Abstract
Depending on the circumstances, decision making requires either comparing current sensory information with that showed recently or with that recovered from long-term memory (LTM). In both cases, to learn from past decisions and adapt future ones, memories and outcomes have to be available after the report of a decision. The ventral premotor cortex (PMv) is a good candidate for integrating memory traces and outcomes because it is involved in working-memory, decision-making, and encoding the outcomes. To test this hypothesis we recorded the extracellular unit activity while monkeys performed 2 variants of a visual discrimination task. In one task, the decision was based on the comparison of the orientation of a current stimulus with that of another stimulus recently shown. In the other task, the monkeys had to compare the current orientation of the stimulus with the correct one retrieved from LTM. Here, we report that when the task required retrieval of the stimulus and its use in the following trials, the neurons continue encoding this internal representation together with the outcomes after the monkey has emitted the motor response. However, this codification did not occur when the stimulus was shown recently and updated every trial. These results suggest that the PMv activity represents the information needed to evaluate the consequences of a decision. We interpret these results as evidence that the PMv plays a role in evaluating the outcomes that can serve to learn and thus adapt future decision to environmental demands.
Keywords: decision-making, outcomes, single neural activity, working memory
Decision making is a complex process essential for guiding behavior that involves evaluating past and current events and their consequences. Electrophysiological studies have shown that several cortical areas participate in the decision making process (1–15). Most decisions are made by comparing recent events with current ones. This is what happens in tasks where monkeys are trained to decide on the difference between 2 sensory stimuli (S1 and S2) showed sequentially and separated by a short interval: the continuous discrimination (CD) task (11, 16–18). This has revealed the role played by several cortical areas in decision making (4, 5, 11, 19–21), including the participation of the ventral premotor cortex (PMv) in reporting outcomes and in integrating previous choices with their consequences (12).
Decisions are also made by comparing long-term memorized events with current ones and, to our knowledge, there are few reports of the cortical areas being involved in a decision process when part of the sensory information has to be recovered from long-term memory (11). To evaluate the consequences of these decisions the information about the retrieved sensory evidence has to be available together with the information about previous choices and their outcomes. This process can be studied with the Fixed Discrimination with Implicit Reference task (FDIR), a variant of the CD task, in which S1 was implicit and monkeys had to recover its correct orientation from long-term memory (LTM) and use its internal representation in the following trials (11, 17).
We hypothesized that for the PMv to play a role in decision-making, single neurons had to combine short-term or long-term memorized sensory events with current ones. Moreover, as both the sensory information used to take the decision and the outcomes can play a role in learning and shaping future behavior (12), we also hypothesized that this information has to be available after the behavioral response.
Here we report that in the FDIR task, PMv neurons encode the internal representation of the stimuli recovered from LTM during all task periods and use this information to reach a decision. These neurons continue encoding the memory traces together with the outcomes after the animal had made the motor report. However, these neural responses do not exist when the conditions of the task changed and the 2 stimuli were shown. We propose that the availability of memory traces and the outcomes can serve to learn and adapt future behavior to the environmental demands.
Results
We simultaneously recorded the extracellular single unit activity while 2 monkeys performed in 2 tasks. In the CD task (Fig. 1A), the monkeys had to decide whether the orientation of a current stimulus (S2) was to the right or left of a memorized trace of S1, recently shown in the visual field. Subjects perceived S1, stored a trace of it in working memory (WM) during the delay period, perceived S2, compared the orientation of S2 to the trace of S1 (S2-S1), decided on the direction of the difference in orientation between the 2 [sign(S2-S1)], and communicated the result of the decision by making an eye movement toward one target. The comparison between the orientation of S1 and S2 reflects the direction (left or right) and the magnitude of the difference (S2-S1). The direction of the difference between the 2 stimuli represents the choice [(S2-S1)]. Tasks like these have revealed that in the PMv the memorized traces of S1 were available during the delay and comparison periods so as to decide the orientation of the stimuli (5, 12).
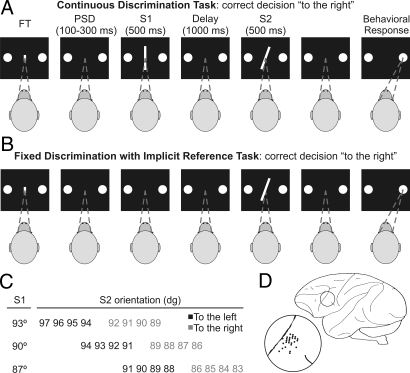
Fig. 1.
