Abstract
In many Asian populations, the commonest form of severe thalassemia results from the coinheritance of HbE and β thalassemia. The management of this disease is particularly difficult because of its extreme clinical diversity; although some genetic and adaptive factors have been identified as phenotypic modifiers, the reasons remain unclear. Because the role of the environment in the course of severe thalassemia has been neglected completely and because malaria due to both Plasmodium falciparum and Plasmodium vivax has been prevalent in Sri Lanka, we carried out a pilot study of patients with HbE β thalassemia that showed high frequencies of antibodies to both parasite species and that 28.6% of the children had DNA-based evidence of current infection with P. vivax. Malarial antibodies then were assessed in patients with HbE β thalassemia compared with those in age-matched controls. There was a significant increase in the frequency of antibodies in the thalassemic patients, particularly against P. vivax and in young children. There was also a higher frequency in those who had been splenectomized compared with those with intact spleens, although in the latter it was still higher than that in the controls. The thalassemic patients showed significant correlations between malaria antibody status and phenotype. Patients with HbE β thalassemia may be more prone to malaria, particularly P. vivax, which is reflected in their clinical severity. Because P. vivax malaria is widespread in Asia, further studies of its interaction with HbE β thalassemia and related diseases are required urgently as a part of ongoing thalassemia control programs.
In excess of 300,000 babies have been estimated recently to be born each year with a serious inherited hemoglobin disorder (1). In sub-Saharan Africa, the main diseases of this type result from HbS or α thalassemia. Throughout the Mediterranean region and the Middle East, α and β thalassemia predominate, although the sickle-cell gene occurs in the oasis populations of Saudi Arabia and extends to some of the tribal groups in India, where β thalassemia also occurs at a high frequency (2). In the eastern parts of the Indian subcontinent, Bangladesh, Myanmar, Thailand, and in other parts of Southeast Asia, HbE is by far the most common hemoglobin variant (2), although both α and β thalassemia also occur at variable frequencies. Because HbE is synthesized at a reduced rate, it behaves phenotypically like a mild form of β thalassemia (3). Because of its extremely high frequency, reaching a 70% carrier rate in some populations, it often is found in the compound heterozygous state with β thalassemia, a condition called HbE β thalassemia. This is the most common severe form of thalassemia in many Asian countries; in Thailand, for example, there are estimated to be ≈100,000 patients with this disease, and in Bangladesh, there are estimated to be twice this number (4, 5).
One of the extraordinary features of HbE β thalassemia, and one that makes its control and management extremely difficult, is its remarkable clinical diversity (2). This is well exemplified in Sri Lanka, where it accounts for one-third of the cases of severe thalassaemia (6). Despite the fact that the common β thalassemia mutations are all of the severe variety that are associated with very limited or no β chain production (6), this interaction results in a spectrum of patients ranging from those who are transfusion-dependent for life to others who, despite moderately severe anemia, grow and develop normally (7, 8). Detailed analysis of these patients over the last 10 years has made possible the definition of mild and severe phenotypes and the determination of at least some of the genetic and adaptive factors that may be responsible for this wide variation in phenotype (7, 8). However, like similar studies in other populations (9, 10), these findings only account for ≈30% of the phenotypic heterogeneity.
There have been no studies reporting the interaction of malaria with severe forms of thalassemia. Because environmental factors of this kind have been neglected in the study of the phenotypic variation of the thalassemias and because until recently both Plasmodium vivax and Plasmodium falciparum malaria have been a serious health burden in Sri Lanka (11, 12), particularly because the increasingly severe spectrum of disease caused by P. vivax malaria only has been appreciated in recent years (13, 14), studying the potential interaction between these forms of malaria and HbE β thalassemia seemed important.
Results
Pilot Study.
A preliminary assessment of the magnitude of exposure to malaria was made in blood samples collected during clinic visits of 93 patients with HbE β thalassemia between 2002 and 2003. Blood samples were analyzed for malarial antibodies to P. falciparum and P. vivax using an immunofluorescent antibody test (IFAT). Plasmodium falciparum antigen blood spots were prepared from cell cultures of P. falciparum IT04 clone cultured in group O Rhesus positive red blood cells. Plasmodium vivax antigen blood spots were prepared from blood samples collected from a chimpanzee infected with the Salvador 1 strain of P. vivax. The DNA samples from the younger group also were tested for P. vivax or P. falciparum by PCR.
In 52 patients aged over 15 years, 40 (76.9%) were positive for antibodies to P. vivax by IFAT, and 33 (63.5%) were positive for antibodies to P. falciparum by IFAT. In 38 patients aged <15 years, IFAT results showed that 31 (81.6%) and 21 (55.2%) were positive for antibodies to P. vivax and P. falciparum, respectively. Among the younger patients, 10 of 35 (28.6%) were positive for P. vivax, and 0 of 35 (0%) were positive for P. falciparum by PCR. The results reflect the first sample obtained from each patient, a procedure used to avoid potential bias due to repeated sampling that was adhered to in all subsequent analyses. However, 27 patients were analyzed on more than one occasion during this period, and the findings were remarkably consistent; three children became seropositive for P. falciparum antibodies, and one child became seropositive for P. vivax antibodies during the study, one child tested negative for P. falciparum antibodies in the second sample, six children were P. vivax PCR positive in the first sample and negative in the second sample, and three children were P. vivax PCR negative in the first sample and positive in the second sample.
