Abstract
Genetic and biochemical studies have led to the identification of the Stat3-Interacting Protein StIP1. The preferential association of StIP1 with inactive (i.e., unphosphorylated) Stat3 suggests that it may contribute to the regulation of Stat3 activation. Consistent with this possibility, StIP1 also exhibits an affinity for members of the Janus kinase family. Overexpression of the Stat3-binding domain of StIP1 blocks Stat3 activation, nuclear translocation, and Stat3-dependent induction of a reporter gene. These studies indicate that StIP1 regulates the ligand-dependent activation of Stat3, potentially by serving as a scaffold protein that promotes the interaction between Janus kinases and their Stat3 substrate. The ability of StIP1 to associate with several additional members of the signal transducer and activator of transcription family suggests that StIP1 may serve a broader role in cytokine-signaling events.
The ability of IFN-α to rapidly induce new genes led to the identification of the signal transducer and activator of transcription (STAT) family of transcription factors. Subsequent studies determined that IL-6, as well as all other cytokines, transduce vital signals through members of the STAT family (1, 2). On binding ligand, receptor-associated kinases from the Janus kinase (JAK) family become activated. STATs are in turn recruited to the receptor, where they become activated in a JAK-dependent tyrosine phosphorylation event. Activated STATs are released from the receptor and dimerize. These dimers then translocate to the nucleus and bind to members of the interferon-gamma activation site (GAS) family of enhancers.
One member of the STAT family, Stat3, has been implicated in a wide range of biological processes including nephrogenesis, gliogenesis, hepatogenesis, T cell proliferation, inflammation, and oncogenesis (3–9). Many of these Stat3-dependent responses are triggered by members of the IL-6 family of cytokines, which transduce their signals through a common gp130 receptor chain. Consistent with these pleiotropic effects, Stat3 knockout mice exhibit an embryonic lethal phenotype (10). More recent studies have begun to identify additional molecules that regulate Stat3 signaling. Some of these molecules potentiate the transcriptional activity of Stat3 (11, 12) whereas others antagonize Stat3-dependent signals (13, 14).
Stat3 consists of several conserved domains that are important for STAT function. This includes well-characterized DNA binding, linker, Src homology 2, and transcriptional activation domains (15, 16). In addition, there are two well-conserved amino-terminal domains whose functions are less well characterized. The more amino proximal domain has been shown to promote cooperativity in DNA binding (17). The larger and more amino distal domain has been shown to form a coiled coil that projects laterally from the core STAT structure (15, 16). This generates a large exposed surface area. Consistent with this, several recent studies have identified proteins that interact with the coiled coil of Stat3 and/or other STATs (12, 18–20).
In an effort to characterize the conserved coiled-coil domain in Stat3, a yeast two-hybrid screen was performed. This led to identification of StIP1. This protein encodes 12 WD40 repeats, which are known to mediate protein–protein interactions (21). Biochemical studies suggest that StIP1 may regulate the activation of Stat3 as well as other STATs.
Materials and Methods
Cell Culture and Transfections.
293T, M1, HepG2, NIH 3T3, and SF9 cells were acquired from the American Type Culture Collection and grown as reported (22–24). Before stimulation with IL-6 (15 ng/ml; PeproTech, Rocky Hill, NJ) or IFN-γ (66 units/ml for 15 min; Pestka Research Labs, New Brunswick, NJ), cells were starved in 0.1% serum. SF9 insect cells were infected with a baculovirus encoding Stat3 and Jak1 as described (22). HepG2 and 293T cells were transfected by the calcium phosphate method after a 5-min pretreatment with 25 mM Chloroquine (Sigma) as described (24). The cells were stimulated with IL-6 24 h later.
Yeast Two-Hybrid Assay.
Two-hybrid screens were performed as described in Y153 cells with a “bait” plasmid (pAS-Stat3) encoding amino acids 103–330 of murine Stat3 (25, 26). A mouse myelomonocytic cDNA library (cloned into pGADNOT) served as the “prey” (a generous gift from S. Goff, Columbia University, New York; ref. 27). His+/β-galactosidase+ prey plasmids underwent secondary screens, including retransformation into Y153 cells with pAS1 (empty vector) or a Gal4-Cyclophillin fusion (a generous gift from J. Luban, Columbia University, New York) to eliminate false positives. To confirm interactions, prey plasmids were retransformed into CTY10–5d cells along with a bait plasmid, where the DNA-binding domain had been changed to that of LexA (pSH2–1; ref. 28).
DNA and RNA.
Full-length StIP1 cDNAs were isolated from a murine library (25). StIP-1 glutathione S-transferase (GST) fusion proteins were generated by cloning the appropriate restriction fragments into pGEX-2T and confirmed by sequencing. GST-StIP1A contains the original sequence identified in the yeast two-hybrid screen (StIP1 amino acids 511–831; Fig. 1 and see Fig. 3D). Additionally, GST-StIP1 fusion proteins included: GST-StIP1B, amino acids 511–724; GST-StIP1C, amino acids 511–683; GST-StIP1D, amino acids 554–683; GST-StIP1E, amino acids 554–634; GST-StIP1G, amino acids 254–593; and GST-StIP1H, amino acids 254–395. GST-StIP1G and GST-StIP1H were derived from the cDNA missing WD40 repeat number 7. A dominant-negative StIP1 mutant, pCGN0.8 (amino acids 554–789) was amino terminally hemagglutinin-tagged (pCGN, a generous gift from S. Goff, Columbia University, New York; ref. 27). RcCMV-Stat3 and pMT2T-Jak1K896R (“kinase dead”) expression constructs were generous gifts from J. Darnell (The Rockefeller University, New York) (25) and J. Krolewski (University of California, Irvine) (29). RNA was prepared by guanidium thiocyanate lysis (30). For reverse transcription–PCR, RNA was reverse transcribed (AMV-RT; Roche Molecular Biochemicals) with a StIP1-specific oligonucleotide primer corresponding to amino acids 384–391 and then amplified with primers flanking WD40 repeat number 7 (i.e., amino acids 296–305 and 378–383; see Fig. 3D).
