Abstract
Legionella pneumophila is a gram-negative bacterial pathogen that replicates in host macrophages and causes a severe pneumonia called Legionnaires' Disease. The innate immune response to L. pneumophila remains poorly understood. Here we focused on identifying host and bacterial factors involved in the production of type I interferons (IFN) in response to L. pneumophila. It was previously suggested that the delivery of L. pneumophila DNA to the host cell cytosol is the primary signal that induces the type I IFN response. However, our data are not easily reconciled with this model. We provide genetic evidence that two RNA-sensing proteins, RIG-I and MDA5, participate in the IFN response to L. pneumophila. Importantly, these sensors do not seem to be required for the IFN response to L. pneumophila DNA, whereas we found that RIG-I was required for the response to L. pneumophila RNA. Thus, we hypothesize that bacterial RNA, or perhaps an induced host RNA, is the primary stimulus inducing the IFN response to L. pneumophila. Our study also identified a secreted effector protein, SdhA, as a key suppressor of the IFN response to L. pneumophila. Although viral suppressors of cytosolic RNA-sensing pathways have been previously identified, analogous bacterial factors have not been described. Thus, our results provide new insights into the molecular mechanisms by which an intracellular bacterial pathogen activates and also represses innate immune responses.
Author Summary
Initial detection of invading microorganisms is one of the primary tasks of the innate immune system. However, the molecular mechanisms by which pathogens are recognized remain incompletely understood. Here, we provide evidence that an immunosurveillance pathway (called the RIG-I/MDA5 pathway), thought primarily to detect viruses, is also involved in the innate immune response to an intracellular bacterial pathogen, Legionella pneumophila. In the response to viruses, the RIG-I/MDA5 immunosurveillance pathway has been shown to respond to viral RNA or DNA. We found that the RIG-I pathway was required for the response to L. pneumophila RNA, but was not required for the response to L. pneumophila DNA. Thus, one explanation of our results is that L. pneumophila RNA may access the host cell cytosol, where it triggers the RIG-I/MDA5 pathway. This is unexpected since bacteria have not previously been thought to translocate RNA into host cells. We also found that L. pneumophila encodes a secreted bacterial protein, SdhA, which suppresses the RIG-I/MDA5 pathway. Several viral repressors of the RIG-I/MDA5 pathway have been described, but bacterial repressors of RIG-I/MDA5 are not known. Thus, our study provides novel insights into the molecular mechanisms by which the immune system detects bacterial infection, and conversely, by which bacteria suppress innate immune responses.
Introduction
The intracellular bacterium Legionella pneumophila has become a valuable model for the study of immunosurveillance pathways. L. pneumophila is a motile gram-negative bacterium that is the cause of a severe pneumonia called Legionnaires' Disease [1]. In the environment, L. pneumophila is believed to replicate in various species of freshwater amoebae. In humans, L. pneumophila causes disease by replicating within alveolar macrophages in the lung [2]. Replication in macrophages and amoebae requires a type IV secretion system that the bacterium uses to inject effector proteins into the host cell cytosol [3]. These effectors are believed to orchestrate the creation of an intracellular vacuole in which L. pneumophila can replicate. Interestingly, there appears to be considerable redundancy among the effectors, and there are few examples of single effector mutations that have a large effect on intracellular replication of L. pneumophila. One L. pneumophila effector required for intracellular replication is SdhA [4], but the mechanism by which SdhA acts on host cells remains uncertain [4].
A variety of immunosurveillance pathways that detect L. pneumophila infection have been described [5],[6],[7],[8]. The best characterized cytosolic immunosurveillance pathway requires the host proteins Naip5 and Ipaf to detect the cytosolic presence of L. pneumophila flagellin, leading to activation of caspase-1, rapid pyroptotic macrophage death, and efficient restriction of bacterial replication [9],[10],[11],[12],[13]. L. pneumophila has also been observed to induce transcriptional activation of type I interferon (IFN) genes in macrophages and epithelial-like cell lines by a mechanism that remains incompletely characterized [14],[15]. Induction of type I IFNs by L. pneumophila is independent of the flagellin-sensing pathway [16], but also appears to contribute to restriction of bacterial replication in macrophages [16],[17] and epithelial-like cell lines [14].
Type I IFNs are an important class of cytokines that orchestrate diverse immune responses to pathogens [18]. Encoded by a single IFNβ gene as well as multiple IFNα and other (e.g., IFNε, κ, δ, ζ) genes, type I IFNs are transcriptionally induced by a number of immunosurveillance pathways, including Toll-like receptors (TLRs) and a variety of cytosolic sensors [19]. For example, cytosolic RNA is recognized by two distinct helicase and CARD-containing sensors, RIG-I and MDA5 [20], that signal through the adaptor IPS-1 (also called MAVS, CARDIF, or VISA) [21],[22],[23],[24],[25]. The cytosolic presence of DNA also induces type I IFNs, but this phenomenon is less well understood [15],[26]. Studies with Ips-1-deficient mice have indicated that cytosolic DNA can signal independently of Ips-1 in many cell types, including macrophages [25]. However, cytosolic responses to DNA appear to require IPS-1 in certain cell types, including 293T cells [26],[27]. Indeed, two recent reports have described a pathway by which AT-rich DNA can signal via IPS-1 [28],[29]. In this pathway, DNA is transcribed by RNA polymerase III to form an RNA intermediate that can be sensed by RIG-I. The RNA Pol III pathway appears to be operational in macrophages, but is redundant with other DNA-sensing pathways in these cells. A couple of reports have proposed that DAI (also called ZBP-1) is a cytosolic DNA-sensor [30],[31], but Zbp1-deficient mice appear to respond normally to cytosolic DNA [32], consistent with the existence of multiple cytosolic sensors for DNA. Other small molecule compounds, such as cyclic-di-GMP and DMXAA, can also trigger cytosolic immunosurveillance pathways leading to induction of type I IFNs, but these remain to be fully characterized [33],[34],[35].
Type I IFNs are typically considered antiviral cytokines that act locally to induce an antiviral state and systemically to induce cellular innate and adaptive immune responses [19]. Mice deficient in the type I IFN receptor (Ifnar) are unable to respond to type I IFNs, and are highly susceptible to viral infections. Interestingly, most bacterial infections also trigger production of type I IFNs, but the physiological significance of type I IFNs in immune defense against bacteria is complex. Type I IFN appears to protect against infection with group B Streptococcus [36], but this is not the case for many other bacterial infections. For example, the intracellular gram-positive bacterium Listeria monocytogenes induces a potent type I IFN response [37],[38], but Ifnar-deficient mice are actually more resistant to L. monocytogenes infection than are wildtype mice [39],[40],[41]. Many bacterial pathogens, including Francisella tularensis, Mycobacterium tuberculosis, Brucella abortus, and group B Streptococcus, induce type I IFN production by macrophages via a cytosolic TLR-independent pathway [42],[43],[44],[45], but the bacterial ligands and host sensors required for the interferon response of macrophages to these bacteria remain unknown.
It was demonstrated that induction of type I IFN by L. pneumophila in macrophages did not require bacterial replication or signaling through the TLR-adaptors MyD88 or Trif, but did require the bacterial Dot/Icm type IV secretion system [15]. Because the IFN response could be recapitulated with transfected DNA [15],[26] and because Dot/Icm system has been shown to conjugate DNA plasmids to recipient bacteria [46], it was proposed that perhaps L. pneumophila induced type I IFN via a cytosolic DNA-sensing pathway [15]. Another report used RNA interference to implicate the signaling adaptor IPS-1 (MAVS) in the IFN response to L. pneumophila in human A549 epithelial-like cells [14]. However, the significance of this latter finding is unclear since RNAi-mediated knockdown of RIG-I and MDA5, the two sensor proteins directly upstream of IPS-1, did not have an effect on induction of type I IFN by L. pneumophila [14]. Moreover, the A549 response to L. pneumophila may be distinct from the macrophage or in vivo response.
Recently, one report proposed that L. pneumophila DNA was recognized in the cytosol by RNA polymerase III [29], resulting in the production of an RNA intermediate that triggered IFN production via the IPS-1 pathway. Apparently consistent with this proposal, Ips-1-deficient mouse macrophages did not produce type I IFN in response to L. pneumophila [29]. Moreover, since Pol III acts preferentially on AT-rich substrates, it is plausible that Pol III would recognize the L. pneumophila genome, which has a high proportion (62%) of A:T basepairs. However, the response to L. pneumophila DNA was not investigated [29]. In addition, the same report, as well as others [28],[34], observed that the type I IFN response to AT-rich (or any other) DNA is not Ips-1-dependent in mouse cells. Thus, if L. pneumophila DNA was reaching the cytosol, the simplest prediction would be that the resulting type I IFN response would be independent of Ips-1, instead of Ips-1-dependent, as was shown [29]. Thus, the mechanism of IFN induction by L. pneumophila remains unclear.
In the present study, we sought to define bacterial and host factors controlling the macrophage type I IFN response to L. pneumophila. In agreement with previous studies [14],[29], we find that Ips-1 is required for optimal induction of type I IFN in response to L. pneumophila infection in vitro. We extend this observation by demonstrating that Ips-1 also contributes to the type I IFN response in an in vivo model of Legionnaires' Disease. Furthermore, we provide the first evidence that two RNA sensors upstream of Ips-1, Rig-i and Mda5, are involved in the macrophage interferon response to L. pneumophila. Importantly, however, we did not observe a role for the Pol III pathway in the type I IFN response to L. pneumophila. Instead, we found that L. pneumophila genomic DNA stimulates an Ips-1/Mda5/Rig-i-independent IFN response in macrophages, which contrasts with the Ips-1-dependent response to L. pneumophila infection. On the other hand, we found that L. pneumophila RNA stimulated a Rig-i-dependent IFN response. Thus, our data are consistent with a model in which L. pneumophila RNA, or host RNA, rather than L. pneumophila DNA, is the primary ligand that stimulates the host IFN response. We also investigated whether bacterial factors that modulate the host type I IFN response. Although numerous viral proteins that interfere with IFN signaling have been described [19], similar bacterial proteins have not been documented. It is therefore interesting that we were able to identify a secreted bacterial effector, SdhA, as an inhibitor of the Ips-1-dependent IFN response to L. pneumophila. Taken together, our findings provide surprising evidence that cytosolic RNA-sensing pathways are not specific for viral infections but can also respond to bacterial infections, and moreover, our data provide a specific example of a bacterial factor that suppresses the host IFN response.
Results
The cytosolic RNA-sensing pathway is involved in the macrophage response to L. pneumophila
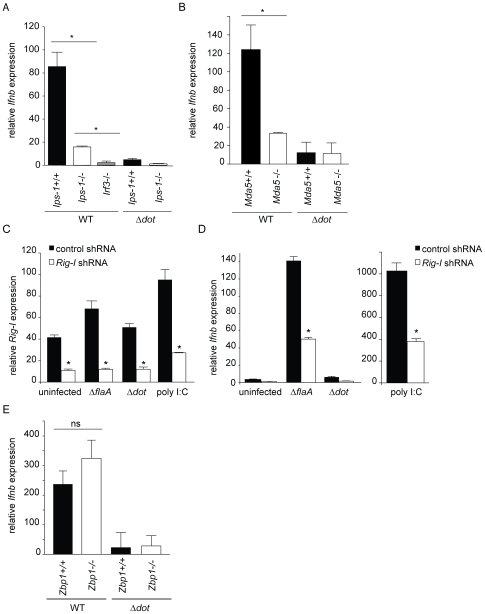
We hypothesized that a cytosolic innate immune sensing pathway controls the type I IFN response to L. pneumophila. To test this hypothesis, we determined whether macrophages deficient in known cytosolic RNA and DNA sensing pathway components can induce type I IFNs in response to L. pneumophila. Macrophages were infected with L. pneumophila at a multiplicity of infection (MOI) of 1 and induction of interferon beta (Ifnb) message was analyzed by quantitative RT-PCR after 4 hours (Figure 1A–D). As previously reported [29], Ips-1 −/− macrophages showed a significantly reduced induction of Ifnb in response to infection with wild type L. pneumophila compared to Ips-1+/+ macrophages (p<0.05; Figure 1A). Induction of Ifnb was not completely eliminated in Ips-1 −/− macrophages, however, as Irf3 −/− macrophages exhibited an even lower induction of Ifnb compared to Ips-1 −/− (p<0.05; Figure 1A). Consistent with previous reports [15], we found that the Dot/Icm type IV secretion system was required to elicit the macrophage type I interferon response since Δdot L. pneumophila did not induce a robust type I interferon response (Figure 1A). These results suggest that L. pneumophila induces type I IFN via a cytosolic RNA immunosurveillance pathway that involves the adaptor Ips-1.