Because in some parts of Asia blood-transfusion-related malarial infection is common (15), plasma from 90 local donor samples and 88 patients with β thalassemia major from the same clinic who were receiving monthly transfusions was analyzed by PCR for P. vivax and P. falciparum. Only two positive samples for P. vivax and no positive samples for P. falciparum were found in the blood bank donors; three samples positive for P. vivax and two samples positive for P. falciparum were found in the patients with β thalassemia major. In addition, antibody seropositivity in the patients with HbE β thalassemia was similar in those with mild disease (class 1) (8) who had not received blood transfusion and those with severe disease (class 5) who had received regular transfusions. In the IFAT assay for P. vivax, 9 of 13 (69.2%) of the class 1 patients were seropositive compared with 18 of 27 (66.7%) of the class 5 patients. The corresponding results for the P. falciparum IFAT were 5 of 13 (38.5%) and 12 of 27 (44.4%). Therefore, the very high rates of infection with malaria in the patients with HbE β thalassemia seem very unlikely to be related to transfusion.
Frequency of Malarial Antibodies in Patients with HbE β Thalassemia Compared with That of Age-Matched Controls.
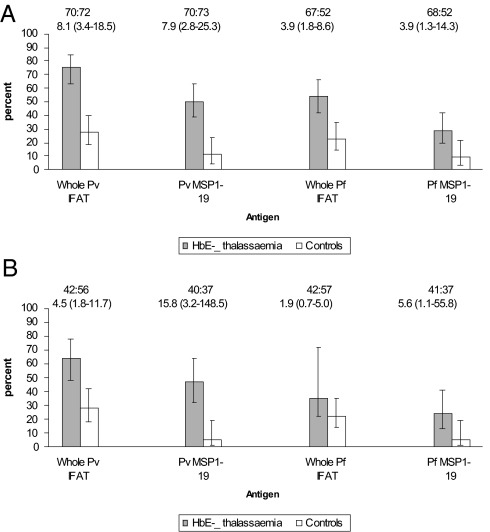
Between 2004 and 2006 malarial antibody levels were compared between 75 patients with HbE β thalassemia and a similar number of age-matched controls admitted at the same time to the same hospital and from the same region as the patient population. Malaria antibodies were measured by IFAT as described above. Antibodies also were measured by ELISA to P. falciparum crude schizont antigens and to the merozoite surface protein 1–19 (MSP1–19) of P. vivax and P. falciparum. The results are summarized in Fig. 1A. A highly significant increase in the frequency of malaria antibodies to both species of parasites was observed in the HbE β thalassemia patient population compared with that in age-matched controls. There was close agreement between the IFAT and ELISA assays using the crude P. falciparum schizont antigen. Differences in antibody positivity between the two populations were more marked for P. vivax than those for P. falciparum. Furthermore, in HbE β thalassemia, antibody positivity was significantly more frequent for P. vivax than that for P. falciparum: odds ratio (95% C.I.) for IFAT 2.63 (1.20–5.77) and MSP1–19 2.47 (1.15–5.35). In contrast, among the controls, seropositivity was similar for P. vivax and P. falciparum: IFAT 1.27 (0.56–2.86) and MSP1–19 1.00 (0.29–5.45). These differences in antibody responses appeared to be established early in life; broadly similar frequencies and patterns of responses were seen when only individuals aged ≤15 years were compared (Fig. 1B). In the younger group, although the numbers were smaller, the differences were also greater for P. vivax than those for P. falciparum in both assays.
Fig. 1.
Comparison of malarial antibody prevalence between patients with HbE β thalassemia and the control population: all ages (A), aged <15 years (B). Figures show the percentage of individuals with positive serology. Error bars are 95% C.I. for a proportion. The number of people tested and odds ratio (95% C.I.) are shown above each graph.
In terms of current infection, although PCR positivity was greater in the HbE β thalassemia patients than that in the controls for P. vivax [9 of 74 (12.1%) and 5 of 79 (6.3%), respectively; P = 0.33] and P. falciparum [12 of 74 (16.2%) and 5 of 79 (6.3%), respectively; P = 0.092], these differences were not statistically significant. These low levels of current infection presumably reflect the reduction in the level of malaria transmission over the period of study (11).
Effect of Splenectomy on Malarial Antibody Levels.
