Abstract
Rationale: Induced mainly by cigarette smoking, chronic obstructive pulmonary disease (COPD) is a global public health problem characterized by progressive difficulty in breathing and increased mucin production. Previously, we reported that acrolein levels found in COPD sputum could activate matrix metalloproteinase-9 (MMP9).
Objectives: To determine whether acrolein increases expression and activity of MMP14, a critical membrane-bound endopeptidase that can initial a MMP-activation cascade.
Methods: MMP14 activity and adduct formation were measured following direct acrolein treatment. MMP14 expression and activity was measured in human airway epithelial cells. MMP14 immunohistochemistry was performed with COPD tissue, and in acrolein- or tobacco-exposed mice.
Measurements and Main Results: In a cell-free system, acrolein, in concentrations equal to those found in COPD sputum, directly adducted cysteine 319 in the MMP14 hemopexin-like domain and activated MMP14. In cells, acrolein increased MMP14 activity, which was inhibited by a proprotein convertase inhibitor, hexa-d-arginine. In the airway epithelium of COPD subjects, immunoreactive MMP14 protein increased. In mouse lung, acrolein or tobacco smoke increased lung MMP14 activity and protein. In cells, acrolein-induced MMP14 transcripts were inhibited by an epidermal growth factor receptor (EGFR) neutralizing antibody, EGFR kinase inhibitor, metalloproteinase inhibitor, or mitogen-activated protein kinase (MAPK) 3/2 or MAPK8 inhibitors, but not a MAPK14 inhibitor. Decreasing the MMP14 protein and activity in vitro by small interfering (si)RNA to MMP14 diminished the acrolein-induced MUC5AC transcripts. In acrolein-exposed mice or transgenic mice with lung-specific transforming growth factor-α (an EGFR ligand) expression, lung MMP14 and MUC5AC levels increased and these effects were inhibited by a EGFR inhibitor, erlotinib.
Conclusions: Taken together, these findings implicate acrolein-induced MMP14 expression and activity in mucin production in COPD.
Keywords: cigarette smoke, acrolein, erlotinib, mucous cell metaplasia, chronic obstructive pulmonary disease
AT A GLANCE COMMENTARY
Scientific Knowledge on the Subject
Acrolein is a component of cigarette smoke and also can be endogenously generated in the airways of persons with chronic obstructive pulmonary disease (COPD).
What This Study Adds to the Field
Low-level acrolein concentrations (equivalent to those present in COPD sputum) activated and increased matrix metalloproteinase-14 (MMP14) transcripts, protein, and activity. MMP14 immunostaining increased in the airway epithelium of subjects with COPD. Inhibition of MMP14 induction, by epidermal growth factor receptor kinase inhibitors, reduced acrolein-induced mucin levels in mouse lung. Thus, local pharmacological inhibition of MMP14 in the airway epithelium could be useful in the treatment of COPD-related mucin overproduction.
A leading cause of morbidity (>14 million cases) and mortality (>110,000 deaths/yr) in the United States (1), chronic obstructive pulmonary disease (COPD) is marked by excessive mucin production, chronic cough, shortness of breath, and labored breathing (2–4). The pathogenesis of COPD involves proteinase/antiproteinase imbalance that leads to disruption of the alveolar structure (emphysema) and alteration of the airway architecture (bronchitis) (3, 5), the latter is marked by decreased ciliated and Clara cells and increased mucin-producing cells. The etiology of COPD has been studied extensively and it is clearly linked to cigarette smoking and other environmental exposures (2–4, 6). Cigarette smoke contains numerous irritants but none stronger than acrolein (7–10), a potent inducer of excessive mucin production in laboratory animals (11–13). Excessive mucin production in more advanced COPD is associated with rapid declines in lung function and more frequent exacerbations (including hospitalization and death) (5, 14).
In the lung, the major proteinases include the matrix metalloproteinases (MMPs) and a disintegrin and metallopeptidase domain proteins (including ADAM17 [a disintegrin and metalloproteinase domain-17], also called TACE [tumor necrosis factor-α converting enzyme]) (15–18). Secreted MMPs are typically inactive zymogens (pro-MMPs) and are activated through initial cleavage by the other MMPs or serine endopeptidases (including neutrophil elastase) and subsequent autocatalytic cleavage. However, certain pro-MMPs lack sequences susceptible to proteolytic activation (19) and are activated by the membrane-bound MMP14 (also known as membrane type 1-MMP), an event that triggers an MMP activation cascade (20, 21). Unlike secreted MMPs, MMP14 is activated by proprotein convertases in the trans-Golgi network, allowing cell-specific control of secreted MMP activation. The cell surface localization of MMP14 permits targeting protease activation to pericellular regions. The expression of MMP14 and subsequent activation of MMPs is seen in normal lung fibroblasts exposed to cigarette smoke extract in vitro (22).
Previously, MMP9 and ADAM17 have been found to mediate increased mucin production, especially mucin 5AC, oligomeric mucus/gel-forming (MUC5AC) (12, 23, 24), through mobilization of epidermal growth factor (EGF) family ligands that bind to and activate receptor-type protein tyrosine kinases, including epidermal growth factor receptor (EGFR) (18, 23–30). However, small interfering RNA (siRNA) directed against ADAM17 and MMP9 did not completely inhibit the acrolein-induced increase in MUC5AC transcripts. In addition, gene-targeted mice lacking MMP9 had only a partial reversal of phenotype (24), suggesting a role of other MMPs in increased MUC5AC transcript levels. Inasmuch as cell surface mobilization of EGF ligands requires localized protease activation and because MMP14 activation can initiate MMP activation cascades at the cell surface, we sought to examine whether MMP14 transcripts are increased in airway epithelial cells and the role of MMP14 activation in acrolein-induced MUC5AC expression.
METHODS
Experimental Design
In a cell-free system, MMP14 activity was measured after submicromolar acrolein exposures at concentrations similar to those found in COPD sputum and acrolein protein adducts were measured by mass spectrometry. In human airway epithelial cells, MMP14 protein activity was measured and the role of proprotein convertase processes evaluated after treatment with an inhibitor, hexa-d-arginine. Immunoreactive MMP14 protein and periodic acid–Schiff staining for mucus glycoprotein was measured in the airway of subjects with COPD and control subjects. Immunoreactive MMP14 protein was measured in the airway of mice exposed to acrolein or cigarette smoke. To determine the role of EGFR and mitogen-activated protein kinase (MAPK) signaling in MMP14 expression, MMP14 transcripts were measured in human airway cells treated with neutralizing antibody (LA1), EGFR kinase inhibitor (AG1478), metalloproteinase inhibitor (GM6001), or MAPK3/2 (PD98059), MAPK8 (SP600125), or MAPK14 (ML3403) inhibitor. In human airway epithelial cells, siRNA to MMP14 was used to determine whether increases in acrolein-induced MUC5AC transcript levels were mediated by MMP14. Last, acrolein-exposed FVB/NJ (nontransgenic) strain mice or doxycycline-regulatable transgenic mice with induced lung-specific transforming growth factor-α (an EGFR ligand) expression were treated with vehicle (control) or with an EGFR kinase inhibitor, erlotinib, and lung MMP14 and MUC5AC transcript levels measured. Values are presented as means ± standard errors and were considered significant when P < 0.05 as determined by analysis of variance using an all-pairwise multiple-comparison procedure (Holm-Sidak method) (SigmaStat 3.5; Systat Software, Inc., San Jose, CA). See the online supplement for additional details of the methods used.
