Abstract
The major light-harvesting chlorophyll a/b complex (LHCII) of the photosynthetic apparatus in plants self-organizes in vitro. The recombinant apoprotein, denatured in dodecyl sulfate, spontaneously folds when it is mixed with its pigments, chlorophylls, and carotenoids in detergent solution, and assembles into structurally authentic LHCII in the course of several minutes. Pulse EPR techniques, specifically double-electron-electron resonance (DEER), have been used to analyze protein folding during this process. Pairs of nitroxide labels were introduced site-specifically into recombinant LHCII and shown not to affect the stability and function of the pigment-protein complex. Interspin distance distributions between two spin pairs were measured at various time points, one pair located on either end of the second transmembrane helix (helix 3), the other one located near the luminal ends of the intertwined transmembrane helices 1 and 4. In the dodecyl sulfate-solubilized apoprotein, both distance distributions were consistent with a random-coil protein structure. A rapid freeze-quench experiment on the latter spin pair indicated that 1 s after initiating reconstitution the protein structure is virtually unchanged. Subsequently, both distance distributions monitored protein folding in the same time range in which the assembly of chlorophylls into the complex had been observed. The positioning of the spin pair spanning the hydrophobic core of LHCII clearly preceded the juxtaposition of the spin pair on the luminal side of the complex. This indicates that superhelix formation of helices 1 and 4 is a late step in LHCII assembly.
Keywords: assembly kinetics, DEER, LHCII
The major light-harvesting complex LHCII largely increases the efficiency of the photosynthesis process by collecting light energy and conducting it to a photosynthetic reaction center where light-driven charge separation takes place. The apoprotein of LHCII is one of the most abundant membrane proteins on Earth, but even so, our knowledge is fragmentary of how the LHCII apoprotein folds and assembles with pigments. For studying these questions, LHCII offers several advantages. Its crystal structure is known (1), its apoprotein can be recombinantly expressed in Escherichia coli (2), and the protein spontaneously folds and assembles with pigments in detergent solution (2, 3). This spontaneous self-organization can be triggered by mixing the apoprotein solubilized in the ionic detergent dodecyl sulfate with a non-ionic detergent solution of the pigments. The assembly process can then easily be monitored by time-resolved fluorescence spectroscopy using the Chls as built-in fluorescence labels. Such experiments showed that protein folding is dependent on the binding of pigments, and that LHCII formation in vitro occurred in at least two apparent phases, a faster one in the range of some tens of seconds and a slower one taking several minutes (4, 5). The faster step could be assigned to the binding of mostly Chl a, whereas the slower one represents Chl b binding exclusively (6, 7). On the other hand, CD spectroscopy showed the formation of α-helical secondary structure during both kinetic phases (8).
The fluorescence and CD data mentioned above give information about pigment binding and about the overall secondary structure of the apoprotein during LHCII assembly but not about structural changes of individual protein domains. Such information can be gathered by various techniques of EPR in combination with site-directed spin labeling (SDSL). Double-electron-electron resonance (DEER) spectroscopy allows the measurement of distances between two spin-labeled residues in the range of 2–6 nm, in favorable cases even 8 nm (9, 10). This technique is especially useful for the prediction of protein structure (11, 12) as well as for measuring structure dynamics (13, 14). Electron spin echo envelope modulation (ESEEM) spectroscopy, also a pulse EPR technique, yields additional structural information by determining the water accessibility of singly spin-labeled protein domains (15). Continuous-wave (CW) spectroscopy is a widely used technique to monitor changes in the structure of both membrane proteins and water-soluble proteins (16–18) and additionally in time-resolved studies to analyze the process of protein folding (19).
DEER recently provided insight into the pattern of helix movement in rhodopsin due to light activation (13) and has been used to determine intra- and intermolecular distance distributions between individual protein domains in LHCII monomers and trimers, respectively (11). Additional information about the accessibility of single residues in various domains of LHCII monomers was obtained by comparison of conventional CW and pulsed EPR measurements (15).
In the present work, we used DEER to obtain information about LHCII assembly by monitoring the change of distances between two spin label pairs. Double-labeled mutant versions of lithium dodecyl sulfate (LDS)-denatured LHCII apoprotein were refolded using a modified reconstitution protocol, flash-frozen in liquid nitrogen after various folding times, and the intramolecular distances were measured. Based on the kinetic data, we compared the folding in vitro of LHCII with that of other membrane proteins.
Results
Functional and Structural Characterization of Spin-Labeled LHCII.
