Abstract
Urinary biomarkers for the detection of bladder cancer have been developed, but no similar markers exist for prediction of clinical outcomes after receiving chemotherapy. Here we evaluate an approach that combines genomic, proteomic, and therapeutic outcome datasets to identify novel putative urinary biomarkers of clinical outcome after neoadjuvant methotrexate, vinblastine, doxorubicin, and cisplatin (MVAC). Using this method, we identified γ-glutamyl hydrolase (GGH), emmprin, survivin, and diazepam-binding inhibitor (DBI). Interestingly, GGH is a protein associated with methotrexate resistance, whereas emmprin, survivin, and DBI had been previously identified as predictors of outcome after platinum-containing chemotherapeutic regimens when assessed on tumor tissue. Using disease-free survival as a marker for clinical outcome, we evaluated the ability of GGH, emmprin, survivin, and DBI expression in tumor tissue to stratify 27 patients treated with neoadjuvant MVAC. DBI (P = 0.046) but not GGH (P = 0.190), emmprin (P = 0.066), or survivin (P = 0.393) successfully stratified patients. When GGH was used with DBI the significance of stratification improved (P = 0.024), whereas the addition of survivin or emmprin to this latter two-gene model reduced its significance (P = 0.036 and P = 0.040, respectively). Although these predictive results were obtained on tumor tissues, the presence of GGH and DBI in urine serves as a rationale for developing them as urinary markers of clinical outcomes for patients treated with neoadjuvant MVAC.
In 2008 approximately 69,000 individuals in the United States were diagnosed with bladder cancer, and 14,000 will ultimately die as a result of the disease.1 Most of the patients who die initially present with muscle-invasive disease that later metastasizes.2 Randomized studies have shown that neoadjuvant chemotherapy with MVAC (methotrexate, vinblastine, doxorubicin, and cisplatin) in patients with muscle-invasive disease improves median survival from 46 to 77 months. However, despite these promising results, only a subgroup of patients benefit from this approach and determining who they are is not currently possible. Hence, biomarkers that predict such outcomes would be of great practical utility, allowing only patients who are likely to derive the greatest benefit to be treated.
Despite the fact that bladder cancer is ideally suited for noninvasive molecular evaluation based on analysis of urine, no urinary biomarkers have been found to robustly predict clinical outcomes after chemotherapy. Recently, tissue expression of two genes, emmprin and survivin, was found to independently predict outcome in patients with bladder cancer treated with cisplatinum-containing regimens.3 Interestingly, both emmprin and survivin have been identified in urine.4,5 In addition, a recent study described a 14-gene signature predictive of therapeutic response in patients with bladder cancer treated with neoadjuvant MVAC.6,7 One of the 14 genes in this model, diazepam-binding inhibitor (DBI), is found in urine.8
Taken together, these findings suggested it may be possible to develop a general method that can identify genes encoding proteins that are both predictors of clinical outcomes and are found in urine. Here we accomplish this and in the process provide a scientific rationale for the development of detection assays for two proteins in urine as noninvasive biomarkers of clinical outcomes in urothelial cancer.
Materials and Methods
Patient Tissue Samples and Microarray Datasets
All patient samples were obtained at the University of Virginia School of Medicine using protocols approved by the University of Virginia Institutional Review Board (HIC 9179). Twenty-five bladder tissue samples were collected from surgical pathology specimens including 6 normal urothelial samples and 19 urothelial tumor samples.9
Five other bladder cancer expression microarray datasets were used in our study. The first evaluated 48 normal bladder samples and 109 bladder carcinoma samples.10 The second evaluated 14 normal bladder samples, 27 superficial transitional cell carcinoma samples, and 13 invasive transitional cell carcinomas.11 Data from 30 microarray chips profiling bladder cancer downloaded from the Gene Expression Omnibus (accession number GSE3167)12,13,14 were also used. A fourth study6,7 on 45 patients with urothelial carcinoma of the bladder treated with neoadjuvant MVAC followed by radical cystectomy, transurethral resection of the bladder tumor, or radiotherapy was used to evaluate disease-free survival outcomes in patients. Finally, a fifth study provided disease-free survival status in 16 patients treated with gemcitabine and cisplatin (GC), another standard chemotherapy regimen for bladder cancer.3
Genoproteomic Method
We used the Affymetrix NetAffx web tools (Affymetrix, Inc, Santa Clara, CA) to match proteins identified by Adachi et al8 as being present in the urine proteome to transcripts queried on the human U133A Affymetrix array. Next, to determine whether any of these transcripts are up-regulated in bladder cancer compared with benign tissue, we examined our microarray data in our cohort of 25 bladder samples described above. A two-tiered approach was used in the identification of up-regulated transcripts. First, using the present, marginal, and absent calls, we screened out genes that did not exhibit at least 75% present or marginal calls in at least one group (tumor or normal). Second, we used the Kolmogorov-Smirnov test to better characterize the degree of overlap between the two distributions and the direction of change. We specifically focused on genes whose expression was increased in bladder cancer compared with that in benign urothelium. The resulting nominal P values from each comparison were corrected for multiple testing using a false discovery rate of <0.01. The probe sets that passed these criteria were ordered based on fold change of cancer compared with benign urothelium.
