Abstract
Clinical and experimental observations indicate a role for VEGF secreted by the retinal pigment epithelium (RPE) in the maintenance of the choriocapillaris (CC). VEGF in mice is produced as three isoforms, VEGF120, VEGF164, and VEGF188, that differ in their ability to bind heparan sulfate proteoglycan. RPE normally produces the more soluble isoforms, VEGF120 and VEGF164, but virtually no VEGF188, reflecting the fact that molecules secreted by the RPE must diffuse across Bruch's membrane (BrM) to reach the choriocapillaris. To determine the role of RPE-derived soluble VEGF on the choriocapillaris survival, we used mice that produce only VEGF188. VEGF188/188 mice exhibited normal choriocapillaris development. However, beginning at 7 months of age, we observed a progressive degeneration characterized by choriocapillaris atrophy, RPE and BrM abnormalities, culminating in areas of RPE loss and dramatic choroidal remodeling. Increased photoreceptor apoptosis in aged VEGF188/188 mice led to a decline in visual acuity as detected by electroretinogram (ERG). These changes are reminiscent of geographic atrophy (GA) and point to a role for RPE-derived VEGF in the maintenance of the choriocapillaris.
Keywords: age-related macular degeneration, Bruch's membrane, geographic atrophy, retinal pigmented epithelium
The vascular endothelial growth factor (VEGF) family is comprised of a number of closely related ligands, including VEGF-A, B, C, D, E, and placental growth factor (PlGF) (1). VEGF-A (hereafter referred to as VEGF) is a critical angiogenic factor associated with several ocular pathologies, including wet age-related macular degeneration (AMD) and proliferative diabetic retinopathy. In AMD, increased VEGF expression leads to proliferation of pathological and highly permeable vessels into the subretinal space, resulting in severe vision loss. The clear involvement of VEGF in proliferative AMD has led to the development of multiple anti-VEGF drugs, with two—Macugen (OSI/Eyetech Pharmaceuticals) and Lucentis (Genentech)—approved by the Food and Drug Administration for the treatment of choroidal neovascularization.
VEGF binds with high affinity to the tyrosine kinase receptors, VEGFR1 and VEGFR2; VEGFR2 appears to be the major signal transducer of VEGF in noncirculating cells, whereas VEGFR1 is considered as a natural decoy for VEGF by regulating its ability to bind VEGFR2. In contrast to VEGFR1, VEGF binding to VEGFR2 leads to a robust autophosphorylation and the induction of various signaling cascades including, MAPK, PLCγ, Akt, and Src, critical for endothelial cell proliferation, migration, and survival (2). VEGFR2 activity is not only regulated by the availability of VEGF but also by the presence of the coreceptors neuropilins (NP-1) Binding of VEGF to NP-1 mediates formation of complexes between it and VEGFR2, potentiating the effects of VEGF (3).
Although the function of VEGF during development and pathology is well established, its role in the adult is only beginning to be appreciated. Analysis of VEGF expression in adult has yielded some insight into its postdevelopmental role. The use of VEGF-LacZ mice revealed that VEGF is expressed in virtually every tissue (4). The highest level of VEGF expression was localized to the epithelia adjacent to the fenestrated vessels including the choriocapillaris (CC), kidney, and choroid plexus.
Studies in rodents have begun to elucidate the function of VEGF in quiescent tissues. Inhibition of VEGF or its receptors has been shown to lead to vessel regression in a number of tissues. In the kidney, VEGF neutralization resulted in glomerular endotheliosis and proteinuria (5–7). Vessel regression was also noted in the pancreas (8, 9), trachea, thyroid, and small intestine (8). Recently, we have reported that systemic inhibition of VEGF using adenoviral expression of sFlt-1 leads to nonperfusion of vessels in the choroid plexus (10). The CC of the eye has been shown to be similarly vulnerable to VEGF inhibition. Decreased fenestrations and vessel occlusion were observed after intravitreal injection of anti-VEGF (bevacizumab) in primate eyes (11).
In addition, there is growing acknowledgment of a role for VEGF on nonvascular cells (12), including a neurotrophic role in the brain (13). Systemic VEGF neutralization in mice led to a breakdown of the ventricular barrier function, and a direct role for VEGF as a survival factor for ependymal cells was indicated by their expression of VEGFR2 (10). We recently demonstrated a paracrine relationship between Müller cell-derived VEGF and VEGFR2 expressing photoreceptors in the murine retina (14). The physiologic importance of this relationship was evidenced by a significant loss of photoreceptors following systemic VEGF inhibition (11).
In mice, VEGF is produced as three isoforms (VEGF120, VEGF164, and VEGF188) by alternative splicing of a single gene (15). The isoforms differ in their inclusion of exons that encode for charged domains that mediate binding to heparan sulfate or to NP-1 which, in turn, determines the extracellular localization of VEGF. Upon secretion, VEGF120, which lacks heparan sulfate binding, is freely diffusible, whereas VEGF188, which contains two heparan sulfate binding sites, is sequestered on the cell surface or in the extracellular matrix, and VEGF164, with one heparan sulfate binding site, has intermediate distribution. Evidence that individual VEGF isoforms serve specific functions was provided by mice engineered to express single VEGF isoforms (16). Consistent with the high levels of VEGF188 in the lung (17), mice expressing only VEGF120 displayed defective pulmonary development (16, 18).
