Abstract
Dietary restriction (DR) is a widely conserved intervention leading to lifespan extension. Despite considerable effort, the mechanisms underlying DR remain poorly understood. In particular, it remains unclear whether DR prolongs life through conserved mechanisms in different species. Here, we show that, in the most common experimental conditions, lifespan extension by DR is abolished by providing Drosophila with ad libitum water, without altering food intake, indicating that DR, as conventionally studied in flies, is fundamentally different from the phenomenon studied in mammals. We characterize an alternative dietary paradigm that elicits robust lifespan extension irrespective of water availability, and thus likely represents a more relevant model for mammalian DR. Our results support the view that protein:carbohydrate ratio is the main dietary determinant of fly lifespan. These findings have broad implications for the study of lifespan and nutrition.
Keywords: aging, caloric restriction, dehydration, longevity, nutrition
Dietary restriction (DR), classically defined as a reduction in nutrient availability short of malnutrition, can extend the lifespan of organisms ranging from yeast to mice (1, 2). In rodents and primates, DR delays the onset of age-related pathologies, such as cancer, cardiovascular disease, and diabetes (3–5). Chronic DR also elicits a number of physiological changes, including decreased circulating glucose, insulin, and cholesterol levels; reduced body mass; and compromised reproductive function (5–8). Despite the evident biomedical interest in DR, its mechanistic basis remains largely unknown, and it is unclear whether DR extends lifespan in different species through similar mechanisms (9, 10). This issue is of fundamental importance, since invertebrate model systems are especially valued for their ability to provide mechanistic clues to be tested in mammals.
In mammals, food restriction is imposed by feeding the DR cohort a fraction of that ingested by the ad libitum group (2). Due to the difficulty of controlling feeding rates in invertebrates, more ingenious, albeit potentially problematic, techniques are used. Drosophila DR is commonly achieved by total food dilution (11) and carried out in the absence of a separate water source, unlike with other species (12–16). Hence, fly food is simultaneously the source of nutrients* and water. This setup prevents flies from independently regulating nutrient and water intake, leaving room for the possibility that any effects of food dilution are mediated by changes in hydration.
Our results show that lifespan extension by typical DR regimes (17–20) can be entirely abolished by providing flies with free access to water. Water supplementation does not affect food consumption, suggesting that DR, as typically applied, does not impact longevity through reduced nutrient intake. Furthermore, we characterize a regime that elicits robust lifespan extension independent of water supplementation. Our findings suggest that most of the work done on Drosophila DR has been confounded by changes in hydration. In conditions where water intake is not limiting, lifespan modulation by DR can be explained by the protein:carbohydrate ratio.
Results and Discussion
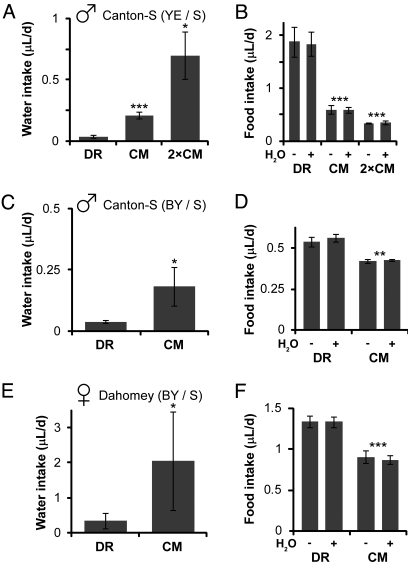
We measured the water consumption of flies fed two different concentrations (dietary restriction, DR; concentrated medium, CM) of yeast extract/sucrose (YE/S) medium by providing ad libitum water labeled with a radioactive tracer (21–23). Flies were housed in population cages containing separate food and water sources of similar surface area. CM-fed flies drank five times as much as those on DR, and this trend was maintained on an even richer medium (Fig. 1A). The difference in water content of the food (CM = 0.86 ± 0.04 and DR = 0.98 ± 0.06 mL H2O/mL medium, respectively) seems mild compared with the dramatic difference in water ingestion. We reasoned that compensatory feeding, the ability of flies to regulate their intake in response to changes in food concentration (24), might play a causal role. Thus, animals that restrict their intake to compensate for the high concentration of CM would consequently ingest less liquid and require an independent water source. Food dilution indeed had a strong phagostimulatory effect (Fig. 1B). Isotope accumulation was near linear for several days (Fig. S1A), and flies fed diluted food were not less efficient in eliminating or metabolizing the label (Fig. S1B), supporting the validity of radioactivity measurements (25, 26). Notably, food intake was unaffected by water access (Fig. 1B). These results were independently confirmed using the Capillary Feeder (CAFE) assay, which directly measures consumption (Fig. S1C) (27). Similar results were observed with both genders, as well as with different media, fly strains, and enclosures (Fig. 1 C–F). These experiments span the most common paradigms of Drosophila DR.
