Myotonic dystrophy type 1 (DM1) is one of a number of microsatellite expansion diseases in which the causative mutation is an aberrant expansion of a 3-nt repeat (1). The DM1 mutation was identified in 1992, and by 2001 it was established that the primary cause of pathogenesis is toxicity of the repeat-containing RNA transcribed from the expanded allele. One mechanism by which the RNA induces pathogenesis is through direct binding and sequestration of the RNA binding protein, muscleblind-like 1 (MBNL1). MBNL1 regulates a subset of alternative splicing events, and its depletion from the nucleoplasm results in misregulation of these events, causing features of the disease (2). In this issue of PNAS, Warf et al. (3) screened a relatively small number of nucleic acid binding compounds to identify those that could disrupt MBNL1–RNA interactions. The results add to the growing list of agents targeting the repeat-containing RNA of DM1 and are the first small molecules to show promising reversal of splicing defects in a DM1 mouse model.
DM1 is caused by a CTG expansion in the 3′ UTR of the DMPK gene. Unaffected individuals have <38 CTG repeats, whereas expansions associated with DM1 range from 80 to >2,500 repeats. Repeat length correlates directly with disease severity and inversely with the age of onset. DM1 is dominantly inherited and multisystemic; the primary causes of mortality and morbidity are progressive muscular dystrophy, cardiac-related sudden death, and CNS dysfunction (4). Therefore, systemic delivery of therapeutic compounds is a particularly attractive approach to provide benefit to the multiple organ systems affected.
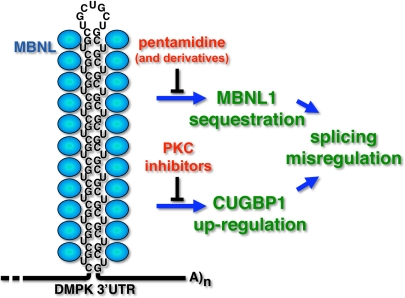
The RNA expressed from the expanded DMPK allele (CUGexp RNA) has at least two pathogenic consequences (Fig. 1). First, it forms a hairpin containing C-G base pairs and bulged U residues that binds MBNL1 with high affinity. In fact, there is a reciprocal relationship in which CUGexp RNA binds and sequesters MBNL1 within nuclear foci whereas MBNL1 enhances formation of the foci and traps CUGexp RNA in the nucleus (5, 6). A second component of pathogenicity is aberrant activation of protein kinase C (PKC) by CUGexp RNA that leads to hyperphosphorylation, stabilization, and up-regulation of a second RNA binding protein, CUG-binding protein 1 (CUGBP1) (7). MBNL1 and CUGBP1 are coregulators of a developmental program of alternative splicing transitions that is disrupted in DM1, resulting in misexpression of embryonic splicing patterns in adult tissues. Some features of the disease are caused by the failure of embryonic splicing patterns to fulfill the functional requirements of adult tissues (8).
Fig. 1.
Small molecules to combat the multiple toxic effects of CUGexp RNA. CUGexp RNA directly binds and sequesters MBNL1 and leads to up-regulation of CUGBP1 caused by PKC activation. Warf et al. (3) identified the nucleic acid binding compound, pentamidine, as an inhibitor of MBNL1-CUGexp RNA interactions. PKC inhibitors have been shown to blunt the effects of CUGexp RNA in a DM1 mouse model (19).