Real-Time PCR in Urothelial Tissues
Microarray gene expression data were validated by quantitative real-time PCR using the SYBR Green SuperMix protocol (Bio-Rad Laboratories, Hercules, CA) with standard curves on an iCycler (Bio-Rad Laboratories). RNA was from 7 of the bladder tumor tissue samples and 3 of the normal urothelium samples that remained from the original 25 surgical pathology specimens used in expression profiling above. RNA was converted to cDNA using the iScript Reverse Transcription kit (Bio-Rad Laboratories). The PTC-200 Peltier thermal cycler (Bio-Rad Laboratories) was used for reverse transcription-PCR reactions. Quantitative real-time PCR reactions were performed in 96-well plates with 12.5 μl of SYBR Green, 0.5 μl of primer, and cDNA in a total reaction volume of 25 μl. GGH was amplified using the following primers: forward 5′-ACTGCCAATTTCCATAAG-3′ and reverse 5′-TGCCATCTGTATTTGTAGTTA-3′.
Statistical Evaluation of Gene Expression as a Function of Tumor Stage and Clinical Outcome after Chemotherapy
We used data from 23 of the 25 aforementioned local samples, as well as 30 microarray chips from GSE3167. The robust microarray average method was used to quantile-normalize, background-adjust, and summarize the gene expression values from these arrays.15,16,17 Microarray profiles were classified according to the type of tissue hybridized to the array including 15 chips with RNA from normal urothelium, 14 chips with non-muscle-invasive bladder tumors (stage Ta or T1 tumors and grade 1 to 2), and 24 chips with muscle-invasive bladder tumors (stage T2 or greater, any grade). We measured the correlation of gene expression (HG-U133A probe set 203560_at) with invasiveness as classified in three groups: normal, non-muscle-invasive tumors, and muscle-invasive tumors. Correlation of gene expression with invasiveness was evaluated using Pearson correlation Spearman rank-based correlation approaches.
Genes discovered by the Genoproteomic method or those found in the literature as predictors of clinical response to cisplatinum-containing chemotherapeutic regimens and found in urine were evaluated as predictors of clinical outcomes to MVAC6,7 or GC.3 For each gene candidate, we used a weighted-voting technique18 to generate response predictions in each patient. Kaplan-Meier curves were used to assess the ability of each gene or gene combinations to stratify disease-free survival. All P values were determined using the log-rank test.
Results
Identification of Urinary Proteins Up-Regulated at the mRNA Level in Urothelial Cancer Tissue
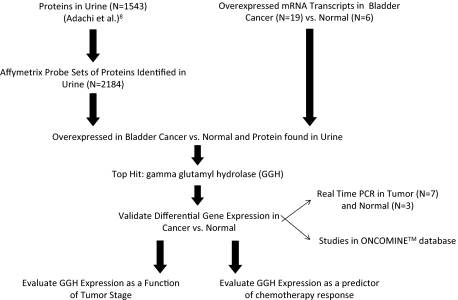
To identify proteins that are present in urine and up-regulated in bladder cancer we used a multifaceted genoproteomic methodology shown in Figure 1. Adachi et al8 identified 1543 proteins in the urine proteome. The proteins identified as being present in the urine proteome were matched to 2184 probe sets in the human HG-U133A Affymetrix array. After specifically selecting for probe sets in our microarray data that matched identified proteins in the urine proteome, we identified 22 probe sets that had significant differential expression between normal and cancer tissue samples (Table 1). GGH, a gene associated with methotrexate resistance,19,20,21,22,23 had the highest overall fold expression in the bladder cancer samples compared with normal bladder samples at 5.5-fold.
Figure 1.
Genoproteomic method. Outline of the approach used to determine that GGH is up-regulated in bladder cancer, detected in the urine proteome, and positively correlated to tumor invasiveness.
Table 1.
