Abstract
AIM: To investigate the effect and mechanism of electro-acupuncture (EA) at ST25 and ST37 on irritable bowel syndrome (IBS) of rats.
METHODS: A total of 21 male Sprague-Dawley rats were randomly divided into normal group, model group and EA group. A rat model of IBS was established by constraining the limbs and distending the colorectum of rats. Rats in EA group received bilateral EA at ST25 and ST37 with a sparse and intense waveform at a frequency of 2/50 Hz for 15 min, once a day for 7 d as a course. Rats in normal and model groups were stimulated by distending colorectum (CR). An abdominal withdrawal reflex (AWR) scoring system was used to evaluate improvements in visceral hypersensitivity. Toluidine blue-improved method, immunohistochemistry and radioimmunoassay were used to observe mucosal mast cells (MC), changes of substance P (SP) and substance P receptor (SPR) in colon and change of corticotropin-releasing hormone (CRH) in hypothalamus.
RESULTS: The threshold of visceral sense was significantly lower in model group than in normal group, and significantly higher in EA group than in model group. The number of mucosal MC was greater in model group than in normal group and significantly smaller in EA group than in model group. The CRH level in hypothalamus of rats was significantly higher in model group than in normal group, which was remarkably decreased after electro-acupuncture treatment. The SP and SPR expression in colon of rats in model group was decreased after electro-acupuncture treatment.
CONCLUSION: EA at ST25 and ST37 can decrease the number of mucosal MC and down-regulate the expression of CRH in hypothalamus, and the expression of SP and SPR in colon of rats with IBS.
Keywords: Electro-acupuncture, Corticotropin-releasing hormone, Irritable bowel syndrome, Substance P, Substance P receptor
INTRODUCTION
Irritable bowel syndrome (IBS) is a common bowel disorder characterized by recurrent abdominal pain or discomfort associated with altered bowel habits in the absence of structural pathology[1]. Since IBS is diagnosed based on its symptoms and its pathophysiology is unclear, its treatment outcome remains unsatisfactory[2,3]. Our previous study showed that electro-acupuncture (EA) is effective against IBS[4]. However, its mechanism of action needs to be further studied.
IBS patients often describe a correlation between stressful life events and the onset or exacerbation of their gastrointestinal symptoms, and seem more susceptible to stressful events in daily life[5]. The central nervous system response to stressful events modulates the autonomic nervous system outflow and activates the hypothalamic-pituitary-adrenal axis[6]. Dysfunction of these systems has been proposed to be an etiological factor for IBS[7,8]. It has been reported that there is a difference in hormone level involving stress response between IBS patients and healthy subjects[8]. Central release of corticotropin-releasing hormone (CRH) plays an important role in the stress response[9], inducing a higher adrenocorticotropic hormone (ACTH) level and a more profound enhancement of colonic motility in IBS patients than in healthy controls[10]. It has been shown that CRH increases rectal sensitivity[11]. Thus, alterations in neuroendocrine response to stress may be of importance in the pathophysiology of IBS[12].
Visceral hypersensitivity is highly prevalent in IBS patients, and activation of intestinal mast cells (MC) may play a role in visceral hypersensitivity because they are in close proximity to gastrointestinal mucosal sensory nerve terminals containing neuropeptides, and a bidirectional pathway connecting the central nervous system, gut and MC have been demonstrated. MC at the ileocecal junction may be a mediator of the gut and nervous system in IBS[13] and substance P (SP) is a gastrointestinal peptide hormone. Both of them reside in the gastrointestinal tract and central nervous system. SP is also an interactive signaling molecule between the nervous and immune systems[14] and can modulate the function of intestinal mucosal MC by regulating neurosecretion and paracrine secretion.
This study was to explore the effect of EA at ST25 and ST37 on IBS by observing the MC count, the CRH level in hypothalamus, and the expression of SP and SPR in colon of rats.
