Abstract
UV-irradiated skin and UV-induced tumors overexpress the inducible isoform of cyclooxygenase-2 (Cox-2), and Cox-2 inhibition reduces photocarcinogenesis. To evaluate photoprotective effects of Polypodium leucotomos extract (PL), hairless Xpc+/− mice were fed for 10 days with PL (300 mg/kg) or vehicle then UV-irradiated, once. By 24 hours, UV-induced Cox-2 levels were increased in vehicle-fed and PL-fed mice, whereas by 48 and 72 hours, Cox-2 levels were four- to fivefold lower in PL-fed mice (P < 0.05). p53 expression/activity was increased in PL-fed versus vehicle-fed then UV-irradiated mice. UV-induced inflammation was decreased in PL-fed mice, as shown by ∼60% decrease (P < 0.001) in neutrophil infiltration at 24 hours, and macrophages by ∼50% (<0.02) at 24 and 48 hours. By 72 hours, 54 ± 5% cyclobutane pyrimidine dimers remained in vehicle-fed versus 31 ± 5% in PL-fed skin (P < 0.003). The number of 8-hydroxy-2′-deoxyguanosine–positive cells were decreased before UV irradiation by ∼36% (P < 0.01), suggesting that PL reduces constitutive oxidative DNA damage. By 6 and 24 hours, the number of 8-hydroxy-2′-deoxyguanosine–positive cells were ∼59% (P < 0.01) and ∼79% (P < 0.03) lower in PL-fed versus vehicle-fed mice. Finally, UV-induced mutations in PL-fed-mice were decreased by ∼25% when assessed 2 weeks after the single UV exposure. These data demonstrate that PL extract supplementation affords the following photoprotective effects: p53 activation and reduction of acute inflammation via Cox-2 enzyme inhibition, increased cyclobutane pyrimidine dimer removal, and reduction of oxidative DNA damage.
Skin cancer accounts for at least 40% of all human malignancies, more than 1 million cases annually in the United States.1,2 Several pathobiologic processes are responsible for increased cancer incidence in UV irradiated skin.3 Some of the harmful UV responses include, but are not limited to: immunosuppression, which may allow tumor cells to escape apoptosis; inflammation and erythema, which produce reactive oxygen radicals that may promote tumor growth; and up-regulation of Cox-2.4,5,6
Cox-2 protein is reported to be up-regulated in human keratinocytes at 6 hours with peak expression at 24 hours post-UV exposure.7 Cox-2 is also reported to be actively involved in the processes of cell differentiation and apoptosis.8 In addition, inhibition of Cox-2 expression leads to suppression of epidermal cell growth.8 A critical role of Cox-2 in UV-induced carcinogenesis is further confirmed by approximately 55% to 90% decreases in the incidence of UV-induced skin tumors after treatment of mice ad libidum with specific Cox-2 inhibitors such as celecoxib and nonsteroidal anti-inflammatory drugs (NSAIDs).9,10 Multiple endogenous and exogenous factors such as UVB, interleukin-1α, epidermal growth factor, transforming growth factor β, tumor necrosis factor α, and androgens can induce Cox-2 expression.11,12 In addition to specific inhibitors of Cox-2 (celecoxibs and other new generation coxibs),13 there are also several known suppressors of Cox-2 including but not limited to p53 tumor suppressor gene, antioxidants, estrogen, and fish oil.14
Considerable data demonstrate the ability of various prostaglandin synthesis inhibitors, such as indomethacin and celecoxib, to reduce growth rate in vivo and prevent UV-induced carcinogenesis through down-regulation of Cox-29. Moreover, oral administration of extracts of natural compounds such Polypodium leucotomos (PL) had been reported to induce photoprotective mechanisms through reduction of UV-induced reactive oxygen species and formation of free radicals, which in turn reduces post-UV inflammation, photodamage, and phototoxicity.5,15
The mouse homologue of the human gene xeroderma pigmentosum group C (Xpc) has been cloned, and subsequently, knockout and heterozygote mice have been generated.16 The Xpc heterozygote mice display a skin cancer proneness that is highly comparable with that observed in mild human XP syndromes, which may also model age-associated increase in skin cancer predisposition due to decline in DNA repair capacity,17 as well as exaggerated inflammation secondary to UV exposure.18 Therefore, Xpc heterozygote mouse model appears appropriate to study the effects of natural antioxidants, such as PL on the prevention of skin cancer through inhibition of UV-induced inflammation and possibly improved removal of UV-induced DNA damage. We report in this manuscript that oral administration of PL extract activate tumor suppressor p53, inhibits UV-induced Cox-2 expression, reduces inflammation, enhances the removal of UV-induced photoproducts, such as cyclobutane pyrimide dimers (CPDs), as well as reduces oxidative DNA damage and decreased UV-induced mutagenesis.
