Abstract
Rationale: Silent gastroesophageal reflux (GER) is common in patients with asthma, but it is unclear whether GER is associated with worse asthma symptoms or reduced lung function.
Objectives: To determine in patients with poorly controlled asthma, whether proximal or distal esophageal reflux is associated with asthma severity, symptoms, physiology, or functional status.
Methods: Baseline asthma characteristics were measured in patients with asthma enrolled in a multicenter trial assessing the effectiveness of esomeprazole on asthma control. All participants underwent 24-hour esophageal pH probe monitoring. Lung function, methacholine responsiveness, asthma symptoms, and quality-of-life scores were compared in subjects with and without GER.
Measurements and Main Results: Of 304 participants with probe recordings, 53% had reflux. Of 242 participants with recordings of proximal pH, 38% had proximal reflux. There was no difference in need for short-acting bronchodilators, nocturnal awakenings, dose of inhaled corticosteroid, use of long-acting β-agonists, lung function, or methacholine reactivity between individuals with and without proximal or distal GER. Participants with GER reported more use of oral corticosteroids and had worse asthma quality of life and subjects with proximal GER had significantly worse asthma quality of life and health-related quality of life compared with participants without GER.
Conclusions: Asymptomatic GER is not associated with distinguishing asthma symptoms or lower lung function in individuals with suboptimal asthma control who are using inhaled corticosteroids. Patients with proximal reflux report significantly worse asthma and health-related quality of life despite lack of physiologic impairment or increase in asthma symptoms.
Clinical trial registered with www.clinicaltrials.gov (NCT00069823).
Keywords: lung function, nocturnal symptoms, asthma, gastroesophageal reflux
AT A GLANCE COMMENTARY
Scientific Knowledge on the Subject
Gastroesophageal reflux has been considered a possible trigger for poor asthma control. Studies of the effect of acid suppressor therapy have shown conflicting effects on asthma control.
What This Study Adds to the Field
We demonstrate that asymptomatic GER is not associated with distinguishing demographic or clinical features in patients with asthma and suboptimal control despite treatment with inhaled corticosteroids, but is associated with poorer health-related quality of life.
Gastroesophageal reflux (GER) is common in patients with asthma, particularly in those with difficult-to-control asthma, with a reported prevalence of 32 to 84% (1–3). GER often occurs in the absence of symptoms such as heartburn (4–6). It has been suggested that acid reflux has a negative impact on asthma control and asthma symptoms, and in particular nocturnal symptoms (5). Accordingly, current guidelines recommend evaluation and treatment for GER in patients with difficult-to-control asthma, regardless of the presence of GER symptoms (7). Despite these recommendations, studies of the effect of acid suppressor agents on asthma control and lung function have been inconclusive. A Cochrane review of 12 randomized controlled clinical trials concluded that treatment of GER was inconsistently associated with a beneficial effect on asthma outcomes (8). A recent trial of 207 patients with moderate to severe asthma with symptomatic GER showed a reduction in exacerbations and improvement in asthma quality of life after treatment of GER with proton pump inhibitors (PPIs), but no change in other asthma outcomes (9). Another study of 770 patients with asthma reported no overall improvement in peak expiratory flow (PEF), exacerbations, or asthma symptoms after 16 weeks of treatment with esomeprazole compared with placebo (10). However, the subgroup of patients who had both symptoms of GER and nocturnal awakenings from asthma did show improvement in PEF with treatment.
Recently, The American Lung Association Asthma Clinical Research Centers conducted a double-masked, placebo-controlled, randomized study of the effect of proton pump inhibition on asthma outcomes in the Study of Acid Reflux and Asthma in patients with inadequately controlled asthma despite treatment with inhaled corticosteroids (ICS) (11). Overall, there was no benefit of PPIs on asthma control in subjects with or without pH probe evidence of GER. This study was the first large-scale trial to conduct pH probe monitoring to document whether GER was present in patients with suboptimal asthma control and minimal or no symptoms of acid reflux. Moreover, a substantial number of patients also underwent dual channel testing to determine whether proximal reflux was present.
The actual effect of GER on asthma severity and symptoms has not been reported systematically in a large series of patients. The mechanism by which acid reflux might affect asthma control is controversial (12–18). It may also be important to know whether proximal reflux into the upper esophagus affects patients differently from distal acid reflux.
Studies to interpret the effect of GER on asthma control may be complicated by reliance on self-report of GER. Indeed, a significant portion of patients with asthma with symptoms of GER do not have GER documented by pH probe measurements, whereas up to 60% of patients with asthma with no symptoms of reflux have GER measured by esophageal pH probe (6). Thus, important questions remain regarding the clinical diagnosis of distal and proximal GER and the effect on patients with poorly controlled asthma.
Accordingly, the baseline data from the Study of Acid Reflux and Asthma trial provides an ideal dataset to address the following questions in a population of patients with inadequately controlled asthma:
(1) Do poorly controlled asthmatics with asymptomatic proximal or distal reflux have distinguishing clinical or demographic characteristics from those without reflux?
(2) What is the concordance between distal and proximal reflux assessed by ambulatory pH probe measurements?
METHODS
Nineteen clinical study centers participating in the American Lung Association Asthma Clinical Research Centers enrolled participants in the study. Eligible participants were nonsmoking individuals with inadequately controlled asthma despite the use of moderate or higher doses of inhaled corticosteroids. Inclusion criteria were: age 18 years or older, physician diagnosis of asthma supported by either a positive methacholine challenge test (for subjects with FEV1 >70% predicted) or a 12% increase in FEV1 with bronchodilators, 8 weeks of stable use of an inhaled corticosteroid equivalent to 400 μg/d or greater of fluticasone, and poor asthma control defined by Juniper Asthma Control Questionnaire score of 1.5 or greater (19), or more than one acute episode of asthma requiring unscheduled medical care in the past year. Participants were excluded if they had smoked cigarettes within 6 months or had 10 or more pack-years of smoking, had an FEV1 less than 50% predicted, had antireflux or peptic ulcer surgery, or had clinical indications for acid suppression treatment (i.e., two or more episodes per wk of heartburn requiring antacids). All participants signed written informed consent statements that had been approved by the local Institutional Review Board and by the Data Coordinating Center.
