Abstract
Members of the Wnt family are secreted glycoproteins that trigger cellular signals essential for proper development of organisms. Cellular signaling induced by Wnt proteins is involved in diverse developmental processes and human diseases. Previous studies have generated an enormous wealth of knowledge on the events in signal-receiving cells. However, relatively little is known about the making of Wnt in signal-producing cells. Here, we describe that Gpr177, the mouse orthologue of Drosophila Wls, is expressed during formation of embryonic axes. Embryos with deficient Gpr177 exhibit defects in establishment of the body axis, a phenotype highly reminiscent to the loss of Wnt3. Although many different mammalian Wnt proteins are required for a wide range of developmental processes, the Wnt3 ablation exhibits the earliest developmental abnormality. This suggests that the Gpr177-mediated Wnt production cannot be substituted. As a direct target of Wnt, Gpr177 is activated by β-catenin and LEF/TCF-dependent transcription. This activation alters the cellular distributions of Gpr177 which binds to Wnt proteins and assists their sorting and secretion in a feedback regulatory mechanism. Our findings demonstrate that the loss of Gpr177 affects Wnt production in the signal-producing cells, leading to alterations of Wnt signaling in the signal-receiving cells. A reciprocal regulation of Wnt and Gpr177 is essential for the patterning of the anterior-posterior axis during mammalian development.
Keywords: A-P axis, β-catenin, developmental deformities, primitive streak, Wnt production
Members of the Wnt family are secreted glycoproteins which trigger cellular signals essential for proper development of organisms (1, 2). Aberrant regulation of an evolutionary conserved Wnt signal transduction pathway has been implicated in a variety of cancers and congenital diseases (3, 4). There is no question that Wnt signaling is intimately involved in human health and disease. Genetic analysis in mice has revealed the essential role of different Wnt proteins in a wide range of developmental processes (http://www.stanford.edu/∼rnusse/wntwindow.html). Wnt3 deficiency appears to cause the earliest abnormality during embryogenesis, suggesting the importance of Wnt signaling in axis determination (5). Three body axes develop sequentially to generate embryo orientations (6–8). At the egg cylinder stage, the dorsal-ventral axis is the first to form. The anterior-posterior (A-P) axis is established to form the primitive streak at the posterior end before gastrulation. Lastly, the left-right asymmetry is formed, followed by embryo turning. Wnt signaling is critical for initiation of the embryonic axes in early development (9–11). Disruptions of key molecules necessary for Wnt signaling regulation lead to defects in axial patterning (12–15).
Studies in the past have generated an enormous wealth of knowledge on the events in signal-receiving cells. Before initiating their effects on the signal-receiving cells, Wnt proteins undergo proper modification, sorting and secretion processes in the signal-producing cells (16–19). Recent identification of Wntless (Wls/Evi/Srt) (20–22), a regulator for Wnt production in Drosophila, has directed attention to the maturation, sorting and secretion processes in signal-producing cells. Given the extensive Wnt family in higher organisms, it is not clear how many Wls genes are present and whether Wls is essential for Wnt production. This study describes Gpr177, the mouse orthologue of Drosophila Wls, required for embryogenesis. Disruption of Gpr177 disturbs axial patterning, a phenotype resembling the loss of Wnt3. This disruption not only affects Wnt production, but also interferes with Wnt signaling. As a Wnt transcriptional target, Gpr177 is elevated to promote Wnt production in a positive feedback loop. Our results indicate that a reciprocal regulation of Wnt and Gpr177 is essential for the establishment of the mammalian A-P axis.
Results
Gpr177 Is Essential for Mouse Embryogenesis.
We investigated how many Wls exist, and whether Wls regulates the Wnt pathway essential for mammalian development. By protein sequence analysis (NCBI HomoloGene) we found that Gpr177, which shows high percentages of identity with Wls of human (96.1%), Drosophila (44.9%), and C. elegans (40.9%), is likely the mouse orthologue. No additional gene product shared significant homology with fly and human counterparts. We then performed whole mount RNA in situ hybridization to examine Gpr177 expression in early embryogenesis. Gpr177 was expressed in the proximal epiblast at the junction between the embryonic and extraembryonic tissue, and later was more restricted to the primitive streak and mesoderm extending to the distal tip of the embryo (Fig. 1 A–D). Its strong presence was found in both posterior visceral endoderm and epiblast at the prestreak, but switched to the mesoderm at late-streak (Fig. 1 E–H). The expression pattern of Gpr177 is reminiscent of Wnt3, which is required for axial patterning (5, 23).
Fig. 1.
Disruption of Gpr177 impairs embryogenesis in mice. (A–H) In situ hybridization in whole mounts (A–D) and sections (E–H) reveals the expression of Gpr177 in E6.25 (A, E, and F) and E7 (B), and E7.25 (C, D, G, and H) embryos. The approximate positions of E–H are shown by the dotted line in A and C. Gross morphological analysis of Gpr177+/+ (I and K) and Gpr177-/- (J and L) embryos at E7.5 (I and J) and E8.5 (K and L). Sections of the Gpr177+/+ (M, N, P–R, and T) and Gpr177-/- (O, S, and U), E7.5 (M–S), and E8.5 (T and U) embryos were analyzed by histology (M–O, T, and U) and immunostaining of Gpr177 (P–S). Arrowheads and arrows indicate the anterior and posterior mesoderm, respectively. AVE, anterior visceral endoderm; Ch, chorion; Ect, ectoderm; Epc, ectoplacental cone; NE, neural ectoderm; PS, primitive streak; VE, visceral endoderm; XEct, extraembryonic ectoderm. [Scale bars, 300 μm (A–D, I, and J); 100 μm (E–H and P–S); 500 μm (K and L); and 200 μm (M–O, T, and U).]
