie other causes such as folate deficiency and myelodysplastic syndrome (Table 2).
Table 2.
Differential diagnosis of PA: other causes of macrocytic anemia and cobalamin deficiency
| Other causes of macrocytic anemia | Other causes of cobalamin deficiency |
| Folate deficiency due to decreased intake, impaired absorption or increased requirements | Gastric causes of impaired absorption/mal-digestion: |
| Drugs (e.g. methotrexate, azathioprine, 6-mercaptopurine, acyclovir, 5-fluorouracil, phenobarbital) | Gastrectomy |
| Accelerated erythropoiesis: hemolytic anemia, response to hemorrhage | Corpus-predominant H pylori gastritis |
| Liver disease (alcoholic, advance cirrhosis, poor dietary intake) | Long-term proton pump inhibitor therapy |
| Hypoplastic anemia, myelodysplastic syndrome | Intestinal causes of impaired absorption: |
| Chronic obstructive pulmonary disease | Ileal disease or resection |
| Blind loop syndrome | |
| Fish tapeworm | |
| Severe pancreatic insufficiency | |
| Decreased intake due to vegetarianism |
In this context, it should be kept in mind that, in order to diagnose vitamin B12 deficiency, total vitamin B12 measurement is used cost-effectively as the parameter of choice, but it has limited sensitivity and specificity, especially in persons with vitamin B12 concentrations in the lower reference range (< 400 pmol/L). As an alternative, modern biomarkers for early diagnosis of vitamin B12 deficiency, such as holotranscobalamin, also known as active B12, and methyl malonic acid as a functional B12 marker, have been proposed[56]. Figure 2 shows a proposed diagnostic work-up when the presence of PA is suspected.
CLINICAL MANAGEMENT OF PATIENTS WITH PA
The clinical management of patients with PA has two different aspects: firstly, the treatment of cobalamin deficiency and the monitoring of onset of iron deficiency; and secondly, the surveillance of these patients, to detect early the long-term consequences of PA, such as gastric cancer and carcinoids.
Treatment of cobalamin deficiency and monitoring of iron deficiency
Cobalamin replacement treatment is able to correct the anemia, whereas the neurological complications may be corrected only if the replacement treatment is given soon after their onset. The therapeutic recommendations for PA with regard to dosage and administration of vitamin B12 substitution treatment are divergent[57]. In the United States, patients usually receive vitamin B12 injections of 1 mg/d in their first week of treatment; in the following month, they receive weekly injections and then monthly injections[58]. In Denmark, patients receive injections of 1 mg/wk cyanocobalamin during the first month and every 3 mo subsequently, or 1 mg hydroxycobalamin every other month[59]. According to our protocol, a higher dosage of cobalamin is used to prevent early relapse of cobalamin deficiency: firstly patients receive intramuscular injection of 5 mg/d cyanocobalamin for 5 d, which replenishes the cobalamin body stores; then, vitamin B12 stores are maintained by intramuscular injection of 5 mg cyanocobalamin every 3 mo.
Furthermore, according to our protocol, PA patients are monitored at least yearly by complete blood count, and serum cobalamin and ferritin levels, to monitor the replacement treatment and to detect early the eventual onset of iron deficiency. Also patients with ABG with iron deficiency anemia or without hematological alterations are monitored in the same way, to detect early the eventual onset of cobalamin deficiency. Finally, PA patients are monitored by at least a yearly clinical interview, to verify the onset of new symptoms that are suspicious of long-term consequences of PA, such as dysphagia, epigastric pain, dyspeptic symptoms, loss of body weight, and/or iron-deficiency, which require immediate gastroscopic investigation.
Long-term consequences of PA
Although PA is substantially a benign disorder for a large number of patients, it is epidemiologically and biologically linked to the development of intestinal-type gastric adenocarcinoma and gastric carcinoid type I[60,61]. Hypergastrinemia, secondary to hypochlorhydria in PA patients, is a well-known risk factor for ECL cell hyperplasia and gastric carcinoids[62,63], and it has been reported that one in 25 patients with PA develops gastric carcinoids[64]. Moreover, the crucial role of hypochlorhydria, as a consequence of atrophy of the oxyntic mucosa, in the development of gastric cancer, has been highlighted[65]. Hypochlorhydria leads to overgrowth of nitrosamine-producing bacteria with potential carcinogen activity[66]. Also ascorbic acid, the main redox agent in the gastric juice with protective action against reactive oxygen species, is reduced in the presence of atrophy of the oxyntic mucosa. It has been described previously that the level of ascorbic acid in the gastric juice is reduced in patients with ABG, with an indirect correlation between ascorbic acid level and intragastric pH[67].
