Abstract
Intrahepatic cholangiocarcinoma (ICC) is a relatively rare and highly fatal neoplasm that arises from the biliary epithelium. Prognosis is generally poor and survival is limited to a few months. Here we present a case of advanced ICC successfully treated by chemosensitivity test-guided systemic chemotherapy combining S-1 and cisplatin (CDDP). A 65-year-old woman with a liver tumor was referred to our hospital on November 21, 2007. Abdominal ultrasonography and computed tomography (CT) showed low-density masses of 50 and 15 mm in diameter, respectively in segment VIII of the liver and in the enlarged lymph node in the para-aorta. Ultrasonography-guided fine needle biopsy diagnosed the tumors as ICC. Since the patient was inoperable for lymph node metastasis, she underwent systemic chemotherapy with gemcitabine. Six months after initiation of chemotherapy, CT revealed ICC progression in the liver and pleural dissemination with pleural effusion. The patient was admitted to our hospital for anticancer drug sensitivity testing on June 9, 2008. Based on the sensitivity test results, we elected to administer systemic chemotherapy combining S-1 and CDDP. Two months into the second chemotherapy treatment, CT revealed a reduction of the tumors in the liver and lymph node and a decrease in pleural effusion. After eight cycles of the second chemotherapy, 17 mo after ICC diagnosis, she is alive and well with no sign of recurrence. We conclude that chemosensitivity testing may effectively determine the appropriate chemotherapy regimen for advanced ICC.
Keywords: Chemosensitivity testing, Cholangiocarcinoma, Cisplatin, Liver neoplasms, Gemcitabine, S-1, Systemic chemotherapy
INTRODUCTION
Intrahepatic cholangiocarcinoma (ICC) is a relatively rare and highly fatal neoplasm that arises from the biliary epithelium. Radical surgery is currently the optimal therapy for ICC with curative potential. However, most patients present with advanced disease at the time of diagnosis. Prognosis in these patients is poor and survival is limited to a few months[1].
Biliary tract carcinoma (BTC) has traditionally been divided into cancers of the gallbladder, the extrahepatic bile ducts, ampulla of Vater, whereas ICC has been classified as liver cancer. Lately, however the term BTC has been used to include the gallbladder, the extrahepatic bile ducts, ICC and the ampulla of Vater.
Chemotherapy has been performed in cases of unresectable advanced ICC and postoperative recurrence of ICC. However, a standard chemotherapeutic regimen has not yet been established for ICC. There are phase II trials that support the following combinations: gemcitabine/cisplatin (CDDP), gemcitabine/oxaliplatin, gemcitabine/capecitabine, and 5-fluorouracil in unresectable or metastatic ICC[2].
Chemosensitivity testing using surgical material is an established method to evaluate tumor response prior to chemotherapy[3-5]. Sensitivity, specificity and accuracy of chemosensitivity testing were reportedly 82.7%, 70.7% and 73.6%, respectively[6]. Several methods are established to measure cancer cell viability[7]. Recently, chemosensitivity testing for gastric cancer treatment has been approved in Japan. However, it has seldom been performed for ICC, since ICC occurs more rarely than other gastrointestinal malignancies. The adenosine triphosphate (ATP) assay is a highly sensitive and precise method for measuring cell viability, and only a few dozen cells are necessary for the ATP assay[8,9]. Very few reports of chemosensitivity testing used surgical material from ICC. This is the first report concerning chemosensitivity testing using the ATP assay for patients with unresectable ICC. We present a case of advanced ICC successfully treated by chemosensitivity test-guided systemic chemotherapy combining S-1 and CDDP.
CASE REPORT
A 65-year-old woman was examined in a follow-up visit at a local hospital for fatty liver 1 year post-diagnosis on October 30, 2007. Although she exhibited no symptoms, abdominal ultrasonography revealed tumors in the liver, and she was referred to our hospital on November 21, 2007. Her past medical and family histories were not remarkable. She did not consume alcohol. On admission, her conjunctivae were not jaundiced, and heart and respiratory sounds were normal. The liver, spleen and tumor were not palpable.