To determine whether Gpr177 regulates Wnt production and this regulation is essential for mouse development, we created a mutant strain Gpr177lacZ, carrying an insertion of β-geo into the ninth intron of Gpr177 (Fig. S1 A and B). The transgene insertion disrupted the seven transmembrane domain which results in generation of a fusion transcript (Fig. S1C). PCR analyses further confirmed that the Gpr177 locus was altered by transgene-mediated mutagenesis (Fig. S1D). The mutant lacking the carboxyl-terminal region of Gpr177 disabled its function as the truncation interrupts its subcellular distribution and protein interaction (see below). Mice heterozygous for Gpr177lacZ appeared normal and were fertile. We were not able to recover Gpr177 homozygous (Gpr177-/-) newborns or embryos after E10.5, suggesting that they died during early embryogenesis. From E6.5 to E8.5, Gpr177+/+ and +/- embryos consisted of three germ layers and underwent gastrulation to form organized structures, including extraembryonic ectoderm, chorion, allantois, head folds, and primitive streak (Fig. 1 I, K, M, N, and T). However, Gpr177-/- embryos did not exhibit distinct structures but remained to grow as egg cylinders (Fig. 1 J and O). The mutants consisted of two layers of tissue, ectoderm and visceral endoderm. In addition, the mesoderm and primitive streak were missing and ectoderm was composed of a thick layer of cells. Later, the ectoderm and visceral endoderm continue to grow and the excess ectoderm becomes irregular and folded (Fig. 1 L and U). To examine the Gpr177 protein, we generated antibodies recognizing its carboxyl terminus, which was deleted in the mutants. Immunostaining detected a strong presence of Gpr177 in the control mesoderm at E7.5 (Fig. 1 P–R). However, the Gpr177-positive mesoderm was absent in the mutants (Fig. 1S).
Disruption of Gpr177 Impairs Development of the Body Axis.
We next analyzed Gpr177 mutants for the expression of specific markers during gastrulation (Fig. 2). BMP4 is expressed in the extraembryonic ectoderm where its signals induce the proximal epiblast to acquire posterior cell fates and restrict the formation of distal visceral endoderm (DVE), which are precursors of anterior visceral endoderm (AVE) to the distal end (7, 24). The expression of BMP4 apparently is not affected by the mutation, implying proper positioning of extraembryonic ectoderm during the proximal-distal (P-D) axis formation (Fig. 2 A and B; n = 5). To examine the formation of AVE, we examined the expression of Cer1 (25), an AVE marker in early to mid-streak stage embryos and later in the definitive endoderm emanating from the node (Fig. 2C). In Gpr177-/- embryos, we detected Cer1 in the visceral endoderm although its expansion into the definitive endoderm was absent, suggesting that AVE was established during initial regional patterning (Fig. 2D; n = 3). However, the subsequent development of the anterior ectoderm did not occur. Otx2 is expressed ubiquitously in all germ layers at the early streak-stage, but maintained only in the anterior region at the mid-streak stage (Fig. 2E). We detected Otx2 throughout the entire ectoderm of Gpr177 mutants (Fig. 2F; n = 4). In addition, the expression of Hesx1 was not affected in the AVE (Fig. 2 G and H; n = 3), but was altered in the forebrain of Gpr177-/- embryos. The uniform presence of Otx2 reflected an undifferentiated ectoderm rather than expansion of anterior neural cell fates. These data indicate that the posterior development of the embryo might also be defective. Gsc was expressed in the node adjacent to the anterior region of primitive streak and the newly formed axial mesoderm (Fig. 2I). At the late-streak stage, the Gsc-expressing progenitors of the notochord, identified in the node located at the anterior end of the primitive streak, possess the capability necessary (26) and sufficient to induce the neural axis (27, 28). However, Gsc expression was abolished in the mutant, suggesting the lack of gastrulation organizer activity in the Gpr177 mutant (Fig. 2J; n = 3). Furthermore, mesoderm specification requires T (Brachyury), which is expressed in the primitive streak and axial mesoderm (Fig. 2K). Its expression in the most anterior streak region is dependent on Wnt signaling (29). At E7.5, we did not detect its presence in the mutant, confirming the lack of primitive streak and mesoderm formation (Fig. 2L; n = 3).
Fig. 2.