Genes with Up-Regulated Expression Levels in Bladder Cancer Tissue Samples for Which Protein Was Found in Urine
| Probe set | Gene | Avg. normal expression | 95% CI (LL) | 95% CI (UL) | Avg. tumor expression | 95% CI (LL) | 95% CI (UL) | Fold change |
|---|---|---|---|---|---|---|---|---|
| 203560_at | GGH | 70.60 | 7.42 | 11.14 | 391.80 | 104.86 | 157.28 | 5.55 |
| 204333_s_at | AGA | 35.80 | 9.54 | 14.30 | 106.70 | 23.74 | 35.60 | 2.98 |
| 202854_at | HPRT1 | 197.90 | 34.82 | 52.22 | 527.50 | 92.62 | 138.94 | 2.67 |
| 53071_s_at | FLJ22222 | 69.60 | 8.32 | 12.48 | 164.00 | 34.57 | 51.85 | 2.36 |
| 201349_at | SLC9A3R1 | 131.90 | 28.48 | 42.72 | 289.80 | 58.81 | 88.21 | 2.20 |
| 211725_s_at | BID | 183.60 | 36.42 | 54.62 | 393.30 | 65.61 | 98.41 | 2.14 |
| 218923_at | CTBS | 44.00 | 8.06 | 12.10 | 92.30 | 17.44 | 26.16 | 2.10 |
| 202118_s_at | CPNE3 | 231.60 | 49.54 | 74.32 | 482.30 | 80.10 | 120.16 | 2.08 |
| 217871_s_at | MIF | 1354.30 | 297.46 | 446.20 | 2754.50 | 307.05 | 460.57 | 2.03 |
| 217346_at | LOC128192 | 106.60 | 17.92 | 26.88 | 213.80 | 24.57 | 36.85 | 2.01 |
| 219215_s_at | SLC39A4 | 151.10 | 22.53 | 33.79 | 275.00 | 25.46 | 38.20 | 1.82 |
| 207986_x_at | CYB561 | 115.50 | 22.66 | 33.98 | 209.30 | 30.03 | 45.05 | 1.81 |
| 201180_s_at | GNAI3 | 481.90 | 41.54 | 62.30 | 851.60 | 131.80 | 197.70 | 1.77 |
| 201098_at | COPB2 | 501.00 | 66.63 | 99.95 | 859.90 | 118.78 | 178.16 | 1.72 |
| 200039_s_at | PSMB2 | 507.20 | 65.35 | 98.03 | 850.20 | 115.46 | 173.20 | 1.68 |
| 217733_s_at | TMSB10 | 3242.20 | 291.95 | 437.93 | 5358.90 | 745.40 | 1118.10 | 1.65 |
| 201524_x_at | UBE2N | 663.80 | 91.28 | 136.92 | 1083.70 | 143.30 | 214.96 | 1.63 |
| 208805_at | PSMA6 | 1145.60 | 82.18 | 123.28 | 1848.50 | 254.74 | 382.12 | 1.61 |
| 213011_s_at | TPI1 | 1494.50 | 134.62 | 201.92 | 2404.40 | 266.01 | 399.01 | 1.61 |
| 201268_at | NME1-NME2 | 1975.90 | 294.07 | 441.11 | 2965.70 | 260.61 | 390.91 | 1.50 |
| 211978_x_at | PPIA | 5646.70 | 426.45 | 639.67 | 7513.60 | 634.72 | 952.08 | 1.33 |
| 214143_x_at | RPL24 | 4523.30 | 185.50 | 278.26 | 5699.70 | 233.53 | 350.29 | 1.26 |
From the analysis in Figure 1, 22 genes showed up-regulation of mRNA transcripts in bladder cancer tissue versus normal bladder tissue. Genes were ranked in order of fold change in bladder cancer versus normal urothelium samples.
CI, confidence interval; LL, lower limit; UL, upper limit.
GGH mRNA Expression Is Increased in Human Urothelial Cancer
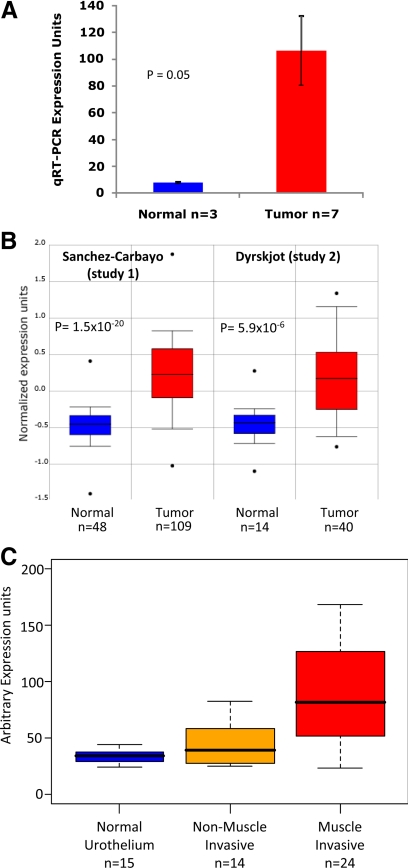
To verify the microarray results indicating that GGH is increased in bladder cancer, quantitative real-time PCR was performed on 3 normal bladder tissue samples and 7 bladder cancer tissue samples remaining from the 25 tissue samples used in the original microarray study. The bladder cancer tissue samples showed increased expression of GGH across various tumor stages at 13.9-fold greater than normal tissue samples (Figure 2A).10,11 Two independent microarray data sets10,11 that evaluated GGH expression as a function of stage were found in the ONCOMINE database and were used to confirm our results (Figure 2B).10,11
Figure 2.