In the literature, the annual incidence of gastric cancer in PA patients ranges from 0.1% to 0.5%[62,64,68]. A recent follow-up study of patients with ABG has reported an annual incidence risk of 0.14% for developing gastric cancer, during an observation period of 6.7 years[69]. To date, the need and cost-effectiveness of endoscopic/histological surveillance in patients with PA have not been established definitively[4]. One previous study[64] that has considered the relatively benign nature of gastric carcinoids in patients with PA has concluded that follow-up is indicated at 5-year intervals only in patients with ECL hyperplasia. As for gastric cancer, the same authors have concluded that the first gastroscopic follow-up after diagnosis of PA should be performed relatively soon, and that only PA patients with preneoplastic lesions and those with gastrointestinal symptoms should undergo endoscopic surveillance[64]. Another study has concluded that follow-up should be performed at 3-year intervals only in PA patients aged < 60 years[70]. A more recent study has compared the usefulness of 2- and 4-year follow-up in patients with ABG, and has shown that the first follow-up performed 4 years after the diagnosis seems to be safe and convenient for early detection of potentially neoplastic lesions[71]. As a result of the lack of other prospective data, and considering the risk for developing neoplastic lesions over time in some PA patients, in our unit, PA patients are monitored regularly by gastroscopy with antral and corporal biopsies at 4-year intervals.
CONCLUSION
PA is an often silent and under-diagnosed autoimmune disease, because its onset and progression are very slow and patients may become used to their complaints. Nevertheless, the clinical consequences of undiagnosed PA may be serious, including gastric neoplastic lesions. Thus, gastroenterologists should increase their awareness of this disorder, whose definite histological diagnosis may be preceded by reliable noninvasive serological screening.
Footnotes
Supported by Funds of the Italian Ministry for University and Research (PRIN 2007) and by funds of the University “La Sapienza”, Rome, Italy
Peer reviewer: Alberto Piperno, Professor, Department of Clinical Medicine and Prevention, Clinical Medicine, San Gerardo Hospital, Via Pergolesi 33, 20052, Monza, Italy
S- Editor Li LF L- Editor Kerr C E- Editor Zheng XM
References
- 1.Zittoun J. [Biermer's disease] Rev Prat. 2001;51:1542–1546. [PubMed] [Google Scholar]
- 2.Meecham J, Jones EW. Addison's disease and Addisonian anaemia. Lancet. 1967;1:535–538. doi: 10.1016/s0140-6736(67)92114-9. [DOI] [PubMed] [Google Scholar]
- 3.Wintrobe MM, Lee GR, Boggs DR, Bithell TC, Foerster J, Athens JW, Lukens JN. Megaloblastic and nonmegaloblastic macrocytic anemias. In: Wintrobe MM, Lee GR, Boggs DR, Bithell TC, Foerster J, et al., editors. Clinical hematology. 8th ed. Philadelphia: Lea & Febiger; 1981. pp. 559–604. [Google Scholar]
- 4.Lee EL, Feldman M. Gastritis and other gastropathies. In: Feldman M, Friedman LS, Sleisenger MH, et al., editors. Sleisenger & Fordtran's gastrointestinal and liver disease: pathophysiology, diagnosis, management. 7th ed. Philadelphia: Saunders; 2002. pp. 810–827. [Google Scholar]
- 5.Babior BM. Erythrocyte disorders: Anemias related to disturbance of DNA synthesis (megaloblastic anemias) In: Williams JW, Beutler E, Erslev AJ, Lichtman MA, et al., editors. Hematology. 4th ed. New York: McGraw-Hill; 1998. pp. 453–481. [Google Scholar]