RESULTS
Acrolein Increases Airway Epithelial MMP14 Transcript, Protein, and Activity
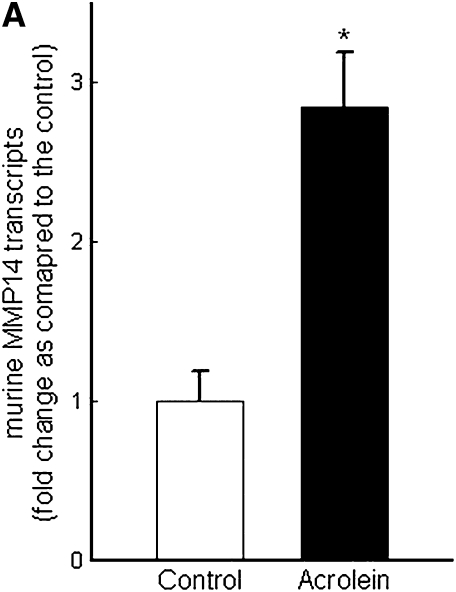
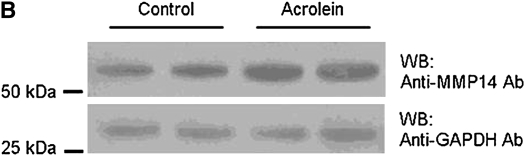
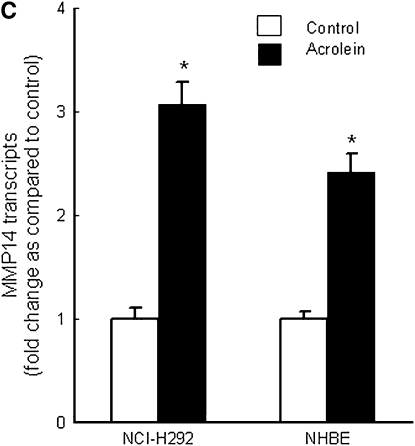
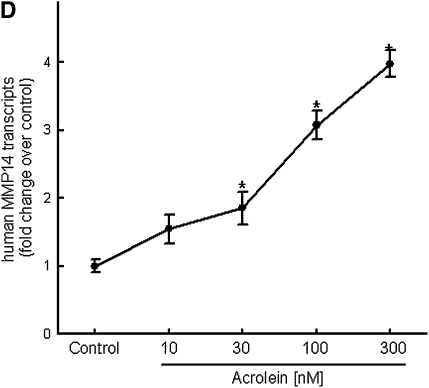
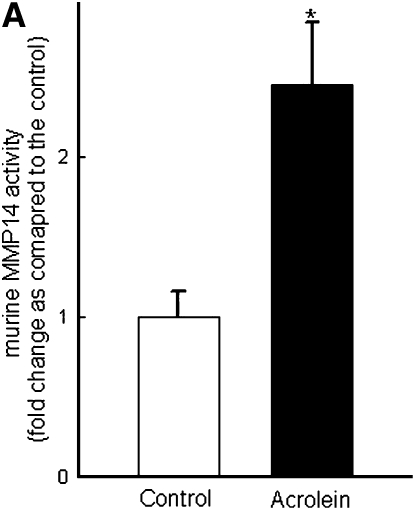
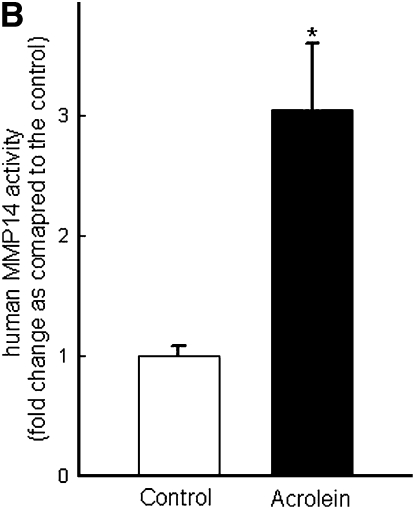
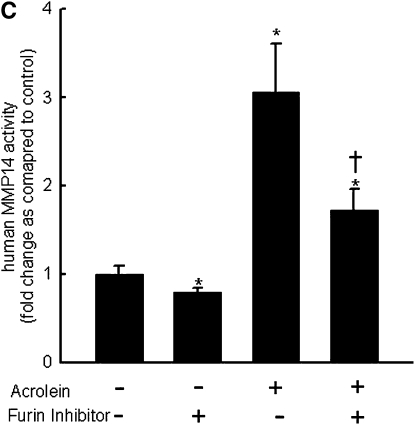
Repetitive acrolein exposure increased murine MMP14 transcript levels in FVB/NJ mouse lung (Figure 1A). MMP14 protein in the whole lung homogenates in acrolein-exposed FVB/NJ mice increased as compared with control (unexposed) mice (Figure 1B). MMP14 transcripts increased in acrolein-treated normal bronchial epithelial (NHBE) cells (Figure 1C) and acrolein increased MMP14 transcripts in airway epithelial (NCI-H292) cells in a concentration-dependent manner (Figure 1D). Thus MMP14 transcripts and protein increased after acrolein treatment. In addition, MMP14 activity also increased in acrolein-treated FVB/NJ mouse lung (1.6 ± 0.43 ng/ml) as compared with control mouse lung (0.52 ± 0.043 ng/ml) (Figure 2A). MMP14 activity increased in acrolein-treated NCI-H292 cells after acrolein exposure (8.78 ± 0.24 ng/ml) as compared with the control (2.87 ± 0.24 ng/ml) (Figure 2B). Pretreatment with a proprotein convertase (furin) inhibitor, hexa-d-arginine, diminished the acrolein-induced increase in MMP14 activity (Figure 2C).
Figure 1.
Acrolein increases matrix metalloproteinase-14 (MMP14) transcripts and protein levels. (A) Lung MMP14 transcript levels increased in FVB/NJ mice exposed to acrolein compared with control mice. FVB/NJ mice were exposed to acrolein (2.0 ppm × 6 h/d × 5 d/wk × 4 wk) or filtered air (control mice) and lung MMP14 levels were measured by quantitative real-time polymerase chain reaction (qRT-PCR). The results are expressed as fold change in the level of transcript after normalizing to glyceraldehyde-3-phosphate dehydrogenase (GAPDH). (B) Lung MMP14 protein levels increased in FVB/NJ mice exposed to acrolein. MMP14 protein level as determined by Western blot increased in acrolein-exposed FVB/NJ mouse lung after acrolein exposure. Each lane was loaded with 60 μg of protein obtained from an individual mouse that is representative of each group (n = 5 mice per treatment). (C) MMP14 transcript levels increased in NCI-H292 cells or normal bronchial epithelial (NHBE) cells treated with 100 nM acrolein (4 h). The level of MMP14 transcript was determined by qRT-PCR. Results are expressed as fold change in the level of MMP14 transcripts after normalizing to ribosomal protein L32 (RPL32). (D) MMP14 transcripts increased in NCI-H292 cells in a concentration-dependent manner after acrolein treatment. Confluent serum-starved NCI-H292 cells or NHBE cells were treated (4 h, 37°C) with 10–300 nM acrolein. Values represent means ± SEM (n = 5–9). *Significantly different from control mice, using an all-pairwise multiple-comparison analysis of variance procedure (Holm-Sidak method).
Figure 2.
Acrolein increases matrix metalloproteinase-14 (MMP14) activity. (A) Acrolein increased MMP14 activity in mouse lung. FVB/NJ mice were exposed to acrolein (2.0 ppm × 6 h/d × 5 d/wk × 4 wk) or filtered air (control). MMP14 activity was determined by capturing active MMP14 with anti-MMP14 antibody, which was then used to activate a modified prourokinase detection enzyme to cleave a chromomeric peptide substrate and the resultant color was read spectrophotometrically at 405 nm. A serial dilution of known sample of active MMP14 was run simultaneously to obtain a reference curve. Each sample was analyzed in the linear portion of the curve and the relative amount of active MMP14 was determined by comparing the OD405 of each sample against the standard curve. (B) Acrolein increased MMP14 activity in NCI-H292 cells. Confluent serum-starved NCI-H292 cells were treated with acrolein (300 nM, 4 h, 37°C) and MMP14 activity was determined. (C) A proprotein convertase inhibitor, hexa-d-arginine, inhibited acrolein-induced MMP14 activity by 61 ± 8%. Confluent serum-starved NCI-H292 cells were pretreated with 0.625 μM hexa-d-arginine and incubated with acrolein (300 nM, 4 h, 37°C), and MMP14 activity was determined. Values represent means ± SEM (n = 5–10). *Significantly different from control (P < 0.05), using an all-pairwise multiple-comparison analysis of variance (ANOVA) procedure (Holm-Sidak method). †Significantly different from acrolein treatment (P < 0.05), using an all-pairwise multiple-comparison ANOVA procedure (Holm-Sidak method).
Acrolein Binds to Cysteine-319 of MMP14 Protein
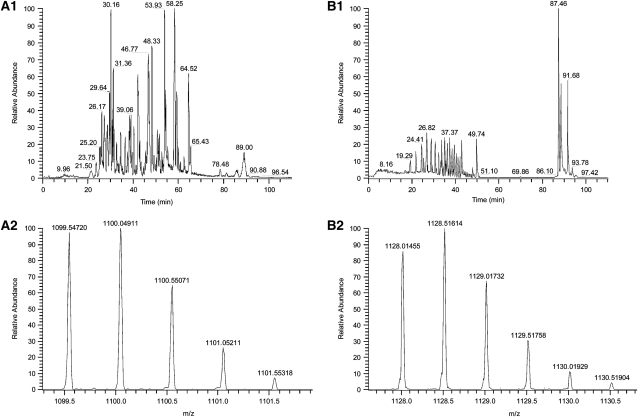
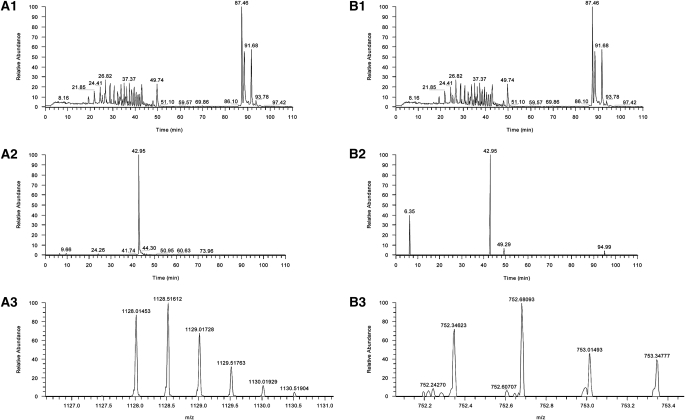
The mass spectral data generated from the full-scan analysis of the digestion of MMP14 were searched against the Uniprot-T database, subset human (Uniprot-T Consortium). This search resulted in the identification of 60 different peptides, including the peptide NPTYGNICDGFDTVAMLR that corresponds to amino acid residues 311 to 328 of the sequence of MMP14. The mass spectral data collected from the analysis of acrolein-exposed MMP14 was searched, using the same database. Twenty-four peptides were identified, and again the peptide NPTYGNICDGFDTVAMLR was identified. When these peptides were compared, the 2+ charge states displayed a mass difference of 28.51067 Da, a mass corresponding to an addition of acrolein to the peptide (Figure 3). The acrolein treatment of MMP14 was repeated and analyzed by single-ion monitoring mass spectrometry. When the mass ranges corresponding to the 2+ and 3+ charge states were scanned, in each case a base peak was observed at 42.95 minutes. This peak eluted at the same time as a peak in the full-scan mass spectrum and displayed mass spectra of the doubly and triply charged acrolein–adducted MMP14 (Figure 4). These data are consistent with an acrolein adduction occurring at the position 319 cysteine. Thus, acrolein not only increases MMP14 production and activity but directly conjugates to a cysteine residue known to form a disulfide bond within the hemopexin domain of MMP14.
Figure 3.
