Abstract
Conflicting reports of the prognostic value of HER4 in breast cancer may be explained by distinct activities of the HER4 intracellular domain, 4ICD. Here, immunohistochemical 4ICD staining of archival invasive breast cancers (n = 923) was scored separately for nuclear and cytosolic expression, and these data were tested for associations with clinicopathological markers, disease-free survival, and disease-specific survival. By univariate analysis, cytosolic 4ICD expression was independently associated with estrogen receptor and progesterone receptor expression and tumor cell apoptosis. Nuclear 4ICD inversely correlated with tumor grade and tumor mitosis. In multivariate analyses cytosolic, but not nuclear 4ICD, significantly correlated with disease-free survival (P = 0.035) and disease-specific survival (P < 0.004) in lymph node-negative patients. Our results demonstrate for the first time that cytosolic 4ICD has significant positive prognostic value in node-negative breast cancer patients. At present, tumor grade and size are the primary clinicopathological parameters commonly used to guide decision making in these patients. Our results suggest that cytosolic 4ICD has important pathological functions and may be used to identify node-negative breast cancer patients at low risk of relapse and an improved survival, thereby avoiding systemic overtreatment of these patients. Our results also suggest that pan-receptor tyrosine kinase inhibitors, currently in clinical trials, or HER4 antagonists, which disengage 4ICD signaling, may have untoward activity in patients whose tumors express cytosolic 4ICD.
Members of the epidermal growth factor receptor (EGFR)-family of receptor tyrosine kinases have a significant influence on the development and progression of breast cancer. For example, the HER2/ERBB2/Neu proto-oncogene is amplified and overexpressed in 20% to 40% of breast cancers and is a negative prognostic factor in patients with lymph node-positive disease.1 The prognostic value of HER2 in lymph node-negative patients remains controversial. The HER2 protein is also the molecular target for trastuzumab therapy and HER2 has become an established clinical marker identifying patients likely to benefit from trastuzumab. Although the independent prognostic value of EGFR or HER3 expression in breast cancer remain unclear, these receptors may cooperate with HER2 to promote tumor progression2 and therapeutic resistance.3 In contrast, breast tumor expression of HER4 has been shown to inversely correlate with HER2 expression4,5 implicating distinct activities for HER4 during breast carcinogenesis. However, a specific role for HER4 expression in breast carcinogenesis has not been defined and its prognostic value is controversial.6
A lack of a consensus on the biological impact of HER4 expression in breast cancer may in part reflect the mechanistically and functionally unique nature of HER4 signaling. Unlike other members of the EGFR-family, activated HER4 receptor undergoes proteolytic cleavage at the cell surface to release a multifunctional and soluble HER4 intracellular domain (4ICD).7 Once released from the plasma membrane, 4ICD may localize in the cytosol to the mitochondria where it initiates tumor cell apoptosis mediated through an intrinsic BH3 cell-killing domain.8,9,10 4ICD may also localize to the nucleus, where it functions as a potent transcriptional coactivator for estrogen receptor (ER)α and signal transducer and activator of transcription 5A. In the later scenario, 4ICD coactivation of ERα promotes estrogen stimulated breast tumor cell proliferation11 or 4ICD may cooperate with signal transducer and activator of transcription 5A to promote cellular differentiation.12,13,14 The subcellular fate of soluble 4ICD, and subsequent cellular responses, are differentially regulated by the agonist promoting HER4 processing,8,9,11,13,14 4ICD interactions with transcription factors,11,14 or treatment with the selective ER modulator tamoxifen.9 For example, tamoxifen disrupts the ERα/4ICD nuclear complex and subsequent mitochondrial accumulation of the untethered 4ICD BH3-only protein mediates tamoxifen-induced tumor cell apoptosis. Significantly, breast cancer patients with tumor expression of nuclear 4ICD all respond to tamoxifen, underscoring the clinical impact of 4ICD activity.9 Interestingly, a physiologically relevant function unique to membrane associated HER4 remains to be confirmed. In fact, HER4 is a relatively impotent signaling receptor, only interacting weakly with a small number of recruited proteins,15 raising doubts about the relevance of membrane HER4 signaling.
With recent descriptions of unique and clinically important functions of an independently signaling 4ICD,7,9 conclusions inferred from previous HER4 expression studies should be viewed with caution. These studies often used methodologies that failed to account for 4ICD localization and activity, including detection of HER4 gene amplification, HER4 mRNA, or extracellular HER4.6 In addition, many of the expression studies to date have been limited by cohort size and the absence of robust multivariate statistical analyses. These limitations not only reduce the significance of the findings, but also prevent application of the results within the general breast cancer patient population.
In this communication, we used an immunohistochemical approach that allowed us to independently score 4ICD expression within nuclear and/or cytosolic subcellular compartments. Because of our large number of study cases and the prolonged clinical follow-up interval, we have sufficient statistical power to detect meaningful differences. We show for the first time that 4ICD expression has independent prognostic value in breast cancer patients. If validated, these data suggest that the analysis of 4ICD expression may be useful to guide management of patients with lymph node-negative breast cancer, a patient population that poses unique clinical challenges.