Internal and external validation of increased GGH gene expression in bladder cancer. A: Quantitative real-time PCR (qRT-PCR) expression of GGH mRNA in normal bladder mucosa (n = 3) and Ta, T2, T3, and T4 bladder tumors (n = 7). Tissue was obtained from original samples used in microarray analysis. Columns, average expression of normal and tumor tissue using quantitative real-time PCR; bars, 95% confidence interval of measured expression across each category (P = 0.05). Fold change from normal to tumor is 13.9. B: Box plots taken from the ONCOMINE database that show up-regulation of GGH in bladder cancer from two published microarray studies.10,11 Both datasets have been log-2 transformed with the median set to 0.0 and SD set to 1. Study 1 and study 2 have P values of 1.5E-20 and 5.9E-6 respectively. C: Relative microarray-derived GGH expression values obtained from 23 of our 25 patients and 30 others (Gene Expression Omnibus accession number GSE3167) as described in Materials and Methods were calculated among three cohorts of patients, as stratified into normal (n = 15), non-muscle-invasive tumors (n = 14), and muscle-invasive tumors (n = 24). Center bar is median for each cohort. Boxes are bound by first and third quartiles of GGH expression in each cohort.
GGH mRNA Expression Is Higher in Muscle-Invasive than in Noninvasive Urothelial Cancer
To determine whether increased GGH expression was associated with muscle invasiveness in human urothelial carcinoma of the bladder, we examined GGH expression as a function of stage in microarray data from 23 of our 25 patients and 30 others (Gene Expression Omnibus accession number GSE3167) (Figure 2C).10,11 We measured the correlation coefficient of GGH expression compared with the invasiveness of the tumor scored as 1 for normal urothelial tissues, 2 for non-muscle-invasive tumors and 3 for muscle-invasive tumors. We found that GGH expression was significantly correlated with invasiveness (Pearson correlation = 0.572, P value = 7.47 × 10−6, Spearman rank-based correlation = 0.614, P value = 9.87 × 10−7).
GGH and DBI Stratify Clinical Outcome after Systemic Chemotherapy
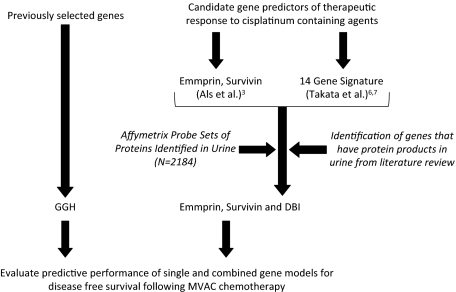
In prior studies, DBI, survivin, and emmprin expression was found to predict outcomes after platinum-based bladder cancer chemotherapy and were present in human urine3,4,5,6,7,8 (Figure 3). Hence, together with GGH, which is known to be associated with methotrexate resistance, we labeled these genes as candidate urinary biomarkers of clinical outcomes after chemotherapy.
Figure 3.
Algorithm used to determine whether selected genes were predictive of clinical outcomes in response to MVAC. Outline of the approach that was used to determine whether GGH, DBI, emmprin, or survivin either alone or in combination could be predictive of clinical outcomes after MVAC chemotherapy. Disease-free survival was used as the measure of clinical outcome.
We used the gene-weighted voting technique of Golub and Slonim18 to predict whether GGH, DBI, survivin, or emmprin expression correlated with clinical outcome as assessed by tumor response and/or disease-free survival after treatment with the MVAC6,7 or GC3 chemotherapy regimens. In the studies of Takata et al,6,7 “responders” are defined as those patients who achieved tumor downstaging after receiving two courses of MVAC neoadjuvant chemotherapy and “nonresponders” are defined as those patients who did not achieve tumor downstaging after receiving two courses of MVAC neoadjuvant chemotherapy. The metric of downstaging was determined by analyzing bladder tissue specimens obtained during cystectomy and/or radiological imaging using computed tomography or magnetic resonance imaging.