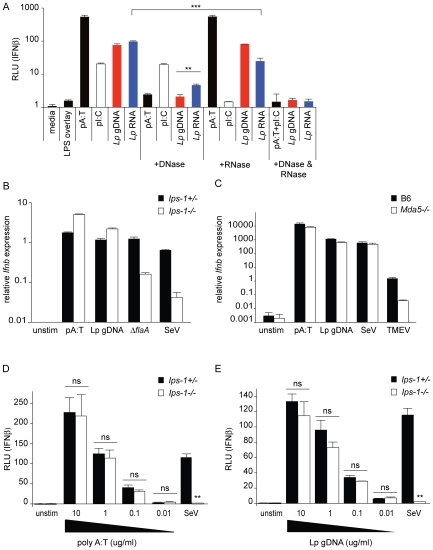
Figure 1. The cytosolic RNA-sensing pathway is involved in the host type I interferon response to L. pneumophila.
(A) Induction of interferon beta (Ifnb) by L. pneumophila is largely dependent on Ips-1. Bone marrow derived Ips-1+/+, Ips-1 −/−, and Irf3 −/− macrophages were infected with wild type and Δdot L. pneumophila at a multiplicity of infection (MOI) of 1. Ifnb induction was analyzed by quantitative RT-PCR 4 hours post infection. Ifnb message was normalized to ribosomal protein rps17 levels. Differences in Ifnb transcript induction were statistically significant in Ips-1+/+versus Ips-1 −/− macrophages (*, p<0.05) and Ips-1 −/− versus Irf3 −/− (*, p<0.05, Student's t-test) when infected with wild type L. pneumophila. (B) Induction of Ifnb by L. pneumophila is partially dependent on Mda5. Bone marrow derived Mda5 +/+ and Mda5 −/− macrophages were infected with wild type and Δdot L. pneumophila at a multiplicity of infection (MOI) of 1. Ifnb induction was assessed by quantitative RT-PCR as in (A). Differences in Ifnb induction were statistically significant (*, p<0.05, Student's t-test) between Mda5 +/+ and Mda5 −/− infected with wild type L. pneumophila. (C) Retroviral transduction of a Rig-i shRNA, but not the control shRNA, knocks down expression of Rig-i in MyD88 −/− Trif −/− immortalized macrophages. Stable transduction of MyD88 −/− Trif −/− immortalized macrophages was performed with a retroviral vector containing a control and Rig-i shRNA. Level of Rig-i knockdown was determined by quantitative RT-PCR under uninfected, infected, and poly I:C stimulation conditions. Differences in Rig-i transcript levels were statistically significant (*, p<0.05, Students t-test) under resting, infected, and ligand-stimulated conditions. (D) Rig-i is involved in the host type I interferon response to infection with L. pneumophila. Rig-i knockdown leads to reduced Ifnb expression in response to infection with ΔflaA L. pneumophila, as well as stimulation with poly I:C. Quantitative RT-PCR was carried out 4 hours post infection. Control knockdown macrophages induced a statistically significant (*, p<0.05) higher level of Ifnb transcript in response to ΔflaA L. pneumophila and poly I:C. No significant difference was found in uninfected or Δdot L. pneumophila infected macrophages. (E) Induction of Ifnb by L. pneumophila is independent of Zbp-1 (Dai). Bone marrow derived Zbp-1+/+ and Zbp-1 −/− macrophages were infected with L. pneumophila strains and analyzed for Ifnb induction as in (A) and (B). Differences in Ifnb transcript levels between Zbp-1+/+ and Zbp-1 −/− macrophages infected with L. pneumophila were not statistically significant (ns, p>0.1, Student's t-test).
We hypothesized that a cytosolic RNA sensor that functions upstream of Ips-1 could be involved in the type I interferon host response to L. pneumophila. However, knockdown experiments in A549 cells previously failed to reveal a role for the known sensors (MDA5 and RIG-I) upstream of IPS-1 [14]. Therefore, we tested Mda5 −/− knockout macrophages (Figure 1B) and found reduced induction of Ifnb message as compared to control Mda5+/+ macrophages. Importantly, however, Dot-dependent induction of type I IFN was not completely abolished in Mda5 −/− macrophages, implying that other redundant pathways are also involved.
Rig-i knockout mice die as embryos, so we were unable to obtain Rig-i −/− knockout macrophages. To circumvent this problem, we stably transduced immortalized macrophages with a retrovirus expressing an shRNA to knock down Rig-i expression. Quantitative RT-PCR demonstrated that the knockdown was effective, even in infected macrophages (Figure 1C), and that Rig-i knockdown had a significant effect on the induction of type I interferon by L. pneumophila (Figure 1D). In the experiments in Figures 1C and 1D we used the ΔflaA strain of L. pneumophila, but similar results were obtained with wildtype, and it was previously shown that flagellin is not required for the IFN response to L. pneumophila [14],[16]. It is unusual, but not unprecedented, that a pathogen would stimulate both the RIG-I and MDA5 RNA-sensing pathways [47].
At present, only one candidate cytosolic DNA sensor involved in the IFN response has been described [30],[31]. To determine whether this sensor, called Dai (or Zpb1), is involved in the type I interferon response to L. pneumophila, we tested whether Zbp1 −/− macrophages respond to L. pneumophila. We observed similar levels of Ifnb induction in Zbp1+/+ and Zbp1 −/− macrophages (Figure 1E). Taken together, these results imply that the RNA sensors Rig-i and Mda5, but not the DNA sensor Zbp1, are involved in sensing L. pneumophila infection.
We tested whether loss of signaling through the RNA sensing components Ips-1 or Mda5 could mimic the previously observed permissiveness of Ifnar −/− macrophages [16]. However, neither Ips-1 −/− nor Mda5 −/− macrophages were permissive to L. pneumophila, suggesting that the low levels of IFNβ produced in the absence of Ips-1 or Mda5 are sufficient to restrict L. pneumophila growth (Figure S1).
The type IV secreted effector SdhA suppresses induction of interferon by L. pneumophila
To identify bacterial components that modulate the type I interferon response to L. pneumophila, we conducted a transposon mutagenesis screen. The LP02 strain of L. pneumophila was mutagenized with a mariner transposon as described previously [12]. Individual transposon mutants were used to infect MyD88 −/− Trif −/− bone marrow-derived macrophages at an MOI of 1, and after approximately 16 hours, supernatants were collected and overlayed on type I IFN reporter cells [48]. Induction of type I IFN was compared to wild type (LP02) and Δdot L. pneumophila controls. We tested approximately 2000 independent mutants and isolated eight mutants that were confirmed to be defective in induction of type I IFN. All these mutants harbored insertions in genes required for the function of the Dot/Icm apparatus (e.g., icmB, icmC, icmD, icmX, icmJ), thereby validating the screen.
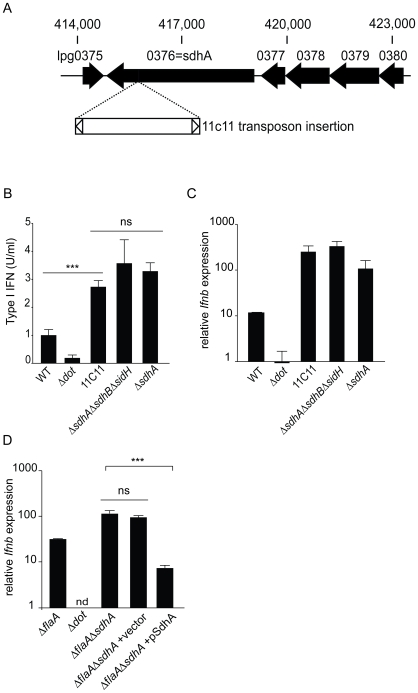
Interestingly, a single transposon mutant, 11C11, was found that consistently hyperinduced the type I interferon response. The transposon insertion mapped to the 3′ end (nucleotide position 3421 of the open reading frame) of a gene, sdhA, that was previously shown [4] to encode a type IV secreted effector protein of 1429 amino acids (166kDa) (Figure 2A). SdhA has previously been shown to be essential for bacterial replication in macrophages [4], but a connection to type I IFNs was not previously noted.
Figure 2. The type IV secreted effector SdhA suppresses induction of interferon by L. pneumophila.
(A) The 11C11 mutant harbors a transposon insertion in sdhA. The transposon insertion site is in the 3′ end of the open reading frame of the sdhA locus at nucleotide position 3421. (B) A clean deletion mutant of sdhA recapitulates the 11C11 transposon mutant and hyperinduces type I interferon. Bone marrow derived Myd88 −/− Trif −/− macrophages were infected with stationary phase L. pneumophila strains at a MOI of 1. Cell supernatants were harvested 8 hours post infection and assayed for type I interferon induction by an L929-ISRE luciferase bioassay. Type I interferon levels were determined by generating a standard curve with recombinant IFNβ An unmarked clean deletion of sdhA was compared to wild type, Δdot, the transposon mutant 11C11, and a triple deletion of sdhA and the two L. pneumophila paralogs, sidH and sdhB. Differences in IFNβ induction were statistically significant between WT L. pneumophila and the transposon mutant 11C11 (***, p<0.0005, Student's t-test). Differences between 11C11, ΔsdhA and ΔsdhAΔsdhBΔsidH were not statistically significant (ns, p>0.05, Student's t-test). (C) A clean deletion mutant of sdhA recapitulates the 11C11 transposon mutant and hyperinduces transcriptional activation of Ifnb. Bone marrow derived Myd88 −/− Trif −/− macrophages were infected with wild type, Δdot, ΔsdhA, 11C11, ΔsdhAΔsdhBΔsidH stationary phase L. pneumophila and transcriptional induction of Ifnb was analyzed by quantitative RT-PCR. (D) Complementation of the sdhA mutant results in loss of the Ifnb hyperinduction phenotype. MyD88 −/− Trif −/− BMDM were infected at an MOI of 1 with ΔflaA, Δdot, ΔflaAΔsdhA and ΔflaAΔsdhA L. pneumophila carrying vector or a plasmid expressing full length SdhA. Expression of Ifnb message was assessed by quantitative RT-PCR 4 hours post infection.
To confirm that the hyperinduction of type I interferon was due to mutation of sdhA, the 11C11 transposon mutant was compared to an unmarked clean deletion of sdhA (Figure 2B). Both the 11C11 mutant and ΔsdhA L. pneumophila showed similar levels of hyperinduction of type I interferon. The L. pneumophila genome contains 2 paralogs of sdhA, called sidH and sdhB. A triple knockout strain, ΔsdhAΔsdhBΔsidH, was compared to single deletion of sdhA to determine if either paralog regulated the induction of type I IFNs. Similar levels of IFNβ were induced ΔsdhAΔsdhBΔsidH and ΔsdhA (Figure 2B). Similar results were obtained when induction of Ifnb was assessed by quantitative RT-PCR (Figure 2C). A role for sdhA in regulating the interferon response was further confirmed by complementing the ΔsdhA mutation with an sdhA expression plasmid [4]. As expected, the complemented strain induced significantly less type I IFN than the control ΔsdhA strain harboring an empty plasmid (Figure 2D). These results indicate that SdhA functions, directly or indirectly, to repress the induction of type I IFN by L. pneumophila.