AMD is a pathology of the retinal pigment epithelium (RPE)-Bruch's membrane (BrM)-CC complex. In the dry AMD, accumulation of extracellular material or drusen under the RPE and within the BrM leads to RPE dysfunction, and in some cases, the loss of RPE, regression of the underlying CC, and death of the overlying photoreceptors, a pathology known as geographic atrophy (GA). In about 10% of cases, breaks in BrM lead to invasion of the CC into the retinal space. The association between RPE and CC loss in GA points to a role for the RPE in the maintenance of the CC. Because the cells are separated by the BrM, the RPE influence on the CC must be mediated by a diffusible factor. A role for VEGF is strongly supported by the expression pattern of VEGF and its main signaling receptor VEGFR2. VEGF is expressed by RPE throughout the ocular development and into adulthood (19). In adults, RPE appears as the only source of VEGF in the back of the eye (19). In vitro studies demonstrated that differentiated RPE secrete VEGF in a polarized fashion toward the basolateral side (20, 21) and that VEGFR2 is preferentially expressed by the CC on the side facing the RPE (19, 20). VEGFR3, which in the adult is normally restricted to lymphatic endothelium, is also expressed by the CC (20). While VEGF does not bind VEGFR3, it is activated by VEGF-C and VEGF-D, both expressed by RPE cells in culture (21, 22). However, their expression by native RPE in vivo has not been demonstrated, and the function of VEGFR3 in the CC remains unknown.
We have previously observed that RPE in the adult produce VEGF164 and VEGF120 (about 75 and 25%, respectively), but not VEGF188 (19), and we speculate that the production of soluble VEGF isoforms by the RPE is consistent with the presence of a barrier posed by the BrM, which lies between the RPE and the target endothelium of the CC. Accordingly, we hypothesized that mice producing only VEGF188, and no soluble VEGF isoforms, might exhibit a choroidal phenotype. We found that the absence of the diffusible VEGF isoforms (120 and 164) leads to an age-dependent degeneration of the RPE-CC that is similar in many respects to dry/atrophic AMD.
Results
Age-Dependent Degeneration of the Choroidal Vasculature and Outer Retina in VEGF188/188 Mice.
We evaluated embryonic ocular development and vascularization in mice expressing only VEGF188 and found no apparent anomalies (Fig. S1). Similarly, light microscopic and ultrastructural evaluation of the outer retina and choroidal tissues of young adult VEGF188/188 mice revealed no changes in the organization compared to wild-type (wt) controls (Fig. 1A and Fig. S2).
Fig. 1.
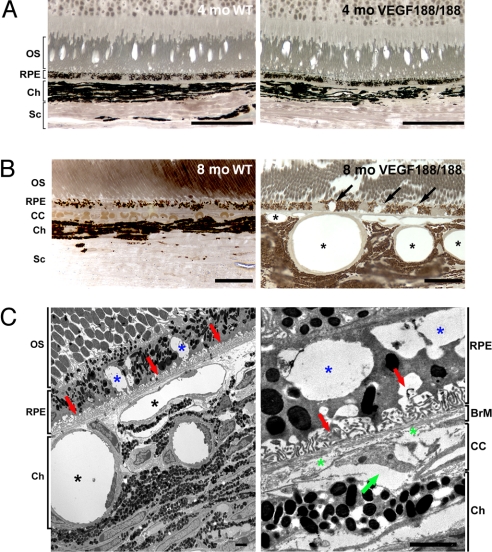
Abnormal choroidal vasculature and RPE defects in aged VEGF188/188 mice. (A and B) One-micrometer-thin epon sections of 4- and 8-month-old wt (right panel) and VEGF188/188 (left panel) mice were stained with para-phenylenediamine for light microscopy (A and B) or were visualized by TEM (C). (A) No changes in the organization of the retina layers were observed in 4-month-old VEGF188/188 mice. (B) An 8-month-old, VEGF188/188 mouse eye displays major abnormalities in the RPE and choroidal vessels. Larges vacuoles were observed within the RPE (arrows). The choroidal vessels appear enlarged, and the CC is replaced by discontinuous vessels wrapped by pericytes (asterisk). (C) Electron micrograph of the mid-periphery of an 8-month-old VEGF188/188 mouse retina. Note the significant enlargement of the choroidal vessels (black asterisk). RPE cells displayed large vacuoles containing membranous debris (blue asterisk). Regression of the RPE basal infoldings is associated with accumulation of materials (red arrow). Higher magnification (right) showing discontinuous collagen and elastin layers in the BrM (green asterisk) and a regressing CC vessel with an entrapped erythrocyte (green arrow). [Scale bar, 50 μm (A); scale bar, 20 μm (B); scale bar, 2 μm (C).] OS, outer segment; BrM, Bruch's membrane; CC, choriocapillaris; Ch, choroid; Sc, sclera.
