Abstract
Regio- and stereoselective oxidation of an unactivated C–H bond remains a central challenge in organic chemistry. Considerable effort has been devoted to identifying transition metal complexes, biological catalysts, or simplified mimics, but limited success has been achieved. Cytochrome P450 mono-oxygenases are involved in diverse types of regio- and stereoselective oxidations, and represent a promising biocatalyst to address this challenge. The application of this class of enzymes is particularly significant if their substrate spectra can be broadened, selectivity controlled, and reactions catalyzed in the absence of expensive heterologous redox partners. In this study, we engineered a macrolide biosynthetic P450 mono-oxygenase PikC (PikCD50N-RhFRED) with remarkable substrate flexibility, significantly increased activity compared to wild-type enzyme, and self-sufficiency. By harnessing its unique desosamine-anchoring functionality via a heretofore under-explored “substrate engineering” strategy, we demonstrated the ability of PikC to hydroxylate a series of carbocyclic rings linked to the desosamine glycoside via an acetal linkage (referred to as “carbolides”) in a regioselective manner. Complementary analysis of a number of high-resolution enzyme-substrate cocrystal structures provided significant insights into the function of the aminosugar-derived anchoring group for control of reaction site selectivity. Moreover, unexpected biological activity of a select number of these carbolide systems revealed their potential as a previously unrecorded class of antibiotics.
Keywords: cytochrome P450 mono-oxygenase, PikC, RhFRED, substrate engineering
The superfamily of cytochrome P450 enzymes (mono-oxygenases) is involved in diverse oxidative processes, including xenobiotic catabolism, steroid synthesis, and biosynthetic tailoring of diverse natural products (1–3). Among various reactions catalyzed by P450 enzymes, the regio- and stereoselective oxidation of an unactivated sp3 C–H bond represents a central challenge in organic chemistry (4–8). Considerable effort has been devoted to identifying biological catalysts or simpler mimics that function by mechanisms typically involving a metal oxo-reactive site (9). Alternatively, transition metal complexes have been identified for C–H bond oxidations that proceed through mechanisms completely distinct from biological systems. A key challenge in developing useful C–H oxidation procedures is the control of site-selectivity among similar C–H bonds. Successful approaches have typically involved either relying on the inherent reactivity differences of various C–H bonds, based on steric and electronic considerations (10–15), or the incorporation of directing groups that orient the catalyst active site toward a specific C–H bond (16–18). The selective oxidation of a C–H bond that is neither inherently more reactive than alternate sites nor positioned adjacent to a directing group poses the most difficult application in site-selective C–H bond functionalization.
Recent reports have shown that supramolecular organometallic assemblies can provide some success in this challenge for synthetic chemistry (19–24). Alternatively, biological catalysts may provide unique potential to selectively oxidize bonds that are chemically similar, yet remote from directing influences. Thus, we were drawn to investigate the potential role of biosynthetic P450 mono-oxygenases, despite their fundamental dependence on substrate-enzyme complementarity, which might limit their application in synthetic chemistry (25). A number of previous efforts have sought to overcome this limitation by employing protein engineering strategies, including scanning chimeragenesis (26, 27) and directed-evolution (28–31) to generate nonnatural cytochrome P450s (e.g., P450BM3) with desired substrate specificities and abilities to selectively oxidize target substrates.
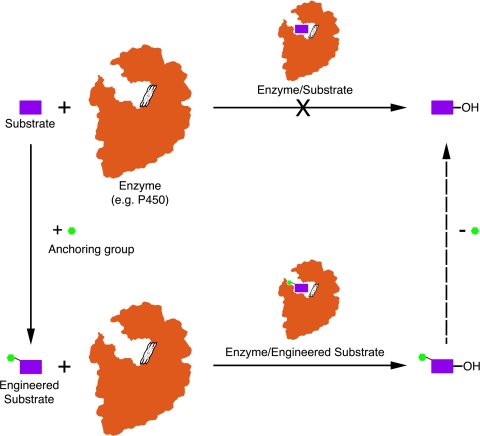
Based on a series of investigations involving P450 mono-oxygenases with remarkable substrate flexibility in macrolide biosynthetic systems (32, 33), we were motivated to explore their potential value in selective C–H bond activation reactions (25). Their unique requirement for a sugar-appended substrate led us to assess an under-explored strategy involving “substrate engineering” (Fig. 1) that offers another way to broaden the substrate landscape of biological catalysis (34, 35). Our anchoring/tethering approach was inspired by an earlier observation that certain functional groups facilitate enzyme-substrate interactions, where a direct functional group linkage (termed “anchoring group” in this study) to previously inaccessible compounds enables selective oxidation of the modified substrates in vivo (36, 37). However, because of the complexity of the cell-based system, the precise mechanism behind the substrate engineering approach has remained unclear. An initial application (36) hypothesized that the anchoring group might be involved in substrate recognition, productive binding, or control of binding orientation in the active site of certain oxidative enzymes (e.g., mono-oxygenases).
Fig. 1.
Schematic strategy of substrate engineering.
Here, we report in vitro implementation of substrate engineering for selective C–H bond oxidations by using an optimized form of the macrolide P450 mono-oxygenase PikC (32, 38). For this study, a series of carbocyclic rings linked to the desosamine glycoside (referred to as “carbolides”) were effectively hydroxylated in a regioselective manner. Furthermore, analysis of a series of high-resolution enzyme-substrate cocrystal structures provided significant insights into the function of the aminosugar-derived anchoring group for control of reaction site selectivity. Finally, unexpected biological activity of a select number of these carbolide systems revealed their potential as an unusual class of antibiotics.
Results and Discussion
Engineered PikCD50N-RhFRED Is Capable of Hydroxylating Carbolides Effectively and Regioselectively.