Laboratory findings on admission were as follows: aspartate aminotransferase 24 IU/L (normal, 13-33 IU/L), alanine aminotransferase 39 IU/L (normal, 6-27 IU/L), γ-glutamyl transpeptidase 26 IU/L (normal, 10-47 IU/L) and alkaline phosphatase 240 IU/L (normal, 115-359 IU/L). Assays for hepatitis B surface antigen and hepatitis C virus antibody were negative. All tumor markers tested showed normal values: specifically, 1.8 ng/mL for CEA (criterion, < 5.0), 5.8 U/mL for CA 19-9 (criterion, < 37), 6.2 ng/mL for AFP (criterion, < 10.0), 10 mAU/mL for PIVKAII (criterion, < 40).
Abdominal ultrasound and computed tomography (CT) scan with contrast enhancement revealed a low-density mass, measuring 50 and 15 mm in diameter, in segment VIII of the liver. The tumor did not show enhancement during the arterial phase but did show peripheral rim enhancement during the portal phase. The CT scan also revealed an enlarged lymph node in the para-aorta (Figure 1). The portal vein was intact until the third branch. Chest X-ray and a CT scan revealed no pleural metastasis.
Figure 1.
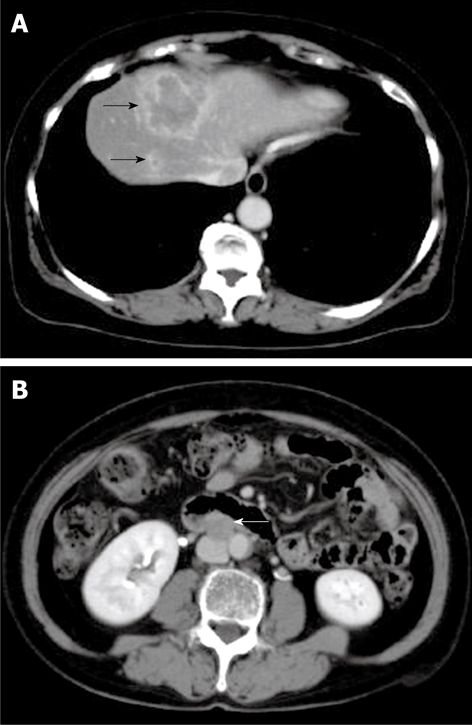
Computed tomography (CT) of the tumor on first admission. CT shows a low-density lesion with rim enhancement in segment VIII of the liver (black arrows) (A) and enlarged lymph node in the para-aorta (white arrow) (B).
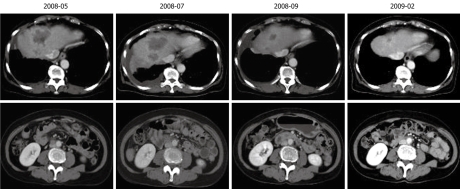
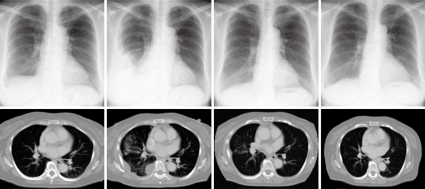
Ultrasonography-guided fine needle biopsy was performed at the main tumor of the liver on November 22, 2007. Microscopic examinations showed tubular adenocarcinoma (Figure 2). Gastroendoscopy and colonoscopy did not show any other malignancy. Subsequently, the tumor was diagnosed as ICC with intrahepatic metastasis and lymph node metastasis. Since the patient was inoperable for lymph nodes metastasis, she underwent systemic chemotherapy with gemcitabine (800 mg/m2, 30 min iv infusion), which was administered on days 1, 8 and 15, and repeated every 4 wk. Six months after initiation of chemotherapy, CT revealed ICC progression in the liver and pleural dissemination with pleural effusion (Figures 3 and 4). The patient was admitted to our hospital for anticancer drug sensitivity testing on June 9, 2008. Cultures and ATP assays were performed as described previously[5]. Cell viability was evaluated by measuring the intracellular ATP level using bioluminescence as described by Kangas et al[10]. Based on the sensitivity test results, we elected to administer systemic chemotherapy combining S-1 and CDDP (S-1 80 mg/m2 per day orally administered for 3 wk, CDDP 60 mg/m2 iv infusion administered on day 8, and repeated every 5 wk) (Figure 5). She had symptoms of cough in July 2008. Two months into the second chemotherapy treatment, she had no symptoms and CT revealed a reduction in the tumors of the liver and lymph node and a decrease in pleural effusion (Figures 3 and 4). Side effects and complications of chemotherapy were tolerable, and she experienced only grade-1 nausea. CDDP added to S-1 might worsen nausea. After eight cycles of the second chemotherapy regime, 17 mo after ICC diagnosis, she is alive and well with no sign of recurrence.