MATERIALS AND METHODS
Animals
Twenty-one male Sprague-Dawley rats (SPF class), weighing 185-215 g, were supplied by Experimental Animal Center of Shanghai University of TCM, and randomly divided into normal group, model group and EA group according to their weights, 7 in each group. All rats were housed at a constant temperature and a humidity environment with free access to food and water. All studies were performed in accordance with the proposals of the Committee for Research and Ethical Issues of the International Association and approved by the Committee on the Use of Human and Animal Subjects in Teaching and Research, Shanghai University of TCM.
Establishment of rat model of IBS
An experimental rat model of IBS was established as previously described[15,16]. On the second day after the rats were fasted, experiment was begun. Rats in the normal group were given grabbing around the anus, while rats in the other two groups were stimulated by distending colorectum (CR). Limbs of the rats were fixed with medical adhesive tapes to limit their movements. The fixed rats could crawl without using their rear limbs. CR was distended before the limbs of rats were constrained and after the medical adhesive tapes were removed. The finger of a disposable rubber glove was tightly fixed onto the end of a polyethylene tube with 4 holes (0.5 cm apart) using medical silk thread as a 4 cm-long balloon. The other end of the tube was connected to a 10 cm-long rubber tube with a tri-channel valve connected to a syringe and sphygmomanometer. Vaseline was smeared on surface of the balloon which was slowly inserted into 5 cm of the rat anus along the physical curve of CR. The fixed time was 2 h each day, and CR was distended for 3 min, once every other day for 8 d. The whole modeling time was 15 d.
Treatment
Rats in the EA group were treated with EA at bilateral ST 25 and ST 37, once a day for 7 d as a course. Needles were pricked 0.3 cm in depth with a dense-sparse waveform at a frequency of 2/50 Hz and retained for 15 min. Rats in the normal and model groups received no EA treatment.
Contraction reaction in rat abnormal scoring test
The abdominal withdrawal reflex (AWR) scoring criteria[14] are shown in Table 1. Rats in the model group were fasted in afternoon of the previous day. Vaseline was smeared on surface of the balloon which was slowly inserted into 5 cm of the rat anus according to the physical curve of CR and retained for 5 min. The test was begun when the rats became adapted.
Table 1.
AWR[14] scoring criteria
| Score 0 | No behavioral response to CRD |
| Score 1 | Immobile during distension of CR and occasional clicking the head at onset of the stimulus |
| Score 2 | A mild contraction of abdominal muscles, but no lifting of abdomen off the plattorm |
| Score 3 | A strong contraction of abdominal muscles and lifting of abdomen off the platform, no lifting of pelvic structure off the platform |
| Score 4 | Arching body and lifting of pelvic structure and scrotum |
AWR: Abdominal withdrawal reflex; CRD: Colorectal distension; CR: Colorectum.
After air was added into the balloon with a syringe, the rat rectum was stimulated and different degrees of contraction reaction were observed. The pressure (mmHg) during behavior response scored as 1, 2, 3, and 4 was recorded and expressed as the threshold of sensitivity. Each score was tested three times, and each rat was tested by two persons not participating in this research. Means were calculated (6 values in total). Three-minute intervals were set between each two tests for the full adaptation of rats.
Observation using toluidine blue-improved method
Samples were taken from the descending colon (5 cm above anus) and cecum, cleaned with normal saline, fixed with 10% formalin, dehydrated, paraffin-embedded, cut into sections and stained with toluidine blue-improved (TBI) method, deparaffinized and rehydrated, dipped in toluidine blue for 30 min. Two or three drops of glacial acetic acid were added into the samples until the presence of pretty clear nuclei and granulation. The samples were dried with cold air, cleaned in xylene, mounted onto Permount or Histoclad, and observed under a microscope (Olympus-BH2, × 100 and × 400). Three high-power fields (× 400) were randomly selected and the number of MC was counted and expressed as mean.