For our evaluation and model comparisons we used 27 of the 45 patients in the Takata et al MVAC study.6,7 We did not use all the patients because the first 18 of the 45 patients were used to train the original 14-gene prediction model. In comparison with the original 14-gene prediction model that was generated by Takata et al neither GGH, DBI, emmprin, or survivin alone or in combination was able to better predict a response to MVAC neoadjuvant chemotherapy than the 14-gene prediction model (Supplemental Table 1 see http://ajp.amjpathol.org).6,7,25,26 In addition, the remaining genes that are up-regulated in bladder cancer and secreted in urine (Table 1) were found to be weaker at predicting response to neoadjuvant MVAC compared with the Takata et al 14-gene model whether alone or in combination with GGH (Supplemental Table 2, see http://ajp.amjpathol.org).6,7,18,25,26,27
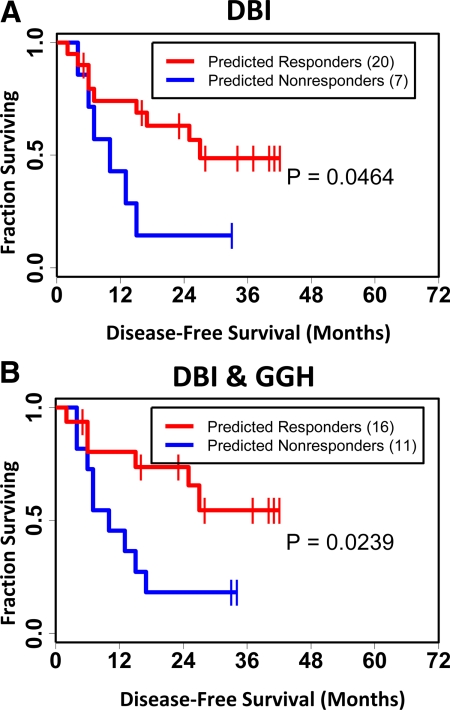
When looking at stratification of disease-free survival after MVAC, we found that the GGH + DBI prediction model (P = 0.024) has greater statistical significance than the DBI model (P = 0.046) alone and had visually better separation of the survival curves (Figure 4, A and B).6,7,18,27 Importantly, the GGH model (P = 0.190), Takata et al 14-gene model (P = 0.077), survivin model (P = 0.393), or the emmprin model (P = 0.066) did not provide any statistically significant stratification of disease-free survival (Supplemental Figure 1, A–D, see http://ajp.amjpathol.org). In addition, when survivin alone, emmprin alone, or survivin and emmprin together are added to the GGH/DBI prediction model, the significance of the two-gene model in stratifying disease-free survival after MVAC therapy is decreased (P = 0.036, P = 0.040, and P = 0.025, respectively) (Supplemental Figure 2, A–C, see http://ajp.amjpathol.org). The combination of survivin and emmprin together (P = 0.205) provided no statistical significance in disease-free survival (data not shown). The remaining genes from Table 1 that are up-regulated in bladder cancer and secreted in urine were also found to be weaker at stratifying disease-free survival than the GGH/DBI prediction model whether alone or in combination with GGH (Supplemental Table 2, see http://ajp.amjpathol.org).6,7,18,25,26,27 The study of Als et al3 only offered us the ability to evaluate the stratification of disease-free survival in patients treated with neoadjuvant GC; the GGH prediction models showed poor significance (data not shown).
Figure 4.
Kaplan-Meier analysis of gene models as predictors of disease-free survival for 27 of the 45 patients in the MVAC study of Takata et al.6,7 The first 18 of the 45 patients6,7 were used to train their original 14-gene prediction model and thus were excluded from the current analysis. The gene-weighted voting technique as described by Golub et al18 was used to predict response to treatment for several different gene prediction models. Kaplan-Meier estimators for disease-free survival were generated for the respective gene prediction models: red curves describe predicted responders to treatment, blue curves describe predicted nonresponders to treatment. A: Gene-weighted voting technique applied for DBI alone. B: Gene-weighted voting technique applied for GGH and DBI in combination. P values were determined using the log-rank test and describe the statistical significance of stratification of disease-free survival based on predicted response.27
Discussion
Our data implicate GGH as a novel biomarker for bladder cancer. When evaluated alone on tumor tissue, it does not significantly predict disease-free survival after MVAC chemotherapy, but its use in conjunction with DBI provides stratification superior to that of DBI alone. Interestingly, this two-gene combination predicts disease-free survival better than a previously described 14-gene prediction model6,7 of therapeutic response after treatment with the MVAC regimen. GGH is an intracellular lysosomal glycoprotein that hydrolyzes folyl- and anti-folylpolyglutamates.28 It is known to hydrolyze the polyglutamate derivatives of folates and anti-folates using both endopeptidase and exopeptidase activity.29 GGH requires reduced thiols for its activity.30 Secretion of GGH in cancer cells was initially established by experiments that showed increased secretion of GGH in H35 hepatoma cells compared with rat hepatocytes.31
GGH has been proposed to act as a prognostic biomarker for acute leukemias in response to methotrexate therapy21 and also to act as a diagnostic biomarker in regard to pulmonary neuroendocrine tumors.33 Our study focused on the interaction of GGH with MVAC chemotherapy treatment. The role of GGH as a predictor of outcome after this treatment is of particular interest because methotrexate is a component of this regimen. The ratio between GGH, which removes terminal glutamates from methotrexate, and folylpolyglutamate synthetase, which adds glutamates to methotrexate metabolites, has been shown to effectively predict methotrexate accumulation in both myeloid and lymphoid blasts.20,21 Inhibition of GGH at the molecular level has been shown to increase methotrexate metabolites in human sarcoma cell lines as well as leukemic cell lines.22,23 Interestingly, methylation of the CpG1 and CpG2 regions in the GGH promoter was shown to significantly reduce GGH mRNA expression and GGH catalytic activity, which resulted in higher accumulation of methotrexate metabolites in acute lymphoblastic leukemic cells.19
Our study showed a weak correlation with marginal significance (P = 0.190) between GGH expression and outcome after methotrexate-containing MVAC chemotherapy treatment regimens. At first glance these data seemingly contradict previous studies that show a strong and significant reduction of methotrexate activity with increased GGH expression. We speculate that the other agents in the MVAC regimen (vinblastine, doxorubicin, and cisplatin) may have the ability to diminish the effects of GGH activity inside the cell, thus increasing the viability of methotrexate within the cell, or that they are more efficacious than methotrexate and thus drive the clinical outcome to a much greater degree.