However, distinct changes were evident by 7–8 months of age (Fig. 1). In 8-month-old VEGF188/188 mice, the CC, which normally form a continuous plexus beneath the BrM, were replaced by enlarged and discontinuous vessels (Fig. 1B). The prominent dilatation of feeder choroidal vessels observed in 7- to 9-month-old VEGF188/188 mice may reflect a response to pressure changes that result from the loss of CC. Changes in the choroidal vasculature were associated with RPE dysfunction, evidenced by the presence of large vacuoles (Fig. 1B). The RPE and choroidal lesions were predominantly in the mid-periphery of the retina. Ultrastructural analysis of the outer retina of 8-month-old VEGF188/188 mice revealed capillary atrophy with enlarged and nonfenestrated feeder vessels abutting the BrM (Fig. 1C). RPE vacuolization was associated with regression of the basal infoldings and accumulation of membranous debris (Fig. 1C). This degeneration was restricted to the outer retina; no change in the retina architecture or thickness were detected (Fig. S3)
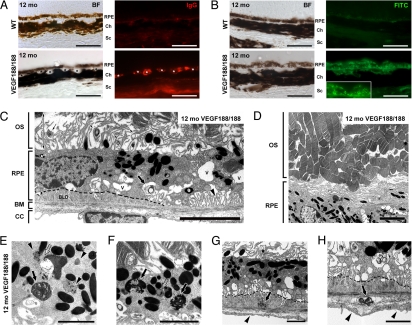
At 12 months of age, immunodetection for mouse IgG revealed intense staining of the CC, indicative of immune complex accumulation and possible local inflammation (Fig. 2A). Lipofuscin, which has been associated with development of AMD (23), was also detected as autofluorescent clusters in the degenerated RPE of VEGF188/188 mice (Fig. 2B). Transmission electron microscopy (TEM) examination revealed RPE and choroidal vessel dysfunction in 12-month-old VEGF188/188 mice (Fig. 2 C–H). Degenerating RPE contained numerous vacuoles and displayed reduced basal infoldings and accumulation of continuous basal deposits (Fig. 2 C, G, and H). Signs of defective phagocytosis including lipofuscin pigment associated with lipid droplets, undigested rod outer segments(OS), and abnormal melanosomes were frequently observed (Fig. 2 D and E).
Fig. 2.
Early AMD features in aged VEGF188/188 mice. (A) IgG staining (red) of the choroids of 12-month-old mice reveal the deposition of IgG in the walls of the choroidal vessel of the transgenic mice (asterisks) but not in aged-matched controls. (B) Increased lipofuscin autofluorescence was detected with an FITC filter. Multiple bright dots and clusters were evident in the RPE layer of the VEGF188/188 mice, especially in the clumped cells (see insert). (C–H) Representative electron micrographs showing RPE and BrM anomalies in 12-month-old VEGF188/188 mice. (C) Unhealthy RPE cells with reduced and disorganized basal infoldings (arrowhead) displaying larges vacuoles (V) and membranous debris (large arrow). Formation of continuous subRPE deposits is outlined by a dotted line. Note the loss of intimate association between the RPE and the OS. The OS were separated from the RPE by a large space (double-headed arrow) containing disorganized RPE apical digitations and lamellar bodies (small arrows). (D) Lower magnification showing highly disorganized OS. (E and F) Higher magnification showing lipofuscin granules (arrowheads), abnormal phagosomes containing melanin (arrows in panel E), and undigested photoreceptor outer-segments in VEGF188/188 RPE (arrow in panel F). Lipid droplets were also observed in the lipofuscin bodies (open arrows). (G and H) Basal laminar deposits were associated with dense and membranous debris in the subRPE space or in the outer layers of the BrM (arrows in panels G and H, respectively). Note the loss of fenestrations and thickening of the endothelium membrane in the CC underlying the subretinal lesions. OS, outer segment; BrM, Bruch's membrane; CC, choriocapillaris; BLD, basal laminar deposits. [Scale bar, 50 μm (A and B); scale bar, 5 μm (C and D; scale bar, 2 μm (E–H).]
Abnormal interactions between the RPE and the retina were evidenced by reduced association between the RPE and the photoreceptor OS (Fig. 2 C and D). RPE apical digitations appeared disorganized, and numerous lamellar bodies were observed. Impaired RPE phagocytosis was suggested by deposits of amorphous material in the subRPE space and the outer layers of the BrM, which led to a significant thickening of the BrM (Fig. S4). The endothelial cells of the CC underlying these basal laminar deposits (BLD) were also thickened and had lost their characteristic fenestrations (Fig. 2 G and H).
Drusen, a hallmark of early AMD, were observed by fundus examination in aged VEGF188/188 mice beginning at 10 months and predominant at 14 months when numerous subretinal yellowish deposits were detected in the mid-periphery of the retina (Fig. S5 A and B). Histological and ultrastructure analysis of these regions revealed degenerative and rounded RPE separated from BrM by large amounts of heterogeneous debris (Fig. S5 C and D). Beneath the lesions, CC appeared atrophic and was replaced by numerous melanocytes (Fig. S5 C and D).
Aged VEGF188/188 Mice Display Increased Photoreceptor Apoptosis and Altered Visual Function.