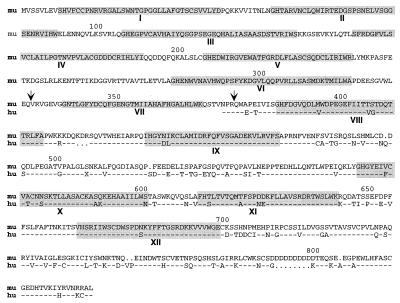
Figure 1.
The StIP1 sequence. Comparison of conceptually translated murine (mu) and human (hu) StIP1 cDNA sequences. WD40 motifs are shaded and labeled 1–12. Dots represent absence and dashes represent identity of corresponding amino acids. Arrows indicate borders of the spliced exon in Δ7 StIP1. Murine StIP1 has a predicted molecular mass of 93 kDa.
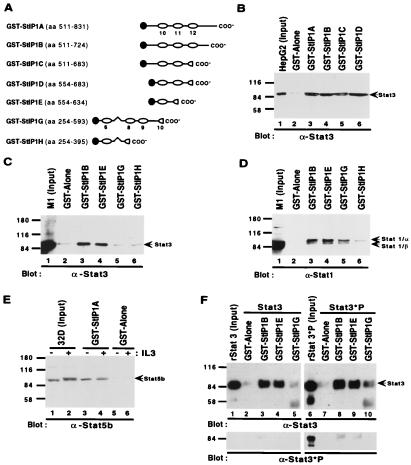
Figure 3.
In vitro association of StIP1 with Stat1, Stat3, and Stat5. (A) Schematic diagram of seven GST-StIP1 fusion proteins (GST-StIP1A–GST-StIP1H). (B) GST-StIP1 fusion proteins (lanes 3–6) or GST alone (lane 2) were incubated with HepG2 extracts. Bound proteins were detected with a Stat3-specific antibody (C-20; Santa Cruz Biotechnology). Lane 1 represents 1/20 of the input material for each pulldown. The mobility of Stat3 is indicated. (C) Extracts prepared from unstimulated M1 cells were incubated with indicated GST-StIP1 fusion proteins (lanes 3–6) or GST alone (lane 2) and detected with a Stat3-specific antibody (C-20; Santa Cruz Biotechnology). Lane 1 represents 1/3 of the input material for each pulldown. (D) The filter from C was reprobed with a Stat1-specific antibody (M-22; Santa Cruz Biotechnology). (E) Extracts from unstimulated (lanes 1, 3, and 5) or IL-3-stimulated (lanes 2, 4, and 6) 32D cells were incubated with GST-StIP1A (lanes 3 and 4) or GST alone (lane 5), and then detected with a Stat5b-specific antibody (C-17; Santa Cruz Biotechnology). Input extracts were loaded directly onto the gel in lanes 1 and 2. Lane 2 shows the appearance of a more slowly migrating band corresponding to the IL-3-stimulated tyrosine-phosphorylated isoform of Stat5b (confirmed by antiphosphotyrosine blotting; data not shown). (F) Recombinant Stat3 either inactive/nontyrosine phosphorylated (rStat3; lanes 1–5) or active/tyrosine phosphorylated (rStat3*P; lanes 6–10) was prepared from insect cells infected either with a Stat3 or Stat3 plus Jak1 baculovirus. These recombinant proteins were incubated with the indicated GST-StIP1 fusion proteins (lanes 3–5 and 8–10) or GST alone (lanes 2 and 7) and then detected by Western blotting with a Stat3-specific antibody (C-20; Santa Cruz Biotechnology). The filter then was reprobed with a phosphotyrosine-specific Stat3 antibody (Lower; New England Biolabs).
Cell Extracts and Protein Assays.
GST pulldown assays were performed as described (22). Immobilized GST fusion proteins (i.e., bound to glutathione agarose; Sigma) then were incubated for 2–16 h with whole-cell extracts from HepG2 and M1 cells (23, 31) or recombinant Stat3 from insect cells infected with Stat3 and/or Jak1 baculoviruses (22). Bound proteins were eluted either with 25 mM glutathione or by boiling in SDS/Laemmli buffer, fractionated on 7–8% SDS/PAGE, and then immunoblotted with Stat3 (C-20; Santa Cruz Biotechnology), phospho-specific Stat3 (New England Biolabs), or Jak1 (Upstate Biotechnology, Lake Placid, NY) antibodies. Immunoblots were visualized by enhanced chemiluminescence (Amersham Pharmacia). Coimmunoprecipitations were preformed as described (22) with Stat3 (C20; Santa Cruz Biotechnology), Jak1 (Upstate Biotechnology), Jak2 (Upstate Biotechnology), Tyk2 (a generous gift of S. Pellegrini, Institut Pasteur, Paris) antibodies, or with a StIP1-specific antibody. The StIP1 antibody was generated by immunizing rabbits with purified GST-StIP1C fusion protein (amino acids 511–683; see Fig. 3D; ref. 30; Covance, Inc., Princeton). It was used at 1:3000 in Western blots and 1:100 in immunoprecipitations. Electrophoretic mobility shift assays were performed with an IFN regulatory factor 1 (IRF-1) GAS probe (gatcGATTTCCCCGAAAT; Oligos Etc., Guilford, CT) as described (32, 33). Reporter assays used a GAS-driven luciferase reporter and were standardized by evaluating “null promoter”-driven renilla luciferase activity (Dual Luciferase; Promega) in a Turner Luminometer.