Figure 2.
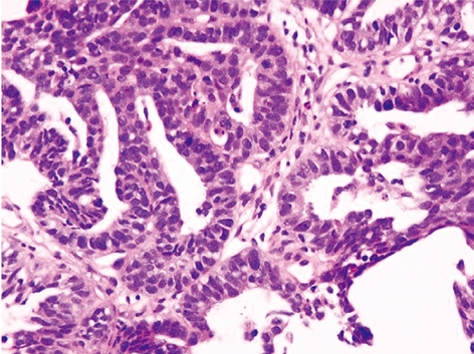
Hematoxylin-eosin staining. Cuboidal cancer cells with chromatin-rich nuclei had proliferated invasively, forming indistinct glandular structures.
Figure 3.
Reduction in tumors in the liver and lymph node following chemotherapy.
Figure 4.
Decreased pleural metastases following chemotherapy.
Figure 5.
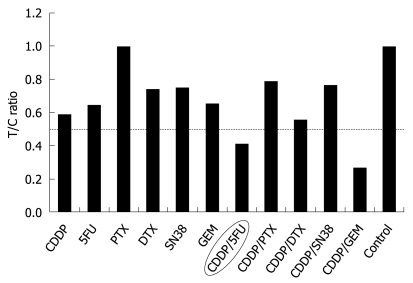
Cell viability evaluated by measuring the intracellular ATP level using bioluminescence. The T/C (treated/control) ratio, or the ratio of ATP quantity of a tumor sample treated with anticancer drugs to that of the control, was used as the index of chemosensitivity.
DISCUSSION
Although radical surgery is considered the most effective therapy for ICC, only 20% of patients present with resectable disease[11]. Without surgery, ICC is a rapidly fatal disease with a 5-year survival rate of less than 5%[12], while in curative resections the 5-year survival rate approaches 20%-35% in cases with negative surgical margins[13]. In the present case, to our surprise, the patient with unresectable ICC survived more than 17 mo with chemotherapy. The role of systemic chemotherapy in unresectable ICC is undefined. Although chemotherapy has been reported to be more beneficial than the best supportive care[14], no standard chemotherapy regimen has yet been identified. Most promising approaches involve the use of single agents such as gemcitabine, which has been shown to be an effective therapy for BTC in phase II trials[15,16]. Response rates for gemcitabine ranged from 8% to 36% and overall survival times from 6.3 to 16 mo. S-1 is a novel oral fluoropyrimidine agent, which contains tegafur, gimeracil and oteracil potassium. Gimeracil is a competitive inhibitor of dihydropyrimidine dehydrogenase and achieves higher concentrations of 5-fluorouracil in plasma and tumor tissues[17]. Fluoropyrimidines have known synergistic effects with CDDP[18], and combinations of S-1 and CDDP are reportedly effective therapies for patients with advanced BTC in phase II trials[19]. In the present case, we selected gemcitabine as first line chemotherapy, because gemcitabine has been extensively evaluated in patients with metastatic BTC.