Mass spectrum of matrix metalloproteinase-14 (MMP-14) or acrolein-treated MMP-14 tryptic digests. Mass spectrum of MMP14 before and after acrolein treatment demonstrates a change in the 2+ charge state of the NPTYGNICDGFDTVAMLR peptide consistent with acrolein adduct formation. (A1) Full scan of MMP-14. (A2) Mass spectrum of the peptide NPTYGNICDGFDTVAMLR, 2+ charge state. (B1) Full scan of MMP-14 that has been exposed to acrolein. (B2) Mass spectrum of the peptide NPTYGNICDGFDTVAMLR with the adduction of acrolein, 2+ charge state. The mass spectral data generated from the full-scan analysis of the digestion of MMP-14 were searched against the Uniprot-T database, subset human (Uniprot-T Consortium).
Figure 4.
Mass spectrum of matrix metalloproteinase-14 (MMP14) or acrolein-treated MMP-14 tryptic digests. Mass spectrum before and after acrolein treatment demonstrates that adduct formation occurs at cysteine-319 in the hemopexin domain of MMP14. (A1) Full scan of acrolein-treated MMP14. (A2) Extracted ion scan for masses 1127.99 to 1128.03 Da, showing the presence of a single peak at 42.95 minutes. (A3) Mass spectrum of the peak eluting at 42.95 minutes, which is the 2+ charge state of the mass corresponding to acrolein adduction to MMP14. (B1) Full scan of acrolein-treated MMP14. (B2) Extracted ion scan for masses 752.34 to 752.36 Da, showing the presence of a base peak at 42.95 minutes. (B3) Mass spectrum of the peak eluting at 42.95 minutes, which is the 3+ charge state of the mass corresponding to acrolein adduction to MMP14. Analyses were performed by single-ion monitoring mass spectrometry.
MMP14 Mediates Increased MUC5AC after Acrolein Exposure
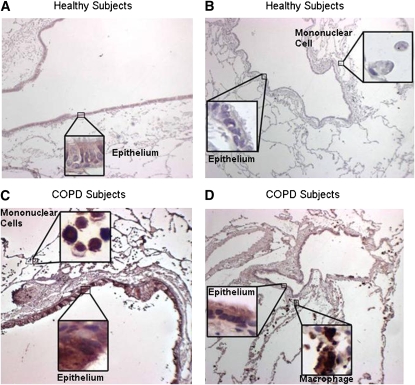
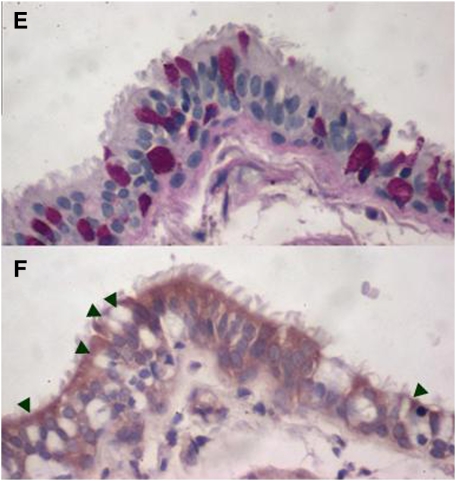
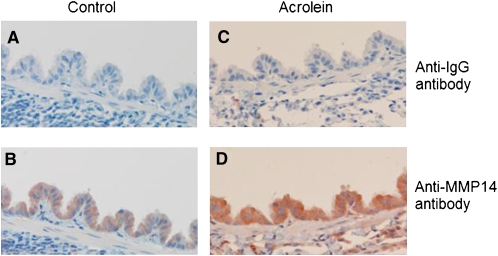
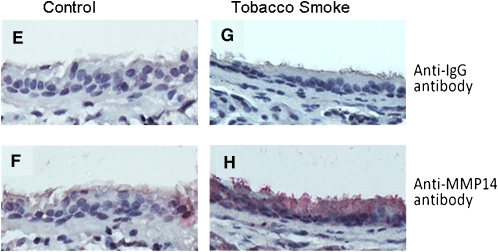
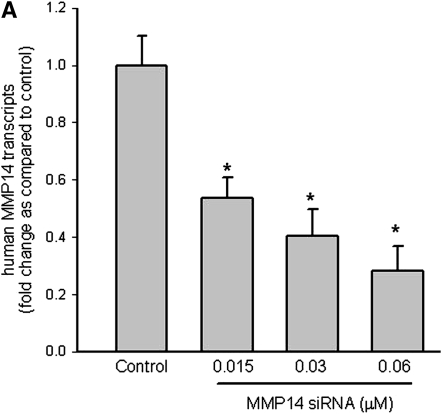
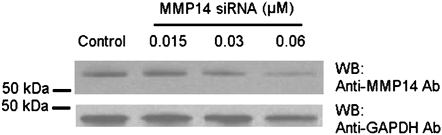
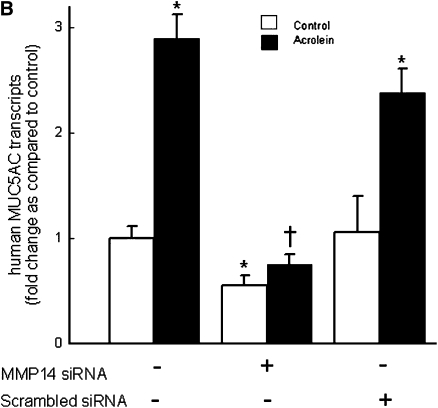
Immunostaining for MMP14 protein increased in the airways of subjects with COPD (Figures 5A–5D) and MMP14 staining accompanied increased mucus granule–containing (goblet) cells in the airway epithelium (Figures 5E and 5F). MMP14 immunostaining also increased in the airway epithelium of acrolein-exposed mice (Figures 6A–6D) or tobacco smoke-exposed mice (Figures 6E–6H). To determine the role of MMP14 in acrolein-induced MUC5AC increase, we transfected NCI-H292 cells with siRNA directed against MMP14. MMP14 siRNA decreased MMP14 transcript and protein levels, and activity (Figure 7A). NCI-H292 cells transfected with MMP14 siRNA had reduced levels of constitutive MUC5AC transcripts and demonstrated no increase in MUC5AC transcripts after acrolein treatment (Figure 7B). In contrast, nontransfected cells and cells transfected with scrambled siRNA demonstrated a normal response to acrolein (Figure 7B). Thus, NCI-H292 cells transfected with MMP14 siRNA responded less to acrolein treatment, supporting the hypothesis that acrolein-induced increases in MUC5AC are mediated by MMP14.
Figure 5.
Matrix metalloproteinase-14 (MMP14) immunostaining increases in subjects with chronic obstructive pulmonary disease (COPD). Lung specimens were obtained from human subjects undergoing lung transplant surgery for COPD treatment under institutional review board–approved protocols at the Washington University Medical Center (St. Louis, MO) and immunostaining with anti-MMP14 was performed. Immunostaining with anti-MMP14 antibody (red stain) increased in the lungs from (C and D) subjects with COPD as compared with (A and B) healthy subjects. The localization was notable for the presence of MMP14 in columnar airway epithelial cells, mononuclear cells in the alveolus, including pigmented macrophages (insets). In subjects with COPD, areas of mucous cell metaplasia were present when stained for (E) periodic acid–Schiff-positive mucus glycoprotein (red-purple) and corresponded with (F) translucent-appearing unstained cells (arrowheads) when immunostained with anti-MMP14 antibody in serial sections.
Figure 6.
Matrix metalloproteinase-14 (MMP14) immunostaining increases in FVB/NJ mouse airway epithelium after acrolein or tobacco smoke exposure. FVB/NJ mice were exposed to (A, B, E, and F) filtered air (control), (C and D) acrolein (2.0 ppm × 6 h/d × 5 d/wk × 4 wk), or (G and H) tobacco smoke (100 mg/m3 total suspended particulates × 6 h/d × 5 d/wk × 13 wk) and lung sections were incubated with (A, C, E, and G) control anti-IgG (diluted 1:100) or (B, D, F, and H) anti-MMP14 (diluted 1:100) antibody. In each image, the airway lumen is above the epithelium, which stains red-brown in the presence of MMP14. Endogenous peroxidase activity was quenched and specimens were incubated with horseradish peroxidase–labeled goat anti-mouse secondary antibody (diluted 1:5,000) in antibody dilution buffer, twice rinsed with phosphate-buffered saline (PBS), incubated with chromogen 3,3′-diaminobenzidine tetrachloride (0.05% in PBS), and counterstained with hematoxylin. The sections were visualized with a SPOT 2000 microscope (×40 objective) and the images were captured with a cooled charge-coupled device camera.
Figure 7.
Matrix metalloproteinase-14 (MMP14) mediates acrolein-induced increases in mucin 5AC, oligomeric mucus/gel-forming (MUC5AC) transcripts in human airway epithelial (NCI-H292) cells. (A) Top: MMP14 transcript levels were diminished in NCI-H292 cells transfected with small interfering RNA (siRNA) directed against MMP14 as compared with cells transfected with scrambled siRNA (negative control) or untransfected cells. Bottom: To determine whether siRNA-diminished transcript levels were accompanied by decreased MMP14 protein and activity, protein was isolated and subjected to Western blotting. (B) Acrolein-induced MUC5AC transcripts were diminished in NCI-H292 cells transfected with siRNA against MMP14 as compared with cells transfected with scrambled (nonsense) siRNA. NCI-H292 cells (40% confluent) were transfected with siRNA against MMP14 or scrambled siRNA (negative control) and compared with cells not transfected with siRNA. Cells were incubated (37°C, 36 h) and then treated with vehicle or acrolein (300 nM, 4 h, 37°C). RNA was isolated and the level of MUC5AC transcript was determined by quantitative real-time polymerase chain reaction. The results are expressed as fold change in the level of MMP14 or MUC5AC transcripts after normalizing to ribosomal protein L32 (RPL32). Values represent means ± SEM (n = 6–9). *Significantly different from control (P < 0.05), using analysis of variance (ANOVA) with all-pairwise multiple-comparison ANOVA procedure (Holm-Sidak method). †Significantly different from acrolein treatment (P < 0.05), using an all-pairwise multiple-comparison ANOVA procedure (Holm-Sidak method).