Radioimmunoassay for CRH
Sample preparation: All rats were killed by dislocating cervical vertebra, with their brain taken out and hypothalamus isolated in ice bath. The hypothalamus was rinsed with 0.9% sodium chloride and restored in a liquid nitrogen container. The hypothalamus was taken out from the liquid nitrogen container, weighed and labeled. One milliliter 1 mol/L glacial acetic acid was added and homogenized for 100 min, then 0.8 mL 1 mol/L NaOH was added and centrifuged for 20 min at 4000 r/min. The supernatant was stored at -20°C for radioimmunoassay. The sample (50 μL) was incubated for 24 h at 4°C. Then, 500 μL separating-medium was added into each tube, incubated at room temperature for 45 min, centrifuged for 20 min at 4000 r/min. The supernatant was aspirated and the results were calculated.
Immunohistochemistry for SP/SPR: Sample sections were deparaffinized in xylene for 10 min, and dehydrated in 95%, 90%, 70% ethanol for 2 min. Primary antibody was bound to the specific rabbit anti-rat antigen diluted at 1:150, at 4°C for 18 h. The samples stained with the envision immunohistochemistry method served as a positive control, while PBS-alternated primary antibody served as a negative control. Brown and dark brown granulation was observed with a background of purple blue. The positive expressing areas of SP and SPR under three fields were averaged.
Statistical analysis
Experimental data were expressed as mean ± SD. Statistical analyses were performed using SPSS 13.0 (SPSS Inc. Wacker Drive, Chicago, Illinois). Differences in mean were compared by one way ANOVA. P < 0.05 was considered statistically significant.
RESULTS
Contraction reaction in rat abnormal scoring test
The threshold pressure was remarkably lower in model group than in normal group, and obviously higher in EA group than in model group (P < 0.01, Table 2).
Table 2.
Threshold pressure of rat contraction reaction in different groups (n = 7) (mean ± SD)
| Group |
Threshold pressure (mmHg) |
|||
| Score 1 | Score 2 | Score 3 | Score 4 | |
| Normal group | 23.38 ± 3.15 | 41.26 ± 3.58 | 68.00 ± 8.97 | 86.35 ± 10.01 |
| Model group | 15.50 ± 3.25b | 23.76 ± 3.91b | 37.43 ± 6.75b | 57.95 ± 5.45b |
| EA group | 21.81 ± 1.93d | 34.02 ± 3.87bd | 50.50 ± 7.28bd | 63.23 ± 6.24bd |
P < 0.01 vs normal group;
P < 0.01 vs model group.
Effect of EA on CRH in hypothalamus of rats
The CRH level was significantly higher in hypothalamus of rats in model group than in normal group (P < 0.05), which was significantly decreased after EA treatment (P < 0.05). No significant difference was found in CRH level between normal and EA groups (Table 3).
Table 3.
Corticotropin-releasing hormone level in hypothalamus of rats and MC count in colonic membrane of rats in different groups (n = 7) (mean ± SD)
| Group | CRH level (pg/mg) | MC count in each visional field |
| Normal group | 42.68 ± 4.39 | 2.19 ± 0.31 |
| Model group | 66.63 ± 18.19a | 10.0 ± 1.21a |
| EA group | 42.81 ± 7.44c | 4.81 ± 0.63ac |
P < 0.05 vs normal group;
P < 0.05 vs model group. EA: Electro-acupuncture; MC: Mast cells..
MC in rat colonic membrane
The number of MC was greater in model group than in normal group (P < 0.05, Table 3) and smaller in EA group than in model group (P < 0.05). The plasma of MC was stained purple, while nuclei were stained dark blue, scattered in mucous and submucous layers, or gathered into groups or lined up. The cells were round, oval, shuttle-like, and erose in shape. Small cells had little plasma and were clear in shape, while big cells had more plasma and were unclear in shape.
SP and SPR expression in colon tissue of rats
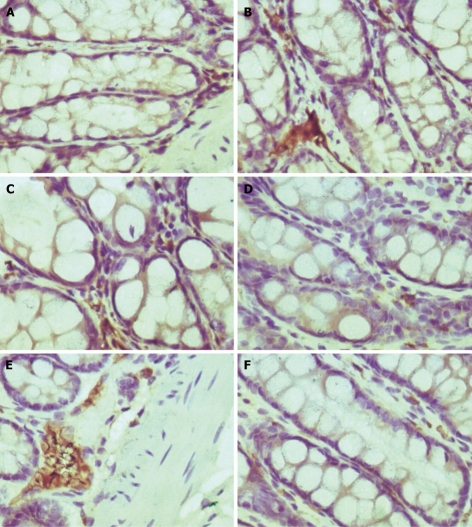
The expression level of SP and SPR was higher in model group than in normal group (P < 0.05), which was decreased after EA treatment (P < 0.05, Table 4, Figure 1).
