Abstract
Abnormal blood pressure response to exercise is reported to occur in up to a third of hypertrophic cardiomyopathy (HCM) cases and is associated with an increased risk of death, particularly in the young, but it is not known whether the HCM-causing mutation influences blood pressure response to exercise. The purpose of this article is to ascertain whether the blood pressure response to exercise differs among carriers of the R92W mutation in the cardiac troponin T gene (TNNT2), which has been associated with an increased risk of sudden cardiac death in young males; carriers of mutations in the cardiac β-myosin heavy chain gene (MYH7); and their noncarrier relatives. Thirty R92WTNNT2 carriers, 51 MYH7 mutation carriers, and 68 of their noncarrier relatives were subjected to bicycle ergonometric exercise testing to assess blood pressure response to, as well as heart rate recovery after, exercise. Additional echocardiographic and demographic details were documented for all participants. R92WTNNT2 carriers demonstrated significantly more abnormal blood pressure responses to exercise (P = .021; odds ratio 3.03; confidence interval 1.13–8.12) and smaller increases in systolic blood pressure than MYH7 mutation carriers or related noncarrier control individuals. Although abnormal blood pressure response occurred at similar frequencies in males in all groups (23%–26%), the percentage of R92WTNNT2 females with abnormal blood pressure response was 64%, compared with 25% for MYH7 and 22% for noncarriers. Therefore, these results show that blood pressure response to exercise is influenced by genotype and gender in patients with HCM.
Keywords: Hypertrophic cardiomyopathy, Abnormal blood pressure response, Survival, Troponin T, Beta-myosin, Genetic mutation
Abbreviations: Ca2+, calcium; CR, chronotropic response; DBP, diastolic blood pressure; ECG, electrocardiogram; HCM, hypertrophic cardiomyopathy; HR, heart rate; LV, left ventricle; LVM, left ventricular mass; maxLVWT, maximum left ventricular wall thickness; METs, metabolic equivalents; MYH7, beta cardiac myosin heavy chain gene; SBP, systolic blood pressure; SCD, sudden cardiac death; TNNT2, cardiac troponin T gene
Introduction
Hypertrophic cardiomyopathy (HCM), a primary cardiac muscle disease with 0.2% prevalence,1 is characterized primarily by thickening of the ventricular wall in the absence of other hypertrophy-predisposing conditions1 and by an increased risk for sudden cardiac death (SCD), which may relate to changes in cardiac energy and/or Ca2+ homeostasis or histopathological changes in the myocardium.2 HCM is typically caused by mutations in genes encoding protein components of the cardiac sarcomere (http://genetics.med.harvard.edu/∼seidman/cg3/index.html), with the three most common causes being mutations in the genes encoding beta cardiac myosin heavy chain (MYH7), cardiac troponin T (TNNT2), and myosin binding protein C. Although the phenotypes resulting from these genetic defects vary greatly, mutations in TNNT2 in humans3,4 and in animal models5,6 have frequently resulted in an increased susceptibility to SCD, often in the presence of mild or absent cardiac hypertrophy. We have previously reported an increased frequency of SCD, affecting young males in particular, in HCM families in which the R92W mutation in TNNT2 segregates,7 although the reason for this poor prognosis was not investigated at the time.
Blood pressure response to exercise testing is commonly used as an indicator of risk for SCD in patients with HCM. The failure of blood pressure to increase appropriately in response to exercise has been recorded in 8%–33% of ungenotyped patients with HCM.8–10 Abnormal blood pressure responses have been associated with an increase in cardiovascular mortality8,11 and have a reported 15% positive predictive value for SCD in HCM.11 It is not known whether the blood pressure response to exercise in HCM is influenced by genotype.
We hypothesized that the R92W mutation, which confers a high rate of SCD, may be associated with an abnormal blood pressure response to exercise. Here we investigated this hypothesis by comparing the blood pressure response to exercise in carriers of the R92WTNNT2 mutation with that of carriers of HCM-causing mutations in the cardiac β-MYH7 mutations (A797T and R403W) and noncarrier relatives.12
Methods
Subjects
The study was approved by the Institutional Review Board of the University of Stellenbosch Health Sciences Faculty (N04/03/062). Individuals belonging to consecutively identified HCM families in which either of three founder HCM-causing mutations segregate (R92WTNNT2, A797TMYH7, R403WMYH7)13 who did not have pacemakers, who were in sinus rhythm at the time of the exercise test, and who gave written informed consent were included in this study.
A medical and family history was taken, and age, sex, height, and weight as well as sudden death events that had occurred in family members were recorded for each participant. Only sudden deaths occurring in genotyped mutation carriers, or obligate heterozygotes, or individuals with a diagnosis of HCM, or with a history of syncope were considered as SCDs. Blood pressure was taken twice in the sitting position, after 5 minutes of bed rest, and the second measurement was used. Individuals were coded as hypertensive if they on more than one occasion had systolic blood pressure (SBP) ≥140 mmHg or diastolic blood pressure (DBP) ≥90 or were on antihypertensive medication.