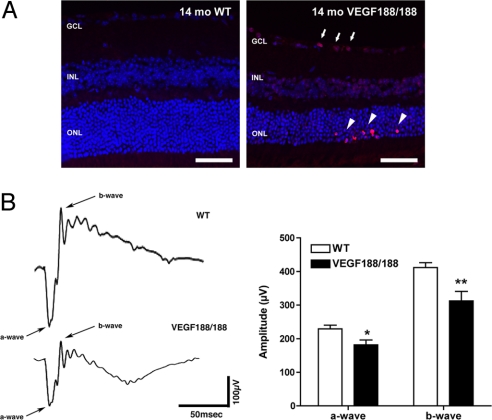
To determine if the focal disruptions in RPE-retina interactions were associated with cell death, retinal sections were stained for apoptotic cells (Fig. 3A). Clusters of TUNEL-positive cells were detected in the ONL of retinas of 14-month-old VEGF188/188 mice but not in the aged-matched wt mice. Apoptotic cells in the GCL were frequently observed in the same location as the dying photoreceptors (Fig. 3A). Clustering of apoptotic photoreceptors has been reported in advanced GA where expanding areas of RPE loss leads to photoreceptor death at the edge of the atrophic zone (24). Visual function in VEGF188/188 mice was assessed using electroretinogram (ERG) recordings, which revealed a mild but significant reduction of both a- and b-wave amplitude (Fig. 3B). Reduction of the a-wave amplitude is indicative of a loss of photoreceptor function and is consistent with our previous observations. The reduction of the b-wave is a sign of an altered photoreceptor postsynaptic cascade that would accompany the increased photoreceptor cell death. A similar decrease in the amplitude of the foveal ERG response has been reported in patients with AMD (25).
Fig. 3.
Increased photoreceptor apoptosis and abnormal ERGs in VEGF188/188 mice. (A) TUNEL staining revealed a markedly increased apoptosis in the ONL of 14-month-old VEGF188/188 mice. Apoptotic photoreceptors appeared as clusters in the outermost part of the ONL layer (arrowhead) and were often associated with increased cell death in the GCL (arrows). (B) Scotopic ERG recordings of 14-month-old mice using a flash intensity of +10 dB revealed a marked reduction of both a- and b-wave amplitudes in VEGF188/188 mice (n = 14 for wt and n = 12 for VEGF188/188). GCL, ganglion cell layer; INL, inner nuclear layer; ONL, outer nuclear layer. (Scale bars, 50 μm.)
Altered RPE Barrier Function in VEGF188/188 Mice.
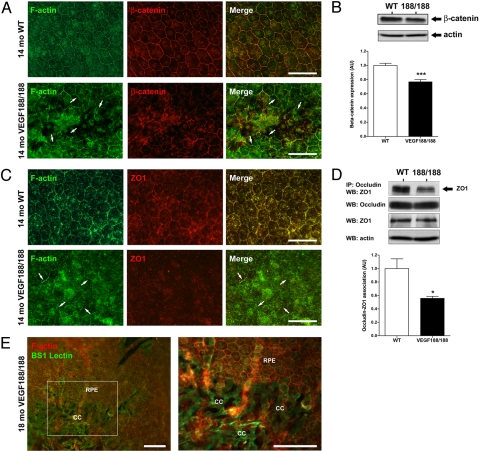
RPE barrier function is determined primarily by tight junctions, which limit diffusion from the extracellular space through the RPE monolayer and regulate transepithelial flow (26). RPE-blood barrier is also controlled by adherens junctions, which mediate intercellular adhesion and preservation of the epithelial morphology (26). RPE junctional integrity in aged VEGF188/188 mice was evaluated by co-immunostaining RPE flat-mounts for the adherens junction protein β-catenin and F-actin. β-catenin, which is normally membrane-associated and colocalizes with F-actin, showed an aberrant distribution at the site of RPE lesions in 14-month-old VEGF188/188 eyes (Fig. 4A). Western blot analysis demonstrated a modest but significant reduction in the level of β-catenin expression in RPE lysates from VEGF188/188 mice (Fig. 4B). Since β-catenin localization was altered only at the sites of RPE lesions, the moderate change at the protein level suggests a more drastic loss at the site of RPE damage.
Fig. 4.
RPE dystrophy is associated with barrier function loss in VEGF188/188 mice. (A) Flat-mount preparation of 14-month-old wt mice and VEGF188/188 showed abnormal distribution of the junctional proteins in RPE layer of transgenic mice. In wt, RPE formed a regular honeycomb pattern. In VEGF188/188, the RPE organization is disturbed and atrophic revealing the choroidal vessel underneath (CC). In the RPE lesions, the gap-junction protein β-catenin has lost its characteristic membranous association (arrows). (B) Abnormal β-catenin distribution was accompanied by a reduction of protein expression showed by Western blot on 16-month-old choroid-RPE samples (n = 6). (C) Aberrant ZO1 localization was also observed in the VEGF188/188 mice. (D) No changes in ZO1 protein level were detected. Immunoprecipitation for occludin followed by ZO1 Western blotting revealed a 45% reduction in association between ZO1 and occludin (n = 3). (E) Over time, the limited RPE lesions observed at 14 months (see panels A and C) expanded significantly leading to a major loss in the integrity of the RPE layer of 18-month-old VEGF188/188 mice. [Scale bar, 50 μm (A and C); scale bar, 100 μm (E).]