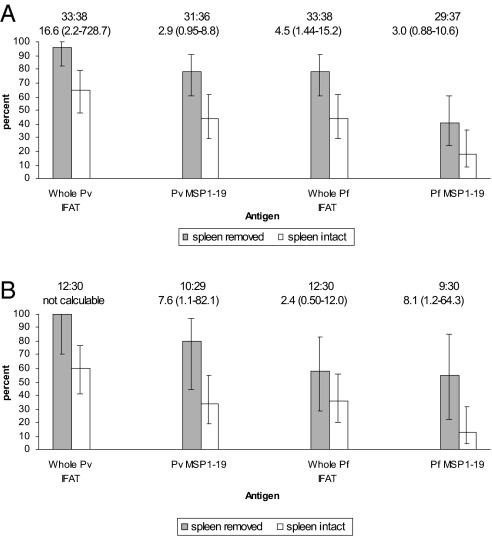
Because splenectomy was, until recently, a very common therapeutic option for the management of HbE β thalassemia in Sri Lanka, comparing the results of malarial antibody estimations between 35 splenectomized and 40 nonsplenectomized patients with this disease was possible. The results are shown in Fig. 2A. Antibody positivity was greater in the splenectomized compared with that in the nonsplenectomized patients; this was marked for the IFAT but of borderline statistical significance for MSP1–19 antibodies. The greatest difference between the groups was observed in the P. vivax IFAT. The higher relative frequency of antibody positivity in splenectomized patients and pattern of responses also were observed in the younger individuals (Fig. 2B). Interestingly, the differences in responses to the MSP1–19 antigens between the younger splenectomized patients and the controls with intact spleens were statistically significant.
Fig. 2.
Comparison of malarial antibody prevalence between patients with HbE β thalassemia who have undergone splenectomy and those with intact spleens: all ages (A), aged <15 years (B). Figures show the percentage of individuals with positive serology. Error bars are 95% C.I. for a proportion. The number of people tested and odds ratio (95% C.I.) are shown above each graph.
Is Splenectomy the Reason for the Relatively High Levels of Malarial Antibodies in HbE β Thalassemia?
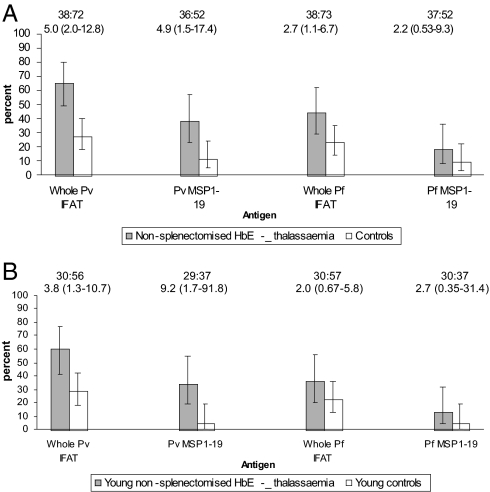
To address this question, the levels of antibodies were compared between nonsplenectomized HbE β thalassemic patients and age-matched controls. The results are summarized in Fig. 3A. Overall, there is a significant increase in the occurrence of antibodies in the nonsplenectomized HbE β thalassemic patients compared with that in the control population; again the results are more impressive for P. vivax malaria. This is particularly clear in the case of the HbE β thalassemic patients aged <15 years in whom there is a highly significant increase in the frequency of antibodies to P. vivax MSP1–19, whereas the differences for P. falciparum are, again, not significant (Fig. 3B).
Fig. 3.
Comparison of malarial antibody prevalence between nonsplenectomized patients with HbE β thalassemia and controls: all ages (A), patients and controls aged <15 years (B). Bar graphs show the percentage of individuals with positive serology. Error bars are 95% C.I. for a proportion. The number of people tested and odds ratio (95% C.I.) are shown above each graph.
Is the High Frequency of Malarial Antibodies in HbE β Thalassemia Patients a Nonspecific Disease-Related Phenomenon?
Because a high proportion of individuals in Sri Lanka receive immunization against tetanus, to address this question duplicate IgG antibody levels against tetanus were measured by ELISA in 42 patients with HbE β thalassemia aged 1–16 years and 42 age-matched controls. The median tetanus IgG levels were 1.77 international units per milliliter (interquartile range 0.69–3.95, range 0.01–5.1) for the controls and 1.71 international units per milliliter (interquartile range 0.33–4.28, range 0.02–5.1) for the patients with HbE β thalassemia. The mean levels of antibody in the patients and controls were not significantly different (Z = 0.47, P = 0.64).
Does Exposure to Malaria in Early Life Affect the Phenotype of HbE β Thalassemia?
Although both diseases have an extremely complex pathophysiology, they share the common feature of causing a variable degree of splenomegaly. To assess whether there was any interaction between malaria and HbE β thalassemia in determining the maximum spleen size a group of 37 children aged ≤15 years was studied, 14 of whom had spleens that measured <7 cm below the costal margin (range 2–6 cm, mean 3.6 cm) and 23 of whom had spleens >7 cm below the costal margin (range 7–26 cm, mean 13.9 cm). The mean age of the children with spleens of <7 cm was 6.3 years, and the mean age of those with spleens >7 cm was 7.2 years. In the former group, 6 of 14 (42.8%) children were positive for P. vivax antibodies, whereas in the latter group 20 of 24 (83.3%) children were positive (χ2 = 4.962, P = 0.0259). In the case of P. falciparum, 1 of 14 (7.1%) children with spleens <7 cm were positive, and 16 of 24 (66.6%) children with spleens >7 cm were positive (χ2 = 10.378, P = 0.0012). These findings suggest that exposure to malaria contributes to the degree of splenomegaly in HbE β thalassaemia in early life.
