Abstract
Diabetes is a major risk factor for ischemic disease. Treatment options for diabetic patients with peripheral arterial disease when revascularization is not possible are limited, resulting in a high incidence of limb amputation. We evaluated the therapeutic potential of AdCA5, an adenovirus encoding a constitutively active form of HIF-1α, in a diabetic model of critical limb ischemia. Diabetic db/db and nondiabetic db/+ mice were subjected to unilateral femoral artery ligation. Limb perfusion, tissue viability, and motor function were more severely impaired in db/db mice. Intramuscular injection of AdCA5 into the ischemic limb of db/db mice increased the recovery of limb perfusion and function, reduced tissue necrosis, rescued the diabetes-associated impairment of circulating angiogenic cells, enhanced endothelial nitric oxide synthase activation, and increased vessel density and luminal area in the ischemic limb.
Keywords: angiogenesis, gene therapy, vascularization
Critical limb ischemia (CLI), the most severe form of peripheral vascular disease in which blood flow is not sufficient to maintain tissue viability, is the leading cause of nontraumatic amputation (1). The relative risk of amputation is 40 times greater in the diabetic population (2). Many CLI patients are not candidates for currently available surgical or endovascular procedures due to complex vascular occlusions and/or the presence of other surgical contraindications. The identification of molecular and cellular mechanisms underlying vascular responses to ischemia (3) has provided potential opportunities for the treatment of such patients by therapeutic angiogenesis.
Ischemia induces both angiogenesis, which is the sprouting of new capillaries from preexisting vessels, and arteriogenesis, which is the remodeling of existing collateral vessels to increase luminal diameter and thereby increase blood flow. Both angiogenesis and arteriogenesis are triggered by the hypoxia-induced expression of angiogenic cytokines, such as vascular endothelial growth factor (VEGF) and stromal-derived factor 1 (SDF-1) (3, 4). One of the important roles of these cytokines is to increase the number of circulating angiogenic cells (CACs), a term that is used here to encompass a heterogeneous population of cells, including endothelial progenitor cells (EPCs), mesenchymal and hematopoietic stem cells, and myeloid cells, which are mobilized from bone marrow and recruited to sites of ischemia where they promote vascularization (5–9). The production of VEGF, SDF-1, and other angiogenic factors is mediated by hypoxia-inducible factor 1 (HIF-1), a transcriptional activator that functions as a master regulator of responses to tissue hypoxia/ischemia (4). HIF-1 is a heterodimer composed of a constitutively expressed HIF-1β subunit and an O2-regulated HIF-1α subunit (10). Vascularization fails in Hif1a−/− (homozygous HIF-1α-null) mouse embryos (11, 12) and ischemia-induced vascularization is impaired in Hif1a+/− adult mice (9).
Ischemia-induced vascularization is impaired in diabetes (13). Levels of CACs are significantly reduced in patients with type 1 and type 2 diabetes (14–16). CACs, e.g., cells coexpressing the progenitor marker CD34 and VEGF receptor 2 (VEGFR2), are also decreased in nondiabetic patients with peripheral vascular disease and the combination of peripheral vascular disease and type 2 diabetes is associated with lower levels of CACs than either condition alone (17). Rats with streptozocin-induced diabetes have impaired mobilization of CACs in response to limb ischemia (18). Leptin-receptor deficient (db/db) mice are hyperglycemic and insulin-resistant as early as 6 weeks of age and represent a model of type 2 diabetes (19). Expression of HIF-1α protein and mRNAs encoding VEGF and other angiogenic growth factors are dramatically reduced in skin wounds of db/db mice and increased HIF-1α expression by pharmacological or gene therapy promotes cutaneous wound healing and angiogenesis (20–22). In this study, we investigated the role of HIF-1 in a mouse model of diabetic CLI.
Results
Vascular Responses to Ischemia Are Impaired in db/db Mice.