- 6.Toh BH, Gleeson PA, Whittingham S, van Driel IR. Autoimmune gastritis and pernicious anemia. In: Rose NR, Mackay IR, et al., editors. The autoimmune diseases. 3rd ed. St. Louis, MO: Academic Press; 1998. pp. 459–476. [Google Scholar]
- 7.D'Elios MM, Appelmelk BJ, Amedei A, Bergman MP, Del Prete G. Gastric autoimmunity: the role of Helicobacter pylori and molecular mimicry. Trends Mol Med. 2004;10:316–323. doi: 10.1016/j.molmed.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 8.Toh BH, van Driel IR, Gleeson PA. Pernicious anemia. N Engl J Med. 1997;337:1441–1448. doi: 10.1056/NEJM199711133372007. [DOI] [PubMed] [Google Scholar]
- 9.Peterson WL, Graham DY. Helicobacter pylori. In: Feldman M, Friedman LS, Sleisenger MH, et al., editors. Sleisenger & Fordtran’s gastrointestinal and liver disease: pathophysiology, diagnosis, management. 7th ed. Philadelphia: Saunders; 2002. pp. 732–746. [Google Scholar]
- 10.Annibale B, Lahner E, Bordi C, Martino G, Caruana P, Grossi C, Negrini R, Delle Fave G. Role of Helicobacter pylori infection in pernicious anaemia. Dig Liver Dis. 2000;32:756–762. doi: 10.1016/s1590-8658(00)80351-5. [DOI] [PubMed] [Google Scholar]
- 11.Varis O, Valle J, Siurala M. Is Helicobacter pylori involved in the pathogenesis of the gastritis characteristic of pernicious anaemia? Comparison between pernicious anaemia relatives and duodenal ulcer relatives. Scand J Gastroenterol. 1993;28:705–708. doi: 10.3109/00365529309098277. [DOI] [PubMed] [Google Scholar]
- 12.Appelmelk BJ, Faller G, Claeys D, Kirchner T, Vandenbroucke-Grauls CM. Bugs on trial: the case of Helicobacter pylori and autoimmunity. Immunol Today. 1998;19:296–299. doi: 10.1016/s0167-5699(98)01281-x. [DOI] [PubMed] [Google Scholar]
- 13.Field J, Biondo MA, Murphy K, Alderuccio F, Toh BH. Experimental autoimmune gastritis: mouse models of human organ-specific autoimmune disease. Int Rev Immunol. 2005;24:93–110. doi: 10.1080/08830180590884585. [DOI] [PubMed] [Google Scholar]
- 14.Toh BH, van Driel IR, Gleeson PA. Autoimmune gastritis: tolerance and autoimmunity to the gastric H+/K+ ATPase (proton pump) Autoimmunity. 1992;13:165–172. doi: 10.3109/08916939209001918. [DOI] [PubMed] [Google Scholar]
- 15.Ma JY, Borch K, Mårdh S. Human gastric H,K-adenosine triphosphatase beta-subunit is a major autoantigen in atrophic corpus gastritis. Expression of the recombinant human glycoprotein in insect cells. Scand J Gastroenterol. 1994;29:790–794. doi: 10.3109/00365529409092512. [DOI] [PubMed] [Google Scholar]
- 16.Claeys D, Faller G, Appelmelk BJ, Negrini R, Kirchner T. The gastric H+,K+-ATPase is a major autoantigen in chronic Helicobacter pylori gastritis with body mucosa atrophy. Gastroenterology. 1998;115:340–347. doi: 10.1016/s0016-5085(98)70200-8. [DOI] [PubMed] [Google Scholar]
- 17.Amedei A, Bergman MP, Appelmelk BJ, Azzurri A, Benagiano M, Tamburini C, van der Zee R, Telford JL, Vandenbroucke-Grauls CM, D'Elios MM, et al. Molecular mimicry between Helicobacter pylori antigens and H+, K+ --adenosine triphosphatase in human gastric autoimmunity. J Exp Med. 2003;198:1147–1156. doi: 10.1084/jem.20030530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.D'Elios MM, Amedei A, Azzurri A, Benagiano M, Del Prete G, Bergman MP, Vandenbroucke-Grauls CM, Appelmelk BJ. Molecular specificity and functional properties of autoreactive T-cell response in human gastric autoimmunity. Int Rev Immunol. 2005;24:111–122. doi: 10.1080/08830180590884611. [DOI] [PubMed] [Google Scholar]