Exercise testing
We performed 12-lead electrocardiography (ECG) on a MAC 1200ST machine after 5 minutes of rest in the supine position and from this determined a resting heart rate (HR) and whether normal sinus rhythm was present. All cardioactive medications were discontinued for at least 5 pharmacokinetic half-lives before exercise testing. Exercise testing was performed by practitioners who were blinded to the genotype status of the participants.
Maximum symptom-limited bicycle ergonometric exercise testing was performed according to the following protocol: eight stages over 24 minutes with incremental increase of 25 W every 2 minutes (25–200 W). Concurrent continuous 12-lead ECG monitoring was performed with the Norav Medical System. SBP and DBP were recorded with a mercury sphygmomanometer by a trained nurse (AG). Blood pressure values were recorded at rest, at 1-minute intervals during exercise, and at 1-minute intervals for 5 minutes during the recovery period.
We recorded the maximal workload achieved (in metabolic equivalents [METs]), total exercise time (in minutes and seconds), the maximum SBP and DBP achieved during exercise, the SBP and DBP and HR at peak exercise and at 1-minute intervals during recovery, and the percentage of predicted maximal HR for age and sex achieved by peak exercise. The predicted maximal HR was calculated as 220 minus age for men and 210 minus age for women.14 Chronotropic reserve (CR) was calculated as described elsewhere.15
Normal blood pressure response was defined as an increase of at least 20 mmHg in SBP during exercise, with a gradual decline during recovery.11 Abnormal blood pressure responses were defined as either (1) an initial increase in SBP with a subsequent fall of >20 mmHg compared with the blood pressure value at peak exercise or a continuous decrease in SBP throughout the exercise test of >20 mmHg compared with resting blood pressure (termed hypotensive responses) or (2) an increase of <20 mmHg in SBP from resting state to peak exercise (termed a flat response).
Exercise was terminated for any of the following reasons: reaching target HR, fatigue, severe dyspnea, significant chest pain, near syncope, or development of arrhythmias.
Echocardiography
Echocardiography was performed on all participating individuals, using a standardized procedure, by a single experienced echocardiographer (MR) who was blinded to mutation status. A GE Healthcare Vivid7 cardiovascular ultrasound system was used to determine maximum wall thickness at 16 left ventricular (LV) wall segments on M-mode and two-dimensional images, as described elsewhere.13 Overall maximum LV wall thickness (maxLVWT) was recorded for each individual.
LV mass (LVM) was calculated using the American Society of Echocardiography formula for estimation of LVM from two-dimensional LV linear dimensions as described elsewhere.13 Ejection fraction was determined by the biplane method of discs, using the modified Simpson's rule, while fractional shortening was calculated using LV diastolic and systolic diameters from two-dimensional echocardiography images.16 Stroke volume was calculated as
where LVd = LV end-diastolic diameter and LVs = LV end-systolic diameter. Cardiac output was calculated as CO = (SV × HR)/100, where SV is stroke volume and HR is resting HR.
LV outflow obstruction was considered to be present when a peak instantaneous subaortic gradient ≥30 mmHg was estimated with continuous wave Doppler echocardiography under resting conditions.17 Mitral regurgitation was graded semiquantitatively (1–4+ scale).18
Statistical analyses
Comparisons were made among three groups consisting of noncarriers, carriers of the R92WTNNT2, and carriers of either of the MYH7 mutations. Quantitative variables were quantile normalized before modeling.19 As the distribution of age, gender, body size, and resting hemodynamic parameters varied between groups, these factors were adjusted for in statistical comparisons of the hemodynamic responses during exercise between groups. General (quantitative) and generalized (dichotomous, categorical) linear mixed-effects models were used, as required, with adjustment for family relatedness as random factor (R package). P <.05 was considered statistically significant.
Results
Thirty R92WTNNT2 carriers from seven families, 51 MYH7 mutation carriers (27 A797TMYH7 carriers from 10 families, 24 R403WMYH7 carriers from three families) and 68 of their noncarrier relatives participated in exercise testing. The relevant clinical and demographic characteristics of these individuals are given in Table 1. Total exercise time, workload, and percentage of target HR achieved were similar between groups (Table 2). In the R92WTNNT2 families, SCD had affected predominantly young males (eight males, age at SCD 22.5 ± 10 years [mean ± standard deviation]; one female, 62 years; Table 3). Among MYH7 mutation carriers, five male (mean age at SCD 35.8 ± 17.3 years) and three female (mean age at SCD 32 ± 20.2 years) A797TMYH7 carriers experienced SCD before the study. At least five of the MYH7 and all R92WTNNT2 SCDs had occurred during periods of physical activity or emotional excitement (Table 3). One SCD in each of the R92WTNNT2 and MYH7 groups occurred in females >40 years.