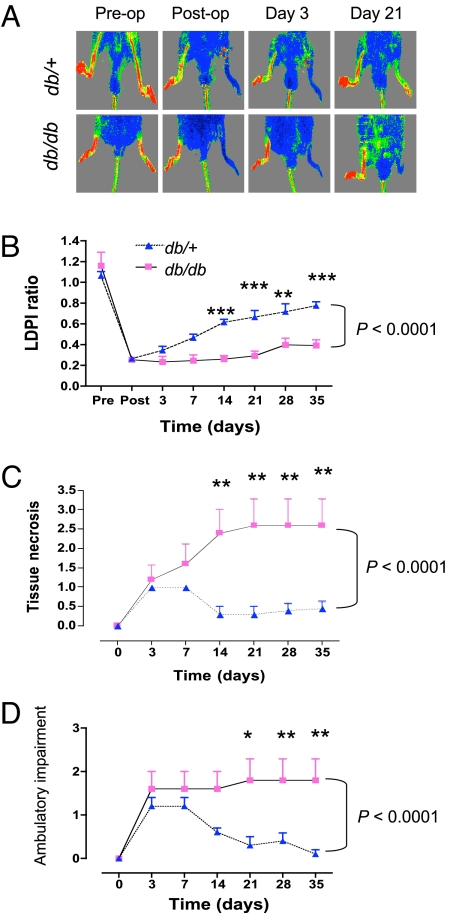
Age- and sex-matched db/db mice and nondiabetic db/+ littermates were subjected to unilateral femoral artery ligation. Recovery of limb perfusion, as determined by serial laser Doppler perfusion imaging (LDPI) (Fig. 1A), was significantly blunted in db/db mice compared with db/+ mice (Fig. 1B). Impaired recovery of perfusion in db/db mice was associated with a marked increase in tissue necrosis (Fig. 1C). Similarly, ambulatory impairment was significantly increased in db/db as compared to db/+ mice (Fig. 1D).
Fig. 1.
Effect of diabetes on recovery after femoral artery ligation. (A) Representative laser Doppler perfusion imaging (LDPI) of diabetic (db/db) and nondiabetic (db/+) mice immediately pre- and postligation and then 3 and 21 days later. The red-to-blue spectrum represents the range of blood flow from highest to lowest, respectively. (B) LDPI was performed at the indicated times after femoral artery ligation and the ratio of perfusion in the ischemic:nonischemic limb was determined. (C and D) Tissue necrosis (C) and ambulatory impairment (D) were scored at each time point using criteria described in Materials and Methods. Data in (B–D) are mean ± SEM (n = 4–5 mice each). Differences between the two groups were assessed by two-way ANOVA with Bonferroni correction (brackets at right); *, P < 0.05, **, P < 0.01, and ***, P < 0.001 for comparison between groups at individual time points.
Flow cytometric analysis of peripheral blood on day 3 after femoral artery ligation revealed a significant reduction in CACs coexpressing chemokine X receptor 4 (CXCR4) and stem cell antigen 1 (Sca-1) in db/db compared to db/+ mice (Fig. S1A). Similarly, the number of CACs coexpressing c-kit (CD117) and VEGFR2 were significantly reduced (Fig. S1B). CD34+/VEGFR2+ CACs were also reduced in db/db compared to db/+ mice but the difference was not significant (Fig. S1C).
Constitutively Active HIF-1α Improves Vascular Recovery in Ischemic db/db Mice.
We previously demonstrated that administration of AdCA5, an adenovirus encoding a constitutively active form of HIF-1α, improves recovery of limb perfusion following unilateral femoral artery ligation in nondiabetic mice (9). To determine whether increased AdCA5 treatment could overcome the diabetes-associated impairment in vascular responses to ischemia, db/db mice received an intramuscular injection of AdCA5 or AdLacZ (a control adenovirus encoding β-galactosidase) along the former course of the excised femoral artery. Quantitative real-time reverse-transcription PCR analysis of HIF-1α mRNA levels in calf muscle on day 3 after femoral artery ligation and gene therapy, using primers that amplified both endogenous mouse HIF-1α and human HIF-1αCA5, revealed 3-fold increased HIF-1α mRNA in the ischemic relative to nonischemic limbs of mice treated with AdLacZ, whereas HIF-1α mRNA levels were 20-fold increased in the ischemic relative to nonischemic limbs of mice treated with AdCA5 (Fig. S2A). Similar results were obtained on day 7 (Fig. S2B). Thus, AdCA5 treatment significantly increased HIF-1α mRNA levels in the ischemic limb for at least 1 week following femoral artery ligation.
By day 3, the recovery of limb perfusion as determined by LDPI (Fig. 2A) was also significantly increased in AdCA5-treated as compared to AdLacZ-treated db/db mice and this difference persisted throughout the observation period (Fig. 2B). By day 21, AdCA5-treated mice had recovered 85% of blood flow compared to only 24% recovery in AdLacZ-treated mice. AdCA5-treated db/db mice also manifested significantly less tissue damage and neurological deficits as compared to AdLacZ-treated littermates (Fig. 2 C and D).
Fig. 2.
