Abstract
The ATP synthase of yeast mitochondria is composed of 17 different subunit polypeptides. We have screened a panel of ATP synthase mutants for impaired expression of Atp6p, Atp8p, and Atp9p, the only mitochondrially encoded subunits of ATP synthase. Our results show that translation of Atp6p and Atp8p is activated by F1 ATPase (or assembly intermediates thereof). Mutants lacking the α or β subunits of F1, or the Atp11p and Atp12p chaperones that promote F1 assembly, have normal levels of the bicistronic ATP8/ATP6 mRNAs but fail to synthesize Atp6p and Atp8p. F1 mutants are also unable to express ARG8m when this normally nuclear gene is substituted for ATP6 or ATP8 in mitochondrial DNA. Translational activation by F1 is also supported by the ability of ATP22, an Atp6p-specific translation factor, to restore Atp6p and to a lesser degree Atp8p synthesis in the absence of F1. These results establish a mechanism by which expression of ATP6 and ATP8 is translationally regulated by F1 to achieve a balanced output of two compartmentally separated sets of ATP synthase genes.
Keywords: F1-ATPase, mitochondria, Saccharomyces cerevisiae, translational regulation
The preservation of functional mitochondria and chloroplasts during cell growth and division depends on a large pool of genetic information resident in the nucleus and of a more limited set of genes present in the genomes of the organelles themselves. The proteins encoded by the chloroplast and mitochondrial genomes interact with partner proteins derived from nuclear genes to form hetero-oligomeric complexes that function, respectively, in photosynthesis and oxidative phosphorylation. This circumstance has necessitated the evolution of mechanisms for insuring a balanced output of the two spatially separate sets of genes. Since mitochondrial and chloroplast proteins are not exported to the cytoplasm, it stands to reason that each organelle must bear the burden of adjusting expression of its genes to the demands imposed by the nucleo-cytoplasmic system.
Regulation of organellar gene expression was first demonstrated by studies of the b6f complex of Chlamydomonas rheinhardtii chloroplasts (1). Translation of the cytochrome f component on chloroplast ribosomes was shown to depend on its association with subunit IV and cytochrome b6, two other subunits of the complex, which unlike cytochrome f, are synthesized on cytoplasmic ribosomes. This regulatory mechanism, termed “control by epistasis of synthesis” (CES), although differing in detail, was shown to operate in the biogenesis of other chloroplast proteins including photosystem II (2). The generality of CES as a means for adjusting the rate of translation of specific organellar gene products to the availability of their imported partners is underscored by the discovery that in Saccharomyces cerevisiae expression of the mitochondrial gene for the Cox1p subunit of cytochrome oxidase is coupled to a downstream assembly event through Mss51p, a translational activator of COX1 mRNA (3).
Currently, little is known about the mechanism by which biogenesis of the mitochondrial ATP synthase (F1-F0 ATPase) is regulated. Assembly of a catalytically active F1 oligomer occurs independently of F0 (4) and entails an initial chaperone-dependent association of the α and β subunits into a hexamer constituting most of the protein mass of F1 (5). Chaperones also play important roles in assembly of F0, having been shown to promote oligomerization of the Atp9p ring (6) and association of the ring with Atp6p (7). Interaction of Atp6p with the Atp9p ring is probably a late assembly event as the resultant complex can cause an unregulated proton leak leading to dissipation of the mitochondrial membrane potential. The incorporation of Atp6p into the complex has, therefore, been inferred to occur at a stage when the structural elements necessary for coupling proton transfer to ATP synthesis or hydrolysis are already in place.
In the present study we present evidence that translation of both Atp6p and Atp8p in yeast mitochondria is contingent on the presence of assembled but not necessarily catalytically active F1. Our evidence, however, indicates that unlike the CES assembly-dependent mechanism for regulation of Cox1p expression, Atp6p and Atp8p translation is subject to direct activation by F1, thus defining an alternate mechanism for self regulation of a mitochondrial enzyme with a dual genetic identity.
Results
Impaired F1 Assembly Affects Expression of ATP6 and ATP8.
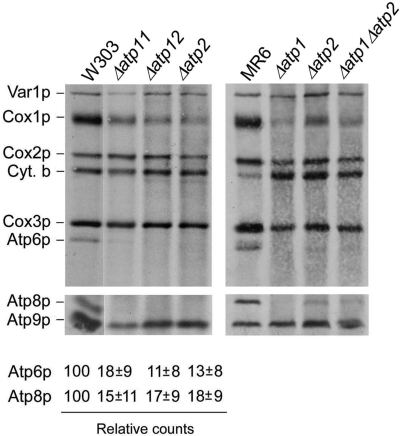
In the absence of the Atp11p and Atp12p chaperone or of their β and α subunit substrates, assembly of the F1 α3β3 hexamer is blocked and the two subunits are deposited as large inactive aggregates in the mitochondrial matrix (5). To assess if F1 affects expression of the mitochondrially encoded subunits of F0, we assayed their translation in vivo in Δatp11, Δatp12, Δatp1, and Δatp2 mutants by pulse-labeling of cells in the presence of cycloheximide to inhibit cytoplasmic protein synthesis. Under these conditions incorporation of radiolabel into Atp6p and Atp8p was 10–18% of that measured in the wild-type strain (Fig. 1).
Fig. 1.