Immunofluorescence.
HepG2 and NIH 3T3 cells were stained as reported (24). Briefly, cells were fixed in 3.7% formaldehyde for 20 min at 22°C, permeabilized in 0.2% Triton X-100 (2–4 min), washed in 1× Tris-buffered saline +0.05% Tween 20 (137 mM NaCl/25 mM Tris⋅HCl, pH 7.4), and blocked in 3% BSA or 10% milk in Tris-buffered saline (30 min). Samples were stained with Stat3 (1:250 dilution of C-20; Santa Cruz Biotechnology) and hemagglutinin epitope tag (1:250 dilution of 12CA5; Roche Molecular Biochemicals) primary antibodies and then Cy3-conjugated (1:500; Jackson ImmunoResearch) and Alexa-conjugated (1:500; Molecular Probes) secondary antibodies. NIH 3T3 cells were stained with the StIP1 antiserum (1:100) and visualized with the Cy3-conjugated secondary antibody. Because the StIP1 antibody does not recognize human StIP1, it was first preabsorbed on HeLa cells. Cells were observed at ×400 under a Nikon Eclipse TE300 microscope after excitation at 495 nm (Alexa) or 550 nm (Cy3).
Results
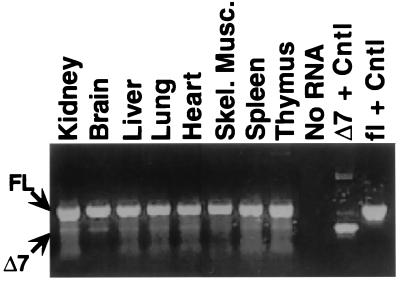
A yeast two-hybrid screen, where the coiled-coil domain of Stat3 (amino acids 103–330) served as “bait”, identified a novel 1.3-kb murine cDNA, referred to as StIP1. Full-length (i.e., ≈3 kb) cDNAs were isolated and found to encode an 831-aa protein whose ORF corresponds to the reading frame identified in the yeast two-hybrid “hit” (Fig. 1). One notable feature of the StIP1 sequence is the presence of 12 WD40 repeat motifs. These motifs form a defined propeller structure that is known to mediate protein–protein interactions in a number of important cellular processes (21). A second cDNA, with an internal 132-bp deletion that leads to a loss of WD40 repeat number 7, also was recovered. Northern blotting studies identified an ≈3-kb transcript in all tissues (data not shown). A subsequent reverse transcription–PCR study with primers flanking the deleted region demonstrated that both full-length and the shorter (i.e., Δ7) transcript are expressed in all examined tissues (Fig. 2). The full-length transcript, however, predominates significantly.
Figure 2.
Expression pattern of StIP1 transcripts. Expression of StIP1 was evaluated by a reverse transcription–PCR assay (Roche Molecular Biochemicals). RNA was prepared from each of the indicated tissues and amplified with primers from repeat 6 and 8 (Fig. 3A). PCR products corresponding to the full-length (FL) or Δ7 transcripts are indicated. Controls samples (Cntl) were amplified from cDNA templates.
A search of the National Center for Biotechnology Information sequence database identified homologous human and nematode expressed sequence tags, but no additional functional domains. The high degree of similarity between the murine StIP1 and an ORF compiled from overlapping human expressed sequence tags indicated that they are likely to encode the human StIP1 homologue (Fig. 1). As is the case with other WD40-containing proteins, the similarity was most striking between corresponding WD40 repeats. Nonrepeat regions were more poorly conserved (21).
StIP1 GST Fusion Proteins Bind STATs.
To further characterize the interaction between StIP1 and Stat3, a series of partially overlapping GST StIP1 fusion proteins were generated (Fig. 3A). When these GST-fusion proteins were incubated with extracts prepared from HepG2 cells, abundant quantities of Stat3 were recovered with both the StIP1 sequences encoded by the original 1.3-kb StIP1 “hit” (i.e., GST-StIP1A), as well as shorter constructs (Fig. 3B). In contrast, GST alone was ineffective at binding Stat3. Studies with additional GST-StIP1 fusion proteins (i.e., GST-StIP1E to GST-StIP1H) determined that WD40 repeat number 10 was most important in mediating the interaction with Stat3, in both human HepG2 cells (data not shown) and murine M1 cells (Fig. 3C).
Next, the ability of StIP1 to interact with other STATs was evaluated. GST-StIP1 fusion proteins were able to recover abundant quantities of Stat1, a close relative of Stat3 (25). The most notable difference was a modest increase in relative affinity for GST-StIP1G. To extend this line of investigation, extracts were prepared from a myeloid cell line that expresses abundant quantities of Stat5, a more distant member of the family (30). Consistent with the previous pulldowns, significant amounts of Stat5 were recovered. However, of the doublet of Stat5b bands normally seen after IL-3 stimulation (Fig. 3E, lane 2), GST-StIP1A appeared to preferentially bind the faster migrating and nontyrosine phosphorylated (30) Stat5 isoform (Fig. 3E, lane 4). The interaction with unphosphorylated Stat5 (i.e., amino acids 71–315) was confirmed in a yeast two-hybrid assay (data not shown). Because a preferential interaction between StIP1 and unphosphorylated STATs also was observed with the M1 extracts (Fig. 3 C and D; data not shown), another set of GST pulldowns was performed with more abundant preparations of Stat3. A panel of GST-StIP1 fusion proteins were either incubated with unphosphorylated or tyrosine-phosphorylated preparations of recombinant Stat3. Again, GST-StIP1B and GST-StIP1E-bound Stat3 avidly, in both unphosphorylated and tyrosine-phosphorylated preparations (Fig. 3F). It is important to note that less than 50% of Stat3 was tyrosine phosphorylated in the activated preparation (data not shown). When these filters were reprobed with an antibody that is specific for tyrosine-phosphorylated Stat3, it was clear that none of the “pulled down” Stat3 was tyrosine phosphorylated, despite adequate quantities of this species being present in the input material (Fig. 3F, lane 6). These observations demonstrate that StIP1 has a strong preferential affinity for the unphosphorylated (i.e., inactive) STATs.
