Abstract
Mutations in the gene DYSF, which codes for the protein dysferlin, underlie Miyoshi myopathy and limb-girdle muscular dystrophy 2B in humans and produce a slowly progressing skeletal muscle degenerative disease in mice. Dysferlin is a Ca2+-sensing, regulatory protein that is involved in membrane repair after injury. To assess the function of dysferlin in healthy and dystrophic skeletal muscle, we generated skeletal muscle–specific transgenic mice with threefold overexpression of this protein. These mice were phenotypically indistinguishable from wild-type, and more importantly, the transgene completely rescued the muscular dystrophy (MD) disease in Dysf-null A/J mice. The dysferlin transgene rescued all histopathology and macrophage infiltration in skeletal muscle of Dysf−/− A/J mice, as well as promoted the rapid recovery of muscle function after forced lengthening contractions. These results indicate that MD in A/J mice is autonomous to skeletal muscle and not initiated by any other cell type. However, overexpression of dysferlin did not improve dystrophic symptoms or membrane instability in the dystrophin-glycoprotein complex–lacking Scgd (δ-sarcoglycan) null mouse, indicating that dysferlin functionality is not a limiting factor underlying membrane repair in other models of MD. In summary, the restoration of dysferlin in skeletal muscle fibers is sufficient to rescue the MD in Dysf-deficient mice, although its mild overexpression does not appear to functionally enhance membrane repair in other models of MD.
The muscular dystrophies (MD) are a diverse group of genetic muscle wasting disorders that typically result in premature death due to cardiac or respiratory failure.1 Most characterized mutations in humans that cause MD result from alterations in structural proteins that connect the underlying contractile proteins to the basal lamina, providing rigidity to the skeletal muscle cell membrane, or in proteins that directly stabilize or repair the cell membrane.1,2 For example, loss of dystrophin in Duchenne’s MD or mutations in other components of the dystrophin-glycoprotein complex (DGC) leads to a fundamental alteration in the physical properties of the sarcolemma, permitting contraction-induced microtears and the unregulated exchange of ions such as Ca2+, leading to necrosis and degeneration of myofibers.1,2 The DGC is a multisubunit complex organized at the sarcolemma that links the underlying contractile proteins to the extracellular matrix, providing the sarcolemma with cytoskeletal support and protecting it from damage incurred during the contractile cycle. The DGC contains structural proteins such as dystrophin, dystroglycans, sarcoglycans, dystrobrevin, sarcospan, and syntrophins, as well as a number of signaling proteins.1,2 That membrane instability and aberrant repair capacity underlie myofiber degeneration in MD was further suggested by the observation that mutations in the putative membrane repair protein dysferlin cause limb girdle muscular dystrophy 2B and Miyoshi myopathy.3 Limb girdle muscular dystrophy 2B/Miyoshi myopathy typically presents in early adulthood or the late teen years, and muscle biopsies from these patients show a striking inflammatory response.3
Dysferlin is a 230 kDa, Ca2+ sensitive protein that participates in membrane resealing events following injury, but does not directly interact with components of the DGC. Mice lacking dysferlin (Dysf) exhibit progressive disease in skeletal muscle and cardiac tissue despite having a functional DGC, which is characterized by myofiber necrosis, cycles of degeneration and regeneration, inflammation, and adipocyte replacement.4,5 Lack of dysferlin also results in the accumulation of vesicles and structural membrane defects as analyzed by electron microscopy, suggesting a role in normal membrane turnover and recycling.4,6
Disease in dystrophic skeletal muscle is dramatically influenced by the inflammatory response, achieved mainly by infiltration of cytotoxic T-lymphocytes and macrophages.7,8 It has been suggested that Dysf-null inflammatory cells may initiate the disease process in the muscles of Dysf-null mice and humans, as these immune cells appear to be hyperactive and abnormal.9,10,11 For example, macrophages and dendritic-T cell activation markers are elevated in the SJL mouse model for Dysf deficiency and in human limb girdle muscular dystrophy 2B.9 Thus, it is unclear whether disease due to dysferlin deficiency is primarily due to an autonomous effect in immune cells or skeletal muscle fibers. To address this issue, we generated transgenic mice that express dysferlin specifically in skeletal muscle and used them to evaluate the necessity of dysferlin within myofibers to initiate muscle disease in Dysf null A/J mice. Moreover, we assessed the ability of increased dysferlin expression to alleviate pathology in a MD model that lacks a component of the DGC, Scgd (δ-sarcoglycan).