In vivo labeling of mitochondrial gene products in wild-type and F1 mutants. The respiratory competent strains W303 and MR6, and F1 mutants with the indicated genotypes were grown in rich galactose medium and labeled for 20 min with [35S]methionine + [35S]cysteine in the presence of cycloheximide. Total cellular extracts were separated by SDS/PAGE in two different polyacrylamide gels prepared with a 30:0.8 ratio of acrylamide and bisacrylamide. Upper gel: 12% polyacrylamide gel containing 4 M urea and 25 glycerol. Lower gel: 17.5% polyacrylamide gel. Proteins were transferred to a nitrocellulose membrane and exposed to X-ray film. The bands corresponding to Atp6p and Atp8p were quantified with a phoshorimager and normalized to Cox3p. The averages of 4–5 independent experiments are expressed as percentage of wild-type.
To exclude the possibility that the reduced labeling of Atp6p and Atp8p is an artifact resulting from the α and/or β subunit aggregates present in the F1 mutants, we also examined a double Δatp1 Δatp2 null mutant, devoid of such aggregates. The results of the in vivo translation assay indicated that the double mutant, like the single α and β subunit mutants, failed to show any significant incorporation of the radioactive precursors into Atp6p and Atp8p (Fig. 1).
The in vivo assays also revealed a significant inhibition of Cox1p synthesis (Fig. 1). This is probably a secondary effect of the ATP synthase deficiency as mutations in Atp6p and other subunits of F0 have been shown to suppress translation of Cox1p (8).
ATP6/ATP8 mRNA Is Normally Transcribed and Processed in F1 Mutants.
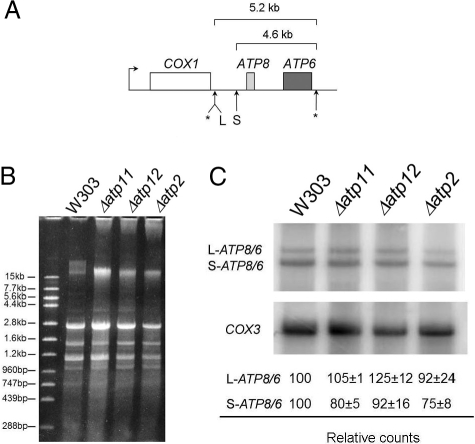
The marked reduction of Atp6p and Atp8p labeling in the F1 mutants could be the product of decreased transcription, mRNA maturation, translation, or enhanced turnover of the newly translated but unassembled subunits. ATP6 and ATP8 are cotranscribed with COX1, the gene for subunit 1 of cytochrome oxidase (Fig. 2A) (9). The primary transcript is processed in several steps to produce two distinct bicistronic ATP8/ATP6 mRNAs of 5.2 and 4.6 kb (10) and a COX1 precursor RNA that undergoes a series of splicing reactions to form the mature mRNA. Some strains of yeast contain the ENS2 gene downstream of ATP6 (11). ENS2 codes for a DNA endonuclease, and when present is part of the ATP8/6 mRNAs. The W303 strain used in the present study lacks this gene.
Fig. 2.
ATP8/6 mRNAs are normally transcribed and processed in F1 mutants. (A) Diagram showing transcription of the primary polycistronic COX1/ATP8/ATP6 RNA from a site upstream of COX1. Cleavage sites that produce the mature messengers are shown by asterisks. The 5.2- and 4.6-kb mRNAs are the result of cleavages at L and S, respectively. COX1, which contains multiple introns in the W303 strain, is not drawn to scale. (B) Mitochondrial RNAs isolated from W303 and Δatp11, Δatp12, and Δatp2 mutants were separated on a 1% agarose gel, stained with ethidium bromide (left panel) and transferred to a Nytran membrane that was hybridized with 32P labeled ATP6- and COX3-specific probes. The ATP6/ATP8 mRNAs was quantified with a phosphorimager and normalized to the COX3 mRNA.
A requirement of F1 for transcription or processing of the COX1/ATP8/ATP6 region was excluded by Northern analysis of total mitochondrial RNAs in the parental respiratory competent strain and in Δatp2, Δatp11, and Δatp12 mutants. The ATP8/ATP6 mRNAs were quantified and normalized to the COX3 mRNA to correct for any differences ascribable to secondary ρ− or ρ0 mutants in the cultures. The Northern hybridizations indicated that the two ATP8/ATP6 mRNAs in the F1 mutants were present at levels comparable to those of the wild-type strain (Fig. 2B).
F1 Upregulates Translation of Atp6p and Atp8p.
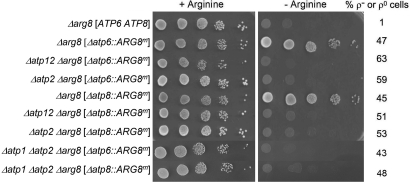
The Northern hybridization results pointed to either reduced translation or enhanced turnover of newly synthesized Atp6p and Atp8p in F1 mutants. To distinguish between these two possibilities, the mitochondrial ATP6 and ATP8 genes were replaced with ARG8m, a version of nuclear ARG8 recoded for expression in mitochondria (12). ARG8 codes for acetylornithine aminotransferase, a mitochondrial protein that functions in arginine biosynthesis (13). The Δatp6::ARG8m (8) or Δatp8::ARG8m (this study) alleles were substituted for wild-type mitochondrial DNA (mtDNA) in Δatp12 Δarg8, Δatp2 Δarg8 double mutants, and in an Δatp1 Δatp2 Δarg8 triple mutant. Expression of mitochondrial ARG8m, detected as arginine-independent growth, should not be affected by mutations in ATP12 or ATP2 if F1 is required for stability of Atp6p and Atp8p. Alternatively, if F1 upregulates translation of ATP6 and ATP8, expression of the ARG8m reporter gene should be compromised in an Δatp2 or Δatp12 mutant.