All subjects were scheduled to have 24-hour pH probe testing before randomization. Dual pH probe testing was performed at some, but not all, centers based on availability of necessary equipment. Calibrated pH probes were placed in the distal esophagus after esophageal manometry, 5 cm above the lower esophageal sphincter. The proximal probe was situated 15 cm above the distal probe. Studies were reviewed at a central reading center. Criteria for an acceptable study included total recording time of at least 16 hours, with at least one meal and 2 hours of recumbency. None of the patients were taking acid-suppressing drugs at the time of the probe study. A study was considered positive for distal GER if the percent of time with distal pH less than 4 was more than 5.5% total time, or more than 8.2% of upright time, or more than 3.5% of supine time (20). Proximal pH probe was considered positive if measured pH in the proximal esophagus was less than 4 more than 1% of the time of measurement, regardless of body position (21). Meal times were excluded in the analysis to avoid false-positive proximal pH data; episodes consistent with pseudoreflux were also excluded from analysis.
During the run-in period of the trial, which lasted 2 to 8 weeks, participants continued their usual asthma medications and recorded daily morning peak flow, asthma symptoms, β-agonist use, and nocturnal asthma awakenings. All participants underwent pulmonary function testing and self-administered the Asthma Symptom Utility Index, Juniper Asthma Control Questionnaire, Juniper mini–Asthma Quality of Life Questionnaire (mini AQLQ), a general health-related quality of life questionnaire (the Medical Outcomes Study SF-36), and a Gastroesophageal Reflux Disease Symptom Assessment Scale at the baseline visit (19, 22–24).
Data were analyzed by the Coordinating Center at Johns Hopkins University. P values were based on chi-square test for categorical variables and the Wilcoxon rank sum test for continuous variables. Cochran-Armitage trend test of categorical variables was used for analysis of effect of acid exposure time on asthma characteristics. The κ statistic was used to evaluate the concordance between proximal and distal pH probe tests for GER. The significance of the κ test statistic was an asymptotic test of the null hypothesis that κ = 0. All analyses were performed in SAS V9 (SAS Institute Inc., Cary, NC). Statistical significance was inferred if P was less than 0.05 without adjustment for multiple comparisons.
RESULTS
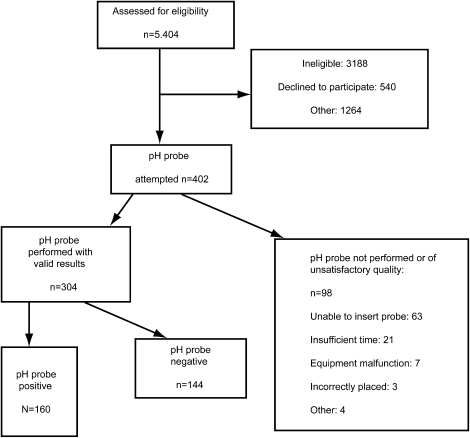
A total of 412 participants completed the run-in and 304 underwent technically successful placement and recordings of esophageal pH. Reasons for exclusion and reasons for inability to collect pH probe results are reported in Figure 1. Of 304 individuals who underwent pH probe testing, 242 had dual (proximal and distal esophageal) pH probe testing performed and 62 had only distal pH probe testing performed. Participants who underwent successful pH probes were similar in baseline demographics to those who did not (data not shown). However, participants who did complete a pH probe test were more likely to report a “burning in throat or sour acid taste” on a questionnaire to assess symptoms of GER than those subjects who did not complete a pH probe test (55 vs. 43%; P = 0.02). Designation of GER status as positive or negative was made according to results of proximal and/or distal pH probe measurements unless otherwise noted. Therefore, if either distal probe or proximal probe results were positive, the subject was considered to have pH probe evidence of GER.
Figure 1.
Flowchart for study participants.
Baseline Demographics
Of the 304 participants who underwent pH probe testing, 160 (53%) had abnormal proximal or distal pH probe results consistent with GER. Distal reflux was present in 123 (40%) of the 304 participants. Ninety-three of the 242 subjects (38%) who had dual probe testing showed evidence of proximal reflux. Demographic characteristics of participants with and without GER are reported in Table 1. No differences in sex, age, or race/ethnicity in subjects with and without GER were evident. Mean body mass index was similar in both groups. There were slightly more obese subjects (body mass index >30) in the GER-positive group compared with the GER-negative group (55 vs. 49%), but the difference was not statistically significant (P = 0.32).
TABLE 1.
DEMOGRAPHIC CHARACTERISTICS IN SUBJECTS WITH AND WITHOUT GASTROESOPHAGEAL REFLUX
| pH Probe Result |
|||
|---|---|---|---|
| Demographic Characteristics | Distal or Proximal Positive | Distal Negative and Proximal Negative or Not Done | P Value* |
| N (%) | 160 (53) | 144 (47) | |
| Mean age at randomization, yr ± SD | 43 ± 13 | 41 ± 14 | 0.29 |
| Males, % of group | 34 | 29 | 0.39 |
| Race or ethnic group, % of group | 0.55 | ||
| White | 51 | 50 | |
| Black | 39 | 38 | |
| Hispanic | 8 | 11 | |
| Other | 3 | 1 | |
| Former smoker, % of group | 17 | 19 | 0.56 |
| BMI | 33 ± 8 | 32 ± 17 | 0.31 |
| Obese (BMI ≥ 30) |
55 |
49 |
0.32 |
Definition of abbreviation: BMI = body mass index.
P values based on chi-square test for categorical variable and Wilcoxon rank sum test for continuous variables.
