Abstract
Rationale: Obesity is considered a relative contraindication to lung transplantation, based on studies that have not accounted for key confounders. Little is known about the risk of death for underweight candidates after transplantation.
Objectives: To examine the associations of pretransplant obesity and underweight with the risk of death after lung transplantation.
Methods: We examined 5,978 adults with cystic fibrosis, chronic obstructive pulmonary disease, and diffuse parenchymal lung disease who underwent lung transplantation in the United States between 1995 and 2003. We used Cox models and generalized additive models to examine the association between pretransplant body mass index and the risk of death after lung transplantation with adjustment for donor and recipient factors.
Measurements and Main Results: The median follow-up time was 4.2 years. Compared with normal weight recipients, the multivariable-adjusted rates of death were 15% higher for underweight recipients (95% confidence interval, 3 to 28%), 15% higher for overweight recipients (95% confidence interval, 6 to 26%), and 22% higher for obese recipients (95% confidence interval, 8 to 39%). These relationships persisted when stratified by diagnosis. The multivariable-adjusted population attributable fraction was 12% at 1 year and 8% at 5 years.
Conclusions: Both obesity and underweight are independent risk factors for death after lung transplantation, contributing to up to 12% of deaths in the first year after transplantation. Primary care providers and pulmonologists should promote a healthy weight for patients with lung disease long before transplantation is considered.
Keywords: anthropometry, body mass index, generalized additive models, lung transplantation, obesity
AT A GLANCE COMMENTARY
Scientific Knowledge on the Subject
Obesity has been linked to higher mortality rates after lung transplantation in studies that have not adequately controlled for potential confounding factors. The impact of underweight after lung transplantation is largely unknown.
What This Study Adds to the Field
Both obesity and underweight are independent risk factors for death after lung transplantation, accounting for up to 12% of deaths in the first year after transplantation.
Lung transplantation is a potentially life-saving treatment for advanced lung disease, yet several broadly defined exclusion criteria restrict lung transplantation to a small, highly selected fraction of the potentially eligible pool of candidates. Existing guidelines for the selection of candidates for lung transplantation are based largely on expert opinion, small observational studies, and limited analyses of registry data (1). Adequately powered studies that account for key confounders may help refine existing selection criteria, strengthen the recommendations of transplant clinicians, and improve posttransplant outcomes.
Obesity (body mass index [BMI] >30 kg/m2) is considered a relative contraindication to lung transplantation (1). Annual registry reports of the International Society of Heart and Lung Transplantation (ISHLT, Addison, TX) have periodically reported associations between higher BMI and greater multivariable-adjusted risks of death at 1 and 5 years after lung transplantation in analyses that were not stratified by diagnosis (2–4). More recent reports have not detected an association between BMI and survival after lung transplantation (5, 6) except among those with diffuse parenchymal lung disease (DPLD) (4, 6). These reports are limited by the exclusion of recipients with missing data, no information about the role of BMI in recipients with cystic fibrosis (CF), year-to-year variation in exposure and covariate specification, and a focus on 1- and 5-year survival rather than overall survival.
Current selection guidelines do not mention underweight as a contraindication to lung transplantation (1). Underweight has been linked to a higher risk of death after lung transplantation in single-center studies (7, 8), but has not been previously reported as a risk factor for 1- or 5-year mortality in ISHLT registry reports. Nevertheless, underweight is a risk factor for poor outcomes after cardiothoracic (9–11) and other major surgical procedures (12). Underweight has yet to be examined as a risk factor for death after lung transplantation in large well-designed studies.
Therefore, we examined the association of BMI with the risk of death after lung transplantation in the United States. We hypothesized that underweight and obesity would be associated with an increased risk of death after transplantation after adjustment for potential confounders. Some of the results of this study have been previously reported in the form of an abstract (13).
METHODS
Study Design
We performed a retrospective cohort study of adults who underwent lung transplantation in the United States between January 1, 1995 and December 31, 2003 with follow-up through November 21, 2008.
Participants
Adults (age ≥18 yr at transplantation) with CF, chronic obstructive pulmonary disease (COPD), or DPLD (recorded as “idiopathic pulmonary fibrosis” by transplant centers) undergoing their first single or bilateral lung transplant procedure from a deceased donor during the study period were eligible for inclusion in the cohort. We chose to restrict the cohort to these three diseases (which account for more than 80% of lung transplant procedures in the United States) to minimize confounding by diagnosis. Exclusion criteria were as follows: use of mechanical ventilation at the time of transplantation, donor age less than 12 years, recipient or donor height less than 138 cm or more than 198 cm, recipient BMI less than 13 or more than 40 kg/m2, and unknown height or weight of either donor or recipient. Recipients with extreme BMI values were excluded because these values were often the result of impossible combinations of height and weight.
Data Sources and Variables
We used data collected by the United Network for Organ Sharing (UNOS, Richmond, VA), which coordinates all solid organ transplants in the United States. Recipient demographics, anthropometrics, and clinical data were collected by clinical staff at 83 U.S. transplant centers using the UNOS Lung Transplant Recipient Registration Worksheet (http://unos.org/data/about/collection.asp). Donor demographics, anthropometrics, and clinical data were collected by organ procurement organization personnel using the UNOS Deceased Donor Registration Worksheet (http://unos.org/data/about/collection.asp). All data were supplied by UNOS as a Standard Transplant Analysis and Research file based on Organ Procurement and Transplantation Network data as of November 21, 2008 supplemented with a coded transplant center identifier.
Body mass index was calculated as weight (kg) divided by height2 (m2), using height and weight reported at the time of transplantation. Measurement of weight and height was not standardized across centers. Recipient factors (demographics, height, weight, diagnosis, human leukocyte antigen type, cytomegalovirus serologic status, diabetes mellitus, hypertension, smoking status, preoperative steroid use, hospitalization at the time of transplantation), donor factors (demographics, height, weight, smoking history, cause of death, presence of bronchopulmonary infection, human leukocyte antigen type, cytomegalovirus serologic status), transplant center, year of transplantation, and allograft ischemic time were provided in the data set. The lung allocation score (LAS) was calculated using data obtained at the time of transplantation (without PaCO2) (http://unos.org/resources/frm_LAS_Calculator.asp). When factors incorporated into the LAS were missing, default values were used as specified by UNOS.