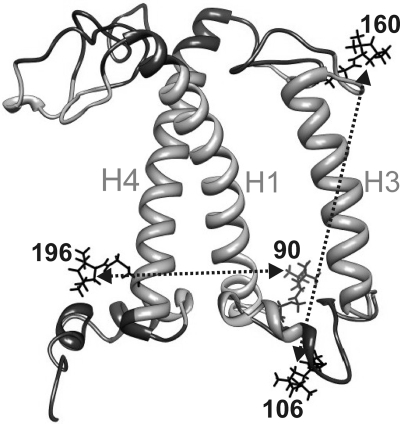
The folding state of Lhcb1 protein upon or during LHCII assembly was measured by monitoring two different intramolecular distances, one between the luminal ends of helices H1 and H4 (90/196, Fig. 1), the other one spanning the hydrophobic center of the complex between the stromal and luminal loops (106/160). The distances were defined by PROXYL spin labels attached to engineered Cys residues replacing two Val in mutant 90/196 and two Ser residues in mutant 106/160. The chosen sites were facing the peptide surface to avoid structural perturbation due to steric clashes but, except for 106, were located inside the detergent micelle (15).
Fig. 1.
Side view of the backbone structure of monomeric LHCII based on the crystal structure (PDB ID code 2BHW). H1, H3, and H4 indicate the three transmembrane α-helices [(1) nomenclature], numbers indicate the PROXYL-labeled amino acid residues, dotted traces show the intramolecular distances measured. Light-gray-colored protein domains reside inside the detergent micelle.
The labeled apoproteins were reconstituted with pigments according to the standard procedure, and the complexes were compared to those reconstituted with the non-labeled WT protein. Neither CD spectra in the visible range nor the intramolecular energy transfer from Chl b to Chl a (20) showed any difference between the two, demonstrating that spin labeling did not affect the structure or function of LHCII (SI Text).
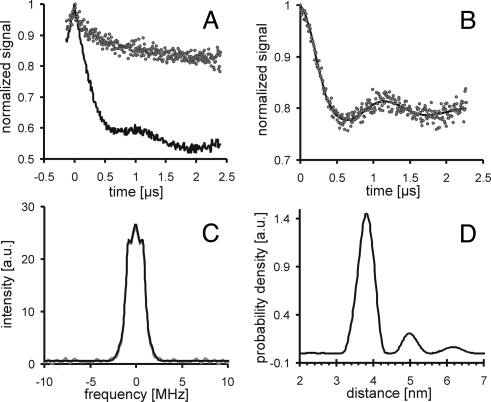
The spin-labeled isolated LHCII was used to measure distance distributions by pulse EPR as shown in Fig. 2. The original data shown in Fig. 2A (90/196, solid black trace) were obtained by a four- pulse DEER experiment monitoring the time evolution of the spin echo intensity (9). To be sure to measure intramolecular distances within the same molecule rather than intermolecular distances between two labeled monomers, a control experiment with a 1:1 mixture of singly spin-labeled mutant 90 and mutant 196 (dots in Fig. 2A) at the same total spin label concentration was performed. In contrast to the doubly spin-labeled mutant no appreciable DEER signal was detected resulting from dipole-dipole interaction between electron spins. Therefore, the background-corrected data for the mutant 90/196 in Fig. 2B originate from two electron spins within the same macromolecule. The corrected data were used to calculate via Fourier transformation the dipolar spectra shown in Fig. 2C and via Tikhonov regularization the distance distribution (Fig. 2D) between two labels within one protein molecule (10).
Fig. 2.
DEER data analysis for LHCII double mutant 90/196. Monomeric LHCII was purified by ultracentrifugation and checked by CD and fluorescence measurements. DEER spectra were scanned with a dipolar evolution time of 2,500 ns, the background correction parameter was 224 ns. (A) Original data (solid black trace) and control experiment with a mixture of singly spin-labeled mutants 90 and 196 (gray dots). (B) Data after background correction (gray dots) and best fit (solid black trace), (C) dipolar spectrum (gray trace) and best fit (black trace). (D) Distance distribution obtained with a regularization parameter α = 100.
Protein folding was monitored by measuring distance distributions between spin pairs at different time points. Labeling sites were preferred (i) where PROXYL labels would face the protein surface to avoid their interference with the pigment-protein structure, and (ii) in protein domains of restricted mobility to keep the expected distance distributions narrow and well reproducible. A number of double mutants carrying pairs of spin labels were screened by producing independent preparations of EPR samples for each mutant and testing their reproducibility by superimposing the DEER dipolar spectra for each mutant. Dipolar spectra for LHCII double mutants were generally well reproducible; only modulation depths and the shapes of broad distance distributions varied in some cases. As shown in Fig. S1, two independent dipolar data sets each of the monomeric 90/196 (Fig. S1A) and 106/160 (Fig. S1B) mutants matched nearly perfectly; therefore, these two mutants were chosen for protein folding kinetics.
Time-Dependent Changes of EPR Signals During LHCII Assembly.
In a stationary DEER experiment, both the LDS-denatured apoprotein and the isolated pigment-protein complex of mutants 90/196 and 106/160 were measured with a long evolution time of 2,500 ns to obtain distance distributions over the entire range of 1.5–6.5 nm.