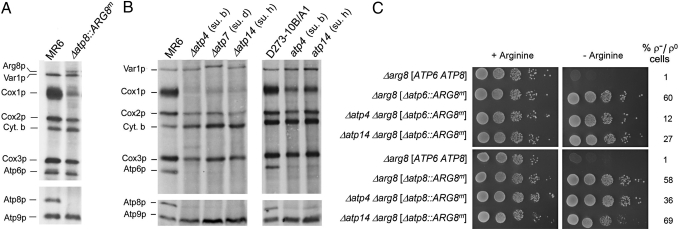
The introduction into MR6, a respiratory competent Δarg8 strain, of mtDNA with the Δatp6::ARG8m or Δatp8::ARG8m allele abolished its arginine requirement, confirming expression of ARG8m from the ATP6 and ATP8 loci (Fig. 3). Neither allele, however, was able to confer arginine-independent growth when combined with Δatp2, Δatp12, or both Δatp1 and Δatp2 mutations (Fig. 3). Since ATP synthase mutants tend to convert at high frequency to ρ− and ρ0 mutants that are deficient in mitochondrial protein synthesis (6, 14), the arginine auxotrophy could also have resulted from a loss of mtDNA. This was excluded by the presence of 40–60% of ρ+ cells in the cultures (Fig. 3). These results confirm that the compromised expression in F1 mutants of Atp6p and Atp8p (or of ARG8m when present at either the ATP6 or ATP8 locus) is a consequence of translational downregulation.
Fig. 3.
F1 mutants do not express ARG8m from the ATP6 or ATP8 locus of mitochondrial DNA. The parental strain MR6 (Δarg8 [ATP6 ATP8]) and the different respiratory deficient mutants with either the mitochondrial Δatp6::ARG8m or Δatp8::ARG8m allele were grown overnight in minimal glucose medium supplemented with auxotrophic requirements including arginine. Serial dilutions were spotted on minimal glucose with or without arginine and incubated at 30 °C for 2 days. The percentage of ρ− or ρ0 mutants in the cultures is indicated next to each strain in the right hand margin. The mitochondrial genotypes are enclosed by the straight brackets.
To determine if overexpression of any single F1 subunit can restore expression of the ATP6 locus in an F1 mutant, the Δatp12 Δarg8 double mutant with the Δatp6::ARG8m allele was transformed with high-copy plasmids containing the α, β, γ, δ, or ε subunit genes. None of the genes were able to suppress the growth requirement for arginine indicating that overexpression of the single F1 subunits is not a sufficient condition for expression of Atp6p.
Translation of Atp6p and Atp8p Does Not Depend on Catalytically Active F1.
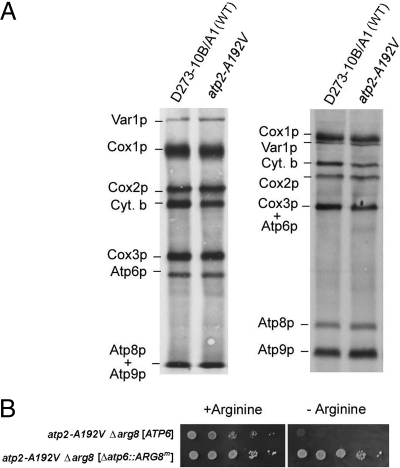
We next asked whether mutants with assembled but catalytically inactive F1 are able to translate Atp6p? The respiratory defective mutant P159 was previously shown to have a loss of function mutation (A192V) in the β subunit that does not interfere with assembly of the F1 oligomer (15). Centrifugation of the solubilized enzyme in a sucrose gradient confirmed that the β subunit of F1 in the mutant sedimented nearly identically to the β-galactosidase size standard, indicative of an assembled F1-F0 complex. P159 was, therefore, likely to have a complete F0 unit with Atp6p and Atp8p. This was confirmed by in vivo labeling of mitochondrial gene products in the mutant. The pattern of labeled proteins in the mutant was identical to that of wild-type with no evidence of diminished translation of the three F0 subunits or of Cox1p of cytochrome oxidase (Fig. 4A).
Fig. 4.
ATP6 and ATP8 are expressed in a catalytic inactive F1 mutant. (A) The parental strain D273–10B/A1 and the point mutants P159 (atp2-A192V) were labeled in vivo as in Fig. 1 and separated by SDS/PAGE on a 12% gel containing 6 M urea and 25% glycerol (left panel) and a 17.5% polyacrylamide gel (right panel). (B) The point mutant (atp2-A192V Δarg8) with wild-type mtDNA and with the Δatp6::ARG8m allele were grown and spotted on minimal glucose with or without arginine as in Fig. 3.
The ability of a catalytically damaged F1 to support Atp6p translation was confirmed by testing expression of ARG8m at the ATP6 locus in the atp2-A192V Δarg8 double mutant. As expected the double mutant with the Δatp6::ARG8m allele was able to grow in the absence of arginine (Fig. 4B) indicating that ATP6/ATP8 expression is not dependent on the hydrolase activity of F1.
Are Soluble But Incompletely Assembled Forms of F1 Sufficient to Activate Translation of Atp6p and Atp8p?
Mitochondrial F1 ATPase consists of five different subunits with an α3β3γδε stoichiometry. In contrast to the Δatp11, Δatp12, Δatp1, and Δatp2 mutants, which accumulate the α and/or β subunits of F1 as bulk aggregates, ε, δ, or γ mutants maintain both α and β subunits as soluble proteins (16–18).