Effect of AdCA5 therapy on the recovery of diabetic mice after ischemia. AdLacZ or AdCA5 was administered immediately after femoral artery ligation. (A) Representative serial LDPI is shown. (B–D) The ratio of perfusion in nonischemic:ischemic limb was determined (B), and tissue necrosis (C) and ambulatory impairment (D) were scored at each time point. Mean ± SEM values are shown (n = 7). Differences between groups were assessed by two-way ANOVA with Bonferroni correction (brackets at right); ***, P < 0.001 at individual time point.
We next examined the histopathology of adductor muscles from the ischemic limb of AdLacZ- and AdCA5-treated mice on day 7. Tissue protection in AdCA5-treated diabetic mice was confirmed by microscopic inspection of hematoxylin/eosin-stained sections (Fig. 3 A–D). AdCA5-treated mice showed a significantly increased area of tissue preservation and a significantly decreased area of inflammatory cell infiltration as compared to AdLacZ-treated mice (Fig. 3K). Infiltration of macrophages, which promote arteriogenesis, was observed in both treatment groups (Fig. 3 E and F). In contrast, infiltration of lymphocytes (Fig. 3 G and H), and neutrophils (Fig. 3 I and J) into ischemic limb was markedly increased in AdLacZ-treated as compared to AdCA5-treated mice.
Fig. 3.
Histological analysis of ischemic adductor muscles from diabetic mice after therapy. (A–D) Hematoxylin-eosin staining of tissue sections on day 7 after femoral artery ligation and treatment with AdLacZ (A) or AdCA5 (B) (original magnification, 40×). Black arrow indicates area infiltrated with inflammatory cells and red arrow indicates area with preservation of normal muscle tissue histology. Boxed areas are shown at higher power (C and D; original magnification, 200×). (E–J) Immunohistochemical staining of infiltrated areas for macrophages (E and F), lymphocytes (G and H), and neutrophils (I and J) using F4/80, CD3 and myeloperoxidase antibodies, respectively (original magnification, 200×). (K) quantification of infiltrated and preserved areas as percentage of total tissue area with mean ± SEM (n = 4 mice each) shown. **, P < 0.01 (Mann–Whitney test).
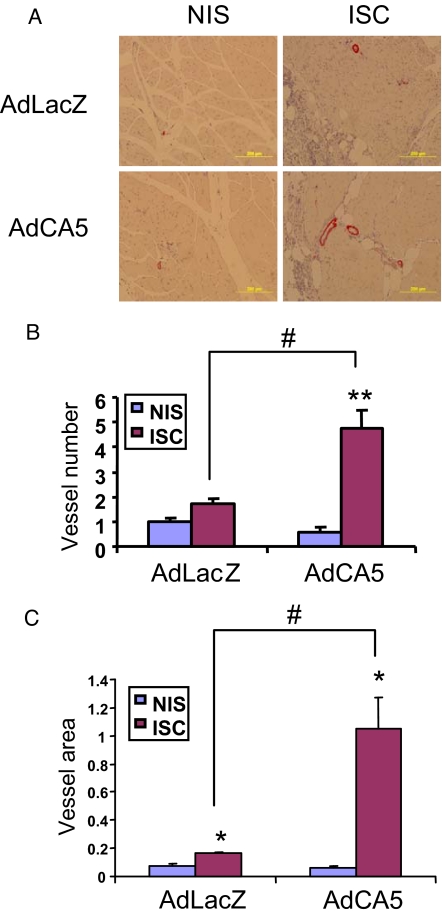
To assess vascularization, adductor muscle sections from ischemic limbs on day 21 were stained for the presence of smooth muscle α-actin (SMA), which is expressed in both pericytes and smooth muscle cells of mature blood vessels (Fig. 4A). Sections from AdCA5-treated ischemic limbs had a significantly increased number of SMA+ vessels (4.75 ± 0.73 per 200× field) (Fig. 4B) as compared to the non-ischemic contralateral limbs (0.60 ± 0.18 per field) or to AdLacZ-treated ischemic limbs (1.70 ± 0.21 per field). Ischemic limbs from both treatment groups showed an increase in vessel luminal area compared to the respective contralateral limbs (AdLacZ, 0.16 ± 0.02% vs. 0.07 ± 0.01% of total area; AdCA5, 1.05 ± 0.22% vs. 0.06 ± 0.01 of total area) (Fig. 4C). However, SMA+ vessel area was significantly increased in AdCA5-treated, as compared to AdLacZ-treated ischemic limbs. The increased number and luminal area of SMA+ vessels provides a histological basis for the increased blood flow and tissue viability in the ischemic limbs of AdCA5-treated db/db mice.
Fig. 4.