Consistency of Malariological Data over the Period of the Study and between Different Antigens.
Studies were carried out on more than one occasion on 39 of the patients with HbE β thalassemia. The findings were consistent across all age groups. Of 20 patients aged ≤15, two became seropositive for both P. vivax and P. falciparum antibodies, two became seropositive for P. vivax antibodies, and three became seropositive for P. falciparum antibodies. Reflecting the low transmission of malaria, six patients were P. vivax PCR negative for the first sample and positive for a second sample, and three children were PCR positive for the first sample and negative for the second sample. In the case of P. falciparum, two patients were PCR negative for the first sample and positive for a second sample, and one was positive for the first sample and negative for the second sample.
Overall, the number of positive results for both P. vivax and P. falciparum antibodies using IFAT tended to be higher than those obtained from MSP1–19 antigens, in both patients with HbE β thalassemia and controls and particularly in young children. These differences may reflect both the low transmission rates and the slower acquisition of antibodies to MSP1–19 compared with crude antigens (16).
Effect of Age on Malariological Data.
This study was carried out in a region of Sri Lanka with a low and decreasing transmission rate for both P. falciparum and P. vivax malaria (11). As judged from the limited PCR data for P. vivax infection, this trend may have continued during the course of this study. However, seropositivity for both parasites was observed from the second year onward in the patients with HbE β thalassemia. To compare the effect of age, the odds ratio for seropositivity in patients for each age stratum was analyzed and combined. The odds ratio of P. vivax antibody positivity was the lowest in the younger age group and became greater with age; the ratio for those aged <5 years was 1.36 (0.3–5.5) and rose to 10.3 (1.0–110) in those aged >15 years (test for trend, P = 0.0005). A similar although less significant trend was observed for P. falciparum. Furthermore, we observed higher titers of antibodies in older patients. For example, the number of young children with maximum antibody titers (1:1,024) to P. vivax was 5 of 51 (9.8%), whereas 23 of 42 (55.48%) of patients aged >15 years achieved this titer.
Discussion
In many developing countries, only after the partial control of malaria and other communicable diseases, together with improvements in public health and nutrition, were the thalassemias recognized as an increasingly important health problem (2). In Sri Lanka, where transmission rates of both P. vivax and P. falciparum malaria have declined over recent years, HbE β thalassemia constitutes approximately one-third of cases of severe β thalassaemia (6, 8). In our study of patients with this disease at the National Thalassaemia Centre in Kurunegala, Sri Lanka, a region of low malarial transmission (11), they have been found to have high frequencies of antibodies to P. vivax and to a lesser degree to P. falciparum from the early years of life, and the levels are significantly higher than those of age-matched controls from the same region. These findings were consistent regardless of whether they were measured by ELISA or IFAT using crude parasite antigens or by antibody responses to MSP1–19, which persist long term once acquired and, therefore, are particularly robust for assessing malaria transmission (16). The results cannot be explained by the fact that some of the patients had undergone splenectomy; although antibody frequencies were significantly higher in splenectomized patients compared with those with intact spleens, the levels in the latter were still significantly higher than those in the age-matched controls.
Considering the low transmission rates for both types of malaria that have been reported in recent years in Sri Lanka (11), at first sight the relatively high values obtained by PCR for P. vivax found in the pilot study and even the lower values found in the later control studies are surprising. However, these data are entirely consistent with a recent report from a low transmission region in Tanzania in which a submicroscopic parasite infection detected by DNA analysis was associated with significantly higher malaria antibody levels than those in control populations that were parasite-free (17). Whether these findings in regions of low transmission reflect repeated submicroscopic infection, relatively long survival of parasite DNA in the circulation, or both is not yet clear.
The reasons for these totally unexpected antibody findings are not absolutely clear. The most likely possibility is that patients with HbE β thalassemia are more susceptible to malaria than normal persons. Particularly in the case of P. vivax, there are compelling reasons why this might be the case. This parasite has a particular predilection for reticulocytes (18, 19). Patients with HbE β thalassemia have enormously expanded erythroid bone marrow, ineffective erythropoiesis, and shortened red-cell survival with significantly increased reticulocyte counts (2). Furthermore, there is an increased rate of infection with P. vivax in young children with α thalassemia in Vanuatu (20) and Papua New Guinea (21); the red cells in this condition also have an increased rate of turnover (22). Although P. falciparum invades red cells of all ages, there is in vitro evidence that it too has a predilection for younger red-cell populations (23).
There are, of course, other possible reasons why children with a severe disease like HbE β thalassemia might be more prone to malaria. For example, there has been considerable interest in the possibility of individual variation in susceptibility to bites by mosquitoes (24). A variety of possibilities have been raised to explain this phenomenon, including body odor and local environmental factors. Children with severe forms of thalassemia are hypermetabolic due to the massive expansion of the bone marrow and increased red-cell turnover and tend to have recurrent attacks of low-grade fever, both of which could modify rates of perspiration and body odor. Splenic dysfunction also is possibly associated with progressive splenomegaly that might contribute to increased susceptibility to infection.
