Abstract
The term biloma describes an encapsulated collection of bile within the abdomen, usually secondary to bile duct disruption. The commonest causes reported in the literature are iatrogenic (secondary to hepatobiliary surgery), trauma or complications due to choledocholithiasis. A few cases have been reported as complications of cholangiocarcinoma or acute cholecystitis. We report the case of a 64-year-old man initially diagnosed with a non-obstructive malignancy of the pancreas, who developed a spontaneous intrahepatic biloma 8 mo later. This was identified following a 1-wk history of fever, rigors and icterus. The biloma was identified on computed tomography and subsequently drained under ultrasound guidance. Forty-eight hours later, a stent was inserted endoscopically into his common bile duct and he made an uneventful in-hospital recovery. We believe this is the first documented case of spontaneous intrahepatic biloma to occur secondary to pancreatic malignancy.
Keywords: Obstructive jaundice, Endoscopic retrograde cholangiopancreatography, Computed tomography, Choledocholithiasis, Bile duct diseases
INTRODUCTION
In 1979, Gould and Patel[1] described the case of a 32-year-old man who was found to have a large encapsulated collection of bile outside the biliary tree, secondary to a tear in the right hepatic duct. This followed a traumatic insult to the upper abdomen. The patient proceeded to have this collection drained and a T-tube was inserted to permit bile drainage. Since the initial description, there have been a few documented cases of spontaneous biloma formation, usually in the context of choledocholithiasis. The current case is sufficiently exceptional with regard to location, etiology and mode of presentation to merit a report.
CASE REPORT
A 64-year-old man was referred to the gastroenterology clinic by his general practitioner (GP) with constant upper abdominal pain that was worse after eating. In addition, the patient had been suffering from alternating constipation and loose stools. He denied the passage of blood in his motions or any weight loss.
A full blood count revealed normocytic anemia (hemoglobin 123 g/L; mean corpuscular volume: 88.4 fL). Renal and liver function tests were normal. Given the features on presentation, a colonoscopy was suggested, however, he was reluctant to go ahead with the procedure. As an alternative, a computed tomography (CT) pneumocolon was arranged.
Although no significant polyps or other colonic abnormalities were seen, there was an irregular 3 cm × 4.5 cm mass arising from the neck/body of the pancreas, with multiple lymph nodes in the peripancreatic region. Further assessment with a dual-phase CT scan of the pancreas was performed (Figure 1). As a result of encasement of the superior mesenteric artery and portal veins, the tumor was deemed inoperable. An ultrasound-guided biopsy of the lesion was undertaken, and histology confirmed adenocarcinoma of the pancreas.
Figure 1.
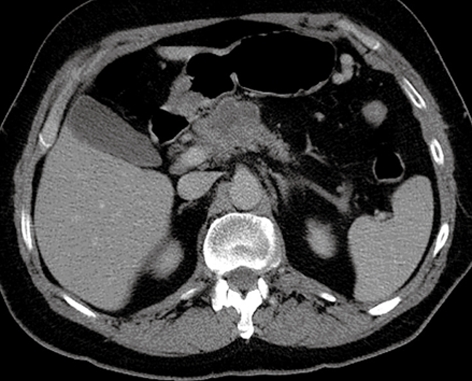
CT pneumocolon, which suggested the presence of advanced pancreatic malignancy.
The patient went on to complete three courses of gemcitabine chemotherapy but further staging CT demonstrated slowly progressive disease over a 3-mo period.
Six months later, the patient was referred by his GP to the on-call medical team with a week-long history of nausea, anorexia and new-onset jaundice. Upon examination, he was tender in the epigastrium and right upper quadrant, with no overt peritonism. His blood tests were as follows: bilirubin, 164 μmol/L; alanine aminotransferase, 84 IU/L; alkaline phosphatase, 5567 IU/L; albumin, 35 g/L; C-reactive protein (CRP), > 160 mg/L; prothrombin time, 16.3 s; and white blood cell count, 12.9 × 109/L.
Repeat CT of the pancreas was undertaken, which demonstrated a dilated common bile duct (CBD) with external compression from enlargement of the pancreatic tumor, and a large, intrahepatic cystic lesion that measured 12 cm × 9 cm, adjacent to the gallbladder (Figure 2).
Figure 2.