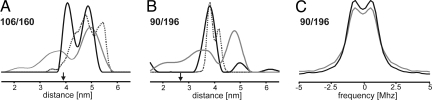
The denatured samples of both mutants (Fig. 3 A and B, gray trace), expected to adopt a random coiled conformation with some α-helical structure (8), yielded broad distance distributions covering the range of 1.5–6 nm. The individual peaks in these distributions may not be significant but rather result from noise-related distortions introduced during conversion of primary DEER data to distance distributions (21). The fully folded and pigmented proteins showed significant narrowing of their distance distributions and a change in the mean distance. For the span between the stromal and luminal loops in 106/160, the obtained broad peak at 4.1 nm is near the distance between the Cα atoms in the crystal structure of LHCII trimers (3.9 nm, indicated by the black arrow) of trimeric LHCII (1). In contrast, the second peak at 4.9 nm as well as the distances between the labels at the luminal ends of helices H1 and H4 (3.85 nm) were all larger than the distances measured between Cα atoms in the crystal structure (2.8 nm for mutant 90/196). As seen in the distance distributions determined from a rotamer library-based simulation of both mutants, this deviation disappears when the labeled side groups point away from one another and the distances are measured between midpoints of the N-O bonds at the side group termini.
Fig. 3.
Distance distributions of LDS-denatured apoprotein and upon LHCII assembly. Data were acquired with a maximum dipolar evolution time of 2,500 ns, the background correction time for 106/160 and 90/196 was 632 and 396 ns, respectively. (A and B) Changes in distance distribution for mutants 106/160 and 90/196, respectively. (C) The dipolar spectra for 90/196 obtained from data cut off at a dipolar evolution time of 1,400 ns and using exponential background correction as in analyzing folding kinetics (vide infra). Gray lines represent the LDS-denatured apoprotein and black lines isolated pigment-protein complexes. The dotted gray traces (A and B) show distance distributions determined from a rotamer library-based simulation. Black arrows (A and B) indicate the distances taken from the crystal structure of trimeric LHCII (1) between the Cα atoms in the labeling positions.
In kinetic EPR measurements of LHCII protein folding, the protein concentration was reduced by a factor of 7.5 compared to the steady-state measurements described above. Thus it was necessary to shorten the dipolar evolution times from 2,500 to 1,500 ns. This partially suppresses long distances due to imperfect background correction. Therefore, protein folding was monitored by focusing on the distance distribution at short distances, where both spin pairs show differences between the unfolded and folded states. In dipolar spectra this corresponds to the large dipolar frequencies, which can be reliably detected even at short evolution times. DEER measurements of the folded double mutants 106/160 and 90/196 measured with dipolar evolution times of 2,500 and 1,500 ns resulted in virtually identical dipolar spectra. Furthermore, unlike computation of distance distributions, the computation of dipolar spectra does not amplify noise-related distortions. Although continuous changes of the distance distribution during folding are also observable, quantification of kinetics from dipolar spectra is thus more reliable, and the remaining influence of noise can be precisely estimated.
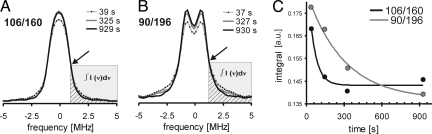
Therefore, the change in the EPR dipolar spectra was measured during assembly of LHCII. The folding process was triggered by manually mixing the protein and pigment solutions. The reaction was quenched by shock freezing after the desired reaction times in the range between 40 s and 15 min. As shown for both mutants in Fig. 4 A and B (106/160 and 90/196, respectively), the normalized dipolar spectra exhibit an increase of low-frequency contributions in the center and a decrease of high frequencies of approximately ± 1.5 to ± 5 MHz in the flanks. This corresponds to a narrowing of the distance distributions and an increase in the mean distance as seen before (Fig. 3).
Fig. 4.
Assembly of LHCII as monitored by the change in DEER dipolar spectra for different LHCII mutants (A 106/160 and B 90/196). Spectra were scanned with a dipolar evolution time of 1,500 ns. Processing parameters were a 404 ns starting time for background correction and 1,400 ns cut off time for mutant 106/160 and a 184 ns starting time for background correction and 1,400 ns cut off time for mutant 90/196. (A and B) DEER-dipolar spectra at three different folding times. The isosbestic points are marked by arrows, frequencies higher than that at the isosbestic point are shaded and the integrated areas hatched. (C) Time-dependent decrease of the high-frequency integral in dipolar spectra obtained at four different folding times for each mutant (106/160 and 90/196, black and gray line, respectively). Lines are exponential fits of the experimentally determined data points.