The other surprising finding in this study was the higher level of malarial antibodies in the patients who had been splenectomized compared with that for patients with intact spleens. Although the spleen is known to have important red-cell remodeling, parasite clearance, and immune functions in malarial infection (25–27), its role in protection against the disease is still not clearly defined. Although doubts have been expressed (28), the absence of a spleen is believed generally to be associated with more severe disease, at least in the case of P. falciparum malaria. Therefore, the higher levels of antibody in the splenectomized children possibly reflect the occurrence of more severe or frequent attacks or persistence of malarial antigen in the circulation for longer. In this context, in the present study even those patients with intact spleens had higher malarial antibody levels than those of the control population. Although splenectomy in children with severe forms of thalassemia is well established to be associated with an increased risk of bacterial infection (2), very little is known about the effect of the gradual increasing splenomegaly characteristic of these diseases on susceptibility to bacterial or parasitic infection. Given the extensive anatomical abnormalities of these enlarged spleens (2), defective splenic function also possibly may contribute to the increased susceptibility to malaria in children with HbE β thalassemia.
Progressive enlargement of the spleen is a major factor in the evolution of the more severe phenotypes of HbE β thalassemia, leading to progressive anemia due to red-cell sequestration and expanded plasma volume, growth retardation, and other complications (2, 4). Therefore, in this study positivity for antibodies against both P. falciparum and P. vivax is of importance to be significantly higher in those with spleens >7 cm below the costal margin, a size at which features of severe hypersplenism occur frequently (2).
Some cross-reactivity is well recognized to occur between malaria species in the IFAT assay, and this is relevant to our study population, because both P. falciparum and P. vivax occur in the Sri Lankan population. We selected a positive cutoff value of 1 in 32 serum dilution to minimize the effect of cross-reactivity. We consider that the IFAT identified malarial species with reasonable accuracy, because the same significant differences in seropositivity were demonstrated by the MSP 1–19 ELISA, which shows no evidence of cross-reactivity between species. In addition, the differences in seropositivity according to age and HbE β thalassemia status cannot be accounted for by low species specificity in the IFAT. Hence, although recognizing the possibility of cross-reaction in the IFAT assay, the data suggest that, although the patients with HbE β thalassemia appear to be more prone to both types of parasite, susceptibility to P. vivax is more marked.
The finding of increased susceptibility to malaria, P. vivax in particular, in children with HbE β thalassemia also raises some new questions regarding the evolutionary biology of the malaria-susceptibility polymorphisms in Asia. Children who are carriers of α thalassemia have been found to be more susceptible to P. vivax infection (20, 21), suggesting that, because of potential cross-immunity between parasite species, this could render them more resistant to P. falciparum later in development (20). Because the hematologic phenotypes of β thalassemia and HbE carriers are similar to those of α thalassemia, particularly in the case of HbE, and in their compound heterozygous states they appear to show an increased susceptibility to P. vivax, these traits alone possibly may be more susceptible. There is strong evidence that both HbE and β thalassemia traits confer resistance to P. falciparum, although the cellular mechanisms are not clear (29); preimmunization by P. vivax offers a possible mode of protection in populations in which both forms of malaria occur at high frequencies (14, 20). The overall effects on the frequency of these susceptibility genotypes also will depend on whether there are protective polymorphisms for susceptibility to P. vivax; epidemiological studies in Sri Lanka suggest that ≈10–15% of variation in P. vivax infection may have a genetic basis, although the genes involved have not been identified (30).
Over recent years there has been a growing awareness of the clinical importance of P. vivax malaria, which affects millions of people in parts of South and Southeast Asia, where HbE β thalassemia is also extremely common. Currently, because of emerging drug resistance and difficulties associated with prophylaxis or obtaining a radical cure for P. vivax malaria, its control and management are under intense investigation (14). Because significant numbers of patients with HbE β thalassemia can grow and develop satisfactorily at relatively low hemoglobin levels (7, 8) further studies of this kind are vital to be carried out in regions where this disease and related forms of thalassemia are common and particularly where there are high transmission rates of malaria. Hitherto, the interplay between different forms of malaria and the more serious varieties of thalassemia has been neglected; malaria prophylaxis or radical cure likely will have to become an integral part of thalassemia control programs in many countries in Asia.
Materials and Methods
Patients.
Blood samples were collected from patients treated at the National Thalassemia Centre in Kurunegala, Sri Lanka, as part of their routine clinical follow-up. The diagnosis and severity categorization of HbE β thalassemia were made by clinical and hematological analysis, hemoglobin analysis, and identification of the underlying mutations in the β globin genes, as described in refs. 6–8. The age-matched control populations were patients with any other disorder who were being treated in the same hospital and from the same region and socioeconomic group. For the pilot study, additional samples were obtained from a local blood bank, and patients with thalassemia major were patients who were receiving regular transfusions in the same clinic.
Malarial Antibodies.