Sequence of events during the discrimination tasks. (A) CD task; the fixation target (FT) and the 2 circles appeared simultaneously in the center and at both sides of the monitor screen, respectively. The monkey initiated the trial by fixating the FT. Fixation had to be maintained during the trial otherwise it was aborted. When the FT disappeared and after a variable prestimulus delay (PSD) (100–300 ms) 2 stimuli (oriented lines S1, S2) each of 500 ms duration, appeared in sequence separated by a delay of 1 s. Once S2 had disappeared, the monkey made an eye movement to one of the two circles to indicate whether the orientation of S2 was to the left or to the right of S1. Correct discriminations were rewarded. Masking white noise was present during the trial. S1 and S2 changed randomly from trial to trial. (B) FDIR task ; same temporal sequence and stimuli set as in the CD task, except that S1 was not shown (i.e., it was implicit) and had to be recovered from LTM by trial and error. The implicit stimulus changed from block to block of about 90 trials each and only S2 changed trial by trial. The interval between trials changed randomly in the 2 tasks between 1.5–3.5s. (C) Distribution of the orientation of the stimuli used in the CD and FDIR tasks. We used 3 S1 and 8 S2 for each S1. (D) Sketch of the brain showing the localization of the recording area.
Since that decision can be made using sensory information retrieved from LTM, we used a variant of the CD task to study this process. In the FDIR task (Fig. 1B), S1 was implicit and remained the same in a block of about 90 trials, only S2 changed. Subjects had to retrieve, at the beginning of each block, the correct S1 by trial and error. In fact, the percentage of errors diminished from the first trial (16% mean) and stabilized from the seventh trial onwards (5% mean). From then on the decision process continued as in the CD task. The most parsimonious explanation is that S1 is retrieved from LTM and maintained in WM (11, 16, 17). The behavioral results show that the monkeys' performance in the 2 tasks is close to their psychophysical thresholds and is based on the comparison of the 2 stimuli, regardless of whether they were recently shown or retrieved from LTM (Fig. S1 A and B). The study of the same PMv neurons in the 2 tasks (n = 105) revealed that the activity represents the monkeys' decisions regardless the orientation of S1 was shown recently in the visual world or retrieved from LTM (SI Results and Figs. S2 and S3). Monkeys performed on a control task (12) in which the motor component was the same as that in the CD and FDIR tasks; the results showed that the motor component of the task couldn't explain the differential responses as a function of the choice (SI Results and Fig. S4).
The Availability of the Memory Traces Depends on Task Demands.
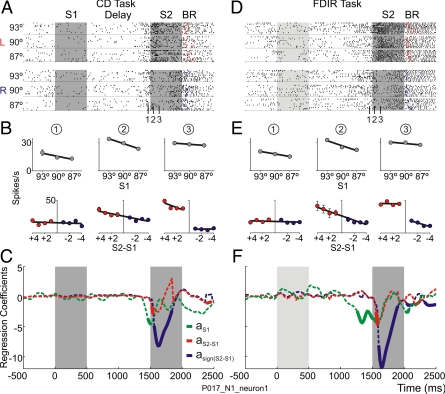
In our tasks, to perform the comparison process, the trace of S1 has to be available during the presentation of S2 and the neural activity has to reflect the comparison between the trace of S1 and S2 (S2-S1). The firing rate of the neuron of Fig. 2 A and D has been sorted by S1 and the choice. By the end of the delay period, the firing rate is modulated by the traces of S1. During the comparison period, the firing rate is modulated by the traces of S1 and by the choice regardless of whether the stimuli were recently showed or retrieved from LTM. The events that constitute the decision process evolve very rapidly. By the end of the delay, the neuron encodes the traces of S1 (Fig. 2 B and E) (1). During the first 50 ms of the comparison period, the neuron continues encoding S1 at the same time as it codifies the comparison between S2 and S1 (S2-S1, Fig. 2 B and E) (2). From the next 60 ms onwards, the neuron now codifies the orientation of S2 to the left and to the right of S1 [sign(S2-S1), Fig. 2 B and E] (3).
Fig. 2.