Hyperinduction of type I IFN by the sdhA mutant involves the cytosolic RNA-sensing pathway
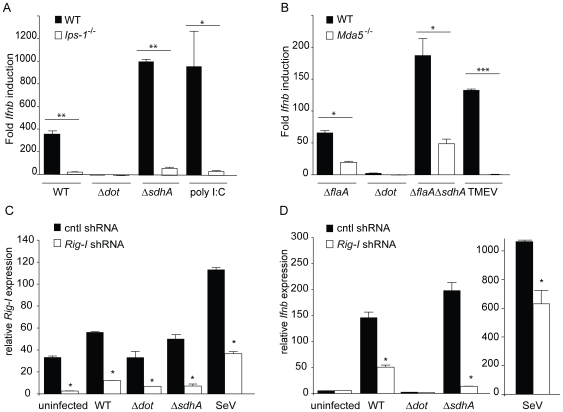
It was possible that ΔsdhA mutants hyperinduced type I IFN via a pathway distinct from the normal cytosolic RNA-sensing pathway that responds to wildtype L. pneumophila. Therefore, to determine whether hyperinduction of type I interferon by ΔsdhA occurs through the same pathway that responds to wild type L. pneumophila, we infected Ips-1 −/− and Mda5 −/− macrophages with ΔsdhA L. pneumophila. Induction of Ifnb message was determined by quantitative RT-PCR. The hyperinduction of Ifnb seen in Ips-1+/+ macrophages was almost abolished in Ips-1 −/− macrophages (p<0.001; Figure 3A). As a control, induction of Ifnb by poly I:C, a double-stranded synthetic RNA analog, was also Ips-1-dependent as expected. Similarly, the hyperinduction of Ifnb was also reduced in Mda5 −/− macrophages (p<0.01; Figure 3B). However, the Mda5 −/− macrophages still induced significant amounts of Ifnb, suggesting that the requirement for Mda5 is not complete. We also tested the ΔsdhA mutant in Rig-i knockdown macrophages. Rig-i knockdown appeared to be effective (Figure 3C) and specifically diminished Ifnb expression (Figure 3D). Thus, the residual Ifnb induction in Mda5 −/− may be due to Rig-i, or to another uncharacterized pathway. As a control, Theiler's virus (TMEV) induced Ifnb in a completely Mda5-dependent manner, as expected (Figure 3B).
Figure 3. Hyperinduction of type I IFN by sdhA mutants involves cytosolic RNA sensing pathway components Ips-1, Rig-i, and Mda5.
(A) Hyperinduction of Ifnb by ΔsdhA L. pneumophila is largely dependent on Ips-1. Bone marrow derived Ips-1+/+ and Ips-1 −/− macrophages were infected with wild type, Δdot, and ΔsdhA L. pneumophila at an MOI of 1. Ips-1+/+ and Ips-1 −/− macrophages were transfected with 1.0 µg/ml poly I:C. 4 hours post infection and stimulation, macrophages were harvested and assessed for Ifnb induction as in Figure 1. Ips-1+/+ infected with WT L. pneumophila induced statistically significant higher levels of Ifnb transcript than Ips-1 −/− (**, p<0.005, Student's t-test). The same phenotype was seen in Ips-1+/+ infected with ΔsdhA L. pneumophila (**, p<0.005) and transfected with poly I:C (*, p<0.05) when compared to Ips-1 −/−. (B) Hyperinduction of Ifnb by ΔsdhA L. pneumophila is partially dependent on Mda5. Bone marrow derived Mda5+/+ and Mda5 −/− macrophages were infected with ΔflaA, Δdot, and ΔflaAΔsdhA L. pneumophila at an MOI of 1. Theiler's virus (TMEV) was overlaid onto Mda5+/+ and Mda5 −/− macrophages. 4 hours post bacterial and viral infection, macrophages were harvested and assessed for Ifnb induction by qRT-PCR as in Figure 1. Ifnb message was induced statistically significantly in Mda5+/+ macrophages infected with ΔflaA L. pneumophila versus Mda5 −/− (*, p<0.05, Student's t-test). Mda5+/+ also responded statistically significantly to ΔflaAΔsdhA L. pneumophila over Mda5 −/− (*, p<0.05, Student's t-test), while Theiler's virus elicited a robust Ifnb response from Mda5+/+ not seen in Mda5 −/− (***, p<0.005, Student's t-test). (C) Retroviral transduction of a Rig-i shRNA, but not the control shRNA, knocks down expression of Rig-i. MyD88 −/− Trif −/− immortalized macrophages were stably transduced with retroviral vector containing a control and Rig-i shRNA. Level of Rig-i knockdown was determined by quantitative RT-PCR under uninfected and infected conditions. Differences in Rig-i transcript levels were statistically significant (*, p<0.05, Students t-test) under resting and infected conditions. (D) Rig-i is involved in the hyperinduction of type I interferon by ΔsdhA L. pneumophila. Rig-i knockdown leads to reduced Ifnb expression in response to infection with WT and ΔsdhA L. pneumophila, as well as Sendai virus. Quantitative RT-PCR was carried out 4 hours post infection. Control knockdown macrophages induced a statistically significant (*, p<0.05) higher level of Ifnb transcript in response to WT and ΔsdhA L. pneumophila and Sendai virus. No significant difference was found in uninfected or Δdot L. pneumophila infected macrophages.
The effects of the ΔsdhA mutant are independent of caspase-1 activation
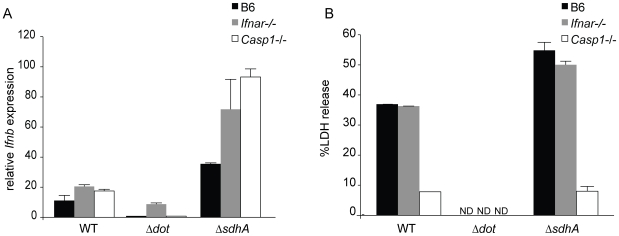
It was previously shown that ΔsdhA mutants induce a rapid death of infected macrophages that is dependent upon activation of multiple cell death pathways [4]. Consequently, we hypothesized that the hyperinduction of type I IFN by the ΔsdhA mutant might be due to the release of molecules from dying cells, such as DNA, that could induce Ifnb expression. To rule out this explanation, we infected Casp1 −/− macrophages, which are resistant to cell death at the early timepoints examined (e.g., 4h post infection), and asked whether type I interferon was still hyperinduced in response to ΔsdhA L. pneumophila. In fact, we found that Casp1 −/−macrophages infected with the ΔsdhA mutant hyperinduced Ifnb to levels above that observed in B6 macrophages (Figure 4A). We suspect that the increased Ifnb induction seen in Casp1 −/− cells was an indirect consequence of the lower levels of cell death in these cells, and was not due to a specific suppression of type I interferon transcription by Casp1 activation. In any case, our results indicated that the hyperinduction of type I IFN by the ΔsdhA mutant was not due to increased cell death induced by the mutant. As a control, we confirmed that Casp1 −/− macrophages were resistant to cell death at the 4h timepoint tested (Figure 4B).
Figure 4. The effects of the sdhA mutation on type I interferon induction are independent of caspase-1 activation and the type I interferon receptor.
(A) Type I interferon receptor signaling and caspase1-dependent pyroptotic cell death are not required for superinduction of Ifnb by the sdhA mutant. Bone marrow derived C57BL/6, Ifnar −/− and Casp1 −/− macrophages were infected with wild type, Δdot, and ΔsdhA L. pneumophila at an MOI of 1. Ifnb message was analyzed by qPCR from macrophage RNA harvested 4 hours post infection. (B) Caspase1-dependent pyroptotic cell death occurs independently of the type I interferon receptor. Bone marrow derived C57BL/6, Ifnar −/− and Casp1 −/− macrophages were infected with wild type, Δdot, and ΔsdhA L. pneumophila at an MOI of 1 and release of lactate dehydrogenase (LDH) in cell supernatants was measured 4 hours post infection. Specific cell lysis was calculated as a percentage of detergent lysed cells with spontaneous LDH release subtracted. No statistically significant difference was found between B6 and Ifnar −/− macrophages infected with ΔsdhA L. pneumophila (p>0.1, Student's t-test). ND, not detected.
SdhA acts independently of the type I IFN receptor
Induction of Ifnb is often regulated by a positive feedback loop in which initial production of IFNβ results in signaling through the type I IFN receptor (Ifnar) and synergistically stimulates the production of additional type I IFN. We therefore examined whether the hyperinduction of Ifnb by the ΔsdhA mutant might be due to positive feedback through the type I IFN receptor. To test this possibility we examined induction of Ifnb by the ΔsdhA mutant in Ifnar −/− macrophages. We found that hyperinduction of Ifnb by ΔsdhA L. pneumophila occurs even in the absence of signaling from the type I interferon receptor, since Ifnar −/− macrophages hyperinduce Ifnb in response to infection with ΔsdhA L. pneumophila (Figure 4A).
The mechanism by which the ΔsdhA mutant induces cell death remains unclear [4]. Studies with the intracellular bacterial pathogen Francisella tularensis have demonstrated the existence of a type I IFN-inducible caspase-1-dependent cell death pathway [43]. Therefore, we sought to establish if caspase-1-dependent cell death occurred in the absence of Ifnar signaling in response to wild type and ΔsdhA L. pneumophila. Ifnar −/− macrophages were infected at an MOI of 1 and assayed for release of the intracellular enzyme lactate dehydrogenase (LDH) 4 hours post infection. Ifnar −/− macrophages exhibited similar LDH release as B6 macrophages, whether infected with WT or ΔsdhA L. pneumophila, and this LDH release was dependent upon caspase-1 activation (Figure 4B). These data demonstrate that caspase1-dependent pyroptotic death occurs independently of the type I interferon receptor during infection with wild type and ΔsdhA L. pneumophila.
Since growth of the ΔsdhA mutant is severely attenuated in macrophages [4], we hypothesized that hyperinduction of type I interferon might contribute to the restriction of replication of the ΔsdhA mutant. To test this hypothesis, we infected lfnar −/− macrophages with luminescent strains of L. pneumophila at an MOI of 0.01 and monitored bacterial replication over a 72 hour time period. As previously reported [16], lfnar −/− macrophages were more permissive to WT and ΔflaA L. pneumophila as compared to C57BL/6 macrophages (Figure S2A, C). However, the ΔsdhA or ΔflaAΔsdhA L. pneumophila strains were still significantly restricted in Ifnar −/− macrophages (Figure S2B, D). Thus, SdhA is required for bacterial replication in macrophages primarily via a mechanism independent of its role in suppressing type I IFN. As expected, Δdot L. pneumophila did not replicate in WT or Ifnar −/− macrophages (Figure S2E).
Since SdhA is a secreted effector, we hypothesized that SdhA may act in the host cell cytosol, rather than in the bacterium, to repress Ifnb induction. To test this hypothesis, we co-expressed SdhA with MDA5 or RIG-I, by transient transfection of HEK293T cells, and assessed interferon expression with an IFNβ-luciferase reporter. Expression of either MDA5 or RIG-I robustly induced the IFNβ-luc reporter upon stimulation with poly I:C (Figure S3). When SdhA was co-expressed with MDA5, a dose-dependent repression of the IFNβ-luc reporter was observed (Figure S3A). Co-expression of SdhA also resulted in a dose-dependent repression of RIG-I-dependent induction of the IFNβ-luc reporter (Figure S3B). However, SdhA co-expression did not affect TRIF-dependent induction of the IFNβ-luc reporter (Figure S3C), arguing against the possibility that SdhA expression has non-specific effects on IFNβ-luc induction. These results must be interpreted with caution since the 293T IFNβ-luc reporter system is highly artificial; moreover, we have not demonstrated a direct interaction of SdhA with signaling components in the RNA-sensing pathway. In fact, the reported effects of SdhA on mitochondria [4] suggest the effect may be somewhat indirect (see Discussion). Nevertheless, the 293T transfection results suggest that SdhA can act in the host cytosol to specifically repress induction of the RIG-I/MDA5 pathway.