- 19.Albert LJ, Inman RD. Molecular mimicry and autoimmunity. N Engl J Med. 1999;341:2068–2074. doi: 10.1056/NEJM199912303412707. [DOI] [PubMed] [Google Scholar]
- 20.Ungar B, Mathews JD, Tait BD, Cowling DC. HLA-DR patterns in pernicious anaemia. Br Med J. 1981;282:768–770. doi: 10.1136/bmj.282.6266.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fernando MM, Stevens CR, Walsh EC, De Jager PL, Goyette P, Plenge RM, Vyse TJ, Rioux JD. Defining the role of the MHC in autoimmunity: a review and pooled analysis. PLoS Genet. 2008;4:e1000024. doi: 10.1371/journal.pgen.1000024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fong TL, Dooley CP, Dehesa M, Cohen H, Carmel R, Fitzgibbons PL, Perez-Perez GI, Blaser MJ. Helicobacter pylori infection in pernicious anemia: a prospective controlled study. Gastroenterology. 1991;100:328–332. doi: 10.1016/0016-5085(91)90199-u. [DOI] [PubMed] [Google Scholar]
- 23.Haruma K, Komoto K, Kawaguchi H, Okamoto S, Yoshihara M, Sumii K, Kajiyama G. Pernicious anemia and Helicobacter pylori infection in Japan: evaluation in a country with a high prevalence of infection. Am J Gastroenterol. 1995;90:1107–1110. [PubMed] [Google Scholar]
- 24.Sari R, Ozen S, Aydogdu I, Yildirim B, Sevinc A. The pathological examinations of gastric mucosa in patients with Helicobacter pylori-positive and -negative pernicious anemia. Helicobacter. 2000;5:215–221. doi: 10.1046/j.1523-5378.2000.00033.x. [DOI] [PubMed] [Google Scholar]
- 25.Valle J, Kekki M, Sipponen P, Ihamäki T, Siurala M. Long-term course and consequences of Helicobacter pylori gastritis. Results of a 32-year follow-up study. Scand J Gastroenterol. 1996;31:546–550. doi: 10.3109/00365529609009126. [DOI] [PubMed] [Google Scholar]
- 26.Karnes WE Jr, Samloff IM, Siurala M, Kekki M, Sipponen P, Kim SW, Walsh JH. Positive serum antibody and negative tissue staining for Helicobacter pylori in subjects with atrophic body gastritis. Gastroenterology. 1991;101:167–174. doi: 10.1016/0016-5085(91)90474-y. [DOI] [PubMed] [Google Scholar]
- 27.Annibale B, Negrini R, Caruana P, Lahner E, Grossi C, Bordi C, Delle Fave G. Two-thirds of atrophic body gastritis patients have evidence of Helicobacter pylori infection. Helicobacter. 2001;6:225–233. doi: 10.1046/j.1083-4389.2001.00032.x. [DOI] [PubMed] [Google Scholar]
- 28.Annibale B, Lahner E, Santucci A, Vaira D, Pasquali A, Severi C, Mini R, Figura N, Delle Fave G. CagA and VacA are immunoblot markers of past Helicobacter pylori infection in atrophic body gastritis. Helicobacter. 2007;12:23–30. doi: 10.1111/j.1523-5378.2007.00467.x. [DOI] [PubMed] [Google Scholar]
- 29.Rose NR, Bona C. Defining criteria for autoimmune diseases (Witebsky's postulates revisited) Immunol Today. 1993;14:426–430. doi: 10.1016/0167-5699(93)90244-F. [DOI] [PubMed] [Google Scholar]
- 30.Carmel R. Prevalence of undiagnosed pernicious anemia in the elderly. Arch Intern Med. 1996;156:1097–1100. [PubMed] [Google Scholar]
- 31.Pedersen AB, Mosbech J. Morbidity of pernicious anaemia. Incidence, prevalence, and treatment in a Danish county. Acta Med Scand. 1969;185:449–452. [PubMed] [Google Scholar]
- 32.Hershko C, Ronson A, Souroujon M, Maschler I, Heyd J, Patz J. Variable hematologic presentation of autoimmune gastritis: age-related progression from iron deficiency to cobalamin depletion. Blood. 2006;107:1673–1679. doi: 10.1182/blood-2005-09-3534. [DOI] [PubMed] [Google Scholar]