MMP14 Transcript Levels Increase after Acrolein Treatment through EGFR/MAPK3/2/MAPK8 Signaling
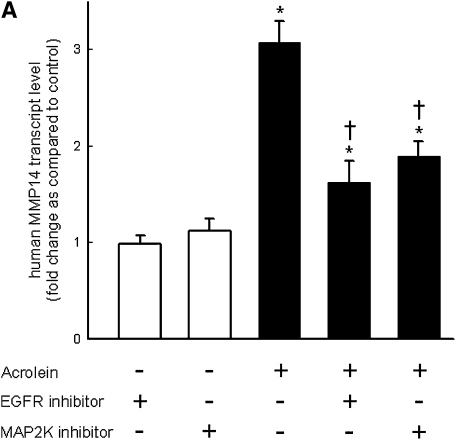
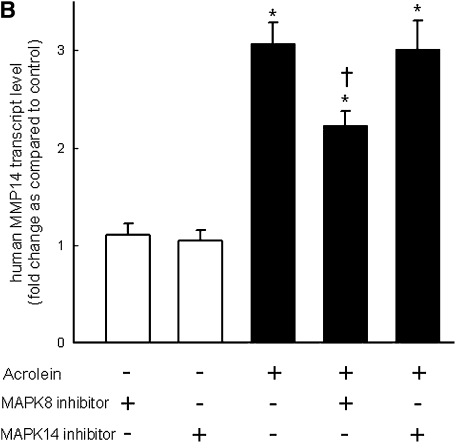
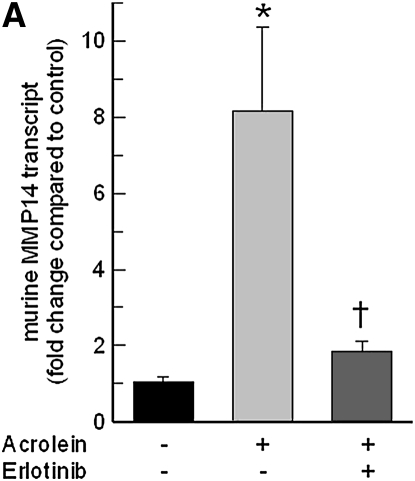
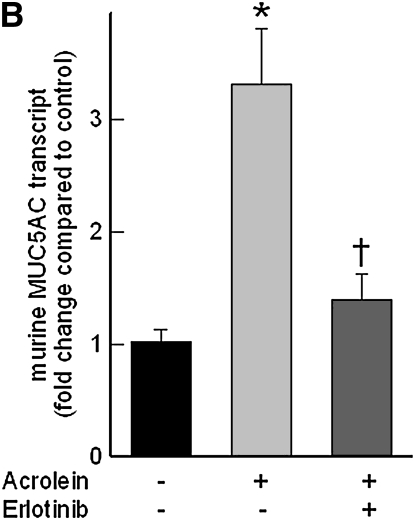
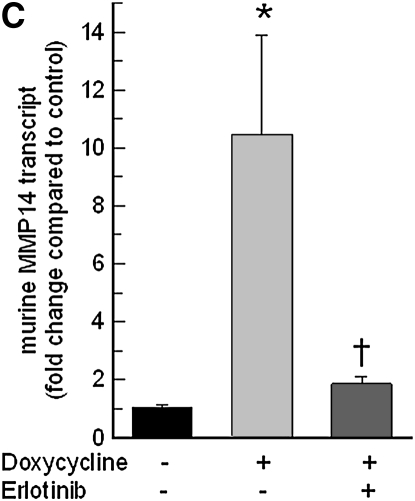
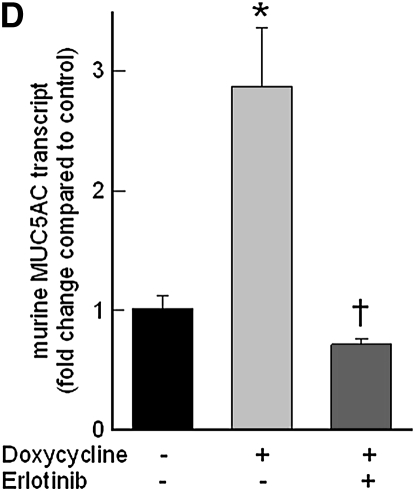
Pretreatment with EGFR tyrosine kinase inhibitor (AG1478) (Figure 8A) or LA1 (see Figure E1 in the online supplement), a neutralizing antibody against EGFR, diminished the acrolein-induced increase in MMP14 transcripts in NCI-H292 cells. EGFR activation leads to activation of downstream mitogen-activated protein kinase (MAPK). Pretreating the cells with MAPK3/2 inhibitor PD98059 (Figure 8A), with MAPK8 (c-Jun N-terminal kinase or JNK) inhibitor SP600125 (Figure 8B), or with metalloproteinase inhibitor GM6001 (Figure E1) diminished the acrolein-induced increase in MMP14 transcripts. Pretreatment with an MAPK14 (p38) inhibitor had no effect on the acrolein-induced increase in MMP14 transcripts (Figure 8B). Previously, we found that an EGFR antagonist, erlotinib, inhibited acrolein-induced MUC5AC transcripts and immunoreactive mucin protein in FVB/NJ mice (24). Lung MMP14 transcripts increased in acrolein-exposed mice and this effect was inhibited by erlotinib (Figure 9A). Likewise, acrolein-induced increased MUC5AC was inhibited by erlotinib (Figure 9B). In addition, lung MMP14 (Figure 9C) and MUC5AC (Figure 9D) transcripts increased with conditional doxycycline-inducible transforming growth factor-α, an EGFR ligand, and erlotinib attenuated this effect in doxycycline-treated mice. Thus, increased MMP14 and MUC5AC transcript levels in acrolein-treated airway epithelial cells are accompanied by metalloproteinase-mediated, EGFR ligand–dependent MAPK3/2 and MAKP8 signaling.
Figure 8.
Acrolein-induced increases in matrix metalloproteinase-14 (MMP14) transcript levels are mediated by epidermal growth factor receptor (EGFR) and mitogen-activated protein kinase (MAPK) signaling in human airway epithelial (NCI-H292) cells. (A) Acrolein-induced increases in transcript levels of MMP14 were diminished by an EGFR kinase inhibitor (0.250 μM AG1478) or an MAPK3/2 inhibitor (5 μM PD98059), by 72 and 57%, respectively. (B) MMP14 transcript levels were also decreased in cells treated with an MAPK8 (also called c-Jun N-terminal kinase, JNK) inhibitor (5 μM SP600125) by 41%, but not in cells treated with MAPK14 (also called p38 MAPK) inhibitor (5 μM ML3403) by 2%. Confluent NCI-H292 cells were pretreated (37°C, 1 h) with inhibitor and then incubated with vehicle or acrolein (100 nM, 4 h, 37°C). RNA was isolated and the level of MMP14 transcript was determined by quantitative real-time polymerase chain reaction. The results are expressed as fold change in the level of MMP14 transcripts after normalizing to ribosomal protein L32 (RPL32). Values represent means ± SEM (n = 4–6). *Significantly different from control (P < 0.05), using an all-pairwise multiple-comparison ANOVA procedure (Holm-Sidak method). †Significantly different from acrolein treatment (P < 0.05), using an all-pairwise multiple-comparison ANOVA procedure (Holm-Sidak method).
Figure 9.
Epidermal growth factor receptor (EGFR) inhibition diminishes acrolein- or transforming growth factor (TGF)-α–induced increases in matrix metalloproteinase-14 (MMP14) or mucin 5AC, oligomeric mucus/gel-forming (MUC5AC) transcripts in FVB/NJ mouse lung. (A) Acrolein-induced increases in MMP14 transcripts were diminished in mice pretreated with an EGFR inhibitor (erlotinib) by 89% compared with mice treated with sterile vehicle control. FVB/NJ mice were pretreated with erlotinib (100 mg/kg/d by gavage) or vehicle and exposed to acrolein (2.0 ppm × 6 h/d × 5 d/wk × 4 wk). (B) Acrolein-induced increases in MUC5AC transcripts were diminished by 86% in mice pretreated with an EGFR inhibitor (erlotinib) compared with mice treated with vehicle control. (C) TGF-α induced increases in MMP14 transcripts in conditional transgenic mice after doxycycline induction as compared with the littermate controls maintained without doxycycline for 8 weeks. This effect was inhibited by 91% in mice pretreated with an EGFR inhibitor (erlotinib, 100 mg/kg/d). (D) TGF-α induced increases in MUC5AC transcripts in conditional transgenic mice after doxycycline induction as compared with the littermate controls maintained without doxycycline for 8 weeks. This effect was inhibited about 100% in mice pretreated with an EGFR inhibitor (erlotinib). RNA was isolated and the levels of MMP14 or MUC5AC transcripts were determined by quantitative real-time polymerase chain reaction. The results are expressed as fold change in the level of MMP14 or MUC5AC transcripts after normalizing to ribosomal protein L32 (RPL32). Values represent means ± SEM (n = 4–9 mice per group). *Significantly different from control (P < 0.05), using an all-pairwise multiple-comparison analysis of variance (ANOVA) procedure (Holm-Sidak method). †Significantly different from acrolein or TGF-α treatment (P < 0.05), using an all-pairwise multiple-comparison ANOVA procedure (Holm-Sidak method).