Asthma Symptoms, Medication Use, and Lung Function
Table 2 lists baseline asthma characteristics during the final 2 weeks of the run-in period for patients with and without pH probe evidence of GER. Most baseline characteristics, including frequency of use of short-acting bronchodilators and nocturnal awakenings from asthma, were similar in both groups. Subjects with evidence of distal or proximal GER reported significantly more use of oral corticosteroids for asthma in the previous year (56 vs. 43%; P = 0.03). Most (91%) enrolled subjects had a Juniper Asthma Control Questionnaire greater than or equal to 1.5, with no difference between GER-positive and GER-negative groups (P = 0.78). About half (48%) of all subjects reported more than one unscheduled visit for asthma in the previous year, with no difference between GER-positive and GER-negative subjects (P = 0.54).
TABLE 2.
BASELINE ASTHMA CHARACTERISTICS FOR SUBJECTS WITH AND WITHOUT GASTROESOPHAGEAL REFLUX
| pH Probe Result |
|||
|---|---|---|---|
| Asthma characteristics | Distal or Proximal Positive | Distal Negative and Proximal Negative or Missing | P Value* |
| N (%) | 160 (53) | 144 (47) | |
| Mean age of asthma onset, yr ± SD | 16 ± 17 | 17 ± 17 | 0.14 |
| Use of inhaled short-acting β-agonist (MDI/Neb) ≥ 2 times/wk, % of group | 81 | 78 | 0.55 |
| ≥2 Unscheduled health care visit in past yr, % | 64 | 57 | 0.54 |
| ACQ ≥ 1.5, % | 92 | 91 | 0.78 |
| Oral corticosteroids for asthma in past yr, % | 56 | 43 | 0.03 |
| Night awakenings due to asthma in past 2 wk, % | 18 | 10 | 0.08 |
| Daily use of ICS, % | 100 | 100 | |
| Daily dose of ICS, μg | 663 ± 23.4 | 647 ± 21.7 | 0.31 |
| Mean ACS (↓) (score range: 0–6) ± SD | 1.9 ± 0.9 | 1.8 ± 0.8 | 0.25 |
| Mean ASUI (↑) (score range: 0–1) ± SD | 0.73 ± 0.18 | 0.77 ± 0.15 | 0.08 |
| Mean AQL (↑) (score range: 1–7) ± SD | 4.4 ± 1.3 | 4.8 ± 1.2 | 0.01 |
| SF-36 quality of life (↑) (score range: 0–100), N | 160 | 143 | |
| Mean physical score ± SD | 41 ± 10 | 43 ± 10 | 0.06 |
| Mean emotional score ± SD | 48 ± 11 | 49 ± 11 | 0.56 |
| Mean pulmonary function measures, N | 160 | 144 | |
| Pre-BD FEV1, % predicted ± SD† | 76 ± 15 | 77 ± 14 | 0.84 |
| Pre-BD FVC, % predicted ± SD† | 87 ± 15 | 88 ± 13 | 0.56 |
| FEV1, % change post-BD ± SD | 10 ± 9 | 11 ± 11 | 0.15 |
| FVC, % change post-BD ± SD | 5 ± 8 | 6 ± 8 | 0.37 |
| Peak expiratory flow rate (% predicted ± SD) | 79 ± 18 | 80 ± 17 | 0.40 |
| Methacholine contraindicated (% of group) | 60 | 55 | 0.39 |
| PC20, mg/ml, N, mean ± SD |
61, 3.4 ± 4.0 |
64, 4.3 ± 5.1 |
0.94 |
Definition of abbreviations: ACS = Asthma Control Score; AQL = Asthma Quality of Life; ASUI = Asthma Symptom Utility Index; BD = bronchodilator; GERD = gastroesophageal reflux disease; ICS = inhaled corticosteroid; MDI/Neb = metered-dose inhaler/nebulizer; PC20 = provocative concentration causing 20% fall in FEV1; SF-36 = RAND 36-item health survey; ↑ = higher score is better; ↓ = lower score is better.
P values based on chi-square test for categorical variable and Wilcoxon rank sum test for continuous variables.
Predicted values for FEV1 and FVC by permission from Reference 36.
The dose of inhaled corticosteroids was similar in participants with positive and negative pH probe results. The mean daily dose of inhaled corticosteroids in the probe-positive versus probe-negative group was 663 ± 23.4 and 647 ± 21.7 μg, respectively (P = 0.31). No differences were observed in prebronchodilator lung function (mean prebronchodilator FEV1 76 ± 15% predicted in GER positive versus 77 ± 14% predicted in GER negative; P = 0.84) or bronchodilator reversibility in individuals with and without GER. The group with GER had a significantly lower (worse) score on the Asthma Quality of Life questionnaire compared with subjects without GER (4.4 ± 1.3 vs. 4.8 ± 1.2; P = 0.01), although the clinical importance of this difference is not certain. Scores on the Asthma Symptom Utility Index, and Medical Outcome Study SF-36 Quality of Life were also worse (lower) in the group with GER but did not reach statistical significance (P = 0.08 and P = 0.06, respectively). The Asthma Control Score, which incorporates measures of frequency of asthma symptoms and lung function, was similar in both groups (Table 2).
Response to the question on the mini-AQLQ related to frequency of feeling bothered by cough was similar in subjects with and without GER (4.4 ± 1.8 vs. 4.8 ± 1.8, respectively; P = 0.06, on a scale of 1 to 7 where 1 reflects symptoms “all of the time” and 7 reflects symptoms “none of the time”) (see Table E1 in the online supplement). Response to the cough-related question on the Asthma Symptom Utility Index was also similar in the GER-positive and GER-negative subjects (P = 0.41) (Table E1). Symptoms, activities, and emotional domain scores on the AQLQ were all statistically significantly worse in the participants with GER compared with those without GER (P = 0.02, 0.007, and 0.05, respectively; Table E2). The only individual item on the SF-36 with a significant difference in score between GER-positive and GER-negative individuals related to physical roles, such as work or daily activities (61 ± 40 vs. 50 ± 42, respectively; P = 0.01).