Table 4.
SP and SPR expression in colonic membrane of rats in different groups (n = 7) (mean ± SD)
| Group |
SP expression |
SPR expression |
||
| Optical density | Expressing area (μm2) | Optical density | Expressing area (μm2) | |
| Normal group | 14.21 ± 0.64 | 1772.77 ± 176.34 | 14.86 ± 0.48 | 362.65 ± 41.96 |
| Model group | 18.21 ± 1.07a | 3157.31 ± 304.95a | 16.36 ± 1.14a | 532.83 ± 105.60a |
| EA group | 16.29 ± 0.95ac | 2020.09 ± 116.31ac | 13.71 ± 0.70ac | 340.02 ± 29.61c |
P < 0.05 vs normal group;
P < 0.05 vs model group. SP: Substance P; SPR: Substance P receptor.
Figure 1.
Expression of substance P and its receptor in colonic tissue of rats in normal group (A, D), model group (B, E) and EA group (C, F) (× 400).
DISCUSSION
IBS is a prevalent functional gastrointestinal (GI) disorder characterized by chronic or recurrent abdominal pain or discomfort associated with altered bowel habits[16].
IBS is presumed to be a disorder of the brain-gut link[17]. Psychological stress induces colonic segmental contractions which are exaggerated in IBS patients[18,19]. Stress can alter GI function. However, the mechanism underlying stress-induced intestinal response is still unclear. Epidemiological data show that psychological stress is one of the most important etiological factors for IBS. Mental stress is one of the factors for the induction or aggravation of the symptoms of IBS[20]. Visceral hypersensitivity and dysregulation of central pain perception in the brain-gut axis play a pivotal role in the pathophysiology of IBS.
The central nervous system response to stress modulates the autonomic nervous system outflow and activates the hypothalamic-pituitary-adrenal axis[6]. Dysfunction of these systems has been proposed to be an etiological factor for IBS[7,8]. In addition, CRH, which plays an important role in the stress response[9], induces a higher adrenocorticotropic hormone (ACTH) level and a more profound enhancement of colonic motility in IBS patients than in healthy controls[10]. It has been shown that CRH increases rectal sensitivity[11]. Thus, alterations in neuroendocrine response to stress may be of importance in the pathophysiology of IBS[12].
It was reported that CRH injected into the intracerebral ventricle of rats exerts a stimulatory effect on colonic motor function by inducing spike burst activities in the proximal colon, accelerating transit, and inducing defecation[21-23]. Use of nonselective CRH1 (NBI-359565) receptor antagonists showed that colonic motor function induced by CRH is delayed in rats, suggesting that central CRH combined with CRH1 receptor can regulate the colon function[23]. In the study by Fukudo et al[10], the descending colon motility induced by CRH was greater in IBS patients than in health subjects, CRH produced duodenal phase III motor activities in 80% of healthy subjects and duodenal dysmotility in 40% of IBS patients, the time of abdominal symptoms evoked by CRH was significantly longer in IBS patients than in healthy subjects, the plasma ACTH level induced by CRH was significantly higher in IBS patients than in healthy subjects, indicating that human intestinal motility is probably modulated by exogenous CRH. The brain-gut in IBS patients may have an exaggerated response to CRH. Intravenous injection of CRH can promote the viscera sensibility in rats, which can be inhibited by CRH1 receptor antagonists[23]. This study showed that the CRH expression level in hypothalamus of rats was significantly higher in model group than in normal group (P < 0.05), which was remarkably decreased (P < 0.05) after EA treatment. No distinct difference in CRH expression was found between normal and EA groups, suggesting that EA therapy can inhibit the expression of CRH in hypothalamus of rats.