Plasma and cell pellets from EDTA samples and sera were stored at −20 °C before shipping on dry ice to Oxford, United Kingdom. The IgG-specific antibodies to P. falciparum schizont antigens and IgG antibodies to the C termini of P. falciparum merozoite MSP1–19 and P. vivax MSP1–19 in serum were measured by ELISA, as described in refs. 31 and 32. The IgG antibodies to P. vivax and P. falciparum in serum were identified by IFAT using cell cultures of P. falciparum IT04 clone cultured in group O Rhesus positive red blood cells and blood samples collected from chimpanzees infected with the Salvador 1 strain of P. vivax. Nested PCR was used to detect P. vivax and P. falciparum DNA sequences (33, 34). All tests were carried out in duplicate and included positive and negative controls, including sera from 30 European controls not previously exposed to malaria.
Plasmodium falciparum-Specific IgG ELISA.
Plasmodium falciparum schizont antigens were obtained from frozen cultures of the ITO A4 clone in O Rhesus positive red cells. Uninfected Rhesus positive cells were used as controls. Schizont antigens and control cells were diluted 1:10,000 in PBS, and serum samples were diluted 1:500 for the assay. The OD was measured at 492 nm using a Multiskan Ascent microplate reader. The OD for the control well of each sample was subtracted from that of the corresponding antigen well to give a corrected OD value. Plasmodium falciparum-specific IgG positive samples were those with a corrected OD more than two standard deviations above the mean OD of 30 European negative control sera.
Plasmodium falciparum MSP1–19 and Plasmodium vivax MSP 1–19 IgG ELISA.
This assay used recombinant antigens P. falciparum MSP1–19 GST [(Wellcome genotype) or P. vivax MSP1–19 GST (P. vivax Belem strain cloned in a similar manner to Babon et al. (35)]. Antigens were coated at 0.5 μg/mL in carbonate buffer, pH 9.6, and serum samples were diluted at 1:1,000 for the assay. Positivity for IgG antibodies to MSP1–19 was defined as a mean OD more than three standard deviations above the mean OD of the 30 European sera.
IFAT for IgG Antibodies to P. vivax and P. falciparum.
Frozen antigen multispot slides prepared from P. vivax-infected chimpanzees were provided by William Collins (Center for Disease Control and Prevention, Atlanta, GA), and frozen cultures of the P. falciparum ITO A4 clone in O Rhesus positive red cells were used to prepare P. falciparum antigen slides. Serial dilutions (1:16 to 1:1,024) of each sample in PBS were added to a correspondingly labeled antigen spot on a multispot slide. Slides were incubated at 37 °C for 30 min in a moisture chamber, then washed in PBS with 0.05% Tween 20 and 1% Marvel for 15 min, and allowed to dry. A 1:1,000 dilution of goat anti-human IgG (Fc-specific) FITC conjugate in PBS and 0.05% Evans Blue was added to each spot. The slides were incubated for 30 min in the dark at room temperature in a moisture chamber. Slides were washed, dried, mounted with a coverslip, and examined under a fluorescent microscope using both FITC and triple filters for the presence of fluorescent antibody bound to parasite antigens. Seropositivity was defined as the detection of fluorescently labeled antibodies in samples diluted 1:32 or greater. The antibody titer was defined as the weakest dilution at which fluorescently-labeled antibody could be observed for each sample. For those samples in which fluorescence was still observed at a 1:1,024 dilution, a further series of serial dilutions (1:1,024 to 1:32,768) were prepared, and the test was repeated.
Nested PCR for P. vivax and P. falciparum.
Plasmodium genus- and species-specific primers were used to amplify a 121-bp DNA product of the small subunit 18S rRNA gene specific to P. vivax and a 205-bp DNA product of the small subunit 18S rRNA gene specific to P. falciparum. DNA was extracted from plasma (DNA blood mini kit; Qiagen) and resuspended in 50 μL of double-distilled water. Five microliters of DNA template was used for the first round of the nested PCR. Positive and negative control DNA samples were included for all tests. Briefly, each nest 1 reaction contained DNA, PCR gold buffer (Applied Biosystems), 3.5 mM magnesium chloride, 250 μM each dNTP, 50 pmol of each Plasmodium genus-specific primer (sense, 5′ TTA AAA TTG TTG CAG TTA AAA CG 3′; antisense, 5′ CCT GTT GTT GCC TTA AAC TTC 3′), 2.5 units of Amplitaq Gold (Applied Biosystems), and double-distilled water to a final volume of 50 μL. Each reaction was overlaid with 10 μL of mineral oil. The PCR cycling conditions were an initial denaturation step at 95 °C for 5 min followed by 35 cycles of denaturation at 94 °C for 30 s, annealing at 58 °C for 1 min and extension at 72 °C for 1 min, and a final extension step at 72 °C for 5 min (34).
A P. falciparum and a P. vivax nest 2 PCR was set up for each sample using 3 μL of PCR product generated from the nest 1 reaction and species-specific primers for P. falciparum and P. vivax [for P. falciparum, sense, 5′ TTA AAC TGG TTT GGG AAA ACC AAA TAT ATT 3′; antisense, 5′ ACA CAA TGA ACT CAA TCA TGA CTA CCC GTC 3′; for P. vivax, sense, 5′ CGC TTC TAG CTT AAT CCA CAT AAC TGA TAC 3′; antisense, 5′ ACT TCC AAG CGC AAG CAA AGA AAG TCC TTA 3′ (33)]. All other reaction and PCR cycling conditions were as described above. Nest 2 PCR products were run on 3.0% agarose gels, stained with ethidium bromide, and visualized with a UV transilluminator. A 121-bp DNA product specific to P. vivax or a 205-bp DNA product specific to P. falciparum indicated a positive result.