Fig. 1.
Food and water intake assayed by medium radiolabeling (22). (A and B) Yeast extract/sucrose (YE/S)-fed Canton-S males maintained in demography cages. (C and D) Brewer's yeast/sucrose (BY/S)-fed Canton-S males maintained in demography cages. (E and F) BY/S-fed Dahomey females maintained in vials. Flies drink greater volumes of water and consume less of the food as concentrations of YE/S (A and B) or BY/S (C–F) increase. Results are expressed as an average (± SD) of 2–6 trials, each containing 6–16 flies. Food composition, YE/S: DR = 2.5% YE + 2.5% S; CM = 10% YE + 10% S; 2×CM = 20% YE + 20% S; BY/S: DR = 10% BY + 5% S; CM = 20% BY + 10% S (all wt/vol). Statistical significance was determined by nonpaired, two-tailed Student's t tests between results on DR and CM or CM and 2×CM media: *, P < 0.05; **, P < 0.01; ***, P < 0.001. (B, D, and F) The presence of water did not affect food intake on any medium (P > 0.05).
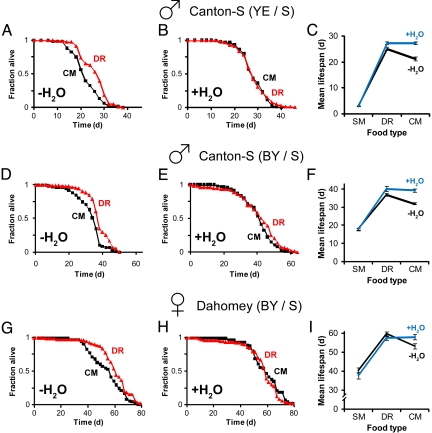
Collectively, our results indicate that, faced with the common food/water sources used in DR, flies give priority to regulating their nutrient intake via compensatory feeding, at the expense of optimal hydration. Since flies exposed to richer media are significantly thirstier than controls (Fig. 1 A, C, and E), we asked if this state of chronic dehydration affects longevity. Lifespan was measured on DR and CM with and without water supplementation. In the control group, the aqueous medium (1% agar) was covered by a nylon mesh, preventing access to the water source while ensuring identical humidity. Strikingly, ad libitum water access prolonged the survival of CM-fed flies to the level of their DR cohorts, whereas the latter experienced only a mild benefit in the presence of water (Fig. 2 A and B). As a result, DR extended lifespan in the absence, but not in the presence, of the aqueous source (Fig. 2C and Table S1). The nonadditive effect of DR and water supplementation on lifespan is not due to an absolute lifespan “ceiling” or maximum, since other conditions imparted greater longevity (Table S2).
Fig. 2.
Ad libitum water supplementation abolishes lifespan extension by dietary restriction (DR). (A–C) Yeast extract/sucrose (YE/S)-fed Canton-S males aged in demography cages. Lifespan curves without (A) and with (B) water supplementation of flies maintained on concentrated (CM) or DR medium. (C) Mean lifespan (± SEM) of flies on diets of CM, DR, or a heavily diluted starvation medium (SM) representing malnourishment. (D–F) Lifespan curves and mean lifespan, as in (A–C), respectively, of brewer's yeast/sucrose (BY/S)-fed Canton-S males aged in demography cages. (G–I) Lifespan curves and mean lifespan, as in (A–C), respectively, of BY/S-fed Dahomey females aged in vials. In the absence of a water source, the DR diet extended lifespan compared with flies fed CM in all conditions tested (A, P = 1.3 × 10−4; D, P = 2.4 × 10−6, G, P = 2.5 × 10−3; log-rank test). (B, E, and H) DR had no effect (P > 0.05, log-rank test) on lifespan upon water supplementation. Food composition is described in Fig. 1; YE/S: SM = 0.1% YE + 0.1% S; BY/S: SM = 1% BY + 0.5% S (all wt/vol). n = 68–156 flies per trial. Statistics of Cox proportional hazards analysis, demonstrating the greatly reduced effect of DR upon water supplementation, are shown in Table S1.