Materials and Methods
Animals
A/J mice, a strain with a homozygous retrotransposon insertion in the Dysf gene described previously,12 were purchased from Jackson Laboratories (Bar Harbor, ME). To generate dysferlin transgenic mice (Dysf-TG) we fused human dysferlin cDNA (88% identical to mouse) to the human α-skeletal actin promoter with an upstream troponin I slow fiber-type enhancer (generously provided by Edna C. Hardeman, University of Sydney). Dysferlin transgenic mice (C57BL/6 background) were bred with A/J and Scgd null mice and both male and female littermates were used for all analyses. Crossing A/J mice with Dysf-TG mice generated heterozygous F1 mice that were subsequently intercrossed (F2) to generate homozygous Dysf-null mice, Dysf-null mice with the transgene, and Dysf wild-type controls with and without the transgene (A/J-C57BL/6 background). Genotyping for the Dysf mutant allele was performed exactly as described previously.12 All animal experiments were approved by the Institutional Animal Care and Use Committee.
Western Blotting and Immunohistochemistry
Expression of the dysferlin transgene was detected in immunoblots of extracts of muscle proteins prepared by homogenization in cell lysis buffer (20 mmol/L Tris [pH 7.4], 137 mmol/L NaCl, 25 mmol/L β-glycerophosphate, 2 mmol/L sodium pyrophosphate, 2 mmol/L EDTA, 1 mmol/L sodium orthovanadate, 1% Triton X-100, 10% glycerol, 1 mmol/L phenylmethanesulphonylfluoride, 5 μg/ml leupeptin, 5 μg/ml aprotinin, and 2 mmol/L benzamide). Extracts were centrifuged at 13,000 × g for 10 minutes and 20 μg of protein were separated on a SDS-5% polyacrylamide gel, for subsequent Western blotting by chemiluminescence (GE Health Care Life Sciences). A dysferlin polyclonal antibody from Orbigen (San Diego, CA) was used at 1:500. Immunohistochemistry was performed on 5 μm cryosections using the same dysferlin antibody at 1:50 dilution. Immunostaining for macrophage content with Mac-3 antibody (1:100, BD Pharmingen, San Jose, CA) and TO-PRO-3 iodine nuclei blue-labeling (Molecular Probes, Carlsbad, CA) was performed on 5 μm paraffin sections. A secondary antibody conjugated to fluorescein isothiocyanate was used to visualize Mac-3 (green).
Muscle Weights and Histological Analyses
Muscle weights were normalized to tibia length. Muscles were paraffin-embedded and sections (5 μm) were cut at the center of the muscle and stained with either H&E or Masson’s trichrome. H&E-stained sections were analyzed using ImageJ software for the number of centrally placed nuclei. At least 400 fibers were counted for each muscle from every animal. Fibrosis was quantitated using MetaMorph analysis of blue staining in Masson’s trichrome sections.
Exercise and Evan’s Blue Dye Uptake
Evan’s blue dye (EBD, 10 mg/ml) was injected i.p. (0.1 ml/10g body weight) and the mice were sacrificed approximately 18 hours later. Quadriceps were embedded in optimal cutting temperature compound (Tissue-Tek) and snap-frozen in liquid nitrogen. Sectioning, staining, and viewing were done exactly as described previously.13 Wheat-germ agglutinin conjugated to fluorescein isothiocyanate (Sigma) was used to visualize membranes (green). To stress the sarcolemma and enhance leakiness, animals were exercised by free access to running wheels for 6 days. On the sixth day, mice were injected with EBD as described above, allowed to run overnight, then sacrificed 18 hours postinjection. Serum was also sampled at this time for levels of creatine kinase (CK). All groups of mice underwent similar levels of exercise, assessed by counting the number of revolutions during the night before harvest.