Gpr177 is required for Wnt production and signaling in patterning of A-P axis. (A–N and P–T) Molecular marker analysis of control (+/+ and +/-) and Gpr177 mutant (-/-) littermates at E6.5 (A and B) and E7.0–7.5 (C–N and P–T) determines the role of Gpr177 in early embryogenesis using in situ hybridization of BMP4 (A and B), Cer1 (C and D), Otx2 (E and F), Hesx1 (G and H), Gsc (I and J), Brachyury (T) (K and L), Wnt3 (M and N), and Axin2 (P and Q), and GFP analysis of Axin2 (R–T). The control embryos are shown with the anterior facing to the left. (O) Gpr177 is essential for Wnt production and signaling. Immunoblot analysis of E6.5 and E7.5 embryos shows the level of Gpr177, Wnt3/3a, and β-catenin proteins affected by the Gpr177 mutation. Actin level is used as a loading control. The number represents the relative protein level of Wnt3/3a and β-catenin between Gpr177+/+, Gpr177+/-, and Gpr177-/-. (R–T) Axin2GFP mouse strain, expressing GFP in the Axin2-expressing cells, was used to examine the activation of Wnt/β-catenin signaling in the Gpr177+/+ (R and S) and Gpr177-/- (T) embryos during gastrulation. AVE, anterior visceral endoderm; DE, definitive endoderm; PS, primitive streak. [Scale bars, 200 μm (A and B) and 300 μm (C–N and P–T).]
Gpr177 Deficiency Alters Wnt Production and Signaling.
The above phenotypes are highly reminiscent of the ablation of Wnt3, which is required for establishment of the primitive streak (5). We therefore examined the expression of Wnt3 whose transcripts were found in the primitive streak, proximal epiblast, and visceral endoderm, at the junction between the embryonic and extraembryonic ectoderm with higher levels localized to the posterior region of both Gpr177+/+ (Fig. 2M) and -/- embryos (Fig. 2N; n = 5). Although the expression pattern of Wnt3 transcript does not seem to be altered, its protein production and signaling effect might be affected by Gpr177 deficiency. We therefore determined whether Gpr177 is essential for Wnt3 production and signaling in early embryogenesis. Previous reports indicated that β-catenin signaling is critical for the establishment of the A-P axis (13, 30, 31). Immunoblot analysis of E6.5 and E7.5 embryos showed that cellular levels of β-catenin were drastically reduced by the mutation (Fig. 2O). Using an antibody recognizing Wnt3/3a, the protein level was unaffected at E6.5 (Wnt3 only) but accumulated at E7.5 (Wnt3 and Wnt3a). Note that the mutants were fairly normal at the prestreak where Wnt3a is not present. Because of technical limitation, we could not examine the expression pattern of Wnt3/3a proteins in the developing embryo. Nonetheless, the results suggest that Wnt expression was not affected in the embryos. However, β-catenin was not activated likely due to a secretion defect. Furthermore, Axin2 is regulated by β-catenin and LEF/TCF-dependent transcription, and has been widely used as a downstream target of Wnt (32, 33). Its expression in the posterior region of the E7.5 embryo (Fig. 2P) appeared to be missing in the mutant (Fig. 2Q; n = 2). Using the Axin2GFP mouse strain to label the Axin2-expressing cells, we detected GFP signals in the Gpr177+/+ but not Gpr177-/- embryos during gastrulation, indicating that the canonical Wnt pathway is affected by the mutation (Fig. 2 R–T; n = 2). Our findings suggest that Gpr177 acts downstream of Wnt3 and regulates its signaling in early patterning of the A-P axis. In addition to Wnt production, Gpr177 is required for Wnt signaling during embryonic axis formation.
Subcellular Distribution of Gpr177.
To determine the role of Gpr177 in production of Wnt, we examined its localization within the cell. First, immunostaining showed that endogenous Gpr177 is mainly localized to cellular vesicles in the majority of cell types (n = 10) examined with an exception of neural stem cells (Fig. 3 A–D). In neural epithelial progenitors (NEP) and neurosphere cells, isolated from E12.5 neural tube and forebrain, respectively, Gpr177 was highly concentrated in the perinuclear area resembling Golgi in addition to the vesicles (Fig. 3 A and B). Consistent with a previous study of Wls in Drosophila (34), the endogenous Gpr177 was co-localized with a Golgi marker GM130 (Fig. 3 E–I) in cut view analysis. Using a combined 3-D imaging and co-localization analysis, Gpr177 was also co-localized with GM130 on Golgi (Fig. 3 J–L). In serial sectioned views of the 3-D images (Fig. 3 M–O), the co-localization signal (white color) appeared to be on the Golgi apparatus between the Gpr177-positive vesicles (green color) and Golgi (red color). The budding of the Gpr177 containing vesicles seems to occur on the surface of Golgi and continue their intracellular trafficking routes.
Fig. 3.
Wnt regulates cellular distribution of Gpr177. (A–D) Three-dimensional images of immunostained cells reveal differential localizations of endogenous Gpr177 in NEP (A), Neurosphere cells (B), MSC (C), and C57MG (D). Cut view (E–I) and 3-D imaging (J–O) analyses show that Gpr177 co-localizes with a Golgi marker GM130 using superimposed imaging (H and L) or pseudocoloring of the co-localization signal in white (I and M–O). NEP cells were immunostained with Gpr177 (E and J) and GM130 (F and K), and counterstained with DAPI (G–O). Three serial sections along the front-view (Co Level 1, 2, and 3) reveal the co-localization signal on the surface of Golgi (M–O). (P–T) High levels of Gpr177 lead to its accumulations in Golgi. Three-dimensional imaging of the endogenous Gpr177 in the vesicles of C3H10T1/2 cells (P). Expression of a myc-tag full length Gpr177 (MT-Gpr177) shows alteration in subcellular distribution of Gpr177 (Q), co-localizing with GM130 (R–T). Co-immunostaining of a MT-Gpr177 mutant lacking the carboxyl terminal region (U) and Calnexin (V) reveals their co-localization (W).