Differences in acid exposure time in the distal esophagus were evaluated with respect to asthma outcomes. Subjects with exposure time less than 5.5% (n = 199), 5.5 to 10% (n = 56), and greater than 10% (n = 49) demonstrated no differences across groups in any of the baseline asthma characteristics (Table E3). Acid exposure time in the proximal esophagus was also evaluated in relation to asthma outcomes. There were no differences in most asthma outcomes when comparing subjects with proximal acid exposure time of less than 0.5%, 0.5 to 1%, or greater than 1%. However, mean physical score on SF-36 decreased significantly as acid exposure time increased (P = 0.02) (Table E4).
Of subjects taking long-acting β-agonists, the proportion of patients with a positive pH probe (54%) was similar to those with a negative pH probe (46%), P = 0.24. No other differences were observed in baseline asthma characteristics between pH probe–negative versus PH probe-positive groups after stratifying by long-acting β-agonists use (data not shown). The prevalence of eczema, sinusitis, rhinitis, and food allergies was the same in subjects with and without pH probe evidence of GER (Table 3).
TABLE 3.
GASTROESOPHAGEAL REFLUX SYMPTOM SCORES IN SUBJECTS WITH AND WITHOUT GASTROESOPHAGEAL REFLUX
| Distal or Proximal Positive | Distal Negative and Proximal Negative or Missing | P Value* | |
|---|---|---|---|
| GERD Symptom Assessment Scale, N | 160 (53) | 144 (47) | |
| Mean number of symptoms (0–15) ± SD | 7 ± 3.5 | 6.8 ± 3.5 | 0.57 |
| Burning or acid taste, % of group | 56 | 55 | 0.89 |
| Mean distress score (↓) (score range: 0–3) ± SD | 0.60 ± 0.49 | 0.56 ± 0.47 | 0.48 |
| Other conditions, N | 160 | 144 | |
| Self-reported GERD, % of group | 18 | 11 | 0.11 |
| Eczema, % of group | 14 | 12 | 0.75 |
| Sinusitis, % of group | 42 | 34 | 0.16 |
| Rhinitis, % of group | 64 | 56 | 0.15 |
| Food allergies, % of group | 22 | 17 | 0.25 |
| Allergies worsen asthma, % of group |
78 |
76 |
0.72 |
Definition of abbreviations: GERD = gastroesophageal reflux disease; ↓ = lower score is better.
P values compare positive on distal or proximal probe result to negative distal result and negative or missing on proximal results based on chi-square test for categorical variable and Wilcoxon rank-sum test for continuous variables.
Comparison of the subset of individuals with both pH probe evidence of GER and self-reported diagnosis of GER (n = 28) also demonstrated no differences in baseline asthma characteristics compared with all others. However, these individuals were more likely to be obese and had higher gastric distress scores (data not shown).
GER Symptoms
GER-positive and GER-negative groups reported similar GER symptoms at baseline. The mean number of GER-related symptoms and Gastroesophageal Symptom Assessment Score was similar in both groups (Table 3). However, when results of distal pH probe test were analyzed by acid exposure time, number of subjects with self-report of GER increased significantly as distal acid exposure time increased, (P = 0.0003; Table E3) (25).
Proximal Reflux
Separate analysis of the 242 subjects who had proximal esophageal pH measured demonstrated a high prevalence of proximal reflux (38%) (Table 4). Concordance between proximal pH probe results and distal pH probe results was moderate to poor (κ = 0.33; 95% confidence interval: 0.21–0.46; P < 0.001). Seventy-five percent of participants with a negative distal probe test were also negative on the proximal probe; 58% of subjects who were positive on the distal probe also had a positive proximal probe test. Of interest, 25% (37 of 146) of participants who would be classified as GER negative based on the distal esophageal pH probe showed evidence of abnormal proximal reflux. There were no differences in the demographic characteristics of subjects with a positive proximal pH probe versus a negative one (data not shown). Individuals with proximal reflux had a significantly lower score on asthma quality of life as measured by the mini-AQLQ (mean 4.4 ± 1.3 vs. 4.8 ± 1.2; P = 0.02) and significantly lower mean physical score on the SF-36 (44 ± 10 vs. 40 ± 10, respectively; P = 0.005) than the individuals with no evidence of proximal reflux (Table 4). Response to the cough-specific question on the mini-AQLQ was similar in the proximal GER–positive versus proximal GER–negative groups (4.2 ± 1.9 vs. 4.7 ± 1.6; P = 0.08). Response to the cough-related question of the Asthma Symptom Utility Index was also similar in the two groups (P = 0.19; Table E1). There were no differences in pre- or postbronchodilator lung function, methacholine responsiveness, nocturnal awakenings, use of short-acting bronchodilators, dose of inhaled corticosteroids, or emergency visits for asthma between the proximal GER-positive and GER-negative groups. Nor were there differences in GER symptoms, Gastroesophageal Reflux Disease Symptom Assessment Scale score, or comorbidities between those with and without evidence of proximal reflux.
TABLE 4.