Table 1.
Clinical characteristics of subjects
| MYH7 | R92WTNNT2 | Controls | |
|---|---|---|---|
| N | 51 | 30 | 68 |
| Males, % (n) | 35 (69) | 12 (41) | 31 (46) |
| Age, years | 38 (17–72) | 34 (14–66) | 34 (17–63) |
| Body surface area, kg/m2 | 1.9 (1.3–2.5) | 1.7 (1.3–2.1) | 1.8 (1.3–2.3) |
| LVM, g | 182 (71–477) | 126 (48–290) | 122 (58–272) |
| maxLVWT, mm | 13.8 (7.7–38.2) | 11.6 (6.7–30.0) | 10.1 (7.1–27.0) |
| Left atrial diameter, mm | 35.8 (20.0–65.0) | 32.9 (21.0–49.0) | 30.6 (22.0–45.0) |
| LV end-diastolic diameter, mm | 44.0 (32.0–52.0) | 40.5 (33.0–48.0) | 44.0 (37.0–60.0) |
| LV end-systolic diameter, mm | 26.8 (17.0–38.0) | 22.7 (16.1–33.2) | 28.0 (21.0–44.0) |
| Ejection fraction, % | 70 (57–81) | 71 (61–79) | 65 (52–75) |
| SV, mL | 83 (33–124) | 78 (43–103) | 80 (48–133) |
| Cardiac output at rest | 6.2 (2.0–11.3) | 5.3 (3.3–8.9) | 6.0 (4.2–10.9) |
| Resting HR, bpm | 75 (60–145) | 72 (55–118) | 78 (48–123) |
| SBPresting, mmHg | 120 (80–140) | 110 (90–130) | 115 (90–160) |
| DBPresting, mmHg | 80 (60–100) | 70 (60–90) | 80 (60–100) |
| Outflow tract gradient >30 mmHg | 0 (0) | 0 (0) | 0 (0) |
| Systolic anterior motion of the mitral valve | 4 (8) | 0 (0) | 0 (0) |
| MR = 1 | 18 (36) | 4 (15) | 4 (7) |
| MR = 2 | 1 (2) | 1 (4) | 1 (2) |
| NYHA 2 | 8 (16) | 3 (11) | 2 (3) |
| Atrial fibrillation | 2 (4) | 2 (7) | 0 (0) |
| Hypertension diagnosis | 7 (14) | 0 (0) | 2 (3) |
| maxLVWT ≥30 mm | 4 (8) | 1 (3) | 0 (0) |
| Syncope | 4 (8) | 3 (10) | 2 (3) |
| % Families with SCD | 39 | 57 | NA |
Note: Values are given as median (range) for continuous variables and as numbers (percentage) for categorical variables. No individuals were above New York Heart Association (NYHA) class 2 or mitral regurgitation (MR) score 2 (according to Zhogbi et al.33 2003). NA = not applicable.
Table 2.
Blood pressure and HR response to exercise in R92WTNNT2 and MYH7 mutation carriers and noncarrier controls
| MYH7 | R92WTNNT2 | Controls | P | |
|---|---|---|---|---|
| Total exercise time, seconds | 528 (96–1104) | 379 (266–937) | 467 (171–1319) | .339 |
| MET, kcal/min | 6.5 (2.3–12.2) | 6.5 (3.9–14.7) | 6.2 (2.9–12.1) | .660 |
| % HR achieved | 96 (74–104) | 89 (56–104) | 94 (75–108) | .417 |
| %CR | 88.0 (32.3–141.2) | 83.7 (24.8–143.1) | 89.4 (54.1–118.5) | .487 |
| HRpeak, bpm | 171 (129–203) | 169 (98–211) | 170 (123–197) | .449 |
| ΔHR, bpm | 92.0 (10–140) | 92.0 (28–136) | 90.5 (44–137) | .499 |
| Abnormal blood pressure response, any (%) | 12 (24) | 14 (48) | 16 (24) | .021 |
| Flat (%) | 10 (20) | 14 (48) | 11 (16) | .018 |
| Hypotensive (%) | 2 (4) | 0 (0) | 5 (8) | .357 |
| SBPpeak, mmHg | 160 (120–230) | 140 (100–190) | 160 (110–220) | .064 |
| ΔSBP, mmHg | 35 (0–100) | 30 (10–60) | 40 (5–100) | .020 |
| DBPpeak, mmHg | 90 (70–120) | 90 (50–100) | 90 (60–120) | .589 |
| ΔDBP, mmHg | 10 (−20–40) | 10 (−10–30) | 10 (−20–40) | .732 |
Note: Values are given as median (range) for continuous variables and as numbers (percentage) for categorical variables. P-values reflect differences between groups for quantile normalized data, adjusted for age, sex, resting mean arterial pressure, and resting HR. Δ = change in parameter between resting and peak exercise; peak = parameter at peak exercise; % HR achieved = % of predicted maximal HR achieved by peak exercise.