Recently, the role of probiotics in intestinal ecosystems has received great attention because of their beneficial effects on human and animal gut health[24]. It has been shown that probiotics can improve inflammation in some IBS patients and alleviate IBS symptoms such as pain[25]. It has been demonstrated in animal studies that neonatal intervention with probiotics can protect against short and long term consequences of impaired intestinal barrier function and gut-associated immune dysfunction induced by neonatal stress, reduce elevated corticosterone levels in pups with early psychological trauma (maternal deprivation), suggesting that normalization of HPA-axis activity is mediated by the effect of probiotics on gut function[26-30]. Further study is needed to explore the relation between acupuncture and probiotics used in treatment of IBS.
The pathological mechanism of IBS is not clear, but it is believed to be associated with alterations in mentality, GI motility, and visceral sensitivity, etc. Recently, researchers have suggested the role of inflammatory cells in the pathogenesis of IBS[31]. Mucosal MC are located throughout the gut in close proximity to enteric nerves, and secrete numerous inflammatory substances including histamine, cytokines, proteases, and eicosanoids that are known to sensitize visceral sensory nerve fibers. That is why some researchers have become interested in them.
SP is closely related with the pathological change in IBS, which plays a role in stress, intestinal infection, and visceral hypersensitivity in the development of IBS[31,32]. Meanwhile, SP is a gastrointestinal peptide hormone existing in the central nervous system and gastrointestinal tract, and a signaling molecule connecting the nervous system to the immune system. Wang et al[33] reported that the expression of SP and c-fos protein in the enteric and central nervous systems of the rat model of constipation-predominant IBS is abnormal, suggesting that abnormal changes in SP may be involved in the pathogenesis of IBS, and SP containing the neural pathway may be one of the neural pathways that play an important role in the regulation of gastrointestinal function.
SP in the intestinal tract is mainly produced by nerve terminal and endocrine cells such as MC. SP in combination with its receptor exerts its effect on the homologous effector cells of stomach and intestine, leading to complicated physiologic functions such as gastrointestinal motility, sensibility, secretion and absorption. In the enteric nervous system, SP, as an enteric nervous system of neurotransmitters, can increase gastrointestinal motility, promote contraction of alimentary tract smooth muscle, reinforce colon progradation, and stimulate water and electrolyte secretion in small intestine and colon. Some researchers believe that mucosal MC can restore the function and paresthesia of intestinal tract, while others hold that there is an amplifying ring among SP, MC and sensory neurofibra. Releasing of neuropeptides from sensory nerve ending, such as SP, has a direct effect on target organs. SP in combination with its special receptor on the surface of mucosal MC can activate and degranulate MC, releasing histamine and influencing sensorineural function, which promotes SP and local blood vessels to release nerve growth factor. In this study, the increased expression of SP was closely related with the number of MC in lamina propria of rats with IBS. It was reported that MC are associated with neurofibra by membrane-membrane touch[34]. The number of MC and degranulated MC is greater in IBS patients than in healthy subjects and the activated MC are adjacent to the inner-intestinal neuroplexus[13,35]. Our previous study showed that MC in colonic mucosa and c-fos positive cells are significantly increased, EA at ST-25 and Tegaserod injected into stomach can inhibit the proliferation and activation of MC in the colon, and regulate the secretion of SP, SPR, VIP, and VIPR, but the effect of EA is obviously better than that of Tegaserod[36]. In this study, the number of MC, the optical density and positive expressing areas of SP, SPR were greater in rats with IBS than in normal rats, indicating that MC, SP and SPR are closely related with the development of IBS (P < 0.05). However, MC, SP and SPR were decreased after EA treatment (P < 0.05), suggesting that EA at ST25 and ST37 can effectively adjust the dysfunction of MC and down-regulate the expression of SP and SPR.
In conclusion, dysfunction of the central and enteric nervous systems leads to IBS. EA at ST25 and ST37 can decrease the number of MC, the expression of SP and SPR in colon, and the CRH level in hypothalamus of rats.