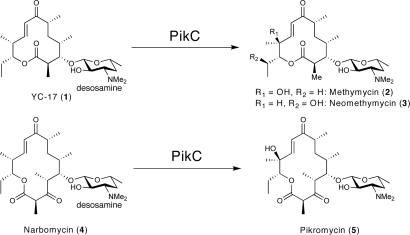
PikC is the cytochrome P450 involved in pikromycin biosynthesis from Streptomyces venezuelae (32, 39). The physiological function of this mono-oxygenase is to hydroxylate both the 12-membered ring macrolide YC-17 (structure 1) and the 14-membered ring macrolide narbomycin (structure 4), giving rise to methymycin/neomethymycin (structures 2 and 3) and pikromycin (structure 5), respectively, as major products (Fig. 2). Recent analysis of X-ray cocrystal structures of PikC (40, 41) involving endogenous substrates revealed that the macrolactone ring contacts the active site residues entirely via nonspecific hydrophobic interactions, likely accounting for the tolerance of PikC toward the variant macrolactone ring size and functionalization. In contrast, the desosamine sugar employs two distinct binding pockets and anchors the substrate through a number of hydrogen bonds and ionic interactions, in particular, a unique salt bridge between the protonated dimethylamino group of desosamine and a glutamate residue, either Glu-94 or Glu-85 in the B/C loop region. Based on these previously recognized molecular interactions that specify substrate binding affinity and orientation in the binding pocket, we reasoned that desosamine could be an effective anchoring group to direct positioning of various unnatural molecules in the active site of PikC for selective C–H bond hydroxylations.
Fig. 2.
Major physiological reactions catalyzed by PikC.
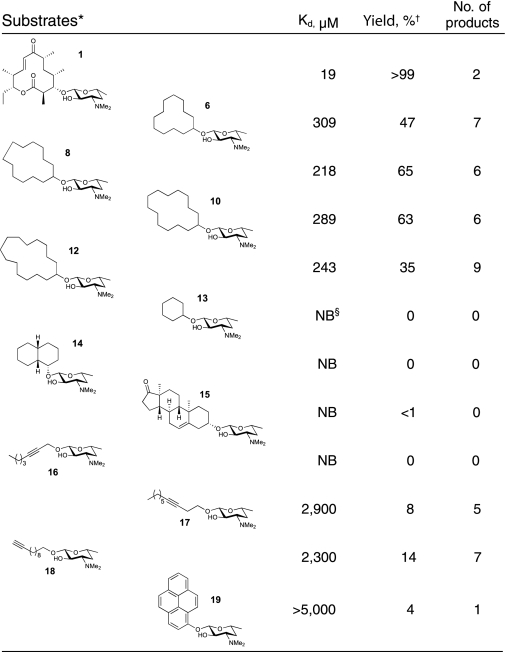
To test this hypothesis, we synthesized the unnatural cyclic carbolide substrate desosaminyl cyclododecane (structure 6) to mimic the structure of the natural substrate YC-17 (structure 1) using a recently developed synthetic strategy (33), which was subsequently used as a general approach to derivatize diverse alcohols with desosamine (Fig. S1 in SI Appendix). An enzyme-substrate analysis showed that structure 6 binds to wild type PikC (PikCwt) with a dissociation constant (Kd) of 1,379 μM, ≈12× higher than the Kd value (116 μM) of structure 1. The decreased binding affinity of structure 6 could result from: (i) an entropic penalty upon binding because of high conformational freedom of the saturated ring system; (ii) lack of hydrophobic interactions between the functional groups on the macrolactone ring of structure 1 and PikC active site residues; and (iii) loss of some specific interactions with desosamine, as observed in the cocrystal structure with PikC (see below). When using the more active PikCD50N mutant (40, 41), the binding affinities of both carbolide (structure 6) and macrolide (structure 1) were shown to be ≈4× higher, with Kd values of 390 and 32 μM, respectively. Moreover, we recently engineered a self-sufficient fusion enzyme PikC-RhFRED (38) that displayed ≈4-fold enhanced catalytic activity (kcat) compared to PikCwt. Combining these two beneficial properties, the resulting engineered form of the P450 enzyme PikCD50N-RhFRED (kcat/Km = 7.44 μM−1·min−1 for structure 1) (Fig. S2 in SI Appendix) is ≈13× more active than PikCwt (38). Interestingly, structure 1 and structure 6 bound to this mutant enzyme with slightly improved Kd values of 19 and 309 μM (Table 1), respectively. Because of enhanced substrate conversion and ease of use in the absence of expensive exogenous redox partners, we elected to employ PikCD50N-RhFRED to hydroxylate carbolide (structure 6) and all other substrates for this work.
Table 1.
The activity of PikCD50N-RhFRED toward various substrates
*Racemic cis-decahydro-1-naphthol was used in preparation of structure 14, leading to the structure shown and its diastereomer. The mixture of diastereomers was subjected to the PikC oxidation reaction.
†The yield shown is based on product formation calculated with AUCtotal products/(AUCtotal products + AUCunreacted substrate) by assuming ionization efficiency of substrate and various hydroxylated products are the same, because the ionization site of this series of desosaminyl derivatives should be the dimethylamino group. The calculated yield is fairly close (difference < ±3%) to the substrate conversion ratio calculated by 1 − AUCunreacted substrate/AUCtotal substrate (AUCtotal substrate is derived from the control reaction using boiled enzyme under identical conditions), indicating the assumption is correct. (AUC: area under curve of the ion count chromatograms, as shown in Fig. 3).
‡The number of products refers to the number of distinct peaks seen in the liquid chromatography-mass spectrometry (LC-MS). Comparison to synthetic standards illustrated a few cases where regioisomers or diastereomers were not distinguished by LC-MS, so the number provided is a lower limit. See SI Appendix for details.
§NB, no binding.