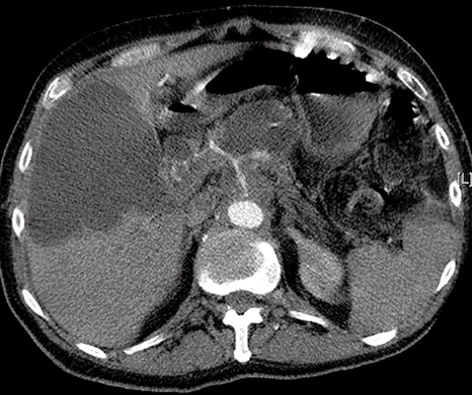
Large intrahepatic cystic lesion with bile duct compression caused by enlargement of the pancreatic neoplasm.
Ultrasound-guided drainage was performed and yielded frank bile in keeping with an intrahepatic biloma. Microbiological analysis of the fluid did not reveal the presence of any pathogenic organisms. Endoscopic retrograde cholangiopancreatography (ERCP) demonstrated a smooth stricture of the distal CBD and a dilated common hepatic duct above. Contrast material was seen to be flowing into the biloma, which implied a connection with the intrahepatic ducts (Figure 3). The stricture was stented and drainage from the biloma ceased within 48 h following insertion.
Figure 3.
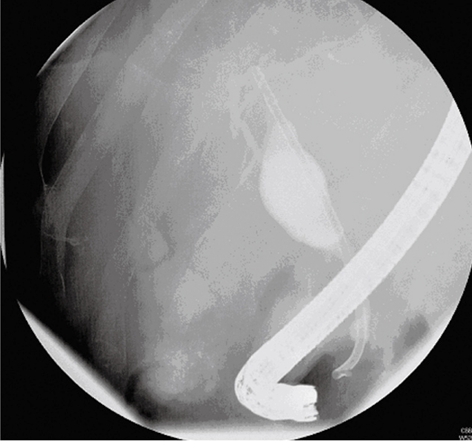
ERCP demonstrating a smooth stricture in the distal CBD, with dilated common hepatic ducts above. Contrast material was seen to flow within the biloma collection, which implied communication with the intrahepatic ducts.
DISCUSSION
The first reported case of a biloma was reported by Gould and Patel in 1979[1]. They reported extrahepatic bile leakage post trauma to the right upper quadrant of the abdomen. The bile accumulated in an encapsulated form. Although originally described as a bilious collection outside the liver, the term biloma has been extended to include any such lesion that may be intrahepatic but anatomically outside the biliary tree[2]. The majority of bilomas are iatrogenic and follow transhepatic cholangiography, liver biopsy, ERCP or cholecystectomy. Biloma also has been recognized to arise following external trauma[3]. Spontaneous biloma is exceedingly rare, and the majority occur secondary to choledocholithiasis or cholangiocarcinoma[4]. Rarer causes have been reported in the context of sickle cell disease[5] or as a complication of hepatic infarction and abscess formation. To the best of our knowledge, biloma that occurs secondary to primary pancreatic malignancy has not been reported previously in the literature.
As diagnostic techniques continue to evolve, an increasing number of cases have been identified, but the exact mechanism behind spontaneous biloma formation is still unknown. Postulated pathogenic mechanisms are Sphincter of Oddi spasm, CBD tumor or calculus obstruction that results in increased intraductal pressure, bile duct necrosis and rupture of the bile duct. As a result of the relatively slow onset of ductal obstruction that occurs in the context of a pancreatic neoplasm, such an acute elevation in biliary pressure is unusual (cf. impacted CBD stone). The size and location of biloma is influenced by the cause of rupture, location and size of bile leak, and rate of absorption by the peritoneum. Most bilomas are secondary to CBD rather than hepatic duct perforation[6].