To determine whether the differential localization of Gpr177 is attributed to neural stem cell specificity, we expressed a myc-tagged Gpr177 (MT-Gpr177) protein in a variety of cell types (n = 6). In all six cell types examined, including C3H10T1/2, MC3T3, C57MG, 293T, mouse embryonic fibroblasts, and mouse mesenchymal stem cells, MT-Gpr177 displayed localization to Golgi in addition to cellular vesicles (Fig. 3 P–T). Furthermore, a MT-Gpr177 mutant, lacking the carboxyl-terminal region similar to the Gpr177-lacZ fusion, exhibited a dislocated distribution pattern. Co-localization of an ER marker Calnexin with this mutant suggests that it fails to enter the secretory pathway (Fig. 3 U–W). The results suggest that the Golgi accumulation of Gpr177 is not due to cell-type specificity. The expression level of Gpr177 might dictate its cellular distribution. It is conceivable that neural stem cells, expressing high levels of Gpr177, are the only Wnt-secreting cell type we have examined. Cells with Gpr177 elevation are likely to be the signal-secreting cells because of its role in assisting the production of Wnt proteins.
Gpr177 Is a Transcriptional Target of Wnt/β-catenin Signaling.
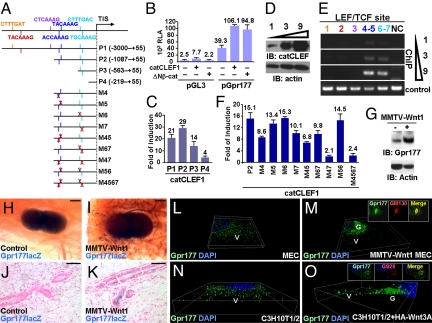
To test whether expression of Wnt might alter the cellular level and distribution of Gpr177, we studied the transcriptional regulation of Gpr177. We isolated and characterized the Gpr177 regulatory region which contains 7 potential LEF/TCF binding sites to determine whether the Gpr177 transcription is regulated by Wnt (Fig. 4A). The results indicated that Gpr177 transcription is stimulated by the dominant-activated mutant, ΔNβ-cat or catCLEF1 (Fig. 4B). Deletion analysis of the Gpr177 regulatory region further showed that four potential LEF/TCF-binding sites (nos. 4–7) might be responsible for the transcriptional stimulation (Fig. 4C). Chromatin immunoprecipitation (ChIP) analysis demonstrated that the expressed catCLEF1 bound to these four LEF/TCF consensus sites in cells (Fig. 4 D and E).
Fig. 4.
Gpr177 is regulated by the canonical Wnt pathway. (A) Graphs illustrate the luciferase reporter constructs for Gpr177 promoter with the wild-type or mutation (cross) of LEF/TCF binding consensus sequences. (B) Expression of dominant activated catCLEF1 or Δβ-cat protein stimulates the transcription activity of a 3-kb Gpr177 promoter (P1) in 293T cells. Relative luciferase activity (RLA) determined the transcriptional activation of the Gpr177 promoter-luciferase construct. The analysis of pGL3, a parent vector, shows background activity. (C) Fold of induction shows the effect of catCLEF1 on transcriptional activation of the deletion mutants. (D) 293T cells were transfected by increasing amounts of DNA plasmid (1, 3, and 9 μg) to express catCLEF1, analyzed by immunoblot (IB). (E) ChIP analysis reveals high affinity LEF/TCF binding sites (nos. 4–5 and 6–7) in the Gpr177 promoter. The order number of these sites is color coded with those shown in A. NC is a negative control, which analyzes the regulatory region of Gpr177 without LEF/TCF binding sequence. The controls are direct PCR analyses of LEF/TCF binding sites without ChIP. (F) Analysis of the promoter constructs containing point mutations further reveals the consensus sites required for β-catenin and LEF/TCF-dependent transcription. (G) IB analysis indicates the Gpr177 level elevated in the primary MEC cells by the MMTV-Wnt1 transgene. The expression level of Actin was analyzed as a loading control. (H–K) β-gal staining of the virgin 2-month mammary glands in whole mounts (H and I) and sections (J and K) reveals the Wnt-dependent activation of Gpr177 in the mammary glands. The reporter expression from the Gpr177-lacZ knock-in allele was detected in the MMTV-Wnt1 transgenics (I and K) but not the controls (H and J). Three-dimensional imaging of the immunostained control (L) and MMTV-Wnt1 transgenic (M) MEC reveals distinct localization patterns of endogenous Gpr177. The endogenous Gpr177 distribution in C3H10T1/2 cells (N) is also altered by high levels of HA-Wnt3A (O). The insets show co-immunostaining of Gpr177 with Golgi markers, GM130 (M) and GS28 (O). Immunostained cells were counterstained by DAPI (blue). G, Golgi; V, vesicle. [Scale bars, 500 μm (H and I) and 50 μm (J and K).]