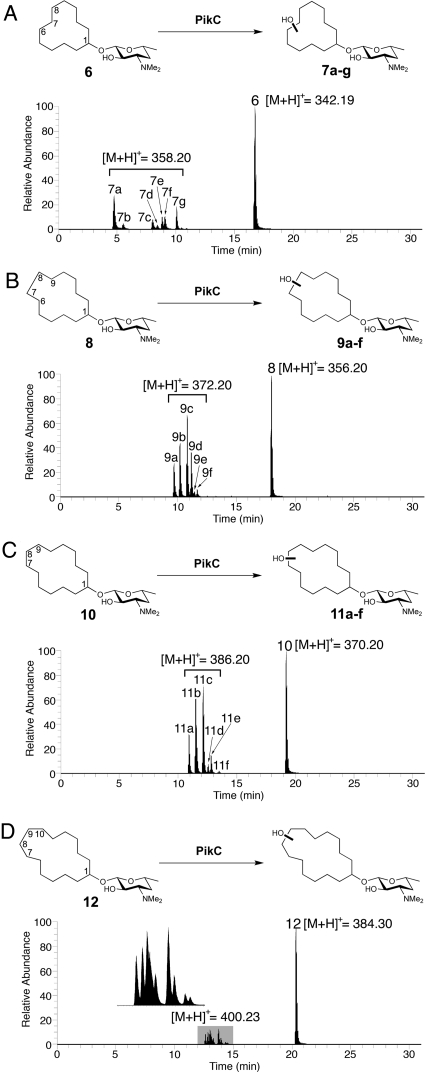
Liquid chromatography-mass spectrometry (LC-MS) analysis (Fig. 3A) of the extract obtained following reaction with PikCD50N-RhFRED showed that 47% of carbolide (structure 6) was converted into seven different monohydroxylated products (structures 7a–7g) (no multihydroxylated products were observed) with expected m/z = 358.19 for structure 6 + OH + H+ using 5 μM PikCD50N-RhFRED in 3 h (the conversion can be driven further by increasing enzyme concentration or reaction time). All product ions displayed the same MS/MS spectra (Fig. S3 in SI Appendix) at m/z = 158.02, corresponding to desosamine–OH+. The unmodified desosamine moiety indicates that all hydroxylations occur on the cyclododecane ring. In contrast, cyclododecanol lacking an appended desosamine was unable to serve as a substrate for PikC P450 under identical conditions. Therefore, it is evident that desosamine is indispensable for this biochemical transformation. Notably, PikCwt, PikCD50N, and PikCwt-RhFRED generated similar product profiles compared to PikCD50N-RhFRED, albeit with lower efficiency. These results indicate that neither the point mutation nor the C-terminal RhFRED-fusion with PikC has a significant impact on the binding mode of structure 6.
Fig. 3.
LC-MS analysis of PikCD50N-RhFRED catalyzed reactions using different cyclized carbolides as substrates. (Ion count chromatograms are shown.) (A) Desosaminyl cyclododecane (structure 6) reaction; structures 7b and 7f correspond to two diastereomers generated by C7 hydroxylation; structures 7a, 7c, 7e, and 7g correspond to four diastereomers generated by C6/C8 hydroxylation. (B) Desosaminyl cyclotridecane (structure 8) reaction; structures 9a, 9d, and 9f correspond to diastereomers arising from C6/C9 hydroxylation; structures 9b and 9c correspond to diastereomers originated from C7/C8 or C6/C9 hydroxylation. The number of products that peak 9c contains is undetermined because of product overlap. (C) Desosaminyl cyclotetradecane (structure 10) reaction. (D) Desosaminyl cyclopentadecane (structure 12) reaction. The details of product assignment for structures 6 and 8 based on correlation with synthesized authentic standards regarding retention time and coinjection confirmation are shown in the SI Appendix.
To assess the regio- and stereoselectivity of PikCD50N-RhFRED toward the selected substrate, we sought to obtain detailed structural information on the reaction products. Challenging preparative-scale separations, as well as the high similarity of the methylene groups on the cyclododecane ring, complicated structure determination of the individual compounds by NMR analysis. Because of the large number of potential isomers (23 total) that could result from oxidation of the 12-membered ring of structure 6, it was impractical to synthesize all possible products. Therefore, guided by the 2.0-Å cocrystal structure of mutant PikCD50N enzyme with structure 6 (Fig. 4), we synthesized a series of authentic standards with a hydroxyl group installed at the C7 and C6/C8 position (the two diastereotopic carbons were numbered differently for clarity) that were likely to be the major reaction products. During the chemical synthesis, but before desosamine installation, racemic mixtures of two diastereomers of a monoprotected diol leading to C6/C8-oxidized authentic materials were prepared. Glycosylation, with the glycosyl fluoride of acetate-protected desosamine followed by deprotection, provided the four diastereomers that correspond to C6/C8 oxidized products (see SI Appendix). Similarly, the two diastereomers of C7-oxidized authentic material were obtained.
Fig. 4.
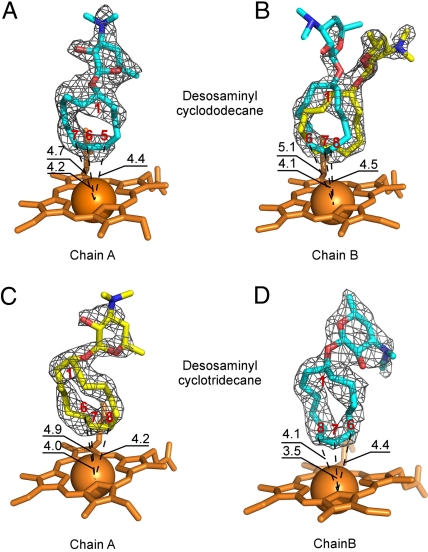
Multiple binding modes of desosaminyl cycloalkanes. Orientations of structure 6 in the active site of PikCD50N (A) in chain A and (B) in chain B, and orientations of structure 8 in the active site of PikCD50N (C) in chain A and (D) in chain B, as defined by the fragments of the electron density map (gray mesh) contoured at 0.8 σ are shown. In (B), structure 6 is docked in the flipped-over orientations, allowing hydroxylation on the both sides of the ring. In (C) and (D), structure 8 is in flipped-over orientations. Heme is shown in orange. Oxygen atoms are in red, nitrogen in blue, iron in orange (shown as a Van der Waals sphere). Atoms of the cycloalkane ring are labeled in red. Distances are in Angstroms. Images are generated using PYMOL.