Materials and Methods
Patient Population
The patient population used for this study has been described in detail elsewhere.16 A total of 923 archived formalin fixed paraffin-embedded breast tumors from patients diagnosed at Massachusetts General Hospital (Boston, MA) between 1976 and 1983 were graded using the Nottingham combined histological grading system.17 Follow-up for a mean of 15.6 years was used to determine associations between patient outcome and 4ICD expression. Additional immunohistochemical, clinical, and pathological details of this cohort have been described elsewhere.2,16,18
HER4/4ICD Immunohistochemistry
HER4/4ICD immunohistochemistry was performed as described previously8,9 using HER4 Ab-4 (Neomarkers, Clone HFR-1), directed against the carboxyl terminus of human HER4 at a concentration of 4.0 μg/ml. Each patient sample was scored by visual estimation of the percentage of 4ICD positive invasive tumor cells in each independent cytosolic and nuclear subcellular compartment. A tumor was scored positive for nuclear or cytosolic 4ICD if ≥10% or ≥25% of invasive tumor cells exhibited positive immunohistochemical staining, respectively.
Statistical Methods
Data for clinical and biological parameters were calculated using continuous variables for most analyses. Pearson product moment correlations were used to express correlations between nuclear or cytoplasmic 4ICD expressed as continuous variables and clinical, pathological and immunohistochemical variables. Mann- Whitney tests were used to correlate nuclear or cytoplasmic HER4 expression with chemotherapeutic and hormone treatment.
For univariate survival and Kaplan-Meier survival curves factors were dichotomized or trichotomized based on previously established cut points. Nuclear 4ICD was dichotomized at <10% vs. ≥10% while cytoplasmic 4ICD was dichotomized at <25% vs. ≥25% of tumor cells positive because this dichotomization gave the best difference in survival. Mitoses/1000 cells counted was dichotomized at <2.3 vs. ≥2.3 and apoptosis/1000 cells was dichotomized at <3.58 vs. ≥3.58 similar to our previous study.16 HER2 was dichotomized at <10% vs. ≥10% of tumor cells reflecting the existing cut point analysis developed for the HerceptTest (Dako Corp. Carpentaria CA). Other markers were dichotomized or trichotomized at the same cut points as have previously been used for this dataset.2,16,18 Outcomes for this study included disease-free survival (DFS) defined as the number of months between the diagnosis date and the date of the first failure (local, regional, or distant) and disease-specific overall survival (DSS) defined as the number of months between the diagnosis date and the date of death from breast cancer. Patients who were alive, but whose status was unknown were excluded from survival analyses (n = 2), patients whose death was not related to breast disease were censored at the date of death, and deaths from unknown causes (n = 2) were excluded from DSS analyses.
For multivariate survival, the number of positive lymph nodes was transformed by taking the 1 minus the log of the number of positive lymph nodes because this gave a more linear relationship between nodes and risk. Patient age, tumor size, tumor grade, estrogen receptor, progesterone receptor, proliferation (mitoses/1000), apoptotic information, HER2, and EGFR were entered as continuous variables. Nuclear 4ICD and cytoplasmic 4ICD were entered both as continuous variables and also as dichotomized variables based on their performance in the univariate model. All calculations were performed using either Stat View 5.1 or Stata 9 statistical programs. Membrane associated HER4 was not analyzed because this compartment was masked by high levels of cytosolic 4ICD expression.
Results
4ICD Is Expressed in Multiple Subcellular Compartments in Breast Tumors
We have previously demonstrated through cell fractionation experiments that 4ICD may localize in the cytosol within mitochondria and/or the cell nucleus, whereas full-length HER4 is excluded from these subcellular compartments.8,9,10,11,14 These preclinical studies were confirmed using antibodies, including HFR-1, the monoclonal antibody used in this study, directed against the carboxyl-terminus of HER4. Consistent with other studies, analysis of invasive breast carcinomas using the HFR-1 antibody identified 4ICD expression in cytosolic and nuclear subcellular compartments.19,20,21,22 Membrane staining of HER4, if present, is difficult to quantitate because the signal is masked by high levels of cytosolic 4ICD. Membrane HER4 was therefore excluded from our analysis as an independent expression compartment. Examples of HFR-1 immunohistochemistry for detection of HER4 and 4ICD in the current patient cohort have been published elsewhere.9
Of the 923 invasive breast tumors analyzed, 4ICD expression, regardless of subcellular compartment, was detected in 630 patients (68%). Of these 4ICD-positive cases, 583 (63%) expressed 4ICD within the cytosolic compartment, 214 (23%) expressed 4ICD in the nuclear compartment, and 167 (18%) expressed 4ICD in both the cytosolic and nuclear compartments. In all cases, including those that expressed both cytosolic and nuclear 4ICD, the levels of 4ICD expression within each cytosolic or nuclear compartment was scored and analyzed as an independent variable. The cutoffs used to quantitate cytosolic and nuclear 4ICD positivity were ≥25% and ≥10%, respectively as described in the Methods section. Pearson product moment correlation coefficient analysis of associations between cytosolic and nuclear 4ICD expression and common clinicopathological variables are summarized in Table 1. Separate analyses of cytosolic or nuclear 4ICD identified distinct associations with clinicopathological variables. Cytosolic 4ICD was significantly associated with various positive prognostic factors ER (r = 0.013; P = 0.0019) and progesterone receptor (PgR) (r = 0.078; P = 0.018). Consistent with the cell-killing activity of mitochondrial localized 4ICD,8,10 cytosolic 4ICD was significantly associated with tumor cell apoptosis (r = 0.101; P = 0.020). In contrast, nuclear 4ICD was significantly inversely associated with grade tumor (r = −0.073; P = 0.028), and consistent with a role for nuclear 4ICD in cellular differentiation13 nuclear 4ICD was inversely associated with mitotic count (r = −0.105; P = 0.013).