To further study the utility of GGH in predicting outcome after MVAC activity we looked at the ability of GGH to predict MVAC treatment outcomes when paired with other proteins that are both found in urine and have been shown to predict response to MVAC. A study conducted by Takata et al6,7 identified a 14-gene model to predict clinical outcome after MVAC chemotherapy for bladder cancer. This model contains DBI, a gene of interest for our study because, like GGH, it was identified in the urine proteome.8 DBI was initially identified as an endogenous ligand that is recognized by the β-carboline/benzodiazepine recognition site located in the GABAA receptor.35 Besides the central nervous system DBI is expressed in peripheral tissues such as the liver, adrenal gland, testis, and kidney, primarily in the mitochondrial membrane.36 We show that addition of DBI to the GGH prediction model has the ability to better stratify disease-free survival than the 14-gene model of Takata et al, the DBI model, or the GGH model alone. In addition to DBI, emmprin and survivin were included in the chemotherapy response and prognostic outcome prediction model as they are both found in urine4,5 and have both been found to be independent prognostic markers for outcome in patients with bladder cancer treated with cisplatinum-containing regimens such as MVAC and GC.3 Addition of emmprin and survivin to the GGH/DBI models weakened their ability to stratify disease-free survival after MVAC. Interestingly, the GGH/DBI prediction model has not shown an ability to predict outcomes to GC therapy. From these results, we speculate that the GGH/DBI model is specific for the three drugs used in the MVAC regimen with the exception of cisplatinum and has little predictive ability for gemcitabine.
The ability of the GGH/DBI model to significantly stratify and predict disease-free survival in patients treated with MVAC has potential for clinical prognostication in patients who undergo this treatment regimen. In addition, the genoproteomic approach supports the rationale for pursuit and development of enzyme-linked immunosorbent assay; assays for urine GGH/DBI that would allow noninvasive prediction of therapeutic outcomes to MVAC based purely on urine evaluation. However, several additional steps would be required to bring this concept to clinical utility. For example, once enzyme-linked immunosorbent assay tools are developed for the leading markers, GGH and DBI, we would propose to quantitate them in urine of patients undergoing neoadjuvant chemotherapy before cystectomy for invasive bladder cancer. A receiver operating characteristic curve analysis would then be performed with the marker level and the incidence of pathological P0 stage at cystectomy. Because the presence of P0 stage at cystectomy indicates chemotherapy benefit and enhanced survival,37 we would use this analysis to “calibrate” the levels of GGH and DBI to provide a reflection of this important pathological outcome. As patients are followed after cystectomy, the marker levels would be further calibrated in a second stage using actual survival outcomes, which, although predicted well by P0 stage, are the standard outcome measure.37 This aim of this future study is to demonstrate “proof of principle” of a new method of defining urinary markers that may be used in combination with assays such as ELISA to predict chemotherapy response.
Supplementary Material
Acknowledgments
We thank members of the Theodorescu Laboratory for their suggestions and assistance.
Footnotes
Address reprint requests to Dan Theodorescu, Department of Molecular Physiology, University of Virginia, PO Box 800422, Charlottesville, VA 22908. E-mail: dt9d@virginia.edu.
Supported by the National Institutes of Health (grants 5-T32-DK069-264-02 to P.W. and CA075115 to D.T.).
Supplemental material for this article can be found on http://ajp.amjpathol.org.