Injury and Assessment of Contractile Function
We studied the loss and recovery of contractile function after inducing an injury to the ankle dorsiflexors by large strain lengthening contractions performed in situ as described previously.14
Statistical Analysis
Results are presented as means ± SEM. For all examinations a one-way analysis of variance was used with a Student-Newman-Kuels post hoc test. We considered values significant when P < 0.05.
Results
Generation and Characterization of Dysf-TG Mice
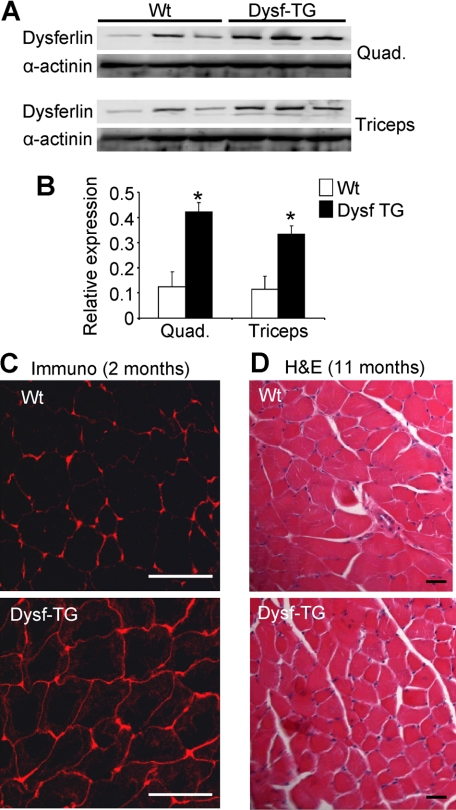
Human dysferlin was expressed under the control of the human skeletal α-actin promoter.15 One usable line was generated from transgene injections, which showed 3- to 3.5-fold overexpression in the quadriceps and triceps muscles (Figure 1, A and B). Increased dysferlin expression was not detected in nonskeletal muscle tissues such as kidney, liver, brain, lungs, spleen, and heart (data not shown). Dysferlin has been shown to reside at the plasma membrane in skeletal muscle, as well as some association with cytoplasmic vesicles.4,16 Immunohistochemistry for dysferlin in sections of quadriceps muscle showed the protein to be predominantly localized at and near the sarcolemma of Dysf-TG mice, similar to wild-type (Figure 1C). Routine H&E staining of the quadriceps of Dysf-TG mice at 11 months of age showed no histopathology associated with mild dysferlin overexpression (Figure 1D). In addition, no tissue pathology was observed in the diaphragm, gastrocnemius, tibialis anterior (TA), triceps, or soleus at ages ranging from 6 weeks to 11 months (data not shown). Dysf-TG mice also showed identical muscle weights to nontransgenic controls, indicating that the transgene did not induce skeletal muscle hypertrophy or atrophy (data not shown). Thus, mild overexpression of dysferlin in skeletal muscle was achieved with no detectable histological untoward effects.
Figure 1.
Characterization of dysferlin transgenic mice. A: Western blot analysis from triceps and quadriceps for dysferlin protein in wild-type (Wt) and dysferlin (Dysf) transgenic (TG) mice. α-Actinin was used as a loading control. B: Quantitation of protein levels from the Western blots shown in (A). *P < 0.05 versus wild-type. C: Immunohistochemistry for dysferlin showing increased membrane staining in Dysf-TG mice, as compared with wild-type. Scale bars = 100 μm. D: H&E-stained quadriceps muscle sections from the indicated mice at 11 months of age, revealing no baseline disease in Dysf-TG mice. Scale bars = 50 μm.