Upon superimposing the dipolar spectra obtained at different times, isosbestic points (black arrows in Fig. 4) became visible for both mutants at approximately 1 MHz. The appearance of isosbestic points confirms the expectation that Fourier analyses of the data are a robust approach and indicates that folding can be described as a two-state process. The integral intensity at frequencies higher than the one of the isosbestic point is a measure for conformations with short distances that exist predominantly in the unfolded ensemble and are depopulated during folding. Thus progress of the protein folding process was quantified by plotting the integral area (hatched area in Fig. 4 A and B) versus the folding time. Fig. 4C indicates fits of the kinetic data to be single-exponential. With four data points, this cannot be strictly proven. However, apparent first-order kinetics is consistent with a two-state process suggested by the occurrence of isosbestic points. Furthermore, single-exponential fits allow for a rough comparison of the time scales of structure generation between the two spin-labeled residues. For mutant 106/160 (Fig. 4C, black line) in which the distance was measured along the transmembrane helix H3, the fit yielded a time constant τH3 of 50–60 s. However, this time constant is only an upper limit since within experimental uncertainty the dipolar spectrum already corresponds to the folded state at the second time point of 146 s. The formation of the superhelical structure defined by the juxtaposition of the luminal ends of helices H1 and H4 (90/196) is slower with a time constant τH1/H4 of about 300 s (Fig. 4C, gray line). Both time constants were in the same range as those seen in time-resolved fluorescence measurements for pigment binding during LHCII assembly (Table S1) (7, 22).
To obtain information within the dead time of about 40 s of the manual mixing procedure, a sample of double mutant 90/196 with a folding time of 1 s was prepared with a rapid freeze-quench apparatus. Due to the unavoidable dead volume of the apparatus and losses in transferring the sample from the cold brass funnel into the EPR tube, such experiments require a much larger amount of protein and pigments. Furthermore, at the same concentration the protein density in the active volume of the EPR resonator is significantly lower due to voids in packing the small shock-frozen particles. Although the ESE spectrum indicated good sample quality, the distance measurements could only be performed with a dipolar evolution time of 800 ns. However, after compensating for the slightly different modulation depth within these 800 ns the primary DEER data superimpose almost perfectly with both the data for the unfolded double mutant 90/196 and the data measured after 37 s (Fig. S2). As this indicates no substantial structural changes of the unfolded protein due to the detergent replacement, no further material-intensive experiments were performed with the rapid freeze-quench setup.
Discussion
DEER as a Monitor in Membrane Protein-Folding Analysis.
In this work we used the techniques of SDSL and DEER distance mapping to study apoprotein folding during the assembly of LHCII. SDSL in combination with CW EPR had been used to measure the kinetics of structural changes in membrane and water-soluble proteins (16, 18, 19). Time-resolved studies on LHCII assembly had yielded information about the kinetics of pigment binding by measuring energy transfer between Chls (4), and about the kinetics of global secondary structure formation in the apoprotein by measuring CD spectra in the UV domain (8). By using pulsed EPR combined with SDSL in the present study, we were able to monitor individual LHCII protein domains during the folding process by assessing distances between these domains.
Two intramolecular distances were defined by the labeling positions in two double-labeled LHCII derivatives, one along the axis of the middle transmembrane helix H3 (106/160) and one between the luminal ends of the two intertwined helices H1/H4 (90/196). The labeled LHCII versions were carefully checked and found to exhibit the same stability as native LHCII.
Recombinant LHCII forms 2-D crystals very similar to those from native LHCII (23). DEER (11) and ESEEM (15) measurements of spin-labeled LHCII mutants indicated a similar structure for monomeric LHCII, solubilized in detergent micelles, permitting us to compare intramolecular distances measured by EPR with those predicted from the crystal structure. The distance distribution measured for the 106/160 label pair in isolated labeled LHCII was rather broad with peaks at 4.1 and 4.9 nm (Fig. 3). The distance between Cα atoms taken from the crystal structure (1) is 3.9 nm. PROXYL spin labels modeled into the crystal structure by using a rotamer library predicted a distance distribution between 3 and 5 nm, in agreement with the measured distribution. In the rotamer model the PROXYL label in position 106 has a substantial amount of motional freedom whereas the one in position 160 is partially restricted in its mobility by its close vicinity to Chl 11. This notion is supported by recent ESEEM measurements, indicating that the label in position 160 is buried in the micellar phase (15). The distance peaks at 4.1 and 4.9 nm are consistent with preferred label orientations parallel to or away from another, respectively. The DEER-measured distance distribution in the 90/196 derivative is remarkably narrow, peaking at 3.85 nm. This is in good agreement with the distance range between 3.6 and 4.2 nm predicted by a rotamer library approach. Both label positions are spatially restricted by neighboring protein side chains (position 90) or pigments (Chls 3, 4, and 12 in position 196), forcing the labels to point away from one another. This explains both the narrow distance distribution and its deviation from the distance between Cα atoms taken from the crystal structure (2.8 nm).