DISCUSSION
It is clear that acrolein can initiate mucin overproduction in vivo. Animals exposed repeatedly to acrolein develop histological changes including epithelial damage, mucous cell metaplasia, and bronchiolitis, accompanied by excessive macrophage accumulation in the airways (11, 31, 32). Acrolein exposure increased mucus-producing cells in airways and increased MUC5AC transcripts in the lungs of Sprague-Dawley rats (11) and MUC5AC and immunoreactive mucin in the lungs of FVB/NJ mice (12, 24). MMPs have been proposed to play an important role in pathogenesis of COPD with several articles describing the role of MMPs in various lung pathologies (33). In this study, we examine whether acrolein alters expression and activation of MMP14, a critical membrane-bound endopeptidase that can initiate an MMP activation cascade.
The acrolein levels used in this study (submicromolar in vitro and 2 ppm in vivo) are relevant to common human exposures. Acrolein levels in second-hand tobacco smoke are elevated compared with mainstream smoke, because concentrations are increased in side-stream smoke due to altered tobacco combustion at lower temperatures (34–36). More than 30 million nonsmokers in the United States are exposed to acrolein concentrations in indoor air ranging from 0.8 to 1.5 ppm and levels between 0.1 and 10 ppm have been detected in bars and restaurants (35, 37–39). Acrolein is also generated by biomass fuel combustion and high-temperature cooking with oils (especially in woks) and is the major irritant in grassland and forest fires, and diesel exhaust (34, 35, 40, 41). In addition to exogenous exposure, acrolein is endogenously generated in inflamed tissues from threonine by myeloperoxidase activation (42–45), spermine or spermidine by amine oxidase–mediated catabolism (46–50), or possibly membrane fatty acids by oxidative degradation (35, 51–53).
Because it forms a highly reactive zwitterion (+CH2CH=CHO−) through electron rearrangement of the α,β-unsaturated bond, acrolein readily reacts with various molecules on the airway surface and thus it is nearly completely retained in the respiratory epithelium (10, 54). Acrolein readily attacks nucleophiles, especially thiol-containing proteins (10, 53, 55, 56). Of all the α,β-unsaturated 2-alkenals, acrolein is among the strongest electrophiles (51), the most irritating (i.e., concentrations as low as 0.06 ppm can cause eye irritation within 5 min) (7, 57, 58), and share in the ability to covalently modify macromolecules, which disrupt critical cellular functions or cause mutations (51, 59–62). Acrolein–protein adducts accumulate in ischemic tissue (52, 63) and in atherosclerotic lesions (45, 64), and we found that acrolein can directly bind to and activate MMP14 in this study.
Previously we reported a role for MMP9 and MMP12 in acrolein-induced MUC5AC expression (12, 23, 24). Acrolein increased MUC5AC transcripts and mucin protein in strain-matched control Mmp9+/+ mice more than gene-targeted Mmp9−/− mice (23, 24). Similarly, acrolein increased MUC5AC transcripts and macrophage accumulation in lungs of strain-matched control Mmp12+/+ mice more than in gene-targeted Mmp12−/− mice (12). Acrolein increased the transcript levels of MUC5AC in NCI-H292 cells (31) and normal bronchial epithelial (NHBE) cells (23, 24). This increase in MUC5AC transcripts is mediated through an EGFR–MAPK pathway that is initiated by ectodomain shedding of EGFR ligands mediated by metalloproteinases ADAM17 and MMP9 (23). However, siRNA directed against ADAM17 and MMP9 did not completely inhibit the acrolein-induced increase in MUC5AC transcripts. Similarly, the inhibition of acrolein-induced MUC5AC increase was partial in the lungs of gene-targeted Mmp9−/− as compared with Mmp9+/+ mice, suggesting a role for another MMP in acrolein-induced MUC5AC increase. Previously, Ning and colleagues (22) demonstrated that cigarette smoke extract increased MMP14 transcript levels in human lung fibroblasts, so MMP14 was a reasonable candidate for further study in human airway epithelial cells and in vivo in mice.
Unlike secreted MMPs, MMP14 is associated with the cell surface through a type 1 transmembrane domain (65). MMP14 is important in lung development as evidenced by a defect in formation of alveolar septae in Mmp14−/− mice (66, 67). MMP14 can activate pro-MMP2 (68) and pro-MMP13 (69), which in turn can cleave pro-MMP9 (70). Here we found that acrolein increased MMP14 transcript (Figure 1A), protein (Figure 1B), and activity (Figure 2A) in the lungs of FVB/NJ mice. The inhaled acrolein concentration (2 ppm × 6 h/d) is estimated to deliver an acrolein dose to the lung equivalent to 0.5–1.0 cigarette pack per day. Acrolein increased MMP14 transcripts in NHBE and NCI-H292 cells (Figure 1C). Moreover, the acrolein concentration necessary to increase MMP14 was as low as 30 nM (Figure 1D), which is a concentration well within the concentrations we previously measured in sputum from subjects with COPD (131 ± 24 nM) (24).
MMP14 activity is tightly controlled at the transcriptional and posttranslational levels. MMP14 is produced as a latent propeptide that keeps the enzyme latent through the interaction of a cysteine residue with a zinc ion in the catalytic domain. MMP14 has a unique regulatory mechanism in which the active enzyme undergoes a series of processing steps, either autocatalytic (71, 72) or mediated by other proteases (73, 74), initially to an enzymatically active (∼56-kD) species and ultimately to an inactive membrane-tethered (∼44-kD) species lacking the entire catalytic domain, thereby regulating the activity and nature of MMP14 proteins at the cell surface and at the pericellular space. MMP14 contains an RXK/RR proprotein convertase enzyme recognition motif between the propeptide and catalytic domain, which can be activated by intracellular subtilisin-type serine proteinases (e.g., furin) before MMP14 reaches the cell surface (73). Pretreatment with a furin inhibitor partially decreased the acrolein-induced increase in MMP14 activity (Figure 2C), suggesting the presence of an additional mechanism for increased MMP14 activity after acrolein treatment.
Inhaled or endogenously generated acrolein reacts directly with protein and nonprotein sulfhydryl groups, mainly at the cell surface, and with primary and secondary amines found in the intracellular proteins (34, 35). In lungs, MMP14 is expressed on surface epithelial cells (75, 76) (Figures 5 and 6). Conjugation of the carbon of acrolein with sulfhydryl groups by a Michael addition reaction is rapid and essentially irreversible (35, 77). Cysteine residues near the transmembrane domain or in the catalytic domain could potentially interfere with the autocatalytic processing and thus increase the amount of active MMP14 present on the cell surface and thus potentially increase MMP14 activity. When MMP14 was treated with acrolein, we identified a cysteine-319 adduct (Figures 3 and 4), which is contained within a hemopexin-like domain and not the conserved “cysteine switch” domain that is cleaved by proprotein convertases. Hemopexin domains are usually involved in substrate recognition of large matrix molecules at sites distant from the catalytic domain (78, 79). However, MMP14 is membrane localized and hemopexin domains appear to be critical for MMP14 dimerization. One process that would require MMP14 self-interaction is the major form of enzyme inactivation by autocatalytic cleavage (72, 74, 79). We propose that our data suggest that MMP14 surface activity is preserved by interference with hemopexin domain–mediated dimerization and autocatalytic inactivation resulting in persistence of active MMP14 on the cell surface of acrolein-treated airway epithelial cells.
MMP14 activity is also regulated at the transcriptional level and can be controlled at the protein level via anti-proteinase inhibitors (80). Acrolein treatment increased the transcript levels of MMP14 in NCI-H292 cells and NHBE cells (Figure 1C). Cytokines (including IL-2, IL-8, and monocyte chemokine protein-1) (81, 82) and growth factors (including EGF [83], fibroblast growth factor-1 [84], vascular endothelial growth factor [85], and insulin-like growth factor-1 [86]) can induce MMP14 expression in various cell lines. Previously, MMP14 has been found to be expressed on rabbit surface airway epithelial cells (75) and alveolar type II cells (76) and in human adenocarcinoma cells (87). We found that MMP14 transcripts increased in the lungs of FVB/NJ mice exposed to acrolein (Figure 1A). Immunostaining for MMP14 increased in the lungs of FVB/NJ mice exposed to acrolein or tobacco smoke (Figure 6) and in the airways of human subjects with COPD (Figure 5). It is important to note that the subjects with COPD were not current smokers, which suggests that increased MMP14 can be persistent (possibly due to endogenously generated acrolein).
We used siRNA to confirm the role of MMP14 transcripts in acrolein-induced MUC5AC increase (Figure 7). siRNA directed against MMP14 efficiently decreased the transcript and protein levels. NCI-H292 cells transfected with siRNA had lower constitutive levels of MUC5AC transcripts as compared with untransfected cells or cells transfected with scrambled siRNA. Transcript levels of MUC5AC in NCI-H292 cells transfected with MMP14 siRNA after acrolein treatment were not significantly different from control cells. Untransfected cells responded appropriately to acrolein treatment. These results indicate that MMP14 plays a critical role in acrolein-induced MUC5AC increase. As noted previously, MMP14 can activate MMP13 and MMP2, which in turn could activate MMP9. Thus, several MMPs are likely to contribute to MUC5AC increases.