Dysf-TG Mice Rescue Muscle Disease in Dysferlin Lacking A/J Mice
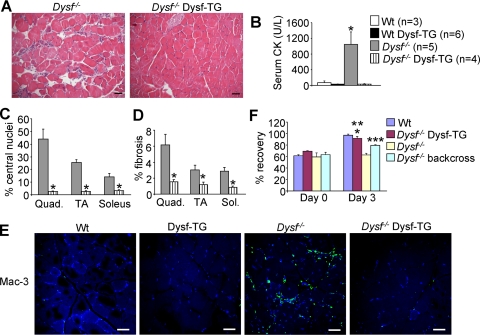
It has been hypothesized that hyperactivation of inflammatory/immune cells may underlie skeletal muscle disease in Dysf deficient MD.9,10 To directly test this hypothesis we crossed the skeletal muscle-specific Dysf-TG mouse (C57Bl/6 background) with A/J mice, a mouse strain that develops MD due to a mutation in the Dysf gene, which results in the absence of expression of the dysferlin protein.12 To assess skeletal muscle disease in these mice, we quantified a number of common dystrophic parameters, such as central nucleation, fibrosis, distribution of myofiber cross-sectional areas, macrophage infiltration, functional recovery from injury, and total serum CK levels (Figure 2). At 8 months of age, histological analysis from H&E-stained sections showed abundant pathology in the quadriceps of Dysf−/− mice, moderate pathology in the TA, and low but detectable pathology in the soleus (Figure 2, A, C, and D), as evidenced by central nucleation and fibrosis. By comparison, no central nucleation or fibrosis was detected in the quadriceps, TA, or soleus of Dysf−/− Dysf-TG mice at 8 months of age (Figure 2, A, C, and D), similar to C57Bl/6 wild-type muscle (data not shown). Careful analysis of cross-sectional areas also showed a significant increase in the percentage of small myofibers in the quadriceps of Dysf−/− mice compared with controls and the “rescued” values in Dysf−/− Dysf-TG mice (P < 0.05, data not shown). Since higher levels of smaller fibers are associated with disease due to ongoing regeneration, it further suggested that the Dysf-TG mitigated disease in Dysf−/− mice. Indeed, plasma levels of muscle-specific CK were dramatically elevated in Dysf−/− mice and completely normalized to wild-type levels in Dysf−/− Dysf-TG mice at 8 months of age (Figure 2B). We repeated these analyses at 11 months of age, also including another proximal muscle (triceps), which once again showed a near complete rescue of skeletal muscle histopathology and CK serum levels in Dysf−/− mice that contained the Dysf-TG compared with Dysf−/− alone (see supplemental Figure S1, http://ajp.amjpathol.org). Moreover, the prominent infiltration of macrophages (green staining) prominently observed in Dysf−/− skeletal muscle was prevented by the skeletal muscle-specific Dysf-TG (Figure 2E).
Figure 2.
Dysf-TG mice rescue pathology in Dysf−/− A/J mice. A: H&E-stained sections from quadriceps in Dysf−/− and Dysf−/− Dysf-TG mice at 8 months of age. Scale bars = 50 μm. B: Levels of creatine kinase (CK) in the indicated cohorts of mice at 8 months of age. *P < 0.05 versus wild-type (Wt). C: Central nucleation, shown as a percentage of total fibers, from the quadriceps, TA, and soleus in Dysf−/− and Dysf−/− Dysf-TG mice. *P < 0.05 versus Dysf−/−. D: Percentage of fibrosis in the indicated muscles from Dysf−/− and Dysf−/− Dysf-TG mice. *P < 0.05 versus Dysf−/−. E: Immunostaining for Mac-3 antibody (green) and TO-PRO-3 nuclei staining (blue) from the quadriceps of the indicated groups of mice at 11 months of age. Scale bars = 100 μm. F: Dorsiflexor muscles were injured by 15 lengthening contractions at day 0, showing a loss of torque in all four groups shown (graphed as % of maximum before injury). Three days later Dysf−/− A/J mice remained compromised in function, yet the Dysf-TG fully rescued function in the null background. *P < 0.05 versus Dysf−/− backcross or Dysf−/− A/J; **P < 0.05 versus day 0; ***P < 0.05 versus day 0 Dysf−/− backcross.