ZO1 and occludin are membrane-associated proteins whose association account for a majority of the blood retinal barrier (27). Abnormal ZO1 localization was also observed at the site of RPE lesions in aged VEGF188/188 mice (Fig. 5C). However, no changes in total ZO1 and occludin protein levels were detected (Fig. S6). Immunoprecipitation of occludin followed by Western blotting for ZO1 revealed a 45% reduction in association in VEGF188/188 RPE (Fig. 4D). Local RPE atrophy observed in 14-month-old VEGF188/188 mice (Fig. 5A and Fig. S7) expanded over time to form large, discreet areas of RPE loss that were associated with the involution of the underlying CC (Fig. 4E).
Fig. 5.
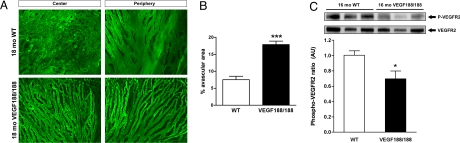
CC atrophy is associated with decreased VEGFR2 phosphorylation in aged VEGF188/188 mice. (A) Choroidal vasculature of 18-month-old wt and VEGF188/188 mice was immunostained by cardiac perfusion of FITC-labeled Bandeiraea simplicifolia (BS-1) isolectin. In wt animals, flat-mounted choroids revealed a typical dense honeycomb vascular network. However, the choroidal vessels of VEGF188/188 animals appeared poorly branched and atrophied. The reduced vascular density was observed in both the central and the peripheral choroidal vasculature. (Scale bar, 200 μm.) (B) Quantification of the avascular area in the CC 15- to 16-month-old VEGF188/188 mice (n = 5) compared to aged-matched wt (n = 5) (P < 0.0001). (C) Level of VEGFR2 phosphorylation in choroid-RPE protein lysates of 16-month-old mice was quantified by Western blotting using [pY1214]-VEGFR2 specific antibody. The level of phosphorylated VEGFR2 was normalized for total VEGFR2. VEGF188/188 RPE-choroid complex displayed a significant reduction of the phosphorylation level of VEGFR2 compared to wt. (Scale bar, 100 μm.)
Choroidal Atrophy in Aged VEGF188/188 Animals.
Choroidal atrophy secondary to RPE degeneration is a key feature of GA (28). FITC-lectin cardiac perfusion followed by flat-mount preparation of the posterior eyecup was used to visualize the choroidal vasculature. Compared to the dense vascular plexus of the wt choroid, the CC of 18-month-old VEGF188/188 mice was severely atrophied, with widened intercapillary spaces and reduced branching both in the central and peripheral retina (Fig. 5A). Choroidal atrophy was reflected by a significant increase in the avascular area of VEGF188/188 choroids (Fig. 5B). A reduction in the ratio of phosphorylated VEGFR2 to total VEGFR2 in the RPE-choroid complex of VEGF188/188 mice demonstrated that the absence of soluble VEGF resulted in a reduced availability of VEGF for the CC and led to age-dependent choroidal involution (Fig. 5C).
Discussion
VEGF isoform mRNA levels vary among tissues (17), with the pattern of isoform expression reflecting the association between the source of the VEGF and its target cells. For instance, in the lung where VEGF188 is the predominant isoform (17), VEGF is produced by type 2 pneumocytes, which are separated from the alveolar capillary endothelial cell by a very thin basement membrane, a arrangement that maximizes oxygen diffusion. On the other hand, in the choroid plexus of the brain, the epithelium produces primarily VEGF120, which supports not only the underlying fenestrated microvasculature, but diffuses into the cerebral spinal fluid to act on the VEGFR2-expressing ependymal cell ventricular lining (10). The fact that RPE express only the soluble VEGF isoforms, VEGF120 and VEGF164, is consistent with the physical barrier posed by the presence of BrM elastic lamina between the VEGF-producing RPE cells and the target CC.
Here, we demonstrate that the absence of soluble VEGF isoforms in mice leads to age-dependent, degenerative changes in the RPE-choroid complex that recapitulate the classical features of dry AMD such as signs of RPE dysfunction, including increased autofluorescence, accumulation of drusen-like basal deposits, and loss of barrier proprieties. These changes preceded the development of both focal choroidal atrophy and RPE attenuation that evolved into larger regions of RPE loss, a progression similar to the natural course of GA (28). The loss of RPE and CC led to photoreceptor death and reduced visual function, again recapitulating the course of human GA.
In embryos and young adult VEGF188/188 mice up to 4 months of age, the RPE-choroid complex appeared normal, an observation that was initially surprising since it had been previously shown that RPE-specific deletion of VEGF leads to defective choroidal development (29). However, understanding the time frame of BrM development provides an explanation. In mice, BrM is not fully formed until around 6 weeks of age, so that the development of the choroidal vasculature, which is initiated as early as E10.5 (19), would be unaffected by the reduced VEGF bioavailability in the VEGF188/188 mice. However, once BrM is in place, the diffusion of VEGF188 to the CC would be significantly decreased, explaining the RPE-choroid complex in aged VEGF188/188 mice.