Temporal evolution of neural activity depends on the trace left by the first stimuli—regardless of whether it was recently shown in the visual world or retrieved from LTM—on the comparison between the first and the second stimuli and on the choice. (A and D) Rasters of the same neuron sorted by S1 (93°, 90° and 87°) and the choice (L, to the left; R, to the right). Red and blue dots signal the behavioral response (BR) in each trial, to the left and to the right, respectively. (B and E) Temporal evolution of the average firing rates fitted as a function of: S1 and the relative orientation of S2 (S2-S1) during the delay [1] and the comparison [2] and [3] periods. (C and F) Stepwise Linear Regression coefficients, aS1, aS2-S1, and asign(S2-S1), as a function of time; continuous traces indicate significant coefficients.
Significant coefficients corresponding to S1 (aS1) appear by the end of the delay period and, during the first 50 ms of the comparison period, together with S2-S1 (aS2-S1). The significant choice coefficient (asign(S2-S1)) partially overlaps S1 and S2-S1 coefficients and continues for the rest of the comparison period (Fig. 2 C and F). These analyses reveal that the information from the first stimulus was used to determine the orientation of S2 also when S1 was retrieved from LTM. These findings suggest that the PMv plays a role in decision-making by combining short-term or long-term memorized events with current ones.
Access to Memory Resources and Availability of Their Traces Depends on the Behavioral Tasks.
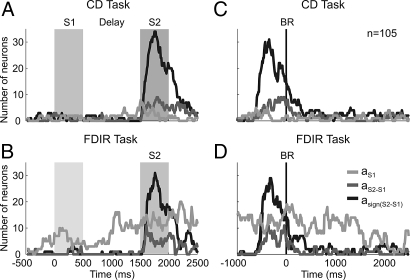
The CD task required maintaining the memory traces of S1 during the delay and comparison periods only. However, the FDIR task required retrieving the correct S1 from LTM and using it for the following trials and therefore the memory traces of S1 should be available during all task periods and after the behavioral report. In fact, these differences between the CD and FDIR tasks are revealed in the dynamics of the PMv population response both until the behavioral report and from the behavioral report onwards. This dynamics is shown in Fig. 3 where the numbers of neurons with significant SLR aS1, aS2-S1 and asign(S2-S1) coefficients are displayed as a function of time. See Table S1 and Table S2.
Fig. 3.
Dynamics of PMv population response during the CD and FDIR tasks. Number of neurons with significant SLR coefficients as a function of time with respect to S1 (A and B) and to the BR (C and D).
The number of neurons that encode S1, the comparison (S2-S1) or the choices [sign(S2-S1)] varies depending on the task. In the CD task, we found that 20 neurons encoded S1 during the delay and the comparison periods, 26 neurons encoded S2-S1 during the comparison period and 54 neurons encoded the choice during the comparison period. However, in the FDIR task the main difference is that the traces of S1 were maintained from the beginning of the trial until the comparison period (n = 59) while the dynamics of both S2-S1 (n = 27) and the choice (n = 45) were the same as in the CD task. In the CD and FDIR tasks, few neurons carried information about the comparison (S2-S1) or the choices (sign(S2-S1)) after the behavioral response (CD, n = 21, n = 26; FDIR, n = 17, n = 29, respectively). However, in the FDIR but not in the CD task a large number of neurons did carry the memory traces of S1 after the behavioral response (FDIR, n = 48; CD, n = 10). This information may be useful for assessing the outcomes and for adapting future decisions if necessary.
Availability of Stimuli Traces and Outcomes After the Decision Report.
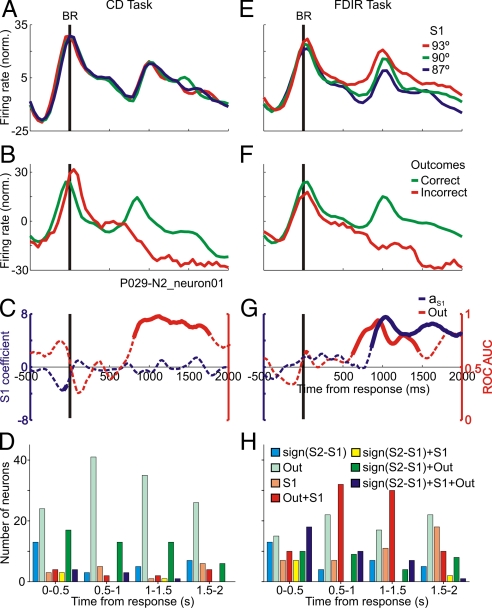
We further investigated the codification of the memory traces and outcomes after the behavioral report was made. Single neuron activity (Fig. 4A, E, C, and G) revealed that after the report of the decision, neurons significantly encoded the S1 traces in the FDIR task. This codification does not happen in the CD task because S1 was showed in the next trial and therefore its availability during this time period is useless. In both tasks, the single neural activity after the behavioral report reflected the correctness and incorrectness of the choice that had been made (Figs. S5 and S6). To assess how the decision process has evolved and to learn from the errors, in the FDIR task the representation of memory traces of S1 is not enough and a concurrent representation with the outcomes is needed. In fact, after the behavioral response in the FDIR task, single neurons encode S1 memory traces and carry significant information about the correctness and incorrectness of the decision that had been made (Fig. 4 B, F, C, and G). This double codification did not happen in the CD task most probably because as the first stimulus was shown in every trial this information was useless and keeping it was unnecessary.