Table 1.
Pearson Product Moment Correlation Coefficients Between Cytosolic 4ICD, Nuclear 4ICD, and Clinicopathological Variables for All Patients
| Factor | Number of patients | Cytosolic 4ICD (≥25%)
|
Nuclear 4ICD (≥10%)
|
||||
|---|---|---|---|---|---|---|---|
| Correlation coefficient (r) | 95% CI | P | Correlation coefficient (r) | 95% CI | P | ||
| Patient age | 923 | −0.004 | −0.07 to 0.06 | 0.91 | 0.005 | −0.06 to 0.07 | 0.89 |
| Tumor grade | 895 | −0.024 | −0.09 to 0.04 | 0.47 | −0.073 | −0.14 to −0.01 | 0.028 |
| Tumor size | 922 | −0.029 | −0.09 to 0.04 | 0.38 | 0.004 | −0.06 to 0.07 | 0.90 |
| No. pos. LN | 923 | −0.043 | −0.11 to 0.02 | 0.19 | −0.027 | −0.09 to −0.04 | 0.42 |
| ER | 923 | 0.103 | 0.04 to 0.17 | 0.0019 | 0.047 | −0.02 to 0.11 | 0.16 |
| PgR | 923 | 0.078 | 0.01 to 0.14 | 0.018 | 0.022 | −0.04 to 0.09 | 0.50 |
| EGFR | 919 | −0.031 | −0.10 to 0.03 | 0.35 | −0.033 | −0.10 to 0.03 | 0.32 |
| HER2 | 923 | 0.028 | −0.04 to 0.09 | 0.40 | 0.022 | −0.04 to 0.09 | 0.50 |
| Mitosis* | 529 | 0.035 | −0.05 to 0.12 | 0.42 | −0.105 | −0.19 to −0.02 | 0.013 |
| Apoptosis* | 531 | 0.101 | 0.02 to 0.18 | 0.020 | −0.025 | −0.11 to 0.06 | 0.57 |
Mitosis and apoptosis recorded as number of events per 1000 tumor cells.
Bold text indicates significant values where P < 0.05 is present.
Cytosolic 4ICD Expression Is Associated with Improved Prognosis
By a univariate analysis, factors significantly associated with worse DFS and DSS in the entire patient cohort included: higher tumor grade (HR = 2.0 for both), larger tumor size (HR = 1.9 and 1.7, respectively), a greater number of affected lymph nodes (HR = 4.2 and 4.9, respectively) or the presence of lymph node involvement (HR = 2.6 and 2.8, respectively), lack of ER (HR = 0.53 and 0.54, respectively) or PgR expression (HR = 0.54 and 0.80, respectively), HER2 overexpression (HR = 1.7 and 1.9, respectively), increased mitoses/1000 cells (HR = 1.6 and 1.8, respectively), and higher tumor apoptosis (HR = 1.4 for both) (Table 2). Factors that were associated with worse DFS, but not DSS, included absence of cytosolic 4ICD (HR = 0.79) and younger patient age (HR = 0.76). In lymph node-negative patients, significant adverse prognostic factors included absence of cytosolic 4ICD or PgR expression, higher tumor grade, larger tumor size, and increased mitoses (Table 3). Lower patient age was associated with disease recurrence but not survival. In the lymph-node positive patient population, variables associated with a worse DFS and DSS have been previously described2 and included: higher tumor grade, number of affected lymph nodes, absence of ER or PgR expression, and expression of EGFR or HER2. Cytosolic 4ICD was not associated with prognosis in the lymph node-positive patient population. Nuclear 4ICD failed to emerge as a prognostic factor in any of the patient population subgroups examined.
Table 2.