A thickening of BrM and an alteration in its permeability are part of the normal human aging (30, 31). The accumulation of lipids in BrM observed in aging eyes, and particularly in patients with AMD, is suspected to limit the diffusion of water-soluble proteins like VEGF (32) and has led to the concept that a reduction in the diffusion of RPE-derived factors such as VEGF could be a causal in the development of GA (20, 33). Consistent with this notion, COX2-deficient mice, which exhibit progressive choroidal degeneration, have been reported to display reduced VEGF expression (34). Inhibition of VEGF signaling has been shown to be associated with rapid loss of fenestration and EC apoptosis in a variety of tissues (6, 8, 10, 35). Our observation of CC atrophy in mice expressing only VEGF188 is consistent with our hypothesis that the reduced ability of VEGF188 to diffuse through the BrM would impair CC survival. The lower level of VEGFR2 phosphorylation in the choroid of VEGF188/188 mice is strong evidence of the limited availability of VEGF188 to the choroidal capillary EC.
Our characterization of the ocular phenotype of VEGF188/188 mice, has not definitively identified the site of primary degeneration. Histological analyses revealed anomalies in both the RPE and the CC at the earliest time studied. We have recently shown that whereas embryonic RPE do not express VEGFR2, the receptor is expressed by adult, differentiated RPE (19). The expression of both VEGF and VEGFR2 by adult RPE suggests the existence of an autocrine function for VEGF in vivo, an observation previously shown for RPE in vitro (36). Thus, a direct effect of the absence of soluble VEGF isoforms on the RPE themselves cannot be excluded, although this seems unlikely, since there is no reason to suspect that VEGF188 produced by RPE would not have access to VEGFR2 on RPE. Due to technical difficulties encountered in the production of recombinant VEGF188, few studies looked at VEGF188 specific signaling and functions. Human VEGF189 stimulates endothelial proliferation, migration, and VEGFR2 up-regulation in vitro to levels comparable to VEGF165 (37). In addition, our preliminary data suggest that VEGF188 is binds NRP-1, as would be expected based on the presence of NRP binding domains (Fig. S8).
Our data strongly support the hypothesis that RPE-derived VEGF is necessary for the maintenance of RPE-complex integrity and suggest that reduced VEGF signaling, secondary to RPE dysfunction and/or BrM thickening, may contribute to the pathogenesis of GA. Anti-VEGF is currently the first line therapy for wet macular degeneration, and it is being increasingly used in the treatment of a number of other ocular pathologies characterized by edema and/or neovascularization, including diabetic macular edema, proliferative diabetic retinopathy, retinal vein occlusion, and the retinopathy of prematurity. In light of the postulated essential role for RPE-derived VEGF in the maintenance of CC, the chronic use of anti-VEGF therapies in the eye should be closely monitored. However, the relatively short intraocular half-life of both Avastin and Lucentis indicate that VEGF neutralization is neither total nor chronic and thus may achieve neutralization of the VEGF involved in pathologic vessel growth without influencing endogenous levels.
Methods
Materials.
C57BL/6J (Jackson Laboratories) and C57BL/6 VEGF188/188 (38) were used in this study. All animal protocols were approved by the Schepens Eye Research IACUC. Embryos were generated by breeding VEGF188/+ mice. Littermates VEGF188/188 and wt were used for our study.
Immunohistochemistry, Histology, and Electron Microscopy.
Whole embryos or enucleated eyes were processed and stained using previously published protocols (14) as described in the SI Materials and Methods.
Characterization of RPE and Choroid.
Mice were perfusion via the aorta with 10 mL 4% paraformaldehyde in PBS followed by 5 mL FITC-conjugated Griffonia simplicifolia isolectin B4 (Vector Laboratories) and washed by 10 mL PBS to remove nonspecific binding. Flat-mounted posterior segment of the eye were immunostained using a rabbit anti mouse-ZO1 (Zymed), rabbit anti-mouse β-catenin (Upstate) antibodies, or FITC-labeled phalloidin (Molecular Probe). For analysis of the choroidal vessels, the RPE layer was removed by gentle brushing. To quantify the avascular area, six photographs were taken in the mid-periphery region of each flat-mounted choroid (representing a total area of analysis of 2.03 mm2) and analyzed using ImageJ software. Each photograph was converted to grayscale, then based on pixel brightness values ranging from 0 to 255, blood vessels stained with the isolectin were distinguished from background by using empirically determined threshold values that included only blood vessels. The avascular area was calculated as the area represented by pixels having a brightness value lower than the corresponding threshold and expressed as a percentage of the total area. The mean value was calculated for each mouse.
Western Blot Analysis and Immunoprecipitation.
The choroid-RPE tissue from wt and VEGF188/188 mice were separated from the retina and homogenized in lysis buffer (Cell Signaling). Each sample represented one mouse. Protein concentration was quantified using a BCA assay (Bio-Rad Laboratories). For Western blot analysis, identical amount of protein was separated by SDS-PAGE under reducing condition and transferred to Immobilon-P membrane (Millipore). Membranes were incubated overnight at 4 °C with β-catenin (1: 500; Upstate) or actin (1:1,000; Santa Cruz Biotechnology) antibodies. Quantification of the level of VEGFR2 phosphorylation in the RPE-choroid lysates was conducted as previously published (19). Details can be found in the SI Materials and Methods.