Table 3.
Characteristics of individuals who experienced SCD
| Families | Sex | Age, years | Circumstances of SCD |
|---|---|---|---|
| R92WTNNT2: | |||
| Ped 100 | F | 62 | Physical exertion |
| Ped 100 | M | 16 | Physical exertion |
| Ped 100 | M | 14 | Physical exertion |
| Ped 100 | M | 36 | Physical exertion |
| Ped 109 | M | 23 | Physical exertion |
| Ped 137 | M | 17 | Physical exertion |
| Ped 137 | M | 15 | Physical exertion |
| Ped 139 | M | 40 | Physical exertion |
| Ped 139 | M | 19 | Physical exertion |
| A797TMYH7: | |||
| Ped 101 | F | 55 | Washing dishes |
| Ped 101 | F | 24 | Upon return from work |
| Ped 101 | F | 17 | Emotional exertion |
| Ped 101 | M | 22 | Physical exertion |
| Ped 104 | M | 40 | Physical exertion |
| Ped 131 | M | 23 | Sedentary |
| Ped 147 | M | 30 | Physical exertion |
| Ped 158 | M | 64 | Physical exertion |
Note: F = female; M = male.
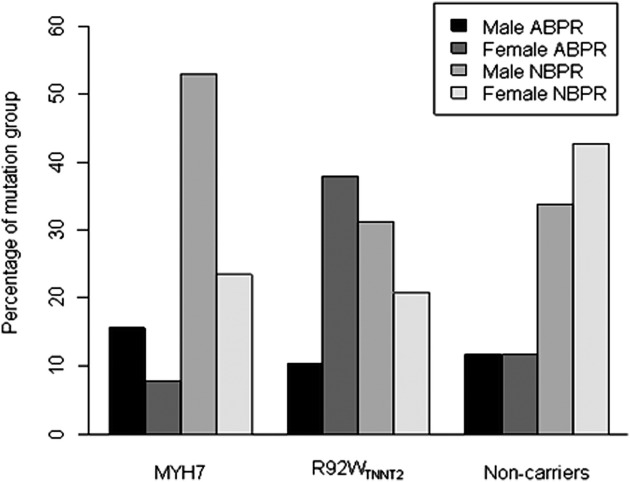
Whereas abnormal blood pressure response to exercise occurred in about a quarter of control individuals or individuals carrying either MYH7 mutation, this phenomenon was present in significantly more, that is, nearly half, of R92WTNNT2 carriers (Table 2). No individuals demonstrated a continuous decrease in SBP throughout the exercise test. Flat abnormal blood pressure responses predominated in all groups but were the only type of abnormal blood pressure response in R92WTNNT2 carriers (Table 2). Statistical analysis indicated that R92WTNNT2 carriers were 3 times as likely to demonstrate an abnormal blood pressure response to exercise compared with either MYH7 mutation carriers or noncarrier control individuals (Table 4). This was particularly noticeable for female R92WTNNT2 carriers (Figure 1); the percentage of females with abnormal blood pressure response for R92WTNNT2 was 64%, for MYH7 25%, and for noncarriers 22%. In contrast, the percentage of males with abnormal blood pressure response was similar for the three groups (R92WTNNT2 24%; MYH7 23%; noncarriers 26%).
Table 4.
P-values and estimated odds ratio (OR) for abnormal blood pressure response in R92WTNNT2 compared with MYH7 carriers and noncarrier control individuals, adjusted for age, sex, resting mean arterial pressure, resting HR, and family relatedness
| Groups | OR | 95% Confidence interval | P |
|---|---|---|---|
| R92WTNNT2 | |||
| MYH7 | 3.03 | 1.13, 8.12 | .029 |
| R92WTNNT2 | |||
| Control | 3.03 | 1.20, 7.68 | .021 |
| MYH7 | |||
| Control | 1.00 | 0.42, 2.37 | 1.000 |
Figure 1.
Percentage of males and females within each group demonstrating normal and abnormal blood pressure responses to exercise.
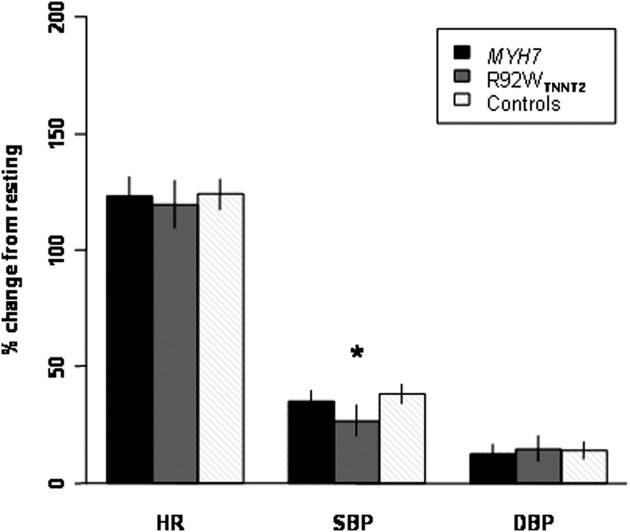
After adjustment for age, sex, resting mean arterial pressure, and resting HR, the effect of exercise on HR and DBP parameters (Table 2) was not different between groups. However, R92WTNNT2 carriers achieved a significantly lower mean SBP at peak exercise, as well as a smaller mean change (Table 2) and a smaller percentage of change in SBP (Figure 2) between resting and peak exercise, than did noncarriers and MYH7 mutation carriers.