Finally, we also examined functional recovery from injury to the ankle dorsiflexor muscles following 15 successive lengthening contractions, a protocol that shows no recovery in A/J mice lacking dysferlin after 3 days, but full recovery in wild-type mice in the closely related strain A/WySnJ (Figure 2F). Replacement of dysferlin in the A/J background with the transgene gave full recovery of muscle function after 3 days (Figure 2F). The backcross between the A/J mice and the Dysf-TG (C57Bl/6) produced a hybrid background that showed partial recovery at 3 days, although recovery was still significantly depressed as compared with wild-type A/WySnJ background controls (Figure 2F). These results strongly suggest that loss of dysferlin in muscle itself is the primary cause of MD in Dysf-deficient A/J mice, and that other Dysf-deficient cells present in these crossed mice, such as macrophages and T-cells, do not initiate MD.
Dysferlin Transgenesis Does Not Improve MD in Scgd−/− Mice
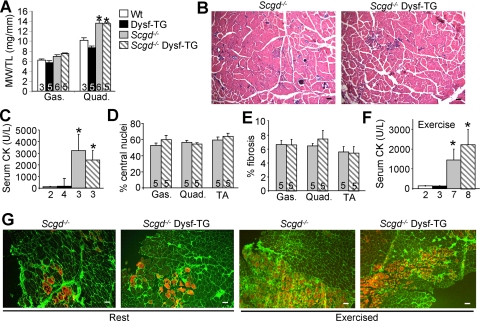
Many forms of MD are caused by mutations in proteins that reside within the DGC, resulting in sarcolemma instability and rupture with contraction. We hypothesized that increasing the repair capacity of the sarcolemmal might mitigate disease in forms of MD associated with defects in the DGC. Overexpression of dysferlin appeared to be a logical approach given its putative function in membrane repair and its ability to rescue disease in A/J mice. Hence, we crossed Dysf-TG mice with mice lacking Scgd to assess the potential protective effect of dysferlin in DGC-dependent MD. In contrast to our prediction, dysferlin transgenesis did not alleviate any dystrophic features in Scgd−/− mice. For example, at 6 months of age, the pseudohypertrophy (a process largely attributed to the excessive inflammation, regeneration, and connective tissue replacement) remained unchanged in Scgd−/− and Scgd−/− Dysf-TG quadriceps (Figure 3A). Histological analysis of pathology also showed no differences in any muscle at 6 weeks or 6 months of age (Figure 3B, and data not shown). Plasma CK levels, measured at 6 months of age, were also similarly increased in Scgd−/− mice and in Scgd−/− mice expressing the dysferlin transgene (Figure 3C). Finally, the percentage of fibers containing central nuclei and the fibrotic index did not differ in the gastrocnemius, quadriceps, and TA from the two experimental groups (Figure 3, D and E). Wild-type and Dysf-TG showed essentially no central nucleation or fibrosis in any skeletal muscle examined (data not shown).
Figure 3.
Scgd−/− mice do not show improvement with dysferlin transgenesis. A: Muscle weight to tibia length ratios of gastrocnemius and quadriceps at 6 months of age. *P < 0.05 versus wild-type (Wt). B: Representative H&E-stained quadriceps histological sections from 6-month-old Scgd−/− and Scgd−/− Dysf-TG. Scale bars = 100 μm. C: Serum CK levels in 6-month-old mice. *P < 0.05 versus wild-type. D: Percentage of fibers containing central nuclei in the gastrocnemius, quadriceps, and TA from Scgd−/− and Scgd−/− Dysf-TG mice. E: Percent fibrosis in the same groups of mice as in D. F: Levels of CK in plasma after exercise in the indicated groups of mice based on the legend shown in A. *P < 0.05 versus wild-type. Numbers below the graphs represent the number of animals studied. G: Uptake of Evan’s blue dye (red) in quadriceps of Scgd−/− and Scgd−/− Dysf-TG at rest and after exercise. Green corresponds to membrane/ECM staining with wheat germ agglutinin-fluorescein isothiocyanate. Scale bars = 100 μm.