StIP1 Coimmunoprecipitates with Stat3.
To determine whether StIP1 and Stat3 associate within the cell, an antiserum specific for murine StIP1 was prepared. Consistent with the predicted molecular weight (Fig. 1), this antibody recognized a doublet of bands of ≈92 kDa (Fig. 4A). It is not known whether these two isoforms arise by pre- or posttranslational processing. To confirm that StIP1 preferentially interacts with inactive Stat3, extracts were prepared from M1 cells both before and after stimulation with IL-6. As anticipated, the StIP1 antibody was able to coimmunoprecipitate Stat3 (Fig. 4A Top). Notably, none of the coprecipitated Stat3 was tyrosine phosphorylated, indicating that StIP1 has a much higher avidity for unphosphorylated Stat3 (Fig. 4A Middle). Likewise, in a reciprocal immunoprecipitation, Stat3 antibodies coprecipitated StIP1, but considerably more robustly in the unstimulated samples (Fig. 4A Top). These studies verify that StIP1 exhibits a strong binding preference for the inactive (unphosphorylated) isoform of Stat3.
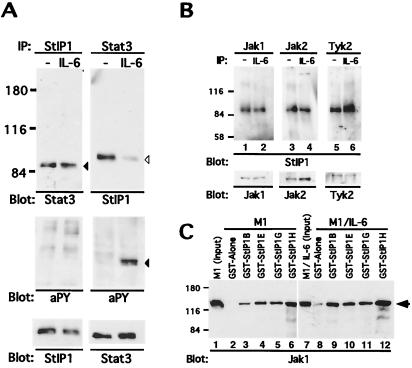
Figure 4.
Association of StIP1 with Stat3 and JAKs. (A) Extracts were prepared from M1 cells either before or after stimulation with IL-6 (200 units/ml). They were immunoprecipitated either with a StIP1 or Stat3 (C-20, Santa Cruz Biotechnology) antibody and subsequently immunoblotted with either a Stat3 (◂; C-20, Santa Cruz Biotechnology), StIP1 (▹), or antiphosphotyrosine (4G10; Upstate Biotechnology) antibodies as indicated. (B) M1 extracts were immunoprecipitated with Jak1 (lanes 1 and 2; Upstate Biotechnology), Jak2 (lanes 3 and 4; Upstate Biotechnology), or Tyk2 (lanes 5 and 6) antibodies and sequentially immunoblotted with StIP1 or the appropriate JAK antibodies as indicated. (C) Extracts from unstimulated (lanes 1–6) and IL-6-stimulated (lanes 7–12) M1 cells were incubated with GST-StIP1 fusion proteins (lanes 3–6 and 9–12) or GST alone (lanes 2 and 8) and bound proteins detected with a Jak1-specific antibody (←; Signal Transduction Laboratories, Lexington, KY).
StIP1 Associates with JAKs.
Because WD40 repeats are known to mediate the formation of multiprotein complexes (21), the ability of StIP1 to interact with another major component of the JAK-STAT signaling pathway was evaluated. Antibodies directed against all three JAKs implicated in IL-6 signaling (i.e., Jak1, Jak2, and Tyk2; ref. 34) were able to coimmunoprecipitate StIP1 from both unstimulated and IL-6-stimulated M1 cell extracts (Fig. 4B). In reciprocal experiments, StIP1 antibodies were able to coimmunoprecipitate JAK kinases (data not shown). To further characterize the interactions between StIP1 and JAKs, an additional set of GST pulldowns were performed and then evaluated with our most informative JAK antibody, α-Jak1. The pattern of Jak1 binding to these GST-StIP1 fusion proteins was quite different from that observed for Stat3, with GST-StIP1H demonstrating the highest affinity (Fig. 4C). No differences were observed between unstimulated and stimulated extracts. Analogous results were obtained with recombinant Jak1 (data not shown). Hence, StIP1 interacts with JAKs in a pattern that is distinct from that observed with Stat3.
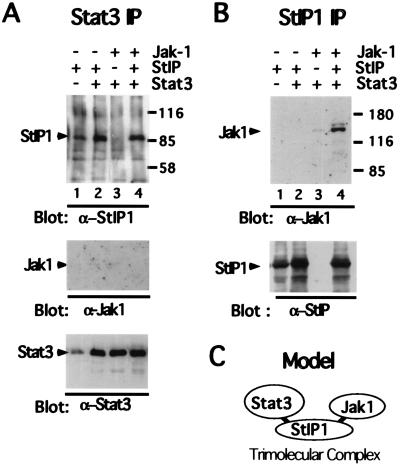
The ability of StIP1 to associate with both inactive Stat3 and JAKs suggested that StIP1 might serve as a scaffold protein, potentiating the functional interaction between kinase and substrate. A number of signaling pathways recently have been shown to use scaffold proteins (35–37). To determine whether a stable complex between Stat3, StIP1, and Jak1 can be identified (see model in Fig. 5C), 293T cells were transfected with cDNAs for Stat3 (25), StIP1, and kinase-dead Jak1 (29). Kinase-dead Jak1 was selected to increase the chance of “trapping” Stat3 in a putative trimolecular complex. As anticipated, abundant quantities of StIP1 were recovered when Stat3 was immunoprecipitated from cells transfected with those corresponding cDNAs (Fig. 5A, lanes 1, 2, and 4). Hence, StIP1 and Stat3 stably associated in these extracts (see model in Fig. 5C). However, overexpression of Stat3 led to only a modest increase in recovered StIP1, most likely because the Stat3 antibody was rate limiting. (Stat3 also was readily recovered in the appropriate StIP-1 immunoprecipitations; data not shown.) Likewise, when StIP1 was immunoprecipitated, abundant quantities of Jak1 were recovered in cells transfected with those corresponding cDNAs (Fig. 5B lane 4). Hence, StIP1 and Jak1 stably associated in these extracts (see model in Fig. 5C). To determine whether a stable trimolecular complex exists, Stat3 immunoprecipitates were probed for associated Jak1. None was identified, suggesting that these molecules do not form a stable trimolecular complex in 293T cells (Fig. 5A Middle).