L. pneumophila genomic DNA does not appear to stimulate an Ips-1-dependent IFN response
Based on our observation that the host type I IFN response requires the L. pneumophila Dot/Icm type IV secretion system and was at least partly Ips-1, Rig-i, and Mda5-dependent, we hypothesized that L. pneumophila nucleic acids (RNA, DNA or both) might gain access to the macrophage cytosol via the type IV secretion system and induce a host type I interferon response. To test if L. pneumophila nucleic acids are sufficient to induce type I interferon, we transfected MyD88 −/− Trif −/− macrophages with purified L. pneumophila genomic DNA or total RNA and determined the induction of type I interferons by bioassay. Poly(dA-dT):poly(dA-dT) (abbreviated as pA:T) was used as a non-CpG containing DNA control and poly I:C was used as an RNA control. Nucleic acid preparations were treated with DNase and/or RNase to eliminate contaminating nucleic acids. Both purified L. pneumophila DNA and the crude RNA preparation induced IFNβ (Figure 5A). L. pneumophila RNA treated with RNase also induced IFNβ, presumably due to (contaminating) DNA in the preparation (Figure 5A). However, L. pneumophila RNA treated with DNase induced type I interferon to a level above that induced by L. pneumophila RNA treated with both RNase and DNase, suggesting that L. pneumophila RNA alone can induce type I interferon production (Figure 5A). The induction of type I IFN by L. pneumophila RNA was modest, possibly because bacterial RNA is less stable than DNA. Nevertheless, these results suggest that both L. pneumophila RNA and DNA can induce a type I interferon host response.
Figure 5. L. pneumophila DNA and RNA stimulate type I IFN production in macrophages.
(A) Purified genomic DNA and RNA from L. pneumophila induces type I interferon independently of MyD88 and Trif. Bone marrow derived Myd88 −/− Trif −/− macrophages were stimulated by transfection of 3.3 µg/ml purified L. pneumophila DNA, L. pneumophila RNA, pA:T (DNA), and pI:C (RNA). Nucleic acids were treated with DNase and/or RNase A before transfection. Macrophage supernatants were harvested 8 hours post stimulation and analyzed for IFNβ levels by L929-ISRE luciferase bioassay. IFNβ production by DNase-treated L. pneumophila RNA was statistically significantly higher compared to DNase-treated L. pneumophila DNA (**, p<0.005). In addition, RNase A-treated L. pneumophila RNA produced statistically significant lower levels of IFNβ (***, p<0.0001, Student's t-test) than L. pneumophila RNA and RNase-treated L. pneumophila DNA. (B) Genomic L. pneumophila DNA does not induce type I interferon in a Ips-1-dependent manner. Ips-1 −/− and heterozygous littermate bone marrow derived macrophages were stimulated by transfection of 1.0 µg/ml pA:T and purified genomic L. pneumophila DNA. Macrophages were infected with ΔflaA L. pneumophila at an MOI of 1. Sendai virus (SeV) was overlaid onto Ips-1 −/− and heterozygous littermate macrophages. Transcriptional activation of Ifnb was determined by quantitative RT-PCR as described in Figure 1. (C) The viral RNA sensor Mda5 is not required for induction of type I interferon by L. pneumophila DNA. WT (C57BL/6) and Mda5 −/− bone marrow derived macrophages were stimulated by transfection of 1.0 µg/ml pA:T and purified genomic L. pneumophila DNA. Sendai virus (SeV) and Theiler's virus (TMEV) were overlaid onto WT and Mda5 −/− macrophages. Quantitative RT-PCR was used to determine Ifnb gene expression. (D) Non-CpG containing DNA (pA:T) does not induce Ips-1-dependent Ifnb at all concentrations tested. Ips-1 −/− and heterozygous littermate bone marrow derived macrophages were stimulated with a titration of pA:T by transfection of 10, 1.0, 0.1, 0.01 µg/ml pA:T. The difference between Ips-1 +/− and Ips-1 −/− macrophages transfected with pA:T was not statistically significant (ns, p>0.1, Student's t-test). Sendai virus (SeV) was overlaid onto Ips-1 −/− and heterozygous littermate macrophages (**, p<0.005). Cell supernatants were collected 8 hours post stimulation/infection. Induction of type I interferon was determined by L929-ISRE luc bioassay. Units are relative light units (RLU). (E) Genomic L. pneumophila DNA induces type I interferon independently of Ips-1 at all concentrations tested. Ips-1 −/− and heterozygous littermate bone marrow derived macrophages were stimulated with a titration of purified genomic L. pneumophila DNA by transfection of 10, 1.0, 0.1, 0.01 µg/ml L. pneumophila DNA. No statistically significant difference was found between Ips-1 +/− and Ips-1 −/− macrophages transfected with genomic L. pneumophila DNA (ns, p>0.1, Student's t-test). Sendai virus (SeV) was overlaid onto Ips-1 −/− and heterozygous littermate controls (**, p<0.005). Macrophage supernatants were collected 8 hours post stimulation/infection. Type I interferon levels were determined by L929-ISRE luc bioassay, units are relative light units (RLU).
Next, we determined if L. pneumophila nucleic acids could induce type I interferon in an Ips-1-dependent manner in macrophages. In certain cell types, though not mouse macrophages [34], AT-rich DNA has been shown to induce type I IFN via IPS-1 [26],[27],[28],[29]. It was important to assess whether L. pneumophila DNA, in particular, might signal in an Ips-1-dependent manner since the L. pneumophila type IV secretion system has previously been shown to translocate DNA [46]. Ips-1 +/− and Ips-1 −/− macrophages were transfected with pA:T and L. pneumophila DNA, as well as infected with Sendai virus, a virus previously determined to induce an Ips-1-dependent IFN response. Stimulation with pA:T or L. pneumophila DNA failed to induce Ifnb in an Ips-1-dependent manner, whereas Sendai virus induced significantly more Ifnb in Ips-1 +/− versus Ips-1 −/− macrophages (Figure 5B). Similar results were obtained in Mda5 −/− macrophages: induction of type I IFN with pA:T or L. pneumophila genomic DNA showed no requirement for Mda5, whereas a control simulation, Theiler's Virus, showed Mda5-dependent induction of IFNβ, as expected (Figure 5C). It was possible that at high concentrations of DNA, an Ips-1-independent DNA-sensing pathway overwhelmed any putative Ips-1-dependent recognition of DNA. However, induction of Ifnb was independent of Ips-1 even when titrated amounts of pA:T or L. pneumophila genomic DNA were transfected into macrophages (Figure 5D, 5E). Thus, these results suggest that while transfected L. pneumophila DNA robustly induces type I interferon, L. pneumophila genomic DNA does not appear to induce the Ips-1-dependent IFN response that is characteristic of L. pneumophila infection.
L. pneumophila RNA stimulates type I interferon via Rig-i
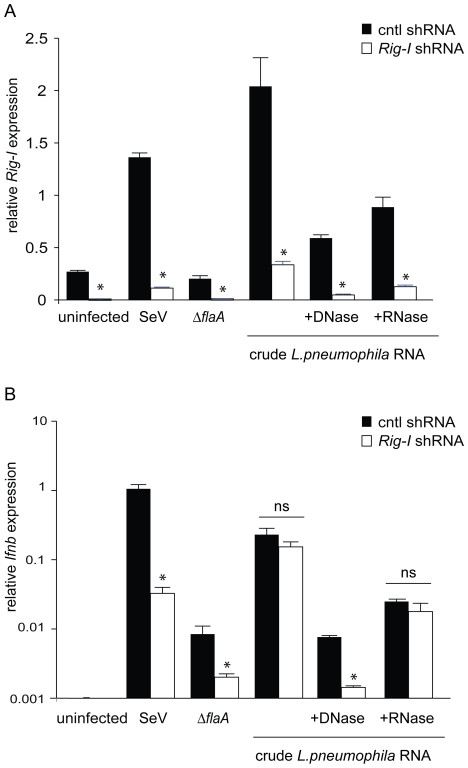
To determine whether L. pneumophila RNA could be recognized by Rig-i, we transfected L. pneumophila RNA into macrophages in which Rig-i expression had been stably knocked down. Importantly, the Rig-i knockdown was performed in immortalized bone-marrow-derived macrophages that lack MyD88 and Trif, in order to avoid potential activation of known RNA-sensing TLRs. Knockdown of Rig-i was effective under our transfection conditions, as Rig-i message was significantly lower in macrophages transduced with a Rig-i shRNA compared to a control shRNA (p<0.05; Figure 6A). Crude L. pneumophila RNA (which also contains genomic DNA contaminants) induced Ifnb robustly in both control shRNA and Rig-i shRNA macrophages, even upon treatment with RNase A (Figure 6B). However, transfection of DNase-treated L. pneumophila nucleic acids induced significantly less Ifnb in Rig-i knockdown macrophages as compared to control knockdown macrophages (p<0.05; Figure 6B.) This result suggests that L. pneumophila RNA can induce Rig-i-dependent type I interferon. It was not possible to perform a similar experiment in the Ips-1 −/− macrophages because these macrophages were MyD88/Trif+ and exhibited background interferon, presumably due to TLR3 signaling.
Figure 6. L. pneumophila RNA induces type I interferon via Rig-i.
(A) The efficiency of Rig-i knockdown was determined by quantitative RT-PCR under uninfected, viral and bacterial infected, and transfected conditions. Differences in Rig-i transcript levels were statistically significant (*, p<0.05, Students t-test) under resting, infected, and transfected conditions. (B) Rig-i is involved in the host type I interferon response to L. pneumophila RNA. Rig-i knockdown leads to reduced Ifnb expression upon transfection with DNase-treated L. pneumophila RNA. Quantitative RT-PCR was carried out 4 hours post stimulation. Control knockdown macrophages induced a statistically significant (*, p<0.05) higher level of Ifnb transcript in ΔflaA L. pneumophila and Sendai virus infected macrophages. No significant difference was found in response to untreated and RNase-treated L. pneumophila nucleic acids.
RNA polymerase III does not appear to be required for the IFN response to L. pneumophila
A recent report found that an inhibitor of RNA polymerase III, ML-60218 [49], blocked the type I IFN response to L. pneumophila [29]. It was proposed that L. pneumophila DNA is translocated into macrophages and transcribed by Pol III into a ligand that could be recognized by RIG-I [29]. In contrast, we did not see an effect of ML-60218 on induction of type I IFN by L. pneumophila in bone marrow-derived macrophages (Figure 7A). The lack of an effect does not appear to be due to redundant recognition by another DNA sensor in macrophages because the interferon induction was still largely Ips-1-dependent (Figure 7A). Because our results with the Pol III inhibitor were negative, we cannot rule out the possibility that the Pol III inhibitor fails to function in macrophages. However, we also tested 293T cells, which express only the Pol III pathway for cytosolic recognition of DNA [28],[29]. As expected, 293T cells responded to pA:T in an ML-60218-inhibitable manner, but did not respond well to L. pneumophila genomic DNA (Figure 7B), again suggesting that L. pneumophila genomic DNA is not an efficient substrate for the Pol III pathway. The Pol III inhibitor also appeared to have little effect on L. pneumophila replication in bone-marrow macrophages (Figure 7C–E). This latter result was expected, since we found that even Ips-1 −/− macrophages exhibit normal restriction of L. pneumophila replication (Figure S1), despite significantly reduced IFN induction.
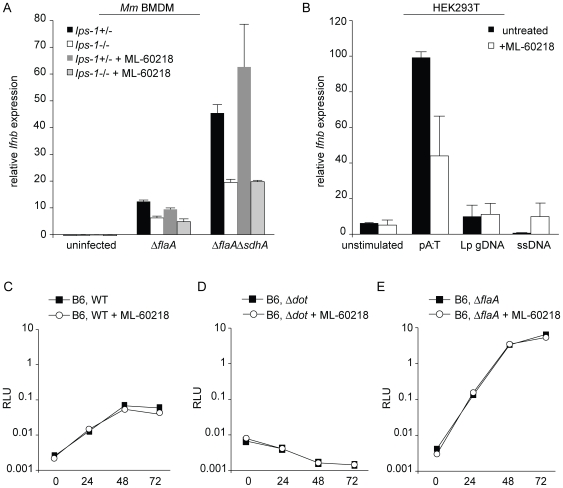
Figure 7. The Pol III pathway does not appear to recognize L. pneumophila DNA or affect L. pneumophila replication.