- 33.Annibale B, Capurso G, Chistolini A, D'Ambra G, DiGiulio E, Monarca B, DelleFave G. Gastrointestinal causes of refractory iron deficiency anemia in patients without gastrointestinal symptoms. Am J Med. 2001;111:439–445. doi: 10.1016/s0002-9343(01)00883-x. [DOI] [PubMed] [Google Scholar]
- 34.Marignani M, Delle Fave G, Mecarocci S, Bordi C, Angeletti S, D'Ambra G, Aprile MR, Corleto VD, Monarca B, Annibale B. High prevalence of atrophic body gastritis in patients with unexplained microcytic and macrocytic anemia: a prospective screening study. Am J Gastroenterol. 1999;94:766–772. doi: 10.1111/j.1572-0241.1999.00949.x. [DOI] [PubMed] [Google Scholar]
- 35.Annibale B, Lahner E, Negrini R, Baccini F, Bordi C, Monarca B, Delle Fave G. Lack of specific association between gastric autoimmunity hallmarks and clinical presentations of atrophic body gastritis. World J Gastroenterol. 2005;11:5351–5357. doi: 10.3748/wjg.v11.i34.5351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carmel R, Weiner JM, Johnson CS. Iron deficiency occurs frequently in patients with pernicious anemia. JAMA. 1987;257:1081–1083. [PubMed] [Google Scholar]
- 37.Eisenbarth GS, Gottlieb PA. Autoimmune polyendocrine syndromes. N Engl J Med. 2004;350:2068–2079. doi: 10.1056/NEJMra030158. [DOI] [PubMed] [Google Scholar]
- 38.Tosetti C, Stanghellini V, Tucci A, Poli L, Salvioli B, Biasco G, Paparo GF, Levorato M, Corinaldesi R. Gastric emptying and dyspeptic symptoms in patients with nonautoimmune fundic atrophic gastritis. Dig Dis Sci. 2000;45:252–257. doi: 10.1023/a:1005439905134. [DOI] [PubMed] [Google Scholar]
- 39.De Block CE, De Leeuw IH, Van Gaal LF. Autoimmune gastritis in type 1 diabetes: a clinically oriented review. J Clin Endocrinol Metab. 2008;93:363–371. doi: 10.1210/jc.2007-2134. [DOI] [PubMed] [Google Scholar]
- 40.Feldt-Rasmussen U, Bech K, Bliddal H, Høier-Madsen M, Jørgensen F, Kappelgaard E, Nielsen H, Lanng Nielsen J, Ryder LP, Thomsen M. Autoantibodies, immune complexes and HLA-D in thyrogastric autoimmunity. Tissue Antigens. 1983;22:342–347. doi: 10.1111/j.1399-0039.1983.tb02263.x. [DOI] [PubMed] [Google Scholar]
- 41.Lahner E, Centanni M, Agnello G, Gargano L, Vannella L, Iannoni C, Delle Fave G, Annibale B. Occurrence and risk factors for autoimmune thyroid disease in patients with atrophic body gastritis. Am J Med. 2008;121:136–141. doi: 10.1016/j.amjmed.2007.09.025. [DOI] [PubMed] [Google Scholar]
- 42.Centanni M, Gargano L, Canettieri G, Viceconti N, Franchi A, Delle Fave G, Annibale B. Thyroxine in goiter, Helicobacter pylori infection, and chronic gastritis. N Engl J Med. 2006;354:1787–1795. doi: 10.1056/NEJMoa043903. [DOI] [PubMed] [Google Scholar]
- 43.Friedlander RD. Racial factor in pernicious anaemia. Am J Med Sci. 1934;187:634. [Google Scholar]
- 44.Carmel R, Johnson CS. Racial patterns in pernicious anemia. Early age at onset and increased frequency of intrinsic-factor antibody in black women. N Engl J Med. 1978;298:647–650. doi: 10.1056/NEJM197803232981203. [DOI] [PubMed] [Google Scholar]
- 45.WHO/UNICEF/UNU. Iron deficiency anaemia: assesment, prevention, and control. Vol. 298. Geneva: World Health Organization; 2001. p. (WHO/NHD/01.3). [Google Scholar]