ERG Recordings.
The ERGs of VEGF188/188 and wt mice ERG were assessed using a UTAS-E3000 recording system (LKC Technologies) (n = 4) using previously published protocols (14) as described in the SI Materials and Methods.
Additional methods are described in the SI Materials and Methods.
Supplementary Material
Acknowledgments.
We thank Drs. Elena Geretti and Michael Klagsbrun (Children's Hospital, Boston, MA) for conducting the binding competition assay (Fig. S8), Dr. Tony Walshe for his critical review of the manuscript, Ms. Christine Bagley for her editorial assistance, and Ms. Patricia Pearson for her help with the electron microscopy. The endomucin antisera was generously provided by Dietmar Vestweber (Max Planck Institute, Bad Nauheim, Germany). This work was supported by National Institutes of Health Grants EY015435 (to P.A.D.) and EY05318 (to P.A.D.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0905010106/DCSupplemental.
References
- 1.Holmes DI, Zachary I. The vascular endothelial growth factor (VEGF) family: Angiogenic factors in health and disease. Genome Biol. 2005;6:209. doi: 10.1186/gb-2005-6-2-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Olsson AK, Dimberg A, Kreuger J, Claesson-Welsh L. VEGF receptor signalling—in control of vascular function. Nat Rev Mol Cell Biol. 2006;7:359–371. doi: 10.1038/nrm1911. [DOI] [PubMed] [Google Scholar]
- 3.Soker S, Miao HQ, Nomi M, Takashima S, Klagsbrun M. VEGF165 mediates formation of complexes containing VEGFR-2 and neuropilin-1 that enhance VEGF165-receptor binding. J Cell Biochem. 2002;85:357–368. doi: 10.1002/jcb.10140. [DOI] [PubMed] [Google Scholar]
- 4.Maharaj AS, Saint-Geniez M, Maldonado AE, D'Amore PA. Vascular endothelial growth factor localization in the adult. Am J Pathol. 2006;168:639–648. doi: 10.2353/ajpath.2006.050834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eremina V, Quaggin SE. The role of VEGF-A in glomerular development and function. Curr Opin Nephrol Hypertens. 2004;13:9–15. doi: 10.1097/00041552-200401000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Maynard SE, et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003;111:649–658. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eremina V, et al. VEGF inhibition and renal thrombotic microangiopathy. N Engl J Med. 2008;358:1129–1136. doi: 10.1056/NEJMoa0707330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kamba T, et al. VEGF-dependent plasticity of fenestrated capillaries in the normal adult microvasculature. Am J Physiol Heart Circ Physiol. 2006;290:H560–H576. doi: 10.1152/ajpheart.00133.2005. [DOI] [PubMed] [Google Scholar]
- 9.Lammert E, et al. Role of VEGF-A in vascularization of pancreatic islets. Curr Biol. 2003;13:1070–1074. doi: 10.1016/s0960-9822(03)00378-6. [DOI] [PubMed] [Google Scholar]
- 10.Maharaj AS, et al. VEGF and TGF-beta are required for the maintenance of the choroid plexus and ependyma. J Exp Med. 2008;205:491–501. doi: 10.1084/jem.20072041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peters S, et al. Ultrastructural findings in the primate eye after intravitreal injection of bevacizumab. Am J Ophthalmol. 2007;143:995–1002. doi: 10.1016/j.ajo.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 12.D'Amore PA. Vascular endothelial cell growth factor-a: Not just for endothelial cells anymore. Am J Pathol. 2007;171:14–18. doi: 10.2353/ajpath.2007.070385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zachary I. Neuroprotective role of vascular endothelial growth factor: Signalling mechanisms, biological function, and therapeutic potential. Neurosignals. 2005;14:207–221. doi: 10.1159/000088637. [DOI] [PubMed] [Google Scholar]
- 14.Saint-Geniez M, et al. Endogenous VEGF is required for visual function: Evidence for a survival role on muller cells and photoreceptors. PLoS One. 2008;3:e3554. doi: 10.1371/journal.pone.0003554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shima DT, et al. The mouse gene for vascular endothelial growth factor. Genomic structure, definition of the transcriptional unit and characterization of transcriptional and post-transcriptional regulatory sequences. J Biol Chem. 1996;271:3877–3883. doi: 10.1074/jbc.271.7.3877. [DOI] [PubMed] [Google Scholar]
- 16.Carmeliet P, et al. Impaired myocardial angiogenesis and ischemic cardiomyopathy in mice lacking the vascular endothelial growth factor isoforms VEGF164 and VEGF188. Nat Med. 1999;5:495–502. doi: 10.1038/8379. [DOI] [PubMed] [Google Scholar]
- 17.Ng Y-S, Rohan R, Sunday M, deMello DE, D'Amore PA. Differential expression of VEGF isoforms in mouse during development and in the adult. Dev Dyn. 2001;220:112–121. doi: 10.1002/1097-0177(2000)9999:9999<::AID-DVDY1093>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 18.deMello DE, Reid LM. Embryonic and early fetal development of human lung vasculature and its functional implications. Pediatr Dev Pathol. 2000;3:439–449. doi: 10.1007/s100240010090. [DOI] [PubMed] [Google Scholar]