The primary outcome was recipient survival, calculated as the number of days from the date of transplantation to the date of death. Recipients who subsequently underwent retransplantation during the study were kept in all analyses and were not censored on retransplantation. Survival time was censored on loss to follow-up or on November 21, 2008 (the last date of follow-up in the UNOS data set). The primary cause of death was reported to UNOS by transplant center personnel. Dates of death were determined using the Social Security Death Master File and the UNOS data set. When these two sources reported discordant vital status, recipients were assumed to have died on the date provided.
Statistical Analysis
For descriptive purposes, the cohort was stratified by BMI, using the World Health Organization classification scheme: underweight, less than 18.5 kg/m2; normal weight, 18.5–24.9 kg/m2; overweight, 25–29.9 kg/m2; obese, at least 30 kg/m2 (14).
We estimated hazard ratios for death after transplantation using stratified Cox proportional hazard models with strata for transplant center, transplant procedure (bilateral vs. single), diagnosis, and year of transplant (dichotomized at 1999) and adjusted for potential confounders. We selected mechanistically plausible covariates for inclusion in multivariable models regardless of their statistical significance in either univariate or multivariable models. We also estimated odds ratios for early death (at 1 yr) and late death (at 5 yr conditional on 1-yr survival), using generalized additive models (GAMs) with loess smoothing functions for continuous variables. GAMs allow for the flexible specification of the relationship between BMI and the risk of death and help minimize misspecification of potential confounding variables (see the online supplement) (15). We performed analyses stratified by diagnosis, regardless of the significance of interaction terms with BMI. Population attributable fractions were calculated using adjusted hazard ratios from Cox models (16). Multiple imputation was used to account for missing covariate data in both Cox models and GAMs as described in the online supplement. Predicted survival curves and plots of continuous associations of BMI and the risk of death were generated from models containing indicator variables for missing data. Statistical significance was defined as two-tailed P values less than 0.05. Analyses were performed with SAS 9.1 (SAS Institute, Cary, NC) and the gam function in R 2.6 (R Foundation, Vienna, Austria) (17).
RESULTS
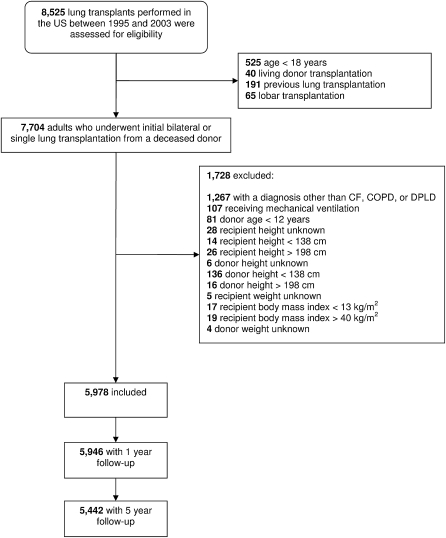
Of the 8,525 lung transplant procedures performed in the United States during the study period, 5,978 met our criteria and were included in all analyses (Figure 1). In all, 3,671 single-lung transplant recipients (2,735 with COPD, 930 with DPLD, and 6 with CF) and 2,307 double-lung transplant recipients (1,031 with COPD, 316 with DPLD, and 960 with CF) were included. The median age was 54 years (interquartile range, 45 to 60 yr), and 53% were men. The median LAS was 32.8 (interquartile range, 31.6 to 35.0). Underweight recipients tended to be younger and were more likely to be female, have CF, and undergo bilateral transplantation (Table 1). Overweight and obese recipients were more likely to have DPLD and undergo single-lung transplantation.
Figure 1.
Study participants.
TABLE 1.
RECIPIENT, DONOR, AND PROCEDURE CHARACTERISTICS AT THE TIME OF TRANSPLANTATION
| Underweight | Normal Weight | Overweight | Obese | |
|---|---|---|---|---|
| No. | 862 | 2,864 | 1,644 | 608 |
| Recipient characteristics | ||||
| Age, years | 42 (27–56) | 54 (45–60) | 57 (51–61) | 56 (50–60) |
| Male | 353/862 (41%) | 1,489/2,864 (52%) | 1,012/1,644 (62%) | 338/608 (56%) |
| Height, cm | 168 (160–173) | 169 (163–177) | 173 (163–178) | 170 (160–178) |
| Lung allocation score, au | 34 (33–36) | 33 (32–35) | 32 (31–35) | 32 (31–(35) |
| Waiting list urgency score, days | 342 (332–350) | 349 (338–355) | 350 (330–356) | 350 (328–358) |
| Posttransplant survival score, days | 330 (328–331) | 328 (327–329) | 327 (309–328) | 318 (303–228) |
| Diagnosis | ||||
| Cystic fibrosis | 410/862 (48%) | 519/2,864 (18%) | 34/1,644 (2%) | 3/608 (<1%) |
| COPD | 411/862 (48%) | 1,976/2,864 (69%) | 1,078/1,644 (66%) | 301/608 (50%) |
| DPLD | 41/862 (5%) | 369/2,864 (13%) | 532/1,644 (32%) | 304/608 (50%) |
| Hospitalized | 86/854 (10%) | 137/2,844 (5%) | 43/1,640 (3%) | 16/605 (3%) |
| Corticosteroid use | 326/799 (41%) | 1312/2,623 (50%) | 846/1,517 (56%) | 333/560 (59%) |
| Diabetes | 94/819 (11%) | 184/2,726 (7%) | 89/1,587 (6%) | 71/588 (12%) |
| Hypertension | 51/815 (6%) | 315/2,709 (12%) | 255/1,574 (16%) | 126/584 (22%) |
| Ever smoker | 340/793 (43%) | 1,723/2,651 (65%) | 1,134/1,554 (73%) | 414/575 (72%) |
| Year of transplantation | ||||
| 1995–1999 | 487/862 (56%) | 1,410/2,864 (49%) | 738/1,644 (45%) | 265/608 (44%) |
| 2000–2003 | 375/862 (44%) | 1,454/2,864 (51%) | 906/1,644 (55%) | 343/608 (56%) |
| Cytomegalovirus mismatch (D+/R−) | 97/389 (25%) | 330/1,504 (22%) | 163/904 (18%) | 63/334 (19%) |
| >3 human leukocyte antigen mismatches | 585/707 (83%) | 1,929/2,340 (82%) | 1,121/1,350 (83%) | 410/480 (85%) |
| Donor characteristics | ||||
| Age, years | 27 (19–42) | 29 (20–43) | 29 (20–43) | 29 (20–43) |