Figure 5.
Stat3, Jak1, and StIP1 do not form a stable trimolecular complex. (A) 293T cells were cotransfected with 5 μg each of RcCMV-Stat3 (25), pMT2T-Jak1K896R (29), and pCNG0.8 as indicated. Whole-cell extracts (10 μl), prepared after 24 h, were diluted to 500 μl and incubated with 5 μl of α-Stat3 (C-20; Santa Cruz Biotechnology). Precipitates were immunoblotted with either α-StIP1 (Top), α-Jak1 (Middle; Upstate Biotechnology), or α-StIP1 (Bottom). Mobilities of StIP1, Jak1, and Stat3 are indicated in the left margin and Mr in the right margin. (B) Samples, prepared as in A, were immunoprecipitated with 5 μl of α-StIP1 and immunoblotted with α-Jak1 (Upper) or α-StIP1 (Lower). Mobilities of Jak1 and StIP1 are indicated in the left margin and Mr in the right margin. (C) Model of the putative Stat3-StIP1-Jak1 trimolecular complex.
Dominant-Negative Mutants of StIP1 Block Stat3 Activation.
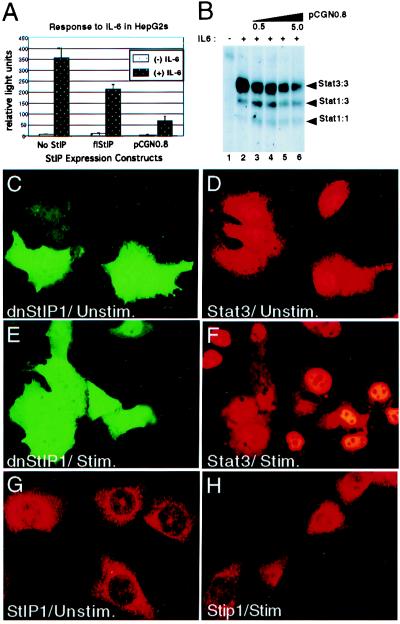
To determine whether the interactions between StIP1 and STATs (or JAKs) are important for signal transduction, the effect of overexpressing the STAT-binding domain of StIP1 on IL-6 signaling was evaluated in a reporter assay. As anticipated, IL-6 stimulated a robust induction of our IRF-1 GAS-driven reporter in HepG2 cells (Fig. 6A). However, cotransfection with a StIP1 construct expressing the WD40 repeats that bind STATs (i.e., repeats 10–12; pCGN0.8), led to a substantial (and dose-dependent; data not shown) reduction of IL-6-dependent luciferase expression (Fig. 6A). We speculated that pCGN0.8 functioned as an effective dominant-negative mutant, binding to and sequestering unphosphorylated Stat3. Cotransfection of full-length StIP1 lead to a more modest reduction in IL-6-dependent luciferase expression. The failure of full-length StIP1 to potentiate signaling is consistent with recent observations on other overexpressed scaffold proteins, presumably because scaffold proteins exhibit a relatively high affinity for individual components of their respective signaling cascade (12, 38, 39).
Figure 6.
Overexpression of StIP1 mutants blocks cytokine signaling. (A) HepG2 cells were cotransfected with an IRF-1 GAS-driven reporter (32) and either empty vector, full-length StIP1, or pCGN0.8. Extracts were assayed for luciferase activity either before (open bars) or after (filled bars) treatment with IL-6. Data represent results from one experiment done in triplicate, but similar results were obtained in three independent studies. Firefly luciferase activity was normalized to renilla luciferase activity expressed by a control vector, pRLnull (Promega). (B) 293T cells were cotransfected with 1 μg of a Stat3 expression vector (25) and increasing amounts (i.e., 0.5, 1.0, 2.0, and 5.0 μg; lanes 3–6) of a pCGN0.8 expression construct with a His-epitope tag, as indicated. DNA-binding activity was evaluated with an IRF-1 GAS probe either before or after stimulation with IL-6 (200 Units/ml) plus soluble IL-6 receptor (0.5 μg/ml; a generous gift from F. Horn, RWTH, Aachen, Germany; ref. 34). The mobility of STAT DNA-binding complexes is indicated in the right margin. (C–F) HepG2 cells were transfected with 5.0 μg of pCGN0.8 and then evaluated by dual immunostaining with anti-hemagglutinin (C and E; 1° antibody = 12CA5, Roche Molecular Biochemicals; and 2° antibody = goat anti-mouse-Alexa, Molecular Probes), and anti-Stat3 (D and F; 1° antibody = C20, Santa Cruz Biotechnology; and 2° antibody = donkey anti-rabbit-Cy3, Jackson ImmunoResearch), either before (C and D) or after an 8-min stimulation with IL-6 (E and F; 15 ng/ml, PeproTech, Rocky Hill, NJ). (G and H) The localization of endogenous StIP1 in NIH 3T3 cells was evaluated by immunostaining with a murine-specific StIP1 antibody either before or after stimulation with ligand.
