Abstract
Accumulation of amyloid β-peptide (Aβ) and tau aggregates, possibly linked to age-associated deficiencies in protein homeostasis, appear to cause Alzheimer's disease. Schiff-base formation between Aβ and the aldehyde-bearing cholesterol oxidation product 3-β-hydroxy-5-oxo-5,6-secocholestan-6-al is known to increase Aβ amyloidogenicity. Here, we synthesized Aβ variants site-specifically modified with the cholesterol aldehyde at Asp-1, Lys-16, or Lys-28, rather than studying mixtures. These distinct modifications have a similar effect on the thermodynamic propensity for aggregation, enabling aggregation at low concentrations. In contrast, the modification site differentially influences the aggregation kinetics; Lys-16-modified Aβ formed amorphous aggregates fastest and at the lowest concentration (within 2 h at a concentration of 20 nM), followed by the Lys-28 and Asp-1 conjugates. Also, the aggregates resulting from Aβ Lys-16 cholesterol aldehyde conjugation were more toxic to primary rat cortical neurons than treatment with unmodified Aβ under identical conditions and at the same concentration. Our results show that Aβ modification by cholesterol derivatives, especially at Lys-16, renders it kinetically and thermodynamically competent to form neurotoxic aggregates at concentrations approaching the physiologic concentration of Aβ.
Keywords: Aβ, amyloid, oxidative stress, oxidized metabolite, protein misfolding
Aging, associated with decreasing protein homeostasis (proteostasis) capacity and increasing oxidative stress, is a prominent amyloid disease risk factor (1, 2). The hallmark of these maladies is tissue-selective deposition of amorphous and/or fibrillar cross-β-sheet-rich protein assemblies called amyloid (3). Alzheimer's disease (AD) (4) is the most common amyloid disease, afflicting >5 million people over age 65 in the United States (5). AD appears to be exacerbated by, if not caused by, defects in proteostasis that lead to intra (6) and extracellular amyloid β-peptide amyloidogenesis (Aβ; 39–43-residue peptides produced by endoproteolytic processing of the amyloid precursor protein) (4), and to intracellular aggregation of tau (7). The 40-residue form of Aβ (Aβ40) is most prevalent (8), whereas longer variants, especially the 42-residue form (Aβ42), are the most amyloidogenic (9). The longer variants dominate Aβ deposits in the brains of patients with AD (10) and their levels are elevated in some familial forms of AD (11).
An important concept in the thermodynamics of amyloidogenesis is the critical concentration, the concentration below which amyloid cannot form (12). The Aβ40 critical concentration has been reported to be in the range of 1–30 μM (9, 13, 14). However, the physiologic concentration of Aβ40 in CSF is in the low nanomolar range (8). As mentioned above, Aβ42 is more amyloidogenic than Aβ40, but its concentration is ≈10-fold lower than that of Aβ40 (8), so its critical concentration is still >100-fold higher than its physiologic concentration. How Aβ40 and Aβ42 aggregate in vivo when their physiologic concentrations are lower than their critical concentrations is one of the many mysteries of AD.
A related issue involves the kinetics of amyloid formation. Aβ is thought to undergo amyloidogenesis by a nucleated polymerization mechanism, which has two phases: a lag phase involving nucleation, followed by a fibril growth phase (12). The duration of the lag phase increases exponentially as concentration decreases, becoming very long when the concentration is close to the critical concentration (12). Thus, Aβ amyloidogenesis has both thermodynamic and kinetic barriers in vivo.
We have proposed that reversible covalent modification of Aβ by small molecule oxidation products, in combination with factors like adsorption to the extracellular matrix or membranes (15), can explain the ability of Aβ to form amyloid at physiologic concentrations (16–18). Small molecule oxidation products are generated when reactive oxygen species react with double bonds, including those of hydrophobic membrane components (19). The concentrations of the resulting hydrophobic aldehydes that can modify proteins through reversible Schiff base formation increase during aging and peak during oxidative stress (20). Both aging and oxidative stress are risk factors for AD (4, 19); in fact, small molecule oxidation products are found at elevated levels in the brains of individuals with AD (16, 19, 21, 22).
We and others have found that the naturally occurring small molecule oxidation products 4-hydroxynonenal (23) and 3β-hydroxy-5-oxo-5,6 secocholestan-6-al (see 1 in Fig. 1A), derived from cholesterol, and the aldol product of the latter (see 2 in Fig. 1A) (24), covalently modify Aβ and increase its amyloidogenicity (16–18, 25–27). Prior data indicated that cholesterol oxidation products 1 and 2, denoted 1(2) hereafter because they interconvert via an aldol/retro-aldol reaction (16, 18, 24), react with Aβ via Schiff base formation at the N-terminal α-amine of Asp-1 (D1) and the side-chain ε-amines of Lys-16 (K16) and Lys-28 (K28) (18). Compounds 1 and 2 have been detected by us (18, 28) and others (29) in ex vivo human and rat brain samples at combined concentrations of up to 400 pg/mg of wet brain (≈1 μM concentration). Scheinost et al. (27) recently suggested that K16 is the “hot spot” for Aβ aggregation induced by 1(2) modification. We previously reported that adduct formation between Aβ40 and 1(2) decreased the aggregation critical concentration to <100 nM (the limit of detection of the method used) (18), changed the aggregation mechanism from a nucleated to a downhill polymerization (17), and resulted in the formation of spherical aggregates when incubated quiescently (17, 18), which are reported to be neurotoxic (30). Thus, a spike in the concentration of these hydrophobic aldehydes (e.g., caused by trauma or inflammation) could trigger Aβ aggregation and then become traceless if the aldehyde concentrations later decrease because of the reversibility of Schiff base formation. However, significant questions about the modification of Aβ by 1(2) persist, including: Why is K16 a hot spot for covalent-modification-induced Aβ aggregation? Does modification by 1(2) lower the critical concentration of Aβ aggregation into the physiologic concentration range? Can Aβ modified by 1(2) aggregate at low concentrations on a biologically relevant time scale? And, are the aggregates formed by Aβ-1(2) conjugates toxic to primary neurons and what is their toxicity relative to unmodified Aβ? We address these questions here.