COMMENTS
Background
Irritable bowel syndrome (IBS) is a common disorder in clinical practice, but its pathophysiology has not been completely elucidated, which makes its treatment difficult. The authors’ previous study showed that the general therapeutic rate of electro-acupuncture (EA) at ST-25 for IBS is 84.90%. However, the regulatory effect of EA on IBS is still unknown.
Research frontiers
More and more data show that IBS is closely related with the brain-gut axis, which has becoming a hot spot of study.
Innovations and breakthroughs
The results of the authors’ study have proved that EA at ST25 and ST37 is effective against IBS. EA at ST25 and ST37 exerts its effect on IBS by decreasing the number of mucosal mast cells and down-regulating the expression of substance P, substance P receptor in colon and corticotropin-releasing hormone in hypothalamus of rats.
Applications
The experimental and clinical data can be used in further study on EA in treatment of IBS.
Peer review
This study is interesting. Its findings are useful for the treatment of IBS.
Footnotes
Supported by Open Fund of Key Laboratory of Acupuncture Combined with Medication (Nanjing University of TCM), Ministry of Education, No. KJA200809; Shanghai Rising-Star Program, No. 08QA14064; Shanghai Leading Academic Discipline Project, No. S30304
Peer reviewers: Mohammad Abdollahi, Professor, Faculty of Pharmacy, Tehran University of Medical Sciences, Tehran 1417614411, Iran; Yvan Vandenplas, Professor, Department of Pediatrics, AZ-VUB, Laarbeeklaan 101, Brussels 1090, Belgium
S- Editor Tian L L- Editor Wang XL E- Editor Yin DH
References
- 1.Jones J, Boorman J, Cann P, Forbes A, Gomborone J, Heaton K, Hungin P, Kumar D, Libby G, Spiller R, et al. British Society of Gastroenterology guidelines for the management of the irritable bowel syndrome. Gut. 2000;47 Suppl 2:ii1–ii19. doi: 10.1136/gut.47.suppl_2.ii1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Torii A, Toda G. Management of irritable bowel syndrome. Intern Med. 2004;43:353–359. doi: 10.2169/internalmedicine.43.353. [DOI] [PubMed] [Google Scholar]
- 3.Talley NJ. Irritable bowel syndrome. Intern Med J. 2006;36:724–728. doi: 10.1111/j.1445-5994.2006.01217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu HR, Yang Y, Wu HG. Clinical Study on Acupuncture in Treating Diarrhea-predominant Irritable Bowel Syndrome. J Acupunct Tuina Sci. 2008;6:360–362. [Google Scholar]
- 5.Whitehead WE, Crowell MD, Robinson JC, Heller BR, Schuster MM. Effects of stressful life events on bowel symptoms: subjects with irritable bowel syndrome compared with subjects without bowel dysfunction. Gut. 1992;33:825–830. doi: 10.1136/gut.33.6.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mayer EA. The neurobiology of stress and gastrointestinal disease. Gut. 2000;47:861–869. doi: 10.1136/gut.47.6.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aggarwal A, Cutts TF, Abell TL, Cardoso S, Familoni B, Bremer J, Karas J. Predominant symptoms in irritable bowel syndrome correlate with specific autonomic nervous system abnormalities. Gastroenterology. 1994;106:945–950. doi: 10.1016/0016-5085(94)90753-6. [DOI] [PubMed] [Google Scholar]
- 8.Munakata J, Mayer EA, Chang L, Schmulson M, Liu M, Tougas G, Kamath M, Naliboff B. Autonomic and neuroendocrine responses to recto-sigmoid stimulation. Gastroenterology. 1998;114:A808. [Google Scholar]