We tested the effect of water access on lifespan in different genders, fly strains, media, and animal enclosures. Water supplementation mimicked the longevity-enhancing effect of DR in all conditions tested (Fig. 2 D–I, and Tables S1 and S2), with no significant effect on food intake (Fig. 1 B, D, and F). The magnitude of lifespan extension by DR in the absence of water is in agreement with reported results (17). Notably, one of our experiments (Fig. 2 G–I) faithfully replicated the conditions recently proposed as the most appropriate for DR experiments on the basis of their effects on lifespan and fecundity (17). The simplest explanation for these findings is that the typical conditions used in Drosophila DR and water supplementation extend lifespan through largely overlapping mechanisms.
Our findings contradict a recent study that found no effect on lifespan when water was provided in a pipette tip, although water consumption was not confirmed (17). The substantially larger water surface used here may enhance access or counter crowding effects, microorganism growth, accumulation of excreta, or other undetermined factors.
Although DR has been established on yeast extract/sucrose/cornmeal (YE/S/C) upon varying yeast alone (28, 29), this paradigm is much less commonly used. In contrast to all other regimes tested, YE/S/C elicited negligible water consumption both in its low- and high-yeast forms (Fig. S2A), indicating that YE/S/C is unique in its ability to satisfy the animals' water needs. Interestingly, reducing yeast levels in YE/S/C stimulated food ingestion (Fig. S2B), demonstrating that compensatory feeding does not necessarily result in dehydration, and suggesting that water needs are determined by an interplay between feeding behavior and the specific nutrient source.
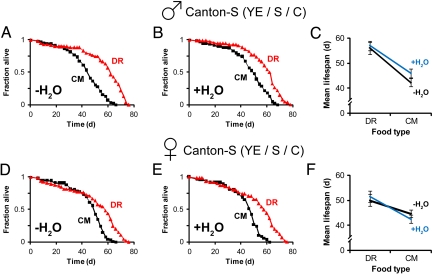
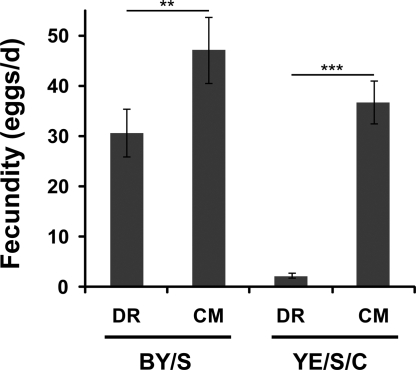
In the YE/S/C paradigm, DR prolonged lifespan in males and females irrespective of water access (Fig. 3). The small increase in lifespan of CM-fed males with water access was not reproducible (Fig. S3). Importantly, female fecundity directly correlated with yeast levels (Fig. 4), arguing against a toxic effect of yeast extract (9). Hence, we have established a paradigm where nutrient manipulation has a clear impact on longevity irrespective of water availability.
Fig. 3.
Water supplementation does not affect lifespan extension upon reducing yeast alone on yeast extract/sucrose/cornmeal (YE/S/C). (A–C) YE/S/C-fed Canton-S males aged in demography cages. Lifespan curves without (A) and with (B) water supplementation of flies maintained on concentrated medium (CM) or dietary restriction (DR). (C) Mean lifespan (± SEM) of flies on CM or DR. (D–F) Lifespan curves and mean lifespan, as in (A–C), respectively, of YE/S/C-fed Canton-S females aged in demography cages. In this paradigm, reducing yeast extended lifespan in both the absence (A and D) and presence (B and E) of water (P < 10−6, log-rank test). Food composition, YE/S/C: DR = 0.25% YE + 5% S + 8.6% C; CM = 5% YE + 5%S + 8.6% C (all wt/vol). n = 60–86 flies per trial. Statistics of Cox proportional hazards analysis are shown in Table S1.
Fig. 4.
Effect of food concentration on fecundity in different dietary paradigms. Increasing nutrient concentration in BY/S and YE/S/C diets stimulates the fecundity of Dahomey females. Results are comparable to the estimated lifetime fecundity on these foods (17). Mated Dahomey females (2–4 days old) maintained on the indicated medium were transferred daily and eggs counted for 12 days. Results represent the average number of eggs laid per day, per fly from days 2–12 and are expressed as the average (± SD) of 5 replicates containing 2 flies each. Medium composition is described in Figs. 1 and 3. Statistical significance was determined by nonpaired, two-tailed Student's t tests between DR and CM: **, P < 0.01; ***, P < 0.001.