Univariate Analysis of Factors Associated with DFS and DSS for All Patients
| Factor | Number of patients | DFS
|
DSS
|
||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | ||
| Cytosolic 4ICD | |||||||
| <25% | 339 | 1 | 1 | ||||
| ≥25% | 583 | 0.787 | 0.63 to 0.98 | 0.034 | 0.787 | 0.61 to 1.02 | 0.07 |
| Nuclear | |||||||
| 4ICD | |||||||
| <10% | 708 | 1 | 1 | ||||
| ≥10% | 214 | 0.881 | 0.68 to 1.15 | 0.35 | 0.810 | 0.59 to 1.11 | 0.19 |
| Patient age | |||||||
| <50 | 215 | 1 | 1 | ||||
| ≥50 | 707 | 0.764 | 0.60 to 0.98 | 0.030 | 0.898 | 0.67 to 1.21 | 0.48 |
| Tumor grade | |||||||
| 1 | 62 | 1 | 1 | ||||
| 2 | 462 | 1.179 | 0.72 to 1.93 | <0.0001 | 1.099 | 0.62 to 1.95 | <0.0001 |
| 3 | 370 | 2.039 | 1.25 to 3.32 | 2.029 | 1.16 to 3.57 | ||
| Tumor size | |||||||
| <2 cm | 473 | 1 | 1 | ||||
| ≥2 cm | 448 | 1.872 | 1.49 to 2.35 | <0.0001 | 1.706 | 1.31 to 2.23 | <0.0001 |
| Lymph nodes | |||||||
| Negative | 495 | 1 | 1 | ||||
| Positive | 427 | 2.588 | 2.06 to 3.26 | <0.0001 | 2.816 | 2.14 to 3.71 | <0.0001 |
| No. positive lymph nodes | |||||||
| 0 | 495 | 1 | 1 | ||||
| 1–3 | 237 | 1.704 | 1.29 to 2.26 | <0.0001 | 1.636 | 1.16 to 2.31 | <0.0001 |
| 4+ | 190 | 4.162 | 3.21 to 5.39 | 4.944 | 3.65 to 6.70 | ||
| ER status | |||||||
| Negative | 332 | 1 | 1 | ||||
| Positive | 586 | 0.532 | 0.45 to 0.70 | <0.0001 | 0.537 | 0.41 to 0.70 | <0.0001 |
| PgR status | |||||||
| Negative | 321 | 1 | 1 | ||||
| Positive | 601 | 0.540 | 0.43 to 0.67 | <0.0001 | 0.797 | 0.38 to 0.64 | <0.0001 |
| HER2 | |||||||
| <10% | 713 | 1 | 1 | ||||
| ≥10% | 209 | 1.699 | 1.34 to 2.16 | <0.0001 | 1.880 | 1.43 to 2.47 | <0.0001 |
| Mitosis | |||||||
| <2.3 | 251 | 1 | 1 | ||||
| ≥2.3 | 277 | 1.604 | 1.25 to 2.06 | 0.0002 | 1.783 | 1.33 to 2.39 | <0.0001 |
| Apoptosis | |||||||
| <3.58 | 261 | 1 | 1 | ||||
| ≥3.58 | 269 | 1.370 | 1.07 to 1.76 | 0.013 | 1.372 | 1.03 to 1.83 | 0.030 |
Bold text indicates significant values where P < 0.05 is present.
Table 3.
Univariate Analysis of Factors Associated with DFS and DSS for Lymph Node-Negative Patients
| Factor | Number of patients | DFS
|
DSS
|
||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | ||
| Cytosolic 4ICD | |||||||
| <25% | 185 | 1 | 1 | ||||
| ≥25% | 310 | 0.667 | 0.6 to 0.97 | 0.032 | 0.537 | 0.34 to 0.85 | 0.007 |
| Nuclear | |||||||
| 4ICD | |||||||
| <10% | 374 | 1 | 1 | ||||
| ≥10% | 121 | 0.842 | 0.54 to 1.31 | 0.44 | 0.673 | 0.38 to 1.19 | 0.17 |
| Patient age | |||||||
| <50 | 101 | 1 | 1 | ||||
| ≥50 | 394 | 0.620 | 0.41 to 0.93 | 0.020 | 0.713 | 0.43 to 1.18 | 0.19 |
| Tumor grade | |||||||
| 1 | 35 | 1 | 1 | ||||
| 2 | 269 | 0.664 | 0.32 to 1.37 | <0.0001 | 0.853 | 0.34 to 2.12 | 0.004 |
| 3 | 173 | 1.668 | 0.83 to 3.37 | 1.859 | 0.76 to 4.54 | ||
| Tumor size | |||||||
| <2 cm | 293 | 1 | 1 | ||||
| ≥2 cm | 202 | 2.227 | 1.53 to 3.25 | <0.0001 | 1.983 | 1.25 to 3.15 | 0.003 |
| ER status | |||||||
| Negative | 164 | 1 | 1 | ||||
| Positive | 328 | 0.687 | 0.47 to 1.00 | 0.05 | 0.761 | 0.48 to 1.21 | 0.25 |
| PgR status | |||||||
| Negative | 161 | 1 | 1 | ||||
| Positive | 334 | 0.539 | 0.37 to 0.78 | 0.001 | 0.485 | 0.31 to 0.77 | 0.002 |
| HER2 | |||||||
| <10% | 401 | 1 | 1 | ||||
| ≥10% | 94 | 1.201 | 0.77 to 1.87 | 0.41 | 1.166 | 0.69 to 1.99 | 0.57 |
| Mitosis | |||||||
| <2.3 | 146 | 1 | 1 | ||||
| ≥2.3 | 111 | 1.694 | 1.11 to 2.59 | 0.014 | 1.672 | 1.00 to 2.80 | 0.048 |
| Apoptosis | |||||||
| <3.58 | 138 | 1 | 1 | ||||
| ≥3.58 | 119 | 1.377 | 0.90 to 2.11 | 0.14 | 1.023 | 0.61 to 1.71 | 0.93 |
Bold text indicates significant values where P < 0.05 is present.