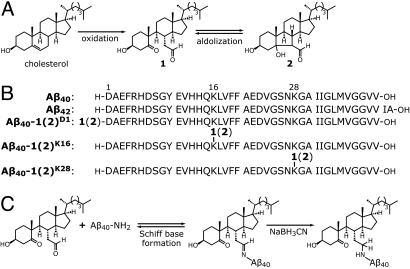
Fig. 1.
Oxidized cholesterol metabolites and their Aβ conjugates. (A) Cholesterol can be oxidized in vivo to form 1 and converted into 2 by a reversible aldol reaction. Compounds 1 and 2 can interconvert, so they are collectively denoted 1(2). (B) WT Aβ40 and Aβ42 sequences, and the sequences of the Aβ40-1(2) conjugates studied here. (C) Cholesterol metabolites 1(2) can attach to an amine of Aβ (D1, K16, or K28) by Schiff base formation. Stable analogs were produced by reducing the Schiff bases with NaBH3CN.
Results
Synthesis of Aβ Peptides Site-Specifically Modified by 1(2).
To investigate the aggregation energetics of Aβ40 modified by 1(2) at specific sites, we chemically synthesized Aβ40 modified with 1(2) at the α-amine of D1 [Aβ40-1(2)D1], or the ε-amine of K16 [Aβ40-1(2)K16] or K28 [Aβ40-1(2)K28] (Fig. 1B) using solid phase peptide synthesis (for detailed procedures, see SI Materials and Methods). Note that the hydrolytically unstable Schiff-base linkage between 1(2) and Aβ was reduced to a secondary amine in each peptide-1(2) conjugate (Fig. 1C). Although this permanent covalent linkage through a secondary amine is less conformationally constrained than a Schiff base, it retains the positive charge at neutral pH and the ability to adopt a Schiff base-equivalent conformation of the hydrophobic appendage enabling aggregation at low concentrations.
Monomerization of Aβ40, Aβ42, and the Aβ40-1(2) Conjugates.
Before studying the amyloidogenesis of Aβ40-1(2) conjugates, they must be monomerized. Because the Aβ40-1(2) conjugates are extremely aggregation prone, the high-pH pretreatment method of Fezoui et al. (31) that we used previously to monomerize Aβ40 (17, 18, 23) was not effective (Fig. S1). Instead, we monomerized the Aβ40-1(2) conjugates by dissolving them in 8 M guanidine hydrochloride (GuHCl) in phosphate buffer [50 mM sodium phosphate (NaPi), pH 7.5]. Solutions of monomeric Aβ variants in 8 M GuHCl were prepared for use by one of three methods. (i) Solutions of Aβ variants for equilibrium aggregation experiments were simply filtered through a 0.2-μm filter and diluted to the desired peptide and GuHCl concentrations. (ii) Solutions of Aβ variants for kinetic experiments, requiring rigorously monomeric Aβ, were obtained by preparative size exclusion chromatography (SEC) using 8 M GuHCl as the mobile phase. (iii) GuHCl-free solutions of Aβ variants were prepared by passing the GuHCl solution through a short SEC column eluted with phosphate buffer (for further details, see SI Materials and Methods).
Critical Concentrations of the Aβ40-1(2) Conjugates.
The critical concentration for aggregation, cagg, is equivalent to the concentration of the monomeric protein left in solution when aggregation reaches equilibrium (12, 32). In principle, cagg can be determined by allowing aggregation to reach equilibrium and measuring the concentration of Aβ40 monomer remaining in solution (32). However, the cagg values of Aβ40-1(2) conjugates are well below the detection thresholds of most protein concentration determinations (18), so direct measurements are challenging, if not impossible.
Instead, we used a chaotrope denaturation strategy to estimate the cagg values of Aβ40-1(2) conjugates. As suggested by Narimoto et al. (33), the free energy of aggregation, ΔGagg, like the free energy of protein folding, should depend linearly on GuHCl concentration, as should the natural logarithm of cagg, because ΔGagg = −RT ln cagg (R is the gas constant and T is the temperature). Thus, cagg should depend on GuHCl concentration as follows:
where [GuHCl] is the concentration of GuHCl, cagg,0 is the critical concentration at 0 M GuHCl, and magg/RT is the slope of a plot of ln cagg vs. [GuHCl]. According to Eq. 1, cagg,0 can be determined by measuring cagg at a series of GuHCl concentrations, plotting ln cagg vs. [GuHCl], and extrapolating to 0 M GuHCl.