Next, the idea that overexpressed StIP1 domains block signaling by preventing Stat3 activation was evaluated. Expression constructs for Stat3 (25) and pCGN0.8 (our most potent dominant-negative StIP1 mutant) were introduced into 293T cells. These cells were selected because they express modest levels of endogenous Stat3 and can be transfected efficiently. The cells then were stimulated with IL-6 and evaluated for Stat3 activation by a sensitive electrophoretic mobility shift assay (Fig. 6B). IL-6 potently induced Stat3 homodimer DNA binding. This activity was inhibited in a dose-dependent manner by the coexpression of pCGN0.8. Stat1 homodimer binding, which arose predominantly from the ≈65% nontransfected cells, served as a control in these studies. These results are consistent with the reporter assays and indicated that the expression of pCGN0.8 blocked ligand-dependent Stat3 activation.
The requirement of tyrosine phosphorylation for STAT nuclear translocation provided an additional assay to evaluate pCGN0.8 under more physiological conditions. HepG2 cells were transfected with pCGN0.8 and then stimulated with IL-6 (Fig. 6 C–F). As reported, when unstimulated cells were stained for Stat3, the protein was predominately cytoplasmic, with modest levels in the nucleus (Fig 6D; ref. 31). Stimulation with IL-6 promoted a dramatic increase in nuclear Stat3 (Fig. 6F). Because the protein encoded by pCGN0.8 was hemagglutinin-tagged, it was possible to evaluate its expression immunohistochemically as well. Consistent with the cellular distribution of endogenous (Fig. 6 G and H) or overexpressed StIP1 (data not shown), pCGN0.8 was expressed predominately in the cytoplasm of both unstimulated and stimulated cells (Fig. 6 C and E). Importantly, IL-6-stimulated nuclear translocation of endogenous Stat3 was blocked in cells expressing pCGN0.8 (Fig. 6F). These studies provide further evidence that expression of pCGN0.8 blocks IL-6-stimulated activation of Stat3.
Discussion
The ability of protein kinases to change the activation state of substrates has led to their exploitation in signaling cascades. Both the number and complexity of kinases has increased markedly during the evolution of more complex organisms. In many cases, a single kinase has evolved to regulate more than one signaling pathway. A classic example of this is STE11, a yeast mitogen-activated protein kinase homologue, which plays a critical role in both the pheromone mating response and osmoregulation (35, 36). Notably, these two pathways function completely independently. This is achieved through the use of two distinct scaffolding proteins, STE5 and Pbs2, respectively. In each case, the scaffold proteins nucleate the formation of a specific multiprotein “transducisome” that consists of STE11 and the appropriate downstream targets. Not only does this facilitate signaling, but it also prevents the activation of inappropriate pathways. More recently, analogous scaffold proteins have been identified for mammalian mitogen-activated protein kinases (JIP-1 and MP1; refs. 39 and 40), as well as for several other important mammalian kinases including c-Src, Raf, IκB kinase, and protein kinase A (38, 41–43). However, to date, no scaffold proteins have been reported for the STAT signaling cascade.
To identify proteins involved in the regulation of STAT signaling, a yeast two-hybrid screen was set up for Stat3. The conserved coiled-coil domain, whose function is poorly understood, was selected as “bait.” Its large exposed surface area suggested that it was likely to mediate protein interactions. Moreover, recent studies have suggested that this domain undergoes conformational changes during activation, potentially regulating the interaction with other proteins (24, 44). Consistent with these observations, several groups recently have identified proteins that interact with STAT coiled-coil domains. The best characterized is IRF-9 (p48), which binds to the coiled-coil domains of Stat1 and Stat2 (18, 19). Additional interacting proteins include p300, members of the protein inhibitor of activated STAT family, and Nmi (12, 20, 45). Our yeast two-hybrid screen identified a novel Stat3-interacting protein, StIP1. Subsequent studies have determined that StIP1 has a similar affinity for other members of the STAT family (e.g., Stat1, Stat3, and Stat5; Fig. 3), suggesting StIP1 may play a more general role in STAT signaling. However, we were unable to detect a stable interaction with Stat2 (data not shown). This is reminiscent of studies demonstrating that Nmi associates with the coiled-coil domain of all STATs, except Stat2 (12). These observations suggest that the Stat2 coiled-coil domain may exhibit some unique structural properties. Consistent with this possibility, recent studies suggest that the STAT coiled-coil domain may regulate DNA-binding activity (C. Leung and C.S., unpublished observation). Perhaps the inability of Stat2 to bind DNA (2) is related to a structurally divergent coiled-coil domain.
StIP1 is a novel protein that consists of 12 WD40 repeats. These repeats have been shown to mediate the formation of multiprotein complexes that play important roles in the regulation of signal transduction, transcription, and targeted proteolysis (21). To determine whether StIP1 might interact with other components of the JAK-STAT signaling cascade, three JAKs that are important in IL-6 signaling were evaluated. Not only did these studies confirm an interaction with these JAKs, but domain-mapping studies also indicate that they exhibit a distinct pattern of binding to StIP1 (i.e., vs. Stat3). These observations, along with the strong preference of StIP1 for unphosphorylated Stat3, suggest that StIP1 plays a role early in the ligand-dependent activation of Stat3. Consistent with this, overexpression of StIP1, or StIP1 domains that bind inactive Stat3, leads to a block in IL-6-dependent Stat3 activation. Moreover, the affinity of StIP for other STATs suggests that it may serve a similar role for other STATs.