Immunohistochemical analysis of tissue vascularization, using an anti-SMA antibody. (A) Representative sections of adductor muscles from nonischemic (NIS) and ischemic (ISC) limbs harvested on day 21 after ligation and treatment with AdLacZ or AdCA5 (original magnification, 200×). (B) Mean number of SMA+ vessels per 200× field in adductor muscle sections. Data are mean ± SEM (n = 4 mice each). **, P < 0.01 vs. contralateral NIS limb; #, P < 0.01 vs. AdLacZ-treated ISC limb (Student's t-test). (C) Vessel luminal area was determined as a percentage of the total tissue area of the field. *, P < 0.05 vs. contralateral NIS limb; #, P < 0.01 vs. AdLacZ-treated ISC limb (Mann–Whitney test).
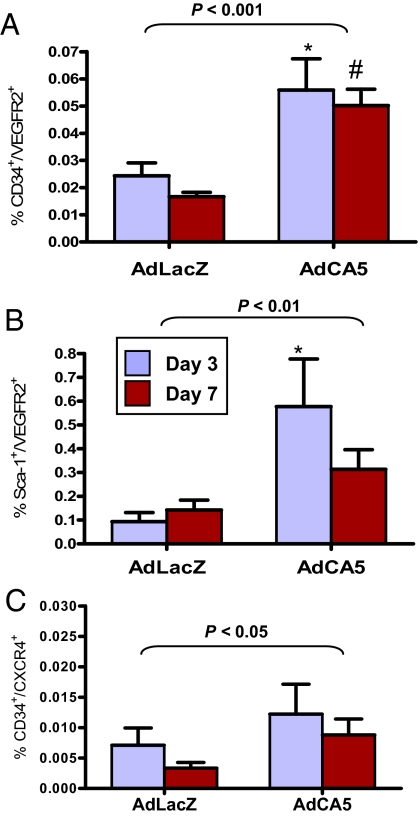
AdCA5 Gene Therapy Increases the Number of CACs in Ischemic db/db Mice.
Analysis of peripheral blood on days 3 and 7 after femoral artery ligation revealed significantly increased numbers of CD34+/VEGFR2+ cells on days 3 and 7 (Fig. 5A) and Sca-1+/VEGFR2+ cells on day 3 (Fig. 5B) in AdCA5-treated as compared to AdLacZ-treated mice. Overall, CD34+/CXCR4+ cells were significantly increased in AdCA5-treated as compared to AdLacZ-treated mice, although the differences at individual time points were not significant (Fig. 5C).
Fig. 5.
Effect of AdCA5 treatment on the number of CACs in peripheral blood of diabetic mice following femoral artery ligation. AdCA5 or AdLacZ was injected into the ischemic limb immediately after surgery. After 3 or 7 days, peripheral blood was collected and analyzed by flow cytometry to detect the following populations of CACs: CD34+/VEGFR2+ (A), Sca-1+/VEGFR2+ (B), and CD34+/CXCR4+ (C). Mean ± SEM (n = 4–5 each) is shown. Statistical significance was assessed by two-way ANOVA with Bonferroni correction (brackets at top); *, #, P < 0.05 at individual time point vs. AdLacZ.
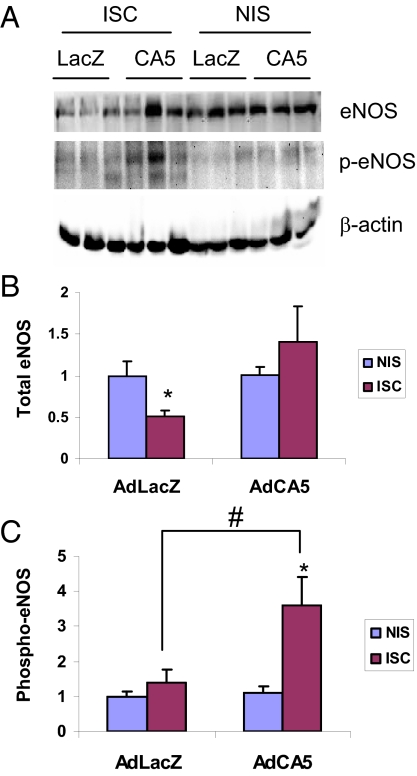
AdCA5 Gene Therapy Rescues eNOS Signaling.