- 9.Taché Y, Martinez V, Million M, Rivier J. Corticotropin-releasing factor and the brain-gut motor response to stress. Can J Gastroenterol. 1999;13 Suppl A:18A–25A. doi: 10.1155/1999/375916. [DOI] [PubMed] [Google Scholar]
- 10.Fukudo S, Nomura T, Hongo M. Impact of corticotropin-releasing hormone on gastrointestinal motility and adrenocorticotropic hormone in normal controls and patients with irritable bowel syndrome. Gut. 1998;42:845–849. doi: 10.1136/gut.42.6.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lembo T, Plourde V, Shui Z, Fullerton S, Mertz H, Tache Y, Sytnik B, Munakata J, Mayer E. Effects of the corticotropin-releasing factor (CRF) on rectal afferent nerves in humans. Neurogastroenterol Motil. 1996;8:9–18. doi: 10.1111/j.1365-2982.1996.tb00237.x. [DOI] [PubMed] [Google Scholar]
- 12.Mayer EA, Naliboff BD, Chang L, Coutinho SV. V. Stress and irritable bowel syndrome. Am J Physiol Gastrointest Liver Physiol. 2001;280:G519–G524. doi: 10.1152/ajpgi.2001.280.4.G519. [DOI] [PubMed] [Google Scholar]
- 13.Yang Y, Zhou D, Zhang W. [Mast cells of ileocecal junction in irritable bowel syndrome] Zhonghua Neike Zazhi. 1997;36:231–233. [PubMed] [Google Scholar]
- 14.Ansel JC, Brown JR, Payan DG, Brown MA. Substance P selectively activates TNF-alpha gene expression in murine mast cells. J Immunol. 1993;150:4478–4485. [PubMed] [Google Scholar]
- 15.Al-Chaer ED, Kawasaki M, Pasricha PJ. A new model of chronic visceral hypersensitivity in adult rats induced by colon irritation during postnatal development. Gastroenterology. 2000;119:1276–1285. doi: 10.1053/gast.2000.19576. [DOI] [PubMed] [Google Scholar]
- 16.Williams CL, Villar RG, Peterson JM, Burks TF. Stress-induced changes in intestinal transit in the rat: a model for irritable bowel syndrome. Gastroenterology. 1988;94:611–621. doi: 10.1016/0016-5085(88)90231-4. [DOI] [PubMed] [Google Scholar]
- 17.Videlock EJ, Chang L. Irritable bowel syndrome: current approach to symptoms, evaluation, and treatment. Gastroenterol Clin North Am. 2007;36:665–685, x. doi: 10.1016/j.gtc.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 18.Fukudo S, Suzuki J. Colonic motility, autonomic function, and gastrointestinal hormones under psychological stress on irritable bowel syndrome. Tohoku J Exp Med. 1987;151:373–385. doi: 10.1620/tjem.151.373. [DOI] [PubMed] [Google Scholar]
- 19.Fukudo S, Nomura T, Muranaka M, Taguchi F. Brain-gut response to stress and cholinergic stimulation in irritable bowel syndrome. A preliminary study. J Clin Gastroenterol. 1993;17:133–141. doi: 10.1097/00004836-199309000-00009. [DOI] [PubMed] [Google Scholar]
- 20.Frank L, Kleinman L, Rentz A, Ciesla G, Kim JJ, Zacker C. Health-related quality of life associated with irritable bowel syndrome: comparison with other chronic diseases. Clin Ther. 2002;24:675–689; discussion 674. doi: 10.1016/s0149-2918(02)85143-8. [DOI] [PubMed] [Google Scholar]
- 21.Okano S, Nagaya H, Ikeura Y, Natsugari H, Inatomi N. Effects of TAK-637, a novel neurokinin-1 receptor antagonist, on colonic function in vivo. J Pharmacol Exp Ther. 2001;298:559–564. [PubMed] [Google Scholar]
- 22.Martínez V, Wang L, Rivier JE, Vale W, Taché Y. Differential actions of peripheral corticotropin-releasing factor (CRF), urocortin II, and urocortin III on gastric emptying and colonic transit in mice: role of CRF receptor subtypes 1 and 2. J Pharmacol Exp Ther. 2002;301:611–617. doi: 10.1124/jpet.301.2.611. [DOI] [PubMed] [Google Scholar]