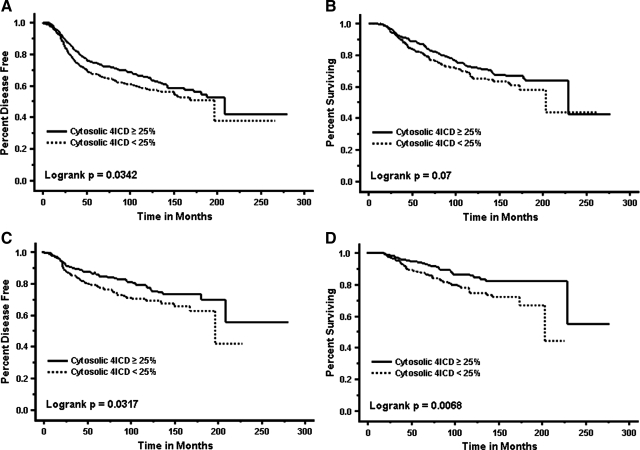
To illustrate the impact of cytosolic or nuclear 4ICD expression on patient outcome, we generated Kaplan-Meier curves of different patient populations. For all patients (n = 923), cytosolic 4ICD expression was significantly associated with an increased time to first recurrence (P = 0.032) but had no impact on patient survival (Figure 1, A and B). Patients with high nuclear 4ICD expression had DFS and DSS similar to patients with low 4ICD expression altogether (data not shown). In lymph node-negative patients (n = 496), high cytosolic 4ICD expression was significantly associated with an extended time to disease progression (P = 0.0317) and patient survival (P = 0.0068) (Figure 1, C and D). Nuclear 4ICD had no significant impact on DFS or DSS at any cut point tested. In the lymph node-positive subset, neither nuclear nor cytoplasmic 4ICD were associated with DFS or DSS.
Figure 1.
Kaplan Meier survival curves for disease-free survival and disease-specific survival. A and B: All patients: cytosolic 4ICD positive (solid line; n = 582) and cytosolic 4ICD negative (dashed line; n = 339). C and D: Lymph node-negative patients: cytosolic 4ICD positive (solid line; n = 310) and cytosolic 4ICD negative (dashed line; n = 186).
Cytosolic 4ICD Expression Is an Independent Prognostic Marker
In a multivariate analysis of variables associated with outcome for all patients, the best-fit baseline model for DFS included the significant variables: number of positive lymph nodes, tumor size, ER status, and EGFR expression. For DSS the significant variables included: number of positive lymph nodes, tumor size, PgR status and HER2 expression. When added to these baselines 4ICD expression failed to add significance to this patient group (Table 4). Expression of 4ICD also failed to add significance to the lymph node-positive subgroup of patients (Table 4).
Table 4.
Multivariate Analysis of Factors Associated with DFS and DSS
| Factors | Number of patients | Number of events | X2 | ΔX2 | P |
|---|---|---|---|---|---|
| All patients, DFS | 909 | 317 | 185.68 | 0.53 | 0.47 |
| Base = logLN + Size + ER + EGFR | 186.21 | 0.41 | 0.52 | ||
| Base + cytosolic 4ICD ≥25% | 186.09 | ||||
| Base + nuclear 4ICD ≥10% | |||||
| All patients, DSS | 912 | 228 | 160.53 | 1.06 | 0.30 |
| Base = logLN + size + PgR + HER2 | 161.58 | 0.01 | 0.92 | ||
| Base + cytosolic 4ICD ≥25% | 160.54 | ||||
| Base + nuclear 4ICD ≥10% | |||||
| Lymph node-negative patients, DFS | 257 | 86 | 23.93 | 4.47 | 0.035 |
| Base = size + mitosis/1000 cells | 28.40 | 0.19 | 0.66 | ||
| Base + cytosolic 4ICD ≥25% | 24.12 | ||||
| Base + nuclear 4ICD ≥10% | |||||
| Lymph node-negative patients, DSS | 481 | 74 | 18.24 | 8.41 | <0.004 |
| Base = size + grade | 23.79 | 2.04 | 0.15 | ||
| Base + cytosolic 4ICD ≥25% | 19.72 | ||||
| Base + nuclear 4ICD ≥10% | |||||
| Lymph node-positive patients, DFS | 425 | 206 | 87.24 | 0.01 | 0.93 |
| Base = logLN + size + EGFR + HER2 | 87.28 | 0.27 | 0.61 | ||
| Base + cytosolic 4ICD ≥25% | 88.74 | ||||
| Base + nuclear 4ICD ≥10% | |||||
| Lymph node-positive patients, DSS | 424 | 154 | 86.64 | 0.99 | 0.32 |
| Base = logLN + EGFR + HER2 | 87.63 | 0.27 | 0.60 | ||
| Base + cytosolic 4ICD ≥25% | 86.91 | ||||
| Base + nuclear 4ICD ≥10% |
Bold text indicates significant values where P < 0.05 is present.