Endothelial nitric oxide synthase (eNOS) activity is essential for EPC mobilization from bone marrow to peripheral blood and for effective ischemia-induced vascularization (23, 24). Moreover, activation of eNOS, as determined by its phosphorylation at serine residue 1,177, is impaired in diabetic bone marrow, resulting in depressed EPC mobilization (25). We determined the levels of S1177-phosphorylated eNOS (phospho-eNOS) and total eNOS protein in ischemic calf muscles on day 7 by immunoblot assays (Fig. 6A). Total eNOS protein levels were decreased in AdLacZ-treated ischemic limbs compared to the contralateral nonischemic limbs, whereas AdCA5 treatment restored total eNOS protein in ischemic limbs to levels similar to those in contralateral nonischemic limbs (Fig. 6B). Phospho-eNOS protein levels were significantly increased in AdCA5-treated ischemic muscle compared to contralateral nonischemic muscle or to AdLacZ-treated ischemic muscle (Fig. 6C).
Fig. 6.
Analysis of eNOS activity in the calf muscles of diabetic mice. Immunoblot assays (A) were performed to quantify the levels of total eNOS protein (B) and eNOS phosphorylated at Ser-1177 (p-eNOS, C) in ischemic (ISC) and nonischemic (NIS) muscles on day 7 after femoral artery ligation. The expression of eNOS or p-eNOS was normalized to the β-actin level in the same lysate and the ISC:NIS ratio was determined. *, P < 0.05 vs. contralateral NIS limbs; #, P < 0.05 vs. AdLacZ-treated ISC limbs (Mann-Whitney test). Mean ± SEM is shown (n = 5–6).
Discussion
Diabetes is a major risk factor for limb ischemia, often resulting in neuropathy, tissue ulceration, and, ultimately, lower extremity amputation. Therapeutic angiogenesis holds promise as a treatment modality to prevent amputation in diabetic patients with CLI who are not candidates for currently available revascularization procedures. We have investigated the therapeutic efficacy of AdCA5 administration in a preclinical model of diabetic CLI. Two limitations of this model should be noted. First, CLI in diabetic patients is the result of a chronic and progressive process in contrast to the acute arterial occlusion that occurs in mice subjected to femoral artery ligation. Second, whereas db/db mice may model the role of genetic predisposition, mice maintained on a high fat diet may better model the role of nongenetic risk factors for human type 2 diabetes.
In this study we observed impaired tissue perfusion, severe tissue necrosis, and a marked degree of ambulatory impairment following femoral artery ligation in db/db mice. We demonstrated that AdCA5 treatment significantly improved limb tissue perfusion, viability, and function by inducing therapeutic angiogenesis, as manifested by increased numbers of CACs in peripheral blood, as well as increased eNOS activation and increased vessel density/luminal area in ischemic limb tissue. We have demonstrated that HIF-1α mRNA levels remain significantly elevated for at least 7 days after AdCA5 administration. It appears that this first week following arterial occlusion represents a critical window of time, during which sufficient blood flow must be restored to maintain tissue viability. Our data indicate that AdCA5 provides a therapeutic stimulus that dramatically increases the recovery of perfusion in db/db mice.
CACs are mobilized from the bone marrow and other tissues in response to ischemia and contribute to the vascular remodeling of ischemic tissue. CACs are reduced in diabetic patients, which is thought to contribute to impaired angiogenesis (26). In the present work, we have shown that the mobilization of CACs after femoral artery ligation is markedly impaired in db/db mice. CD117+/VEGFR2+ and CXCR4+ cell numbers are decreased in peripheral blood of type 2 diabetic patients and the number of CXCR4+ cells is further decreased in diabetic patients with vascular complications (27). Diabetic patients with ischemic foot lesions due to end–stage peripheral vascular disease have a greater reduction of CD34+ and CD34+/VEGFR2+ cells as compared with diabetic patients with peripheral vascular disease, but without foot lesions (17). These data highlight the importance of our finding that AdCA5 treatment significantly increased the number of CD34+/VEGFR2+, Sca-1+/VEGFR2+, and CD34+/CXCR4+ CACs in diabetic mice with CLI.
Compared to controls, patients with type 2 diabetes have decreased HIF-1β mRNA levels in pancreatic islet tissue, which suggests that changes in HIF-1 activity may contribute to the pathogenesis of diabetes (28). A human genetic polymorphism that results in the substitution of serine for proline at residue 582 of HIF-1α is associated with type 2 diabetes (29). The P582S polymorphism is also associated with absence of coronary collaterals in patients with ischemic heart disease (30). Taken together with our data, these studies provide a link between HIF-1, diabetes, and impaired vascularization in humans and mice.