- 23.Williams CL, Peterson JM, Villar RG, Burks TF. Corticotropin-releasing factor directly mediates colonic responses to stress. Am J Physiol. 1987;253:G582–G586. doi: 10.1152/ajpgi.1987.253.4.G582. [DOI] [PubMed] [Google Scholar]
- 24.Barouei J, Adams MC, Hodgson DM. Prophylactic role of maternal administration of probiotics in the prevention of irritable bowel syndrome. Med Hypotheses. 2009;73:764–767. doi: 10.1016/j.mehy.2009.04.023. [DOI] [PubMed] [Google Scholar]
- 25.Andresen V, Camilleri M. Irritable bowel syndrome: recent and novel therapeutic approaches. Drugs. 2006;66:1073–1088. doi: 10.2165/00003495-200666080-00004. [DOI] [PubMed] [Google Scholar]
- 26.Gareau MG, Jury J, MacQueen G, Sherman PM, Perdue MH. Probiotic treatment of rat pups normalises corticosterone release and ameliorates colonic dysfunction induced by maternal separation. Gut. 2007;56:1522–1528. doi: 10.1136/gut.2006.117176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.García-Ródenas CL, Bergonzelli GE, Nutten S, Schumann A, Cherbut C, Turini M, Ornstein K, Rochat F, Corthésy-Theulaz I. Nutritional approach to restore impaired intestinal barrier function and growth after neonatal stress in rats. J Pediatr Gastroenterol Nutr. 2006;43:16–24. doi: 10.1097/01.mpg.0000226376.95623.9f. [DOI] [PubMed] [Google Scholar]
- 28.Gareau MG, Jury J, Perdue MH. Neonatal maternal separation of rat pups results in abnormal cholinergic regulation of epithelial permeability. Am J Physiol Gastrointest Liver Physiol. 2007;293:G198–G203. doi: 10.1152/ajpgi.00392.2006. [DOI] [PubMed] [Google Scholar]
- 29.Eutamene H, Bueno L. Role of probiotics in correcting abnormalities of colonic flora induced by stress. Gut. 2007;56:1495–1497. doi: 10.1136/gut.2007.124040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barbara G, De Giorgio R, Stanghellini V, Cremon C, Corinaldesi R. A role for inflammation in irritable bowel syndrome? Gut. 2002;51 Suppl 1:i41–i44. doi: 10.1136/gut.51.suppl_1.i41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ringel Y, Sperber AD, Drossman DA. Irritable bowel syndrome. Annu Rev Med. 2001;52:319–338. doi: 10.1146/annurev.med.52.1.319. [DOI] [PubMed] [Google Scholar]
- 32.Dong WZ, Zou DW, Li ZS, Zou XP, Zhu AY, Xu GM, Yin N, Gong YF, Sun ZX, Man XH. Study of visceral hypersensitivity in irritable bowel syndrome. Chin J Dig Dis. 2004;5:103–109. doi: 10.1111/j.1443-9573.2004.00168.x. [DOI] [PubMed] [Google Scholar]
- 33.Wang WF, Yang YS, Peng LH, Sun G. Alternation of substance P-containing neural pathways in a rat model of irritable bowel syndrome with rectal distension. Chin J Dig Dis. 2006;7:211–218. doi: 10.1111/j.1443-9573.2006.00273.x. [DOI] [PubMed] [Google Scholar]
- 34.Dong WZ, Zhou XP, Zhou DW, Li ZS, Xu GM, Zhou AY, Gong YF. The relationship between mast cell and neuropeptide fibre of colonic mucous membrane in irritable bowel syndrome. Dier Junyi Daxue Xuebao. 2003;24:147–150. [Google Scholar]
- 35.Park CH, Joo YE, Choi SK, Rew JS, Kim SJ, Lee MC. Activated mast cells infiltrate in close proximity to enteric nerves in diarrhea-predominant irritable bowel syndrome. J Korean Med Sci. 2003;18:204–210. doi: 10.3346/jkms.2003.18.2.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu HG, Jiang B, Zhou EH, Shi Z, Shi DR, Cui YH, Kou ST, Liu HR. Regulatory mechanism of electroacupuncture in irritable bowel syndrome: preventing MC activation and decreasing SP VIP secretion. Dig Dis Sci. 2008;53:1644–1651. doi: 10.1007/s10620-007-0062-4. [DOI] [PubMed] [Google Scholar]