| Male | 471/862 (55%) | 1,799/2,864 (63%) | 1,108/1,644 (68%) | 394/608 (65%) |
| Height, cm | 170 (165–178) | 173 (168–180) | 175 (168–183) | 175 (168–183) |
| Body mass index, kg/m2 | 23 (21–26) | 24 (21–26) | 24 (21–27) | 24 (22–27) |
| Smoking >20 pack-years | 243/847 (29%) | 815/2,843 (29%) | 473/1,620 (29%) | 172/594 (29%) |
| Bronchopulmonary infection | 134/862 (16%) | 426/2,864 (15%) | 284/1,644 (17%) | 111/608 (18%) |
| Diabetes | 22/855 (3%) | 85/2,848 (3%) | 50/1,637 (3%) | 18/602 (3%) |
| Cause of death | ||||
| Head trauma | 488/862 (57%) | 1,637/2,864 (57%) | 928/1,644 (56%) | 348 (57%) |
| Cerebrovascular | 303/862 (35%) | 1,055/2,864 (35%) | 588/1,644 (36%) | 208/608 (34%) |
| Anoxic brain injury | 43/862 (5%) | 142/2,864 (5%) | 81/1,644 (5%) | 30/608 (5%) |
| CNS tumor | 11/862 (1%) | 35/2,864 (1%) | 17/1,644 (1%) | 7,608 (1%) |
| Missing | 17/862 (2%) | 45/2,864 (2%) | 30/1,644 (2%) | 15/608 (2%) |
| Procedure characteristics | ||||
| Bilateral lung transplantation | 516/862 (60%) | 1,189/2,864 (42%) | 1,188/1,644 (26%) | 462/608 (24%) |
| Ischemic time, hours | 4.6 (3.5–6) | 4.3 (3.3–5.3) | 4.2 (3.3–5.3) | 4.0 (3.1–5.1) |
| n = 756 |
n = 2,463 |
n = 1,408 |
n = 527 |
|
Definition of abbreviations: au = arbitrary units; CNS = central nervous system; COPD = chronic obstructive pulmonary disease; DPLD = diffuse parenchymal lung disease.
Data represent median (interquartile range) and frequency/total (%). Percentages may not add to 100% because of rounding. Continuous variables all had complete data (n = 5,978) except for ischemic time as noted.
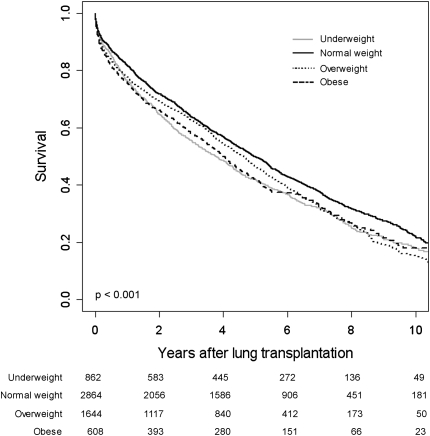
Figure 2 shows predicted survival curves for underweight, normal weight, overweight, and obese lung transplant recipients adjusted for recipient and donor factors, transplant procedure, diagnosis, transplant year, and additional potential confounders (see model 4 in the footnote to Table 2; likelihood ratio test P < 0.001 compared with the full model without BMI categories). Unadjusted survival curves (Kaplan-Meier method) showed similar results (log-rank P < 0.001; see Figure E1 in the online supplement). The median (unadjusted) survival time for the entire cohort was 4.8 years (interquartile range, 1.4 to 9.2 yr).
Figure 2.
Multivariable-adjusted survival curves for underweight, normal weight, overweight, and obese lung transplant recipients. Survival estimates are adjusted for the covariates in model 4 listed in the footnote to Table 2. Bottom: Numbers indicate the number of surviving lung transplant recipients at each time point.
TABLE 2.
ASSOCIATIONS BETWEEN BODY MASS INDEX AND THE RISK OF DEATH AFTER LUNG TRANSPLANTATION
| Body Mass Index Category |
|||||
|---|---|---|---|---|---|
| Underweight | Normal Weight | Overweight | Obese | P Value | |
| No. transplanted | 862 | 2,864 | 1,644 | 608 | |
| Median follow-up time, years | 4.1 | 4.5 | 4.1 | 3.7 | |
| Person-years | 3,760 | 13,041 | 6,640 | 2,336 | |
| Overall mortality | |||||
| Mortality rate per 100 person-years (95% CI) | 15.6* (14.4 to 17.0) | 14.0 (13.4 to 14.7) | 17.1† (16.1 to 18.1) | 18.4† (16.7 to 20.2) | <0.001 |
| Hazard ratios for death (95% CI) | |||||
| Model 1 | 1.16† (1.04 to 1.29) | 1.0 | 1.14† (1.05 to 1.24) | 1.20† (1.06 to 1.36) | <0.001 |
| Model 2 | 1.16† (1.04 to 1.29) | 1.0 | 1.15† (1.06 to 1.25) | 1.22† (1.07 to 1.38) | <0.001 |
| Model 3 | 1.15* (1.03 to 1.28) | 1.0 | 1.17† (1.07 to 1.27) | 1.25† (1.10 to 1.42) | <0.001 |
| Model 4 | 1.15* (1.03 to 1.28) | 1.0 | 1.15† (1.06 to 1.26) | 1.22† (1.08 to 1.39) | <0.001 |
| Death 1 year after transplantation | |||||
| No. with 1-year follow-up | 860 | 2,848 | 1,633 | 605 | |
| Risk of death at 1 year (95% CI) | 21.1%* (20.6 to 21.7%) | 17.7% (17.4 to 17.9%) | 22.8%† (22.3 to 23.2%) | 25.2%† (24.4 to 26.1%) | |
| Odds ratios for death at 1 year (95% CI) | |||||
| Model 1 | 1.37† (1.12 to 1.68) | 1.0 | 1.21* (1.03 to 1.42) | 1.29* (1.03 to 1.60) | 0.004 |
| Model 2 | 1.37† (1.12 to 1.68) | 1.0 | 1.23* (1.05 to 1.44) | 1.32* (1.06 to 1.64) | 0.002 |
| Model 3 | 1.34† (1.09 to 1.64) | 1.0 | 1.26† (1.07 to 1.48) | 1.38† (1.10 to 1.73) | 0.001 |
| Model 4 | 1.34† (1.09 to 1.64) | 1.0 | 1.27† (1.08 to 1.49) | 1.40† (1.11 to 1.75) | 0.001 |
| Death 5 years after transplantation conditional on 1-year survival | |||||
| No. with 5-year follow-up | 806 | 2,592 | 1,491 | 553 | |
| No. of 1-year survivors with 5-year follow-up | 626 | 2,088 | 1,118 | 401 | |
| Risk of death at 5 years (95% CI) | 54.0%† (52.2 to 55.9%) | 49.2% (48.3 to 50.1%) | 54.8%† (53.4 to 56.1%) | 60.1%† (57.7 to 62.5%) | |
| Risk of death at 5 years conditional on 1-year survival (95% CI) | 41.9%* (40.3 to 43.5%) | 38.3% (37.6 to 39.1%) | 41.4% (40.3 to 42.6%) | 46.7%† (44.5 to 49.0%) | |
| Odds ratios for death at 5 years conditional on 1-year survival (95% CI) | |||||
| Model 1 | 1.26* (1.04 to 1.53) | 1.0 | 1.04 (0.89 to 1.21) | 1.32* (1.06 to 1.66) | 0.02 |
| Model 2 | 1.25* (1.03 to 1.52) | 1.0 | 1.03 (0.89 to 1.21) | 1.33* (1.06 to 1.67) | 0.02 |
| Model 3 | 1.23* (1.01 to 1.50) | 1.0 | 1.05 (0.90 to 1.22) | 1.35† (1.08 to 1.62) | 0.02 |
| Model 4 |
1.22* (1.01 to 1.49) |
1.0 |
1.04 (0.89 to 1.22) |
1.29* (1.02 to 1.63) |
0.07 |
Definition of abbreviation: CI = confidence interval.