Through comparison of LC-MS retention times and confirmation by coinjections (Fig. S4 in SI Appendix), six of the seven PikC-derived hydroxylated products of structure 6 were assigned as the C7 and C6/C8 oxidized materials. Because authentic samples of oxidized products were prepared as diastereomeric pairs, hydroxyl positioning was determined, but the precise stereostructure was not. The ratio of C7 oxidized products (structure 7b and structure 7f) to C6/C8 oxidized products (structure 7a, structure 7c, structure 7e, and structure 7g) was ≈1:4 (see SI Appendix for product assignment). Thus, it is evident that PikC-catalyzed hydroxylation occurs primarily at sites most remote from the desosamine-anchoring group, as predicted by the crystal structure (see Fig. 4). The C7 and C6/C8 oxidized compounds account for 95% of the mass of monohydroxylated material, and the only unidentified minor product (structure 7d) (5%) might be one of the C5 hydroxylated products. Considering the abundance of secondary C–H bonds on the 12-membered ring with almost equal reactivities, this regioselectivity is considerable, but not as strict as that observed toward the native macrolide substrates structure 1 (YC-17) and structure 4 (narbomycin).
We next sought to determine if C–H hydroxylation still occurs at the sites remote from desosamine in the cases of larger hydrocarbon rings, thus the 13-membered ring carbolide structure 8 was synthesized and treated with PikCD50N-RhFRED in a similar way to structure 6. Consistently, the major peaks (structures 9a–9d) (accounting for 94% of all products) (Fig. 3B) were determined to be C7/C8 or C6/C9 monohydroxylated products (Figs. S5 and S6 in SI Appendix). However, because of high structural similarity between these molecules, some synthetic hydroxylated authentic standards containing different diastereomeric pairs were not distinguishable from one another because of identical retention times (e.g., Fig. S6 D and E in SI Appendix), which prevented determination of the exact number of diastereomers generated in the reaction.
Furthermore, two larger cyclic carbolides, including the 14-membered ring (structure 10) and 15-membered ring (structure 12) were investigated. The reactivity of desosaminyl cyclotetradecane (structure 10) was similar to that of 13-membered ring carbolide (structure 8), with 65% substrate converted to hydroxylation products (structures 11a–11f) (Fig. 3C and Fig. S7 in SI Appendix). In contrast, the desosaminyl cyclopentadecane (structure 12), although having comparable binding affinity with structure 8 and structure 10 (Table 1), showed lower conversion (35%) and decreased selectivity reflected by the increased number of products (Fig. 3D and Fig. S8 in SI Appendix), suggesting that this large cyclic substrate might not be located in a suitable position within the PikC active site.
Analysis of PikCD50N-RhFRED Reactivity Toward Other Types of Desosaminyl Derivatives.
Because of the surprising activity and regioselectivity that PikCD50N-RhFRED showed toward 12-, 13-, and 14-membered ring carbolide derivatives, we decided to investigate the ability of desosamine to function as an anchor for other classes of compounds. Thus, the activity of PikCD50N-RhFRED toward additional cyclic and linear synthetic substrates (see Table 1) was assessed. The results revealed that PikC only marginally hydroxylated cyclic derivatives (structures 13 to 15), suggesting that these small ring systems might not be accommodated within the active site, or cannot reach the iron-oxo center for catalysis.
Surprisingly, the linear derivatives (structures 17 and 18) were oxidized to form multiple mono-hydroxylated products with 8% and 14% yields, respectively (see Table 1, Figs. S9 and S10 in SI Appendix). The predominant hydroxylation site for structure 18 was identified as the C9 propargylic position (Fig. S11 in SI Appendix). When aromatic derivative (structure 19) was used as substrate (Fig. S12 in SI Appendix), we observed a single oxidized product determined to be the 8-hydroxy desosaminyl pyrene (Table S1 in SI Appendix), albeit with a conversion of 4%. This observation suggests that rigidity of the substrate might be a key factor to gain high selectivity when using PikC as a biocatalyst for oxidation. The low level of conversion might result from its poor binding or higher C–H bond dissociation energy in this aromatic ring system. Taken together, these data reveal that PikC-catalyzed oxidation occurs primarily at the site most remote from the desosamine anchoring group when linear or aromatic substrates are used, suggesting a similar mechanism for control of regioselectivity compared to the carbolides.
Structural Basis for Regioselectivity of PikC Toward 12- and 13-Membered Ring Carbolides.
To understand the structural basis for the regioselectivity of PikC and predict the hydroxylation sites of the 12- and 13-membered ring carbolides to direct the synthesis of authentic standards, we determined the crystal structures (Table S2 in SI Appendix, and see Figs. 4 and 5) of PikCD50N in complex with unnatural substrates (structures 6 and 8).
Fig. 5.