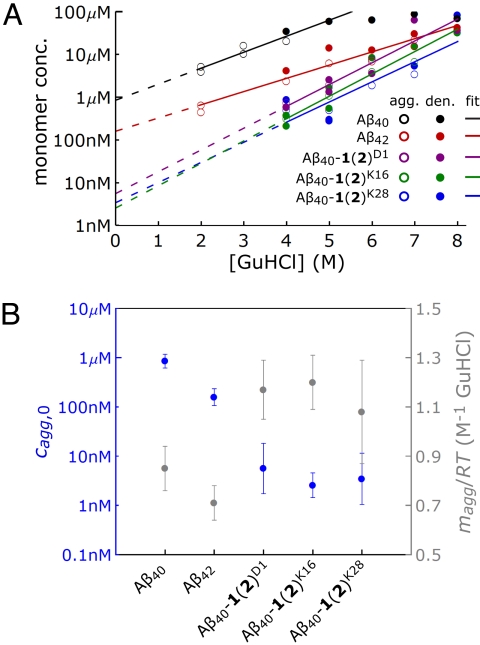
The aggregate denaturation method described above was implemented as follows. Aβ40, Aβ42, and the Aβ40-1(2) conjugates were monomerized by method i. Solutions of each variant were diluted to make a set of solutions with increasing GuHCl concentrations but constant Aβ concentration. These sets of solutions were incubated at 37 °C under constant agitation for at least 5 days, after which they were filtered through a 0.2-μm filter, and the value of cagg was determined by measuring the monomer concentration using the integrated peak intensities in a size exclusion chromatogram. To ensure that denaturation was reversible, preformed aggregates were also placed in increasing concentrations of GuHCl and cagg was determined. The aggregates in question were fibrillar for Aβ40 and Aβ42 and amorphous for the Aβ40-1(2) conjugates. The cagg values determined by approaching the aggregate-monomer equilibrium from the monomer or aggregate direction are plotted together vs. [GuHCl] for the Aβ variants in Fig. 2A. As expected for a true equilibrium, the cagg values from the two methods are consistent and are fit to a single line. Fig. 2B (left axis, solid blue circles) shows the cagg,0 values extrapolated from Fig. 2A. The critical concentration of Aβ40 determined by this method (850 nM) is similar to that determined previously by O'Nuallain et al. (14). The critical concentration of Aβ42 determined by this method is 160 nM, substantially lower than that of Aβ40, as expected (9).
Fig. 2.
Determination of cagg,0 for Aβ variants. (A) Plots of Aβ40 (black), Aβ42 (red), Aβ40-1(2)D1 (purple), Aβ40-1(2)K16 (green), and Aβ40-1(2)K28 (blue) monomer concentration vs. GuHCl concentration, [GuHCl]. Data points from aggregating Aβ variants at a given [GuHCl] (open circles). Data points from denaturing preformed aggregates at a given [GuHCl] (filled circles). Fits of Eq. 1 to the data (lines). Only data points between 2 and 5 M GuHCl were used in the fit for Aβ40, because its cagg is greater than the total peptide concentration when [GuHCl] > 5 M. Extrapolations of the fits to 0 M GuHCl for determining cagg,0 (dashed lines). (B) Blue axis: estimates for the aggregation critical concentrations (cagg,0) at 0 M GuHCl of the Aβ variants (Left). Gray axis: slopes (magg/RT) of the fits of Eq. 1 to the data in A (Right). Error bars are SEM.
The critical concentrations of Aβ40-1(2)D1, Aβ40-1(2)K16, and Aβ40-1(2)K28 determined by the denaturation method (Fig. 2B) are the same within experimental error, averaging ≈4 nM. This value of cagg is >200-fold lower than that of Aβ40 and 40-fold lower than that of Aβ42. The effect of the modification of Aβ40 by 1(2) on Aβ40 aggregation thermodynamics is apparently strong enough to drive cagg,0 of the Aβ40-1(2) conjugates into the physiologic concentration range of Aβ, and is independent of the site of modification.
The slopes of the plots of ln cagg vs. [GuHCl] in Fig. 2A for the Aβ variants are also plotted in Fig. 2B (right axis, solid gray circles). The Aβ40-1(2) conjugates have slopes ≈1.2 M−1 GuHCl (corresponding to magg = 0.7 kcal mol−1 M−1 GuHCl), compared with 0.85 M−1 GuHCl (magg = 0.52 mol−1 M−1 GuHCl) and 0.71 M−1 GuHCl (magg = 0.44 kcal mol−1 M−1 GuHCl) for unmodified Aβ40 and Aβ42, respectively. The value of magg should be proportional to the accessible surface area buried during aggregation (34). Assuming that magg increases by 2.2 × 10−4 kcal mol−1 M−1 GuHCl per Å2 of buried surface area (34), the difference in magg values suggests that the Aβ40-1(2) conjugates bury ≈900 Å2 more surface area on aggregation than unmodified Aβ40, comparable with the accessible surface area of cholesterol (≈670 Å2).
Aggregation Kinetics of Aβ40-1(2) Conjugates Monitored by Light Scattering.
Samples for studying the aggregation rates of the Aβ40-1(2) conjugates were monomerized by method ii, yielding rigorously monomeric Aβ40-1(2). The fractions corresponding to monomeric Aβ40-1(2) were collected, diluted so that the GuHCl concentration was 0.8 M, and the peptide concentration was as desired, and aggregation was monitored (without agitation) by the intensity of the light scattered at 90°, ΔIs (90°).
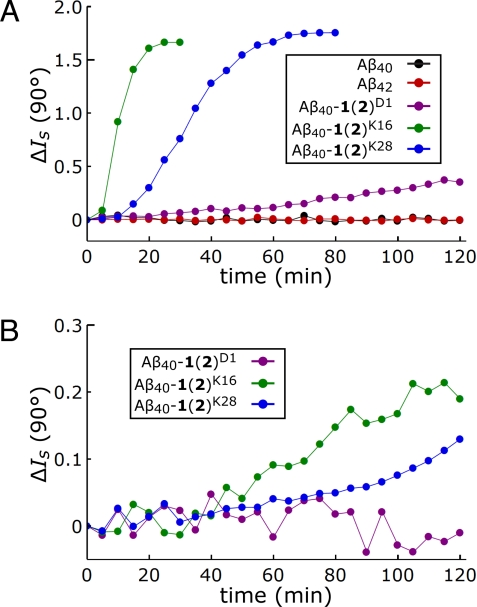
The aggregation time courses of Aβ40, Aβ42, and the Aβ40-1(2) conjugates at 37 °C in phosphate buffer (50 mM NaPi/300 mM NaCl, pH 7.5) with 0.8 M GuHCl present are plotted in Fig. 3A (500 nM) and 3B (100 nM). Unmodified Aβ40 and Aβ42 did not aggregate on the time scale of these experiments (2 h) at concentrations of 500 nM. In contrast, the Aβ40-1(2) conjugates showed evidence of aggregation within 2 h at a concentration of 500 nM (Fig. 3A). Unlike aggregation thermodynamics, the aggregation rates of the Aβ40-1(2) conjugates depended strongly on the modification site. Aβ40-1(2)K16 aggregated faster than Aβ40-1(2)K28, which aggregated faster than Aβ40-1(2)D1. This ordering is maintained at concentrations of 100 nM, except that Aβ40-1(2)D1 no longer aggregated within 2 h (Fig. 3B). Although the light scattering data were noisy at even lower Aβ40-1(2) concentrations, Aβ40-1(2)K16 and Aβ40-1(2)K28 still aggregated at concentrations of 50 nM, the limit of detection (aggregation reactions at 20 nM were examined, but exhibited no signal above the background; see Fig. S2).