Materials and Methods
Animal Model
Hairless Xpc+/− and wild-type-control SKH1 mice were housed and breed at our Laboratory Animal Science Center) for more than 6 years.19 Twenty-five male mice, ages 2 to 3 months, were used in each group. Polypodium Leucotomos (PL) extract was provided by Industrial Farmacéutica Cantabria (Madrid, Spain) as a slightly sweet but otherwise tasteless powder and was given to mice fresh every day with drinking water. Polypodium leucotomos is one of a species of tropical ferns found in Central and South America and is a rich source of polyphenolic compounds, mainly 4-hydroxycinnamic acid (caffeic acid), 3-methoxy-4-hydroxybenzoic acid (vanillic acid), and 3-caffeoylquinic acid (chlorogenic acid) in addition to also being rich in monosaccharides and flavonoids. Fernblock, a standardized extract of the tropical fern Polypodium leucotomos has been used in humans for over 20 years as a dietary supplement in more than 10 countries including Spain, Italy, Austria, Singapore, and New Zealand. To assure proper delivery of the PL in water we supplemented mice (four in one cage) only with 5 ml of water containing PL (30 to 35 mg), a predetermined volume of the water supply for 1 day for 4 mice (the average mouse weight was 22 to 25 grams). We estimated that mice in PL group were given ∼300 mg/kg PL in drinking water per day. Mice in both PL and control groups were fed regular chow and mice in control group were given regular water. All animal protocols were approved by the Institutional Animal Care and Use Committee at Boston University School of Medicine.
UV Irradiation
Mice were irradiated with six fluorescent American Phillips F40 sunlamps permanently mounted above the animal cages. Irradiance was metered with a research radiometer fitted with a UV probe, at 285 ± 5 nm (model IL, 1700 A, International Light, Newburyport, MA), as described.20 Mice received a single dose of UV (25 mJ/cm2) after 10 days of being fed with ∼300 mg/kg of PL. We sacrificed mice before (no UV), immediately after UV (time 0), 6, 24, 48, and 72 hours after exposure.
Western Blot Analysis
Mouse skin was harvested at different times and immediately was snap-frozen and stored at −80°C. Total protein was isolated from mouse skin homogenates, and 25 μg of total protein was run in each lane in a 10% SDS-polyacrylamide gel and was transferred to a polyvinylidene difluoride membrane as described.4 Antibody reactions were performed with the following antibodies: total p53 (p53total) (1:500 dilution) (DO-1, Santa Cruz Biotechnology, CA), phospho-p53 Serine 15 (p53ser15) (1:1000 dilution) (Cell Signaling Technology, MA), Cox-2 (1:250 dilution) (N-20, Santa Cruz Biotechnology, CA), and Actin (1:2000 dilution) (I-19, Santa Cruz Biotechnology, CA).
Histology, Immunohistochemistry, and Immunofluorescence
Tissue sections (6 to 8 μm thick) were stained with H&E and examined by a single dermatopathologist blinded to the treatments. Each section was assigned a number from 0 to 3 indicating an increasing quantity of inflammatory infiltrate, where, score of 0 indicated normal mouse skin; 1 – low inflammatory infiltrate; 2 – moderate inflammatory infiltrate; 3 – significant inflammatory infiltrate. Fresh optimal cutting temperature-embedded and snap-frozen samples of murine skin were cut into 6 to 8 μm thick sections and fixed in ethanol/acetic acid (2:1) at −20°C for 20 minutes. To minimize background staining, the sections were blocked for 30 minutes in goat normal serum (10%). The sections were then incubated overnight at 4°C with antibodies against myeloperoxidase-1 (MPO-1) (1:100 dilution) (Abcam, Cambridge, MA), CD-68 (Santa Cruz Biotechnology, CA), 8-hydroxy-2′-deoxyguanosine (8-ox-dG) (1:250 dilution) (Trevigen, Inc. Gaithersburg, MD), and CPDs (1:3000 dilution) (MBL, Nagoya, Japan). Slides were then incubated with the corresponding fluorescent-labeled secondary antibodies and were later examined using fluorescent microscopy. To control for possible nonspecific staining of primary and secondary antibodies, in all stainings as negative control adjacent sections were either stained with IgG-isotype specific antibodies (control for primary antibody) or by skipping primary antibody staining and using only secondary antibodies (control for nonspecific binding of secondary antibodies). Results were quantified by measuring the percentage of positive cells for each staining using Image-J 1.34S computer software (Wayne-Rasband, National Institutes of Health, Bethesda, MD).