SELECTED BASELINE ASTHMA CHARACTERISTICS IN SUBJECTS WITH AND WITHOUT PROXIMAL GASTROESOPHAGEAL REFLUX
| Proximal PH Probe Result |
|||
|---|---|---|---|
| Asthma symptoms | Positive | Negative | P Value* |
| N (%) | 93 (38) | 149 (62) | |
| Use of inhaled short-acting β-agonist ≥ 2 times/wk, % | 80 | 80 | 0.96 |
| ≥ 2 Unscheduled health care visit in past yr, % | 63 | 58 | 0.39 |
| Oral corticosteroids for asthma in past year, % | 56 | 47 | 0.18 |
| Long-acting β-agonist use, % | 75 | 81 | 0.33 |
| Night awakenings due to asthma in past 2 wk | 16 | 11 | 0.22 |
| Mean ACS (↓) (score range: 0–6) ± SD | 1.9 ± 0.9 | 1.7 ± 0.7 | 0.12 |
| Mean ASUI (↑) (score range: 0–1) ± SD | 0.72 ± 0.19 | 0.76 ± 0.15 | 0.12 |
| Mean AQL (↑) (score range: 1–7) ± SD | 4.4 ± 1.3 | 4.8 ± 1.2 | 0.02 |
| SF-36 Quality of Life (↑) | |||
| Mean physical score ± SD | 40 ± 10 | 44 ± 10 | 0.005 |
| Mean emotional score ± SD | 48 ± 10 | 49 ± 11 | 0.32 |
| Mean pulmonary function measures | |||
| Pre-BD FEV1, % predicted ± SD | 76 ± 16 | 77 ± 14 | 0.49 |
| Pre-BD FVC, % predicted ± SD | 87 ± 16 | 88 ± 12 | 0.43 |
| FEV1, % change post-BD ± SD | 10 ± 9 | 11 ± 10 | 0.33 |
| FVC, % change post-BD ± SD | 4 ± 7 | 6 ± 8 | 0.06 |
| Peak expiratory flow rate, % predicted ± SD | 80 ± 20 | 81 ± 16 | 0.89 |
| PC20 mg/ml, n, mean ± SD |
37, 3.4 ± 4.3 |
67, 3.9 ± 4.7 |
0.62 |
Definition of abbreviations: ACS = Asthma Control Score; AQL = Asthma Quality of Life; ASUI = Asthma Symptom Utility Index; BD = bronchodilator; PC20 = provocative concentration causing 20% fall in FEV1; SF-36 = RAND 36-item health survey; ↑ = higher score is better; ↓ = lower score is better.
P values are based on chi-square test for categorical variables and Wilcoxon rank-sum test for continuous variables.
Analysis of only distal pH probe results showed no difference in baseline asthma symptoms, lung function, methacholine responsiveness, and quality-of-life measures between subjects with and without evidence of distal reflux (data not shown).
DISCUSSION
The main finding of this study is that there are no clear clinical or demographic characteristics that can be used to differentiate between poorly controlled patients with asthma with or without ambulatory pH probe–documented acid reflux. These results indicate that although asymptomatic GER frequently accompanies poorly controlled asthma, it is not associated with lower lung function, worse asthma control, or increased airway responsiveness, but is associated with significantly worse asthma quality of life. In addition, the concordance between lower (distal) esophageal reflux and upper (proximal) reflux was only moderate; 25% of subjects had one without the other. Discordance between proximal and distal reflux measurements are likely due to the established normal values used for presence of acid at each of these sites. Because the distal esophagus is more frequently exposed to gastric acid, a normal total time with pH less than 4 is 5.5% in the distal esophagus, compared with less than 1% in the proximal esophagus (21, 26). In patients who are undergoing esophageal pH probe monitoring for evaluation of disorders such as chronic cough, it seems a reasonable extrapolation to suggest that dual-probe studies are necessary to exclude proximal acid reflux.
Previously published studies have shown lack of association between probe-documented GER and asthma outcomes (6, 27). However, because of small sample size, these studies had limited ability to identify asthma characteristics associated with reflux. The present study is the largest to use ambulatory pH probe monitoring and confirms these findings in a larger group of patients with asthma without significant symptoms of GER.
The 2007 National Asthma Education and Prevention Program Guidelines for the diagnosis and management of asthma recommend that clinicians consider treatment of reflux to improve asthma control in patients with poorly controlled asthma (7). In our study of 304 subjects with asthma who had ambulatory pH probe monitoring performed, we have demonstrated no effect of reflux on lung function, airway hyperresponsiveness, acute care visits for asthma, asthma symptoms, nocturnal symptoms, dose of asthma maintenance therapy, or need for rescue therapy. Subjects with GER did report significantly worse Asthma Quality of Life, though the clinical significance of this modest effect is uncertain. Our finding of greater previous use of oral corticosteroids among subjects with GER may suggest that patients with GER have more acute episodes of worsening of asthma, which may affect quality of life.
Individuals with proximal reflux did not demonstrate worse asthma symptoms, lower lung function, increased airway responsiveness, or rescue bronchodilator use, but did have substantially poorer asthma quality of life and generic health-related quality of life compared with those participants without proximal GER. Specifically, there was a trend toward increase in feeling bothered by cough on the mini-AQLQ in subjects with proximal GER and the SF-36 showed a significantly worse physical activity score. This finding suggests that proximal reflux may be more relevant to subjective evaluation of asthma symptoms rather than physiologic impairment of lung function. In this regard, Ferrari and colleagues studied 17 patients with asthma and proximal reflux. They found that omeprazole reduced the cough sensitivity to capsaicin but did not alter airways reactivity to methacholine (28). Interestingly, previous studies have demonstrated that proximal acid reflux is predictive of a favorable response to acid suppressor therapy, although we did not find this in our trial (2, 29).
Nocturnal asthma symptoms are frequently present in patients with difficult-to-control asthma, raising the suggestion that GER contributes to both nocturnal symptoms and poor asthma control. Kiljander and colleagues reported that in patients with asthma with combined symptoms of GER and nocturnal asthma, treatment with esomeprazole resulted in a modest improvement in morning and evening peak flow (10). One may speculate that nocturnal asthma symptoms are a marker of proximal esophageal reflux, which in our study is associated with worsened asthma quality of life. In support of this, Tomonaga and colleagues demonstrated that nocturnal cough was associated with proximal, but not distal, esophageal reflux (30). Our study however, did not demonstrate a difference in nocturnal asthma symptoms between those individuals with and without proximal reflux. It is possible that asymptomatic GER may disrupt sleep in more subtle ways, thus leading to impaired quality of life without significant differences in asthma control. In a large study from Taiwan, Chen and colleagues found that asymptomatic esophagitis was associated with poorer sleep quality and shorter sleep duration (31).