In LDS-denatured LHCII apoprotein, DEER-measured distance distributions were broad and quite different from those in the isolated pigment-protein complex (Fig. 3), making DEER an useful monitor for protein folding. The distance distribution for the 90/196 spin pair has a somewhat shorter mean distance compared to the one of the 106/160 pair, while the opposite may be expected in a random coil because of the larger separation of the 90/196 pair in the primary protein sequence. Most likely, the assumption of a random coil structure is an over-simplification since hydrophobic stretches of the protein presumably are sequestered in the hydrophobic interior of detergent micelles (24). Moreover, it is known that dodecyl sulfate-dissolved LHCII apoprotein exhibits about half of the α-helical structure seen in the fully folded protein (8). The same has been observed in other proteins such as bacteriorhodopsin, leading to the proposal that dodecyl sulfate stabilizes a folding intermediate of membrane proteins (25). It should be noted, however, that LHCII protein can also be refolded from a guanidinium hydrochloride (Gnd) solution in which no α-helical structure is seen; the latter therefore is no prerequisite for successfully folding this protein (26). In this work, we chose the dodecyl sulfate solution of the protein as a starting condition since folding yields are considerably higher compared to experiments starting from the Gnd-solubilized protein.
Events During the First 30 s of LHCII Apoprotein Folding.
Very short T2 (spin-spin) relaxation times were observed in mock reconstitution experiments that involved the same detergent exchange as during proper reconstitution in the absence of pigments. This effect could be traced back to protein aggregation, with high local spin concentrations of such aggregates being verified by instantaneous diffusion measurements and distortions in the line shape of the ESE spectrum (Fig. S3). However, these aggregates formed in the absence of pigments clearly constitute a kinetic trap, since subsequent addition of pigments never induced any detectable protein folding. For double mutant 90/196, a refolding experiment under standard conditions with rapid freeze quench after about 1 s led to an undistorted ESE spectrum and to a DEER decay function that matched both the decay function of LDS-denatured apoprotein and the one observed after 37 s of folding. This virtual coincidence of all three data sets is consistent with the folding time constant of 300 s observed for this mutant. The data indicate that mere detergent exchange, which is expected to be faster than 1 s, does not cause significant changes in the random coil ensemble of unfolded LHCII protein. In the presence of pigments, folding is induced and changes occur on the time scales observed with manual freeze quench. In the absence of pigments, unfolded apoprotein is not well-solubilized by the detergent mixture used for reconstitution and aggregates, probably on a time scale comparable to the folding time scale.
LHCII Protein Folds in Two Apparent Phases in the Range of Less than 1 Min and Several Minutes.
Following the dead time of manual freeze-quench experiments of about 30 s, the folding of the LHCII apoprotein was traced by DEER measurements at various time points. While at optimum spin concentrations (Fig. 3) reliable distance distributions can be obtained, under the non-ideal conditions of refolding experiments (Fig. 4) the dipolar evolution time had to be shortened, resulting in suppression of longer distances. In LHCII, this problem is not serious as maximum distances measured in the denatured state and the compact intermediate were shorter than 6 nm. As a consequence of the low signal-to-noise ratio for such samples poorly predictable noise-related distortions are introduced in the distance distributions. This problem is not encountered in computing dipolar spectra from the primary data, because here the influence of noise can be estimated precisely. The integrated area under dipolar spectra at high frequencies declined during LHCII assembly, reflecting the diminishment of very short distances in the distance distribution. These integrals were used as a monitor for assessing the kinetics of LHCII protein folding (Fig. 4).
The two spin label pairs yielded distinctly different kinetics. The distance distribution between positions 106/160, spanning the length of the middle helix (H3), changed between time points 40 and 150 s and remained unchanged thereafter, defining an upper limit for the apparent time constant of 50–60 s (Fig. 4). The data points for the distance distribution between positions 90/196 (H1/H4), describing the distance between the two intertwined transmembrane helices, could be fitted with a single exponential yielding an apparent time constant of 294 s. These two time windows correspond to the ones seen in time-resolved fluorescence measurements of LHCII assembly, monitoring the assembly of Chls into the complex. Under the reaction conditions used for the EPR measurements, apparent time constants were obtained of (26 ± 11) and (178 ± 114) s (SI Text, Fig. S4, and Table S1). Earlier experiments had shown that the faster phase represents the binding mostly of Chl a, whereas the slower one reflects the binding of Chl b exclusively. The former phase leads to an instable intermediate that dissociates upon dilution while the slower Chl b binding coincides with the final stabilization of the complex (6). We cannot at this point prove that the similar kinetic phases detected by EPR and fluorescence reflect the same molecular events. However, it would be conceivable that the slow juxtaposition of the two intertwined helices is connected with Chl b binding and the stabilization of the LHCII structure (Fig. 5). The faster positioning of the labels on either end of helix H3 then coincides with the faster binding step in which the Chl a binding sites are filled. The distance between these two labels (106/160) is probably defined by the formation (or completion) of helix H3. Earlier CD measurements had revealed that α-helix formation in LHCII apoprotein (beyond the helical structure preformed in dodecyl sulfate solution) is dependent on pigment binding (27), and that most but not all of it happens during the faster reaction phase in the range of 10 s to 1 min. The helix formation positioning labels 106/160 is not likely to be triggered by Chls binding locally to this helical domain, since these are all Chl b binding sites whose occupation takes place during the slower kinetic phase of several minutes. The nearest Chl a binding site is that of Chl 6. Although its central Mg2+ binds to Gly 78 in H1, Chl 6 is also close to a short β-sheet and the short amphiphilic helix H2 in the luminal loop. This interaction could trigger the formation of secondary and tertiary structure in the luminal loop and, thus, promote the completion of helix H3.