The main goal of invertebrate research is to generate insights into the mechanisms of human biology, and thus an ideal fly DR model should bear analogy to the mammalian phenomenon. Since DR in mammals is generally conducted in the presence of ad libitum water (12, 13) and thus impacts longevity in hydrated animals, one would expect the fly paradigm to also be dependent on nutrient, rather than water ingestion. However, our findings show that the bulk of Drosophila DR studies (11, 17–20) have dissected a form of lifespan extension that is entirely dependent on water availability, and therefore differs fundamentally from the phenomenon studied in mammals. The YE/S/C water-independent paradigm more closely resembles mammalian DR and, therefore, likely represents a more relevant model of DR in higher organisms. Two observations lend further support to this view. First, maximum lifespan, arguably a better indicator of aging rate than average longevity (30), is robustly extended upon DR in rodents (4) and in the water-independent regime (Fig. 3 and Table S2), but not in the classical Drosophila DR paradigms (Fig. 2 and Table S2). Second, lifespan extension on YE/S/C was accompanied by a dramatic reduction in female fecundity that is reminiscent of the reproductive diapause seen in restricted rodents (31–33), whereas DR on water-dependent media has only a mild effect [Fig. 4 and (17)].
Our findings are consistent with the view that the protein:carbohydrate (P:C) ratio is the main dietary determinant of fly longevity (34), a fact that may have been classically obscured by the hydration confound. As predicted by this model, classical DR paradigms, based on whole medium dilution and thus maintaining a constant P:C ratio, have only a mild, nutrient-independent effect on lifespan, whereas the YE/S/C paradigm used here, based on varying yeast alone and thus altering the P:C ratio, impacts longevity more dramatically, and in a nutrient-dependent manner. Notably, the low-yeast form of YE/S/C has a P:C ratio of 1:15, similar to the 1:16 described by Lee et al. as optimal for longevity (34). Other DR paradigms that alter the P:C ratio within the appropriate range without causing dehydration should be functionally equivalent to YE/S/C. Our results also demonstrate the remarkable plasticity of Drosophila feeding rate, in agreement with the finding that fly lifespan is determined by the interplay between P:C ratio and food intake (34). The lesson gleaned from these observations is that quantitative measurements of steady-state food intake are indispensable for any study aiming to understand the effects of nutrition on lifespan.
Our results directly contradict the long-held assumption that food manipulation affects fly lifespan solely through changes in nutrient ingestion. Since this erroneous view has pervaded the field since its inception (11), our observations warrant a careful reexamination of the entire body of work of Drosophila DR. Any insights stemming from work on fruit flies (18–20, 24, 35, 36) are potentially confounded by changes in hydration and thus difficult to extrapolate to mammalian DR. This caveat extends to the numerous mutants shown to regulate fly DR (e.g., 37, 38). Extensive validation will be required to assess their value as clues to DR and aging in higher organisms. All future work should employ conditions in which ad libitum water is either present or shown not to affect lifespan.
Experimental Procedures
Reagents.
Bacto™ agar and yeast extract were from BD Diagnostic Systems, sucrose from Mallinckrodt Baker, brewer's yeast from MP Biomedicals, and cornmeal (yellow) from Quaker Oats. Drosophila bottles (polypropylene, 8 oz. round bottom) and vials (polystyrene, 25 × 95 mm) were purchased from VWR International. For lifespan measurements in cages (large embryo collection cage, Genesee Scientific), food and water were supplied in compartmentalized dishes (four-section plates, Fisher Scientific).
Food Preparation.
Food compositions are provided in the figure legends. Agar (0.5% if the food contained cornmeal, otherwise 1%, wt/vol) was heated with continuous stirring in ddH2O (10–20% less than the desired final volume) on a hot plate. Upon boiling, food components were added and the heat reduced. After simmering with vigorous stirring for 2 min, food was removed from heat and the final volume adjusted with ddH2O. After cooling to <65°C, a mixture of propionic and phosphoric acids [0.4 and 0.06% (vol/vol) final, respectively] was added and the food dispensed into either vials (2 mL) or two of the four compartments of segmented Petri dishes (7 mL). Ad libitum water was supplied as 1% agar, boiled and cooled to <65°C, and dispensed onto vial walls (400 μL) approximately 3 cm from the bottom. For cages, polypropylene caps from 50-mL conical tubes were filled with 1% agar (2.5 mL) and affixed with double-sided tape to the empty compartments of the Petri dishes. For both vials and cages, the surface area of the water source was approximately 75% of that of the food medium. In half of the cage experiments, the agar-containing caps were covered with nylon mesh to maintain humidity while preventing flies from accessing the water source.