The present study confirms and extends prior work that has emphasized the high prevalence of silent proximal and distal GER in patients with asthma. This is the first large-scale study to use pH probe monitoring to compare severity of asthma symptoms and asthma control in patients with and without documented evidence of acid GER. Because the present study enrolled only patients with minimal or absent GER symptoms, we cannot address the question whether in patients with symptomatic GER, reflux does contribute to asthma symptoms, asthma exacerbations, and poorer lung function. Furthermore, nonacid esophageal reflux (e.g., pepsin, bile acids) has been recognized as a cause of respiratory symptoms such as cough and wheeze (32, 33) and is not detectable with the pH monitoring performed in our study. Our findings therefore cannot be extrapolated to the effect of nonacid reflux on asthma control. Several studies have demonstrated that in patients not taking acid suppressor therapy, combined pH and impedance testing for detection of both acid and nonacid reflux has demonstrated that only 6.3% of reflux events are nonacid. Nonacid reflux appears to play a more significant role in patients who have persistent reflux symptoms despite PPI therapy (34, 35).
Our study does not support the idea that asymptomatic reflux is associated with lower lung function, worse asthma control, increased airway hyperresponsiveness, or increased asthma symptoms. Evaluation for GER using ambulatory pH probes in individuals with poorly controlled asthma with no reflux symptoms is therefore not usually warranted unless atypical symptoms, such as cough or unexplained chest symptoms, might suggest the diagnosis.
Supplementary Material
Supported by NIH-NHLBI 5 U01HL072968 and the American Lung Association. Esomeprazole and placebo were provided through a grant from Astra-Zeneca.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.200904-0625OC on August 6, 2009
Conflict of Interest Statement: E.D. received $5,001–$10,000 in lecture fees from AstraZeneca. J.T.H. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. E.S. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. J.R. received $10,000 from Novartis in industry-sponsored grants from 2005 to 2008. J.R.'s monies from AstraZeneca/TAP were for a speaking honorarium at CME-sponsored panel rounds at individual medical schools and for national AZ meetings. S.N. holds $1,001–$5,000 equity in IRA from Pfizer. N.P. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. J.G.M. is in speaker bureau for GlaxoSmithKline and received less than $10,000 in fees over the past 3 years. J.G.M. received £10,000 in research grants from Pfizer. R.A.W. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
American Lung Association Asthma Clinical Research Centers: Baylor College of Medicine, Houston: N.A. Hanania (principal investigator), M. Sockrider (coprincipal investigator), L. Giraldo (principal clinic coordinator), R. Valdez (coordinator); Columbia University–New York University Consortium, New York: J. Reibman (principal investigator), E. DiMango (coprincipal investigator), C. Cammarata and K. Carapetyan (clinic coordinators at New York University), J. Sormillon and E. Simpson (clinic coordinators at Columbia University); Duke University Medical Center, Durham, NC: L. Williams (principal investigator), J. Sundy (coprincipal investigator), G. Dudek (principal clinic coordinator), R. Newton and A. Dugdale (coordinators); Emory University School of Medicine, Atlanta: W.G. Teague (principal investigator), R. Patel (principal clinic coordinator), J. Peabody, E. Hunter, D. Whitlock (coordinators); Illinois Consortium, Chicago: L. Smith (principal investigator), J. Moy, E. Naureckas, C.S. Olopade (coprincipal investigators), J. Hixon (principal clinic coordinator), A. Brees, G. Rivera, S. Sietsema, V. Zagaja (coordinators); Indiana University, Asthma Clinical Research Center, Indianapolis: M. Busk (principal investigator), F. Leickly, C. Williams (coprincipal investigators), P. Puntenney (principal clinic coordinator); Jefferson Medical College, Philadelphia: F. Leone (principal investigator), M. Hayes-Hampton (principal clinic coordinator); Louisiana State University Health Sciences Center: Ernest N. Morial; Asthma, Allergy, and Respiratory Disease Center, New Orleans: W.R. Summer (principal investigator), C. Glynn and G. Meyaski (clinic coordinators); National Jewish Medical and Research Center, Denver: S. Wenzel and R. Katial (principal investigators), P. Silkoff (coprincipal investigator), R. Gibbs (principal clinic coordinator), L. Lopez, C. Ruis, B. Schoen (coordinators); Nemours Children's Clinic–University of Florida Consortium, Jacksonville: J. Lima (principal investigator), K. Blake (coprincipal investigator), A. Santos (principal clinic coordinator), L. Duckworth, D. Schaeffer, M. McRae (coordinators); North Shore–Long Island Jewish Health System, New Hyde Park, NY: J. Karpel (principal investigator), R. Cohen (coprincipal investigator), R. Ramdeo (principal clinic coordinator); Northern New England Consortium (formerly Vermont Lung Center at the University of Vermont), Colchester, VT: C.G. Irvin (principal investigator), A.E. Dixon, D.A. Kaminsky, E. Kent, T. Lahiri, P. Shapero (coprincipal investigators), S. Lang (principal clinic coordinator), J. Allen, A. Coote, L.M. Doucette, K. Girard, J. Lynn, L. Moon, T. Viola, S. Burns (coordinators); The Ohio State University Medical Center/Columbus Children's Hospital, Columbus: J. Mastronarde (principal