In the lymph node-negative patient subgroup, the baseline for DFS included tumor size and mitosis and the baseline for DSS included the variables tumor size and grade. Interestingly, when added to these baselines to determine significance, cytosolic, but not nuclear, 4ICD expression added significance to both baseline models and emerged as an independent marker for improved DFS (P = 0.035) and DSS (P < 0.004) in lymph node-negative breast cancer patients (Table 4).
Discussion
The biological influence of HER4 expression on the natural history of breast cancer and its impact on the clinical outcome of breast cancer patients remains controversial.6 Interpretation of HER4 expression studies is complicated by HER4 proteolytic processing to generate the independently signaling intracellular domain, 4ICD. 4ICD localizes within multiple tumor cell subcellular compartments and these different 4ICD subpopulations directly regulate divergent cellular responses.7 For example, we have shown that mitochondrial localized 4ICD induces tumor cell apoptosis via an intrinsic pro-apoptotic BH3 domain,8,9,10 whereas nuclear 4ICD functions as a transcriptional coactivator that regulates gene expression to promote cell proliferation or differentiation.11,13 This is the first correlative study to account for the functional diversity of 4ICD signaling activities by examining the impact of 4ICD tumor expression in independent cytosolic and nuclear subcellular compartments. In concordance with our functional data we demonstrate that cytosolic, but not nuclear 4ICD, is associated with tumor cell apoptosis. By a multivariate analysis cytosolic 4ICD emerged as an independent positive prognostic factor in lymph node-negative invasive breast cancer patients.
The management of lymph-node negative breast cancer patients poses significant challenges, as few markers are available to identify which of these patients may need aggressive treatment with chemotherapy. Even patients diagnosed with lymph-node negative cancers with large primary tumors have a 5-year survival rate of 75% or better, therefore, the role of adjuvant systemic chemotherapy in this group remains controversial. Up to 25% of node-negative patients, however, may still benefit from adjuvant systemic chemotherapy.23,24,25 At present, tumor grade and size are the primary clinicopathological parameters commonly used to guide therapeutic decisions in node-negative patients, although recent molecular assays, including OncoTypeDx, may also provide important data to guide decision making in this patient subgroup. Nevertheless, selection of the patient group at high risk for recurrence becomes problematic. Our results suggest that cytosolic expression of 4ICD represents a new prognostic marker in patients with lymph node-negative disease, which can be easily implemented in the breast cancer clinic to further stratify this patient group.
Several reports have suggested conflicting roles for nuclear 4ICD as an indicator of patient outcome, which has led to recent confusion in the literature. Preclinical data indicates that nuclear 4ICD cooperates with signal transducer and activator of transcription 5A or ERα to promote differentiation13,14 or estrogen stimulated breast tumor cell proliferation,11 respectively. However, the nuclear 4ICD activity that predominates during breast carcinogenesis remains unclear. For example, one recent report suggests that nuclear 4ICD predicts a worse patient outcome than membrane/cytosolic HER4/4ICD.19 However, when we examine nuclear 4ICD expression in the context of the general breast cancer population a more accurate and clinically useful representation of the data emerges. In contrast to cytosolic 4ICD, nuclear 4ICD expression failed to provide significant prognostic value by either univariate or multivariate analyses. In fact, in each patient subpopulation examined, nuclear 4ICD expression had a similar insignificant impact on patient relapse and survival as patients lacking HER4/4ICD expression altogether. Our results indicate that nuclear 4ICD fails to provide useful prognostic information.
Although lacking prognostic value, recent evidence suggests that nuclear 4ICD may predict improved outcome for patients treated with adjuvant tamoxifen as a single agent.9 Tamoxifen appears to disrupt the 4ICD/ERα transcriptional complex thereby releasing nuclear 4ICD into the cytosol where the cell killing activity of mitochondrial 4ICD predominates.9 These results corroborate recent studies where HER4 loss emerged as an independent marker for tamoxifen resistance.26 Interestingly, full-length HER4 also appears to localize to the nucleus of breast tumor cells, which, in contrast to nuclear 4ICD, predicts worse outcome for tamoxifen treated patients.22 Although this intriguing observation requires confirmation, it nevertheless implies that full-length HER4 may have important nuclear functions, which differ from nuclear 4ICD. Unfortunately, none of the clinical studies to date effectively address the ability of HER4 or 4ICD expression to predict clinical response to specific therapeutic interventions. In nearly all studies the sample size is too small, discrimination between different functional populations of 4ICD is not adequately addressed, or, as in the case of the current study, the therapeutic regimens used would be considered suboptimal by current standards.
Because breast cancer patients appear to benefit from 4ICD associated apoptosis, the use of pan-EGFR-family inhibitors currently in clinical trials should be approached with caution. More specifically, the impact of suppressed 4ICD signaling needs to be considered in this clinical setting. In fact, our results suggest that the use of drugs that directly or indirectly affect 4ICD signaling may not be beneficial. At best, patients with tumor expression of 4ICD will not receive benefit from HER4 inhibition, but may in fact be at higher risk of disease recurrence and reduced survival as a result of 4ICD inactivation.