Lifespan Analysis.
Flies were raised in bottles containing Lewis medium (39). Groups of enclosed adults (0–3 days old) were transferred to fresh bottles and allowed to mate for 2 days. Males and females were then separated under CO2 anesthesia and randomly allocated to different media (approximately 20 flies per vial or 120–150 flies per cage). All enclosures were maintained at 25°C in a controlled light (12/12-h light/dark cycle) and humidity (>70%) environment. Flies were scored for survival and provided with fresh medium every 2–3 days. Enclosures were placed randomly in the incubator, and positions were rotated after each transfer to minimize the effects of microclimate. Statistical significance of different survivorship curves was determined by log-rank test. Cox proportional hazards analysis was also used to generate a hazard ratio for each experiment in the presence or absence of water (Table S1). When the hazard ratio is close to 1, DR has little effect on survival.
Feeding Rate Measurement.
Feeding assays were performed essentially as described (22). Briefly, adults (2–5 days old, approximately 10 flies/vial) were habituated for 4 days on the food medium being tested, with a transfer to fresh food on the second day. On day 4, flies were transferred to the same medium supplemented with 0.5–4 μCi/mL [α-32P]-dCTP (<1.3 nM, final, MP Biomedicals), allowed to feed for 24 h, and then transferred to empty vials for 30 min. Cold-anesthetized flies were assayed in 10 mL of scintillation fluid (Research Products International) on an LS 5000 TA Liquid Scintillation System (Beckman Coulter). Flies fed nonlabeled food were used as blanks and the values were subtracted from experimental readings. Aliquots of the radioactive tracer were used to calculate food volumes from scintillation counts. Flies accumulated radioactive tracer at a near-linear rate for at least several days (Fig. S1 A and B).
Nutritional Information.
Water content of the media was determined by preparing food as described above and dispensing 10 mL into preweighed containers. After the food had solidified, mass was measured and food density was calculated. Subtraction of the dry weight of the food components provided the water content. Protein and carbohydrate content of food components was taken from manufacturer's information (yeast extract: 51% protein + 16.33% carbohydrate; cornmeal: 7.5% protein + 78.8% carbohydrate, which include simple and complex carbohydrates).
Supplementary Material
Acknowledgments.
We wish to dedicate this work to the life and career of our beloved friend and mentor, Seymour Benzer. We thank A.W. Chang for technical assistance; M. Piper and L. Partridge (University College London) for the Dahomey fly line; and S.M. Lee, S.J. Simpson (University of Sydney), P. Kapahi (Buck Institute for Age Research), and H.-D. Wang (National Tsing Hua University) for comments on the manuscript. This work was supported by a postdoctoral fellowship from the John Douglas French Alzheimer's Foundation (to W.W.J.); a Lawrence L. and Audrey W. Ferguson Fellowship (to G.B.C.); and Grants from the National Institutes of Health (1R15AG027749–01A1 to T.B., 1K99AG030493 to W.W.J., and AG16630, AG24366, and DK070154 to S.B.), National Science Foundation (MCB-0418479 to S.B.), and the Ellison Foundation (to S.B.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Although water is commonly considered a nutrient, we use this term to refer to all nonwater food components (e.g., yeast and sugar) throughout the text.
This article contains supporting information online at www.pnas.org/cgi/content/full/0908016106/DCSupplemental.