Figure 2.
Bar graph of adjusted means and 95% confidence interval of untransformed values, showing percentage change in hemodynamic parameters from resting to peak exercise. *P = .029.
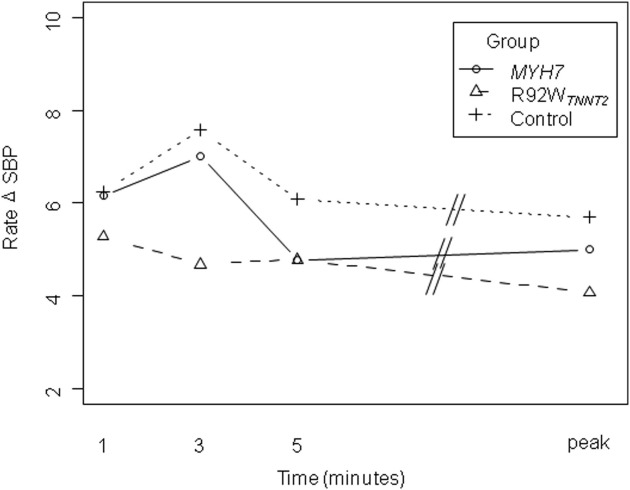
Interestingly, we also observed that, while the mean rate of SBP change increased in the first 3 minutes of exercise in the control and MYH7 groups, R92WTNNT2 carriers on average demonstrated a slight decrease in the rate at which SBP changed during the first 3 minutes of exercise (Figure 3). However, perhaps owing to the small numbers of individuals involved, this difference was not statistically significant.
Figure 3.
Mean rate of change in SBP in the groups during early exercise and at peak exercise. Values were adjusted for age, sex, body surface area, resting HR, and resting SBP.
Discussion
Abnormal blood pressure response to exercise has been associated with HCM-related death, with patients <50 years old showing a fourfold increase in premature HCM-related death.8,11 However, it is unknown whether abnormal blood pressure response is influenced by the HCM-causing genotype. Here we show that individuals carrying the R92WTNNT2 mutation are 3 times as likely to experience abnormal blood pressure response upon exercise compared with those carrying either of two MYH7 mutations or noncarrier controls.
We have previously described an increased frequency of SCD, predominantly affecting young males in their teenage years, in two ancestrally related families in which the R92WTNNT2 mutation segregates.7 In this study, we report SCDs in a further two of five additional R92WTNNT2 families identified (Table 3); these families have been shown by genetic haplotype analysis (data not shown) to be ancestrally related to the original two R92WTNNT2 families.7 While SCD had occurred in four (57%) of the seven R92WTNNT2 families, predominantly affecting young men in their teenage years, SCD in MYH7 families was limited to those in which the A797TMYH7 mutation segregates (five [50%] of 10 A797TMYH7 families, 39% of total MYH7 families) and mostly affected adult individuals (Table 3). Similar to our first report of the clinical picture associated with R92WTNNT2 carriers, overt hypertrophy was a rare occurrence in the R92WTNNT2 group7 but occurred more frequently in the MYH7 group (n = 4, all A797TMYH7 carriers), while syncope occurred at comparable levels in both mutation groups (Table 1).
We found that carriers of the R92WTNNT2 mutation demonstrate abnormal blood pressure response to exercise significantly more frequently (Tables 2 and 4; P = .021, odds ratio = 3.03) and show smaller absolute (ΔSBP, Table 2) and relative (% change from resting SBP, Figure 2) changes in SBP than do carriers of MYH7 mutations or related noncarrier control individuals. Thus, our study of the largest number of cases with an identical mutation in the TNNT2 gene to date provides strong evidence for the influence of genotype on hemodynamic response in HCM.
In R92WTNNT2 carriers, abnormal blood pressure responses were always of the flat type, namely, a failure to increase SBP by >20 mmHg between resting and peak exercise state. This finding, as well as the smaller increases in SBP observed in this study in the R92WTNNT2 group during exercise (Table 2, Figure 1), supports the results of Sakata et al,20 who recently reported that a group consisting of carriers of diverse troponin mutations demonstrated a smaller increase in SBP during exercise than did individuals without troponin mutations.