In summary, our results provide the first clinical evidence that 4ICD expression has independent prognostic value. Specifically, cytosolic 4ICD expression emerged in a multivariate analysis as a positive prognostic factor predicting improved disease-free and overall survival for lymph node-negative breast cancer patients. With recent advances in early screening techniques we anticipate the proportion of women diagnosed with lymph node-negative breast cancer to exceed 60%. The availability of cytosolic 4ICD as an independent tumor marker predicting improved prognosis may allow further stratification of lymph-node positive patients for optimized disease management. Indeed, these patients pose a particular clinical challenge and currently are not stratified for adjuvant systemic chemotherapy when some of these patients would clearly benefit from early aggressive treatment. Because 4ICD expression predicts improved response to hormone therapy,9,26 4ICD expression may be used to identify lymph-node negative and ER-positive patients that would benefit from hormone therapy alone whereas patients lacking tumor expression of 4ICD may be better served with a therapeutic regimen including adjuvant systemic chemotherapy. Our results also suggest that treatment of lymph node-negative patients with tumor expression of 4ICD, with therapeutics that abrogate HER4 or 4ICD signaling may be harmful, potentially increasing recurrences and reducing survival in this patient group. The benefit of sustained 4ICD activity should be an important consideration as pan-EGFR-family and HER4 targeted therapies are introduced into the breast cancer clinic.
Acknowledgments
We thank members of Dr. Jones’ and Dr. Thor’s laboratories for critical input during the development and completion of this project.
This work is dedicated, with the hope of better days to come, to June Allison, who is contending with recurrent node-negative breast cancer.
Footnotes
Address reprint requests to Frank E. Jones, Department of Cell and Molecular Biology, Tulane University, 6400 Freret St., New Orleans, Louisiana, 70118. E-mail: fjones3@tulane.edu.
Supported by National Cancer Institute/National Institutes of Health Grants RO1CA95783 (F.E.J.) and RO1CA96717 (F.E.J.).
References
- Hynes NE, Stern DF. The biology of erbB-2/neu/HER-2 and its role in cancer. Biochim Biophys Acta. 1994;1198:165–184. doi: 10.1016/0304-419x(94)90012-4. [DOI] [PubMed] [Google Scholar]
- DiGiovanna MP, Stern DF, Edgerton SM, Whalen SG, Moore D, 2nd, Thor AD. Relationship of epidermal growth factor receptor expression to ErbB-2 signaling activity and prognosis in breast cancer patients. J Clin Oncol. 2005;23:1152–1160. doi: 10.1200/JCO.2005.09.055. [DOI] [PubMed] [Google Scholar]
- Liu B, Ordonez-Ercan D, Fan Z, Edgerton SM, Yang X, Thor AD. Downregulation of erbB3 abrogates erbB2-mediated tamoxifen resistance in breast cancer cells. Int J Cancer. 2007;120:1874–1882. doi: 10.1002/ijc.22423. [DOI] [PubMed] [Google Scholar]
- Suo Z, Risberg B, Kalsson MG, Willman K, Tierens A, Skovlund E, Nesland JM. EGFR family expression in breast carcinomas. c-erbB-2 and c-erbB-4 receptors have different effects on survival. J Pathol. 2002;196:17–25. doi: 10.1002/path.1003. [DOI] [PubMed] [Google Scholar]
- Witton CJ, Reeves JR, Going JJ, Cooke TG, Bartlett JM. Expression of the HER1–4 family of receptor tyrosine kinases in breast cancer. J Pathol. 2003;200:290–297. doi: 10.1002/path.1370. [DOI] [PubMed] [Google Scholar]
- Sundvall M, Iljin K, Kilpinen S, Sara H, Kallioniemi OP, Elenius K. Role of ErbB4 in breast cancer. J Mammary Gland Biol Neoplasia. 2008;13:259–268. doi: 10.1007/s10911-008-9079-3. [DOI] [PubMed] [Google Scholar]
- Jones FE. HER4 intracellular domain (4ICD) activity in the developing mammary gland and breast cancer. J Mammary Gland Biol Neoplasia. 2008;13:247–258. doi: 10.1007/s10911-008-9076-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naresh A, Long W, Vidal GA, Wimley WC, Marrero L, Sartor CI, Tovey S, Cooke TG, Bartlett JMS, Jones FE. The ERBB4/HER4 intracellular domain 4ICD is a BH3-only protein promoting apoptosis of breast cancer cells. Cancer Res. 2006;66:6412–6420. doi: 10.1158/0008-5472.CAN-05-2368. [DOI] [PubMed] [Google Scholar]