Diabetes may also result in changes in HIF-1 activity, as hyperglycemia inhibits the induction of HIF-1α in hypoxic cells (31). Hyperglycemia reduces eNOS expression in hypoxic murine EPCs due to HIF-1α modification by methylglyoxal, a consequence of increased reactive oxygen species formed in hyperglycemia (32). Bone marrow-derived EPCs from db/db mice showed reduced expression of eNOS and phospho-eNOS (33). AdCA5 restored total eNOS levels and significantly increased phosphorylation of eNOS in the ischemic limb. Our data suggest that the restoration of CACs and eNOS signaling that results from AdCA5 treatment contributes to the improved vascular response to ischemia in the diabetic setting. These results provide preclinical data supporting a trial of AdCA5 gene therapy in no-option diabetic patients with CLI.
Materials and Methods
Mice.
Male db/db and db/+ littermates (strain BKS.Cg-m +/+ Leprdb/J) were purchased from The Jackson Laboratory. Animal care and experimental procedures were performed under a protocol approved by the Johns Hopkins University Animal Care and Use Committee. At the start of the experiments, mice were 8 weeks old.
Surgery and Gene Therapy.
Femoral artery ligation was performed as described (9). AdCA5 is a constitutively active form of human HIF-1α that contains a deletion (residues 392–520) and two missense mutations (Pro567Thr and Pro658Gln). Large-scale adenovirus production was performed at the NHLBI Vector Core Facility (University of Pittsburgh). Immediately after femoral artery ligation, AdLacZ or AdCA5 was injected at three sites in the adductor muscle and one site in the gastrocnemius muscle of the left hindlimb of db/db mice. A total of 2 × 108 plaque-forming units were injected in each mouse.
Blood Flow Analysis and Physical Examination.
Blood flow was measured by Laser Doppler Perfusion Imaging system (Moor Instruments, Inc.) and expressed as signal in the ischemic:nonischemic limb (9). Ambulatory impairment was scored as follows: 0 = plantar/toe flexion to resist gentle tail traction, 1 = plantar but not toe flexion, 2 = no plantar or toe flexion, 3 = no use of foot. Tissue necrosis was scored as follows: 0 = no necrosis, 1 = cyanosis/discoloration, 2 = necrosis/loss of 1–2 toes, 3 = necrosis/loss of 3–5 toes, 4 = necrosis extending to dorsum pedis or higher.
Flow Cytometry.
Blood was subjected to red cell lysis and Fc blockade (CD16/CD32; BD PharMingen). Mononuclear cells were analyzed by flow cytometry (LSRII; Becton Dickinson) using phycoerythrin-conjugated anti-VEGFR2 (Avas 12α1; BD PharMingen) and FITC-conjugated anti-CD34 (RAM34; eBioscience), anti-CD117 (2B8, eBioscience), and anti-Sca-1 (E13–161.7, BD PharMingen) antibodies. Two hundred thousand events were acquired, the live cell population in the forward-scatter vs. side-scatter window was gated, and double-positive cells were expressed as a percentage of total cells.
Quantitative Real-Time Reverse Transcription PCR.
Total RNA was extracted from muscle using TRIzol (Invitrogen), precipitated in isopropanol, and treated with DNase I (Ambion). A 1-μg aliquot of total RNA served as template for cDNA synthesis using the iScript kit (BioRad). Real-time PCR was performed using iQ SYBR Green Supermix and the iCycler Real-time PCR Detection System (BioRad). HIF-1α mRNA relative to RPL13A mRNA was calculated using the comparative ΔCt method (9).
Histology and Immunohistochemistry.
Adductor muscles were harvested, fixed in 10% formalin, paraffin embedded, and 5-μm-thick sections were stained with hematoxylin and eosin. Areas of inflammatory cell infiltration and tissue preservation (normal histology) were quantified using Image J software (National Institutes of Health) and reported as a percentage of total area. For immunohistochemistry, heat-induced antigen retrieval was performed after sections were deparaffinized. Endogenous peroxidase activity was blocked by incubation in H2O2/methanol. Nonspecific binding was blocked with serum-free protein block (DAKO Corporation) or 5% rabbit serum. Sections were incubated with the following primary antibodies (at indicated dilution) for 1 h at room temperature or overnight at 4 °C: rat anti-mouse F4/80 (1:100, Invitrogen), rabbit anti-myeloperoxidase (1:50, Abcam), and rabbit anti-CD3 (1:500, Abcam). Sections were incubated with secondary biotinylated antibodies, washed in PBS, and incubated with Vectastain ABC Elite (Vector Labs). The avidin-biotin complex was visualized using a 3, 3′ diaminobenzidine peroxidase substrate (Vector Labs). SMA staining was performed using a monoclonal antibody against smooth muscle α-actin (1:50, Sigma-Aldrich), ABC-AP kit (Vector Labs) and AP substrate kit (Vector Labs). Sections were counterstained in hematoxylin (Richard-Allen). SMA+ vessel density and vessel area were quantified in 5 random fields per slide at 200× power using Image J software.