The mechanisms underlying abnormal blood pressure response to exercise in HCM are not yet completely understood. While some studies indicate that this response is due to an exaggerated decrease in systemic vascular resistance,9,21 other studies suggest that impaired cardiac output is responsible for exercise hypotension.22,23 Although it remains possible that either or a combination of both mechanisms are at play in subsets of HCM patients,24 Sakata et al20 found that systemic vascular resistance decreased to a similar extent in the TNNT2 and non-TNNT2 groups. They proposed that their findings related to the systolic dysfunction, which would result in decreased cardiac output that developed only in the TNNT2 group during exercise.
The development of abnormal blood pressure response may also be related to autonomic control, as studies of neurotransmitter levels before and after exercise have also previously indicated that HCM patients manifest a sympathoadrenal imbalance during exercise.25,26 The cause of this imbalance is not known, nor is it known whether this imbalance is genotype related, but it has been suggested that the enhanced systolic function that occurs under resting conditions in transgenic mutant TNNT2 mice,27 as well as in prehypertrophic R92WTNNT2 carriers,13 may activate ventricular mechanoreceptors and cause chronic alterations in vagal tone. On the other hand, Kawasaki et al10 recently demonstrated that subendocardial ischemia, which occurs in about 50% of HCM patients upon exercise,23,28 leads to vagal enhancement in patients with HCM, which may be related to the development of abnormal blood pressure response to exercise.
The preponderance of abnormal blood pressure response in females in the R92WTNNT2 group might be explained by vagal enhancement, which results from either mechanism proposed above, occurring on the background of the normally predominantly parasympathetically biased cardiac regulation in females.29 The preponderance of abnormal blood pressure response in female R92WTNNT2 carriers, who experience less SCD than their male counterparts, could suggest that this hemodynamic factor is not causally associated with the high frequency of sudden death with exercise that we have observed in the R92WTNNT2 group. However, in light of previous longitudinal studies indicating an increase in premature HCM-related death among HCM patients demonstrating abnormal blood pressure response to exercise,8,11 the survival and abnormal blood pressure response data in this study may suggest that a parasympathetic shift is not well tolerated in the predominantly sympathetically regulated male hearts.29
Failure to increase SBP appropriately during exercise is indicative of severe coronary artery disease in the general population30,31 and is more marked for those who fail to increase SBP appropriately during the first 3 minutes of exercise rather than later on during exercise.30 This is interesting, given our preliminary observation of the rate of change in SBP, which suggests that individuals with the R92WTNNT2 mutation may fail to increase blood pressure appropriately, particularly early on during exercise (Figure 3).
In contrast to the high rate of abnormal blood pressure response to exercise in the R92WTNNT2 group, the frequency of abnormal blood pressure response to exercise in the MYH7 group was similar to that reported for genotype-unknown HCM patients8,9 and was equal to that observed in the control group of noncarrier relatives (62% from MYH7 and 38% from R92WTNNT2 families). However, the frequency of abnormal blood pressure response to exercise in this control group was much higher than that reported for a non–South African general population (2%–8%).30 The reason for this is not immediately clear; however, half of the control individuals with abnormal blood pressure response in this study were overweight or obese (body mass index >25), perhaps indicating a risk for coronary artery disease and silent myocardial ischemia.32 Other reported causes of an abnormal blood pressure response to exercise include valvular heart disease, orthostatic hypotension, and the use of drugs such as vasodilators, negative inotropic agents, and diuretics.30
Conclusion
Individuals with R92WTNNT2 mutations demonstrate abnormal blood pressure response to exercise significantly more frequently than do individuals with MYH7 mutations or noncarrier controls, indicating that abnormal blood pressure response is influenced by genotype. The preponderance of female R92WTNNT2 carriers with abnormal blood pressure response, with a lower risk of SCD than affected males, supports the suggestion that abnormal blood pressure response may be associated with a parasympathetic shift in the regulation of cardiac function and that parasympathetic shifts are not well tolerated in predominantly sympathetically regulated male hearts. Although implantable defibrillators are the most effective life-saving therapy available for prevention of SCD in HCM, this option is not widely available outside of developed nations owing to cost. Elucidating the molecular mechanisms underlying SCD in HCM may eventually facilitate development of alternative effective therapeutic options.
Acknowledgment
The authors thank Peter Doubell for his help in collating the data.
Footnotes
The first two authors contributed equally to this work.
This study was funded by a Wellcome Trust International Senior Research Fellowship.
The Vivid 7 cardiovascular ultrasound system was provided pro bono by GE Healthcare (Germany) for the duration of the study.
None of the authors have any conflict of interest to disclose.