Past investigations of MMP14 regulation have focused on protein processing (as noted previously), and therefore less is known about the signal transduction pathways involved in increased MMP14 expression in the lung. Inhibition of MAPK3/2 (ERK1/2) decreased MMP14 expression in fibrosarcoma cells (88). MAPK3/2 but not MAPK8 (JNK) or MAPK14 (p38) regulates increased MMP14 expression in rat endothelial cells (89) and lung fibroblasts (22). Moreover, constitutively active MAP2K increased MMP14 expression in MDK cells (90) and an MAP2K1/2 (MEK1/2) inhibitor diminished cigarette smoke extract–induced MMP14 expression in lung fibroblasts (22). Here we report that an EGFR kinase inhibitor diminished the acrolein-induced increase in MMP14 transcripts, confirming the role of EGFR in the acrolein-induced increase in NCI-H292 cell (treated with AG1478) (Figure 8A) and mouse lung (treated with erlotinib) (Figure 9) MMP14 transcripts. Treatment with MAP3/2 inhibitor (PD98059) and the MAPK8 (JNK) inhibitor (SP600125), but not the MAPK14 (p38) inhibitor (ML3403), decreased the acrolein-induced increase in MMP14 transcripts, suggesting that MAPK3/2 (ERK1/2) and MAPK8 (JNK), but not MAPK14 (p38), are involved in the response initiated by acrolein in the airway epithelium. Thus, regulation of MMP14 expression in the airway epithelium (which includes MAPK8) differs from that in lung fibroblasts.
The MMP proteinase activity can be regulated by a counterbalance with antiproteinase. For example, the tissue inhibitors of metalloproteinase proteins (TIMPs) represent a family of at least four 20- to 29-kD secreted proteins (TIMPs 1–4) that reversibly inhibit the MMPs in a 1:1 stoichiometric fashion (91). TIMP1 (92), TIMP2, TIMP3, and TIMP4 (93) are expressed in bronchial epithelium. TIMP2 (80) and TIMP3 (94), but not TIMP1 (95), inhibit MMP14 activity. TIMP3 also has the unique ability to bind via its C-terminal domain to heparin sulfate proteoglycans within the extracellular matrix, thereby concentrating it to specific regions within tissues and basement membranes (96). Unlike other TIMPs, TIMP3 is subject to a high degree of transcriptional regulation (97). Previously, we determined that TIMP3 transcript levels decreased in the lungs of FVB/NJ mice after acrolein exposure (23), which also could contribute to an increase in MMP14 and other proteinase activity.
In summary, these findings implicate acrolein-induced MMP14 expression and activity in mucin production in COPD. Low-level acrolein concentrations (equivalent to those present in COPD sputum) activated and increased MMP14 transcripts, protein, and activity. MMP14 immunostaining increased in the airway epithelium of subjects with COPD. Inhibition of MMP14 induction, by EGFR kinase inhibitors, reduced acrolein-induced mucin levels in mouse lung. Thus, local pharmacological inhibition of MMP14 in the airway epithelium could be useful in the treatment of COPD-related mucin overproduction.
Supplementary Material
Supported by National Institutes of Health grants ES006096, ES010562, ES015036, ES015675, HL058795, HL065213, HL065612, HL077763, HL081270, HL085655, HL086598, and HL091938.
This article has an online supplement, which is accessible from this issue's table of contents online at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.200903-0328OC on August 6, 2009
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Mannino DM, Homa DM, Akinbami LJ, Ford ES, Redd SC. Chronic obstructive pulmonary disease surveillance:–United States, 1971–2000. MMWR Surveill Summ 2002;51:1–16. [PubMed] [Google Scholar]
- 2.Perez-Vilar J. Mucin granule intraluminal organization. Am J Respir Cell Mol Biol 2007;36:183–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hogg JC. Pathophysiology of airflow limitation in chronic obstructive pulmonary disease. Lancet 2004;364:709–721. [DOI] [PubMed] [Google Scholar]
- 4.Rabe KF, Hurd S, Anzueto A, Barnes PJ, Buist SA, Calverley P, Fukuchi Y, Jenkins C, Rodriguez-Roisin R, van Weel C, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2007;176:532–555. [DOI] [PubMed] [Google Scholar]
- 5.Shapiro SD, Ingenito EP. The pathogenesis of chronic obstructive pulmonary disease: advances in the past 100 years. Am J Respir Cell Mol Biol 2005;32:367–372. [DOI] [PubMed] [Google Scholar]
- 6.Celli BR, Halbert RJ, Nordyke RJ, Schau B. Airway obstruction in never smokers: results from the Third National Health and Nutrition Examination Survey. Am J Med 2005;118:1364–1372. [DOI] [PubMed] [Google Scholar]
- 7.Andre E, Campi B, Materazzi S, Trevisani M, Amadesi S, Massi D, Creminon C, Vaksman N, Nassini R, Civelli M, et al. Cigarette smoke–induced neurogenic inflammation is mediated by α,β-unsaturated aldehydes and the TRPA1 receptor in rodents. J Clin Invest 2008;118:2574–2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morris JB, Symanowicz PT, Olsen JE, Thrall RS, Cloutier MM, Hubbard AK. Immediate sensory nerve–mediated respiratory responses to irritants in healthy and allergic airway-diseased mice. J Appl Physiol 2003;94:1563–1571. [DOI] [PubMed] [Google Scholar]
- 9.Nielsen GD, Wolkoff P, Alarie Y. Sensory irritation: risk assessment approaches. Regul Toxicol Pharmacol 2007;48:6–18. [DOI] [PubMed] [Google Scholar]
- 10.Reddy S, Finkelstein EI, Wong PS, Phung A, Cross CE, van der Vliet A. Identification of glutathione modifications by cigarette smoke. Free Radic Biol Med 2002;33:1490–1498. [DOI] [PubMed] [Google Scholar]
- 11.Borchers MT, Wert SE, Leikauf GD. Acrolein-induced MUC5ac expression in rat airways. Am J Physiol 1998;274:L573–L581. [DOI] [PubMed] [Google Scholar]
- 12.Borchers MT, Wesselkamper S, Wert SE, Shapiro SD, Leikauf GD. Monocyte inflammation augments acrolein-induced Muc5ac expression in mouse lung. Am J Physiol 1999;277:L489–L497. [DOI] [PubMed] [Google Scholar]
- 13.Wright JL, Cosio M, Churg A. Animal models of chronic obstructive pulmonary disease. Am J Physiol Lung Cell Mol Physiol 2008;295:L1–L15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vestbo J, Hogg JC. Convergence of the epidemiology and pathology of COPD. Thorax 2006;61:86–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim JH, Lee SY, Bak SM, Suh IB, Shin C, Shim JJ, In KH, Kang KH, Yoo SH. Effects of matrix metalloproteinase inhibitor on LPS-induced goblet cell metaplasia. Am J Physiol Lung Cell Mol Physiol 2004;287:L127–L133. [DOI] [PubMed] [Google Scholar]
- 16.Lemaitre V, D'Armiento J. Matrix metalloproteinases in development and disease. Birth Defects Res C Embryo Today 2006;78:1–10. [DOI] [PubMed] [Google Scholar]
- 17.Nadel JA. Innate immune mucin production via epithelial cell surface signaling: relationship to allergic disease. Curr Opin Allergy Clin Immunol 2007;7:57–62. [DOI] [PubMed] [Google Scholar]
- 18.Shao MX, Nakanaga T, Nadel JA. Cigarette smoke induces Muc5ac mucin overproduction via tumor necrosis factor-α-converting enzyme in human airway epithelial (NCI-H292) cells. Am J Physiol Lung Cell Mol Physiol 2004;287:L420–L427. [DOI] [PubMed] [Google Scholar]
- 19.Nagase H, Suzuki K, Morodomi T, Enghild JJ, Salvesen G. Activation mechanisms of the precursors of matrix metalloproteinases 1, 2 and 3. Matrix Suppl 1992;1:237–244. [PubMed] [Google Scholar]
- 20.Cowell S, Knauper V, Stewart ML, D'Ortho MP, Stanton H, Hembry RM, Lopez-Otin C, Reynolds JJ, Murphy G. Induction of matrix metalloproteinase activation cascades based on membrane-type 1 matrix metalloproteinase: associated activation of gelatinase A, gelatinase B and collagenase 3. Biochem J 1998;331:453–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fridman R, Toth M, Pena D, Mobashery S. Activation of progelatinase B (MMP-9) by gelatinase A (MMP-2). Cancer Res 1995;55:2548–2555. [PubMed] [Google Scholar]
- 22.Ning W, Dong Y, Sun J, Li C, Matthay MA, Feghali-Bostwick CA, Choi AM. Cigarette smoke stimulates matrix metalloproteinase-2 activity via EGR-1 in human lung fibroblasts. Am J Respir Cell Mol Biol 2007;36:480–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deshmukh HS, Case LM, Wesselkamper SC, Borchers MT, Martin LD, Shertzer HG, Nadel JA, Leikauf GD. Metalloproteinases mediate mucin 5AC expression by epidermal growth factor receptor activation. Am J Respir Crit Care Med 2005;171:305–314. [DOI] [PubMed] [Google Scholar]