References
- 1.Guarente L, Kenyon C. Genetic pathways that regulate ageing in model organisms. Nature. 2000;408:255–262. doi: 10.1038/35041700. [DOI] [PubMed] [Google Scholar]
- 2.Guarente L, Picard F. Calorie restriction–the SIR2 connection. Cell. 2005;120:473–482. doi: 10.1016/j.cell.2005.01.029. [DOI] [PubMed] [Google Scholar]
- 3.Colman RJ, et al. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science. 2009;325:201–204. doi: 10.1126/science.1173635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weindruch R, Walford RL, Fligiel S, Guthrie D. The retardation of aging in mice by dietary restriction: Longevity, cancer, immunity and lifetime energy intake. J Nutr. 1986;116:641–654. doi: 10.1093/jn/116.4.641. [DOI] [PubMed] [Google Scholar]
- 5.Longo VD, Finch CE. Evolutionary medicine: From dwarf model systems to healthy centenarians? Science. 2003;299:1342–1346. doi: 10.1126/science.1077991. [DOI] [PubMed] [Google Scholar]
- 6.Heilbronn LK, Ravussin E. Calorie restriction and aging: Review of the literature and implications for studies in humans. Am J Clin Nutr. 2003;78:361–369. doi: 10.1093/ajcn/78.3.361. [DOI] [PubMed] [Google Scholar]
- 7.Walford RL, Harris SB, Gunion MW. The calorically restricted low-fat nutrient-dense diet in Biosphere 2 significantly lowers blood glucose, total leukocyte count, cholesterol, and blood pressure in humans. Proc Natl Acad Sci USA. 1992;89:11533–11537. doi: 10.1073/pnas.89.23.11533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walford RL, Mock D, Verdery R, MacCallum T. Calorie restriction in Biosphere 2: Alterations in physiologic, hematologic, hormonal, and biochemical parameters in humans restricted for a 2-year period. J Gerontol A Biol Sci Med Sci. 2002;57:B211–224. doi: 10.1093/gerona/57.6.b211. [DOI] [PubMed] [Google Scholar]
- 9.Piper MD, Partridge L. Dietary restriction in Drosophila: Delayed aging or experimental artefact? PLoS Genet. 2007;3:e57. doi: 10.1371/journal.pgen.0030057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Masoro EJ. Caloric restriction and aging: Controversial issues. J Gerontol A Biol Sci Med Sci. 2006;61:14–19. doi: 10.1093/gerona/61.1.14. [DOI] [PubMed] [Google Scholar]
- 11.Chapman T, Partridge L. Female fitness in Drosophila melanogaster: An interaction between the effect of nutrition and of encounter rate with males. Proc Biol Sci. 1996;263:755–759. doi: 10.1098/rspb.1996.0113. [DOI] [PubMed] [Google Scholar]
- 12.Ross MH. Length of life and nutrition in the rat. J Nutr. 1961;75:197–210. doi: 10.1093/jn/75.2.197. [DOI] [PubMed] [Google Scholar]
- 13.Kemnitz JW, et al. Dietary restriction of adult male rhesus monkeys: Design, methodology, and preliminary findings from the first year of study. J Gerontol. 1993;48:B17–B26. doi: 10.1093/geronj/48.1.b17. [DOI] [PubMed] [Google Scholar]
- 14.Carey JR, et al. Life history response of Mediterranean fruit flies to dietary restriction. Aging Cell. 2002;1:140–148. doi: 10.1046/j.1474-9728.2002.00019.x. [DOI] [PubMed] [Google Scholar]
- 15.Cooper TM, Mockett RJ, Sohal BH, Sohal RS, Orr WC. Effect of caloric restriction on life span of the housefly. Musca domestica. FASEB J. 2004;18:1591–1593. doi: 10.1096/fj.03-1464fje. [DOI] [PubMed] [Google Scholar]
- 16.Carey JR, et al. Longevity-fertility trade-offs in the tephritid fruit fly, Anastrepha ludens, across dietary-restriction gradients. Aging Cell. 2008;7:470–477. doi: 10.1111/j.1474-9726.2008.00389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bass TM, et al. Optimization of dietary restriction protocols in Drosophila. J Gerontol A Biol Sci Med Sci. 2007;62:1071–1081. doi: 10.1093/gerona/62.10.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mair W, Goymer P, Pletcher SD, Partridge L. Demography of dietary restriction and death in Drosophila. Science. 2003;301:1731–1733. doi: 10.1126/science.1086016. [DOI] [PubMed] [Google Scholar]