References
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics. 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- Grossman HB, Natale RB, Tangen CM, Speights VO, Vogelzang NJ, Trump DL, deVere White RW, Sarosdy MF, Wood DP, Jr, Raghavan D, Crawford ED. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med. 2003;349:859–866. doi: 10.1056/NEJMoa022148. [DOI] [PubMed] [Google Scholar]
- Als AB, Dyrskjøt L, von der Maase H, Koed K, Mansilla F, Toldbod HE, Jensen JL, Ulhøi BP, Sengeløv L, Jensen KME, Ørntoft TF. Emmprin and survivin predict response and survival following cisplatin-containing chemotherapy in patients with advanced bladder cancer. Clin Cancer Res. 2007;13:4407–4414. doi: 10.1158/1078-0432.CCR-07-0109. [DOI] [PubMed] [Google Scholar]
- Guirguis R, Javadpour N, Sharareh S, Biswas C, el-Amin W, Mansur I, Kim JS. A new method for evaluation of urinary autocrine motility factor and tumor cell collagenase stimulating factor as markers for urinary tract cancers. J Occup Med. 1990;32:846–853. doi: 10.1097/00043764-199009000-00017. [DOI] [PubMed] [Google Scholar]
- Smith SD, Wheeler MA, Plescia J, Colberg JW, Weiss RM, Altieri DC. Urine detection of survivin and diagnosis of bladder cancer. JAMA. 2001;285:324–328. doi: 10.1001/jama.285.3.324. [DOI] [PubMed] [Google Scholar]
- Takata R, Katagiri T, Kanehira M, Tsunoda T, Shuin T, Miki T, Namiki M, Kohri K, Matsushita Y, Fujioka T, Nakamura Y. Predicting response to methotrexate, vinblastine, doxorubicin, and cisplatin neoadjuvant chemotherapy for bladder cancers through genome-wide gene expression profiling. Clin Cancer Res. 2005;11:2625–2636. doi: 10.1158/1078-0432.CCR-04-1988. [DOI] [PubMed] [Google Scholar]
- Takata R, Katagiri T, Kanehira M, Shuin T, Miki T, Namiki M, Kohri K, Tsunoda T, Fujioka T, Nakamura Y. Validation study of the prediction system for clinical response of M-VAC neoadjuvant chemotherapy. Cancer Sci. 2007;98:113–117. doi: 10.1111/j.1349-7006.2006.00366.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adachi J, Kumar C, Zhang Y, Olsen JV, Mann M. The human urinary proteome contains more than 1500 proteins, including a large proportion of membrane proteins. Genome Biol. 2006;7:R80. doi: 10.1186/gb-2006-7-9-r80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson BE, Frierson HF, Conaway MR, Seraj JM, Harding MA, Hampton GM, Theodorescu D. Profiling the evolution of human metastatic bladder cancer. Cancer Res. 2004;64:7813–7821. doi: 10.1158/0008-5472.CAN-04-0826. [DOI] [PubMed] [Google Scholar]
- Sanchez-Carbayo M, Socci ND, Lozano J, Saint F, Cordon-Cardo C. ONCOMINE: defining molecular profiles of poor outcome in patients with invasive bladder cancer using oligonucleotide microarrays. J Clin Oncol. 2006;24:778–789. doi: 10.1200/JCO.2005.03.2375. [DOI] [PubMed] [Google Scholar]
- Dyrskjøt L, Kruhøffer M, Thykjaer T, Marcussen N, Jensen JL, Møller K, Ørntoft TF. ONCOMINE: Gene expression in the urinary bladder: a common carcinoma in situ gene expression signature exists disregarding histopathological classification. Cancer Res. 2004;64:4040–4048. doi: 10.1158/0008-5472.CAN-03-3620. [DOI] [PubMed] [Google Scholar]
- Barrett T, Troup DB, Wilhite SE, Ledoux P, Rudnev D, Evangelista C, Kim IF, Soboleva A, Tomashevsky M, Edgar R. NCBI GEO: mining tens of millions of expression profiles—database and tools update. Nucleic Acids Res. 2007;35:D760–765. doi: 10.1093/nar/gkl887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyrskjøt L, Kruhøffer M, Thykjaer T, Marcussen N, Jensen JL, Møller K, Ørntoft TF. Gene expression in the urinary bladder: a common carcinoma in situ gene expression signature exists disregarding histopathological classification. Cancer Res. 2004;64:4040–4048. doi: 10.1158/0008-5472.CAN-03-3620. [DOI] [PubMed] [Google Scholar]
- Edgar R, Domrachev M, Lash AE. Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res. 2002;30:207–210. doi: 10.1093/nar/30.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolstad BM, Irizarry RA, Astrand M, Speed TP. A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics. 2003;19:185–193. doi: 10.1093/bioinformatics/19.2.185. [DOI] [PubMed] [Google Scholar]