One appealing model is that StIP1 functions as a scaffold protein, potentiating the interaction between Stat3 and JAKs. Once phosphorylated, Stat3 loses its affinity for StIP1 and dissociates from the receptor complex. Although we have been unable to isolate a Stat3–StIP1–Jak1 complex, there are several lines of evidence that support this model. First, the interaction between Stat3 and StIP1 depends on the phosphorylation state of Stat3. Second, StIP1 exhibits a distinct pattern of affinity for the JAK kinases. Third, StIP1 is a cytosolic protein. Fourth, overexpression of StIP1 domains block IL-6-stimulated Stat3 activation, preventing dimerization/DNA binding, nuclear translocation, and the induction of a reporter gene.
Acknowledgments
We thank Jeremy Luban and Steve Greenberg for reagents and advice and Bill Kim and Wei Liu for superb technical assistance. These studies were supported by National Institutes of Health Grant HL55413, the Leukemia Society of America, the Lucille P. Markey Foundation, and the Pfizer Postdoctoral Fellowship Program.
Abbreviations
- StIP1
Stat3-interacting protein
- JAK
Janus kinase
- STAT
signal transducer and activator of transcription
- GAS
interferon-gamma activation site
- GST
glutathione S-transferase
- IRF
IFN regulatory factor
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. AF291064).
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.170192197.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.170192197
References
- 1.Darnell J E. Science. 1997;277:1630–1635. doi: 10.1126/science.277.5332.1630. [DOI] [PubMed] [Google Scholar]
- 2.Schindler C, Strehlow I. Cytokines and STAT Signaling. San Diego: Academic; 2000. [DOI] [PubMed] [Google Scholar]
- 3.Barasch, J., Yang, J., Ware, C. B., Taga, T., Yoshida, K., Erdjument-Bromage, H., Tempst, P., Parravicini, E., Malach, S., Aranoff, T., et al.Cell99, 377–386. [DOI] [PubMed]
- 4.Boccaccio C, Ando M, Tamagnone L, Bardelli A, Michieli P, Battistini C, Comoglio P M. Nature (London) 1998;391:285–288. doi: 10.1038/34657. [DOI] [PubMed] [Google Scholar]
- 5.Bonni A, Sun Y, Nadal-Vicens M, Bhatt A, Frank D A, Rozovosky I, Stahl N, Yancopoulos G D, Greenberg M E. Science. 1997;278:477–483. doi: 10.1126/science.278.5337.477. [DOI] [PubMed] [Google Scholar]
- 6.Bromberg J F, Wrzeszczynska M H, Devgan G, Zhao Y, Pestell R G, Albanese C, Darnell J E. Cell. 1999;98:295–303. doi: 10.1016/s0092-8674(00)81959-5. [DOI] [PubMed] [Google Scholar]
- 7.Garcia R, Jove R. J Biomed Sci. 1998;5:79–85. doi: 10.1007/BF02258360. [DOI] [PubMed] [Google Scholar]
- 8.Sano S, Itami S, Takeda K, Tarutani M, Yamaguchi Y, Miura H, Yoshikawa K, Akira S, Takeda J. EMBO J. 2000;18:4657–4668. doi: 10.1093/emboj/18.17.4657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takeda K, Clausen B E, Kaisho T, Tsujimura T, Terada N, Förster I, Akira S. Immunity. 1999;10:39–49. doi: 10.1016/s1074-7613(00)80005-9. [DOI] [PubMed] [Google Scholar]
- 10.Yoshida K, Taga T, Saito M, Suematsu S, Kumanogoh A, Tanaka T, Fujiwara H, Hirata M, Yamagami T, Nakahata T, et al. Proc Natl Acad Sci USA. 1996;93:407–411. doi: 10.1073/pnas.93.1.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wen Z, Zhong Z, Darnell J E. Cell. 1995;82:241–250. doi: 10.1016/0092-8674(95)90311-9. [DOI] [PubMed] [Google Scholar]
- 12.Zhu M-H, John S, Berg M, Leonard W J. Cell. 1999;96:121–130. doi: 10.1016/s0092-8674(00)80965-4. [DOI] [PubMed] [Google Scholar]
- 13.Schmitz J, Weissenbach M, Haan S, Heinrich P C, Schaper F. J Biol Chem. 2000;275:12848–12856. doi: 10.1074/jbc.275.17.12848. [DOI] [PubMed] [Google Scholar]
- 14.Yu C L, Jin Y J, Burakoff S J. J Biol Chem. 2000;275:599–604. doi: 10.1074/jbc.275.1.599. [DOI] [PubMed] [Google Scholar]
- 15.Becker S, Groner B, Müller C W. Nature (London) 1998;394:145–151. doi: 10.1038/28101. [DOI] [PubMed] [Google Scholar]
- 16.Chen X, Winkemeier U, Zhao Y, Jeruzalmi D, Darnell J E, Kuriyan J. Cell. 1998;93:827–839. doi: 10.1016/s0092-8674(00)81443-9. [DOI] [PubMed] [Google Scholar]
- 17.Vinkemeier U, Cohen S L, Moarefi I, Chait B T, Kuriyan J, Darnell J E. EMBO J. 1996;15:5616–5626. [PMC free article] [PubMed] [Google Scholar]
- 18.Martinez-Moczygemba M, Glutch M J, French D L, Reich N C. J Biol Chem. 1997;272:20070–20076. doi: 10.1074/jbc.272.32.20070. [DOI] [PubMed] [Google Scholar]