The CUGexp RNA provides a novel and attractive therapeutic target. Results from an inducible DM1 mouse model demonstrated that the disease phenotype improved when the pathogenic RNA was shut off, suggesting that at least some disease features are reversible (9). RNA also presents an expansive sequence and structural space that is a relatively unexplored target for small-molecule ligands. Several recent reports have applied strategic chemical design and high-throughput screening to identify compounds that block binding of MBNL1 to CUGexp RNA or the CCUGexp RNA of the related disease, DM, type 2 (DM2) (10–13). The significance of the results of Warf et al. (3) is the logical progression from in vitro assays to testing efficacy in a DM1 mouse model. They used a stabilized CUG hairpin RNA and recombinant MBNL1 protein in a gel-shift assay to screen a small library of 26 compounds known to bind to structured RNA and found two compounds, pentamidine and neomycin B, which blocked MBNL1–RNA interactions. They then demonstrated that pentamidine (but not neomycin B) blocked the trans-dominant effects of CUGexp RNA on splicing in cell culture by using a transient transfection assay in which CUGexp RNA was coexpressed with minigene splicing reporters. Importantly, in situ hybridization to detect CUGexp RNA combined with immunofluorescent staining for endogenous MBNL1 demonstrated that pentamidine caused dispersal of the RNA foci and redistribution of MBNL1 to the nucleoplasm. This finding resembles recent results demonstrating that antisense CAG morpholino oligonucleotides that block binding of MBNL1 to CUGexp RNA also caused dissipation of RNA foci, MBNL1 redistribution, and down-regulation of the CUGexp RNA by 50% or more (14, 15). Because morpholino oligos do not cause RNA degradation directly via the RNase H pathway, it is likely that the RNA is degraded after release from the foci. It will be of particular interest to determine whether pentamidine has this added benefit of causing loss of CUGexp RNA.
The next test for efficacy was to administer pentamidine to the HSALR DM1 mouse model that expresses 250 CUG repeats within the 3′ UTR of a human skeletal α-actin transgene and reproduces DM1 splicing abnormalities (16). Pentamidine caused reversion of misregulated splicing from the embryonic toward the normal adult patterns. Although the effects of pentamidine were relatively mild in the mice, the results provide not only a proof of principle but also a chemical scaffold for strategic modifications to increase efficacy, reduce toxicity, and optimize delivery to multiple tissues.
All drugs have “off-target” effects, and in the case of pentamidine, the good news is that several alternative splicing events unaffected by CUGexp RNA were not affected by pentamidine. However, the results also suggested that there will be a subset of MBNL1-regulated splicing events affected by MBNL1-targeted compounds. In addition, agents that target expanded RNA repeats are likely to interact with DNA. Microsatellite expansions are dynamic in that repeat number increases over the lifetime of affected individuals and this somatic instability is thought to contribute to the progressive nature of disease (17). It is therefore important to consider the possibility that compounds targeted to the RNA could also have inadvertent effects (harmful or beneficial) on the propensity of the DNA repeats to expand. Another consideration is that CUGexp RNA has multiple pathological effects. Compounds that promote dissolution of the MBNL1-RNA foci and degradation of CUGexp RNA should have broad beneficial effects by eliminating the primary offending agent. However, approaches that are limited to restoring MBNL1 function are unlikely to address all pathological consequences of CUGexp RNA. For example, PKC activation by CUGexp RNA is likely to be independent of MBNL1 depletion. Recently, administration of PKC inhibitors to a DM1 mouse model ameliorated the effects of CUGexp RNA relevant to CUGBP1 up-regulation (18), suggesting that the divergent effects of the toxic RNA can be addressed by using combinatorial therapeutic approaches (Fig. 1).
Overall, the spate of recent publications supporting a wide variety of therapeutic approaches for DM1, <17 years after identification of the unusual mutation that causes disease, bodes well for the prospects of effective therapies for this and related diseases.
Acknowledgments.
The work in my lab is supported by the National Institutes of Health, Muscular Dystrophy Association, and the Myotonic Dystrophy Foundation.
Footnotes
The author declares no conflict of interest.
See companion article on page 18551.