- 24.Deshmukh HS, Shaver C, Case LM, Dietsch M, Wesselkamper SC, Hardie WD, Korfhagen TR, Corradi M, Nadel JA, Borchers MT, et al. Acrolein-activated matrix metalloproteinase 9 contributes to persistent mucin production. Am J Respir Cell Mol Biol 2008;38:446–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Casalino-Matsuda SM, Monzon ME, Forteza RM. Epidermal growth factor receptor activation by epidermal growth factor mediates oxidant-induced goblet cell metaplasia in human airway epithelium. Am J Respir Cell Mol Biol 2006;34:581–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Basbaum C, Li D, Gensch E, Gallup M, Lemjabbar H. Mechanisms by which gram-positive bacteria and tobacco smoke stimulate mucin induction through the epidermal growth factor receptor (EGFR). Novartis Found Symp 2002;248:171–176; discussion 176–180, 277–182. [PubMed] [Google Scholar]
- 27.Kim HJ, Park YD, Moon UY, Kim JH, Jeon JH, Lee JG, Bae YS, Yoon JH. The role of Nox4 in oxidative stress–induced MUC5AC overexpression in human airway epithelial cells. Am J Respir Cell Mol Biol 2008;39:598–609. [DOI] [PubMed] [Google Scholar]
- 28.Tichelaar JW, Lu W, Whitsett JA. Conditional expression of fibroblast growth factor-7 in the developing and mature lung. J Biol Chem 2000;275:11858–11864. [DOI] [PubMed] [Google Scholar]
- 29.Hardie WD, Korfhagen TR, Sartor MA, Prestridge A, Medvedovic M, Le Cras TD, Ikegami M, Wesselkamper SC, Davidson C, Dietsch M, et al. Genomic profile of matrix and vasculature remodeling in TGF-α induced pulmonary fibrosis. Am J Respir Cell Mol Biol 2007;37:309–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hardie WD, Davidson C, Ikegami M, Leikauf GD, Le Cras TD, Prestridge A, Whitsett JA, Korfhagen TR. EGF receptor tyrosine kinase inhibitors diminish transforming growth factor-α–induced pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol 2008;294:L1217–L1225. [DOI] [PubMed] [Google Scholar]
- 31.Borchers MT, Carty MP, Leikauf GD. Regulation of human airway mucins by acrolein and inflammatory mediators. Am J Physiol 1999;276:L549–L555. [DOI] [PubMed] [Google Scholar]
- 32.Feron VJ, Kruysse A, Til HP, Immel HR. Repeated exposure to acrolein vapour: subacute studies in hamsters, rats and rabbits. Toxicology 1978;9:47–57. [DOI] [PubMed] [Google Scholar]
- 33.Atkinson JJ, Senior RM. Matrix metalloproteinase-9 in lung remodeling. Am J Respir Cell Mol Biol 2003;28:12–24. [DOI] [PubMed] [Google Scholar]
- 34.Faroon O, Roney N, Taylor J, Ashizawa A, Lumpkin M, Plewak D. Acrolein environmental levels and potential for human exposure. Toxicol Ind Health 2008;24:543–564. [DOI] [PubMed] [Google Scholar]
- 35.Stevens JF, Maier CS. Acrolein: sources, metabolism, and biomolecular interactions relevant to human health and disease. Mol Nutr Food Res 2008;52:7–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leikauf GD. Hazardous air pollutants and asthma. Environ Health Perspect 2002;110:505–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nazaroff WW, Singer BC. Inhalation of hazardous air pollutants from environmental tobacco smoke in us residences. J Expo Anal Environ Epidemiol 2004;14:S71–S77. [DOI] [PubMed] [Google Scholar]
- 38.Jermini C, Weber A, Grandjean E. [Quantitative determination of various gas-phase components of the side-stream smoke of cigarettes in the room air as a contribution to the problem of passive-smoking] [author's translation]. Int Arch Occup Environ Health 1976;36:169–181. [DOI] [PubMed] [Google Scholar]
- 39.Weber A, Jermini C, Grandjean E. Irritating effects on man of air pollution due to cigarette smoke. Am J Public Health 1976;66:672–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hales CA, Musto SW, Janssens S, Jung W, Quinn DA, Witten M. Smoke aldehyde component influences pulmonary edema. J Appl Physiol 1992;72:555–561. [DOI] [PubMed] [Google Scholar]
- 41.Tam BN, Neumann CM. A human health assessment of hazardous air pollutants in Portland, OR. J Environ Manage 2004;73:131–145. [DOI] [PubMed] [Google Scholar]
- 42.Vasilyev N, Williams T, Brennan ML, Unzek S, Zhou X, Heinecke JW, Spitz DR, Topol EJ, Hazen SL, Penn MS. Myeloperoxidase-generated oxidants modulate left ventricular remodeling but not infarct size after myocardial infarction. Circulation 2005;112:2812–2820. [DOI] [PubMed] [Google Scholar]
- 43.Anderson MM, Hazen SL, Hsu FF, Heinecke JW. Human neutrophils employ the myeloperoxidase–hydrogen peroxide–chloride system to convert hydroxy-amino acids into glycolaldehyde, 2-hydroxypropanal, and acrolein: a mechanism for the generation of highly reactive α-hydroxy and α,β-unsaturated aldehydes by phagocytes at sites of inflammation. J Clin Invest 1997;99:424–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hazen SL, Hsu FF, d'Avignon A, Heinecke JW. Human neutrophils employ myeloperoxidase to convert α-amino acids to a battery of reactive aldehydes: a pathway for aldehyde generation at sites of inflammation. Biochemistry 1998;37:6864–6873. [DOI] [PubMed] [Google Scholar]
- 45.Shao B, Fu X, McDonald TO, Green PS, Uchida K, O'Brien KD, Oram JF, Heinecke JW. Acrolein impairs ATP binding cassette transporter A1-dependent cholesterol export from cells through site-specific modification of apolipoprotein A-I. J Biol Chem 2005;280:36386–36396. [DOI] [PubMed] [Google Scholar]
- 46.Tabor CW, Tabor H, Bachrach U. Identification of the aminoaldehydes produced by the oxidation of spermine and spermidine with purified plasma amine oxidase. J Biol Chem 1964;239:2194–2203. [PubMed] [Google Scholar]
- 47.Houen G, Bock K, Jensen AL. Hplc and nmr investigation of the serum amine oxidase catalyzed oxidation of polyamines. Acta Chem Scand 1994;48:52–60. [DOI] [PubMed] [Google Scholar]
- 48.Lee Y, Sayre LM. Reaffirmation that metabolism of polyamines by bovine plasma amine oxidase occurs strictly at the primary amino termini. J Biol Chem 1998;273:19490–19494. [DOI] [PubMed] [Google Scholar]
- 49.O'Brien PJ, Siraki AG, Shangari N. Aldehyde sources, metabolism, molecular toxicity mechanisms, and possible effects on human health. Crit Rev Toxicol 2005;35:609–662. [DOI] [PubMed] [Google Scholar]
- 50.Sakata K, Kashiwagi K, Sharmin S, Ueda S, Igarashi K. Acrolein produced from polyamines as one of the uraemic toxins. Biochem Soc Trans 2003;31:371–374. [DOI] [PubMed] [Google Scholar]
- 51.Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic Biol Med 1991;11:81–128. [DOI] [PubMed] [Google Scholar]
- 52.Uchida K, Kanematsu M, Morimitsu Y, Osawa T, Noguchi N, Niki E. Acrolein is a product of lipid peroxidation reaction: formation of free acrolein and its conjugate with lysine residues in oxidized low density lipoproteins. J Biol Chem 1998;273:16058–16066. [DOI] [PubMed] [Google Scholar]
- 53.Furuhata A, Nakamura M, Osawa T, Uchida K. Thiolation of protein-bound carcinogenic aldehyde: an electrophilic acrolein–lysine adduct that covalently binds to thiols. J Biol Chem 2002;277:27919–27926. [DOI] [PubMed] [Google Scholar]
- 54.Egle JL Jr. Retention of inhaled formaldehyde, propionaldehyde, and acrolein in the dog. Arch Environ Health 1972;25:119–124. [DOI] [PubMed] [Google Scholar]
- 55.Kasahara DI, Poynter ME, Othman Z, Hemenway D, van der Vliet A. Acrolein inhalation suppresses lipopolysaccharide-induced inflammatory cytokine production but does not affect acute airways neutrophilia. J Immunol 2008;181:736–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Valacchi G, Pagnin E, Phung A, Nardini M, Schock BC, Cross CE, van der Vliet A. Inhibition of NFκB activation and IL-8 expression in human bronchial epithelial cells by acrolein. Antioxid Redox Signal 2005;7:25–31. [DOI] [PubMed] [Google Scholar]
- 57.Darley EF, Middleton JT, Garber MJ. Plant damage and eye irritation from ozone hydrocarbon reactions. J Agric Food Chem 1960;8:483–485. [Google Scholar]
- 58.Steinhagen WH, Barrow CS. Sensory irritation structure–activity study of inhaled aldehydes in B6C3F1 and Swiss-Webster mice. Toxicol Appl Pharmacol 1984;72:495–503. [DOI] [PubMed] [Google Scholar]
- 59.Cohen SM, Garland EM, St John M, Okamura T, Smith RA. Acrolein initiates rat urinary bladder carcinogenesis. Cancer Res 1992;52:3577–3581. [PubMed] [Google Scholar]
- 60.Izard C, Libermann C. Acrolein. Mutat Res 1978;47:115–138. [DOI] [PubMed] [Google Scholar]
- 61.Feng Z, Hu W, Hu Y, Tang MS. Acrolein is a major cigarette-related lung cancer agent: preferential binding at p53 mutational hotspots and inhibition of DNA repair. Proc Natl Acad Sci USA 2006;103:15404–15409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Haberzettl P, Vladykovskaya E, Srivastava S, Bhatnagar A. Role of endoplasmic reticulum stress in acrolein-induced endothelial activation. Toxicol Appl Pharmacol 2009;234:14–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Luo J, Uchida K, Shi R. Accumulation of acrolein–protein adducts after traumatic spinal cord injury. Neurochem Res 2005;30:291–295. [DOI] [PubMed] [Google Scholar]