Fig. 4.
After the monkey gave the BR the same neuron encoded the outcomes (Out) and the memory traces of S1 depending on the task. (A and E) Averaged firing rates sorted by S1; the memory traces are encoded in the FDIR task only. (B and F) Averaged firing rates sorted by correct and incorrect outcomes; in both tasks the same neuron differentiated correct from incorrect trials. (C and G) ROC AUC for correct vs. incorrect trials and SLR aS1 coefficient, as a function of time; continuous traces indicate significant values. (D and H) Number of neurons that carried significant information (ROC and SLR analysis) about the components of the decision in CD and FDIR task. Time intervals taken from the behavioral response. The same neurons can be represented in more than one time period.
In the CD task the decision process can be evaluated by integrating previous decisions with outcomes; there are neurons that encode the outcomes, the previous choice, or both components of the task simultaneously (Fig. 4D). The performance in the FDIR task depends also on the recovered orientation of S1. Therefore, to evaluate the decision process and adjust future behavior this information has to be combined with the outcomes of the choices. In the FDIR task there are neurons that encode the same components of the task as in the CD task (outcomes, previous choices, and previous choices and outcomes simultaneously) and (a) the recovered orientation of S1 and the outcomes, and (b) the recovered S1 orientation, the previous choices and the outcomes (Fig. 4H).
Discussion
Our main finding is that the activity of the PMv neurons depends on task demands. When the information about the first stimulus is recovered from LTM and maintained trial to trial, its memory trace and the outcome of the decision are available after the motor report. This encoding does not occur when the information about the first stimulus is available in the visual field because it is not necessary to maintain it in memory.
Validation of Our Results.
Decision reports in the CD task were made with vibrotactile and visual stimuli in several cortical areas (4, 5, 11, 12, 19–21). The PMv activity during this process cannot be explained only by a motor component (5, 12). Other discrimination tasks, e.g., the random dot motion, have revealed the role of sensory, associative and motor areas in perceptual decisions by combining perceptive information with previous knowledge (22). The events that take place after the behavioral report have been studied in perceptual decisions (12) and mainly in value-based decisions (23–25). The codification of the correctness and incorrectness of a behavioral result is important to evaluate previous decisions and adapt future behavior (23, 26). Neural activity related to outcomes has been shown in several cortical areas (23, 24, 27–32). To use the information about the outcomes to shape behavior, there has to be an integration of the outcomes with the previous decision (25, 33–35). Decisions and outcomes are represented in different neurons of the caudate nucleus (25) and in the same neurons in the supplementary eye fields (35), in the prefrontal cortex (34, 36), and in PMv (12).
Decisions are based many times on changing sensory information that has to be contrasted with a constant one (FDIR task). Under these circumstances, the constant information is kept after the behavioral report together with the behavioral result. The advantage of this concurrent representation after the behavioral report is that it can be used to evaluate the choice that has been made and if it is necessary to adapt the strategy for the next decision. In the CD task, the codification of the first stimuli after the behavioral report would not have that behavioral meaning because S1 is presented in every trial and subjects do not need to predict which S1 will appear in the following trial and this interpretation is consistent with our results.
Comparison with Other Studies.