Fig. 3.
Aggregation kinetics of Aβ variants at low concentrations monitored by light scattering. (A) Representative light scattering intensity changes, ΔIs (90°), vs. time for Aβ40 (black), Aβ42 (red), Aβ40-1(2)D1 (purple), Aβ40-1(2)K16 (green), and Aβ40-1(2)K28 (blue) at concentrations of 500 nM. (B) Representative ΔIs (90°) vs. time for Aβ40-1(2)D1 (purple), Aβ40-1(2)K16 (green), and Aβ40-1(2)K28 (blue) at concentrations of 100 nM. Analogous data using concentrations <100 nM are shown in Fig. S2.
Immuno-Electron Microscopy of Samples from Low Concentration Light Scattering Experiments.
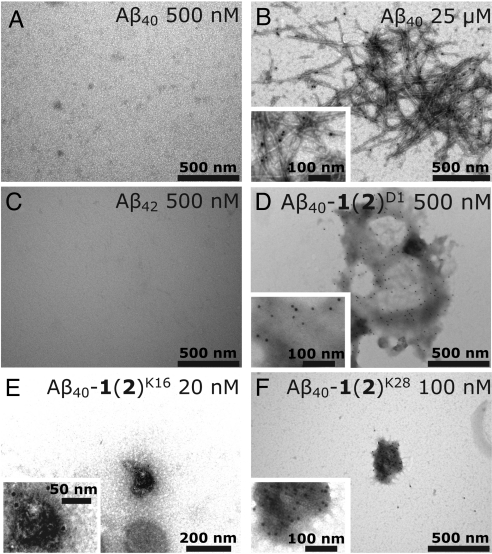
Samples from the low concentration light scattering time courses were examined by immuno-EM to ensure that the aggregates detected were composed of Aβ40-1(2), and to attempt to detect aggregates at even lower concentrations. Aliquots (50 μL) were removed from the aggregation reactions after 2 h and applied to EM grids. These EM grids were incubated with a monoclonal anti-Aβ antibody (6E10) followed by protein A (which binds to 6E10) conjugated to 10-nm gold particles. The resulting EM images are shown in Fig. 4 and Fig. S3 (for experimental details, see SI Materials and Methods). No aggregates were observed in samples of Aβ40 after 2 h of aggregation at a concentration of 500 nM (Fig. 4A), but gold particles were readily visible covering samples of Aβ40 fibrils formed at 25 μM (Fig. 4B). Aβ42 also showed no evidence of aggregation at 500 nM after 2 h (Fig. 4C), despite this concentration being above its apparent critical concentration. The remaining panels of Fig. 4 show images from the lowest concentration samples in which aggregates could be found in the 2-h aggregation reactions of the Aβ40-1(2) conjugates. Amorphous aggregates were detected at 500 nM for Aβ40-1(2)D1 (Fig. 4D), at 20 nM for Aβ40-1(2)K16 (Fig. 4E), and at 100 nM for Aβ40-1(2)K28 (Fig. 4F). Given that these are the minimal concentrations at which aggregates could be detected, the aggregates shown in Fig. 4 D–F were infrequently detected on the EM grids, as expected. Utilization of an Aβ-specific antibody guarantees that the aggregates are composed of Aβ. Also, the fact that aggregates are not observed in the unmodified Aβ samples suggests that they are not spurious. These results extend the light scattering data described above by demonstrating that Aβ40-1(2)K16 aggregates at concentrations below the light scattering detection limit.
Fig. 4.
Immuno-EM images of aggregates formed (or not formed) by Aβ variants. (A) Representative image from a sample in which Aβ40 at a concentration of 500 nM was incubated at 37 °C for 2 h. No Aβ aggregates were observed. (B) Representative image of preformed unmodified Aβ40 fibrils formed in a solution of Aβ40 (25 μM) at 37 °C with agitation. Gold particles (black dots), indicating the presence of Aβ40, can be seen attached to fibrils. (C) As in A, but with Aβ42. (D) As in A, but with Aβ40-1(2)D1 at a concentration of 500 nM. (E) As in A, but with Aβ40-1(2)K16 at a concentration of 20 nM. (F) As in A, but with Aβ40-1(2)K28 at a concentration of 100 nM. Aggregates of Aβ40-1(2) conjugates were not detected by this method in samples at concentrations lower than those noted in D–F. Additional immuno-EM images are shown in Fig. S3.
Aggregation Kinetics of the Aβ40-1(2) Conjugates Monitored by Thioflavin T (TfT) Fluorescence.