Immunoblot Assays.
Protein lysates of ischemic calf muscles were prepared in RIPA buffer containing protease and phosphatase inhibitor cocktails (Complete Protease Inhibitor mixture and PhosSTOP, Roche Diagnostics) and fractionated by SDS/PAGE. Antibodies against eNOS and and phospho-eNOS [Ser-1177] were from Novus Biologicals and Cell Signaling Technology, respectively.
Statistical Analysis.
Results are presented as mean ± SEM. ANOVA followed by Bonferroni post hoc analysis, Student's t-test, or Mann-Whitney U test was used to determine the significance of differences in means between groups.
Supplementary Material
Acknowledgments.
We thank Karen Padgett (Novus Biologicals) for providing anti-eNOS antibodies, Andrea Gambotto and Susan Schoonover (University of Pittsburgh) for large-scale adenovirus preparations, and Ryan Chang for technical assistance. This work was supported by the American Diabetes Association and the Johns Hopkins Institute for Cell Engineering. G.L.S. is the C. Michael Armstrong Professor at the Johns Hopkins University School of Medicine.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0910561106/DCSupplemental.
References
- 1.Novo S, Coppola G, Milio G. Critical limb ischemia: Definition and natural history. Curr Drug Targets Cardiovasc Haematol Disord. 2004;4:219–225. doi: 10.2174/1568006043335989. [DOI] [PubMed] [Google Scholar]
- 2.Nathan DM. Long-term complications of diabetes mellitus. N Engl J Med. 1993;328:1676–1685. doi: 10.1056/NEJM199306103282306. [DOI] [PubMed] [Google Scholar]
- 3.Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nat Med. 2000;6:389–395. doi: 10.1038/74651. [DOI] [PubMed] [Google Scholar]
- 4.Semenza GL. Regulation of oxygen homeostasis by hypoxia-inducible factor 1. Physiology (Bethesda) 2009;24:97–106. doi: 10.1152/physiol.00045.2008. [DOI] [PubMed] [Google Scholar]
- 5.Asahara T, et al. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res. 1999;85:221–228. doi: 10.1161/01.res.85.3.221. [DOI] [PubMed] [Google Scholar]
- 6.Grant MB, et al. Adult hematopoietic stem cells provide functional hemangioblast activity during retinal neovascularization. Nat Med. 2002;8:607–612. doi: 10.1038/nm0602-607. [DOI] [PubMed] [Google Scholar]
- 7.Urbich C, Dimmeler S. Endothelial progenitor cells: Functional characterization. Trends Cardiovasc Med. 2004;14:318–322. doi: 10.1016/j.tcm.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 8.Yoder MC, et al. Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood. 2007;109:1801–1809. doi: 10.1182/blood-2006-08-043471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bosch-Marcé M, et al. Effects of aging and hypoxia-inducible factor-1 activity on angiogenic cell mobilization and recovery of perfusion after limb ischemia. Circ Res. 2007;101:1310–1318. doi: 10.1161/CIRCRESAHA.107.153346. [DOI] [PubMed] [Google Scholar]
- 10.Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci USA. 1995;92:5510–5514. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iyer NV, et al. Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1α. Genes Dev. 1998;12:149–162. doi: 10.1101/gad.12.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ryan HE, Lo J, Johnson RS. HIF-1α is required for solid tumor formation and embryonic vascularization. EMBO J. 1998;17:3005–3015. doi: 10.1093/emboj/17.11.3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Waltenberger J. Impaired collateral vessel development in diabetes: Potential cellular mechanisms and therapeutic implications. Cardiovasc Res. 2001;49:554–560. doi: 10.1016/s0008-6363(00)00228-5. [DOI] [PubMed] [Google Scholar]
- 14.Loomans CJ, et al. Endothelial progenitor cell dysfunction: A novel concept in the pathogenesis of vascular complications of type 1 diabetes. Diabetes. 2004;53:195–199. doi: 10.2337/diabetes.53.1.195. [DOI] [PubMed] [Google Scholar]
- 15.Tamarat R, et al. Impairment in ischemia-induced neovascularization in diabetes: Bone marrow mononuclear cell dysfunction and therapeutic potential of placenta growth factor treatment. Am J Pathol. 2004;164:457–466. doi: 10.1016/S0002-9440(10)63136-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tepper OM, et al. Human endothelial progenitor cells from type II diabetics exhibit impaired proliferation, adhesion, and incorporation into vascular structures. Circulation. 2002;106:2781–2786. doi: 10.1161/01.cir.0000039526.42991.93. [DOI] [PubMed] [Google Scholar]