Our observation that PMv neurons reflect the decision process and the correct and incorrect outcomes suggest that they are associated with reporting the decision and encoding the sensory signals on which the decision is based. Some of our findings in PMv agreed with those reported by Romo et al. (5) with a vibrotactile CD task. They identified neurons involved in the decision process which were driven by vibrotactile stimuli and their traces during the delay and comparison periods. Similar results were described in other cortical areas (4, 5, 11, 18, 21). In the medial premotor cortex, when the decision report was postponed, the neural activity between the end of the stimuli and the motor report reflected again the monkey's choice and the sensory information (18). In our task, we have investigated what happened after the monkeys emitted the motor report, finding that when needed (FDIR task) the neural activity reflected concurrently the outcomes, the memory traces of the stimuli and the choices used to take the decision.
The adaptation of the PMv neural activity to the demands of a perceptual task suggests that this cortical area may use past and current information for assessing the result of the decision and if it is necessary adjust the behavior in the next one.
Materials and Methods
Discrimination Tasks.
Monkeys were trained to perform in 3 tasks, already described in detail: continuous discrimination, control task (12, 17), and fixed discrimination with implicit reference (FDIR, (11, 17)) (SI Methods). Here we report on the same neurons studied in the CD and FDIR (n = 105) (Fig. 1). All these neurons were task-related (Wilcoxon test, P < 0.01). Some of them could be also studied in a motor control task (n = 59; SI Methods). Animals (BM5, 8 kg and BM6, 6 kg) were handled according to the standards of the European Union (86/609/EU), Spain (RD 1201/2005) and the Society for Neuroscience Policies and Use of Animals and Humans in Neuroscience Research. The experimental procedures were approved by the Bioethics Commission of the University of Santiago de Compostela.
Recordings.
Neuronal recordings were obtained with tungsten microelectrodes (1.5–3.5 MΩ) in the posterior bank of the ventral arm of the sulcus arcuatus and adjacent surface in the Premotor ventral cortex in the 4 hemispheres of the 2 monkeys (Fig. 1D). The locations of the penetrations were confirmed through standard histological techniques for the 2 recorded monkeys. Recordings sites changed from session to session.
Data Analysis.
All analyses were performed using custom-made programs in Matlab R2008b with Exlink Toolbox. We considered a neuron's response as task-related if during any of the task periods (PSD, S1, delay, S2, behavioral response, and until 2,000 ms after the behavioral response) the firing rate —averaged across each period— was significantly different from the control period preceding the white noise on at the beginning of each trial (Wilcoxon test, P < 0.01). Neural activity dependences were obtained through Receiving Operating Characteristics (ROC) (37) and Stepwise Linear Regression (SLR) analyses (12, 38). The ROC analysis allows the measure of the degree of overlap between 2 response distributions (13, 21, 39). For each neuron with sufficient data (at least 5 trials for each condition) we computed the area under the ROC curve (ROC AUC) within a time bin of 100 ms that was slid in 20 ms steps until the entire periods of the task had been analyzed. Detailed information is in SI Methods.
Stepwise Linear Regression analysis (SLR) was used to establish the dependence between the firing rate and (a) S1, (b) S2-S1, (c) the choice (sign(S2-S1)) (5, 12, 21, 38). The resulting aS1, aS2-S1 and asign(S2-S1) coefficients were plotted as a time function. The dynamics of those coefficients were calculated using a sliding window of 100 ms moving in 20 ms steps throughout the neuron-firing rate. Coefficients were included in the model if P value for a predictor was lower than 0.02 for at least 2 consecutive bins. The deviation from the mean (2 SD) was used to determine the significant time periods for each coefficient. Detailed information is in SI Methods.
Behavioral analyses have already been described (11, 12) (Fig. S1).
Supplementary Material
Acknowledgments.
The research of C.A. was supported by grants from Ministerio de Ciencia e Innovación, Spain. The work of J.L.P.-V. and V.L. was supported by predoctoral Ministerio de Ciencia e Innovación fellowships.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0910524106/DCSupplemental.