Aβ40, Aβ42, and the Aβ40-1(2) conjugates were monomerized by method iii and diluted to a concentration of 10 μM in phosphate buffer (50 mM NaPi/300 mM NaCl, pH 7.5). Their TfT-monitored aggregation time courses are shown in Fig. S4. Aggregates that yield a TfT fluorescence signal do not necessarily have classic fibrillar morphologies (17, 18, 35, 36), and therefore, will be termed “microfibrillar.” These experiments were performed with mild agitation (5 s of shaking every 10 min) to encourage the transition to microfibrillar aggregates (17). Microfibrillar aggregates appeared fastest for Aβ40-1(2)D1, followed by Aβ40-1(2)K16 and then Aβ40-1(2)K28, although more slowly in every case than the aggregates detected in the light scattering experiments, despite higher concentrations being used. This result suggests that the amorphous aggregates detected by light scattering are TfT-negative (i.e., they are not microfibrillar), and the amorphous aggregates either form first and then convert to microfibrillar aggregates or the amorphous and microfibrillar aggregates form in parallel with the amorphous aggregates appearing somewhat faster. We prefer the first explanation, because the second implies that the TfT-negative amorphous aggregates are off-pathway. The TfT-monitored time courses are not consistent with amyloidogenesis with off-pathway aggregates, because the TfT fluorescence does not increase quadratically with time, as required by off-pathway aggregation (37). Also, we have shown previously that amorphous aggregates formed by Aβ40-1(2) conjugates can convert directly to fibrils on seeding or agitation (17, 18).
Toxicity of the Aggregates Formed by Aβ40-1(2)K16 to Primary Neurons.
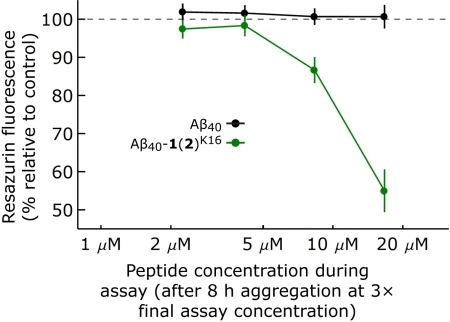
Although modification of Aβ40 with 1(2) dramatically lowers cagg,0 and hastens aggregate formation, whether these aggregates are toxic to primary neurons is unknown. The neurotoxicities of Aβ40 and Aβ40-1(2)K16 were compared by first monomerizing each variant by method iii, except that the peptides were eluted from the gel filtration column with HBSS. Aβ40 and Aβ40-1(2)K16 were incubated in HBSS for 8 h at 6.75 to 50 μM (3× the final toxicity assay concentration) at room temperature under quiescent conditions. After the incubation period, glial-cell-free primary rat cortical neurons (Fig. S5) were incubated for 48 h with varying concentrations of the Aβ peptides (0- to 16.67-μM final concentration) in cell culture media. The solutions of unmodified Aβ40 had little effect on cell viability/metabolic activity relative to buffer control at all concentrations under these aggregation conditions (Fig. 5), as determined by a resazurin assay (38). This result is consistent with our previous observation that rigorously monomerized, unmodified Aβ40 does not aggregate within 8 h when it is not agitated (17, 18). In contrast, the solutions of Aβ40-1(2)K16 were toxic to cells at concentrations >4.17 μM (up to a 45 ± 4% reduction in cell viability at 16.67 μM relative to buffer-treated controls; Fig. 5). The deleterious effect of Aβ40-1(2)K16 aggregates on the neurons are also apparent from the altered cell morphologies. Phase contrast microscopy images of primary rat cortical neurons incubated for 48 h with HBSS or Aβ40 at a final concentration of 16.67 μM revealed healthy neurons that formed interconnected networks (Fig. S6 A and B). However, primary rat cortical neurons incubated for 48 h with Aβ40-1(2)K16 at an identical concentration exhibited atrophy, clumping, and a loss of neural connections (Fig. S6C). These results demonstrate that the Aβ40-1(2) conjugate at K16 forms neurotoxic aggregates under conditions where Aβ40 does not.
Fig. 5.
Neurotoxicity of Aβ40 vs. Aβ40-1(2)K16. Aβ40 (black) and Aβ40-1(2)K16 (green) were subjected to aggregation conditions quiescently for 8 h (6.75 μM to 50 μM, 3× the final concentration used in the toxicity assay) and applied to primary rat cortical neurons at final concentrations ranging from 0 to 16.67 μM. After 48 h of incubation, the viability of the cells was assessed by resazurin fluorescence. Viability is shown as a percentage of the metabolic activity of cells treated with buffer alone. Data are from independent triplicates, and the error bars are for SEM. Fig. S6, which reveals neuron atrophy and disruption of neural connections only in Aβ40-1(2)K16-treated neurons, further supports the toxicity of Aβ40-1(2)K16, but not Aβ40 under these aggregation conditions.
Discussion
We have shown that the Aβ40-1(2) conjugates are thermodynamically and kinetically competent to form amorphous aggregates in vitro at concentrations close to the physiologic concentration of Aβ. However, their kinetic competence, unlike their thermodynamic competence, depends strongly on the site of modification. Aβ40-1(2)K16 forms aggregates most rapidly and at the lowest concentration (within 2 h at a concentration of 20 nM), followed by Aβ40-1(2)K28 and Aβ40-1(2)D1. This ranking matches the local hydrophobicity of the Aβ sequence at the modification site: K16 resides in the most hydrophobic context (…VHHQKLVFF…), followed by K28 (…VGSNKGAII…), and then D1 (DAEFRHDSG…). This correlation between local hydrophobicity and the kinetic effect of metabolite modification suggests that K16 and its surrounding residues were already important for aggregate nucleation and growth, consistent with previous findings (39), and that the increase in hydrophobicity attendant to the attachment of 1(2) to K16 magnifies the influence of this region. Thus, it is a hotspot for hydrophobic metabolite modification-mediated aggregation.