IgG Antibody Levels to Tetanus Toxoid Antigen.
These levels were measured by ELISA, using a commercially-available kit (RE56901; IBL).
Statistical Analysis.
The χ2 test was used for categorical data. Calculation of 95% confidence intervals for proportions was done by Dimension Research, Inc. Calculation of odds ratios was done using EPI INFO6, version 6.04d (Centers for Disease Control and Prevention and World Health Organisation). P < 0.05 was considered statistically significant.
Ethical Permission.
Ethical approval for the research program on HbE β thalassemia was obtained from the Ethical Committee of the College of Paediatricians, Colombo, Sri Lanka, and the Oxford Tropical Research Ethics Committee.
Acknowledgments.
We thank William Collins for kindly providing the P. vivax antigen slides, Tony Holder for reagents, Robert Pinches for the preparation of the P. falciparum schizont antigens, Wendy Bailey and Brett Lowe for their advice on the IFAT assays, Brian Greenwood, Richard Carter, and Nick White for their critical assessment of many aspects of this work, and Jeanne Packer and Liz Rose for help in preparing the manuscript. This work was supported by grants from The Wellcome Trust and March of Dimes.
Footnotes
The authors declare no conflict of interest.
References
- 1.Christianson A, Howson CP, Modell B. March of Dimes Global Report on Birth Defects. White Plains, NY: March of Dimes Birth Defects Foundation; 2006. [Google Scholar]
- 2.Weatherall DJ, Clegg JB. The Thalassaemia Syndromes. 4th Ed. Oxford: Blackwell Scientific; 2001. [Google Scholar]
- 3.Orkin SH, et al. Abnormal RNA processing due to the exon mutation of βE-globin gene. Nature. 1982;300:768–769. doi: 10.1038/300768a0. [DOI] [PubMed] [Google Scholar]
- 4.Fucharoen S, Winichagoon P. Hemoglobinopathies in Southeast Asia: Molecular biology and clinical medicine. Hemoglobin. 1997;21:299–319. doi: 10.3109/03630269709000664. [DOI] [PubMed] [Google Scholar]
- 5.Khan WA, et al. Prevalence of beta thalassemia trait and Hb E trait in Bangladeshi school children and health burden of thalassemia in our population. Dhaku Shishu (Children) Hospital Journal. 2005;21:1–7. [Google Scholar]
- 6.Fisher CA, et al. The molecular basis for the thalassaemias in Sri Lanka. Br J Haematol. 2003;121:662–671. doi: 10.1046/j.1365-2141.2003.04346.x. [DOI] [PubMed] [Google Scholar]
- 7.Olivieri NF, et al. Studies in haemoglobin E beta-thalassaemia. Br J Haematol. 2008;141:388–397. doi: 10.1111/j.1365-2141.2008.07126.x. [DOI] [PubMed] [Google Scholar]
- 8.Premawardhena A, et al. Haemoglobin E β thalassaemia in Sri Lanka. Lancet. 2005;366:1467–1470. doi: 10.1016/S0140-6736(05)67396-5. [DOI] [PubMed] [Google Scholar]
- 9.Rund D, Fucharoen S. Genetic modifiers in hemoglobinopathies. Curr Mol Med. 2008;8:600–608. doi: 10.2174/156652408786241410. [DOI] [PubMed] [Google Scholar]
- 10.Sripichai O, et al. Coinheritance of the different copy numbers of α-globin gene modifies severity of β-thalassemia/Hb E disease. Ann Hematol. 2008;87:375–379. doi: 10.1007/s00277-007-0407-2. [DOI] [PubMed] [Google Scholar]
- 11.Briët OJ, Galappaththy GN, Konradsen F, Amerasinghe PH, Amerasinghe FP. Maps of the Sri Lanka malaria situation preceding the tsunami and key aspects to be considered in the emergency phase and beyond. Malar J. 2005;4:8. doi: 10.1186/1475-2875-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mendis C, et al. Characteristics of malaria transmission in Kataragama, Sri Lanka: A focus for immuno-epidemiological studies. Am J Trop Med Hyg. 1990;42:298–308. doi: 10.4269/ajtmh.1990.42.298. [DOI] [PubMed] [Google Scholar]
- 13.Mendis K, Sina BJ, Marchesini P, Carter R. The neglected burden of Plasmodium vivax malaria. Am J Trop Med Hyg. 2001;64:97–106. doi: 10.4269/ajtmh.2001.64.97. [DOI] [PubMed] [Google Scholar]
- 14.Rogerson SJ, Carter R. Severe vivax malaria: Newly recognised or rediscovered. PLoS Med. 2008;5:e136. doi: 10.1371/journal.pmed.0050136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Choudhury NJ, et al. Post-transfusion malaria in thalassaemia patients. Blut. 1990;61:314–316. doi: 10.1007/BF01732885. [DOI] [PubMed] [Google Scholar]