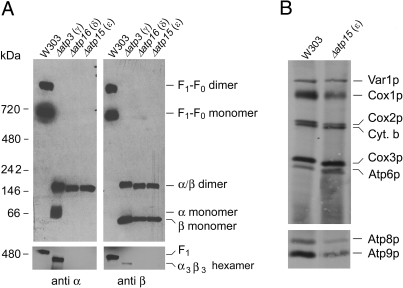
The oligomeric status of the α and β subunits in ε, δ, and γ mutants was examined by BN-PAGE separation of digitonin solubilized mitochondria. The α and β subunit specific antibodies detected the same bands, which differed from F1 and the F1-F0 complex (Fig. 5A). Based on their migration, the two most prominent bands, present in all three mutants, but absent in wild-type, were estimated to have masses of 50 kDa and 150 kDa probably corresponding to the β monomers and the αβ dimer, respectively. The mass of the αβ dimer should be approximately 110 kDa. The tendency of the BN-PAGE system to overestimate the masses of the native ATP synthase complex and of F1 could account for discrepancy in the mass of the αβ dimer. However, it is not excluded that what we identify to be the dimer could have an extra F1 subunit. The γ mutant, and in some experiments the δ and ε mutants as well, had a band detected by the α subunit-specific antibody that migrated slightly slower than the monomeric β subunit. The third band, recognized by both antibodies in the γ subunit mutant, migrated slightly ahead of F1. This band required longer exposure time to be visible. Based on its size and reactivity this band is probably the α3β3 hexamer. At present, it is not possible to say if the αβ dimer and the monomeric subunits are breakdown products of an unstable hexamer or if they are bona fide assembly intermediates of F1.
Fig. 5.
Characterization of F1 and expression of Atp6p and Atp8p in γ, ε, and δ subunit null mutants. (A) Mitochondria were extracted with 2% digitonin and samples representing 250 μg of starting mitochondrial protein were analyzed by BN-PAGE as described in Materials and Methods. (B) The respiratory competent strain W303 and the ε null mutant (Δatp15) were labeled in vivo and separated on two different SDS/PAGE gel systems as in Fig. 1.
It was of interest to ascertain if the partially assembled, soluble forms of the α and β subunits are capable of activating Atp6p and Atp8p translation. Direct measurements of Atp6p and Atp8p translation in the δ and γ subunit mutants were not possible because of the extensive loss of wild-type mtDNA, a hallmark of these strains. For the same reason we were unable to construct δ and γ subunit mutants with the ARG8m substitutions at the ATP6 and ATP8 loci of mtDNA. Although the ε subunit mutant is also unstable, the percentage of ρ+ cells in a freshly grown culture was adequate for detection of radiolabeled mitochondrial gene products by in vivo translation. These assays indicated that the ε subunit is not essential for translation of Atp6p and Atp8p (Fig. 5B), which is consistent with a recent report showing that an ε mutant can assemble a complete ATP synthase complex, albeit at very reduced levels (19).
ATP6/ATP8 Translation in F0 Assembly Mutants.
Mutants blocked in expression of Atp9p retain the ability to translate Atp6p and Atp8p (6). Similarly, the Δatp8 mutation does not affect translation of Atp6p and Atp9p as evidenced by the comparable labeling of Atp6p and Atp9p (relative to Cox3p) in the wild-type and the Δatp8 mutant (Fig. 6A). Earlier studies indicated that Atp8p and Atp9p translation is also not affected in the Δatp6 mutant (8). These results demonstrate that expression of the three mitochondrially encoded subunits of the ATP synthase is not interdependent.
Fig. 6.
Expression of Atp6p, Atp8p in F0 and peripheral stalk mutants. (A) Mitochondrial gene products of the parental strain MR6 and of the Δatp8::ARG8m mutant were labeled in vivo and analyzed by SDS/PAGE as in Fig. 1. (B) The respiratory competent parental strains MR6 and D273–10B/A1, the subunit b, d, and h null mutants (Δatp4, Δatp7, and Δatp14, respectively) and the subunit b and h point mutants (atp4 and atp14) (20) were labeled and analyzed as in Fig. 1. (C) Strains with the indicated genotypes were serially diluted, spotted on minimal glucose with or without arginine and grown as in Fig. 3.
Information bearing on the effect of mutations in peripheral stalk subunits on expression of mitochondrial gene products is scant. We, therefore, studied the synthesis of Atp6p and Atp8p in cells expressing fully assembled and functional F1 but missing subunits of the peripheral stalk. Mitochondrial gene products were labeled in vivo in Δatp4 (subunit b), Δatp7 (subunit d), and Δatp14 (subunit h) null mutants. All three peripheral stalk mutants displayed a deficit of Atp6p and Atp8p (Fig. 6B). The atp4 and atp14 point mutants also showed large reductions in labeling of Atp6p and to a lesser extent Atp8p (Fig. 6B). The inability of Δatp5 mutants to maintain a full length mitochondrial genome precluded assessing the effect of absence of it product, OSCP, the fourth peripheral stalk subunit, on translation of Atp6p and Atp8p.
Decreased in vivo labeling of a mitochondrial gene product is not necessarily indicative of a translation defect as rapid turnover of a newly synthesized but unassembled protein would be expected to elicit the same phenotype. The ARG8m expression assay was used to discriminate between an effect of the peripheral stalk mutations on translation and turnover of Atp6p and Atp8p. The mitochondrial Δatp6::ARG8m and Δatp8::ARG8m alleles were introduced into Δatp4 Δarg8 and Δatp14 Δarg8 double mutants. The ability of these mutants to grow in the absence of arginine indicated that absence of the peripheral stalk subunits did not affect expression of ARG8m from the ATP6 or ATP8 loci of mtDNA (Fig. 6C). The decreased labeling of Atp6p and Atp8p is, therefore, most likely the result of enhanced turnover of the two subunits.
ATP22 Is a High Copy Suppressor of the Atp6p/Atp8p Translation Defect.