Fig. S4 shows that the formation of microfibrillar species is fastest for Aβ40-1(2)D1, followed by Aβ40-1(2)K16, and then by Aβ40-1(2)K28. This ranking matches the proximity of the modification to the N terminus rather than correlating with local hydrophobicity. We posit that the microfibrillar species result from conformational rearrangements within the initially-formed amorphous aggregates, consistent with the expectation that the transition from the TfT-negative amorphous aggregates to microfibrillar aggregates requires larger conformational changes at K28 and K16 than at D1, which is known to be disordered in structural models of fibrillar Aβ (40, 41).
Our observation that Aβ40-1(2)D1 and Aβ40-1(2)K28 are highly aggregation prone appear to contradict the results of Scheinost et al. (27), who reported that Aβ40 variants in which Schiff base formation was blocked at the ε-amine of K16 did not form TfT-positive aggregates in the presence of 1(2), even when the α-amine of D1 and the ε-amine of K28 were available. Our results can be reconciled with those of Scheinost et al. (27) by noting that Schiff base formation is reversible and Schiff bases are unstable in aqueous solution. Therefore, the concentration of Aβ40-1(2) conjugates should be low when Aβ40 is mixed with exogenous 1(2). Our results show that Aβ40-1(2)K16, which aggregates most rapidly at the lowest concentration, should be best able to aggregate under such conditions.
It is likely that no single factor will fully explain the pathogenesis of AD. AD probably results from the balance between Aβ aggregation and aggregate clearance tipping toward the former on aging, leading to aggregate accumulation, neurotoxicity, and memory loss. It is probable that the sum of all of the factors that either promote aggregation or diminish aggregate clearance determine whether an individual will become afflicted with AD. We have shown that Aβ modification by the oxidized cholesterol metabolite 1(2), especially at K16, could be an especially potent promoter of Aβ aggregation. Aβ40-1(2)K16 is, to our knowledge, the only Aβ variant that is kinetically and thermodynamically competent to aggregate at concentrations approaching the physiologic concentration of Aβ. Also, we have demonstrated that the aggregates formed by Aβ40-1(2)K16 are toxic to primary neurons. These observations, especially when considered with the previous demonstration that Aβ40-1(2) conjugates can induce unmodified Aβ to coaggregate (18), suggest that metabolite-initiated amyloid formation could contribute to AD pathogenesis. These data also imply that inhibiting Aβ modification by oxidized metabolites by using aldehyde-sequestering compounds or the equivalent could be a viable preventative strategy against AD (16, 17, 42). However, future efforts will be required to better understand the role of membrane component-derived Schiff base modifications of Aβ in the etiology of AD.
Methods
For detailed descriptions of the synthesis of Aβ variants site-specifically modified with 1(2) and the procedures for monomerization, immuno-EM, TfT-monitored aggregation kinetics, and the primary rat cortical neuron-based toxicity assay, see SI Materials and Methods. The solvent accessible surface area of cholesterol was determined by using ChemBio3D Ultra (probe radius = 1.4 Å).
Estimating Critical Concentrations Using GuHCl Denaturation and SEC.
Solutions of Aβ40, Aβ42, Aβ40-1(2)D1, Aβ40-1(2)K16, and Aβ40-1(2)K28 monomerized by method i (see SI Materials and Methods) were diluted to a peptide concentration of 100 μM and GuHCl concentrations ranging from 2 to 8 M in 1 M increments. These solutions were incubated with agitation using an EchoTherm RT10 rotating mixer (Torrey Pines Scientific) at 20 rpm for >5 days at 37 °C to enable aggregation. Alternately, preformed aggregates of each Aβ variant were prepared by monomerizing the peptides by method i, diluting to a peptide concentration of 100 μM and a GuHCl concentration of 0.8 M, and incubating at 37 °C with agitation as above for >5 days. Preformed aggregates were also prepared by directly dissolving the Aβ variants in buffer and incubating at 37 °C with agitation. Solutions of preformed aggregates, prepared with or without monomerization, were diluted to final peptide concentrations of 10 or 100 μM, respectively, and final GuHCl concentrations between 2 and 8 M to denature aggregates, and then incubated with agitation (20 rpm, 37 °C, >5 days).
Aggregation and aggregate denaturation solutions were filtered through a 0.22-μm syringe filter (Millipore). Samples (100 μL) were injected onto an AKTA FPLC employing a Superdex 75 HR 10/30 SEC column (GE Healthcare) and eluted with 50 mM sodium phosphate (pH 7.5, 0.02% NaN3) containing GuHCl at the same concentration as the injected sample. Aβ variants were detected by absorbance at 280 nm. Concentrations of monomeric Aβ variants were determined from the integrated intensity of the monomer peaks.
Kinetics of Aggregation of Aβ Variants Monitored by Light Scattering.
Seed-free solutions of Aβ40, Aβ42, and Aβ-1(2) conjugates in 8 M GuHCl prepared by method ii (see SI Materials and Methods) were diluted with 8 M GuHCl to 10× the desired final peptide concentration. These solutions were then diluted 10-fold in phosphate buffer (50 mM NaPi/300 mM NaCl, pH 7.5) so that the GuHCl concentration was 0.8 M and the peptide concentration was as desired. Samples (5 mL) were transferred to 25 mL scintillation vials, which were placed in a Dawn EOS light scattering photometer (Wyatt Technology) with a Peltier temperature controller. Light scattering intensity data at 90° offset by the intensity at t = 0 min, or ΔIs (90°), were collected for 2 h at 37 °C.
Acknowledgments.
We thank Prof. Hisakazu Mihara (Tokyo Institute of Technology, Tokyo) for advice on purifying the Aβ40-1(2) conjugates, and Dr. Xinyi Li for his advice on the culturing of primary neurons. This work was supported by the National Institutes of Health Grant NS050636, the Skaggs Institute for Chemical Biology, the Lita Annenberg Hazen Foundation, and the Bruce Ford and Anne Smith Bundy Foundation. K.U. was supported by a Young Scientists fellowship from the Japan Society for the Promotion of Science, and J.F.P. by a fellowship from the Swedish Research Council.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0804758106/DCSupplemental.