- 17.Fadini GP, et al. Circulating endothelial progenitor cells are reduced in peripheral vascular complications of type 2 diabetes mellitus. J Am Coll Cardiol. 2005;45:1449–1457. doi: 10.1016/j.jacc.2004.11.067. [DOI] [PubMed] [Google Scholar]
- 18.Fadini GP, et al. Diabetes impairs progenitor cell mobilisation after hindlimb ischemia-reperfusion injury in rats. Diabetologia. 2006;49:3075–3084. doi: 10.1007/s00125-006-0401-6. [DOI] [PubMed] [Google Scholar]
- 19.Kobayashi K, et al. The db/db mouse, a model for diabetic dyslipidemia: Molecular characterization and effects of Western diet feeding. Metabolism. 2000;49:22–31. doi: 10.1016/s0026-0495(00)90588-2. [DOI] [PubMed] [Google Scholar]
- 20.Mace KA, et al. Sustained expression of HIF-1α in the diabetic environment promotes angiogenesis and cutaneous wound repair. Wound Repair Regen. 2007;15:636–645. doi: 10.1111/j.1524-475X.2007.00278.x. [DOI] [PubMed] [Google Scholar]
- 21.Liu L, et al. Age-dependent impairment of HIF-1α expression in diabetic mice: Correction with electroporation-facilitated gene therapy increases wound healing, angiogenesis, and circulating angiogenic cells. J Cell Physiol. 2008;217:319–327. doi: 10.1002/jcp.21503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Botusan IR, et al. Stabilization of HIF-1α is critical to improve wound healing in diabetic mice. Proc Natl Acad Sci USA. 2008;105:19426–19431. doi: 10.1073/pnas.0805230105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aicher A, et al. Essential role of endothelial nitric oxide synthase for mobilization of stem and progenitor cells. Nat Med. 2003;9:1370–1376. doi: 10.1038/nm948. [DOI] [PubMed] [Google Scholar]
- 24.Murohara T, et al. Nitric oxide synthase modulates angiogenesis in response to tissue ischemia. J Clin Invest. 1998;101:2567–2578. doi: 10.1172/JCI1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gallagher KA, et al. Diabetic impairments in NO-mediated endothelial progenitor cell mobilization and homing are reversed by hyperoxia and SDF-1α. J Clin Invest. 2007;117:1249–1259. doi: 10.1172/JCI29710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fadini GP, Agostini C, Avogaro A. Endothelial progenitor cells and vascular biology in diabetes mellitus: Current knowledge and future perspectives. Curr Diabetes Rev. 2005;1:41–58. doi: 10.2174/1573399052952640. [DOI] [PubMed] [Google Scholar]
- 27.Egan CG, et al. Generalized reduction of putative endothelial progenitors and CXCR4-positive peripheral blood cells in type 2 diabetes. Diabetologia. 2008;51:1296–1305. doi: 10.1007/s00125-008-0939-6. [DOI] [PubMed] [Google Scholar]
- 28.Gunton JE, et al. Loss of ARNT/HIF-1β mediates altered gene expression and pancreatic-islet dysfunction in human type 2 diabetes. Cell. 2005;122:337–349. doi: 10.1016/j.cell.2005.05.027. [DOI] [PubMed] [Google Scholar]
- 29.Yamada N, et al. Genetic variation in the hypoxia-inducible factor-1α gene is associated with type 2 diabetes in Japanese. J Clin Endocrinol Metab. 2005;90:5841–5847. doi: 10.1210/jc.2005-0991. [DOI] [PubMed] [Google Scholar]
- 30.Resar JR, et al. Hypoxia-inducible factor 1α polymorphism and coronary collaterals in patients with ischemic heart disease. Chest. 2005;128:787–791. doi: 10.1378/chest.128.2.787. [DOI] [PubMed] [Google Scholar]
- 31.Catrina SB, et al. Hyperglycemia regulates hypoxia-inducible factor-1α protein stability and function. Diabetes. 2004;53:3226–3232. doi: 10.2337/diabetes.53.12.3226. [DOI] [PubMed] [Google Scholar]
- 32.Ceradini DJ, et al. Decreasing intracellular superoxide corrects defective ischemia-induced new vessel formation in diabetic mice. J Biol Chem. 2008;283:10930–10938. doi: 10.1074/jbc.M707451200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ii M, et al. Endothelial progenitor thrombospondin-1 mediates diabetes-induced delay in reendothelialization following arterial injury. Circ Res. 2006;98:697–704. doi: 10.1161/01.RES.0000209948.50943.ea. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.