The yeast nuclear ATP22 gene codes for an Atp6p-specific translational activator (21). In the present study this gene was isolated based on its ability to suppress the arginine requirement of the Δatp12 Δarg8 double mutant with the Δatp6::ARG8m mitochondrial allele. Transformation of the double mutant with a high-copy yeast genomic plasmid library produced arginine-independent clones, some of which contained plasmids with ATP22. Subcloning confirmed that ATP22 was responsible for restoring arginine-independent growth of the double mutant (Fig. 7A).
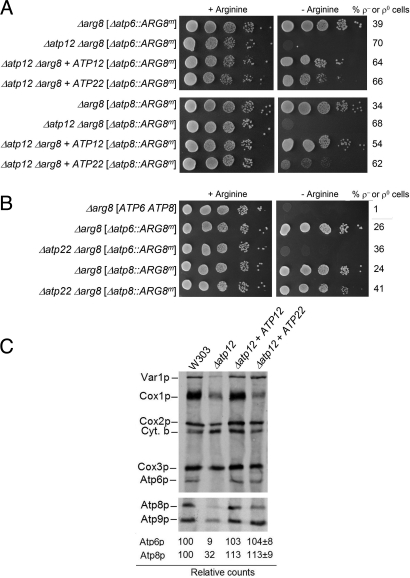
Fig. 7.
Suppression of the Atp6p and Atp8p translation defect in F1 mutants by ATP22. (A) Mutants with the indicated genotypes were transformed with ATP12 and ATP22 cloned in high-copy plasmids and were grown overnight in minimal glucose supplemented with all of the amino acids requirements including arginine. Serial dilutions were spotted on minimal glucose with or without arginine and grown as in Fig. 3. (B) ARG8m is expressed from the ATP8 but not ATP6 locus. The parental strain MR6 (Δarg8 [ATP6 ATP8]) and the different mutants were grown in minimal glucose containing arginine and serial dilutions were spotted on minimal medium with and without arginine as in Fig. 3. (C) ATP22 restores translation of Atp6p and Atp8p in an F1 mutant. The respiratory competent strain W303, the Δatp12 mutant and transformants harboring ATP12 and ATP22 on high copy plasmids were labeled in vivo as in Fig. 1 and separated by SDS/PAGE on a 12% gel containing 6 M urea (upper panel) and a 17.5% polyacrylamide gel (lower panel).
In earlier studies, the function of Atp22p was inferred from the phenotype of atp22 mutants, which are blocked in translation of Atp6p but not Atp8p (21). In view of this, the ability of ATP22 to partially suppress the arginine auxotrophy of Δatp12Δarg8 double mutants with the mitochondrial Δatp8::ARG8m allele was unexpected (Fig. 7A). To further validate this result we constructed an Δatp22 Δarg8 double mutants with either the Δatp6::ARG8m or Δatp8::ARG8m alleles. Growth of the two strains on minimal medium lacking arginine, when compared to the wild-type, indicated that whereas the Δatp22 mutant with the Δatp6::ARG8m allele was auxotrophic for arginine, the Δatp8::ARG8m allele was sufficiently well expressed in the Δatp22 mutant to allow arginine-independent growth, albeit not as well as the wild-type (Fig. 7B). These results are consistent with the only partial rescue of the arginine auxotrophy of the Δatp8::ARG8m allele in the background of the Δatp2 or Δatp12 mutation.
To ascertain if ATP22 also rescues translation of Atp6p and Atp8p in F1 mutants, Δatp12 null mutants containing wild-type mtDNA with and without ATP22 on a high copy plasmid were radiolabeled in the presence of cycloheximide and mitochondrial gene products analyzed in two different gel systems optimized for the resolution of Atp6p and Atp8p (Fig. 7C). Translation of Atp6p in the transformants, when quantified and normalized to Cox3p, was very similar to that of the wild-type. The results of the in vivo translation assay are consistent with the growth phenotype of the Δatp2 and Δatp12 mutants harboring the mitochondrial Δatp6::ARG8m allele (Fig. 7A). Surprisingly, overexpression of ATP22 also rescued translation of Atp8p.
Discussion
Biogenesis of the mitochondrial ATP synthase is a complex process because of its numerous constituent polypeptides that need to be coordinately expressed from two separate genomes. Those derived from mtDNA are more likely to be targets for regulation by their nuclear counterparts, rather than the converse, simply based on the fact that they do not cross the mitochondrial barrier and, therefore, are physically barred from exerting an influence on the transcriptional and translational machineries of the nucleus and cytoplasm.
In this study we show that translation of Atp6p and Atp8p, two subunits vital for the function of the F0 sector, is controlled by F1. In the absence of the α or β subunits of F1, or of their chaperones Atp11p and Atp12p, expression of Atp6p and Atp8p but not of the other gene products of mtDNA is sharply reduced. Although Cox1p synthesis is also lower in F1 mutants, this is a hallmark of ATP synthase strains in general (8, 14). The presence of normal levels of ATP8/ATP6 mRNA in the F1 assembly mutants excludes a defect in either transcription or processing of the COX1/ATP8/ATP6 multicistronic transcript. A high turnover rate of the two subunits is also unlikely. This follows from the finding that expression of ARG8m at the ATP6 or ATP8 locus of mtDNA is also arrested in F1 mutants. The ARG8 product is not normally translated in mitochondria and has no functional or structural relationship to the ATP synthase. It is, therefore, difficult to rationalize how the absence of F1 would affect its stability. Moreover, respiratory deficient mutants, including ATP synthase mutants, are prototrophic for arginine when acetylornithine aminotransferase is expressed from nuclear ARG8 and imported into mitochondria. These results constitute compelling evidence that F1, or components thereof, upregulate translation of Atp6p and Atp8p.