To examine whether increased expression of dysferlin might only be protective in the Scgd−/− mouse when membrane damage was enhanced by exercise, we exposed mice to voluntary wheel running for 7 days, followed by analysis of plasma CK and EBD uptake in skeletal muscle fibers. All animals underwent a similar level of exercise as quantified by total wheel revolutions (data not shown). Plasma CK levels were not statistically different between Scgd−/− and Scgd−/− Dysf-TG mice after exercise, although both groups were elevated compared with wild-type and Dysf-TG mice (Figure 3F). Quadriceps muscles from Scgd−/− mice exhibited a moderate level of EBD uptake at baseline and an elevated amount of uptake after exercise (15% ± 5.2 EBD positive fibers at baseline and 28.1% ± 1.3 after exercise), but no improvement was detected in Scgd−/− Dysf-TG mice (15.2% EBD positive fibers ±3.1 at baseline and 30.6% ± 2.4 after exercise) at baseline or after exercise (Figure 3G). These results indicate that 3- to 3.5-fold overexpression of dysferlin does not enhance membrane repair or reduce dystrophic disease in a DGC-lacking model of MD (see discussion).
Discussion
Many human mutations that cause MD can be linked in one manner or another to an alteration in the stability of the sarcolemma. Loss of dystrophin or other components of the DGC enhance susceptibility to membrane microtears, leading to the release of muscle-specific factors and increasing the permeation of dyes and large molecular weight markers. This instability of the sarcolemma likely promotes myofiber degeneration through increased Ca2+ influx and reactive oxygen species generation, leading to necrosis. We initially hypothesized that mild overexpression of dysferlin would enhance repair of these microtears and improve dystrophic characteristics, given the proposed centrality of dysferlin in resolving membrane disruptions. However, mild overexpression of dysferlin in muscle did not positively or negatively alter disease progression or membrane stability in Scgd−/− mice. In contrast, it did rescue the dystrophic phenotype of Dysf null muscles, showing that the MD caused by the absence of dysferlin is due to its function in skeletal muscle, and not in other tissues or cell types, such as inflammatory cells (see below). This later result also indicates that the human dysferlin protein expressed from the transgene was fully functional in the mouse, which was anticipated given the high degree of sequence conservation between mouse and human dysferlin.
Although the results of our studies in Scgd−/− mice were negative, they do provide some insight into the function of dysferlin in MD disease, and membrane repair in general. It remains possible that the membrane repair complex, which may also contain SNARE proteins and synaptotagmins, is a stoichiometrically defined unit and as such, overexpression of any single component does not generate more total repair complexes. Thus, while loss of dysferlin cripples the repair complex and leads to MD, enhanced expression has no effect.
Another possibility is that dysferlin is not directly involved in membrane repair, but that it indirectly supports membrane health by permitting homeostatic membrane recycling through vesicular shuttling and turnover of damaged membrane proteins. This hypothesis is consistent with ultrastructural analysis of muscle fibers from Dysf-deficient tissue, in which the surface of the cell membrane is irregular, and there is a large accumulation of membrane vesicles under the sarcolemma.4,6 In this model, lack of normal membrane turnover might compromise the function of select structural proteins, ion channels, or signaling complexes that become damaged, leading to disease as they accumulate. Dysferlin interacts with a number of other proteins that could affect the health of the membrane such as annexins, AHNAK, and MG53, which have also been implicated in membrane trafficking and resealing.17,18,19,20 Dysferlin also interacts with affixin, a focal adhesion protein, the L-type calcium channel, caveolin 3, and calpain 3, the later two of which themselves can cause a limb-girdle MD when deficient.21,22,23
Our results clearly show that replacement of dysferlin by transgenesis only in skeletal muscle of A/J mice completely rescued muscle pathology and fully restored functional recovery from injury caused by large strain lengthening contractions. Despite clear abnormalities reported in Dysf-null inflammatory cells, our results suggest that such defects are secondary and not capable of initiating disease in A/J mice. Thus, although Dysf-deficient inflammatory cells and T-cells can exacerbate disease once initiated, it is the loss of dysferlin from skeletal muscle that is the primary cause of disease, demonstrating a myocyte autonomous mechanism.
Supplementary Material
Footnotes
Address reprint requests to Jeffery D. Molkentin, Cincinnati Children’s Hospital Medical Center and Howard Hughes Medical Institute, 240 Albert Sabin Way, MLC7020, Cincinnati OH, 45229. E-mail: jeff.molkentin@cchmc.org.