- 16.Drakeley CJ, et al. Estimating medium- and long-term trends in malaria transmission by using serological markers of malaria exposure. Proc Natl Acad Sci USA. 2005;102:5108–5113. doi: 10.1073/pnas.0408725102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shekalaghe S, et al. Low density parasitaemia, red blood cell polymorphisms and Plasmodium falciparum specific immune responses in a low endemic area in northern Tanzania. BMC Infect Dis. 2009;9:69. doi: 10.1186/1471-2334-9-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Galinski MR, Medina CC, Ingravallo P, Barnwell JW. A reticulocyte-binding protein complex of Plasmodium vivax merozoites. Cell. 1992;69:1213–1226. doi: 10.1016/0092-8674(92)90642-p. [DOI] [PubMed] [Google Scholar]
- 19.Rodriguez LE, et al. Plasmodium vivax MSP-1 peptides have high specific binding activity to human reticulocytes. Vaccine. 2002;20:1331–1339. doi: 10.1016/s0264-410x(01)00472-8. [DOI] [PubMed] [Google Scholar]
- 20.Williams TN, et al. High incidence of malaria in α-thalassaemic children. Nature. 1996;383:522–525. doi: 10.1038/383522a0. [DOI] [PubMed] [Google Scholar]
- 21.Allen SJ, et al. α+-Thalassemia protects children against disease caused by other infections as well as malaria. Proc Natl Acad Sci USA. 1997;94:14736–14741. doi: 10.1073/pnas.94.26.14736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rees DC, Williams TN, Maitland K, Clegg JB, Weatherall DJ. Alpha thalassaemia is associated with increased soluble transferrin receptor levels. Br J Haematol. 1998;103:365–370. doi: 10.1046/j.1365-2141.1998.00971.x. [DOI] [PubMed] [Google Scholar]
- 23.Pasvol G, Weatherall DJ, Wilson RJ. The increased susceptibility of young red cells to invasion by the malarial parasite Plasmodium falciparum. Br J Haematol. 1980;45:285–295. doi: 10.1111/j.1365-2141.1980.tb07148.x. [DOI] [PubMed] [Google Scholar]
- 24.Keystone JS. Of bites and body odour. Lancet. 1996;347:1423. doi: 10.1016/s0140-6736(96)91678-5. [DOI] [PubMed] [Google Scholar]
- 25.Barnwell JW, Howard RJ, Coon HG, Miller LH. Splenic requirement for antigenic variation and expression of the variant antigen on the erythrocyte membrane in cloned Plasmodium knowlesi malaria. Infect Immun. 1983;40:985–994. doi: 10.1128/iai.40.3.985-994.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chotivanich K, et al. Central role of the spleen in malaria parasite clearance. J Infect Dis. 2002;185:1538–1541. doi: 10.1086/340213. [DOI] [PubMed] [Google Scholar]
- 27.Wyler DJ, Oster CN, Quinn TC. In: The Role of the Spleen in the Immunology of Parasitic Diseases. Torrigiani G, editor. Basel: Schwabe; 1979. pp. 183–204. [Google Scholar]
- 28.Looareesuwan S, Suntharasamai P, Webster HK, Ho M. Malaria in splenectomized patients: Report of four cases and review. Clin Infect Dis. 1993;16:361–366. doi: 10.1093/clind/16.3.361. [DOI] [PubMed] [Google Scholar]
- 29.Weatherall DJ. Genetic variation and susceptibility to infection: The red cell and malaria. Br J Haematol. 2008;141:276–286. doi: 10.1111/j.1365-2141.2008.07085.x. [DOI] [PubMed] [Google Scholar]
- 30.Mackinnon MJ, et al. Quantifying genetic and nongenetic contributions to malarial infection in a Sri Lankan population. Proc Natl Acad Sci USA. 2000;97:12661–12666. doi: 10.1073/pnas.220267997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Snow RW, et al. Infant parasite rates and immunoglobulin M seroprevalence as a measure of exposure to Plasmodium falciparum during a randomized controlled trial of insecticide-treated bed nets on the Kenyan coast. Am J Trop Med Hyg. 1996;55:144–149. [PubMed] [Google Scholar]
- 32.Okech BA, et al. Fine specificity of serum antibodies to Plasmodium falciparum merozoite surface protein, PfMSP-119, predicts protection from malaria infection and high-density parasitemia. Infect Immun. 2004;72:1557–1567. doi: 10.1128/IAI.72.3.1557-1567.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Snounou G, et al. High sensitivity of detection of human malaria parasites by the use of nested polymerase chain reaction. Mol Biochem Parasitol. 1993;61:315–320. doi: 10.1016/0166-6851(93)90077-b. [DOI] [PubMed] [Google Scholar]
- 34.Gal S, et al. Detection of Plasmodium falciparum DNA in plasma. Ann NY Acad Sci. 2001;945:234–238. doi: 10.1111/j.1749-6632.2001.tb03891.x. [DOI] [PubMed] [Google Scholar]
- 35.Babon J, et al. Structural studies on Plasmodium vivax merozoite surface protein-1. Mol Biochem Parasitol. 2007;153:31–40. doi: 10.1016/j.molbiopara.2007.01.015. [DOI] [PubMed] [Google Scholar]