Odds ratios are from generalized additive models with loess smoothers for continuous covariates. Hazards ratios are from Cox models without smoothers. Odds ratios are adjusted for recipient transplant procedure (double vs. single) and diagnosis (cystic fibrosis, chronic obstructive pulmonary disease, diffuse parenchymal lung disease). Cox models include strata for transplant procedure, diagnosis, center, and transplant year. P values are from likelihood ratio tests comparing each model with a model that does not include body mass index categories.
Model 1: Adjusted for recipient characteristics (age, sex, height, and diagnosis), transplant procedure, and transplant year. Cox models are also adjusted for transplant center.
Model 2: Model 1 + adjustment for donor characteristics (age, sex, height, body mass index, pulmonary infection, smoking >20 pack-years, and donor cause of death).
Model 3: Model 2 + lung allocation score at the time of transplantation.
Model 4: Model 3 + cardiovascular risk factors (hypertension, smoking status, diabetes mellitus), hospitalization at transplant, cytomegalovirus mismatch status, HLA mismatch, use of steroids before transplant, graft ischemic time.
Wald test P < 0.05 compared with normal weight.
Wald test P < 0.01 compared with normal weight.
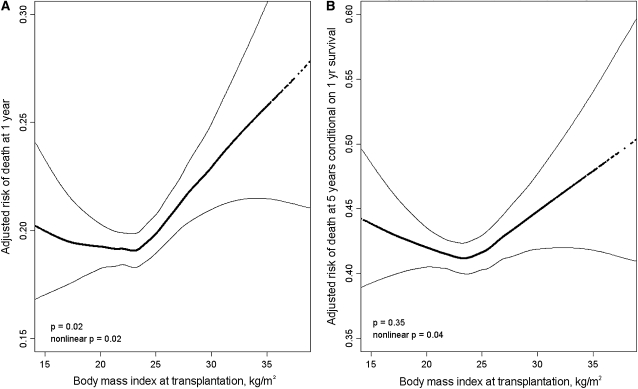
The continuous relationship between BMI and the adjusted risk of dying within 1 year of transplantation was J-shaped (Figure 3A; P = 0.02 for nonlinearity) with an increased predicted probability of death at high BMI and, to a lesser extent, at low BMI. Similarly, the continuous relationship between BMI and the adjusted risk of dying at 5 years among 1-year survivors was U-shaped (Figure 3B; P = 0.04 for nonlinearity) with a higher predicted probability of dying at both low and high BMI.
Figure 3.
Continuous relationships of body mass index and the risk of death (A) at 1 year and (B) at 5 years conditional on 1-year survival after lung transplantation. Thick dotted lines = smoothed regression lines adjusted for the model 4 covariates listed in the footnote to Table 2. Thin solid lines = 95% confidence intervals. In (A), both nonlinear (P = 0.02) and linear (P = 0.02) relationships were statistically significant. In (B), the nonlinear (P = 0.04), but not the linear (P = 0.35), relationship was statistically significant. The significant P values for the smoothed (nonlinear) curves suggest that the relationship between body mass index and the risk of death after lung transplantation is nonlinear, with higher early and late mortality rates for both underweight and obese recipients. The wide confidence intervals at the extremes of body mass index are due to smaller numbers of transplant recipients with these values.
Overweight and Obese Recipients
The adjusted (model 4) mortality rate of overweight recipients was 15% greater than that of normal weight recipients (95% confidence interval, 6 to 26%; P = 0.002; Table 2). The adjusted odds of death were 27% greater at 1 year (95% confidence interval, 8 to 49%; P = 0.004) but only 4% greater at 5 years among 1-year survivors (95% confidence interval, −12 to +22%; P = 0.60) among overweight recipients compared with normal weight recipients.
The adjusted mortality rate of obese recipients was 22% greater than that of normal weight recipients (95% confidence interval, 8 to 39%; P = 0.003). The adjusted odds of death were 40% greater at 1 year (95% confidence interval, 11 to 75%; P = 0.004) and were 29% greater at 5 years among 1-year survivors (95% confidence interval, 2 to 63%; P = 0.03) among obese recipients compared with normal weight recipients.
Underweight Recipients
The adjusted mortality rate of underweight recipients was 15% greater than that of normal weight recipients (95% confidence interval, 3 to 28%; P = 0.02; Table 2). The adjusted odds of death were 34% greater at 1 year (95% confidence interval, 9 to 64%; P = 0.006) and 22% greater at 5 years among 1-year survivors (95% confidence interval, 1 to 49%; P = 0.04) who were underweight compared with normal weight recipients.
Variation by Diagnosis
The association between BMI and death did not appear to vary by diagnosis (P values for interactions with diagnosis were 0.28 for BMI categories and 0.17 for BMI specified as a continuous variable). Smoothed predicted curves for early and late mortality stratified by diagnosis are shown elsewhere (see Figures E2–E4 in the online supplement).