References
- 1.Maron B.J., Gardin J.M., Flack J.M. Prevalence of hypertrophic cardiomyopathy in a general population of young adults: Echocardiographic analysis of 4111 subjects in the CARDIA Study. Coronary Artery Risk Development in (Young) Adults. Circulation. 1995;92:785–789. doi: 10.1161/01.cir.92.4.785. [DOI] [PubMed] [Google Scholar]
- 2.Elliott P., McKenna W.J. Hypertrophic cardiomyopathy. Lancet. 2004;363:1881–1891. doi: 10.1016/S0140-6736(04)16358-7. [DOI] [PubMed] [Google Scholar]
- 3.Watkins H., McKenna W.J., Thierfelder L. Mutations in the genes for cardiac troponin T and alpha-tropomyosin in hypertrophic cardiomyopathy. N Engl J Med. 1995;332:1058–1064. doi: 10.1056/NEJM199504203321603. [DOI] [PubMed] [Google Scholar]
- 4.Varnava A.M., Elliott P.M., Baboonian C. Hypertrophic cardiomyopathy: histopathological features of sudden death in cardiac troponin T disease. Circulation. 2001;104:1380–1384. doi: 10.1161/hc3701.095952. [DOI] [PubMed] [Google Scholar]
- 5.Tardiff J.C., Hewett T.E., Palmer B.M. Cardiac troponin T mutations result in allele-specific phenotypes in a mouse model for hypertrophic cardiomyopathy. J Clin Invest. 1999;104:469–481. doi: 10.1172/JCI6067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ertz-Berger B.R., He H., Dowell C. Changes in the chemical and dynamic properties of cardiac troponin T cause discrete cardiomyopathies in transgenic mice. Proc Natl Acad Sci U S A. 2005;102:18219–18224. doi: 10.1073/pnas.0509181102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moolman J.C., Corfield V.A., Posen B. Sudden death due to troponin T mutations. J Am Coll Cardiol. 1997;29:549–555. doi: 10.1016/s0735-1097(96)00530-x. [DOI] [PubMed] [Google Scholar]
- 8.Olivotto I., Maron B.J., Montereggi A. Prognostic value of systemic blood pressure response during exercise in a community-based patient population with hypertrophic cardiomyopathy. J Am Coll Cardiol. 1999;33:2044–2051. doi: 10.1016/s0735-1097(99)00094-7. [DOI] [PubMed] [Google Scholar]
- 9.Frenneaux M.P., Counihan P.J., Caforio A.L. Abnormal blood pressure response during exercise in hypertrophic cardiomyopathy. Circulation. 1990;82:1995–2002. doi: 10.1161/01.cir.82.6.1995. [DOI] [PubMed] [Google Scholar]
- 10.Kawasaki T., Azuma A., Kuribayashi T. Vagal enhancement due to subendocardial ischemia as a cause of abnormal blood pressure response in hypertrophic cardiomyopathy. Int J Cardiol. 2008;129:59–64. doi: 10.1016/j.ijcard.2007.05.023. [DOI] [PubMed] [Google Scholar]
- 11.Sadoul N., Prasad K., Elliott P.M. Prospective prognostic assessment of blood pressure response during exercise in patients with hypertrophic cardiomyopathy. Circulation. 1997;96:2987–2991. doi: 10.1161/01.cir.96.9.2987. [DOI] [PubMed] [Google Scholar]
- 12.Moolman-Smook J.C., De Lange W.J., Bruwer E.C. The origins of hypertrophic cardiomyopathy-causing mutations in two South African subpopulations: a unique profile of both independent and founder events. Am J Hum Genet. 1999;65:1308–1320. doi: 10.1086/302623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Revera M., Van Der M.L., Heradien M. Troponin T and beta-myosin mutations have distinct cardiac functional effects in hypertrophic cardiomyopathy patients without hypertrophy. Cardiovasc Res. 2008;77:687–694. doi: 10.1093/cvr/cvm075. [DOI] [PubMed] [Google Scholar]
- 14.Bruce R.A., McDonough J.R. Stress testing in screening for cardiovascular disease. Bull N Y Acad Med. 1969;45:1288–1305. [PMC free article] [PubMed] [Google Scholar]
- 15.Nanas S., Nastasiou-Nana M., Dimopoulos S. Early heart rate recovery after exercise predicts mortality in patients with chronic heart failure. Int J Cardiol. 2006;110:393–400. doi: 10.1016/j.ijcard.2005.10.032. [DOI] [PubMed] [Google Scholar]
- 16.Schiller N.B., Shah P.M., Crawford M. Recommendations for quantitation of the left ventricle by two-dimensional echocardiography: American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two-Dimensional Echocardiograms. J Am Soc Echocardiogr. 1989;2:358–367. doi: 10.1016/s0894-7317(89)80014-8. [DOI] [PubMed] [Google Scholar]