- 19.Mair W, Piper MD, Partridge L. Calories do not explain extension of life span by dietary restriction in Drosophila. PLoS Biol. 2005;3:e223. doi: 10.1371/journal.pbio.0030223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Min KJ, Flatt T, Kulaots I, Tatar M. Counting calories in Drosophila diet restriction. Exp Gerontol. 2007;42:247–251. doi: 10.1016/j.exger.2006.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ayaki T, Oshima K, Yoshikawa I. Linear relationship between lethal mutation yield and intake of ethyl methanesulfonate in Drosophila melanogaster. Environ Mutagen. 1985;7:147–153. doi: 10.1002/em.2860070203. [DOI] [PubMed] [Google Scholar]
- 22.Carvalho GB, Kapahi P, Anderson DJ, Benzer S. Allocrine modulation of feeding behavior by the sex peptide of Drosophila. Curr Biol. 2006;16:692–696. doi: 10.1016/j.cub.2006.02.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thompson ED, Reeder BA, Bruce RD. Characterization of a method for quantitating food consumption for mutation assays in Drosophila. Environ Mol Mutagen. 1991;18:14–21. doi: 10.1002/em.2850180104. [DOI] [PubMed] [Google Scholar]
- 24.Carvalho GB, Kapahi P, Benzer S. Compensatory ingestion upon dietary restriction in Drosophila melanogaster. Nat Methods. 2005;2:813–815. doi: 10.1038/nmeth798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carvalho GB, Ja WW, Kapahi P, Benzer S. Reply to “Pitfalls of measuring feeding rate in the fruit fly Drosophila melanogaster”. Nat Methods. 2008;5:215. doi: 10.1038/nmeth0308-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wong R, Piper MD, Blanc E, Partridge L. Pitfalls of measuring feeding rate in the fruit fly Drosophila melanogaster. Nat Methods. 2008;5:214–215. doi: 10.1038/nmeth0308-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ja WW, et al. Prandiology of Drosophila and the CAFE assay. Proc Natl Acad Sci USA. 2007;104:8253–8256. doi: 10.1073/pnas.0702726104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kapahi P, et al. Regulation of lifespan in Drosophila by modulation of genes in the TOR signaling pathway. Curr Biol. 2004;14:885–890. doi: 10.1016/j.cub.2004.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wood JG, et al. Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature. 2004;430:686–689. doi: 10.1038/nature02789. [DOI] [PubMed] [Google Scholar]
- 30.Sohal RS, Weindruch R. Oxidative stress, caloric restriction, and aging. Science. 1996;273:59–63. doi: 10.1126/science.273.5271.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ball ZB, Barnes RH, Visscher MB. The effects of dietary caloric restriction on maturity and senescence, with particular reference to fertility and longevity. Am J Physiol. 1947;150:511–519. doi: 10.1152/ajplegacy.1947.150.3.511. [DOI] [PubMed] [Google Scholar]
- 32.Nelson JF, Gosden RG, Felicio LS. Effect of dietary restriction on estrous cyclicity and follicular reserves in aging C57BL/6J mice. Biol Reprod. 1985;32:515–522. doi: 10.1095/biolreprod32.3.515. [DOI] [PubMed] [Google Scholar]
- 33.Holehan AM, Merry BJ. Modification of the oestrous cycle hormonal profile by dietary restriction. Mech Ageing Dev. 1985;32:63–76. doi: 10.1016/0047-6374(85)90036-3. [DOI] [PubMed] [Google Scholar]
- 34.Lee KP, et al. Lifespan and reproduction in Drosophila: New insights from nutritional geometry. Proc Natl Acad Sci USA. 2008;105:2498–2503. doi: 10.1073/pnas.0710787105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pletcher SD, et al. Genome-wide transcript profiles in aging and calorically restricted Drosophila melanogaster. Curr Biol. 2002;12:712–723. doi: 10.1016/s0960-9822(02)00808-4. [DOI] [PubMed] [Google Scholar]
- 36.Bross TG, Rogina B, Helfand SL. Behavioral, physical, and demographic changes in Drosophila populations through dietary restriction. Aging Cell. 2005;4:309–317. doi: 10.1111/j.1474-9726.2005.00181.x. [DOI] [PubMed] [Google Scholar]
- 37.Clancy DJ, Gems D, Hafen E, Leevers SJ, Partridge L. Dietary restriction in long-lived dwarf flies. Science. 2002;296:319. doi: 10.1126/science.1069366. [DOI] [PubMed] [Google Scholar]
- 38.Rogina B, Helfand SL, Frankel S. Longevity regulation by Drosophila Rpd3 deacetylase and caloric restriction. Science. 2002;298:1745. doi: 10.1126/science.1078986. [DOI] [PubMed] [Google Scholar]
- 39.Lewis EB. A new standard food medium. Drosophila Inf Serv. 1960;34:117–118. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.