References
- 1.Orr HT, Zoghbi HY. Trinucleotide repeat disorders. Annu Rev Neurosci. 2007;30:575–621. doi: 10.1146/annurev.neuro.29.051605.113042. [DOI] [PubMed] [Google Scholar]
- 2.Wheeler TM, Thornton CA. Myotonic dystrophy: RNA-mediated muscle disease. Curr Opin Neurol. 2007;20:572–576. doi: 10.1097/WCO.0b013e3282ef6064. [DOI] [PubMed] [Google Scholar]
- 3.Warf MB, Nakamori M, Matthys CM, Thornton CA, Berglund JA. Pentamidine reverses the splicing defects associated with myotonic dystrophy. Proc Natl Acad Sci USA. 2009;106:18551–18556. doi: 10.1073/pnas.0903234106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harper PS. Myotonic Dystrophy. 3rd Ed. London: Saunders; 2001. [Google Scholar]
- 5.Dansithong W, Paul S, Comai L, Reddy S. MBNL1 is the primary determinant of focus formation and aberrant insulin receptor splicing in DM1. J Biol Chem. 2005;280:5773–5780. doi: 10.1074/jbc.M410781200. [DOI] [PubMed] [Google Scholar]
- 6.Miller JW, et al. Recruitment of human muscleblind proteins to (CUG)n expansions associated with myotonic dystrophy. EMBO J. 2000;19:4439–4448. doi: 10.1093/emboj/19.17.4439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kuyumcu-Martinez MN, Wang GS, Cooper TA. Increased steady-state levels of CUGBP1 in myotonic dystrophy 1 are due to PKC-mediated hyperphosphorylation. Mol Cell. 2007;28:68–78. doi: 10.1016/j.molcel.2007.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cooper TA, Wan L, Dreyfuss G. RNA and disease. Cell. 2009;136:777–793. doi: 10.1016/j.cell.2009.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mahadevan MS, et al. Reversible model of RNA toxicity and cardiac conduction defects in myotonic dystrophy. Nat Genet. 2006;38:1066–1070. doi: 10.1038/ng1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arambula JF, Ramisetty SR, Baranger AM, Zimmerman SC. A simple ligand that selectively targets CUG trinucleotide repeats and inhibits MBNL protein binding. Proc Natl Acad Sci USA. 2009;106:16068–16073. doi: 10.1073/pnas.0901824106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gareiss PC, et al. Dynamic combinatorial selection of molecules capable of inhibiting the (CUG) repeat RNA-MBNL1 interaction in vitro: Discovery of lead compounds targeting myotonic dystrophy (DM1) J Am Chem Soc. 2008;130:16254–16261. doi: 10.1021/ja804398y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee MM, Pushechnikov A, Disney MD. Rational and modular design of potent ligands targeting the RNA that causes myotonic dystrophy 2. ACS Chem Biol. 2009;4:345–355. doi: 10.1021/cb900025w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pushechnikov A, et al. Rational design of ligands targeting triplet repeating transcripts that cause RNA dominant disease: application to myotonic muscular dystrophy type 1 and spinocerebellar ataxia type 3. J Am Chem Soc. 2009;131:9767–9779. doi: 10.1021/ja9020149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wheeler TM, et al. Reversal of RNA dominance by displacement of protein sequestered on triplet repeat RNA. Science. 2009;325:336–339. doi: 10.1126/science.1173110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mulders SA, et al. Triplet-repeat oligonucleotide-mediated reversal of RNA toxicity in myotonic dystrophy. Proc Natl Acad Sci USA. 2009;106:13915–13920. doi: 10.1073/pnas.0905780106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mankodi A, et al. Myotonic dystrophy in transgenic mice expressing an expanded CUG repeat. Science. 2000;289:1769–1773. doi: 10.1126/science.289.5485.1769. [DOI] [PubMed] [Google Scholar]
- 17.Gomes-Pereira M, Monckton DG. Chemical modifiers of unstable expanded simple sequence repeats: What goes up, could come down. Mutat Res. 2006;598:15–34. doi: 10.1016/j.mrfmmm.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 18.Wang GS, et al. PKC inhibition ameliorates the cardiac phenotype of a mouse model for myotonic dystrophy type 1. J Clin Invest. 2009 doi: 10.1172/JCI37976. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]