- 64.Park YS, Taniguchi N. Acrolein induces inflammatory response underlying endothelial dysfunction: a risk factor for atherosclerosis. Ann N Y Acad Sci 2008;1126:185–189. [DOI] [PubMed] [Google Scholar]
- 65.Takino T, Sato H, Yamamoto E, Seiki M. Cloning of a human gene potentially encoding a novel matrix metalloproteinase having a C-terminal transmembrane domain. Gene 1995;155:293–298. [DOI] [PubMed] [Google Scholar]
- 66.Apte SS, Fukai N, Beier DR, Olsen BR. The matrix metalloproteinase-14 (MMP-14) gene is structurally distinct from other MMP genes and is co-expressed with the TIMP-2 gene during mouse embryogenesis. J Biol Chem 1997;272:25511–25517. [DOI] [PubMed] [Google Scholar]
- 67.Oblander SA, Zhou Z, Galvez BG, Starcher B, Shannon JM, Durbeej M, Arroyo AG, Tryggvason K, Apte SS. Distinctive functions of membrane type 1 matrix-metalloprotease (MT1-MMP or MMP-14) in lung and submandibular gland development are independent of its role in pro-MMP-2 activation. Dev Biol 2005;277:255–269. [DOI] [PubMed] [Google Scholar]
- 68.Strongin AY, Collier I, Bannikov G, Marmer BL, Grant GA, Goldberg GI. Mechanism of cell surface activation of 72-kDa type IV collagenase. Isolation of the activated form of the membrane metalloprotease. J Biol Chem 1995;270:5331–5338. [DOI] [PubMed] [Google Scholar]
- 69.Knauper V, Will H, Lopez-Otin C, Smith B, Atkinson SJ, Stanton H, Hembry RM, Murphy G. Cellular mechanisms for human procollagenase-3 (MMP-13) activation: evidence that MT1-MMP (MMP-14) and gelatinase A (MMP-2) are able to generate active enzyme. J Biol Chem 1996;271:17124–17131. [DOI] [PubMed] [Google Scholar]
- 70.Leeman MF, Curran S, Murray GI. The structure, regulation, and function of human matrix metalloproteinase-13. Crit Rev Biochem Mol Biol 2002;37:149–166. [DOI] [PubMed] [Google Scholar]
- 71.Hernandez-Barrantes S, Toth M, Bernardo MM, Yurkova M, Gervasi DC, Raz Y, Sang QA, Fridman R. Binding of active (57 kDa) membrane type 1-matrix metalloproteinase (MT1-MMP) to tissue inhibitor of metalloproteinase (TIMP)-2 regulates MT1-MMP processing and pro-MMP-2 activation. J Biol Chem 2000;275:12080–12089. [DOI] [PubMed] [Google Scholar]
- 72.Lehti K, Lohi J, Valtanen H, Keski-Oja J. Proteolytic processing of membrane-type-1 matrix metalloproteinase is associated with gelatinase A activation at the cell surface. Biochem J 1998;334:345–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pei D, Weiss SJ. Furin-dependent intracellular activation of the human stromelysin-3 zymogen. Nature 1995;375:244–247. [DOI] [PubMed] [Google Scholar]
- 74.Pei D, Weiss SJ. Transmembrane-deletion mutants of the membrane-type matrix metalloproteinase-1 process progelatinase A and express intrinsic matrix-degrading activity. J Biol Chem 1996;271:9135–9140. [DOI] [PubMed] [Google Scholar]
- 75.Fukuda Y, Ishizaki M, Okada Y, Seiki M, Yamanaka N. Matrix metalloproteinases and tissue inhibitor of metalloproteinase-2 in fetal rabbit lung. Am J Physiol Lung Cell Mol Physiol 2000;279:L555–L561. [DOI] [PubMed] [Google Scholar]
- 76.Kunugi S, Fukuda Y, Ishizaki M, Yamanaka N. Role of MMP-2 in alveolar epithelial cell repair after bleomycin administration in rabbits. Lab Invest 2001;81:1309–1318. [DOI] [PubMed] [Google Scholar]
- 77.Esterbauer H, Nohammer G, Schauenstein E, Weber P. [Determination of protein–SH groups with DDD reagent]. Acta Histochem Suppl 1976;16:183–188. [PubMed] [Google Scholar]
- 78.Strongin AY, Collier IE, Krasnov PA, Genrich LT, Marmer BL, Goldberg GI. Human 92 kDa type IV collagenase: functional analysis of fibronectin and carboxyl-end domains. Kidney Int 1993;43:158–162. [DOI] [PubMed] [Google Scholar]
- 79.Cao J, Kozarekar P, Pavlaki M, Chiarelli C, Bahou WF, Zucker S. Distinct roles for the catalytic and hemopexin domains of membrane type 1-matrix metalloproteinase in substrate degradation and cell migration. J Biol Chem 2004;279:14129–14139. [DOI] [PubMed] [Google Scholar]
- 80.Zucker S, Mirza H, Conner CE, Lorenz AF, Drews MH, Bahou WF, Jesty J. Vascular endothelial growth factor induces tissue factor and matrix metalloproteinase production in endothelial cells: conversion of prothrombin to thrombin results in progelatinase A activation and cell proliferation. Int J Cancer 1998;75:780–786. [DOI] [PubMed] [Google Scholar]
- 81.Galvez BG, Genis L, Matias-Roman S, Oblander SA, Tryggvason K, Apte SS, Arroyo AG. Membrane type 1-matrix metalloproteinase is regulated by chemokines monocyte-chemoattractant protein-1/CCL2 and interleukin-8/CXCL8 in endothelial cells during angiogenesis. J Biol Chem 2005;280:1292–1298. [DOI] [PubMed] [Google Scholar]
- 82.Galvez BG, Matias-Roman S, Yanez-Mo M, Vicente-Manzanares M, Sanchez-Madrid F, Arroyo AG. Caveolae are a novel pathway for membrane-type 1 matrix metalloproteinase traffic in human endothelial cells. Mol Biol Cell 2004;15:678–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kheradmand F, Rishi K, Werb Z. Signaling through the EGF receptor controls lung morphogenesis in part by regulating MT1-MMP-mediated activation of gelatinase A/MMP2. J Cell Sci 2002;115:839–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Udayakumar TS, Nagle RB, Bowden GT. Fibroblast growth factor-1 transcriptionally induces membrane type-1 matrix metalloproteinase expression in prostate carcinoma cell line. Prostate 2004;58:66–75. [DOI] [PubMed] [Google Scholar]
- 85.Munaut C, Noel A, Hougrand O, Foidart JM, Boniver J, Deprez M. Vascular endothelial growth factor expression correlates with matrix metalloproteinases MT1-MMP, MMP-2 and MMP-9 in human glioblastomas. Int J Cancer 2003;106:848–855. [DOI] [PubMed] [Google Scholar]
- 86.Zhang D, Brodt P. Type 1 insulin-like growth factor regulates MT1-MMP synthesis and tumor invasion via PI 3-kinase/Akt signaling. Oncogene 2003;22:974–982. [DOI] [PubMed] [Google Scholar]
- 87.Sato H, Seiki M. Regulatory mechanism of 92 kDa type IV collagenase gene expression which is associated with invasiveness of tumor cells. Oncogene 1993;8:395–405. [PubMed] [Google Scholar]
- 88.Tanimura S, Asato K, Fujishiro SH, Kohno M. Specific blockade of the ERK pathway inhibits the invasiveness of tumor cells: down-regulation of matrix metalloproteinase-3/-9/-14 and CD44. Biochem Biophys Res Commun 2003;304:801–806. [DOI] [PubMed] [Google Scholar]
- 89.Boyd PJ, Doyle J, Gee E, Pallan S, Haas TL. MAPK signaling regulates endothelial cell assembly into networks and expression of MT1-MMP and MMP-2. Am J Physiol Cell Physiol 2005;288:C659–C668. [DOI] [PubMed] [Google Scholar]
- 90.Munshi HG, Wu YI, Mukhopadhyay S, Ottaviano AJ, Sassano A, Koblinski JE, Platanias LC, Stack MS. Differential regulation of membrane type 1-matrix metalloproteinase activity by ERK 1/2- and p38 MAPK-modulated tissue inhibitor of metalloproteinases 2 expression controls transforming growth factor-β1–induced pericellular collagenolysis. J Biol Chem 2004;279:39042–39050. [DOI] [PubMed] [Google Scholar]
- 91.Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol 2001;17:463–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yao PM, Lemjabbar H, D'Ortho MP, Maitre B, Gossett P, Wallaert B, Lafuma C. Balance between MMP-9 and TIMP-1 expressed by human bronchial epithelial cells: relevance to asthma. Ann N Y Acad Sci 1999;878:512–514. [DOI] [PubMed] [Google Scholar]
- 93.Thomas P, Khokha R, Shepherd FA, Feld R, Tsao MS. Differential expression of matrix metalloproteinases and their inhibitors in non-small cell lung cancer. J Pathol 2000;190:150–156. [DOI] [PubMed] [Google Scholar]
- 94.Will H, Atkinson SJ, Butler GS, Smith B, Murphy G. The soluble catalytic domain of membrane type 1 matrix metalloproteinase cleaves the propeptide of progelatinase A and initiates autoproteolytic activation: regulation by TIMP-2 and TIMP-3. J Biol Chem 1996;271:17119–17123. [DOI] [PubMed] [Google Scholar]
- 95.Kinoshita T, Sato H, Takino T, Itoh M, Akizawa T, Seiki M. Processing of a precursor of 72-kilodalton type IV collagenase/gelatinase A by a recombinant membrane-type 1 matrix metalloproteinase. Cancer Res 1996;56:2535–2538. [PubMed] [Google Scholar]
- 96.Langton KP, Barker MD, McKie N. Localization of the functional domains of human tissue inhibitor of metalloproteinases-3 and the effects of a Sorsby's fundus dystrophy mutation. J Biol Chem 1998;273:16778–16781. [DOI] [PubMed] [Google Scholar]
- 97.Garcia-Alvarez J, Ramirez R, Checa M, Nuttall RK, Sampieri CL, Edwards DR, Selman M, Pardo A. Tissue inhibitor of metalloproteinase-3 is up-regulated by transforming growth factor-β1 in vitro and expressed in fibroblastic foci in vivo in idiopathic pulmonary fibrosis. Exp Lung Res 2006;32:201–214. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.