References
- 1.Balch WE, Morimoto RI, Dillin A, Kelly JW. Adapting proteostasis for disease intervention. Science. 2008;319:916–919. doi: 10.1126/science.1141448. [DOI] [PubMed] [Google Scholar]
- 2.Cohen E, Bieschke J, Perciavalle RM, Kelly JW, Dillin A. Opposing activities protect against age-onset proteotoxicity. Science. 2006;313:1604–1610. doi: 10.1126/science.1124646. [DOI] [PubMed] [Google Scholar]
- 3.Kelly JW. Alternative conformations of amyloidogenic proteins govern their behavior. Curr Opin Struct Biol. 1996;6:11–17. doi: 10.1016/s0959-440x(96)80089-3. [DOI] [PubMed] [Google Scholar]
- 4.Tanzi RE, Bertram L. Twenty years of the Alzheimer's disease amyloid hypothesis: A genetic perspective. Cell. 2005;120:545–555. doi: 10.1016/j.cell.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 5.Hebert LE, Scherr PA, Bienias JL, Bennett DA, Evans DA. Alzheimer disease in the US population: Prevalence estimates using the 2000 census. Arch Neurol. 2003;60:1119–1122. doi: 10.1001/archneur.60.8.1119. [DOI] [PubMed] [Google Scholar]
- 6.LaFerla FM, Green KN, Oddo S. Intracellular amyloid-β in Alzheimer's disease. Nat Rev Neurosci. 2007;8:499–509. doi: 10.1038/nrn2168. [DOI] [PubMed] [Google Scholar]
- 7.Ballatore C, Lee VM, Trojanowski JQ. Tau-mediated neurodegeneration in Alzheimer's disease and related disorders. Nat Rev Neurosci. 2007;8:663–672. doi: 10.1038/nrn2194. [DOI] [PubMed] [Google Scholar]
- 8.Mehta PD, Pirttila T, Patrick BA, Barshatzky M, Mehta SP. Amyloid β protein 1–40 and 1–42 levels in matched cerebrospinal fluid and plasma from patients with Alzheimer disease. Neurosci Lett. 2001;304:102–106. doi: 10.1016/s0304-3940(01)01754-2. [DOI] [PubMed] [Google Scholar]
- 9.Jarrett JT, Berger EP, Lansbury PT., Jr The carboxy terminus of the β-amyloid protein is critical for the seeding of amyloid formation: Implications for the pathogenesis of Alzheimer's disease. Biochemistry. 1993;32:4693–4697. doi: 10.1021/bi00069a001. [DOI] [PubMed] [Google Scholar]
- 10.Iwatsubo T, et al. Visualization of Aβ 42(43) and Aβ 40 in senile plaques with end-specific Aβ monoclonals: Evidence that an initially deposited species is Aβ 42(43) Neuron. 1994;13:45–53. doi: 10.1016/0896-6273(94)90458-8. [DOI] [PubMed] [Google Scholar]
- 11.Suzuki N, et al. An increased percentage of long amyloid-β protein secreted by familial amyloid-β protein precursor (βAPP717) mutants. Science. 1994;264:1336–1340. doi: 10.1126/science.8191290. [DOI] [PubMed] [Google Scholar]
- 12.Powers ET, Powers DL. The kinetics of nucleated polymerizations at high concentrations: Amyloid fibril formation near and above the “supercritical concentration”. Biophys J. 2006;91:122–132. doi: 10.1529/biophysj.105.073767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hortschansky P, Christopeit T, Schroeckh V, Fandrich M. Thermodynamic analysis of the aggregation propensity of oxidized Alzheimer's β-amyloid variants. Protein Sci. 2005;14:2915–2918. doi: 10.1110/ps.051585905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O'Nuallain B, Shivaprasad S, Kheterpal I, Wetzel R. Thermodynamics of Aβ(1–40) amyloid fibril elongation. Biochemistry. 2005;44:12709–12718. doi: 10.1021/bi050927h. [DOI] [PubMed] [Google Scholar]
- 15.Matsuzaki K. Physicochemical interactions of amyloid beta-peptide with lipid bilayers. Biochim Biophys Acta. 2007;1768:1935–1942. doi: 10.1016/j.bbamem.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 16.Bieschke J, et al. Small molecule oxidation products trigger disease-associated protein misfolding. Acc Chem Res. 2006;39:611–619. doi: 10.1021/ar0500766. [DOI] [PubMed] [Google Scholar]
- 17.Bieschke J, Zhang Q, Powers ET, Lerner RA, Kelly JW. Oxidative metabolites accelerate Alzheimer's amyloidogenesis by a two-step mechanism, eliminating the requirement for nucleation. Biochemistry. 2005;44:4977–4983. doi: 10.1021/bi0501030. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Q, et al. Metabolite-initiated protein misfolding may trigger Alzheimer's disease. Proc Natl Acad Sci USA. 2004;101:4752–4757. doi: 10.1073/pnas.0400924101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sayre LM, Smith MA, Perry G. Chemistry and biochemistry of oxidative stress in neurodegenerative disease. Curr Med Chem. 2001;8:721–738. doi: 10.2174/0929867013372922. [DOI] [PubMed] [Google Scholar]
- 20.Voss P, Siems W. Clinical oxidation parameters of aging. Free Radic Res. 2006;40:1339–1349. doi: 10.1080/10715760600953859. [DOI] [PubMed] [Google Scholar]
- 21.Markesbery WR, Lovell MA. Four-hydroxynonenal, a product of lipid peroxidation, is increased in the brain in Alzheimer's disease. Neurobiol Aging. 1998;19:33–36. doi: 10.1016/s0197-4580(98)00009-8. [DOI] [PubMed] [Google Scholar]
- 22.Montine TJ, Markesbery WR, Morrow JD, Roberts LJ., II Cerebrospinal fluid F2-isoprostane levels are increased in Alzheimer's disease. Ann Neurol. 1998;44:410–413. doi: 10.1002/ana.410440322. [DOI] [PubMed] [Google Scholar]