(A) Inhibition of Pol III had no effect on Ips-1-dependent Ifnb induction by L. pneumophila. Ips-1 +/− and Ips-1 −/− macrophages were pretreated (controls were untreated) with 20 µM ML-60218 10 hours before infection with ΔflaA and ΔflaAΔsdhA L. pneumophila at an MOI of 1. Ifnb induction was analyzed by quantitative RT-PCR 4 hours post infection. Ifnb message was normalized to ribosomal protein rps17 levels. (B) L. pneumophila genomic DNA does not induce IFNB in HEK293T cells. HEK293T cells were pretreated, or left untreated, with 20 µM ML-60218 10 hours before transfection with 1.0 µg/ml pA:T, L. pneumophila genomic DNA, or salmon sperm DNA. Ifnb induction was analyzed by quantitative RT-PCR 4 hours post infection. Ifnb message was normalized to S9 levels. (C) WT (C57BL/6) macrophages were infected at an MOI of 0.01 in the presence or absence of 20 µM ML-60218 and growth of luminescent L. pneumophila strains was determined by RLU at 0, 24, 48, and 72 hours post infection. For inhibitor conditions, macrophages were pretreated with 20 µM ML-60218 10 hours before infection. Macrophages were infected with WT (LP02) L. pneumophila or with isogenic Δdot L. pneumophila (D) ΔflaA L. pneumophila (E).
In vivo role of Ips-1 in the host type I interferon response to L. pneumophila
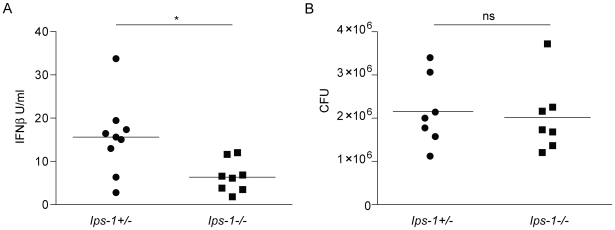
In order to validate our findings in vivo, we infected Ips-1 −/− and littermate Ips-1 +/− mice with L. pneumophila (2.5×106 LP01 ΔflaA per mouse, infected intranasally) and assayed type I interferon production in bronchoalveolar lavage fluid 20 hours post infection by bioassay. Ips-1 +/− mice induced an IFN response that was statistically significantly greater than the response of Ips-1 −/− mice (Student's t-test, p = 0.01; Figure 8A). The difference in IFN production was not explained by a difference in bacterial burden in the Ips-1 +/− and Ips-1 −/− mice, since both genotypes exhibited similar levels of bacterial colonization (p = 0.76, Student's t-test; Figure 8B). The lack of an effect of Ips-1-deficiency on bacterial replication in vivo was not surprising given that we also failed to observe an effect of Ifnar-deficiency on bacterial replication in vivo (data not shown). We suspect that type II IFN (IFNλ), which is not made by macrophages in vitro, or another in vivo pathway, may compensate for loss of type I IFN in vivo. Nevertheless, our results provided an important validation of our in vitro studies and affirm a role for Ips-1 in the in vivo type I interferon response to L. pneumophila. Since Ips-1-deficient mice still mounted a measurable IFN response in vivo, it appears that additional Ips-1-independent pathways (e.g., TLR-dependent pathways, possibly involving other cell types [50]) also play a role in vivo.
Figure 8. Role of Ips-1 in the in vivo response to L. pneumophila.
(A) The type I interferon response to L. pneumophila involves Ips-1 in vivo. Ips-1 −/− and heterozygous littermate mice were infected intranasally with 2.5×106 LP01ΔflaA. Bronchoalveolar lavage with PBS was performed 20 hours post infection. Type I interferon levels in the bronchoalveolar lavage fluid (BALF) were analyzed by bioassay and recombinant IFNβ was used to determine a standard curve. A two-tailed t-test determined the differences in IFNβ levels were statistically significant (*, p<0.01, Student's t-test) upon comparison of Ips-1 +/− and Ips-1 −/− mice. (B) L. pneumophila colony forming units are not significantly different in Ips-1 +/− and Ips-1 −/−. Bronchoalveolar lavage fluid from infected Ips-1 +/− and Ips-1 −/− mice was centrifuged to isolate cells. Hypotonic lysis of cells was performed and CFU were plated on buffered yeast extract charcoal plates with antibiotic selection for L. pneumophila. A two-tailed t-test determined that CFU in Ips-1 +/− and Ips-1 −/− mice 20 hours post infection were not statistically significantly different (ns, p>0.5, Student's t-test).
Discussion
Type I interferons (IFNs) have long been appreciated as critical players in antiviral immune defense, and recent work has identified several molecular immunosurveillance pathways that induce type I IFN expression in response to viruses [18],[19]. In contrast, the roles of type I IFNs in response to bacteria, and the pathways by which bacteria induce type I IFNs, are considerably less well understood. In this study, we sought to characterize the type I IFN response to the gram-negative bacterial pathogen Legionella pneumophila. Our study focused on the type I IFN response mounted by macrophages, since this is the cell type that is believed to be the primary replicative niche in the pathogenesis of Legionnaires' Disease.
In agreement with previous work [15], we found that L. pneumophila induces type I IFNs in macrophages via a TLR-independent pathway that requires expression of the bacterial type IV secretion system. These results suggested that a cytosolic immunosurveillance pathway controls the IFN response in macrophages. In this report we identify the cytosolic RNA-sensing pathway as a key responder to L. pneumophila infection (Figure 1) and, in agreement with previous results using human A549 cells [51], we did not observe a role for Dai (Zbp1), a gene implicated in the response to cytosolic DNA [30],[31]. A previous study using RNA interference in the human A549 epithelial-like cell line also found a role for IPS-1 in the type I IFN response to L. pneumophila [14]. However, knockdown of RIG-I or MDA5 did not appear to affect the IFN response [14], so the role of the IPS-1 pathway was unclear. In our study, we used mice harboring targeted gene deletions to establish a role for Mda5 and Ips-1 in the type I IFN response to L. pneumophila in macrophages, and uncovered a role for Rig-i using an shRNA knockdown strategy. We also found that the cytosolic RNA-surveillance pathway regulated the IFN response in vivo in a mouse model of Legionnaires' Disease.
After our manuscript was submitted, a report published by Chiu and colleagues also concluded that Ips-1 is required for the macrophage type I IFN response to L. pneumophila [29]. However, the report of Chiu et al differs considerably from our current work by proposing that the type I IFN response to L. pneumophila occurs via a novel and unexpected pathway in which L. pneumophila DNA reaches the host cytosol and is transcribed by RNA polymerase III to generate an RNA intermediate that is sensed by RIG-I. Others have found that the Pol III pathway can be activated by viral and AT-rich DNA in certain cell types [28]. Our data, however, are not easily reconciled with a role for the Pol III pathway in recognition of L. pneumophila. First, and perhaps most important, is the observation that the response to DNA (in contrast to the response to L. pneumophila infection) has never been seen to be Ips-1-dependent in macrophages ([34]; Figure 5). This suggests that the response to L. pneumophila is not simply a response to DNA, regardless of the mechanisms by which potentially translocated DNA might be recognized.
We considered the possibility that L. pneumophila DNA exhibits unique properties that cause it to be a particularly efficient substrate for the Pol III pathway. Indeed, the L. pneumophila genome does contain stretches of highly AT-rich DNA, and it has been reported that only highly AT-rich DNA is an efficient substrate for the Pol III pathway [28],[29]. Therefore we tested whether L. pneumophila genomic DNA, unlike other DNA, could induce an Ips-1-dependent response in macrophages. Although L. pneumophila DNA induced a robust IFN response, the response was not Ips-1-dependent (Figure 5B, E). Indeed, even the optimal Pol III substrate poly(dA–dT):poly(dA–dT) (abbreviated as pA:T) does not appear to induce an Ips-1-dependent IFN response in macrophages (Figure 5B, D and [34]). The lack of Ips-1-dependence in the response to pA:T appears to be due to an unidentified Ips-1-independent DNA-sensing pathway that recognizes pA:T and dominates over the Pol III pathway in bone marrow macrophages [28]. Thus, if translocated DNA is the relevant bacterial ligand that stimulates the Ips-1-dependent host type I IFN response, an explanation is required for how the dominant and unidentified DNA-sensing pathway is not activated. While L. pneumophila could selectively inhibit or evade the dominant DNA-sensing pathway, there is at present no evidence to support this mechanism. Moreover, in our hands, the Pol III inhibitor used by Chiu et al (ML-60218) failed to affect IFN induction or bacterial replication in macrophages (Figure 7), in contrast to what would be predicted if the Pol III pathway was selectively activated in response to L. pneumophila infection. Therefore, our data lead us to consider alternative models.
Although Chiu et al primarily used the RAW macrophage-like cell line in their experiments with L. pneumophila, we do not believe that cell-type-specific effects can account for the discrepancy in results. Although it is possible that RAW cells express only the Pol III pathway, this would not change the fact that the proposed model of Chiu et al invokes DNA as the primary IFN-inducing ligand produced by L. pneumophila. The simplest prediction of such a model would be that the response of bone marrow macrophages to L. pneumophila would be Ips-1-independent, as is the response of macrophages to all forms of DNA that have been tested. In contrast, as documented here (Figure 1) and by Chiu et al [29], the response to L. pneumophila is Ips-1-dependent. Moreover, 293T cells, which express only the Pol III DNA-sensing pathway [28],[29], failed to respond significantly to L. pneumophila genomic DNA, despite a robust response to pA:T (Figure 7B). Therefore, our data suggest that recognition of L. pneumophila genomic DNA by Pol III is not responsible for the Ips-1-dependent IFN response to L. pneumophila.
We considered two other models to explain how L. pneumophila induces a type I interferon response. The first is that L. pneumophila translocates RNA into host cells. In support of this model, we demonstrate that L. pneumophila RNA, unlike any form of DNA tested, induced a Rig-i-dependent type I IFN response in macrophages (Figures 5A, 6). However, we did not demonstrate that L. pneumophila RNA species are translocated into host cells, and this will be important to examine in future studies. Interestingly, it was recently reported that purified Helicobacter pylori RNA stimulates RIG-I in transfected 293T cells [52]. A second model to explain type I IFN induction by L. pneumophila is that infection induces a host response that indirectly results in signaling via the MDA5/RIG-I/IPS-1 pathways. L. pneumophila secretes a large number of effectors into the host cytosol and these effectors disrupt or alter a large number of host cell processes [53]. Such disruption may either lead to the generation of host-derived RNA ligands for the RIG-I and MDA5 sensors, or may result in signaling through these sensors in the absence of specific ligands. It was previously proposed that a host nuclease, RNaseL, can generate self-RNA ligands for the RIG-I and MDA5 pathways in response to viral infection [54]. Although we could not observe a role for RNaseL in the response to L. pneumophila (K.M. Monroe, unpublished data), it is conceivable that a different host enzyme can fulfill a similar function.