Fig. 5.
Proposed folding model for LHCII in vitro. After mixing the LDS-denatured spin-labeled Lhcb1 with pigments, folding occurs in two apparent phases taking less than 1 min and several minutes. Formation of secondary and tertiary structure are assigned to the first and second phase, respectively, both triggered by cofactor binding.
The juxtaposition of the intertwined helices during the slower phase of LHCII formation corresponds to stage 2, the positioning of transmembrane helices, in the 2-stage model proposed by Popot and Engelman (28) as a general folding scheme of α-helical membrane proteins. Stage 1 in this model is the faster formation of α-helices. In the case of LHCII protein folding, α-helix formation extends to later stages; this may be attributed to the unusually large number of more than 15 pigments as cofactors (29, 30) which make up roughly one third of the total mass of the final LHCII structure and clearly play a pivotal role in its formation. Folding analysis by DEER allows to focus on individual protein domains and thus opens up the possibility to analyze how groups of pigments modulate the folding procedure. In combination with time-resolved ESEEM measurements of singly spin-labeled domains as well as reconstitution studies using various pigment stoichiometries, DEER has the capability to resolve the assembly process of LHCII in vitro in a detailed manner. Furthermore, if spin label positions and measurement conditions are carefully chosen, the analysis of structural changes by DEER may become sensitive enough to study the folding of LHCII protein or other proteins in environments more similar to their native ones, such as lipid vesicles, the thylakoid, or other biological membranes.
Materials and Methods
Protein Preparation, SDSL, and Reconstitution of LHCII with Pigments.
Proteins used in this study were derivatives of the Lhcb1*2 (AB80) gene from pea (Pisum sativum) (31) with its single Cys in position 79 replaced with Ser. In DEER measurements, two different double mutants were used (106/160 and 90/196), each containing two Cys replacing Ser at positions 106 and 160 and replacing Val in positions 90 and 196. All derivatives have either been described in earlier studies (11) or were constructed by using the QuikChange mutagenesis kit (Stratagene). Bacterial expression of the Lhcb1 gene derivatives (2) and labeling of the protein with PROXYL spin labels [3-(2-iodoacetamido)-PROXYL, Aldrich] were performed as described elsewhere (15).
For steady-state DEER the spin-labeled complexes were reconstituted using the detergent-exchange procedure (henceforward called “standard reconstitution”) as described in (27) except that 10 mM β-mercaptoethanol (β-ME) was used as a reductant. Purification of LHCII and preparation of EPR samples were performed as described in (11).
For time-resolved DEER measurements, reconstitution was initiated by manually mixing protein and pigment solutions containing high reactant concentrations (“reconstitution for EPR measurements”). The reaction was terminated by rapidly adding 40% glycerol as a cryoprotectant and then flash-freezing the sample in liquid nitrogen. Alternatively, the protein and pigment solutions were mixed in a rapid freeze-quench apparatus, and the reaction products were mixed with glycerol and then sprayed into a cold brass funnel as described in the SI Text. Each data point in the EPR kinetic measurements represents an independently mixed protein sample exhibiting a final protein concentration of about 40 μM LHCII.
EPR Spectroscopy.
The X-band (9 GHz) pulse EPR measurements were performed on a Bruker Elexsys EX 580 EPR spectrometer using a Bruker Flexline split-ring resonator ER 4118X_MS3 overcoupled to Q approximately 100. All pulse measurements were performed at 50 K with liquid helium cooling using an Oxford CF935 cryostat with an Oxford ITC4 temperature controller.
Four-pulse DEER experiments were performed as described by Jeschke et al. (11) with interpulse delays τ2 between 800 and 2,500 ns depending on the transverse relaxation times, a repetition time of 6 ms, and a total measurement time between 8 and 24 h, depending on spin label concentration. Sample quality was checked by electron spin echo (ESE) measurements as described in (15).