Supported by grants from the National Institutes of Health (J.D.M.), an award from the Jain Foundation (J.D.M., R.J.B.), and the Howard Hughes Medical Institute (J.D.M.). D.P.M. was supported through the Jain Foundation.
Supplemental material for this article can be found on http://ajp.amjpathol.org.
References
- Durbeej M, Campbell KP. Muscular dystrophies involving the dystrophin-glycoprotein complex: an overview of current mouse models. Curr Opin Genet Dev. 2002;12:349–361. doi: 10.1016/s0959-437x(02)00309-x. [DOI] [PubMed] [Google Scholar]
- Lapidos KA, Kakkar R, McNally EM. The dystrophin glycoprotein complex: signaling strength and integrity for the sarcolemma. Circ Res. 2004;94:1023–1031. doi: 10.1161/01.RES.0000126574.61061.25. [DOI] [PubMed] [Google Scholar]
- Laval SH, Bushby KM. Limb-girdle muscular dystrophies–from genetics to molecular pathology. Neuropathol Appl Neurobiol. 2004;30:91–105. doi: 10.1111/j.1365-2990.2004.00555.x. [DOI] [PubMed] [Google Scholar]
- Bansal D, Miyake K, Vogel SS, Groh S, Chen CC, Williamson R, McNeil PL, Campbell KP. Defective membrane repair in dysferlin-deficient muscular dystrophy. Nature. 2003;423:168–172. doi: 10.1038/nature01573. [DOI] [PubMed] [Google Scholar]
- Han R, Bansal D, Miyake K, Muniz VP, Weiss RM, McNeil PL, Campbell KP. Dysferlin-mediated membrane repair protects the heart from stress-induced left ventricular injury. J Clin Invest. 2007;117:1805–1813. doi: 10.1172/JCI30848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selcen D, Stilling G, Engel AG. The earliest pathologic alterations in dysferlinopathy. Neurology. 2001;56:1472–1481. doi: 10.1212/wnl.56.11.1472. [DOI] [PubMed] [Google Scholar]
- Confalonieri P, Oliva L, Andreetta F, Lorenzoni R, Dassi P, Mariani E, Morandi L, Mora M, Cornelio F, Mantegazza R. Muscle inflammation and MHC class I up-regulation in muscular dystrophy with lack of dysferlin: an immunopathological study. J Neuroimmunol. 2003;142:130–136. doi: 10.1016/s0165-5728(03)00255-8. [DOI] [PubMed] [Google Scholar]
- Gallardo E, Rojas-Garcia R, de Luna N, Pou A, Brown RH, Jr, Illa I. Inflammation in dysferlin myopathy: immunohistochemical characterization of 13 patients. Neurology. 2001;57:2136–2138. doi: 10.1212/wnl.57.11.2136. [DOI] [PubMed] [Google Scholar]
- Kesari A, Fukuda M, Knoblach S, Bashir R, Nader GA, Rao D, Nagaraju K, Hoffman EP. Dysferlin deficiency shows compensatory induction of Rab27A/Slp2a that may contribute to inflammatory onset. Am J Pathol. 2008;173:1476–1487. doi: 10.2353/ajpath.2008.080098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaraju K, Rawat R, Veszelovszky E, Thapliyal R, Kesari A, Sparks S, Raben N, Plotz P, Hoffman EP. Dysferlin deficiency enhances monocyte phagocytosis: a model for the inflammatory onset of limb-girdle muscular dystrophy 2B. Am J Pathol. 2008;172:774–785. doi: 10.2353/ajpath.2008.070327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Luna N, Freixas A, Gallano P, Caselles L, Rojas-Garcia R, Paradas C, Nogales G, Dominguez-Perles R, Gonzalez-Quereda L, Vilchez JJ, Marquez C, Bautista J, Guerrero A, Salazar JA, Pou A, Illa I, Gallardo E. Dysferlin expression in monocytes: a source of mRNA for mutation analysis. Neuromuscul Disord. 2007;17:69–76. doi: 10.1016/j.nmd.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Ho M, Post CM, Donahue LR, Lidov HG, Bronson RT, Goolsby H, Watkins SC, Cox GA, Brown RH., Jr Disruption of muscle membrane and phenotype divergence in two novel mouse models of dysferlin deficiency. Hum Mol Genet. 2004;13:1999–2010. doi: 10.1093/hmg/ddh212. [DOI] [PubMed] [Google Scholar]