There is no difference in the incidence between males and females, but the condition is found more often in the sixth to seventh decades of life. The age predominance may reflect that of the underlying etiological factor rather than that of developing the complication. Presentation is nonspecific, with abdominal pain, usually in the right upper quadrant (although a few reported cases of bile migration to the left subphrenic space have been documented, which has given rise to a predominance of pain on the left side). Fever may be accompanied by jaundice and abdominal distension. Extreme cases that result in bilious ascites also have been reported[7]. In our case, there was no history of recent hepatobiliary intervention (pancreatic biopsy had been performed 6 mo prior to presentation, but no evidence of biloma was seen on interval scanning). There were complaints of fever, rigors, anorexia and scleral icterus. Blood tests may show neutrophil leukocytosis, elevated CRP and obstructive liver function tests. Blood cultures may show Gram-negative bacteremia. Biloma can be picked up on ultrasound, CT or magnetic resonance imaging. Despite advancing imaging modalities, biloma may be difficult to differentiate from large cystic metastasis, seroma, angioma or lymphocele. Ultrasound becomes useful in this situation, with a definitive diagnosis being made following radiologically guided aspiration. Once fluid is obtained, microbiological testing is mandatory to exclude the presence of coexisting infection. ERCP is particularly useful in diagnosing an active leak; this may also allow therapeutic intervention[8]. Precise location of the biloma allows for percutaneous drainage, which negates the need for surgical intervention. Endoscopic intervention includes sphincterotomy with stone extraction, if appropriate, to lower biliary pressure. Placement of a stent in more distal lesions is an option, as this reduces the pressure gradient into the duodenum and facilitates forward flow of bile. This also relieves obstruction from lesions that narrow the biliary tree. In our case, the latter approach was used to overcome the obstruction caused by the large pancreatic tumor.
Surgical management remains contentious but can be useful in cases of ongoing leakage despite endoscopic therapy. The goals are to halt abdominal contamination from bile by means of peritoneal drainage, surgical closure of active leaks, and T-tube drainage[7-9].
Footnotes
Peer reviewer: Dr. Massimo Raimondo, Division of Gastroenterology and Hepatology, Mayo Clinic,4500 San Pablo Road, Jacksonville, FL 32224, United States
S- Editor Li LF L- Editor Kerr C E- Editor Zheng XM
References
- 1.Gould L, Patel A. Ultrasound detection of extrahepatic encapsulated bile: "biloma". AJR Am J Roentgenol. 1979;132:1014–1015. doi: 10.2214/ajr.132.6.1014. [DOI] [PubMed] [Google Scholar]
- 2.Kuligowska E, Schlesinger A, Miller KB, Lee VW, Grosso D. Bilomas: a new approach to the diagnosis and treatment. Gastrointest Radiol. 1983;8:237–243. doi: 10.1007/BF01948126. [DOI] [PubMed] [Google Scholar]
- 3.Vazquez JL, Thorsen MK, Dodds WJ, Quiroz FA, Martinez ML, Lawson TL, Stewart ET, Foley WD. Evaluation and treatment of intraabdominal bilomas. AJR Am J Roentgenol. 1985;144:933–938. doi: 10.2214/ajr.144.5.933. [DOI] [PubMed] [Google Scholar]
- 4.Fujiwara H, Yamamoto M, Takahashi M, Ishida H, Ohashi O, Onoyama H, Takeyama Y, Kuroda Y. Spontaneous rupture of an intrahepatic bile duct with biloma treated by percutaneous drainage and endoscopic sphincterotomy. Am J Gastroenterol. 1998;93:2282–2284. doi: 10.1111/j.1572-0241.1998.00636.x. [DOI] [PubMed] [Google Scholar]
- 5.Middleton JP, Wolper JC. Hepatic biloma complicating sickle cell disease. A case report and a review of the literature. Gastroenterology. 1984;86:743–744. [PubMed] [Google Scholar]
- 6.Kang SB, Han HS, Min SK, Lee HK. Nontraumatic perforation of the bile duct in adults. Arch Surg. 2004;139:1083–1087. doi: 10.1001/archsurg.139.10.1083. [DOI] [PubMed] [Google Scholar]
- 7.Hartle RJ, McGarrity TJ, Conter RL. Treatment of a giant biloma and bile leak by ERCP stent placement. Am J Gastroenterol. 1993;88:2117–2118. [PubMed] [Google Scholar]
- 8.Lilly JR, Weintraub WH, Altman RP. Spontaneous perforation of the extrahepatic bile ducts and bile peritonitis in infancy. Surgery. 1974;75:664–673. [PubMed] [Google Scholar]
- 9.Jackson BT, Saunders P. Perforated choledochus cyst. Br J Surg. 1971;58:38–42. doi: 10.1002/bjs.1800580107. [DOI] [PubMed] [Google Scholar]