Activation of Atp6p and Atp8p translation by F1 does not depend on its hydrolytic activity. This is evident from the ability of mutants with assembled but catalytically incompetent F1 to synthesize Atp6p and Atp8p and to express ARG8m from either the ATP6 and ATP8 loci. We also tried to answer the question of whether the presence of native α and β subunits of F1 is a sufficient condition for translational activation. Native gels of the γ, δ, and ε mutants revealed the presence of soluble monomeric α and β (only in the γ mutant), dimeric αβ and to a much lesser extent of hexameric α3β3 oligomers. The ability of the ε subunit mutant to synthesize Atp6p and Atp8p indicates that assembly of a complete F1 unit is not a precondition for translational regulation. The soluble monomers or αβ dimers present in mutants lacking the minor subunits of F1 could be assembly intermediates or breakdown products of α3β3 hexamers. Since our data do not distinguish between the two, it is uncertain which of these F1 subassemblies functions in translational activation. The fact that translation is reduced in Δatp11 and Δatp12 mutants, which contain aggregated forms the α and β subunits (5), indicates that a minimal requirement for translation activation is that the α and β subunits of F1 be present as soluble proteins. A requirement of γ and δ subunit mutants for translation of Atp6p and Atp8p proved to be difficult to assess directly because of the extensive loss of ρ+ mitochondrial genome in these strains (17, 18). The low level of γ and δ subunits reported in the ε mutant (16), however, favors the notion that neither of these F1 components is essential for translational activation. It is also worth noting that overexpression of different F1 subunits was ineffective in restoring ARG8m expression in a Δatp12 mutant. Taken together these results point to soluble α or β subunit alone, the αβ dimer or the α3β3 hexamer as the minimal structural elements of F1 required for activation of translation.
Deletions of any single mitochondrially encoded subunit of the ATP synthase had no noticeable effect on translation of the other two. This was shown previously for strains deficient Atp6p and Atp9p (6, 8) and in the present study for the Δatp8 null mutant. Interestingly, in vivo translation assays of Δatp4 and Δatp14 mutants lacking, respectively, subunits b and h of the peripheral stalk, disclosed a severe reduction of both Atp6p and Atp8p. We attribute this to turnover rather than lower translation because null alleles of these peripheral stalk genes did not significantly affect expression of ARG8m when it replaced either ATP6 or ATP8. These results argue against a contribution of F0 components in regulating translation of Atp6p and Atp8p.
ATP22, which was previously shown to code for an Atp6p-specific translational activator (21), is a high copy suppressor of the Atp6p translation defect in F1 mutants. This further substantiates the in vivo translation results and the ARG8m expression data indicating that regulation by F1 is exerted at the translational stage. ATP22 was also able to restore translation of Atp8p, although not as effectively as Atp6p. The earlier conclusion that Atp22p is a specific activator of ATP6 (21) is supported by the finding that the Δatp8::ARG8m allele is expressed in an Δatp22 mutant. Growth of the Δatp22 mutant with the Δatp8:::ARG8m allele, however, is somewhat slower than wild-type in the absence of arginine. An explanation for partial effect of Atp22p on translation of the ATP8 locus will require a better understanding of how this translational activator functions.
Activation of Atp6p translation by F1 does not involve the CES mechanism proposed to regulate some chloroplast genes in Chlamydomonas (1, 2) and the mitochondrial COX1 gene in yeast (3). An essential feature of this mechanism is that a factor needed for translation of a specific mRNA also binds to the newly synthesized but unassembled protein product. For example, Mss51p, a COX1-specific translation factor (22) was shown to bind to Cox1p before incorporation of this subunit into cytochrome oxidase (23). The release of the factor from the complex, a necessary step for initiating a new round of translation, occurs after the newly translated subunit interacts with a partner protein imported from the cytoplasm. In this scenario the release of a translational factor (for example Atp22p) from Atp6p and/or Atp8p would be driven by F1. This mechanism is inconsistent with our data showing that F1 mutants are severely compromised in expressing ARG8m. Since Atp6p and Atp8p are not related to acetylornithine aminotransferase, interaction of this arginine biosynthetic enzyme with an ATP6 or ATP8 specific translation factor is highly dubious. Accordingly, translation of the mitochondrial ARG8m mRNA would not be expected to be affected in F1 mutants. This evidence is more consistent with a direct effect of F1 or of a partially assembled F1 on mitochondrial translation of the two ATP synthase subunits. Even though control of Atp6p and Atp8p translation by F1 differs from CES at the mechanistic level, both share the common feature of coupling translation of organellar gene products to assembly of their parent complex. Since the relationship between activation of Atp6p translation by Atp22p and by F1 is not presently know, it is not excluded that a CES regulation may operate in the Atp22p-dependent step of Atp6p translation.
Finally, it is worth pointing out that in addition to achieving a coordinate output of the two compartmentally segregated sets of ATP synthase-related genes, regulation of Atp6p translation by F1 also serves the important physiological function of preventing the accumulation of an Atp6p-Atp9p intermediate complex with a capacity to dissipate the membrane potential of mitochondria.
Materials and Methods
Strains and Growth Media.
The genotypes of the yeast strains used in this study are indicated in the figures. Unless otherwise indicated all of the translation assays were done with mutants in the W303 (MATa or MATα ade2–1 his3–1,15 leu2–3,112 trp1–1 ura3–1) genetic background (24). The mutants used for growth tests were derived from crosses to MR6 (MATa ade2–1 his3–1,15 leu2–3,112 trp1–1 ura3–1 arg8::HIS3), a respiratory competent strains with an arg8 null mutation (8). The compositions of the solid and liquid YPD, YPGal, YPEG, and minimal glucose media have been described previously (18).