- 23.Siegel SJ, Bieschke J, Powers ET, Kelly JW. The oxidative stress metabolite 4-hydroxynonenal promotes Alzheimer protofibril formation. Biochemistry. 2007;46:1503–1510. doi: 10.1021/bi061853s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wentworth P, Jr, et al. Evidence for ozone formation in human atherosclerotic arteries. Science. 2003;302:1053–1056. doi: 10.1126/science.1089525. [DOI] [PubMed] [Google Scholar]
- 25.Komatsu H, Liu L, Murray IV, Axelsen PH. A mechanistic link between oxidative stress and membrane mediated amyloidogenesis revealed by infrared spectroscopy. Biochim Biophys Acta. 2007;1768:1913–1922. doi: 10.1016/j.bbamem.2007.05.026. [DOI] [PubMed] [Google Scholar]
- 26.Murray IV, et al. Membrane-mediated amyloidogenesis and the promotion of oxidative lipid damage by amyloid-β proteins. J Biol Chem. 2007;282:9335–9345. doi: 10.1074/jbc.M608589200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scheinost JC, Wang H, Boldt GE, Offer J, Wentworth P., Jr Cholesterol seco-sterol-induced aggregation of methylated amyloid-β peptides-Insights into aldehyde-initiated fibrillization of amyloid-β. Angew Chem Int Ed Engl. 2008;47:3919–3922. doi: 10.1002/anie.200705922. [DOI] [PubMed] [Google Scholar]
- 28.Bosco DA, et al. Elevated levels of oxidized cholesterol metabolites in Lewy body disease brains accelerate alpha-synuclein fibrilization. Nat Chem Biol. 2006;2:249–253. doi: 10.1038/nchembio782. [DOI] [PubMed] [Google Scholar]
- 29.Karu K, et al. Liquid chromatography-mass spectrometry utilizing multi-stage fragmentation for the identification of oxysterols. J Lipid Res. 2007;48:976–987. doi: 10.1194/jlr.M600497-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klein WL, Stine WB, Jr, Teplow DB. Small assemblies of unmodified amyloid β-protein are the proximate neurotoxin in Alzheimer's disease. Neurobiol Aging. 2004;25:569–580. doi: 10.1016/j.neurobiolaging.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 31.Fezoui Y, et al. An improved method of preparing the amyloid β-protein for fibrillogenesis and neurotoxicity experiments. Amyloid. 2000;7:166–178. doi: 10.3109/13506120009146831. [DOI] [PubMed] [Google Scholar]
- 32.O'Nuallain B, et al. Kinetics and thermodynamics of amyloid assembly using a high-performance liquid chromatography-based sedimentation assay. Methods Enzymol. 2006;413:34–74. doi: 10.1016/S0076-6879(06)13003-7. [DOI] [PubMed] [Google Scholar]
- 33.Narimoto T, et al. Conformational stability of amyloid fibrils of β2-microglobulin probed by guanidine-hydrochloride-induced unfolding. FEBS Lett. 2004;576:313–319. doi: 10.1016/j.febslet.2004.09.024. [DOI] [PubMed] [Google Scholar]
- 34.Myers JK, Pace CN, Scholtz JM. Denaturant m values and heat capacity changes: Relation to changes in accessible surface areas of protein unfolding. Protein Sci. 1995;4:2138–2148. doi: 10.1002/pro.5560041020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Frankenfield KN, Powers ET, Kelly JW. Influence of the N-terminal domain on the aggregation properties of the prion protein. Protein Sci. 2005;14:2154–2166. doi: 10.1110/ps.051434005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hurshman AR, White JT, Powers ET, Kelly JW. Transthyretin aggregation under partially denaturing conditions is a downhill polymerization. Biochemistry. 2004;43:7365–7381. doi: 10.1021/bi049621l. [DOI] [PubMed] [Google Scholar]
- 37.Powers ET, Powers DL. Mechanisms of protein fibril formation: Nucleated polymerization with competing off-pathway aggregation. Biophys J. 2008;94:379–391. doi: 10.1529/biophysj.107.117168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.O'Brien J, Wilson I, Orton T, Pognan F. Investigation of the Alamar Blue (resazurin) fluorescent dye for the assessment of mammalian cell cytotoxicity. Eur J Biochem. 2000;267:5421–5426. doi: 10.1046/j.1432-1327.2000.01606.x. [DOI] [PubMed] [Google Scholar]
- 39.Hilbich C, Kisters-Woike B, Reed J, Masters CL, Beyreuther K. Substitutions of hydrophobic amino acids reduce the amyloidogenicity of Alzheimer's disease βA4 peptides. J Mol Biol. 1992;228:460–473. doi: 10.1016/0022-2836(92)90835-8. [DOI] [PubMed] [Google Scholar]
- 40.Luhrs T, et al. 3D structure of Alzheimer's amyloid-β(1–42) fibrils. Proc Natl Acad Sci USA. 2005;102:17342–17347. doi: 10.1073/pnas.0506723102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Petkova AT, Yau WM, Tycko R. Experimental constraints on quaternary structure in Alzheimer's β-amyloid fibrils. Biochemistry. 2006;45:498–512. doi: 10.1021/bi051952q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Negre-Salvayre A, Coatrieux C, Ingueneau C, Salvayre R. Advanced lipid peroxidation end products in oxidative damage to proteins. Potential role in diseases and therapeutic prospects for the inhibitors. Br J Pharmacol. 2008;153:6–20. doi: 10.1038/sj.bjp.0707395. [DOI] [PMC free article] [PubMed] [Google Scholar]