- Naresh A, Thor AD, Edgerton SM, Torkko KC, Kumar R, Jones FE. The HER4/4ICD estrogen receptor coactivator and BH3-only protein is an effector of tamoxifen-induced apoptosis. Cancer Res. 2008;68:6387–6395. doi: 10.1158/0008-5472.CAN-08-0538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal GA, Naresh A, Marrero L, Jones FE. Presenilin-dependent γ-secretase processing regulates multiple ERBB4/HER4 activities. J Biol Chem. 2005;280:19777–19783. doi: 10.1074/jbc.M412457200. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Sullivan LL, Nair SS, Williams CC, Pandey A, Marrero L, Vadlamudi RK, Jones FE. Coregulation of estrogen receptor by estrogen-inducible ERBB4/HER4 establishes a growth promoting autocrine signal in breast cancer. Cancer Res. 2006;66:7991–7998. doi: 10.1158/0008-5472.CAN-05-4397. [DOI] [PubMed] [Google Scholar]
- Long W, Wagner K-U, Lloyd KCK, Binart N, Shillingford JM, Hennighausen L, Jones FE. Impaired differentiation and lactational failure in ErbB4-deficient mammary glands identify ERBB4 as an obligate mediator of Stat5. Development. 2003;130:5257–5268. doi: 10.1242/dev.00715. [DOI] [PubMed] [Google Scholar]
- Muraoka-Cook RS, Sandahl M, Husted C, Hunter D, Miraglia L, Feng SM, Elenius K, Earp HS., 3rd The intracellular domain of ErbB4 induces differentiation of mammary epithelial cells. Mol Biol Cell. 2006;17:4118–4129. doi: 10.1091/mbc.E06-02-0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams CC, Allison JG, Vidal GA, Burow ME, Beckman BS, Marrero L, Jones FE. The ERBB4/HER4 receptor tyrosine kinase regulates gene expression by functioning as a STAT5A nuclear chaperone. J Cell Biol. 2004;167:469–478. doi: 10.1083/jcb.200403155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones RB, Gordus A, Krall JA, MacBeath G. A quantitative protein interaction network for the ErbB receptors using protein microarrays. Nature. 2006;439:168–174. doi: 10.1038/nature04177. [DOI] [PubMed] [Google Scholar]
- Liu S, Edgerton SM, Moore DH, 2nd, Thor AD. Measures of cell turnover (proliferation and apoptosis) and their association with survival in breast cancer. Clin Cancer Res. 2001;7:1716–1723. [PubMed] [Google Scholar]
- Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long term follow-up. Histopathol. 1991;19:403–410. doi: 10.1111/j.1365-2559.1991.tb00229.x. [DOI] [PubMed] [Google Scholar]
- Thor AD, Liu S, Edgerton S, II DM, Kasowitz KM, Benz CC, Stern DF, DiGiovanna MP. Activation (tyrosine phosphorylation) of ErbB-2 (HER-2/neu): a study of incidence and correlation with outcome in breast cancer. J Clin Oncol. 2000;18:3230–3239. doi: 10.1200/JCO.2000.18.18.3230. [DOI] [PubMed] [Google Scholar]
- Junttila TT, Sundvall M, Lundin M, Lundin J, Tanner M, Harkonen P, Joensuu H, Isola J, Elenius K. Cleavable ErbB4 isoform in estrogen receptor-regulated growth of breast cancer cells. Cancer Res. 2005;65:1384–1393. doi: 10.1158/0008-5472.CAN-04-3150. [DOI] [PubMed] [Google Scholar]
- Kew TY, Bell JA, Pinder SE, Denley H, Srinivasan R, Gullick WJ, Nicholson RI, Blamey RW, Ellis IO. c-erbB-4 protein expression in human breast cancer. Br J Cancer. 2000;82:1163–1170. doi: 10.1054/bjoc.1999.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan R, Gillett CE, Barnes DM, Gullick WJ. Nuclear expression of the c-erbB-4/HER4 growth factor receptor in invasive breast cancers. Cancer Res. 2000;60:1483–1487. [PubMed] [Google Scholar]
- Tovey SM, Dunne B, Witton CJ, Cooke TG, Bartlett JM. HER4 in breast cancer: comparison of antibodies against intra- and extra-cellular domains of HER4. Breast Cancer Res. 2006;8:R19. doi: 10.1186/bcr1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanrahan EO, Valero V, Gonzalez-Angulo AM, Hortobagyi GN. Prognosis and management of patients with node-negative invasive breast carcinoma that is 1 cm or smaller in size (stage 1; T1a,bN0M0): a review of the literature. J Clin Oncol. 2006;24:2113–2122. doi: 10.1200/JCO.2005.02.8035. [DOI] [PubMed] [Google Scholar]
- Mirza AN, Mirza NQ, Vlastos G, Singletary SE. Prognostic factors in node-negative breast cancer: a review of studies with sample size more than 200 and follow-up more than 5 years. Ann Surg. 2002;235:10–26. doi: 10.1097/00000658-200201000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quiet CA, Ferguson DJ, Weichselbaum RR, Hellman S. Natural history of node-negative breast cancer: a study of 826 patients with long-term follow-up. J Clin Oncol. 1995;13:1144–1151. doi: 10.1200/JCO.1995.13.5.1144. [DOI] [PubMed] [Google Scholar]
- Guler G, Iliopoulos D, Guler N, Himmetoglu C, Hayran M, Huebner K. Wwox and Ap2γ expression levels predict tamoxifen response. Clin Cancer Res. 2007;13:6115–6121. doi: 10.1158/1078-0432.CCR-07-1282. [DOI] [PubMed] [Google Scholar]