Next, we determined which of these four sites is most critical for the activation of Gpr177 by the β-catenin and LEF/TCF-dependent transcription. Because the sites 4 and 5, as well as 6 and 7, are closely linked to each other, a point mutation strategy (Fig. 4A) was used to disrupt each of these four sites (M4, M5, M6, and M7). Disruption of number 4 or 7 site, but not 5 or 6, significantly diminished the transcriptional stimulation (Fig. 4F). A combinatorial mutation of number 4 and 5 (M45) or number 6 and 7 (M67) caused a reduction similar to the effect of M4 or M7 (Fig. 4F). However, the loss of both number 4 and 7 sites (M47), but not number 5 and 6 sites (M56) drastically abolished the β-catenin and LEF/TCF-dependent transcription (Fig. 4F). The transcriptional activity of M47 is about the same as M4567 where all four sites are disrupted (Fig. 4F). The results strongly suggest that Gpr177 is a direct Wnt downstream target whose expression is regulated by the β-catenin and LEF/TCF-dependent transcription.
Wnt Expression Alters the Cellular Level and Distribution of Gpr177.
We examined whether the Gpr177 expression is stimulated by Wnt that might provide a feedback mechanism to regulate its production and signaling. Indeed, immunoblot analysis showed that the steady state level of the endogenous Gpr177 increased in the primary mammary epithelial cells (MEC) of MMTV-Wnt1 compared to the controls (Fig. 4G). To test the stimulation of Gpr177 by Wnt in animals, we crossed the MMTV-Wnt1 transgene into mice carrying the Gpr177lacZ allele. This allele, which contains the β-geo reporter controlled by the Gpr177 locus, permits an examination of its endogenous expression activity. In virgin mammary glands heterozygous for the Gpr177lacZ allele, no β-gal staining could be detected (Fig. 4 H and J). In contrast, the MMTV-Wnt1 transgenic littermates showed strong β-gal stained signals, suggesting that the Gpr177 expression is stimulated (Fig. 4 I and K). Next, the cellular localization of Gpr177 affected by Wnt was analyzed to further our investigation on their interactions. We examined primary MEC cells isolated from MMTV-Wnt1 transgenic females and their control littermates. Compared to the controls, Gpr177 was located to Golgi in addition to cellular vesicles in the MMTV-Wnt1 cells (Fig. 4 L and M). Furthermore, transient expression of HA-Wnt3A in C3H10T1/2 cells also led to an accumulation of endogenous Gpr177 in the Golgi (Fig. 4 N and O). These data support the hypothesis that Wnt proteins modulate the cellular level and the expression of Gpr177. As a direct target of Wnt, Gpr177 might facilitate their maturation, sorting, and secretion processes in a feedback regulatory loop.
Interactions of Gpr177 and Wnt Proteins.
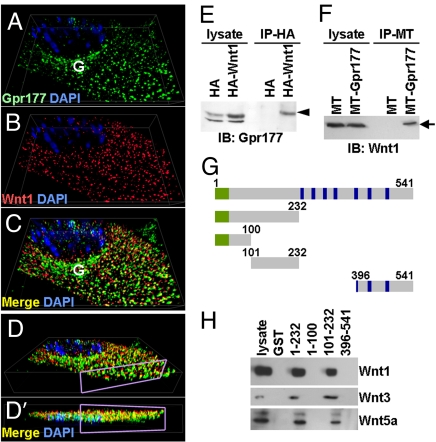
To investigate whether the Wnt-dependent elevation of Gpr177 assists the Wnt production through a feedback regulatory mechanism, we examined their subcellular localizations. Immunostaining showed that the elevated Gpr177, caused by expression of Wnt1 (Fig. 5 A–D), became co-localized together in the Golgi and vesicles. Co-immunoprecipitation further identified complexes containing Gpr177 and Wnt proteins in cells (Fig. 5 E and F). GST pull down assay indicated that Gpr177 associates with Wnt1, Wnt3, and Wnt5a proteins, which are highly expressed in neural stem cells (Fig. 5H). This association was not detectable in other cell types without high levels of Wnt expression. Analysis of the GST-Gpr177 deletion mutants (Fig. 5G) further revealed that a short N-terminal domain (amino acids 101–232) is required for Wnt proteins to associate with Gpr177 (Fig. 5H). Thus, the Wnt-mediated elevation of Gpr177 interacts with Wnt proteins in a feedback loop. Although it remains possible that Gpr177 does not bind directly to Wnt, they appear to regulate each other in a reciprocal manner.
Fig. 5.
Wnt proteins bind to and co-localized with Gpr177. Three-dimensional imaging analysis of immunostained C57MG cells expressing MT-Wnt1 reveals co-localization (C) of endogenous Gpr177 (A) with MT-Wnt1 (B) in both Golgi and vesicles. D and D′ display different angle views of C where the sectioned level is shown by purple rectangle. (E and F) IP-IB analysis identifies protein complexes containing Gpr177 and Wnt in cells transfected by HA-Wnt1 (E) or MT-Gpr177 (F). (G) A scheme of GST-Gpr177 fragments. Numbers indicate positions of amino acids. Endoplasmic reticulum signal sequence and transmembrane domains are highlighted in green and blue, respectively. (H) GST pull-down assay analyzes the association of Gpr177 with the Wnt1, Wnt3, or Wnt5a protein complex.