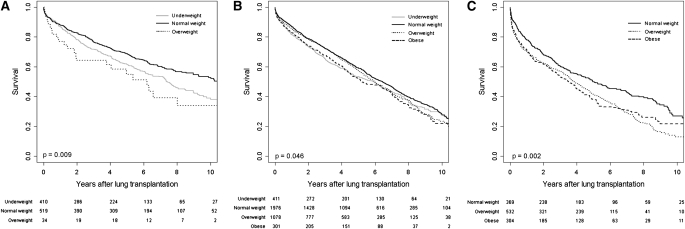
All but 37 recipients with CF had a body mass index less than 25 kg/m2. The adjusted mortality rate for underweight recipients with CF was 25% greater than that of normal weight recipients (95% confidence interval, 4 to 52%; P = 0.02). Interestingly, underweight was associated with late, but not early, mortality among those with CF (Table 3; Figure 4A).
TABLE 3.
ASSOCIATIONS BETWEEN BODY MASS INDEX AND THE RISK OF DEATH AFTER LUNG TRANSPLANTATION, STRATIFIED BY DIAGNOSIS
| Body Mass Index Category |
|||||
|---|---|---|---|---|---|
| Underweight | Normal Weight | Overweight | Obese | P Value | |
| Cystic fibrosis | |||||
| No. | 410 | 519 | 34 | ||
| Mortality rate per 100 person-years (95% CI) | 13.9* (12.2 to 15.7) | 10.2 (9.1 to 11.6) | 16.7† (10.7 to 24.7) | 0.002 | |
| Hazard ratio for death (95% CI) | 1.25† (1.04 to 1.52) | 1.0 | 1.69† (1.10 to 2.60) | 0.009 | |
| Odds ratio for death at 1 year (95% CI) | 1.01 (0.69 to 1.49) | 1.0 | 2.08 (0.92 to 4.70) | 0.24 | |
| Odds ratio for death at 5 years conditional on 1-year survival (95% CI) | 1.58† (1.08 to 2.30) | 1.0 | 1.41 (0.55 to 3.60) | 0.06 | |
| Chronic obstructive pulmonary disease | |||||
| No. | 411 | 1976 | 1078 | 301 | |
| Mortality rate per 100 person-years (95% CI) | 17.1† (15.2 to 19.2) | 14.9 (14.1 to 15.7) | 16.0 (14.9 to 17.2) | 17.3† (15.1 to 19.8) | 0.04 |
| Hazard ratio for death (95% CI) | 1.13 (0.99 to 1.28) | 1.0 | 1.08 (0.99 to 1.19) | 1.17† (1.01 to 1.36) | 0.046 |
| Odds ratio for death at 1 year (95% CI) | 1.64* (1.30 to 2.08) | 1.0 | 1.11 (0.91 to 1.36) | 1.31 (0.96 to 1.80) | 0.002 |
| Odds ratio for death at 5 year conditional on 1-year survival (95% CI) | 1.10 (0.85 to 1.43) | 1.0 | 1.05 (0.88 to 1.26) | 1.22 (0.91 to 1.65) | 0.59 |
| Diffuse parenchymal lung disease | |||||
| No. | 369 | 532 | 304 | ||
| Mortality rate per 100 person-years (95% CI) | 15.4 (13.5 to 17.5) | 20.0* (18.0 to 22.0) | 19.7* (17.2 to 22.6) | 0.002 | |
| Hazard ratio for death (95% CI) | 1.0 | 1.31* (1.09 to 1.57) | 1.32† (1.06 to 1.63) | 0.002 | |
| Odds ratio for death at 1 year (95% CI) | 1.0 | 1.69* (1.24 to 2.32) | 1.75* (1.21 to 2.53) | 0.001 | |
| Odds ratio for death at 5 year conditional on 1-year survival (95% CI) |
1.0 |
0.98 (0.68 to 1.42) |
1.24 (0.81 to 1.90) |
0.39 |
|
Odds ratios are from generalized additive models with loess smoothers for continuous covariates. Hazards ratios are from Cox models without smoothers. All models are adjusted for age, sex, height, donor characteristics (age, sex, height, body mass index, smoking history >20 pack-years, bronchopulmonary infection, cause of death), lung allocation score, hypertension, smoking status, diabetes, hospitalization at transplant, CMV mismatch, HLA mismatch, use of steroids before transplant, ischemic time. Odds ratios are adjusted for recipient transplant procedure (double vs. single). Cox models are stratified by transplant procedure and transplant year. Hazard ratios are not adjusted for center. P values are from likelihood ratio tests comparing each model to a model that does not include BMI categories. P values for interaction with diagnosis (from likelihood ratio tests) were 0.28 for categorical BMI and 0.17 for continuous BMI. CI = confidence interval.
Wald test P < 0.01 compared with normal weight.
Wald test P < 0.05 compared with normal weight.
Figure 4.
Multivariate-adjusted survival curves for underweight, normal weight, overweight, and obese lung transplant recipients with (A) cystic fibrosis, (B) chronic obstructive pulmonary disease, (C) and diffuse parenchymal lung disease. Survival estimates are adjusted for the covariates listed in the footnote to Table 2 (model 4). Bottom: Numbers indicate the number of surviving lung transplant recipients at each time point.
The adjusted mortality rate was 13% higher (95% confidence interval, −1 to 28%; P = 0.06) for underweight recipients with COPD, 8% higher (95% confidence interval, −1 to 19%; P = 0.09) for overweight recipients with COPD, and 17% higher (95% confidence interval, 1 to 36%; P = 0.04) for obese recipients with COPD compared with normal weight recipients with COPD. In contrast to recipients with CF, underweight was associated with early, but not late, mortality among recipients with COPD (Table 3; Figure 4B).
All but 41 recipients with DPLD had a body mass index greater than 18.5 kg/m2. The adjusted mortality rate was 31% higher for overweight recipients with DPLD (95% confidence interval, 9 to 57%; P = 0.004) and 32% higher for obese recipients with DPLD (95% confidence interval, 6 to 63%; P = 0.01) compared with normal weight recipients with DPLD. Overweight and obesity were both strongly associated with early mortality but not with late mortality (Table 3; Figure 4C).
Cause-specific Mortality
Transplant centers reported the cause of death to UNOS for 2,972 of 3,981 decedents (75%) (see Table E1 in the online supplement). Compared with normal weight recipients, underweight recipients were more likely to die from infection and chronic rejection and less likely to die from cardiac causes. Overweight and obese recipients were more likely to die from infection, cardiac disease, and cancer. Overweight recipients were more likely to die from primary graft dysfunction, and obese recipients were more likely to die from respiratory failure.