- 46.Kekki M, Samloff IM, Varis K, Ihamäki T. Serum pepsinogen I and serum gastrin in the screening of severe atrophic corpus gastritis. Scand J Gastroenterol Suppl. 1991;186:109–116. doi: 10.3109/00365529109103997. [DOI] [PubMed] [Google Scholar]
- 47.Väänänen H, Vauhkonen M, Helske T, Kääriäinen I, Rasmussen M, Tunturi-Hihnala H, Koskenpato J, Sotka M, Turunen M, Sandström R, et al. Non-endoscopic diagnosis of atrophic gastritis with a blood test. Correlation between gastric histology and serum levels of gastrin-17 and pepsinogen I: a multicentre study. Eur J Gastroenterol Hepatol. 2003;15:885–891. doi: 10.1097/00042737-200308000-00009. [DOI] [PubMed] [Google Scholar]
- 48.Lahner E, Norman GL, Severi C, Encabo S, Shums Z, Vannella L, Fave GD, Annibale B. Reassessment of intrinsic factor and parietal cell autoantibodies in atrophic gastritis with respect to cobalamin deficiency. Am J Gastroenterol. 2009;104:2071–2079. doi: 10.1038/ajg.2009.231. [DOI] [PubMed] [Google Scholar]
- 49.Toh BH, Alderuccio F. Parietal cell and intrinsic factor autoantibodies. In: Shoenfeld Y, Gershwin ME, Meroni PL, et al., editors. Autoantibodies. 2nd ed. Amsterdam: Elsevier; 2007. pp. 479–486. [Google Scholar]
- 50.Ungar B, Whittingham S, Francis CM. Pernicious anaemia: incidence and significance of circulating antibodies to intrinsic factor and to parietal cells. Australas Ann Med. 1967;16:226–229. doi: 10.1111/imj.1967.16.3.226. [DOI] [PubMed] [Google Scholar]
- 51.Davidson RJ, Atrah HI, Sewell HF. Longitudinal study of circulating gastric antibodies in pernicious anaemia. J Clin Pathol. 1989;42:1092–1095. doi: 10.1136/jcp.42.10.1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Carmel R. Reassessment of the relative prevalences of antibodies to gastric parietal cell and to intrinsic factor in patients with pernicious anaemia: influence of patient age and race. Clin Exp Immunol. 1992;89:74–77. doi: 10.1111/j.1365-2249.1992.tb06880.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Carmel R, Perez-Perez GI, Blaser MJ. Helicobacter pylori infection and food-cobalamin malabsorption. Dig Dis Sci. 1994;39:309–314. doi: 10.1007/BF02090202. [DOI] [PubMed] [Google Scholar]
- 54.Cohen H, Weinstein WM, Carmel R. Heterogeneity of gastric histology and function in food cobalamin malabsorption: absence of atrophic gastritis and achlorhydria in some patients with severe malabsorption. Gut. 2000;47:638–645. doi: 10.1136/gut.47.5.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Annibale B, Capurso G, Delle Fave G. Consequences of Helicobacter pylori infection on the absorption of micronutrients. Dig Liver Dis. 2002;34 Suppl 2:S72–S77. doi: 10.1016/s1590-8658(02)80170-0. [DOI] [PubMed] [Google Scholar]
- 56.Herrmann W, Obeid R. Causes and early diagnosis of vitamin B12 deficiency. Dtsch Arztebl Int. 2008;105:680–685. doi: 10.3238/arztebl.2008.0680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hvas AM, Nexo E. Diagnosis and treatment of vitamin B12 deficiency--an update. Haematologica. 2006;91:1506–1512. [PubMed] [Google Scholar]
- 58.Pruthi RK, Tefferi A. Pernicious anemia revisited. Mayo Clin Proc. 1994;69:144–150. doi: 10.1016/s0025-6196(12)61041-6. [DOI] [PubMed] [Google Scholar]
- 59.Bastrup-Madsen P, Helleberg-Rasmussen I, Nørregaard S, Halver B, Hansen T. Long term therapy of pernicious anaemia with the depot cobalamin preparation betolvex. Scand J Haematol. 1983;31:57–62. doi: 10.1111/j.1600-0609.1983.tb02137.x. [DOI] [PubMed] [Google Scholar]