Our finding that a secreted bacterial effector, SdhA, previously shown to suppress host cell death, also suppresses the IFN response to L. pneumophila, is consistent with a model in which a host cell stress response leads to direct or indirect activation of the cytosolic RNA-sensing pathway. However, the mechanism by which SdhA acts on host cells remains mysterious. Laguna and colleagues provided evidence that SdhA is critical for prevention of mitochondrial disruption that occurs when host cells are infected with the ΔsdhA mutant [4]. Given that Ips-1 localizes to mitochondria and requires mitochondrial localization for its function [21], it is tempting to speculate that SdhA acts on mitochondria in a way that both prevents their disruption and interferes with the function of Ips-1. To provide evidence that SdhA acts specifically on the RIG-I/MDA5 pathway, we used transient transfections of 293T cells. SdhA repressed induction of Ifnb when co-expressed with Mda5 or Rig-I but not Trif (Figure S3). Given these results and the evidence that SdhA is translocated into host cells [4], we favor the idea that SdhA acts within host cells. Mutation of sdhA was reported not to affect translocation of other effectors into host cells [4]; thus, we tend not to support the alternative possibility that SdhA blocks translocation of the putative IFN-stimulatory ligand through the type IV secretion system. SdhA is a large protein of 1429 amino acids, but does not contain domains of known function, except for a putative coiled coil (a.a. 1037–1068). In future studies it will be important to address whether subdomains of SdhA can be identified that are required for suppression of the IFN response. It will also be important to determine whether these subdomains are distinguishable from any putative subdomains required for suppression of host cell death. In fact, our data have suggested that suppression of cell death and the IFN response may be separable functions of SdhA. We found that cell death was not required for hyperinduction of IFN by the ΔsdhA mutant, and conversely, we also found that hyperinduction of type I IFN does not lead to increased cell death (Figure 4).
Our studies demonstrate a partial role for both Mda5 and Rig-i RNA sensors in response to L. pneumophila. Although these sensors are typically thought to respond to distinct classes of viruses, there are indications that they can also function cooperatively in response to certain stimuli, e.g., West Nile Virus [47]. Our results suggest that L. pneumophila produces ligands that can stimulate both Mda5 and Rig-i and that these two sensors cooperatively signal via Ips-1. Fitting with this model, we found that Ips-1-deficiency generally had a more severe impact on type I IFN induction than did Mda5 or Rig-i deficiency.
Cytosolic RNA-sensing pathways are believed to respond exclusively to viral infection, and it is therefore surprising that L. pneumophila appears to trigger these pathways. Other bacterial species, such as Listeria monocytogenes and Francisella tularensis, have been shown to induce an Ips-1-independent cytosolic pathway leading to type I IFN induction [25],[43],[55]. The sensor(s) required for the IFN response to Listeria or Francisella have not yet been identified, but are widely assumed to be identical to the (also unknown) sensor(s) that respond to cytosolic DNA [15],[26].
Ips-1 or Mda5-deficiency, as well as Rig-i knockdown, did not result in a complete elimination of the type I IFN response (Figure 1, Figure 3). Thus, a cytosolic DNA-sensing pathway may also be stimulated in response to L. pneumophila infection. A minor role for a cytosolic DNA-sensing pathway would be consistent with the observation that the L. pneumophila Dot/Icm type IV secretion system can translocate DNA into recipient cells [46]. However, as discussed above, our results with purified L. pneumophila DNA suggest that cytosolic sensing of L. pneumophila DNA does not account for the Ips-1-dependent induction of IFN that we observe (Figure 5). One last possibility that we cannot eliminate is that a non-DNA, non-RNA ligand is translocated into host cells and stimulates the Ips-1 pathway. In fact, in separate work, we have found that a small bacterial cyclic dinucleotide, c-di-GMP, can trigger a type I IFN response in macrophages, but importantly, this response is entirely independent of the Ips-1 pathway [34]. Nevertheless, there may be other small molecules that can be translocated by the Dot/Icm secretion system and signal in host cells via Ips-1.
Taken together, our results lead to new insights into the host immunosurveillance pathways that provide innate defense against bacterial pathogens. We demonstrate an unexpected role for a viral RNA-sensing pathway in the response to L. pneumophila, and identify a secreted bacterial effector, SdhA, that can suppress this response. Our results therefore open new possibilities for immunosurveillance of bacterial pathogens.
Materials and Methods
Ethics statement
Animal experiments were approved by the University of California, Berkeley, Institutional Animal Care and Use Committee.
Mice, cell lines and plasmids
Bone marrow derived macrophages were derived from the following mouse strains: C57BL/6J (B6), Ips-1 −/− [25], Mda5 −/− [56], Ifnar −/− [57], Zbp1 −/− [32], MyD88/Trif −/−, and Casp1 −/− [58]. C57BL/6J mice were purchased from the Jackson Laboratory. Ips-1 −/− mice were from Z. Chen (University of Texas Southwestern Medical Center). Ips-1 −/− were obtained on a mixed B6/129 background and Ips-1 −/− and Ips-1 +/− littermate controls were generated by breeding (Ips-1 −/− x B6) F1 mice to Ips-1 −/−. Mda5 −/− mice were from M. Colonna and S. Gilfillan (Washington University). L929-ISRE IFN reporter cells were from B. Beutler (The Scripps Research Institute). Viruses to immortalize MyD88 −/− Trif −/− immortalized bone marrow derived macrophages were the generous gift of K. Fitzgerald, D. Golenbock (U. Mass, Worcester) and D. Kalvakolanu (U. Maryland). The complementation plasmid (pJB908-SdhA) was generously provided by R. Isberg (Tufts). Expression constructs pEF-BOS-RIG-I and pEF-BOS-MDA5 were generously provided by J. Jung (Harvard Medical School).
Bacterial strains
LP02 is a streptomycin-resistant thymidine auxotroph derivative of Legionella pneumophila strain LP01. LP02ΔsdhA and LP02ΔsdhAΔsdhBΔsidH were a generous gift from R. Isberg (Tufts University). The ΔflaAΔsdhA strain was generated by introducing an unmarked deletion of flaA in LP02ΔsdhA using the allelic exchange vector pSR47S-ΔflaA [12].
Cell culture
L929-ISRE and HEK293T cells were cultured in DMEM supplemented with 10% FBS, 2 mM L-glutamine, 100 µM streptomycin, and 100 U/mL penicillin. Macrophages were derived from bone marrow cells cultured for eight days in RPMI supplemented with 10% FBS, 2 mM L-glutamine, 100 µM streptomycin, 100 U/mL penicillin, and 10% supernatant from 3T3-CSF cells, with feeding on the fifth day of growth. MyD88 −/− Trif −/− immortalized macrophages were cultured in RPMI supplemented with 10% FBS, 2 mM L-glutamine, 100 µM streptomycin, and 100 U/mL penicillin.
Reagents
Poly I:C was from GE Biosciences, pA:T (poly(dA-dT):poly(dA-dT)) was from Sigma, and Sendai Virus was from Charles River Laboratories. Wildtype Theiler's Virus GDVII was from M. Brahic and E. Freundt (Stanford University). Pol III inhibitor (ML-60218) was from Calbiochem.
Isolation of nucleic acids from L. pneumophila
Total bacterial RNA was isolated using RNAprotect Bacterial Reagent (Qiagen) and RNeasy kit (Qiagen). Genomic DNA was isolated by guanidinium thiocyanate followed by phenol:chloroform extraction. Nucleic acids were treated with RQ1 RNase-Free DNase (Promega) and/or RNaseA (Sigma).
Quantitative RT-PCR
Bone marrow derived macrophages were plated at a density of 2×106 per well in 6 well plates and infected with an MOI of 1. Macrophage RNA was harvested 4 hours post infection and isolated with the RNeasy kit (Qiagen) according to the manufacturer's protocol. RNA was DNase treated with RQ1 RNase-Free DNase (Promega) and reverse transcribed with Superscript III (Invitrogen). Quantitative PCR assays were performed on the Step One Plus RT PCR System (Applied Biosystems) with Platinum Taq DNA polymerase (Invitrogen) and EvaGreen dye (Biotium). Gene expression values were normalized to Rps17 (mouse) or S9 (human) levels for each sample. The following primer sequences were used: mouse Ifnb, F, 5′-ATAAGCAGCTCCAGCTCCAA-3′and R, 5′-CTGTCTGCTGGTGGAGTTCA-3′; mouse Rps17, F, 5′-CGCCATTATCCCCAGCAAG-3′ and R, 5′- TGTCGGGATCCACCTCAATG-3′; mouse Rig-i, F, 5′-ATTGTCGGCGTCCACAAAG-3′ and R, 5′-GTGCATCGTTGTATTTCCGCA-3′, human Ifnb, F, 5′-AAACTCATGAGCAGTCTGCA-3′ and R, 5′- AGGAGATCTTCAGTTTCGGAG G-3′; human S9, F, 5′-ATCCGCCAGCGCCATA-3′ and R, 5′-TCAATGTGCTTCTGGGAATCC-3′.
Cell stimulation and transfection
Cell stimulants were transfected with Lipofectamine 2000 (LF2000, Invitrogen) according to the manufacturer's protocol. Nucleic acids were mixed with LF2000 in Optimem (Invitrogen) at a ratio of 1.0 µl LF2000/µg nucleic acid and incubated for 20 minutes at room temperature. The ligand-lipid complexes were added to cells at a final concentration of 3.3 µg/ml (96-well plates) and 1.0 µg/ml (6 well plates). For poly I:C, the stock solution (2.5 mg/ml) was heated at 55°C for 10 minutes and cooled to room temperature immediately before mixing with LF2000. Transfection experiments were incubated for 8 hours, unless otherwise stated. RIG-I, MDA5, TRIF and SdhA expression plasmids, along with an IFNβ-firefly luciferase reporter and TK-Renilla luciferase plasmids, were transfected with FuGENE 6 (Roche) according to the manufacturer's protocol. Nucleic acids were mixed with FuGENE 6 in Optimem at 0.5 µl/96 well and incubated for 15 minutes. Total transfected DNA was normalized to 200 ng per well using an empty pcDNA3 plasmid. Cells were stimulated 20 hours after transfection of expression plasmids.
Type I IFN bioassay and luciferase reporter assay
Cell culture supernatants or bronchoalveolar lavage fluid (BALF) was overlayed on L929-ISRE IFN reporter cells in a 96-well plate format and incubated for 4 hours at 37°C and 5%CO2. L929-ISRE IFN reporter cells and HEK293T cells expressing an IFNβ-firefly luciferase reporter and TK-Renilla luciferase were lysed in Passive Lysis Buffer (Promega) for 5 minutes at room temperature and relative light units were measured upon injection of firefly luciferin substrate (Biosynth) or Renilla substrate with the LmaxII384 luminometer (Molecular Devices). For transient transfection reporter assays, luciferase values were normalized to an internal Renilla control.
Cytotoxicity assays
Cytotoxicity of bacterial strains was determined by measuring lactate dehydrogenase release essentially as previously described [59]. Macrophages were plated at a density of 1×105 in a 96-well plate and infected with stationary phase L. pneumophila at a multiplicity of infection (MOI) of 1. Plates were spun at 400×g for 10 minutes to allow equivalent infectivity of non-motile and motile strains [12]. Plates were re-spun 4 hours post infection and cell culture supernatants were assayed for LDH activity. Specific lysis was calculated as a percentage of detergent lysed cells.
Growth curves
Bacterial growth was determined as previously described [16]. Bone marrow derived macrophages were plated at a density of 1×105 per well in white 96-well plates (Nunc) and allowed to adhere overnight. Macrophages were infected with stationary-phase L. pneumophila at a multiplicity of infection (MOI) of 0.01. Growth of luminescent L. pneumophila strains was assessed by RLU with the LmaxII384 luminometer (Molecular Devices). Nonluminescent bacterial strains were analyzed for colony-forming units on buffered charcoal yeast extract plates.
Transposon mutagenesis
Transposon mutagenesis of LP02 was previously described [12]. Briefly, the pSC123 mariner transposon was mated from E.coli SM10 λpir into the L. pneumophila strain LP02. Matings were plated on buffered yeast extract charcoal plates with streptomycin (100 µg/ml) and kanamycin (25 µg/ml). Single colonies were isolated and grown in overnight cultures and used to infect bone marrow derived MyD88 −/− Trif −/− macrophages. After overnight incubation, levels of type I interferon in the supernatant was determined by bioassay. The site of transposon insertion was determined by Y-linker PCR [60].