Population Attributable Fraction
The population attributable fraction (also known as “population attributable risk” or “etiologic fraction”) is the proportion of deaths related to an exposure of interest and represents the greatest possible proportional reduction in the number of deaths if the exposure of interest were eliminated from the population (18). Underweight, overweight, and obesity (and their related confounding factors) contributed to up to 12% of deaths in the first year after transplantation and up to 8% of deaths by 5 years (Table 4). Among those with DPLD, overweight and obesity contributed to up to 20% of deaths in the first year after transplantation.
TABLE 4.
MULTIVARIABLE-ADJUSTED POPULATION ATTRIBUTABLE FRACTIONS STRATIFIED BY DIAGNOSIS
| PAF at 1 Year |
PAF at 5 Years |
||
|---|---|---|---|
| No. | (%) | (%) | |
| All recipients | 5,978 | 12 | 8 |
| Cystic fibrosis | 966 | 1 | 7 |
| Chronic obstructive pulmonary disease | 3,766 | 11 | 7 |
| Diffuse parenchymal lung disease |
1,246 |
20 |
12 |
Definition of abbreviation: PAF = population attributable fraction.
PAF was estimated using multivariable-adjusted hazard ratios from Cox models containing model 4 covariates and censored at 1 or 5 years. PAF is the proportion of deaths related to an exposure of interest and represents the greatest possible proportional reduction in the number of deaths if the exposure of interest were eliminated from the population. For example, if all lung transplant recipients were of normal weight, up to 12% of deaths might be prevented in the first year after transplantation.
Additional Analyses
Inclusion of those with diagnoses other than CF, COPD, or DPLD (such as pulmonary arterial hypertension and sarcoidosis) resulted in similar effect estimates (see Table E2 in the online supplement). Substitution of the posttransplant survival score (a component of the LAS) instead of LAS, inclusion of pediatric donors, inclusion of those with extreme BMI values, and adjustment for recipient/donor sex interaction did not substantially change the results (Table E2).
DISCUSSION
We found a nonlinear association between recipient BMI and the risk of death after lung transplantation, independent of diagnosis, transplant center, transplant procedure, disease severity at transplantation, donor characteristics, and other potential confounders. Examination of this association stratified by diagnosis showed consistent and informative findings, with higher early mortality rates among obese recipients with DPLD and obese recipients with COPD, higher early mortality rates among underweight recipients with COPD, and higher late mortality rates among underweight recipients with CF.
Obesity
The association between obesity and the risk of death after lung transplantation has been previously examined. Obesity has been reported as a risk factor for death 1 year after lung transplantation in some (2–4) but not all (5, 6) ISHLT registry reports. Only the most recent reports have stratified by pretransplant diagnosis, an important potential confounder of the association between obesity and survival after lung transplantation (4–6). ISHLT analyses from 2007 and 2008 did not detect statistically significant associations between BMI and the risk of death after lung transplantation among those with COPD or CF (5, 6). Greater recipient BMI (4) and body weight (6) have been associated with an increased risk of death at 1 year only among those with DPLD. Our study adds to these data by demonstrating an association between obesity and the risk of death both early and late after lung transplantation across diagnoses even after adjustment for potential confounders.
Three single-center studies have also examined the association between obesity and survival after lung transplantation. Madill and colleagues found that 43 recipients with a BMI greater than 27 kg/m2 had a 5-fold increased risk of death 90 days after lung transplantation (8). Kanasky and colleagues reported a 3-fold increased odds of death among 10 obese lung transplant recipients after adjustment for procedure type and bronchiolitis obliterans syndrome (19). Most obese recipients died within 2 years of transplantation. Similarly, Culver and colleagues found a 3-fold increased odds of death 90 days after lung transplantation among 46 obese recipients (20). Despite their small sample sizes and the influence of unmeasured confounders, these studies are consistent with our finding of a 40% increased odds of death 1 year after lung transplantation among obese recipients.
The mechanisms responsible for the higher risk of early mortality among obese lung transplant recipients remain unclear. Lung transplantation may be more technically demanding in the obese, perhaps leading to longer cold ischemic times and increased use of cardiopulmonary bypass, known risk factors for primary graft dysfunction after lung transplantation (21, 22). In addition, abdominal obesity decreases respiratory system compliance (23, 24), which may contribute to a greater risk of postoperative atelectasis, respiratory failure, and pneumonia. We found a higher risk of death due to respiratory failure among obese recipients and a greater risk of death due to primary graft failure among overweight recipients. Although cause of death reporting is subject to misclassification and missingness, our results are consistent with a greater risk of primary graft dysfunction among obese lung transplant recipients (22).
Another potential mechanism by which obesity might affect early outcomes after lung transplantation is through modulation of the systemic inflammatory response, which may affect graft function. Increased levels of IL-6 and decreased levels of adiponectin, an antiinflammatory cytokine released by adipose tissue and found in decreased circulating levels in obesity, have been described in animal models of myocardial ischemia–reperfusion injury (25, 26). The roles of IL-6, adiponectin, and other adipocytokines in primary graft dysfunction after lung transplantation should be examined in futures studies.
Underweight
Underweight is a risk factor for death among patients with COPD (27) and CF (28), and, in light of its association with a higher risk of death on the waiting list, lower BMI increases the waiting list urgency score component of the LAS (29). Few data exist, however, to suggest an association between underweight and the risk of death after transplantation. Madill and colleagues and Culver and colleagues reported trends toward higher mortality rates among 23 and 43 underweight lung transplant recipients, respectively (8, 20). Plöchl and colleagues reported a higher intensive care unit mortality rate for underweight lung transplant recipients with an intensive care unit length of stay greater than 5 days (7).
In our study, underweight was associated with a higher risk of death after transplantation. Notably, underweight recipients with COPD were at increased risk of death early after transplantation, whereas recipients with CF were at increased risk later after transplantation. Early deaths among underweight patients with COPD might be attributable to diaphragmatic weakness and respiratory failure related to poor nutritional status (30, 31). However, both groups had an increased risk of infection-related deaths, and recipients with CF had an increased risk of death due to chronic allograft dysfunction. The greater risk of infection in underweight transplant recipients could explain the increase in early mortality among the older population of recipients with COPD and the increase in late mortality due to bronchiolitis obliterans among younger recipients with CF.