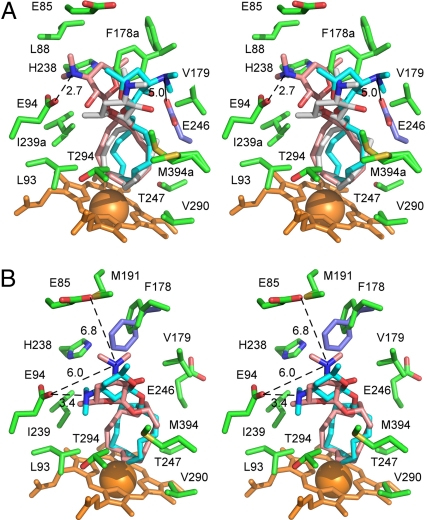
Desosaminyl cycloalkane binding sites. (A) Stereoview of the PikCD50N binding site with the three superimposed structure 6 conformers highlighted (gray) in chain A and (pink and cyan) chain B, surrounded by the chain-A amino acid side chains within 5 Å plus E85 (green). E246 of chain B is highlighted in ice blue. For clarity, V242 was omitted from the drawing. (B) Stereoview of the PikCD50N binding site with two superimposed structure 8 conformers highlighted (pink) in chain A and (cyan) chain B, surrounded by the chain A amino acid side chains within 5 Å plus E85. F178 of chain B is highlighted in ice blue. Heme is shown in orange. Oxygen atoms are in red, nitrogen in blue, iron in orange (shown as a Van der Waals sphere). Distances between tertiary amine and carboxylic groups are in Angstroms. Lowercase “a” in the residue label indicates that alternative conformations are shown.
In the cocrystal structure of PikCD50N and structure 6, the electron density for structure 6 is well defined in one monomer of the asymmetric unit (see Fig. 4A). In contrast, the other monomer showed an unambiguously positioned cyclododecane ring, while the dispersed electron density for desosamine indicated at least two alternative conformations (see Fig. 4B). A satisfactory fit was achieved when structure 6 was docked in two flipped orientations. The carbolide (structure 6) binds in the active site in the L-shaped conformation bringing four cyclododecane carbons most remote from the desosamine anchoring group within 5 Å of the Fe reaction center (see Fig. 4 A and B), revealing that C5, C6, C7, and C8 are the likely hydroxylation sites. The observed pattern of regioselectivity could arise from sporadic contacts of the desosamine moiety of structure 6 with a number of amino acid residues (see Fig. 5A). Indeed, the specific salt-bridge involving E94 is found in only one conformer (pink in Fig. 5A) of structure 6, and a number of the chain A active-site residues (green) (including F178, I239, V242, and M394) adopt alternative conformations indicative of dynamic interactions with the substrate. In chain B, although the salt-bridge to E94 is lost, E246 (ice blue in Fig. 5A) is located within electrostatic distance from the dimethyamino group of the proximal conformer (cyan). However, the E246 side chain is missing from the electron-density map of chain A. In addition, the suboptimal regioselectivity could be caused by the inherent flexibility of the large cycloalkane ring. Because all six diastereomers arising from the C7 and C6/C8 hydroxylated regioisomers were observed, there is clearly a lack of stereoselectivity from this enzymatic transformation. The cocrystal structure suggests that compromised stereoselectivity is likely caused by flipping (or rotating) of the carbolide substrate in the PikC active site, resulting in oxidation on both faces of the ring (see Fig. 4 A and B).
Further inspection of the cocrystal structures revealed that, whereas three orientations are distinguishable for structure 6, only two orientations (one in each protein monomer in the asymmetric unit) are observed for structure 8 in the 2.2-Å X-ray cocrystal structure (see Figs. 4 and 5), suggesting improved complementarity or more limited conformational freedom of the larger ring in the active site. This could explain the increased binding affinity (Kd = 218 μM) and hence reactivity (65% yield) of structure 8 compared to structure 6 (Kd = 309 μM, 47% yield). In one monomer (cyan in Fig. 5B), the desosamine moiety is in salt-bridge contact with E94, while in the other monomer (pink) it is within electrostatic distance from both E94 and E85. Amino acid side chains in the active site are stabilized in a single conformation, with the exception of F178, which is represented by different conformers in chain A (green) and chain B (ice blue) (see Fig. 5B). Similar to structure 6, the cocrystal structure suggests that flipping-over (or rotation) of structure 8 in the active site could enable hydroxylation (likely at C6/C9 or C7/C8) on both faces of the ring. However, a more limited resolution of the product profile in the LC-MS analysis (see Fig. S6 in SI Appendix) (unlike oxidations of compound structure 6, where oxidation products were better distinguished by LC-MS) (see Fig. S4 in SI Appendix) prevents verification of this prediction, or further evaluation of the reaction stereoselectivity.
Despite previously demonstrated dynamics of PikC (40), no induced-fit conformational changes were observed in response to binding of the various carbolides, which could potentially prevent the substrate from wobbling in the active site. This observation contradicts the concept of “conformational plasticity” applied for mammalian P450 enzymes (42), and limits the role of PikC conformational dynamics to substrate access to and product release from the active site.
Antibacterial Activities of Synthetic Desosaminyl Derivatives.
In macrolide antibiotics, desosamine (or a related deoxysugar moiety) has been found to play a crucial role in the interaction of these important compounds with the 23S ribosomal RNA (the major drug target for macrolides) through a number of specific contacts with ribonucleotides in the peptidyl-transferase center (43). Accordingly, we surmised that our series of synthetic desosaminyl derivatives might possess antibacterial activity. As shown in Table S3 in SI Appendix, the cyclic carbolides including structures 6, 8, 10, and 12 displayed some inhibitory activities against selected microbial targets, while their corresponding aglycones were inactive, confirming the significance of desosamine for bioactivity. Remarkably, the aromatic derivative (structure 19) showed similar or even higher bioactivity compared to the natural macrolide antibiotics (structures 2 and 5). We also compared the bioactivity of structure 8 with its synthetic hydroxylated products. Unexpectedly, upon installation of a hydroxyl group at either the C7/C8 or C6/C9 positions (the four authentic standards, each of which contains a pair of diastereomers), the products lost activity. Although hydroxylation of structure 8 had a detrimental impact on biological activity against a limited number of microbial targets, installation of this functional group might enable a useful chemical handle for further functionalization (44) and subsequent generation of more potent therapeutics. Further analysis of these carbolides, and direct investigation of their presumed binding to ribosomal targets, will provide important insights into the impact of PikC-mediated regioselective hydroxylation for development of new small molecules for treatment of microbial pathogens and other human diseases.