Chemosensitivity testing using surgical material is an established method for evaluation of tumor response prior to chemotherapy. Several methods have been established for measurement of cancer cell viability. However, it has seldom been performed in ICC, since ICC occurs more rarely than gastrointestinal malignancies and the prognosis of ICC is poor. There have been few reports of chemosensitivity testing using surgical material for ICC without the ATP assay[20-22]. The ATP assay is a highly sensitive and precise method used to measure cell viability. The ATP assay has been shown to be a good predictor of response to chemotherapy in other tissue types, such as breast cancer, ovarian cancer, colorectal adenocarcinoma, melanoma and lung cancer. The assay predicts that anticancer drugs with a lower treated/control (T/C) ratio (< 0.6) are more sensitive. In the present case, based on the results of chemosensitivity testing, we selected systemic chemotherapy combining S-1 and CDDP. Ultimately, in vitro ATP chemosensitivity testing was useful. Since 5-fluorouracil has considerable toxic effects and entails the inconvenience of continuous iv infusions, we selected S-1 as an alternative to 5-fluorouracil. Although the T/C ratio combining gemcitabine and CDDP was more sensitive than that combining S-1 and CDDP, we did not select the former combination since gemcitabine administration on its own did not halt disease progression.
Footnotes
Peer reviewers: Gerardo Rosati, MD, Medical Oncology Unit, “S. Carlo” Hospita, Via Potito Petrone, 1, Potenza 85100, Italy; Susumu Ohwada, Associate Professor, Department of Surgery, Gunma University Graduate School of Medicine, 3-39-15 Shoma-Machi, Maebashi 371-8511, Japan
S- Editor Tian L L- Editor Cant MR E- Editor Zheng XM
References
- 1.Sirica AE. Cholangiocarcinoma: molecular targeting strategies for chemoprevention and therapy. Hepatology. 2005;41:5–15. doi: 10.1002/hep.20537. [DOI] [PubMed] [Google Scholar]
- 2.Hezel AF, Zhu AX. Systemic therapy for biliary tract cancers. Oncologist. 2008;13:415–423. doi: 10.1634/theoncologist.2007-0252. [DOI] [PubMed] [Google Scholar]
- 3.Furukawa T, Kubota T, Suto A, Takahara T, Yamaguchi H, Takeuchi T, Kase S, Kodaira S, Ishibiki K, Kitajima M. Clinical usefulness of chemosensitivity testing using the MTT assay. J Surg Oncol. 1991;48:188–193. doi: 10.1002/jso.2930480310. [DOI] [PubMed] [Google Scholar]
- 4.Yamaue H, Tanimura H, Noguchi K, Hotta T, Tani M, Tsunoda T, Iwahashi M, Tamai M, Iwakura S. Chemosensitivity testing of fresh human gastric cancer with highly purified tumour cells using the MTT assay. Br J Cancer. 1992;66:794–799. doi: 10.1038/bjc.1992.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kawamura H, Ikeda K, Takiyama I, Terashima M. The usefulness of the ATP assay with serum-free culture for chemosensitivity testing of gastrointestinal cancer. Eur J Cancer. 1997;33:960–966. doi: 10.1016/s0959-8049(97)00075-0. [DOI] [PubMed] [Google Scholar]
- 6.Kondo T, Kubota T, Tanimura H, Yamaue H, Akiyama S, Maehara Y, Tanigawa N, Kitajima M, Takagi H. Cumulative results of chemosensitivity tests for antitumor agents in Japan. Japan Research Society for Appropriate Cancer Chemotherapy. Anticancer Res. 2000;20:2389–2392. [PubMed] [Google Scholar]
- 7.Carmichael J, DeGraff WG, Gazdar AF, Minna JD, Mitchell JB. Evaluation of a tetrazolium-based semiautomated colorimetric assay: assessment of radiosensitivity. Cancer Res. 1987;47:943–946. [PubMed] [Google Scholar]
- 8.Crouch SP, Kozlowski R, Slater KJ, Fletcher J. The use of ATP bioluminescence as a measure of cell proliferation and cytotoxicity. J Immunol Methods. 1993;160:81–88. doi: 10.1016/0022-1759(93)90011-u. [DOI] [PubMed] [Google Scholar]