Deletion and Replacement of ATP8 with ARG8m.
The Δatp8::ARG8m deletion cassette was constructed by PCR amplification of 300 bp of the 5′flanking sequence of ATP8 and sequences with primers 5′- ggctctagatttaataattattaaattatattc and 5′- ggcggatcctatatattattaatttatttattc and 400 bp of the 3′-flanking sequence with primers 5′- ggcggatccttttatatatattttttaataag and 5′-ggcgagctcttattttatatataaatatagg. The two PCR fragments were digested with a XbaI/BamH1 and BamH1/SacI and ligated to pJM2 (25). The plasmid obtained from this ligation was digested with BamH1 and ligated to ARG8m as a BamH1 fragment obtained by PCR amplification of the gene in pDS24 (25) with primers 5′- ggcggatccatgttcaaaagatatttatcatc and 5′-ggcggatccttaagcatatacagc. The resulting plasmid (pATP8/ST12) was introduced into αDFS160, ρ0 strain with a kar1 mutation by biolistic transformation using the Bio-Rad PDS-1000/He particle delivery system as described previously (26). Mitochondrial transformants were identified by their ability to correct a cox2 mutant. A transformant (αDFS160/ATP8/ST12) was crossed to the respiratory competent arg8 mutant MR6 (8). Respiratory defective recombinants with the Δatp8::ARG8m were confirmed to be arginine independent indicating mitochondrial expression of ARG8m.
Nucleic Acid Manipulations.
Standard techniques were used for DNA cloning and for transformation and purification of plasmid DNA from Escherichia coli (27). The LiAc procedure was used for yeast transformation (28). Northern analysis of mitochondrial RNAs was performed as described previously (29).
Miscellaneous Procedures.
Mitochondria were isolated from the cells grown in YPGal at 30 °C by the methods of Herrmann et al. (30). Mitochondrial gene products were labeled in vivo with [35S] Methionine + [35S] Cysteine (1,000 Ci/mmol, MP Biochemicals), transferred to a nitocellulose membrane and visualized by autoradiography as previously described (31). For BN-PAGE mitochondrial proteins were extracted with 2% final concentration of digitonin and separated on a 4–13% linear polyacrylamide gel (32). Proteins were transferred to a PVDF membrane and probed with rabbit polyclonal antibodies against yeast α and β subunits of F1. The antibody complexes were visualized with the Super Signal West Pico Chemiluminescent substrate kit (Pierce Chemical Co.).
Acknowledgments.
This research was supported by National Institutes of Health Research Grant HL-022174.
Footnotes
The authors declare no conflict of interest.
References
- 1.Wollman FA, Minai L, Nechushtai R. The biogenesis and assembly of photosynthetic proteins in thylakoid membranes1. Biochim Biophys Acta. 1999;1411:21–85. doi: 10.1016/s0005-2728(99)00043-2. [DOI] [PubMed] [Google Scholar]
- 2.Minai L, Wostrikoff K, Wollman FA, Choquet Y. Chloroplast biogenesis of photosystem II cores involves a series of assembly-controlled steps that regulate translation. Plant Cell. 2006;18:159–175. doi: 10.1105/tpc.105.037705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barrientos A, Zambrano A, Tzagoloff A. Mss51p and Cox14p jointly regulate mitochondrial Cox1p expression in Saccharomyces cerevisiae. EMBO J. 2004;23:3472–3482. doi: 10.1038/sj.emboj.7600358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schatz G. Impaired binding of mitochondrial adenosine triphosphatase in the cytoplasmic “petite” mutant of Saccharomyces cerevisiae. J Biol Chem. 1968;243:2192–2199. [PubMed] [Google Scholar]
- 5.Ackerman S, Tzagoloff A. Identification of two nuclear genes (ATP11, ATP12) required for the assembly of yeast F1 ATPase. Proc Natl Acad Sci USA. 1990;87:4986–4990. doi: 10.1073/pnas.87.13.4986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zeng X, Barros MH, Shulman T, Tzagoloff A. ATP25, a new nuclear gene of Saccharocmyces cerevisiae required for expression an assembly of the Atp9p subunit of mitochondrial ATPase. Mol Biol Cell. 2008;19:1366–1377. doi: 10.1091/mbc.E07-08-0746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tzagoloff A, Barrientos A, Neupert W, Herrmann H. Atp10p assists assembly of Atp6p into the Fo unit of the yeast mitochondrial ATPase. J Biol Chem. 2004;279:19775–19780. doi: 10.1074/jbc.M401506200. [DOI] [PubMed] [Google Scholar]
- 8.Rak M, et al. Yeast cells lacking the mitochondrial gene encoding the ATP synthase subunit 6 exhibit a selective loss of complex IV and unusual mitochondrial morphology. J Biol Chem. 2007;282:10853–10864. doi: 10.1074/jbc.M608692200. [DOI] [PubMed] [Google Scholar]
- 9.Christianson T, Rabinowitz M. Identification of multiple transcriptional initiation sites on the yeast mitochondrial genome by in vitro capping with guanylyltransferase. J Biol Chem. 1983;258:14025–14033. [PubMed] [Google Scholar]
- 10.Simon M, Faye G. Organization and processing of the mitochondrial oxi3/oli2 multigenic transcript in yeast. Mol Gen Genet. 1984;196:266–274. doi: 10.1007/BF00328059. [DOI] [PubMed] [Google Scholar]