Data Analysis.
DEER data were analyzed using the “DeerAnalysis2006” program (21), which can be downloaded at http://www.epr.ethz.ch/software/index. Processing parameters are given in the SI Text and in Fig. S5, S6, S7, and S8.
Rotamer Library Simulations.
Distance distributions were predicted from the crystal structure (1) with rotamer library modeling of spin label conformations using a modified version of an approach introduced in earlier work (10, 12). Details are given in the SI Text.
Supplementary Material
Acknowledgments.
We thank Hans Wolfgang Spiess for providing access to a pulsed EPR spectrometer and Götz Grundmann for performing time-resolved fluorescence measurements. This work was supported by Deutsche Forschungsgemeinschaft Grant SFB 625/TP B10 (to G.J. and H.P.) and Swiss National Foundation Grant 200021_121579 (to Y.P.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0906462106/DCSupplemental.
References
- 1.Standfuss J, Terwisscha van Scheltinga AC, Lamborghini M, Kühlbrandt W. Mechanisms of photoprotection and nonphotochemical quenching in pea light-harvesting complex at 2.5 Å resolution. EMBO J. 2005;24:919–928. doi: 10.1038/sj.emboj.7600585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Paulsen H, Rümler U, Rüdiger W. Reconstitution of pigment-containing complexes from light-harvesting chlorophyll-a/b-binding protein overexpressed in Escherichia coli. Planta. 1990;181:204–211. doi: 10.1007/BF02411539. [DOI] [PubMed] [Google Scholar]
- 3.Plumley FG, Schmidt GW. Reconstitution of chlorophyll a/b light-harvesting complexes: Xanthophyll-dependent assembly and energy transfer. Proc Natl Acad Sci USA. 1987;84:146–150. doi: 10.1073/pnas.84.1.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Booth PJ, Paulsen H. Assembly of light-harvesting chlorophyll a/b complex in vitro. Time-resolved fluorescence measurements. Biochemistry. 1996;35:5103–5108. doi: 10.1021/bi953053f. [DOI] [PubMed] [Google Scholar]
- 5.Reinsberg D, Ottmann K, Booth PJ, Paulsen H. Effects of chlorophyll a, chlorophyll b, and xanthophylls on the in vitro assembly kinetics of the major light harvesting chlorophyll a/b complex, LHCIIb. J Mol Biol. 2001;308:59–67. doi: 10.1006/jmbi.2001.4573. [DOI] [PubMed] [Google Scholar]
- 6.Horn R, Paulsen H. Early steps in the assembly of light-harvesting chlorophyll a/b complex. J Biol Chem. 2004;279:44400–44406. doi: 10.1074/jbc.M407188200. [DOI] [PubMed] [Google Scholar]
- 7.Horn R, Grundmann G, Paulsen H. Consecutive binding of chlorophylls a and b during the assembly in vitro of light-harvesting chlorophyll a/b protein (LHCIIb) J Mol Biol. 2007;366:1045–1054. doi: 10.1016/j.jmb.2006.11.069. [DOI] [PubMed] [Google Scholar]
- 8.Horn R, Paulsen H. Folding in vitro of light-harvesting chlorophyll a/b protein is coupled with pigment binding. J Mol Biol. 2002;318:547–556. doi: 10.1016/S0022-2836(02)00115-8. [DOI] [PubMed] [Google Scholar]
- 9.Pannier M, Veit S, Godt A, Jeschke G, Spiess HW. Dead-time free measurement of dipole-dipole interactions between electron spins. J Magn Reso. 2000;142:331–340. doi: 10.1006/jmre.1999.1944. [DOI] [PubMed] [Google Scholar]
- 10.Jeschke G, Polyhach Y. Distance measurements on spin-labeled biomacromolecules by pulsed electron paramagnetic resonance. Phys Chem Chem Phys. 2007;9:1895–1910. doi: 10.1039/b614920k. [DOI] [PubMed] [Google Scholar]
- 11.Jeschke G, et al. Localization of the N-terminal domain in light-harvesting chlorophyll a/b protein by EPR measurements. J Biol Chem. 2005;280:18623–18630. doi: 10.1074/jbc.M501171200. [DOI] [PubMed] [Google Scholar]