- 17.Maron M.S., Olivotto I., Betocchi S. Effect of left ventricular outflow tract obstruction on clinical outcome in hypertrophic cardiomyopathy. N Engl J Med. 2003;348:295–303. doi: 10.1056/NEJMoa021332. [DOI] [PubMed] [Google Scholar]
- 18.Sobel E., Sengul H., Weeks D.E. Multipoint estimation of identity-by-descent probabilities at arbitrary positions among marker loci on general pedigrees. Hum Hered. 2001;52:121–131. doi: 10.1159/000053366. [DOI] [PubMed] [Google Scholar]
- 19.Pilia G., Chen W.M., Scuteri A. Heritability of cardiovascular and personality traits in 6,148 Sardinians. PLoS Genet. 2006;2:e132. doi: 10.1371/journal.pgen.0020132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sakata K., Ino H., Scuteri N. Exercise-induced systolic dysfunction in patients with non-obstructive hypertrophic cardiomyopathy and mutations in the cardiac troponin genes. Heart. 2008;94:1282–1287. doi: 10.1136/hrt.2007.116970. [DOI] [PubMed] [Google Scholar]
- 21.Counihan P.J., Frenneaux M.P., Webb D.J. Abnormal vascular responses to supine exercise in hypertrophic cardiomyopathy. Circulation. 1991;84:686–696. doi: 10.1161/01.cir.84.2.686. [DOI] [PubMed] [Google Scholar]
- 22.Ciampi Q., Betocchi S., Lombardi R. Hemodynamic determinants of exercise-induced abnormal blood pressure response in hypertrophic cardiomyopathy. J Am Coll Cardiol. 2002;40:278–284. doi: 10.1016/s0735-1097(02)01950-2. [DOI] [PubMed] [Google Scholar]
- 23.Ciampi Q., Betocchi S., Losi M.A. Abnormal blood-pressure response to exercise and oxygen consumption in patients with hypertrophic cardiomyopathy. J Nucl Cardiol. 2007;14:869–875. doi: 10.1016/j.nuclcard.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 24.Lim P.O., Morris-Thurgood J.A., Frenneaux M.P. Vascular mechanisms of sudden death in hypertrophic cardiomyopathy, including blood pressure responses to exercise. Cardiol Rev. 2002;10:15–23. doi: 10.1097/00045415-200201000-00005. [DOI] [PubMed] [Google Scholar]
- 25.Omodani H., Kinugawa T., Ogino K. Augmented exercise plasma noradrenaline with impaired chronotropic responsiveness in patients with hypertrophic cardiomyopathy. Clin Exp Pharmacol Physiol. 1998;25:1018–1023. doi: 10.1111/j.1440-1681.1998.tb02177.x. [DOI] [PubMed] [Google Scholar]
- 26.Carl I., Ong H., Donnelly R. Exercise in hypertrophic cardiomyopathy is associated with sympatho-adrenal imbalance. Int J Cardiol. 2007;116:124–125. doi: 10.1016/j.ijcard.2006.03.048. [DOI] [PubMed] [Google Scholar]
- 27.Knollmann B.C., Blatt S.A., Horton K. Inotropic stimulation induces cardiac dysfunction in transgenic mice expressing a troponin T (I79N) mutation linked to familial hypertrophic cardiomyopathy. J Biol Chem. 2001;276:10039–10048. doi: 10.1074/jbc.M006745200. [DOI] [PubMed] [Google Scholar]
- 28.Okeie K., Shimizu M., Yoshio H. Left ventricular systolic dysfunction during exercise and dobutamine stress in patients with hypertrophic cardiomyopathy. J Am Coll Cardiol. 2000;36:856–863. doi: 10.1016/s0735-1097(00)00818-4. [DOI] [PubMed] [Google Scholar]
- 29.Kuo T.B., Lin T., Yang C.C. Effect of aging on gender differences in neural control of heart rate. Am J Physiol. 1999;277:H2233–H2239. doi: 10.1152/ajpheart.1999.277.6.H2233. [DOI] [PubMed] [Google Scholar]
- 30.Watson G., Mechling E., Ewy G.A. Clinical significance of early vs late hypotensive blood pressure response to treadmill exercise. Arch Intern Med. 1992;152:1005–1008. [PubMed] [Google Scholar]
- 31.Comess K.A., Fenster P.E. Clinical implications of the blood pressure response to exercise. Cardiology. 1981;68:233–244. doi: 10.1159/000173286. [DOI] [PubMed] [Google Scholar]
- 32.Irace C., Scavelli F., Carallo C. Body mass index, metabolic syndrome and carotid atherosclerosis. Coron Artery Dis. 2009;20:94–99. doi: 10.1097/MCA.0b013e3283219e76. [DOI] [PubMed] [Google Scholar]
- 33.Zoghbi W.A., Enriquez-Sarano M., Foster E. Recommendations for evaluation of the severity of native valvular regurgitation with two-dimensional and Doppler echocardiography. J Am Soc Echocardiogr. 2003;16:777–802. doi: 10.1016/S0894-7317(03)00335-3. [DOI] [PubMed] [Google Scholar]