Conclusions
The work described in this article is based on the innate substrate flexibility of the biosynthetic P450 PikC enzyme that could be harnessed using a substrate engineering strategy in vitro because of its desosamine-anchoring functionality. In contrast to previous chemical approaches that have exploited proximal directing influences or inherent differences in C–H bond strengths, our approach utilizes the interactions of an engineered bacterial P450 enzyme with aminosugar (desosamine)-linked substrates that otherwise lack all of the functionality of the native biosynthetic intermediates. This approach provides the unique opportunity to obtain high-resolution crystallographic insights into the nature of the substrate-catalyst interactions, which has generally not been available with biomimetic and organometallic systems that achieve catalytic oxidation of unactivated C–H bonds. Attachment of desosamine to a number of unnatural aglycones (especially cyclic aglycones) via an acetal linkage leads to productive enzyme binding and considerable regioselectivity in the hydroxylation reactions. However, the level of regio- and stereoselectivity observed for natural macrolide substrate YC-17 (structure 1) and narbomycin (structure 4) was not achieved, which infers synergistic contributions of both desosamine and macrolactone portions of these endogenous substrates. Insights gained from structural analysis in the present study suggest that selectivity could be improved by (i) rigidifying the substrate structure, (ii) limiting conformational freedom by optimizing the complementarity between substrate size and shape and the volume of the active site, and (iii) increasing structural complexity of compounds to improve the specificity of their binding.
Currently, further investigations, including the applicability of this approach in selective oxidation of complex compounds of medicinal significance, the development of alternative and more chemically accessible glycosides (as well as alternative molecular anchors), and the development of diverse linkage groups, are in progress. Additionally, the observed selectivity could be potentially exploited for building libraries of compounds with hydroxyl group functional handles for further synthetic transformations. Moreover, the ability to obtain enzyme/substrate cocrystal structures offers unique mechanistic insights, as well as a means to engineer P450 enzymes that display greater regio- and stereoselectivity. This type of reagent engineering is particularly amenable to biological catalysts, and provides effective approaches to tailor the catalytic outcome toward a desired synthetic goal. Finally, these findings suggest that in addition to PikC, diverse natural product P450s can be further harnessed for a substrate engineering approach to selectively oxidize C–H bonds, and to facilitate further chemical diversification of both synthetic and natural product molecules of biological interest.
Materials and Methods
Preparation of PikCD50N-RhFRED.
Using previously prepared pET28b-pikC-RhFRED as a template (38), site-directed mutagenesis was performed by following the QuikChange (Stratagene) protocol. The primers for mutagenesis were: forward, 5′-CACCCCCGAGGGGAATGAGGTGTGGCTGG-3′; reverse, 5′-CCAGCCACACCTCATTCCCCTCGGGGGTG-3′. Protein expression and purification of PikCD50N-RhFRED followed the procedure developed previously (38).
PikCD50N-RhFRED Assay.
The standard assay contains 5 μM PikCD50N-RhFRED, 0.5 mM substrate, 2.5 mM NADPH, 0.25 Unit of glucose-6-phosphate dehydrogenase, and 5 mM glucose-6-phosphate for NADPH regeneration in 100 μL of reaction buffer (50 mM NaH2PO4, pH 7.3, 1 mM EDTA, 0.2 mM dithioerythritol, and 10% glycerol). The reaction was carried out at 30 °C for 3 h and terminated by extraction using 3 × 200 μL of CHCl3. The resulting organic extract was dried by N2 and redissolved in 120 μL of methanol. The subsequent LC-MS analysis was performed on a ThermoFinnigan LTQ linear ion-trap instrument (Department of Pharmacology, University of Michigan) equipped with electrospray source and Surveyor HPLC system by using an XBridge C18 3.5 μm 150 mm reverse-phase HPLC column under the following conditions: mobile phase (A = deionized water + 0.1% formic acid, B = acetonitrile + 0.1% formic acid), 20% B for 3 min, 20 to 100% B over 25 min, 100% B for 5 min, 100 to 20% B over 1 min, 20% B for 15 min; flow rate, 0.21 mL/min. The substrate binding assays were performed as previously described (38).
Crystallization, Data Collection, and Structure Refinement.
Crystallization conditions for PikCD50N complexes with structures 6 and 8 were identified by using commercial high throughput screening kits available in deep-well format (Hampton Research), a nanoliter drop-setting Mosquito robot (TTP Lab Tech) operating with 96-well plates, and a hanging-drop crystallization protocol. Optimization of conditions was carried out manually in 24-well plates. The protein from the 1 mM stock was diluted to 0.2 mM by mixing with structure 6 or structure 8 dissolved at 2 mM in 10 mM Tris·HCl, pH 7.5. Crystals of PikCD50N-structure 6 complex were obtained from 15% PEG 4000, 0.1 M Tris·HCl, pH 7.5; 200 mM MgCl2. Crystals of the PikCD50N-structure 8 complex were obtained from 12% PEG 8000, 0.1 M sodium cacodilate, pH 6.5, and 200 mM Li2SO4. Before data collection, the crystals were cryo-protected by plunging them into a drop of reservoir solution supplemented with 20% glycerol. Diffraction data were collected at 100 to 110 K at beamline 8.3.1 (Advanced Light Source, Lawrence Berkeley National Laboratory). Data indexing, integration, and scaling were conducted using HKL2000 software suite. Crystal structures were determined by molecular replacement using the atomic coordinates of the PDB ID code 2C6H as a search model (see Table S2 in SI Appendix).
For full experimental details of biochemistry/crystallography and synthetic chemistry, see the SI Appendix.
Supplementary Material
Acknowledgments.