Discussion
This study demonstrated an essential role of Gpr177, the mouse orthologue of Drosophila Wls, in establishment of the A-P axis. Genetic analysis in mice has revealed the requirement of many different Wnt proteins in a wide range of developmental processes. The disruption of Wnt3 seems to exhibit the earliest developmental abnormality. The Gpr177 mutation causes defects in the primitive streak and mesoderm formation, highly resembling the loss of Wnt3 (5). This suggests an indispensible role of Gpr177 in Wnt sorting and secretion. The Gpr177-mediated Wnt production is essential for mammalian development.
Golgi accumulations of endogenous Gpr177 occur in neural stem cells. This is in agreement with previous analyses of exogenous Wls, over-expressed in transfected cells (20, 35–37). The Golgi accumulation is not due to the cell-type specificity, but rather the expression level within the cells. Indeed, high levels of Wnt proteins are found in the neural stem cells where their association with Gpr177 can be detected. The results imply that the cellular localization of Gpr177 might serve as an indicator for Wnt-producing cells. Indeed, cells expressing Wnt exhibit elevated levels of Gpr177, leading to Golgi accumulation. Wnt expression might modulate the distribution of Gpr177 that dictates the trafficking routes in signal-producing cells. This feedback regulatory mechanism ensures proper sorting pathways to be activated for the secretion of Wnt proteins (34–38). In contrast, the main role of Gpr177 expressed at low levels in non Wnt-producing cells could help in generating a morphogen gradient for long range effects through endocytosis and exocytosis. Further analysis on the trafficking routes of Gpr177-containing vesicles will elucidate the mechanism underlying the process of Wnt maturation, sorting and secretion.
We hypothesize that a reciprocal interaction between Wnt and Gpr177 plays a key role in the regulation of their expression, subcellular distribution, binding, and organelle-specific association. The mechanisms underlying the reciprocal regulation in the Wnt-producing cells are necessary for the patterning of the A-P axis. The requirement of Wnt signaling in development of various organs suggests that Gpr177 might be required for these developmental processes (1, 2). Indeed, we have found that Gpr177 is expressed in different tissues, including kidney, lung, skin, intestine, brain, spinal cord, skeleton, eyes, excretion glands, ear, tooth, and palatal shelve. As Wnt signaling is intimately involved in a variety of cancers and congenital diseases in humans (3, 4), Gpr177 might also be essential for these processes. Creation of mouse strains permitting genetic inactivation of Gpr177 in a spatiotemporal-specific fashion promises important insights into the reciprocal regulation of Wnt and Gpr177 in development and disease. As there are 19 different members of Wnt in mammals, it is not known whether Gpr177 is required for all of their productions. Targeted disruption of Gpr177 in cells expressing a specific Wnt is likely to gain knowledge on the generality of its function.
Methods
Details for experimental materials and analyses are described in the SI Methods. In brief, the Gpr177 mutant strain was generated using an ES clone (Bay Genomics). For embryo genotyping, yolk sacs or embryonic materials recovered from paraffin sections were used in PCR analysis. Axin2GFP strain permits inducible expression of GFP in the Axin2-expressing cells (39, 40). Care and use of experimental animals described in this work comply with guidelines and policies of the University Committee on Animal Resources at the University of Rochester. Isolation and culture of primary neurospheres, NEP, MEC, calvarial MSC, and other cell lines are described in the SI Methods (41–44). RNA probes (5, 25) were generated to analyze the gene expression pattern by in situ hybridization (45). Histology, β-gal staining, immunostaining, immunoblot, protein precipitation, chromatin immunoprecipitation, and various DNA vectors used are described in the SI Methods (39, 40, 42, 45–47).
Supplementary Material
Acknowledgments.
We thank Richard Behringer, Edward De Robertis, and Brigid Hogan for reagents, C.-S. Victor Lin and Chris Proschel for technical assistance, and the reviewers for comments and suggestions. This work was supported by National Institutes of Health Grants DE15654 and CA106308 (to W.H.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0904894106/DCSupplemental.