- 12.Hilger D, Polyhach Y, Jung H, Jeschke G. Backbone structure of transmembrane domain IX of the Na+/Proline Transporter PutP of Escherichia coli. Biophys J. 2009;96:217–225. doi: 10.1016/j.bpj.2008.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Altenbach C, Kusnetzow AK, Ernst OP, Hofmann KP, Hubbell WL. High-resolution distance mapping in rhodopsin reveals the pattern of helix movement due to activation. Proc Natl Acad Sci USA. 2008;105:7439–7444. doi: 10.1073/pnas.0802515105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klein JC, et al. Actin-binding cleft closure in myosin II probed by site-directed spin labeling and pulsed EPR. Proc Natl Acad Sci USA. 2008;105:12867–12872. doi: 10.1073/pnas.0802286105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Volkov A, Dockter C, Bund T, Paulsen H, Jeschke G. Pulsed EPR determination of water accessibility to spin-labeled amino acid residues in LHCIIb. Biophys J. 2009;96:1124–1141. doi: 10.1016/j.bpj.2008.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Farahbakhsh ZT, Hideg K, Hubbell WL. Photoactivated conformational changes in rhodopsin: A time-resolved spin label study. Science. 1993;262:1416–1419. doi: 10.1126/science.8248781. [DOI] [PubMed] [Google Scholar]
- 17.Shin YK, Levinthal C, Levinthal F, Hubbell WL. Colicin E1 binding to membranes: Time-resolved studies of spin-labeled mutants. Science. 1993;259:960–963. doi: 10.1126/science.8382373. [DOI] [PubMed] [Google Scholar]
- 18.Steinhoff HJ, et al. Time-resolved detection of structural changes during the photocycle of spin-labeled bacteriorhodopsin. Science. 1994;266:105–107. doi: 10.1126/science.7939627. [DOI] [PubMed] [Google Scholar]
- 19.DeWeerd K, Grigoryants V, Sun Y, Fetrow JS, Scholes CP. EPR-detected folding kinetics of externally located cysteine-directed spin-labeled mutants of iso-1-cytochrome c. Biochemistry. 2001;40:15846–15855. doi: 10.1021/bi011414n. [DOI] [PubMed] [Google Scholar]
- 20.Hobe S, Niemeier H, Bender A, Paulsen H. Carotenoid binding sites in LHCIIb. Relative affinities towards major xanthophylls of higher plants. Eur J Biochem. 2000;267:616–624. doi: 10.1046/j.1432-1327.2000.01060.x. [DOI] [PubMed] [Google Scholar]
- 21.Jeschke G, et al. DeerAnalysis2006 - A comprehensive software package for analyzing pulsed ELDOR data. Appl Magn Reson. 2006;30:473–498. [Google Scholar]
- 22.Reinsberg D, Booth PJ, Jegerschoeld C, Khoo BJ, Paulsen H. Folding, assembly, and stability of the major light-harvesting complex of higher plants, LHCII, in the presence of native lipids. Biochemistry. 2000;39:14305–14313. doi: 10.1021/bi001365z. [DOI] [PubMed] [Google Scholar]
- 23.Hobe S, Prytulla S, Kühlbrandt W, Paulsen H. Trimerization and crystallization of reconstituted light-harvesting chlorophyll a/b complex. EMBO J. 1994;13:3423–3429. doi: 10.1002/j.1460-2075.1994.tb06647.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ibel K, et al. Structure of dodecyl sulfate-protein complexes at subsaturating concentrations of free detergent. Biophys Chem. 1994;53:77–84. doi: 10.1016/0301-4622(94)00078-6. [DOI] [PubMed] [Google Scholar]
- 25.Booth PJ, Curnow P. Membrane proteins shape up: Understanding in vitro folding. Curr Opin Struct Biol. 2006;16:480–488. doi: 10.1016/j.sbi.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 26.Yang C, Horn R, Paulsen H. The light-harvesting chlorophyll a/b complex can be reconstituted in vitro from its completely unfolded apoprotein. Biochemistry. 2003;42:4527–4533. doi: 10.1021/bi0273157. [DOI] [PubMed] [Google Scholar]
- 27.Paulsen H, Finkenzeller B, Kühlein N. Pigments induce folding of light-harvesting chlorophyll a/b-binding protein. Eur J Biochem. 1993;215:809–816. doi: 10.1111/j.1432-1033.1993.tb18096.x. [DOI] [PubMed] [Google Scholar]
- 28.Popot JL, Engelman DM. Membrane protein folding and oligomerization: The two-stage model. Biochemistry. 1990;29:4031–4037. doi: 10.1021/bi00469a001. [DOI] [PubMed] [Google Scholar]
- 29.Popot JL, Engelman DM. Helical membrane protein folding, stability, and evolution. Annu Rev Biochem. 2000;69:881–922. doi: 10.1146/annurev.biochem.69.1.881. [DOI] [PubMed] [Google Scholar]
- 30.Engelman DM, et al. Membrane protein folding: Beyond the two stage model. FEBS Lett. 2003;555:122–125. doi: 10.1016/s0014-5793(03)01106-2. [DOI] [PubMed] [Google Scholar]
- 31.Cashmore AR. Structure and expression of a pea nuclear gene encoding a chlorophyll a/b-binding polypeptide. Proc Natl Acad Sci USA. 1984;81:2960–2964. doi: 10.1073/pnas.81.10.2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.