investigator), K. McCoy (coprincipal investigator), J. Parsons (coinvestigator), J. Drake (principal clinic coordinator), R. Compton, L. Raterman, D. Cosmar (coordinators); University of Alabama at Birmingham, Birmingham: L.B. Gerald (principal investigator), W.C. Bailey (coprincipal investigator), S. Erwin (principal clinic coordinator), H. Young, A. Kelley, D. Laken, B. Martin (coordinators); University of Miami, Miami–University of South Florida, Tampa: A. Wanner (principal investigator, Miami), R. Lockey (principal investigator, Tampa), E. Mendes (principal clinic coordinator for University of Miami), S. McCullough (principal clinic coordinator for University of South Florida), B. Fimbel, M. Grandstaff (coordinators); University of Minnesota, Minneapolis: M.N. Blumenthal (principal investigator), G. Brottman, J. Hagen (coprincipal investigators), A. Decker, D. Lascewski, S. Kelleher (principal clinic coordinators), K. Bachman, M. Sneen (coordinators); University of Missouri, Kansas City School of Medicine, Kansas City: G. Salzman (principal investigator), D. Pyszczynski (coprincipal investigator), P. Haney (principal clinic coordinator); St. Louis Asthma Clinical Research Center: Washington University, St. Louis University and Clinical Research Center, St. Louis: M. Castro (principal investigator), L. Bacharier, K. Sumino (coinvestigators), M.E. Scheipeter and J. Tarsi (coordinators); University of California San Diego: S. Wasserman (principal investigator), J. Ramsdell (coprincipal investigator), J Vitin and T Tucker (clinic coordinators); Chairman's Office, Respiratory Hospital, Winnipeg, Manitoba, Canada: N. Anthonisen (research group chair); Data Coordinating Center, Johns Hopkins University Center for Clinical Trials, Baltimore: R. Wise (center director), J. Holbrook (deputy director), E. Brown (principal coordinator), D. Amend-Libercci, K. Barry, M. Daniel, A. Lears, G. Leatherman, C. Levine, R. Masih, S. Modak, D. Nowakowski, N. Prusakowski, D. Shade, C. Shiflett, E. Sugar; Esophageal pH Probe Quality Control Center, Temple University School of Medicine: J. Richter (center director); Data and Safety Monitoring Board: S. Lazarus (chair), W. Calhoun, P. Kahrilas, B. McWilliams, A. Rogatko, C. Sorkness; Project Office, American Lung Association, New York: E. Lancet, R. Vento (project officers), N. Edelman (scientific consultant), S. Rappaport, G. Pezza; Project Office, National Heart Lung and Blood Institute: V. Taggart (project officer), G. Weinmann (DSMB secretary, airway branch chief); ALA Scientific Advisory Committee: G. Snider (chair), N. Anthonisen, M. Castro, J. Fish, D. Ingbar, S. Jenkinson, D. Mannino, H. Perlstadt, L. Rosenwasser, J. Samet, D. Schraufnagel, J. Smith, L. Smith, T. Standiford, A. Wanner, and T. Weaver.
References
- 1.Sontag SJ, O'Connell S, Khandelwal S, Greenlee H, Schnell T, Nemchausky B, Chejfec G, Miller T, Seidel J, Sonnenberg A. Asthmatics with gastroesophageal reflux: long term results of a randomized trial of medical and surgical antireflux therapies. Am J Gastroenterol 2003;98:987–999. [DOI] [PubMed] [Google Scholar]
- 2.Harding SM, Richter JE, Guzzo MR, Schan CA, Alexander RW, Bradley LA. Asthma and gastroesophageal reflux: acid suppressive therapy improves asthma outcome. Am J Med 1996;100:395–405. [DOI] [PubMed] [Google Scholar]
- 3.Kiljander TO, Laitinen JO. The prevalence of gastroesophageal reflux disease in adult asthmatics. Chest 2004;126:1490–1494. [DOI] [PubMed] [Google Scholar]
- 4.Sontag SJ, O'Connell S, Khandelwal S, Miller T, Nemchausky B, Schnell TG, Serlovsky R. Most asthmatics have gastroesophageal reflux with or without bronchodilator therapy. Gastroenterology 1990;99:613–620. [DOI] [PubMed] [Google Scholar]
- 5.Irwin RS, Curley FJ, French CL. Difficult-to-control asthma. Contributing factors and outcome of a systematic management protocol. Chest 1993;103:1662–1669. [DOI] [PubMed] [Google Scholar]
- 6.Harding SM, Guzzo MR, Richter JE. The prevalence of gastroesophageal reflux in asthma patients without reflux symptoms. Am J Respir Crit Care Med 2000;162:34–39. [DOI] [PubMed] [Google Scholar]
- 7.National Heart Lung and Blood Institute National Asthma Education and Prevention Program Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma. Full report. 2007 [accessed 16 March, 2008]. Available from: http://www.nhlbi.nih.gov/guidelines/asthma/asthgdln.htm.
- 8.Gibson PG, Henry RL, Coughlan JL. Gastro-oesophageal reflux treatment for asthma in adults and children. Cochrane Database Syst Rev 2003:CD001496. [DOI] [PubMed]
- 9.Littner MR, Leung FW, Ballard ED II, Huang B, Samra NK. Effects of 24 weeks of lansoprazole therapy on asthma symptoms, exacerbations, quality of life, and pulmonary function in adult asthmatic patients with acid reflux symptoms. Chest 2005;128:1128–1135. [DOI] [PubMed] [Google Scholar]
- 10.Kiljander TO, Harding SM, Field SK, Stein MR, Nelson HS, Ekelund J, Illueca M, Beckman O, Sostek MB. Effects of esomeprazole 40 mg twice daily on asthma: a randomized placebo-controlled trial. Am J Respir Crit Care Med 2006;173:1091–1097. [DOI] [PubMed] [Google Scholar]
- 11.Mastronarde JG, Anthonisen NR, Castro M, Holbrook JT, Leone FT, Teague WG, Wise RA. Efficacy of esomeprazole for treatment of poorly controlled asthma. N Engl J Med 2009;360:1487–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Richter JE. Asthma and gastroesophageal reflux disease: the truth is difficult to define. Chest 1999;116:1150–1152. [DOI] [PubMed] [Google Scholar]