In vivo studies
Age and sex-matched Ips-1 −/− and littermate Ips-1 +/− mice were infected intranasally with 2.5×106 LP01 ΔflaA in 20 µl PBS. Bronchoalveolar lavage was performed 20 hours post infection via the trachea using a catheter (BD Angiocath 18 g, 1.3×48 mm) and 800 µl PBS. Type I interferon induction was determined by bioassay. Type I interferon amounts were calculated using a 4-parameter standard curve determined by dilution of recombinant IFNβ (R&D Systems). CFUs were determined by hypotonic lysis of cells from the brochoalveolar lavage fluid (BALF). In parallel experiments, it was determined that CFU in the BALF was representative of total CFU in the lung.
shRNA knockdown
Knockdown constructs were generated with the MSCV/LTRmiR30-PIG (LMP) vector from Open Biosystems. shRNA PCR products were cloned into the LMP vector using XhoI and EcoRI sites. Rig-i sequence: 5′-GCCCATTGAAACCAAGAAATT-3′, control shRNA sequence: 5′-TGACAGTGTCTTCGCTAATGAA-3′. MyD88 −/− Trif −/− immortalized bone marrow derived macrophages were transduced with retrovirus as previously described [10]. GFP+ macrophages were sorted with the DAKO-Cytomation MoFlo High Speed Sorter.
Supporting Information
L. pneumophila replication is restricted in Ips-1 −/− and Mda-5 −/− macrophages. Ips-1 +/−, Ips-1 −/−, C57BL/6 (B6) and Mda5 −/− macrophages were infected at an MOI of 0.01 and growth of luminescent L. pneumophila strains was determined by RLU at 0, 24, 48, and 72 hours post infection. (A) Ips-1 +/− and Ips-1 −/− macrophages were infected WT (LP02) L. pneumophila (B) C57BL/6 (B6) and Mda5 −/− macrophages were infected as in A (C) Ips-1 +/− and Ips-1 −/− macrophages were infected with Δdot L. pneumophila (D) C57BL/6 (B6) and Mda5 −/− macrophages were infected as in C (E) Ips-1 +/− and Ips-1 −/− infected with ΔflaA L. pneumophila (F) C57BL/6 (B6) and Mda5 −/− macrophages were infected as in E.
(0.31 MB PDF)
Abrogation of type I interferon receptor signaling alone does not permit growth of ΔsdhA mutant. C57BL/6 (B6) and Ifnar −/− macrophages were infected at an MOI of 0.01 and growth of luminescent L. pneumophila strains was determined by RLU at 0, 24, 48, and 72 hours post infection. (A) C57BL/6 (B6) and Ifnar −/− macrophages were infected WT (LP02) L. pneumophila (B) macrophages were infected as in A but with ΔsdhA L. pneumophila (C) ΔflaA L. pneumophila (D) ΔflaAΔsdhA L. pneumophila (E) Δdot L. pneumophila.
(0.29 MB PDF)
SdhA represses MDA5 and RIG-I induction of interferon. Overexpression of SdhA in HEK293T cells results in repression of interferon induction mediated by MDA5 or RIG-I but not TRIF. (A) HEK293T cells were transfected with plasmids encoding the IFNβ-firefly luciferase reporter, TK-Renilla luciferase reporter (for normalization), full length MDA5 and/or increasing amounts of full length SdhA. At 20 hours post transfection, cells were transfected with poly I:C and then firefly luciferase and Renilla luciferase levels were determined 8 hours later. (B) Transfection and stimulation were performed as in A, except with a RIG-I expression plasmid and/or increasing amounts of full length SdhA expression plasmid. (C) Transfection and stimulation were performed as in A, except with a Trif expression plasmid and/or SdhA.
(0.27 MB PDF)
Acknowledgments
We would like to thank G. Barton, J. Coers and members of the Vance and Barton labs for discussions, G. Lam, K. Sotelo-Troha, and L. Tieu for technical assistance, R. Isberg for sdhA mutants and advice, D. Rookhuizen for help with the mutagenesis screen, K. Fitzgerald, D. Golenbock and D. Kalvakolanu for virus to immortalize macrophages, K. Ishii and S. Akira for Zpb1 −/− bone marrow, M. Colonna and S. Gilfillan for Mda5 −/− mice, D. Portnoy for Ifnar −/− mice, and Z. Chen for Ips-1 −/− mice.
Footnotes
The authors have declared that no competing interests exist.
R.E.V. is supported by a Cancer Research Institute Investigator Award, the Hellman Family Faculty Fund, and NIH grants AI075039 and AI080749. K.M.M is a National Science Foundation Graduate Research Fellow. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Fields BS, Benson RF, Besser RE. Legionella and Legionnaires' disease: 25 years of investigation. Clin Microbiol Rev. 2002;15:506–526. doi: 10.1128/CMR.15.3.506-526.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Horwitz MA, Silverstein SC. Legionnaires' disease bacterium (Legionella pneumophila) multiples intracellularly in human monocytes. J Clin Invest. 1980;66:441–450. doi: 10.1172/JCI109874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Isberg RR, O'Connor TJ, Heidtman M. The Legionella pneumophila replication vacuole: making a cosy niche inside host cells. Nat Rev Microbiol. 2009;7:13–24. doi: 10.1038/nrmicro1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Laguna RK, Creasey EA, Li Z, Valtz N, Isberg RR. A Legionella pneumophila-translocated substrate that is required for growth within macrophages and protection from host cell death. Proc Natl Acad Sci U S A. 2006;103:18745–18750. doi: 10.1073/pnas.0609012103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Archer KA, Roy CR. MyD88-dependent responses involving toll-like receptor 2 are important for protection and clearance of Legionella pneumophila in a mouse model of Legionnaires' disease. Infect Immun. 2006;74:3325–3333. doi: 10.1128/IAI.02049-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hawn TR, Berrington WR, Smith IA, Uematsu S, Akira S, et al. Altered inflammatory responses in TLR5-deficient mice infected with Legionella pneumophila. J Immunol. 2007;179:6981–6987. doi: 10.4049/jimmunol.179.10.6981. [DOI] [PubMed] [Google Scholar]
- 7.Hawn TR, Smith KD, Aderem A, Skerrett SJ. Myeloid differentiation primary response gene (88)- and toll-like receptor 2-deficient mice are susceptible to infection with aerosolized Legionella pneumophila. J Infect Dis. 2006;193:1693–1702. doi: 10.1086/504525. [DOI] [PubMed] [Google Scholar]
- 8.Shin S, Case CL, Archer KA, Nogueira CV, Kobayashi KS, et al. Type IV secretion-dependent activation of host MAP kinases induces an increased proinflammatory cytokine response to Legionella pneumophila. PLoS Pathog. 2008;4:e1000220. doi: 10.1371/journal.ppat.1000220. doi: 10.1371/journal.ppat.1000220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Amer A, Franchi L, Kanneganti TD, Body-Malapel M, Ozoren N, et al. Regulation of Legionella phagosome maturation and infection through flagellin and host IPAF. J Biol Chem. 2006;281:35217–35223. doi: 10.1074/jbc.M604933200. [DOI] [PubMed] [Google Scholar]
- 10.Lightfield KL, Persson J, Brubaker SW, Witte CE, von Moltke J, et al. Critical function for Naip5 in inflammasome activation by a conserved carboxy-terminal domain of flagellin. Nat Immunol. 2008;9:1171–1178. doi: 10.1038/ni.1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Molofsky AB, Byrne BG, Whitfield NN, Madigan CA, Fuse ET, et al. Cytosolic recognition of flagellin by mouse macrophages restricts Legionella pneumophila infection. J Exp Med. 2006;203:1093–1104. doi: 10.1084/jem.20051659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ren T, Zamboni DS, Roy CR, Dietrich WF, Vance RE. Flagellin-deficient Legionella mutants evade caspase-1- and Naip5-mediated macrophage immunity. PLoS Pathog. 2006;2:e18. doi: 10.1371/journal.ppat.0020018. doi: 10.1371/journal.ppat.0020018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zamboni DS, Kobayashi KS, Kohlsdorf T, Ogura Y, Long EM, et al. The Birc1e cytosolic pattern-recognition receptor contributes to the detection and control of Legionella pneumophila infection. Nat Immunol. 2006;7:318–325. doi: 10.1038/ni1305. [DOI] [PubMed] [Google Scholar]
- 14.Opitz B, Vinzing M, van Laak V, Schmeck B, Heine G, et al. Legionella pneumophila induced IFNbeta in lung epithelial cells via IPS-1 and IRF3 which also control bacterial replication. J Biol Chem. 2006;281:36173–36179. doi: 10.1074/jbc.M604638200. [DOI] [PubMed] [Google Scholar]
- 15.Stetson DB, Medzhitov R. Recognition of cytosolic DNA activates an IRF3-dependent innate immune response. Immunity. 2006;24:93–103. doi: 10.1016/j.immuni.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 16.Coers J, Vance RE, Fontana MF, Dietrich WF. Restriction of Legionella pneumophila growth in macrophages requires the concerted action of cytokine and Naip5/Ipaf signalling pathways. Cell Microbiol. 2007;9:2344–2357. doi: 10.1111/j.1462-5822.2007.00963.x. [DOI] [PubMed] [Google Scholar]
- 17.Schiavoni G, Mauri C, Carlei D, Belardelli F, Pastoris MC, et al. Type I IFN protects permissive macrophages from Legionella pneumophila infection through an IFN-gamma-independent pathway. J Immunol. 2004;173:1266–1275. doi: 10.4049/jimmunol.173.2.1266. [DOI] [PubMed] [Google Scholar]
- 18.Stetson DB, Medzhitov R. Type I interferons in host defense. Immunity. 2006;25:373–381. doi: 10.1016/j.immuni.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 19.Pichlmair A, Reis e Sousa C. Innate recognition of viruses. Immunity. 2007;27:370–383. doi: 10.1016/j.immuni.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 20.Yoneyama M, Kikuchi M, Natsukawa T, Shinobu N, Imaizumi T, et al. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat Immunol. 2004;5:730–737. doi: 10.1038/ni1087. [DOI] [PubMed] [Google Scholar]
- 21.Seth RB, Sun L, Ea CK, Chen ZJ. Identification and characterization of MAVS, a mitochondrial antiviral signaling protein that activates NF-kappaB and IRF 3. Cell. 2005;122:669–682. doi: 10.1016/j.cell.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 22.Kawai T, Takahashi K, Sato S, Coban C, Kumar H, et al. IPS-1, an adaptor triggering RIG-I- and Mda5-mediated type I interferon induction. Nat Immunol. 2005;6:981–988. doi: 10.1038/ni1243. [DOI] [PubMed] [Google Scholar]
- 23.Xu LG, Wang YY, Han KJ, Li LY, Zhai Z, et al. VISA is an adapter protein required for virus-triggered IFN-beta signaling. Mol Cell. 2005;19:727–740. doi: 10.1016/j.molcel.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 24.Meylan E, Curran J, Hofmann K, Moradpour D, Binder M, et al. Cardif is an adaptor protein in the RIG-I antiviral pathway and is targeted by hepatitis C virus. Nature. 2005;437:1167–1172. doi: 10.1038/nature04193. [DOI] [PubMed] [Google Scholar]
- 25.Sun Q, Sun L, Liu HH, Chen X, Seth RB, et al. The specific and essential role of MAVS in antiviral innate immune responses. Immunity. 2006;24:633–642. doi: 10.1016/j.immuni.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 26.Ishii KJ, Coban C, Kato H, Takahashi K, Torii Y, et al. A Toll-like receptor-independent antiviral response induced by double-stranded B-form DNA. Nat Immunol. 2006;7:40–48. doi: 10.1038/ni1282. [DOI] [PubMed] [Google Scholar]