We thank Mr. Yousong Ding, Dr. Liangcai Gu, and Dr. Kathleen Noon for assistance with LC-MS analysis, Dr. Patricia Cruz Lopez for assistance with NMR structural analysis, and Mr. Tom McQuade and Ms. Martha J. Larson in the Center for Chemical Genomics, University of Michigan for assistance with automatic antibacterial assays. We also thank the staff members of beamline 8.3.1, James Holton, George Meigs, and Jane Tanamachi, the Advanced Light Source at Lawrence Berkeley National Laboratory for assistance. This work was funded by National Institutes of Health Grants RO1 GM078553 (to D.H.S. and L.M.P.) and RO1 GM57014 (to J.M.). The Advanced Light Source is supported by the Director, Office of Science, Office of Basic Energy Sciences, of the U.S. Department of Energy under Contract No. DE-AC02–05CH11231.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The atomic coordinates and structure factor have been deposited in the Protein Data Bank, www.pdb.org (PDB ID codes 2WI9 and 2WHW)
This article contains supporting information online at www.pnas.org/cgi/content/full/0907203106/DCSupplemental.
References
- 1.Ortiz de Montellano PR. Cytochrome P450: Structure, Mechanism, and Biochemistry. 2nd Ed. New York: Plenum Press; 1995. [Google Scholar]
- 2.Guengerich FP. Common and uncommon cytochrome P450 reactions related to metabolism and chemical toxicity. Chem Res Toxicol. 2001;14:611–650. doi: 10.1021/tx0002583. [DOI] [PubMed] [Google Scholar]
- 3.Coon MJ. Cytochrome P450: Nature's most versatile biological catalyst. Ann Rev Pharmacol Toxicol. 2005;45:1–25. doi: 10.1146/annurev.pharmtox.45.120403.100030. [DOI] [PubMed] [Google Scholar]
- 4.Dick AR, Sanford MS. Transition metal catalyzed oxidative functionalization of C–H bonds. Tetrahedron. 2006;62:2439–2463. [Google Scholar]
- 5.Crabtree RH. Alkane C–H activation and functionalization with homogeneous transition metal catalysts: A century of progress—A new millennium in prospect. J Chem Soc, Dalton Trans. 2001:2437–2450. [Google Scholar]
- 6.Bergman RG. Organometallic chemistry: C–H activation. Nature. 2007;446:391–393. doi: 10.1038/446391a. [DOI] [PubMed] [Google Scholar]
- 7.Labinger JA, Bercaw JE. Understanding and exploiting C–H bond activation. Nature. 2002;417:507–514. doi: 10.1038/417507a. [DOI] [PubMed] [Google Scholar]
- 8.Godula K, Sames D. C–H bond functionalization in complex organic synthesis. Science. 2006;312:67–72. doi: 10.1126/science.1114731. [DOI] [PubMed] [Google Scholar]
- 9.Que L, Jr, Tolman W. Biologically inspired oxidation catalysis. Nature. 2008;445:333–340. doi: 10.1038/nature07371. [DOI] [PubMed] [Google Scholar]
- 10.Chen MS, White CM. A predictably selective aliphatic C–H oxidation reaction for complex molecule synthesis. Science. 2007;318:783–787. doi: 10.1126/science.1148597. [DOI] [PubMed] [Google Scholar]
- 11.Brodsky BH, Du Bois J. Oxaziridine-mediated catalytic hydroxylation of unactivated 3° C–H bonds using hydrogen peroxide. J Am Chem Soc. 2005;127:15391–15393. doi: 10.1021/ja055549i. [DOI] [PubMed] [Google Scholar]
- 12.Wender PA, Hilinski MK, Mayweg AV. Late-stage intermolecular CH activation for lead diversification: A highly chemoselective oxyfunctionalization of the C-9 position of potent bryostatin analogues. Org Lett. 2005;7:79–82. doi: 10.1021/ol047859w. [DOI] [PubMed] [Google Scholar]
- 13.Lee S, Fuchs PL. Chemospecific chromium[VI] catalyzed oxidation of C–H bonds at −40° C1. J Am Chem Soc. 2002;124:13978–13979. doi: 10.1021/ja026734o. [DOI] [PubMed] [Google Scholar]
- 14.Chen H, Schlecht S, Semple TC, Hartwig JF. Thermal, catalytic, regiospecific functionalization of alkanes. Science. 2000;287:1995–1997. doi: 10.1126/science.287.5460.1995. [DOI] [PubMed] [Google Scholar]
- 15.Cook BR, Reinert TJ, Suslick KS. Shape-selective alkane hydroxylation by metalloporphyrin catalysts. J Am Chem Soc. 1986;108:7281–7286. [Google Scholar]
- 16.Desai LV, Hull KL, Sanford MS. Palladium-catalyzed oxygenation of unactivated sp3 C-H bonds. J Am Chem Soc. 2004;126:9542–9543. doi: 10.1021/ja046831c. [DOI] [PubMed] [Google Scholar]
- 17.Dick AR, Hull KL, Sanford MS. A highly selective catalytic method for the oxidative functionalization of C–H bonds. J Am Chem Soc. 2004;126:2300–2301. doi: 10.1021/ja031543m. [DOI] [PubMed] [Google Scholar]
- 18.Dangel BD, Johnson JA, Sames D. Selective functionalization of amino acids in water: A synthetic method via catalytic C–H bond activation. J Am Chem Soc. 2001;123:8149–8150. doi: 10.1021/ja016280f. [DOI] [PubMed] [Google Scholar]
- 19.Das S, Incarvito CD, Crabtree RH, Brudvig GW. Molecular recognition in the selective oxygenation of saturated C–H bonds by a dimanganese catalyst. Science. 2006;312:1941–1943. doi: 10.1126/science.1127899. [DOI] [PubMed] [Google Scholar]
- 20.Das S, Brudvig GW, Crabtree RH. High turnover remote catalytic oxygenation of alkyl groups: How steric exclusion of unbound substrate contributes to high molecular recognition selectivity. J Am Chem Soc. 2008;130:1628–1637. doi: 10.1021/ja076039m. [DOI] [PubMed] [Google Scholar]