References
- 1.Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Ann Rev Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- 2.Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127:469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 3.van Amerongen R, Berns A. Knockout mouse models to study Wnt signal transduction. Trends Genet. 2006;22:678–689. doi: 10.1016/j.tig.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 4.Grigoryan T, Wend P, Klaus A, Birchmeier W. Deciphering the function of canonical Wnt signals in development and disease: Conditional loss- and gain-of-function mutations of beta-catenin in mice. Genes Dev. 2008;22:2308–2341. doi: 10.1101/gad.1686208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu P, Wakamiya M, Shea MJ, Albrecht U, Behringer RR, Bradley A. Requirement for Wnt3 in vertebrate axis formation. Nat Gen. 1999;22:361–365. doi: 10.1038/11932. [DOI] [PubMed] [Google Scholar]
- 6.Beddington RS, Robertson EJ. Axis development and early asymmetry in mammals. Cell. 1999;96:195–209. doi: 10.1016/s0092-8674(00)80560-7. [DOI] [PubMed] [Google Scholar]
- 7.Tam PP, Loebel DA, Tanaka SS. Building the mouse gastrula: Signals, asymmetry and lineages. Curr Opin Genet Dev. 2006;16:419–425. doi: 10.1016/j.gde.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 8.Takaoka K, Yamamoto M, Hamada H. Origin of body axes in the mouse embryo. Curr Opin Genet Dev. 2007;17:344–350. doi: 10.1016/j.gde.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 9.Hamatani T, Carter MG, Sharov AA, Ko MS. Dynamics of global gene expression changes during mouse preimplantation development. Dev Cell. 2004;6:117–131. doi: 10.1016/s1534-5807(03)00373-3. [DOI] [PubMed] [Google Scholar]
- 10.Wang QT, et al. A genome-wide study of gene activity reveals developmental signaling pathways in the preimplantation mouse embryo. Dev Cell. 2004;6:133–144. doi: 10.1016/s1534-5807(03)00404-0. [DOI] [PubMed] [Google Scholar]
- 11.Rossant J, Tam PP. Emerging asymmetry and embryonic patterning in early mouse development. Dev Cell. 2004;7:155–164. doi: 10.1016/j.devcel.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 12.Zeng L, et al. The mouse Fused locus encodes Axin, an inhibitor of the Wnt signaling pathway that regulates embryonic axis formation. Cell. 1997;90:181–192. doi: 10.1016/s0092-8674(00)80324-4. [DOI] [PubMed] [Google Scholar]
- 13.Chazaud C, Rossant J. Disruption of early proximodistal patterning and AVE formation in Apc mutants. Development. 2006;133:3379–3387. doi: 10.1242/dev.02523. [DOI] [PubMed] [Google Scholar]
- 14.Morkel M, et al. Beta-catenin regulates Cripto- and Wnt3-dependent gene expression programs in mouse axis and mesoderm formation. Development. 2003;130:6283–6294. doi: 10.1242/dev.00859. [DOI] [PubMed] [Google Scholar]
- 15.Kemler R, et al. Stabilization of beta-catenin in the mouse zygote leads to premature epithelial-mesenchymal transition in the epiblast. Development. 2004;131:5817–5824. doi: 10.1242/dev.01458. [DOI] [PubMed] [Google Scholar]
- 16.Coudreuse D, Korswagen HC. The making of Wnt: New insights into Wnt maturation, sorting and secretion. Development. 2007;134:3–12. doi: 10.1242/dev.02699. [DOI] [PubMed] [Google Scholar]
- 17.Hausmann G, Banziger C, Basler K. Helping Wingless take flight: How WNT proteins are secreted. Nat Rev. 2007;8:331–336. doi: 10.1038/nrm2141. [DOI] [PubMed] [Google Scholar]
- 18.Takada R, et al. Monounsaturated fatty acid modification of Wnt protein: Its role in Wnt secretion. Dev Cell. 2006;11:791–801. doi: 10.1016/j.devcel.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 19.Willert K, et al. Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature. 2003;423:448–452. doi: 10.1038/nature01611. [DOI] [PubMed] [Google Scholar]
- 20.Banziger C, Soldini D, Schutt C, Zipperlen P, Hausmann G, Basler K. Wntless, a conserved membrane protein dedicated to the secretion of Wnt proteins from signaling cells. Cell. 2006;125:509–522. doi: 10.1016/j.cell.2006.02.049. [DOI] [PubMed] [Google Scholar]
- 21.Bartscherer K, Pelte N, Ingelfinger D, Boutros M. Secretion of Wnt ligands requires Evi, a conserved transmembrane protein. Cell. 2006;125:523–533. doi: 10.1016/j.cell.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 22.Goodman RM, et al. Sprinter: A novel transmembrane protein required for Wg secretion and signaling. Development. 2006;133:4901–4911. doi: 10.1242/dev.02674. [DOI] [PubMed] [Google Scholar]
- 23.Rivera-Perez JA, Magnuson T. Primitive streak formation in mice is preceded by localized activation of Brachyury and Wnt3. Dev Biol. 2005;288:363–371. doi: 10.1016/j.ydbio.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 24.Lawson KA, et al. Bmp4 is required for the generation of primordial germ cells in the mouse embryo. Genes Dev. 1999;13:424–436. doi: 10.1101/gad.13.4.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Belo JA, et al. Cerberus-like is a secreted factor with neutralizing activity expressed in the anterior primitive endoderm of the mouse gastrula. Mech Dev. 1997;68:45–57. doi: 10.1016/s0925-4773(97)00125-1. [DOI] [PubMed] [Google Scholar]