- 27.Cheng G, Zhong J, Chung J, Chisari FV. Double-stranded DNA and double-stranded RNA induce a common antiviral signaling pathway in human cells. Proc Natl Acad Sci U S A. 2007;104:9035–9040. doi: 10.1073/pnas.0703285104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ablasser A, Bauernfeind F, Hartmann G, Latz E, Fitzgerald KA, et al. RIG-I-dependent sensing of poly(dA:dT) through the induction of an RNA polymerase III-transcribed RNA intermediate. Nat Immunol. 2009. [DOI] [PMC free article] [PubMed]
- 29.Chiu YH, Macmillan JB, Chen ZJ. RNA Polymerase III Detects Cytosolic DNA and Induces Type I Interferons through the RIG-I Pathway. Cell. 2009;138:576–591. doi: 10.1016/j.cell.2009.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Takaoka A, Wang Z, Choi MK, Yanai H, Negishi H, et al. DAI (DLM-1/ZBP1) is a cytosolic DNA sensor and an activator of innate immune response. Nature. 2007;448:501–505. doi: 10.1038/nature06013. [DOI] [PubMed] [Google Scholar]
- 31.Wang Z, Choi MK, Ban T, Yanai H, Negishi H, et al. Regulation of innate immune responses by DAI (DLM-1/ZBP1) and other DNA-sensing molecules. Proc Natl Acad Sci U S A. 2008;105:5477–5482. doi: 10.1073/pnas.0801295105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ishii KJ, Kawagoe T, Koyama S, Matsui K, Kumar H, et al. TANK-binding kinase-1 delineates innate and adaptive immune responses to DNA vaccines. Nature. 2008;451:725–729. doi: 10.1038/nature06537. [DOI] [PubMed] [Google Scholar]
- 33.Karaolis DK, Means TK, Yang D, Takahashi M, Yoshimura T, et al. Bacterial c-di-GMP is an immunostimulatory molecule. J Immunol. 2007;178:2171–2181. doi: 10.4049/jimmunol.178.4.2171. [DOI] [PubMed] [Google Scholar]
- 34.McWhirter SM, Barbalat R, Monroe KM, Fontana MF, Hyodo M, et al. A host type I interferon response is induced by cytosolic sensing of the bacterial second messenger cyclic-di-GMP. J Exp Med. 2009;206:1899–1911. doi: 10.1084/jem.20082874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roberts ZJ, Goutagny N, Perera PY, Kato H, Kumar H, et al. The chemotherapeutic agent DMXAA potently and specifically activates the TBK1-IRF-3 signaling axis. J Exp Med. 2007;204:1559–1569. doi: 10.1084/jem.20061845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mancuso G, Midiri A, Biondo C, Beninati C, Zummo S, et al. Type I IFN signaling is crucial for host resistance against different species of pathogenic bacteria. J Immunol. 2007;178:3126–3133. doi: 10.4049/jimmunol.178.5.3126. [DOI] [PubMed] [Google Scholar]
- 37.Leber JH, Crimmins GT, Raghavan S, Meyer-Morse N, Cox JS, et al. Distinct TLR- and NLR-mediated transcriptional responses to an intracellular pathogen. PLoS Pathog. 2008;4:e6. doi: 10.1371/journal.ppat.0040006. doi: 10.1371/journal.ppat.0040006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.O'Riordan M, Yi CH, Gonzales R, Lee KD, Portnoy DA. Innate recognition of bacteria by a macrophage cytosolic surveillance pathway. Proc Natl Acad Sci U S A. 2002;99:13861–13866. doi: 10.1073/pnas.202476699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Auerbuch V, Brockstedt DG, Meyer-Morse N, O'Riordan M, Portnoy DA. Mice lacking the type I interferon receptor are resistant to Listeria monocytogenes. J Exp Med. 2004;200:527–533. doi: 10.1084/jem.20040976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carrero JA, Calderon B, Unanue ER. Type I interferon sensitizes lymphocytes to apoptosis and reduces resistance to Listeria infection. J Exp Med. 2004;200:535–540. doi: 10.1084/jem.20040769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.O'Connell RM, Saha SK, Vaidya SA, Bruhn KW, Miranda GA, et al. Type I interferon production enhances susceptibility to Listeria monocytogenes infection. J Exp Med. 2004;200:437–445. doi: 10.1084/jem.20040712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Charrel-Dennis M, Latz E, Halmen KA, Trieu-Cuot P, Fitzgerald KA, et al. TLR-independent type I interferon induction in response to an extracellular bacterial pathogen via intracellular recognition of its DNA. Cell Host Microbe. 2008;4:543–554. doi: 10.1016/j.chom.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Henry T, Brotcke A, Weiss DS, Thompson LJ, Monack DM. Type I interferon signaling is required for activation of the inflammasome during Francisella infection. J Exp Med. 2007;204:987–994. doi: 10.1084/jem.20062665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Roux CM, Rolan HG, Santos RL, Beremand PD, Thomas TL, et al. Brucella requires a functional Type IV secretion system to elicit innate immune responses in mice. Cell Microbiol. 2007;9:1851–1869. doi: 10.1111/j.1462-5822.2007.00922.x. [DOI] [PubMed] [Google Scholar]
- 45.Stanley SA, Johndrow JE, Manzanillo P, Cox JS. The Type I IFN response to infection with Mycobacterium tuberculosis requires ESX-1-mediated secretion and contributes to pathogenesis. J Immunol. 2007;178:3143–3152. doi: 10.4049/jimmunol.178.5.3143. [DOI] [PubMed] [Google Scholar]
- 46.Vogel JP, Andrews HL, Wong SK, Isberg RR. Conjugative transfer by the virulence system of Legionella pneumophila. Science. 1998;279:873–876. doi: 10.1126/science.279.5352.873. [DOI] [PubMed] [Google Scholar]
- 47.Fredericksen BL, Keller BC, Fornek J, Katze MG, Gale M., Jr Establishment and maintenance of the innate antiviral response to West Nile Virus involves both RIG-I and MDA5 signaling through IPS-1. J Virol. 2008;82:609–616. doi: 10.1128/JVI.01305-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jiang Z, Georgel P, Du X, Shamel L, Sovath S, et al. CD14 is required for MyD88-independent LPS signaling. Nat Immunol. 2005;6:565–570. doi: 10.1038/ni1207. [DOI] [PubMed] [Google Scholar]
- 49.Wu L, Pan J, Thoroddsen V, Wysong DR, Blackman RK, et al. Novel small-molecule inhibitors of RNA polymerase III. Eukaryot Cell. 2003;2:256–264. doi: 10.1128/EC.2.2.256-264.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mancuso G, Gambuzza M, Midiri A, Biondo C, Papasergi S, et al. Bacterial recognition by TLR7 in the lysosomes of conventional dendritic cells. Nat Immunol. 2009;10:587–594. doi: 10.1038/ni.1733. [DOI] [PubMed] [Google Scholar]
- 51.Lippmann J, Rothenburg S, Deigendesch N, Eitel J, Meixenberger K, et al. IFNbeta responses induced by intracellular bacteria or cytosolic DNA in different human cells do not require ZBP1 (DLM-1/DAI). Cell Microbiol. 2008;10:2579–2588. doi: 10.1111/j.1462-5822.2008.01232.x. [DOI] [PubMed] [Google Scholar]
- 52.Rad R, Ballhorn W, Voland P, Eisenacher K, Mages J, et al. Extracellular and intracellular pattern recognition receptors cooperate in the recognition of Helicobacter pylori. Gastroenterology. 2009;136:2247–2257. doi: 10.1053/j.gastro.2009.02.066. [DOI] [PubMed] [Google Scholar]
- 53.Ensminger AW, Isberg RR. Legionella pneumophila Dot/Icm translocated substrates: a sum of parts. Curr Opin Microbiol. 2009;12:67–73. doi: 10.1016/j.mib.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Malathi K, Dong B, Gale M, Jr, Silverman RH. Small self-RNA generated by RNase L amplifies antiviral innate immunity. Nature. 2007;448:816–819. doi: 10.1038/nature06042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Crimmins GT, Herskovits AA, Rehder K, Sivick KE, Lauer P, et al. Listeria monocytogenes multidrug resistance transporters activate a cytosolic surveillance pathway of innate immunity. Proc Natl Acad Sci U S A. 2008;105:10191–10196. doi: 10.1073/pnas.0804170105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gitlin L, Barchet W, Gilfillan S, Cella M, Beutler B, et al. Essential role of mda-5 in type I IFN responses to polyriboinosinic:polyribocytidylic acid and encephalomyocarditis picornavirus. Proc Natl Acad Sci U S A. 2006;103:8459–8464. doi: 10.1073/pnas.0603082103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Muller U, Steinhoff U, Reis LF, Hemmi S, Pavlovic J, et al. Functional role of type I and type II interferons in antiviral defense. Science. 1994;264:1918–1921. doi: 10.1126/science.8009221. [DOI] [PubMed] [Google Scholar]
- 58.Li P, Allen H, Banerjee S, Franklin S, Herzog L, et al. Mice deficient in IL-1 beta-converting enzyme are defective in production of mature IL-1 beta and resistant to endotoxic shock. Cell. 1995;80:401–411. doi: 10.1016/0092-8674(95)90490-5. [DOI] [PubMed] [Google Scholar]
- 59.Decker T, Lohmann-Matthes ML. A quick and simple method for the quantitation of lactate dehydrogenase release in measurements of cellular cytotoxicity and tumor necrosis factor (TNF) activity. J Immunol Methods. 1988;115:61–69. doi: 10.1016/0022-1759(88)90310-9. [DOI] [PubMed] [Google Scholar]
- 60.Kwon YM, Ricke SC. Efficient amplification of multiple transposon-flanking sequences. J Microbiol Methods. 2000;41:195–199. doi: 10.1016/s0167-7012(00)00159-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
L. pneumophila replication is restricted in Ips-1 −/− and Mda-5 −/− macrophages. Ips-1 +/−, Ips-1 −/−, C57BL/6 (B6) and Mda5 −/− macrophages were infected at an MOI of 0.01 and growth of luminescent L. pneumophila strains was determined by RLU at 0, 24, 48, and 72 hours post infection. (A) Ips-1 +/− and Ips-1 −/− macrophages were infected WT (LP02) L. pneumophila (B) C57BL/6 (B6) and Mda5 −/− macrophages were infected as in A (C) Ips-1 +/− and Ips-1 −/− macrophages were infected with Δdot L. pneumophila (D) C57BL/6 (B6) and Mda5 −/− macrophages were infected as in C (E) Ips-1 +/− and Ips-1 −/− infected with ΔflaA L. pneumophila (F) C57BL/6 (B6) and Mda5 −/− macrophages were infected as in E.
(0.31 MB PDF)
Abrogation of type I interferon receptor signaling alone does not permit growth of ΔsdhA mutant. C57BL/6 (B6) and Ifnar −/− macrophages were infected at an MOI of 0.01 and growth of luminescent L. pneumophila strains was determined by RLU at 0, 24, 48, and 72 hours post infection. (A) C57BL/6 (B6) and Ifnar −/− macrophages were infected WT (LP02) L. pneumophila (B) macrophages were infected as in A but with ΔsdhA L. pneumophila (C) ΔflaA L. pneumophila (D) ΔflaAΔsdhA L. pneumophila (E) Δdot L. pneumophila.
(0.29 MB PDF)
SdhA represses MDA5 and RIG-I induction of interferon. Overexpression of SdhA in HEK293T cells results in repression of interferon induction mediated by MDA5 or RIG-I but not TRIF. (A) HEK293T cells were transfected with plasmids encoding the IFNβ-firefly luciferase reporter, TK-Renilla luciferase reporter (for normalization), full length MDA5 and/or increasing amounts of full length SdhA. At 20 hours post transfection, cells were transfected with poly I:C and then firefly luciferase and Renilla luciferase levels were determined 8 hours later. (B) Transfection and stimulation were performed as in A, except with a RIG-I expression plasmid and/or increasing amounts of full length SdhA expression plasmid. (C) Transfection and stimulation were performed as in A, except with a Trif expression plasmid and/or SdhA.
(0.27 MB PDF)