- 21.Breslow R, et al. Remote oxidation of steroids by photolysis of attached benzophenone groups. J Am Chem Soc. 1973;95:3251–3262. doi: 10.1021/ja00791a031. [DOI] [PubMed] [Google Scholar]
- 22.Yang J, Gabriele B, Belvedere S, Huang Y, Breslow R. Catalytic oxidations of steroid substrates by artificial cytochrome P-450 enzymes. J Org Chem. 2002;67:5057–5067. doi: 10.1021/jo020174u. [DOI] [PubMed] [Google Scholar]
- 23.Grieco PA, Stuk TL. Remote oxidation of unactivated carbon-hydrogen bonds in steroids via oxometalloporphinates. J Am Chem Soc. 1990;112:7799–7801. [Google Scholar]
- 24.Groves JT, Neumann R. Regioselective oxidation catalysis in synthetic phospholipid vesicles. Membrane-spanning steroidal metalloporphyrins. J Am Chem Soc. 1989;111:2900–2909. [Google Scholar]
- 25.Urlacher VB, Eiben S. Cytochrome P450 monooxygenases: Perspectives for synthetic application. Trends Biotechnol. 2006;24:324–330. doi: 10.1016/j.tibtech.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 26.Landwehr M, Carbone M, Otey CR, Li Y, Arnold FH. Diversification of catalytic function in a synthetic family of chimeric cytochrome P450s. Chem Biol. 2007;14:269–278. doi: 10.1016/j.chembiol.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sieber V, Martinez CA, Arnold FH. Libraries of hybrid proteins from distantly related sequences. Nat Biotechnol. 2001;19:456–460. doi: 10.1038/88129. [DOI] [PubMed] [Google Scholar]
- 28.Arnold FH. Fancy footwork in the sequence-space shuffle. Nat Biotechnol. 2006;24:328–330. doi: 10.1038/nbt0306-328. [DOI] [PubMed] [Google Scholar]
- 29.Bloom JD, et al. Evolving strategies for enzyme engineering. Curr Opin Struc Biol. 2005;15:447–452. doi: 10.1016/j.sbi.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 30.Kumar S, Chen CS, Waxman DJ, Halpert JR. Directed evolution of mammalian cytochrome P450 2B1. J Biol Chem. 2005;280:19569–19575. doi: 10.1074/jbc.M500158200. [DOI] [PubMed] [Google Scholar]
- 31.Bloom JD, Arnold FH. In the light of directed evolution: pathway of adaptive protein evolution. Proc Natl Acad Sci USA. 2009;106:9995–10000. doi: 10.1073/pnas.0901522106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xue Y, Wilson D, Zhao L, Liu H-w, Sherman DH. Hydroxylation of macrolactones YC-17 and narbomycin is mediated by the pikC-encoded cytochrome P450 in Streptomyces venezuelae. Chem Biol. 1998;5:661–667. doi: 10.1016/s1074-5521(98)90293-9. [DOI] [PubMed] [Google Scholar]
- 33.Anzai Y, et al. Functional analysis of MycG and MycCI, cytochrome P450 enzymes involved in biosyntheis of mycinamicin macrolide antibiotics. Chem Biol. 2008;15:950–959. doi: 10.1016/j.chembiol.2008.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Griengl H, de Raadt A. The use of substrate engineering in biohydroxylation. Curr Opin Biotechnol. 2002;13:537–542. doi: 10.1016/s0958-1669(02)00358-0. [DOI] [PubMed] [Google Scholar]
- 35.Lairson LL, Watts AG, Wakarchuk WW, Withers SG. Using substrate engineering to harness enzymatic promiscuity and expand biological catalysis. Nat Chem Biol. 2006;2:724–728. doi: 10.1038/nchembio828. [DOI] [PubMed] [Google Scholar]
- 36.Braunegg G, et al. The concept of docking/protecting groups in biohydroxylation. Angew Chem Int Ed. 1999;38:2763–2766. [PubMed] [Google Scholar]
- 37.de Raadt A, Griengl H, Weber HJ. The concept of docking and protecting groups in biohydroxylation. Chem Eur J. 2001;7:27–31. doi: 10.1002/1521-3765(20010105)7:1<27::aid-chem27>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 38.Li S, Podust LM, Sherman DH. Engineering and analysis of a self-sufficient biosynthetic cytochrome P450 PikC fused to the RhFRED reductase domain. J Am Chem Soc. 2007;129:12940–12941. doi: 10.1021/ja075842d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xue Y, Zhao L, Liu H-w, Sherman DH. A gene cluster for macrolide antibiotic biosynthesis in Streptomyces venezuelae: Architecture of metabolic diversity. Proc Natl Acad Sci USA. 1998;95:12111–12116. doi: 10.1073/pnas.95.21.12111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sherman DH, et al. The structural basis for substrate anchoring, active site selectivity, and product formation by P450 PikC from Streptomyces venezuelae. J Biol Chem. 2006;281:26289–26297. doi: 10.1074/jbc.M605478200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li S, Ouellet H, Sherman DH, Podust LM. Analysis of transient and catalytic desosamine-binding pockets in cytochrome P-450 PikC from Streptomyces venezuelae. J Biol Chem. 2009;284:5723–5730. doi: 10.1074/jbc.M807592200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Muralidhara BK, Halpert JR. Thermodynamics of ligand binding to P450 2B4 and P450eryF studied by isothermal titration calorimetry. Drug Metab Rev. 2007;39:539–556. doi: 10.1080/03602530701498182. [DOI] [PubMed] [Google Scholar]
- 43.Schlünzen F, et al. Structural basis for the interaction of antibiotics with the peptidyl transferase centre in eubacteria. Nature. 2001;413:814–821. doi: 10.1038/35101544. [DOI] [PubMed] [Google Scholar]
- 44.Rentmeister A, Arnold FH, Fasan R. Chemo-enzymatic fluorination of unactivated organic compounds. Nat Chem Biol. 2009;5:26–28. doi: 10.1038/nchembio.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.