- Irizarry RA, Bolstad BM, Collin F, Cope LM, Hobbs B, Speed TP. Summaries of Affymetrix GeneChip probe level data. Nucleic Acids Res. 2003;31:e15. doi: 10.1093/nar/gng015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irizarry RA, Hobbs B, Collin F, Beazer-Barclay YD, Antonellis KJ, Scherf U, Speed TP. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics. 2003;4:249–264. doi: 10.1093/biostatistics/4.2.249. [DOI] [PubMed] [Google Scholar]
- Golub TR, Slonim DK, Tamayo P, Huard C, Gaasenbeek M, Mesirov JP, Coller H, Loh ML, Downing JR, Caligiuri MA, Bloomfield CD, Lander ES. Molecular classification of cancer: class discovery and class prediction by gene expression monitoring. Science. 1999;286:531–537. doi: 10.1126/science.286.5439.531. [DOI] [PubMed] [Google Scholar]
- Cheng Q, Cheng C, Crews KR, Ribeiro RC, Pui C-H, Relling MV, Evans WE. Epigenetic regulation of human γ-glutamyl hydrolase activity in acute lymphoblastic leukemia cells. Am J Hum Genet. 2006;79:264–274. doi: 10.1086/505645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole PD, Kamen BA, Gorlick R, Banerjee D, Smith AK, Magill E, Bertino JR. Effects of overexpression of γ-glutamyl hydrolase on methotrexate metabolism and resistance. Cancer Res. 2001;61:4599–4604. [PubMed] [Google Scholar]
- Longo GS, Gorlick R, Tong WP, Lin S, Steinherz P, Bertino JR. γ-Glutamyl hydrolase and folylpolyglutamate synthetase activities predict polyglutamylation of methotrexate in acute leukemias. Oncol Res. 1997;9:259–263. [PubMed] [Google Scholar]
- Waltham MC, Li W-W, Gritsman H, Tong WP, Bertino JR. γ-Glutamyl hydrolase from human sarcoma HT-1080 cells: characterization and inhibition by glutamine antagonists. Mol Pharmacol. 1997;51:825–832. doi: 10.1124/mol.51.5.825. [DOI] [PubMed] [Google Scholar]
- Whitehead VM, Kalman TI. Enhanced accumulation of long-chain methotrexate polyglutamates in human T cell acute lymphoblastic leukemia cell lines by avicin. Proc Am Soc Hematol. 1997;80:327a. [Google Scholar]
- Harrington DP, Fleming T. A class of rank test procedures for censored survival data. Biometrika. 1982;69:553–566. [Google Scholar]
- Julious SA. Two-sided confidence intervals for the single proportion: comparison of seven methods by Robert G. Newcombe, Statistics in Medicine 1998; 17:857–872. Stat Med. 2005;24:3383–3384. doi: 10.1002/sim.2164. [DOI] [PubMed] [Google Scholar]
- Wilson EB. Probable inference, the law of succession, and statistical inference. J Am Stat Assoc. 1927;22:209–212. [Google Scholar]
- Galivan J, Ryan TJ, Chave K, Rhee M, Yao R, Yin D. Glutamyl hydrolase: pharmacological role and enzymatic characterization. Pharmacol Ther. 2000;85:207–215. doi: 10.1016/s0163-7258(99)00063-7. [DOI] [PubMed] [Google Scholar]
- Mcguire JJ, Coward JK. Pteroylpolyglutamates: biosynthesis, degradation, and function. Blakley RL, Benkovic SJ, editors. New York: Wiley,; Folates and Pterins. 1984:pp 135–190. [Google Scholar]
- Silink M, Reddel R, Bethel M, Rowe PB. γ-Glutamyl hydrolase (conjugase): purification and properties of the bovine hepatic enzyme. J Biol Chem. 1975;250:5982–5994. [PubMed] [Google Scholar]
- O'Connor BM, Rotundo RF, Nimec Z, McGuire JJ, Galivan J. Secretion of γ-glutamyl hydrolase in vitro. Cancer Res. 1991;51:3874–3881. [PubMed] [Google Scholar]
- He P, Varticovski L, Bowman ED, Fukuoka J, Welsh JA, Miura K, Jen J, Gabrielson E, Brambilla E, Travis WD, Harris CC. Identification of carboxypeptidase E and γ-glutamyl hydrolase as biomarkers for pulmonary neuroendocrine tumors by cDNA microarray. Hum Pathol. 2004;35:1196–1209. doi: 10.1016/j.humpath.2004.06.014. [DOI] [PubMed] [Google Scholar]
- Guidotti A, Forchetti CM, Corda MG, Konkel D, Bennett CD, Costa E. Isolation, characterization, and purification to homogeneity of an endogenous polypeptide with agonistic action on benzodiazepine receptors. Proc Natl Acad Sci USA. 1983;80:3531–3535. doi: 10.1073/pnas.80.11.3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anholt RR, Pedersen PL, De Souza EB, Snyder SH. The peripheral-type benzodiazepine receptor: localization to the mitochondrial outer membrane. J Biol Chem. 1986;261:576–583. [PubMed] [Google Scholar]
- Sonpavde G, Goldman BH, Speights VO, Lerner SP, Wood DP, Vogelzang NJ, Trump DL, Natale RB, Grossman HB, Crawford ED. Quality of pathologic response and surgery correlate with survival for patients with completely resected bladder cancer after neoadjuvant chemotherapy. Cancer. 2009;115:4104–4109. doi: 10.1002/cncr.24466. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.