- 9.Andreotti PE, Cree IA, Kurbacher CM, Hartmann DM, Linder D, Harel G, Gleiberman I, Caruso PA, Ricks SH, Untch M. Chemosensitivity testing of human tumors using a microplate adenosine triphosphate luminescence assay: clinical correlation for cisplatin resistance of ovarian carcinoma. Cancer Res. 1995;55:5276–5282. [PubMed] [Google Scholar]
- 10.Kangas L, Grönroos M, Nieminen AL. Bioluminescence of cellular ATP: a new method for evaluating cytotoxic agents in vitro. Med Biol. 1984;62:338–343. [PubMed] [Google Scholar]
- 11.Jarnagin WR, Fong Y, DeMatteo RP, Gonen M, Burke EC, Bodniewicz BS J, Youssef BA M, Klimstra D, Blumgart LH. Staging, resectability, and outcome in 225 patients with hilar cholangiocarcinoma. Ann Surg. 2001;234:507–517; discussion 517-519. doi: 10.1097/00000658-200110000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Farley DR, Weaver AL, Nagorney DM. "Natural history" of unresected cholangiocarcinoma: patient outcome after noncurative intervention. Mayo Clin Proc. 1995;70:425–429. doi: 10.4065/70.5.425. [DOI] [PubMed] [Google Scholar]
- 13.Patel T, Singh P. Cholangiocarcinoma: emerging approaches to a challenging cancer. Curr Opin Gastroenterol. 2007;23:317–323. doi: 10.1097/MOG.0b013e3280495451. [DOI] [PubMed] [Google Scholar]
- 14.Glimelius B, Hoffman K, Sjödén PO, Jacobsson G, Sellström H, Enander LK, Linné T, Svensson C. Chemotherapy improves survival and quality of life in advanced pancreatic and biliary cancer. Ann Oncol. 1996;7:593–600. doi: 10.1093/oxfordjournals.annonc.a010676. [DOI] [PubMed] [Google Scholar]
- 15.Penz M, Kornek GV, Raderer M, Ulrich-Pur H, Fiebiger W, Lenauer A, Depisch D, Krauss G, Schneeweiss B, Scheithauer W. Phase II trial of two-weekly gemcitabine in patients with advanced biliary tract cancer. Ann Oncol. 2001;12:183–186. doi: 10.1023/a:1008352123009. [DOI] [PubMed] [Google Scholar]
- 16.Tsavaris N, Kosmas C, Gouveris P, Gennatas K, Polyzos A, Mouratidou D, Tsipras H, Margaris H, Papastratis G, Tzima E, et al. Weekly gemcitabine for the treatment of biliary tract and gallbladder cancer. Invest New Drugs. 2004;22:193–198. doi: 10.1023/B:DRUG.0000011797.09549.53. [DOI] [PubMed] [Google Scholar]
- 17.Ueno H, Okusaka T, Ikeda M, Takezako Y, Morizane C. Phase II study of S-1 in patients with advanced biliary tract cancer. Br J Cancer. 2004;91:1769–1774. doi: 10.1038/sj.bjc.6602208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scanlon KJ, Newman EM, Lu Y, Priest DG. Biochemical basis for cisplatin and 5-fluorouracil synergism in human ovarian carcinoma cells. Proc Natl Acad Sci USA. 1986;83:8923–8925. doi: 10.1073/pnas.83.23.8923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim YJ, Im SA, Kim HG, Oh SY, Lee KW, Choi IS, Oh DY, Lee SH, Kim JH, Kim DW, et al. A phase II trial of S-1 and cisplatin in patients with metastatic or relapsed biliary tract cancer. Ann Oncol. 2008;19:99–103. doi: 10.1093/annonc/mdm439. [DOI] [PubMed] [Google Scholar]
- 20.Shimada M, Takenaka K, Kawahara N, Yamamoto K, Shirabe K, Maehara Y, Sugimachi K. Chemosensitivity in primary liver cancers: evaluation of the correlation between chemosensitivity and clinicopathological factors. Hepatogastroenterology. 1996;43:1159–1164. [PubMed] [Google Scholar]
- 21.Ieta K, Tanaka F, Utsunomiya T, Kuwano H, Mori M. CEACAM6 gene expression in intrahepatic cholangiocarcinoma. Br J Cancer. 2006;95:532–540. doi: 10.1038/sj.bjc.6603276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.He XR, Wu XP. Difference in biological characteristics and sensitivity to chemotherapy and radiotherapy between intrahepatic and extrahepatic cholangiocarcinoma cells in vitro. Chin Med Sci J. 2008;23:54–59. doi: 10.1016/s1001-9294(09)60011-0. [DOI] [PubMed] [Google Scholar]