- 19.Horvath C M, Stark G R, Kerr I M, Darnell J E. Mol Cell Biol. 1996;16:6957–6964. doi: 10.1128/mcb.16.12.6957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chung C D, Liao J, Liu B, Rao X, Jay P, Berta P, Shuai K. Science. 1997;278:1803–1805. doi: 10.1126/science.278.5344.1803. [DOI] [PubMed] [Google Scholar]
- 21.Smith T F, Gaitatzes C, Saxena K, Neer E J. Trends Biochem Sci. 1999;24:181–185. doi: 10.1016/s0968-0004(99)01384-5. [DOI] [PubMed] [Google Scholar]
- 22.Gupta S, Yan H, Wong L H, Ralph S, Krolewski J, Schindler C. EMBO J. 1996;15:1075–1084. [PMC free article] [PubMed] [Google Scholar]
- 23.Rothman P, Kreider B, Azam M, Levy D, Wegenka U, Eilers A, Decker T, Horn F, Kashleva H, Ihle J, et al. Immunity. 1994;1:457–468. doi: 10.1016/1074-7613(94)90088-4. [DOI] [PubMed] [Google Scholar]
- 24.Strehlow I, Schindler C. J Biol Chem. 1998;273:28049–28056. doi: 10.1074/jbc.273.43.28049. [DOI] [PubMed] [Google Scholar]
- 25.Zhong Z, Wen Z, Darnell J E. Science. 1994;264:95–98. doi: 10.1126/science.8140422. [DOI] [PubMed] [Google Scholar]
- 26.Durfee T, Becherer K, Chen P, Yeh S, Yang Y, Kilburn A E, Lee W, Elledge S J. Genes Dev. 1993;7:555–569. doi: 10.1101/gad.7.4.555. [DOI] [PubMed] [Google Scholar]
- 27.Dunaief J L, Strober B E, Guha S, Khavari P A, Alin K, Luban J, Begemann M, Crabtree G R, Goff S P. Cell. 1994;79:119–130. doi: 10.1016/0092-8674(94)90405-7. [DOI] [PubMed] [Google Scholar]
- 28.Luban J, Bossolt K L, Franke E K, Kalpana G, Goff S P. Cell. 1993;73:1067–1078. doi: 10.1016/0092-8674(93)90637-6. [DOI] [PubMed] [Google Scholar]
- 29.Krishnan K, Pine R, Krolewski J J. Eur J Biochem. 1997;247:298–305. doi: 10.1111/j.1432-1033.1997.00298.x. [DOI] [PubMed] [Google Scholar]
- 30.Azam M, Erdjument-Bromage H, Kreider B L, Xia M, Quelle F, Basu R, Saris C, Tempst P, Ihle J N, Schindler C. EMBO J. 1995;14:1402–1411. doi: 10.1002/j.1460-2075.1995.tb07126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang X, Blenis J, Li H-C, Schindler C, Chen-Kiang S. Science. 1995;267:1990–1994. doi: 10.1126/science.7701321. [DOI] [PubMed] [Google Scholar]
- 32.Pine R, Canova A, Schindler C. EMBO J. 1994;13:158–167. doi: 10.1002/j.1460-2075.1994.tb06245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Park C, Schindler C. Methods Companion Methods Enzymol. 1998;15:175–188. doi: 10.1006/meth.1998.0622. [DOI] [PubMed] [Google Scholar]
- 34.Lütticken C, Wegenka U M, Yuan J, Buschmann J, Schindler C, Ziemiecki A, Harpur A G, Wilks A F, Yasukawa K, Taga T, et al. Science. 1994;263:89–92. doi: 10.1126/science.8272872. [DOI] [PubMed] [Google Scholar]
- 35.Faux M C, Scott J D. Cell. 1996;85:9–12. doi: 10.1016/s0092-8674(00)81075-2. [DOI] [PubMed] [Google Scholar]
- 36.Whitmarsh A J, Davies R J. Trends Biochem Sci. 1998;23:481–485. doi: 10.1016/s0968-0004(98)01309-7. [DOI] [PubMed] [Google Scholar]
- 37.Zou H, Henzel W J, Liu X, Lutschg A, Wang X. Cell. 1997;90:405–413. doi: 10.1016/s0092-8674(00)80501-2. [DOI] [PubMed] [Google Scholar]
- 38.Cohen L, Henzel W J, Baeuerle P A. Nature (London) 1998;395:292–296. doi: 10.1038/26254. [DOI] [PubMed] [Google Scholar]
- 39.Yasuda J, Whitmarsh A J, Cavanagh J, Sharma M, Davies R J. Mol Cell Biol. 1999;19:7245–7254. doi: 10.1128/mcb.19.10.7245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schaeffer H J, Catling A D, Eblen S T, Collier L S, Krauss A, Weber M J. Science. 1998;281:1668–1674. doi: 10.1126/science.281.5383.1668. [DOI] [PubMed] [Google Scholar]
- 41.Luttrell L M, Ferguson S S G, Daaka Y, Miller W E, Maudsley S, Rocca G J D, Lin F-T, Kawakatsu H, Owada K, Lutrell D K, et al. Science. 1999;283:655–660. doi: 10.1126/science.283.5402.655. [DOI] [PubMed] [Google Scholar]
- 42.Therrien M, Wong A M, Rubin G M. Cell. 1998;95:343–353. doi: 10.1016/s0092-8674(00)81766-3. [DOI] [PubMed] [Google Scholar]
- 43.Schillace R V, Scott J D. J Clin Invest. 1999;103:761–765. doi: 10.1172/JCI6491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sekimoto T, Imamoto N, Nakajima K, Tachibana T, Hirano T, Yoneda Y. EMBO J. 1997;16:7076–7077. doi: 10.1093/emboj/16.23.7067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bhattacharya S, Eckner R, Grossman S, Oldread E, Arany Z, D'Andrea A, Livingston D. Nature (London) 1996;383:344–347. doi: 10.1038/383344a0. [DOI] [PubMed] [Google Scholar]