- 26.Belo JA, Leyns L, Yamada G, De Robertis EM. The prechordal midline of the chondrocranium is defective in Goosecoid-1 mouse mutants. Mech Dev. 1998;72:15–25. doi: 10.1016/s0925-4773(97)00204-9. [DOI] [PubMed] [Google Scholar]
- 27.Sulik K, et al. Morphogenesis of the murine node and notochordal plate. Dev Dyn. 1994;201:260–278. doi: 10.1002/aja.1002010309. [DOI] [PubMed] [Google Scholar]
- 28.Beddington RS. Induction of a second neural axis by the mouse node. Development. 1994;120:613–620. doi: 10.1242/dev.120.3.613. [DOI] [PubMed] [Google Scholar]
- 29.Yamaguchi TP, Takada S, Yoshikawa Y, Wu N, McMahon AP. T (Brachyury) is a direct target of Wnt3a during paraxial mesoderm specification. Genes Dev. 1999;13:3185–3190. doi: 10.1101/gad.13.24.3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kimura-Yoshida C, et al. Canonical Wnt signaling and its antagonist regulate anterior-posterior axis polarization by guiding cell migration in mouse visceral endoderm. Dev Cell. 2005;9:639–650. doi: 10.1016/j.devcel.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 31.Huelsken J, Vogel R, Brinkmann V, Erdmann B, Birchmeier C, Birchmeier W. Requirement for beta-catenin in anterior-posterior axis formation in mice. J Cell Biol. 2000;148:567–578. doi: 10.1083/jcb.148.3.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jho EH, Zhang T, Domon C, Joo CK, Freund JN, Costantini F. Wnt/beta-catenin/Tcf signaling induces the transcription of Axin2, a negative regulator of the signaling pathway. Mol Cell Biol. 2002;22:1172–1183. doi: 10.1128/MCB.22.4.1172-1183.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lustig B, et al. Negative feedback loop of Wnt signaling through upregulation of conductin/axin2 in colorectal and liver tumors. Mol Cell Biol. 2002;22:1184–1193. doi: 10.1128/MCB.22.4.1184-1193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Port F, et al. Wingless secretion promotes and requires retromer-dependent cycling of Wntless. Nat Cell Biol. 2008;10:178–185. doi: 10.1038/ncb1687. [DOI] [PubMed] [Google Scholar]
- 35.Franch-Marro X, et al. Wingless secretion requires endosome-to-Golgi retrieval of Wntless/Evi/Sprinter by the retromer complex. Nat Cell Biol. 2008;10:170–177. doi: 10.1038/ncb1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Belenkaya TY, et al. The retromer complex influences Wnt secretion by recycling wntless from endosomes to the trans-Golgi network. Developmental cell. 2008;14:120–131. doi: 10.1016/j.devcel.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 37.Yang PT, Lorenowicz MJ, Silhankova M, Coudreuse DY, Betist MC, Korswagen HC. Wnt signaling requires retromer-dependent recycling of MIG-14/Wntless in Wnt-producing cells. Dev Cell. 2008;14:140–147. doi: 10.1016/j.devcel.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 38.Pan CL, Baum PD, Gu M, Jorgensen EM, Clark SG, Garriga G. C. elegans AP-2 and retromer control Wnt signaling by regulating mig-14/Wntless. Dev Cell. 2008;14:132–139. doi: 10.1016/j.devcel.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yu HM, Liu B, Chiu SY, Costantini F, Hsu W. Development of a unique system for spatiotemporal and lineage-specific gene expression in mice. Proc Natl Acad Sci USA. 2005;102:8615–8620. doi: 10.1073/pnas.0500124102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yu HM, Liu B, Costantini F, Hsu W. Impaired neural development caused by inducible expression of Axin in transgenic mice. Mech Dev. 2007;124:146–156. doi: 10.1016/j.mod.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yu HM, et al. The role of Axin2 in calvarial morphogenesis and craniosynostosis. Development. 2005;132:1995–2005. doi: 10.1242/dev.01786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu B, Yu HM, Huang J, Hsu W. Co-opted JNK/SAPK signaling in Wnt/beta-catenin-induced tumorigenesis. Neoplasia. 2008;10:1004–1013. doi: 10.1593/neo.08548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grandbarbe L, et al. Delta-Notch signaling controls the generation of neurons/glia from neural stem cells in a stepwise process. Development. 2003;130:1391–1402. doi: 10.1242/dev.00374. [DOI] [PubMed] [Google Scholar]
- 44.Welm BE, Tepera SB, Venezia T, Graubert TA, Rosen JM, Goodell MA. Sca-1(pos) cells in the mouse mammary gland represent an enriched progenitor cell population. Dev Biol. 2002;245:42–56. doi: 10.1006/dbio.2002.0625. [DOI] [PubMed] [Google Scholar]
- 45.Chiu SY, Asai N, Costantini F, Hsu W. SUMO-specific protease 2 is essential for modulating p53-Mdm2 in development of trophoblast stem cell niches and lineages. PLoS Biol. 2008;6:e310. doi: 10.1371/journal.pbio.0060310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu B, Yu HM, Hsu W. Craniosynostosis caused by Axin2 deficiency is mediated through distinct functions of beta-catenin in proliferation and differentiation. Dev Biol. 2007;301:298–308. doi: 10.1016/j.physletb.2003.10.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hsu W, Mirando AJ, Yu HM. Manipulating gene activity in Wnt1-expressing precursors of neural epithelial and neural crest cells. Dev Dyn. 2009 Aug 3; doi: 10.1002/dvdy.22044. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.