- Millay DP, Sargent MA, Osinska H, Baines CP, Barton ER, Vuagniaux G, Sweeney HL, Robbins J, Molkentin JD. Genetic and pharmacologic inhibition of mitochondrial-dependent necrosis attenuates muscular dystrophy. Nat Med. 2008;14:442–447. doi: 10.1038/nm1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche JA, Lovering RM, Bloch RJ. Impaired recovery of dysferlin-null skeletal muscle after contraction-induced injury in vivo. NeuroReport. 2008;19:1579–1584. doi: 10.1097/WNR.0b013e328311ca35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan KJ, Hardeman EC. Quantitative analysis of the human alpha-skeletal actin gene in transgenic mice. J Biol Chem. 1993;268:719–725. [PubMed] [Google Scholar]
- Anderson LV, Davison K, Moss JA, Young C, Cullen MJ, Walsh J, Johnson MA, Bashir R, Britton S, Keers S, Argov Z, Mahjneh I, Fougerousse F, Beckmann JS, Bushby KM. Dysferlin is a plasma membrane protein and is expressed early in human development. Hum Mol Genet. 1999;8:855–861. doi: 10.1093/hmg/8.5.855. [DOI] [PubMed] [Google Scholar]
- Huang Y, Laval SH, van Remoortere A, Baudier J, Benaud C, Anderson LV, Straub V, Deelder A, Frants RR, den Dunnen JT, Bushby K, van der Maarel SM. AHNAK, a novel component of the dysferlin protein complex, redistributes to the cytoplasm with dysferlin during skeletal muscle regeneration. FASEB J. 2007;21:732–742. doi: 10.1096/fj.06-6628com. [DOI] [PubMed] [Google Scholar]
- Lennon NJ, Kho A, Bacskai BJ, Perlmutter SL, Hyman BT, Brown RH., Jr Dysferlin interacts with annexins A1 and A2 and mediates sarcolemmal wound-healing. J Biol Chem. 2003;278:50466–50473. doi: 10.1074/jbc.M307247200. [DOI] [PubMed] [Google Scholar]
- McNeil AK, Rescher U, Gerke V, McNeil PL. Requirement for annexin A1 in plasma membrane repair. J Biol Chem. 2006;281:35202–35207. doi: 10.1074/jbc.M606406200. [DOI] [PubMed] [Google Scholar]
- Cai C, Weisleder N, Ko JK, Komazaki S, Sunada Y, Nishi M, Takeshima H, Ma J. Membrane repair defects in muscular dystrophy are linked to altered interaction between MG53, caveolin-3, and dysferlin. J Biol Chem. 2009;284:15894–15902. doi: 10.1074/jbc.M109.009589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ampong BN, Imamura M, Matsumiya T, Yoshida M, Takeda S. Intracellular localization of dysferlin and its association with the dihydropyridine receptor. Acta Myol. 2005;24:134–144. [PubMed] [Google Scholar]
- Anderson LV, Harrison RM, Pogue R, Vafiadaki E, Pollitt C, Davison K, Moss JA, Keers S, Pyle A, Shaw PJ, Mahjneh I, Argov Z, Greenberg CR, Wrogemann K, Bertorini T, Goebel HH, Beckmann JS, Bashir R, Bushby KM. Secondary reduction in calpain 3 expression in patients with limb girdle muscular dystrophy type 2B and Miyoshi myopathy (primary dysferlinopathies). Neuromuscul Disord. 2000;10:553–559. doi: 10.1016/s0960-8966(00)00143-7. [DOI] [PubMed] [Google Scholar]
- Matsuda C, Kameyama K, Tagawa K, Ogawa M, Suzuki A, Yamaji S, Okamoto H, Nishino I, Hayashi YK. Dysferlin interacts with affixin (beta-parvin) at the sarcolemma. J Neuropathol Exp Neurol. 2005;64:334–340. doi: 10.1093/jnen/64.4.334. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.