Clinical Implications
Our findings do not provide definitive evidence to deny lung transplantation to potential candidates who are obese or underweight. However, both the early and late risks associated with obesity and underweight should be included in the complex balance of risk, benefit, and organ allocation. For instance, although underweight was associated with increased risk of death for recipients with COPD in our study, others have shown that the survival benefit conferred by lung transplantation is greatest in underweight recipients with COPD (32). Likewise, the deleterious effects of underweight on survivorship after lung transplantation for CF appear to occur two or more years after transplantation and therefore might easily be outweighed by the higher mortality rate of underweight patients with CF awaiting transplantation (28). Nevertheless, those at the extremes of BMI may be at particularly high risk of early or late death after transplantation.
Although some centers may choose to use obesity and underweight as litmus tests for transplant candidacy, we suggest an alternative approach. Among exclusion criteria, obesity and underweight are uniquely preventable and treatable conditions, and although we did not show that improvements in BMI are associated with reductions in the risk of death after transplantation, a healthy weight should nevertheless be promoted long before lung transplantation is indicated. The salutary effects of corticosteroid withdrawal and avoidance, nutritional counseling and dietary modification (including enteral nutritional support), pulmonary rehabilitation, and bariatric surgery should be considered by clinicians and studied by investigators with an eye toward improving survivorship after lung transplantation. Professional societies should develop guidelines for primary care physicians and pulmonologists in this regard.
Limitations
Our study has several limitations. First, we retrospectively ascertained height and weight as reported by transplant center personnel. It is likely that some of these height and weight values were measured at the time of listing. Nevertheless, an association between BMI at listing and survival after lung transplantation would still be of great importance to transplant physicians, who routinely make decisions for transplant eligibility at the time of listing. Furthermore, any misclassification of BMI is unlikely to be differential by posttransplant survival time, thereby biasing our effect estimates toward the null. Our findings might therefore underestimate the true impact of BMI on outcomes after lung transplantation.
Second, inclusion of imprecisely measured confounders and failure to include unmeasured confounders in our models could have contributed to some or all of the associations we observed. Our findings, however, were independent of important potential confounders, such as diagnosis, transplant center, height, donor characteristics, and cardiovascular risk factors. Nevertheless, residual confounding by other factors cannot be entirely excluded.
Third, we were unable to examine additional rigorously defined outcomes other than death, such as primary graft dysfunction (PGD) and bronchiolitis obliterans syndrome (BOS). Although BOS stage is reported to UNOS annually after lung transplantation, the timing of BOS onset is not recorded, and BOS onset and death were often reported simultaneously, limiting examination of this competing outcome. In addition, PGD was not consistently recorded. Nevertheless, we were able to examine cause-specific mortality rates, including PGD- and BOS-specific mortality rates. Because we did not use an independent adjudication committee to ascertain cause of death in our study, these results should be interpreted with caution.
Conclusion
In conclusion, we found that pretransplant underweight and obesity were both independently associated with a higher risk of death in a nationwide cohort of lung transplant recipients. Together, obesity and underweight accounted for up to 12% of deaths 1 year after transplantation. Both underweight and obesity may increase the risk of infection-related deaths. Obesity is associated with death due to respiratory failure, particularly among patients with DPLD, and underweight may increase the risk of death due to chronic allograft dysfunction. Prevention of underweight and obesity should be prioritized by primary care physicians and pulmonologists caring for patients with even early stages of these diseases.
Supplementary Material
Acknowledgments
The content is the responsibility of the authors alone and does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. government.
Supported by NIH grant K23 HL086714, the Robert Wood Johnson Physician Faculty Scholars Program, and the Herbert and Florence Irving Scholar Award (D.J.L.). This work was supported in part by Health Resources and Services Administration contract 234–2005–370011C.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.200903-0425OC on July 16, 2009
Conflict of Interest Statement: D.J.L. has received ($1,001–$5,000) for medical consulting from CanAccord Adams and, an industry-sponsored grant from Gilead ($10,001–$50,000), Broncus Technologies ($1,001–$5,000). J.S.W. has received an industry-sponsored grant from XDx ($100,001 and more). F.D. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. M.D.B. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. L.S. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. S.R. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. J.L. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. B.K. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. J.R.S. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. S.M.A. received an industry-sponsored grant from Astellas and APT Therapeutics ($50,001 and more) and Talecris ($100,001and more).
References
- 1.Orens JB, Estenne M, Arcasoy S, Conte JV, Corris P, Egan JJ, Egan T, Keshavjee S, Knoop C, Kotloff R, et al. International guidelines for the selection of lung transplant candidates: 2006 update–a consensus report from the Pulmonary Scientific Council of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant 2006;25:745–755. [DOI] [PubMed] [Google Scholar]
- 2.Hertz MI, Taylor DO, Trulock EP, Boucek MM, Mohacsi PJ, Edwards LB, Keck BM. The Registry of the International Society for Heart and Lung Transplantation: nineteenth official report—2002. J Heart Lung Transplant 2002;21:950–970. [DOI] [PubMed] [Google Scholar]
- 3.Trulock EP, Edwards LB, Taylor DO, Boucek MM, Keck BM, Hertz MI. Registry of the International Society for Heart and Lung Transplantation: twenty-second official adult lung and heart–lung transplant report−2005. J Heart Lung Transplant 2005;24:956–967. [DOI] [PubMed] [Google Scholar]