References
- 1.Kim JN, Shadlen MN. Neural correlates of a decision in the dorsolateral prefrontal cortex of the macaque. Nat Neurosci. 1999;2:176–185. doi: 10.1038/5739. [DOI] [PubMed] [Google Scholar]
- 2.Sugrue LP, Corrado GS, Newsome WT. Matching behavior and the representation of value in the parietal cortex. Science. 2004;304:1782–1787. doi: 10.1126/science.1094765. [DOI] [PubMed] [Google Scholar]
- 3.Platt ML. Neural correlates of decisions. Curr Opin Neurobiol. 2002;12:141–148. doi: 10.1016/s0959-4388(02)00302-1. [DOI] [PubMed] [Google Scholar]
- 4.Romo R, Hernandez A, Zainos A, Lemus L, Brody CD. Neuronal correlates of decision-making in secondary somatosensory cortex. Nat Neurosci. 2002;5:1217–1225. doi: 10.1038/nn950. [DOI] [PubMed] [Google Scholar]
- 5.Romo R, Hernandez A, Zainos A. Neuronal correlates of a perceptual decision in ventral premotor cortex. Neuron. 2004;41:165–173. doi: 10.1016/s0896-6273(03)00817-1. [DOI] [PubMed] [Google Scholar]
- 6.Schall JD. Neural basis of deciding, choosing and acting. Nat Rev Neurosci. 2001;2:33–42. doi: 10.1038/35049054. [DOI] [PubMed] [Google Scholar]
- 7.Glimcher PW. The neurobiology of visual-saccadic decision making. Annu Rev Neurosci. 2003;26:133–179. doi: 10.1146/annurev.neuro.26.010302.081134. [DOI] [PubMed] [Google Scholar]
- 8.Schall JD, Stuphorn V, Brown JW. Monitoring and control of action by the frontal lobes. Neuron. 2002;36:309–322. doi: 10.1016/s0896-6273(02)00964-9. [DOI] [PubMed] [Google Scholar]
- 9.Romo R, Salinas E. Touch and go: Decision-making mechanisms in somatosensation. Annu Rev Neurosci. 2001;24:107–137. doi: 10.1146/annurev.neuro.24.1.107. [DOI] [PubMed] [Google Scholar]
- 10.Leon MI, Shadlen MN. Exploring the neurophysiology of decisions. Neuron. 1998;21:669–672. doi: 10.1016/s0896-6273(00)80584-x. [DOI] [PubMed] [Google Scholar]
- 11.Nacher V, Ojeda S, Cadarso-Suarez C, Roca-Pardinas J, Acuña C. Neural correlates of memory retrieval in the prefrontal cortex. Eur J Neurosci. 2006;24:925–936. doi: 10.1111/j.1460-9568.2006.04964.x. [DOI] [PubMed] [Google Scholar]
- 12.Pardo-Vazquez JL, Leboran V, Acuña C. Neural correlates of decisions and their outcomes in the ventral premotor cortex. J Neurosci. 2008;28:12396–12408. doi: 10.1523/JNEUROSCI.3396-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Britten KH, Shadlen MN, Newsome WT, Movshon JA. The analysis of visual motion: A comparison of neuronal and psychophysical performance. J Neurosci. 1992;12:4745–4765. doi: 10.1523/JNEUROSCI.12-12-04745.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roitman JD, Shadlen MN. Response of Neurons in the lateral intraparietal area during a combined visual discrimination reaction time task. J Neurosci. 2002;22:9475–9489. doi: 10.1523/JNEUROSCI.22-21-09475.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shadlen MN, Newsome WT. Neural basis of a perceptual decision in the parietal cortex (area LIP) of the rhesus monkey. J Neurophysiol. 2001;86:1916–1936. doi: 10.1152/jn.2001.86.4.1916. [DOI] [PubMed] [Google Scholar]
- 16.Hernandez A, Salinas E, Garcia R, Romo R. Discrimination in the sense of flutter: New psychophysical measurements in monkeys. J Neurosci. 1997;17:6391–6400. doi: 10.1523/JNEUROSCI.17-16-06391.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vazquez P, Cano M, Acuña C. Discrimination of line orientation in humans and monkeys. J Neurophysiol. 2000;83:2639–2648. doi: 10.1152/jn.2000.83.5.2639. [DOI] [PubMed] [Google Scholar]
- 18.Lemus L, et al. Neural correlates of a postponed decision report. Proc Natl Acad Sci USA. 2007;104:17174–17179. doi: 10.1073/pnas.0707961104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Romo R, et al. Probing the cortical neuronal correlates of a sensory discrimination process. Arch Ital Biol. 2002;140:253–262. [PubMed] [Google Scholar]