- 19.Saint-Geniez M, Maldonado AE, D'Amore PA. VEGF expression and receptor activation in the choroid during development and in the adult. Invest Ophthalmol Vis Sci. 2006;47:3135–3142. doi: 10.1167/iovs.05-1229. [DOI] [PubMed] [Google Scholar]
- 20.Blaauwgeers HG, et al. Polarized vascular endothelial growth factor secretion by human retinal pigment epithelium and localization of vascular endothelial growth factor receptors on the inner choriocapillaris. Evidence for a trophic paracrine relation. Am J Pathol. 1999;155:421–428. doi: 10.1016/S0002-9440(10)65138-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kannan R, et al. Stimulation of apical and basolateral VEGF-A and VEGF-C secretion by oxidative stress in polarized retinal pigment epithelial cells. Mol Vis. 2006;12:1649–1659. [PubMed] [Google Scholar]
- 22.Ikeda Y, et al. The regulation of vascular endothelial growth factors (VEGF-A, -C, and -D) expression in the retinal pigment epithelium. Exp Eye Res. 2006;83:1031–1040. doi: 10.1016/j.exer.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 23.Delori FC, Fleckner MR, Goger DG, Weiter JJ, Dorey CK. Autofluorescence distribution associated with drusen in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2000;41:496–504. [PubMed] [Google Scholar]
- 24.Dunaief JL, Dentchev T, Ying GS, Milam AH. The role of apoptosis in age-related macular degeneration. Arch Ophthalmol. 2002;120:1435–1442. doi: 10.1001/archopht.120.11.1435. [DOI] [PubMed] [Google Scholar]
- 25.Li J, Tso MO, Lam TT. Reduced amplitude and delayed latency in foveal response of multifocal electroretinogram in early age related macular degeneration. Br J Ophthalmol. 2001;85:287–290. doi: 10.1136/bjo.85.3.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rizzolo LJ. Development and role of tight junctions in the retinal pigment epithelium. Int Rev Cytol. 2007;258:195–234. doi: 10.1016/S0074-7696(07)58004-6. [DOI] [PubMed] [Google Scholar]
- 27.Konari K, et al. Development of the blood-retinal barrier in vitro: Formation of tight junctions as revealed by occludin and ZO-1 correlates with the barrier function of chick retinal pigment epithelial cells. Exp Eye Res. 1995;61:99–108. doi: 10.1016/s0014-4835(95)80063-8. [DOI] [PubMed] [Google Scholar]
- 28.Ambati J, Ambati B, Yoo S, Ianchulev S, Adamis A. Age-related macular degeneration: Etiology, pathogenesis, and therapeutic strategies. Surv Ophthalmol. 2003;48:257–293. doi: 10.1016/s0039-6257(03)00030-4. [DOI] [PubMed] [Google Scholar]
- 29.Marneros AG, et al. Vascular endothelial growth factor expression in the retinal pigment epithelium is essential for choriocapillaris development and visual function. Am J Pathol. 2005;167:1451–1459. doi: 10.1016/S0002-9440(10)61231-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ramrattan RS, et al. Morphometric analysis of Bruch's membrane, the choriocapillaris, and the choroid in aging. Invest Ophthalmol Vis Sci. 1994;35:2857–2864. [PubMed] [Google Scholar]
- 31.Moore DJ, Hussain AA, Marshall J. Age-related variation in the hydraulic conductivity of Bruch's membrane. Invest Ophthalmol Vis Sci. 1995;36:1290–1297. [PubMed] [Google Scholar]
- 32.Bird AC. Bruch's membrane change with age. Br J Ophthalmol. 1992;76:166–168. doi: 10.1136/bjo.76.3.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zarbin MA. Current concepts in the pathogenesis of age-related macular degeneration. Arch Ophthalmol. 2004;122:598–614. doi: 10.1001/archopht.122.4.598. [DOI] [PubMed] [Google Scholar]
- 34.Houssier M, et al. CD36 deficiency leads to choroidal involution via COX2 down-regulation in rodents. PLoS Med. 2008;5:e39. doi: 10.1371/journal.pmed.0050039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Inai T, et al. Inhibition of vascular endothelial growth factor (VEGF) signaling in cancer causes loss of endothelial fenestrations, regression of tumor vessels, and appearance of basement membrane ghosts. Am J Pathol. 2004;165:35–52. doi: 10.1016/S0002-9440(10)63273-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guerrin M, et al. Vasculotropin/vascular endothelial growth factor is an autocrine growth factor for human retinal pigment epithelial cells cultured in vitro. J Cell Physiol. 1995;64:385–394. doi: 10.1002/jcp.1041640219. [DOI] [PubMed] [Google Scholar]
- 37.Herve MA, Buteau-Lozano H, Mourah S, Calvo F, Perrot-Applanat M. VEGF189 stimulates endothelial cells proliferation and migration in vitro and up-regulates the expression of Flk-1/KDR mRNA. Exp Cell Res. 2005;309:24–31. doi: 10.1016/j.yexcr.2005.05.022. [DOI] [PubMed] [Google Scholar]
- 38.Carmeliet P, et al. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature. 1996;380:435–439. doi: 10.1038/380435a0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.