- 13.Herve P, Denjean A, Jian R, Simonneau G, Duroux P. Intraesophageal perfusion of acid increases the bronchomotor response to methacholine and to isocapnic hyperventilation in asthmatic subjects. Am Rev Respir Dis 1986;134:986–989. [DOI] [PubMed] [Google Scholar]
- 14.Wu DN, Tanifuji Y, Kobayashi H, Yamauchi K, Kato C, Suzuki K, Inoue H. Effects of esophageal acid perfusion on airway hyperresponsiveness in patients with bronchial asthma. Chest 2000;118:1553–1556. [DOI] [PubMed] [Google Scholar]
- 15.Cuttitta G, Cibella F, Visconti A, Scichilone N, Bellia V, Bonsignore G. Spontaneous gastroesophageal reflux and airway patency during the night in adult asthmatics. Am J Respir Crit Care Med 2000;161:177–181. [DOI] [PubMed] [Google Scholar]
- 16.Jack CI, Calverley PM, Donnelly RJ, Tran J, Russell G, Hind CR, Evans CC. Simultaneous tracheal and oesophageal ph measurements in asthmatic patients with gastro-oesophageal reflux. Thorax 1995;50:201–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harding SM, Schan CA, Guzzo MR, Alexander RW, Bradley LA, Richter JE. Gastroesophageal reflux-induced bronchoconstriction. Is microaspiration a factor? Chest 1995;108:1220–1227. [DOI] [PubMed] [Google Scholar]
- 18.Harding SM, Richter JE. The role of gastroesophageal reflux in chronic cough and asthma. Chest 1997;111:1389–1402. [DOI] [PubMed] [Google Scholar]
- 19.Juniper EF, Bousquet J, Abetz L, Bateman ED. Identifying ‘well-controlled’ and ‘not well-controlled’ asthma using the asthma control questionnaire. Respir Med 2006;100:616–621. [DOI] [PubMed] [Google Scholar]
- 20.Richter JE. Typical and atypical presentations of gastroesophageal reflux disease. The role of esophageal testing in diagnosis and management. Gastroenterol Clin North Am 1996;25:75–102. [DOI] [PubMed] [Google Scholar]
- 21.Dobhan R, Castell DO. Normal and abnormal proximal esophageal acid exposure: Results of ambulatory dual-probe pH monitoring. Am J Gastroenterol 1993;88:25–29. [PubMed] [Google Scholar]
- 22.Juniper EF, Guyatt GH, Cox FM, Ferrie PJ, King DR. Development and validation of the mini asthma quality of life questionnaire. Eur Respir J 1999;14:32–38. [DOI] [PubMed] [Google Scholar]
- 23.Bousquet J, Knani J, Dhivert H, Richard A, Chicoye A, Ware JE Jr, Michel FB. Quality of life in asthma. I. Internal consistency and validity of the SF-36 questionnaire. Am J Respir Crit Care Med 1994;149:371–375. [DOI] [PubMed] [Google Scholar]
- 24.Damiano A, Handley K, Adler E, Siddique R, Bhattacharyja A. Measuring symptom distress and health-related quality of life in clinical trials of gastroesophageal reflux disease treatment: further validation of the gastroesophageal reflux disease symptom assessment scale (GSAS). Dig Dis Sci 2002;47:1530–1537. [DOI] [PubMed] [Google Scholar]
- 25.Dickman R, Bautista JM, Wong WM, Bhatt R, Beeler JN, Malagon I, Risner-Adler S, Lam KF, Fass R. Comparison of esophageal acid exposure distribution along the esophagus among the different gastroesophageal reflux disease (GERD) groups. Am J Gastroenterol 2006;101:2463–2469. [DOI] [PubMed] [Google Scholar]
- 26.Richter JE, Bradley LA, DeMeester TR, Wu WC. Normal 24-hr ambulatory esophageal pH values. Influence of study center, pH electrode, age, and gender. Dig Dis Sci 1992;37:849–856. [DOI] [PubMed] [Google Scholar]
- 27.Vincent D, Cohen-Jonathan AM, Leport J, Merrouche M, Geronimi A, Pradalier A, Soule JC. Gastro-oesophageal reflux prevalence and relationship with bronchial reactivity in asthma. Eur Respir J 1997;10:2255–2259. [DOI] [PubMed] [Google Scholar]
- 28.Ferrari M, Benini L, Brotto E, Locatelli F, De Iorio F, Bonella F, Tacchella N, Corradini G, Lo Cascio V, Vantini I. Omeprazole reduces the response to capsaicin but not to methacholine in asthmatic patients with proximal reflux. Scand J Gastroenterol 2007;42:299–307. [DOI] [PubMed] [Google Scholar]
- 29.Schnatz PF, Castell JA, Castell DO. Pulmonary symptoms associated with gastroesophageal reflux: use of ambulatory pH monitoring to diagnose and to direct therapy. Am J Gastroenterol 1996;91:1715–1718. [PubMed] [Google Scholar]
- 30.Tomonaga T, Awad ZT, Filipi CJ, Hinder RA, Selima M, Tercero F Jr, Marsh RE, Shiino Y, Welch R. Symptom predictability of reflux-induced respiratory disease. Dig Dis Sci 2002;47:9–14. [DOI] [PubMed] [Google Scholar]
- 31.Chen MJ, Wu MS, Lin JT, Chang KY, Chiu HM, Liao WC, Chen CC, Lai YP, Wang HP, Lee YC. Gastroesophageal reflux disease and sleep quality in a Chinese population. Journal of the Formosan Medical Association = Taiwan yi zhi 2009;108:53–60. [DOI] [PubMed] [Google Scholar]
- 32.Irwin RS, Zawacki JK, Wilson MM, French CT, Callery MP. Chronic cough due to gastroesophageal reflux disease: failure to resolve despite total/near-total elimination of esophageal acid. Chest 2002;121:1132–1140. [DOI] [PubMed] [Google Scholar]
- 33.Tutuian R, Mainie I, Agrawal A, Adams D, Castell DO. Nonacid reflux in patients with chronic cough on acid-suppressive therapy. Chest 2006;130:386–391. [DOI] [PubMed] [Google Scholar]
- 34.Zerbib F, Roman S, Ropert A, des Varannes SB, Pouderoux P, Chaput U, Mion F, Verin E, Galmiche JP, Sifrim D. Esophageal ph-impedance monitoring and symptom analysis in GERD: a study in patients off and on therapy. Am J Gastroenterol 2006;101:1956–1963. [DOI] [PubMed] [Google Scholar]
- 35.Pauwels A, Blondeau K, Dupont L, Sifrim D. Cough and gastroesophageal reflux: from the gastroenterologist end. Pulm Pharmacol Ther 2009;22:135–138. [DOI] [PubMed] [Google Scholar]
- 36.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med 1999;159:179–187. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.