- 20.Romo R, Salinas E. Sensing and deciding in the somatosensory system. Curr Opin Neurobiol. 1999;9:487–493. doi: 10.1016/S0959-4388(99)80073-7. [DOI] [PubMed] [Google Scholar]
- 21.Hernandez A, Zainos A, Romo R. Temporal evolution of a decision-making process in medial premotor cortex. Neuron. 2002;33:959–972. doi: 10.1016/s0896-6273(02)00613-x. [DOI] [PubMed] [Google Scholar]
- 22.Gold JI, Shadlen MN. The neural basis of decision making. Annu Rev Neurosci. 2007;30:535–574. doi: 10.1146/annurev.neuro.29.051605.113038. [DOI] [PubMed] [Google Scholar]
- 23.Schultz W. Behavioral theories and the neurophysiology of reward. Annu Rev Psychol. 2006;57:87–115. doi: 10.1146/annurev.psych.56.091103.070229. [DOI] [PubMed] [Google Scholar]
- 24.Roesch MR, Olson CR. Neuronal activity dependent on anticipated and elapsed delay in macaque prefrontal cortex, frontal and supplementary eye fields, and premotor cortex. J Neurophysiol. 2005;94:1469–1497. doi: 10.1152/jn.00064.2005. [DOI] [PubMed] [Google Scholar]
- 25.Lau B, Glimcher PW. Action and outcome encoding in the primate caudate nucleus. J Neurosci. 2007;27:14502–14514. doi: 10.1523/JNEUROSCI.3060-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sutton RS, Barto AG. Reinforcement learning: An introduction. IEEE Trans Neural Netw. 1998;9:1054. [Google Scholar]
- 27.Roesch MR, Olson CR. Impact of Expected Reward on Neuronal Activity in Prefrontal Cortex, Frontal and Supplementary Eye Fields and Premotor Cortex. J Neurophysiol. 2003;90:1766–1789. doi: 10.1152/jn.00019.2003. [DOI] [PubMed] [Google Scholar]
- 28.Roesch MR, Olson CR. Neuronal activity related to reward value and motivation in primate frontal cortex. Science. 2004;304:307–310. doi: 10.1126/science.1093223. [DOI] [PubMed] [Google Scholar]
- 29.Schultz W. Neural coding of basic reward terms of animal learning theory, game theory, microeconomics and behavioural ecology. Curr Opin Neurobiol. 2004;14:139–147. doi: 10.1016/j.conb.2004.03.017. [DOI] [PubMed] [Google Scholar]
- 30.Schultz W, Tremblay L, Hollerman JR. Reward processing in primate orbitofrontal cortex and basal ganglia. Cereb Cortex. 2000;10:272–284. doi: 10.1093/cercor/10.3.272. [DOI] [PubMed] [Google Scholar]
- 31.Stuphorn V, Taylor TL, Schall JD. Performance monitoring by the supplementary eye field. Nature. 2000;408:857–860. doi: 10.1038/35048576. [DOI] [PubMed] [Google Scholar]
- 32.Ito S, Stuphorn V, Brown JW, Schall JD. Performance monitoring by the anterior cingulate cortex during saccade countermanding. Science. 2003;302:120–122. doi: 10.1126/science.1087847. [DOI] [PubMed] [Google Scholar]
- 33.Lee D, Seo H. Mechanisms of reinforcement learning and decision making in the primate dorsolateral prefrontal cortex. Ann NY Acad Sci. 2007;1104:108–122. doi: 10.1196/annals.1390.007. [DOI] [PubMed] [Google Scholar]
- 34.Seo H, Barraclough DJ, Lee D. Dynamic signals related to choices and outcomes in the dorsolateral prefrontal cortex. Cereb Cortex. 2007;17(Suppl 1):i110–i117. doi: 10.1093/cercor/bhm064. [DOI] [PubMed] [Google Scholar]
- 35.Uchida Y, Lu X, Ohmae S, Takahashi T, Kitazawa S. Neuronal activity related to reward size and rewarded target position in primate supplementary eye field. J Neurosci. 2007;27:13750–13755. doi: 10.1523/JNEUROSCI.2693-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee D. Neural basis of quasi-rational decision making. Curr Opin Neurobiol. 2006;16:191–198. doi: 10.1016/j.conb.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 37.Green DM, Swets JA. Signal Detection Theory and Psychophysics. New York: Wiley; 1966. [Google Scholar]
- 38.Draper NR, Smith H. Applied Regression Analysis. New York: Wiley; 1966. [Google Scholar]
- 39.Wallis JD, Miller EK. From rule to response: Neuronal processes in the premotor and prefrontal cortex. J Neurophysiol. 2003;90:1790–1806. doi: 10.1152/jn.00086.2003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.