- 60.Correa P. Human gastric carcinogenesis: a multistep and multifactorial process--First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer Res. 1992;52:6735–6740. [PubMed] [Google Scholar]
- 61.Wu MS, Chen CJ, Lin JT. Host-environment interactions: their impact on progression from gastric inflammation to carcinogenesis and on development of new approaches to prevent and treat gastric cancer. Cancer Epidemiol Biomarkers Prev. 2005;14:1878–1882. doi: 10.1158/1055-9965.EPI-04-0792. [DOI] [PubMed] [Google Scholar]
- 62.Sjöblom SM, Sipponen P, Miettinen M, Karonen SL, Jrvinen HJ. Gastroscopic screening for gastric carcinoids and carcinoma in pernicious anemia. Endoscopy. 1988;20:52–56. doi: 10.1055/s-2007-1018130. [DOI] [PubMed] [Google Scholar]
- 63.Annibale B, Azzoni C, Corleto VD, di Giulio E, Caruana P, D'Ambra G, Bordi C, Delle Fave G. Atrophic body gastritis patients with enterochromaffin-like cell dysplasia are at increased risk for the development of type I gastric carcinoid. Eur J Gastroenterol Hepatol. 2001;13:1449–1456. doi: 10.1097/00042737-200112000-00008. [DOI] [PubMed] [Google Scholar]
- 64.Kokkola A, Sjöblom SM, Haapiainen R, Sipponen P, Puolakkainen P, Järvinen H. The risk of gastric carcinoma and carcinoid tumours in patients with pernicious anaemia. A prospective follow-up study. Scand J Gastroenterol. 1998;33:88–92. doi: 10.1080/00365529850166266. [DOI] [PubMed] [Google Scholar]
- 65.Uemura N, Okamoto S, Yamamoto S, Matsumura N, Yamaguchi S, Yamakido M, Taniyama K, Sasaki N, Schlemper RJ. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med. 2001;345:784–789. doi: 10.1056/NEJMoa001999. [DOI] [PubMed] [Google Scholar]
- 66.Eisenbrand G, Adam B, Peter M, Malfertheiner P, Schlag P. Formation of nitrite in gastric juice of patients with various gastric disorders after ingestion of a standard dose of nitrate--a possible risk factor in gastric carcinogenesis. IARC Sci Publ. 1984;345:963–968. [PubMed] [Google Scholar]
- 67.Annibale B, Capurso G, Lahner E, Passi S, Ricci R, Maggio F, Delle Fave G. Concomitant alterations in intragastric pH and ascorbic acid concentration in patients with Helicobacter pylori gastritis and associated iron deficiency anaemia. Gut. 2003;52:496–501. doi: 10.1136/gut.52.4.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schafer LW, Larson DE, Melton LJ 3rd, Higgins JA, Zinsmeister AR. Risk of development of gastric carcinoma in patients with pernicious anemia: a population-based study in Rochester, Minnesota. Mayo Clin Proc. 1985;60:444–448. doi: 10.1016/s0025-6196(12)60867-2. [DOI] [PubMed] [Google Scholar]
- 69.Lahner E, Bordi C, Cattaruzza MS, Iannoni C, Milione M, Delle Fave G, Annibale B. Long-term follow-up in atrophic body gastritis patients: atrophy and intestinal metaplasia are persistent lesions irrespective of Helicobacter pylori infection. Aliment Pharmacol Ther. 2005;22:471–481. doi: 10.1111/j.1365-2036.2005.02582.x. [DOI] [PubMed] [Google Scholar]
- 70.Sjöblom SM, Sipponen P, Järvinen H. Gastroscopic follow up of pernicious anaemia patients. Gut. 1993;34:28–32. doi: 10.1136/gut.34.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lahner E, Caruana P, D'Ambra G, Ferraro G, Di Giulio E, Delle Fave G, Bordi C, Annibale B. First endoscopic-histologic follow-up in patients with body-predominant atrophic gastritis: when should it be done? Gastrointest Endosc. 2001;53:443–448. doi: 10.1067/mge.2001.112189. [DOI] [PubMed] [Google Scholar]