- 11.Nakagawa K, Morishima N, Shibata T. A maturase-like subunit of the sequence-specific endonuclease endo. SceI from yeast mitochondria. J Biol Chem. 1991;266:1977–1984. [PubMed] [Google Scholar]
- 12.Bonnefoy N, Fox TD. In vivo analysis of mutated initiation codons in the mitochondrial COX2 gene of Saccharomyces cerevisiae fused to the reporter gene ARG8m reveals lack of downstream reinitiation. Mol Gen Genet. 2000;262:1036–1046. doi: 10.1007/pl00008646. [DOI] [PubMed] [Google Scholar]
- 13.Jauniaux JC, Urrestarazu LA, Wiame JM. Arginine metabolism in Saccharomyces cerevisiae: Subcellular localization of the enzymes. J Bacteriol. 1978;133:1096–1107. doi: 10.1128/jb.133.3.1096-1107.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paul M-F, Velours J, Arselin de Chateaubodeau G, Aigle M, Guerin B. The yeast ATP synthase subunit 4, structure and function. Eur J Biochem. 1989;185:163–171. doi: 10.1111/j.1432-1033.1989.tb15098.x. [DOI] [PubMed] [Google Scholar]
- 15.Liang Y, Ackerman SH. Characterization of mutations in the beta subunit of the mitochondrial F1-ATPase that produce defects in enzyme catalysis and assembly. J Biol Chem. 1996;271:26522–26528. doi: 10.1074/jbc.271.43.26522. [DOI] [PubMed] [Google Scholar]
- 16.Guélin E, Chevallier J, Rigoulet M, Guérin B, Velours J. ATP synthase of yeast mitochondria. Isolation and disruption of the ATP epsilon gene. J Biol Chem. 1993;268:161–167. [PubMed] [Google Scholar]
- 17.Giraud MF, Velours J. ATP synthase of yeast mitochondria. Isolation of the F1 delta subunit, sequence, and disruption of the structural gene. Eur J Biochem. 1994;222:851–859. doi: 10.1111/j.1432-1033.1994.tb18932.x. [DOI] [PubMed] [Google Scholar]
- 18.Paul M-F, Ackerman S, Yue J, Arselin G, Velours J, Tzagoloff A. Cloning of the yeast ATP3 gene coding for the gamma subunit of F1 and characterization of atp3 mutants. J Biol Chem. 1994;269:26158–26164. [PubMed] [Google Scholar]
- 19.Marsy S, Frachon P, Dujardin G, Lombès A, Lemaire C. Respiratory mutations lead to different pleiotropic effects on OXPHOS complexes in yeast and in human cells. FEBS Lett. 2008;582:3489–3493. doi: 10.1016/j.febslet.2008.09.016. [DOI] [PubMed] [Google Scholar]
- 20.Tzagoloff A, Dieckmann CL. PET genes of Saccharomyces cerevisiae. Microbiol Rev. 1990;54:211–225. doi: 10.1128/mr.54.3.211-225.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zeng X, Hourset A, Tzagoloff A. The Saccharomyces cerevisiae ATP22 gene codes for the mitochondrial ATPase subunit 6-specific translation factor. Genetics. 2007;175:55–63. doi: 10.1534/genetics.106.065821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Decoster E, Simon M, Hatat D, Faye G. The MSS51 gene product is required for the translation of the COX1 mRNA in yeast mitochondria. Mol Gen Genet. 1990;224:111–118. doi: 10.1007/BF00259457. [DOI] [PubMed] [Google Scholar]
- 23.Perez-Martinez X, Broadley SA, Fox TD. Mss51p promotes mitochondrial Cox1p synthesis and interacts with newly synthesized Cox1p. EMBO J. 2003;22:5951–5961. doi: 10.1093/emboj/cdg566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thomas BJ, Rothstein R. The genetic control of direct-repeat recombination in Saccharomyces: The effect of rad52 and rad1 on mitotic recombination at GAL10, a transcriptionally regulated gene. Genetics. 1989;123:725–738. doi: 10.1093/genetics/123.4.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Steele DF, Butler CA, Fox TD. Expression of a recoded nuclear gene inserted into yeast mitochondrial DNA is limited by mRNA-specific translational activation. Proc Natl Acad Sci USA. 1996;93:5253–5257. doi: 10.1073/pnas.93.11.5253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bonnefoy N, Fox TD. Directed alteration of Saccharomyces cerevisiae mitochondrial DNA by biolistic transformation and homologous recombination. Methods Mol Biol. 2007;372:153–166. doi: 10.1007/978-1-59745-365-3_11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maniatis T, Fritsch E, Sambrook J. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1982. [Google Scholar]
- 28.Schiestl RH, Gietz RD. High efficiency transformation of intact yeast cells using single stranded nucleic acids as a carrier. Curr Genet. 1989;16:339–346. doi: 10.1007/BF00340712. [DOI] [PubMed] [Google Scholar]
- 29.Barros MH, Myers AM, Van Driesche S, Tzagoloff A. COX24 codes for a mitochondrial protein required for processing of the COX1 transcript. J Biol Chem. 2006;281:3743–5371. doi: 10.1074/jbc.M510778200. [DOI] [PubMed] [Google Scholar]
- 30.Herrmann JM., Fölsch H, Neupert W, Stuart RA. In: Cell Biology: A Laboratory Handbook. Celis D. E., editor. Vol. 1. San Diego: Academic, Inc; 1994. pp. 538–544. [Google Scholar]
- 31.Barrientos A, Korr D, Tzagoloff A. Shy1p is necessary for full expression of mitochondrial COX1 in the yeast model of Leigh's syndrome. EMBO J. 2002;21:43–52. doi: 10.1093/emboj/21.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wittig I, Braun HP, Schägger H. Blue native PAGE. Nat. Protoc. 2006;1:418–428. doi: 10.1038/nprot.2006.62. [DOI] [PubMed] [Google Scholar]