- 4.Trulock EP, Edwards LB, Taylor DO, Boucek MM, Keck BM, Hertz MI. Registry of the International Society for Heart and Lung Transplantation: twenty-third official adult lung and heart–lung transplantation report−2006. J Heart Lung Transplant 2006;25:880–892. [DOI] [PubMed] [Google Scholar]
- 5.Trulock EP, Christie JD, Edwards LB, Boucek MM, Aurora P, Taylor DO, Dobbels F, Rahmel AO, Keck BM, Hertz MI. Registry of the International Society for Heart and Lung Transplantation: twenty-fourth official adult lung and heart–lung transplantation report—2007. J Heart Lung Transplant 2007;26:782–795. [DOI] [PubMed] [Google Scholar]
- 6.Christie JD, Edwards LB, Aurora P, Dobbels F, Kirk R, Rahmel AO, Taylor DO, Kucheryavaya AY, Hertz MI. Registry of the International Society for Heart and Lung Transplantation: twenty-fifth official adult lung and heart/lung transplantation report—2008. J Heart Lung Transplant 2008;27:957–969. [DOI] [PubMed] [Google Scholar]
- 7.Plöchl W, Pezawas L, Artemiou O, Grimm M, Klepetko W, Hiesmayr M. Nutritional status, ICU duration and ICU mortality in lung transplant recipients. Intensive Care Med 1996;22:1179–1185. [DOI] [PubMed] [Google Scholar]
- 8.Madill J, Gutierrez C, Grossman J, Allard J, Chan C, Hutcheon M, Keshavjee SH. Nutritional assessment of the lung transplant patient: body mass index as a predictor of 90-day mortality following transplantation. J Heart Lung Transplant 2001;20:288–296. [DOI] [PubMed] [Google Scholar]
- 9.Potapov EV, Loebe M, Anker S, Stein J, Bondy S, Nasseri BA, Sodian R, Hausmann H, Hetzer R. Impact of body mass index on outcome in patients after coronary artery bypass grafting with and without valve surgery. Eur Heart J 2003;24:1933–1941. [DOI] [PubMed] [Google Scholar]
- 10.Rahmanian PB, Adams DH, Castillo JG, Chikwe J, Bodian CA, Filsoufi F. Impact of body mass index on early outcome and late survival in patients undergoing coronary artery bypass grafting or valve surgery or both. Am J Cardiol 2007;100:1702–1708. [DOI] [PubMed] [Google Scholar]
- 11.Reeves BC, Ascione R, Chamberlain MH, Angelini GD. Effect of body mass index on early outcomes in patients undergoing coronary artery bypass surgery. J Am Coll Cardiol 2003;42:668–676. [DOI] [PubMed] [Google Scholar]
- 12.Windsor JA. Underweight patients and the risks of major surgery. World J Surg 1993;17:165–172. [DOI] [PubMed] [Google Scholar]
- 13.Lederer DJ, Wilt JS, D'Ovidio F, Bacchetta M, Sonett JR, Arcasoy SM. Pretransplant body mass index is associated with late mortality after lung transplantation [abstract]. Am J Respir Crit Care Med 2009;179:A2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.World Health Organization Expert Committee on Physical Status. Physical status: the use and interpretation of anthropometry: report of a WHO expert committee. Geneva, Switzerland: World Health Organization; 1995. [PubMed]
- 15.Hastie TJ, Tibshirani RJ. Generalized additive models. London: Chapman & Hall; 1990.
- 16.Miettinen OS. Proportion of disease caused or prevented by a given exposure, trait or intervention. Am J Epidemiol 1974;99:325–332. [DOI] [PubMed] [Google Scholar]
- 17.R Development Core Team. R: a language and environment for statistical computing. Vienna, Austria: Foundation for Statistical Computing; 2009. Available from: http://www.R-project.org
- 18.Rockhill B, Newman B, Weinberg C. Use and misuse of population attributable fractions. Am J Public Health 1998;88:15–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kanasky WF Jr, Anton SD, Rodrigue JR, Perri MG, Szwed T, Baz MA. Impact of body weight on long-term survival after lung transplantation. Chest 2002;121:401–406. [DOI] [PubMed] [Google Scholar]
- 20.Culver DA, Mazzone PJ, Khandwala F, Blazey HC, Decamp MM, Chapman JT. Discordant utility of ideal body weight and body mass index as predictors of mortality in lung transplant recipients. J Heart Lung Transplant 2005;24:137–144. [DOI] [PubMed] [Google Scholar]
- 21.Gammie JS, Cheul Lee J, Pham SM, Keenan RJ, Weyant RJ, Hattler BG, Griffith BP. Cardiopulmonary bypass is associated with early allograft dysfunction but not death after double-lung transplantation. J Thorac Cardiovasc Surg 1998;115:990–997. [DOI] [PubMed] [Google Scholar]
- 22.Kuntz CL, Hadjiliadis D, Ahya VN, Kotloff RM, Pochetiino A, Lewis J, Christie JD. Risk factors for early primary graft dysfunction after lung transplantation: a registry study. Clin Transplant 2009; Feb 22. [DOI] [PubMed]
- 23.Rochester DF, Enson Y. Current concepts in the pathogenesis of the obesity–hypoventilation syndrome: mechanical and circulatory factors. Am J Med 1974;57:402–420. [DOI] [PubMed] [Google Scholar]
- 24.Leone N, Courbon D, Thomas F, Bean K, Jego B, Leynaert B, Guize L, Zureik M. Lung function impairment and metabolic syndrome: the critical role of abdominal obesity. Am J Respir Crit Care Med 2009;179:509–516. [DOI] [PubMed] [Google Scholar]
- 25.Shibata R, Sato K, Pimentel DR, Takemura Y, Kihara S, Ohashi K, Funahashi T, Ouchi N, Walsh K. Adiponectin protects against myocardial ischemia–reperfusion injury through AMPK- and COX-2–dependent mechanisms. Nat Med 2005;11:1096–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thakker GD, Frangogiannis NG, Bujak M, Zymek P, Gaubatz JW, Reddy AK, Taffet G, Michael LH, Entman ML, Ballantyne CM. Effects of diet-induced obesity on inflammation and remodeling after myocardial infarction. Am J Physiol Heart Circ Physiol 2006;291:H2504–H2514. [DOI] [PubMed] [Google Scholar]
- 27.Landbo C, Prescott E, Lange P, Vestbo J, Almdal TP. Prognostic value of nutritional status in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1999;160:1856–1861. [DOI] [PubMed] [Google Scholar]
- 28.Liou TG, Adler FR, Fitzsimmons SC, Cahill BC, Hibbs JR, Marshall BC. Predictive 5-year survivorship model of cystic fibrosis. Am J Epidemiol 2001;153:345–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Egan TM, Murray S, Bustami RT, Shearon TH, McCullough KP, Edwards LB, Coke MA, Garrity ER, Sweet SC, Heiney DA, et al. Development of the new lung allocation system in the United States. Am J Transplant 2006;6:1212–1227. [DOI] [PubMed] [Google Scholar]
- 30.Arora NS, Rochester DF. Respiratory muscle strength and maximal voluntary ventilation in undernourished patients. Am Rev Respir Dis 1982;126:5–8. [DOI] [PubMed] [Google Scholar]
- 31.Kelsen SG, Ference M, Kapoor S. Effects of prolonged undernutrition on structure and function of the diaphragm. J Appl Physiol 1985;58:1354–1359. [DOI] [PubMed] [Google Scholar]
- 32.Thabut G, Ravaud P, Christie JD, Castier Y, Fournier M, Mal H, Leseche G, Porcher R. Determinants of the survival benefit of lung transplantation in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2008;177:1156–1163. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.