Mutation Analysis
We performed mutation analyses using coded samples from at least 7 to 8 hairless Xpc+/−/lacZ+ (these mice in addition of being Xpc heterozygous were also transgenic for lacZ/pUR288 mutation-indicator gene) mice per treatment condition, 14 days after a single UV exposure similar to a mutagenic protocol previously described for Xpa+/− mice.19 Genomic DNA was isolated by using commercially available kit (Qiagen, Valencia, CA). We isolated 30 to 40 μg of genomic DNA from each of the approximately 1 × 2-cm tissue samples, which is enough to perform mutation analyses of the integrated lacZ gene as originally described by Boerrigter et al.21 The following paragraph is a brief overview of transgenic mouse model for mutagenesis studies based on analysis of mutations in lacZ/pUR288 plasmid incorporated in the mouse genome. The lacZ reporter-indicator gene is located on the plasmids, which are integrated head to tail in the mouse genome. To analyze the frequency of the mutations in the lacZ reporter gene, the plasmids are rescued from the mouse genomic DNA by HindIII digestion and magnetic bead separation. After elution and ligation, the circularized plasmids are transfected to electrocompetent Escherichia coli strain C (Δ lacZ/galE) cells. Two microliters (from 2 ml total) of bacterial suspension are plated on nonselective titer plates, whereas the remainder is plated on selective plates containing phenyl-β-d-galactoside (Pgal, Sigma). The lacZ mutant frequency is calculated by dividing the number of colonies present on the selective plate by those present on the titer plate (for further technical details 21).
Statistical Analysis
Analysis of variance and unpaired t-test was performed using StatView (SAS Institute Inc., Version 5.0). Statistical significance was established at P < 0.05.
Results
Polypodium Leucotomos Increases UV-Induced p53ser15 Expression in Mouse Skin
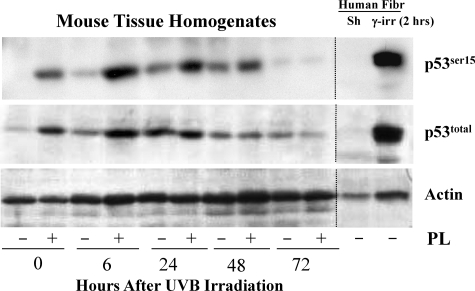
To quantify p53ser15 expression homogenized tissue of mice fed with vehicle or PL was evaluated at 0, 6, 24, 48, and 72 hours after UVB irradiation. Compared with vehicle, in PL-fed then UV-irradiated mice already by time 0 (constitutive protein level) p53total and p53ser15 levels were increased two- to fourfold. These increases were persistent throughout 6 hours for p53total and throughout 48 hours for p53ser15. Maximal fourfold increase in p53total levels was observed 6 hours after UVB irradiation, whereas p53ser15 levels increased four- to sevenfold over levels of vehicle-fed mice, with maximal sevenfold increase at 6 hours after UVB irradiation (Figure 1).
Figure 1.
Total p53total and phospho-p53ser15 expression in mouse skin homogenates at 0, 6, 24, 48, and 72 hours after UV irradiation. Western blot analysis showed marked increase of p53total protein expression in PL-fed versus vehicle-fed mice at 0 and 6 hours after UVB irradiation. p53ser15 protein expression in PL-fed versus vehicle-fed mice were also increased at 0 and 6 hours and remained increased throughout 24 and 48 hours after UVB radiation. By 24 hours for p53total and by 72 hours for p53ser15 levels were similar between PL-fed and vehicle-fed mice. As control for total-p53total and for phospho-p53ser15, we used protein extracts of sham and γ-irradiated (2 hours) human fibroblasts. Protein loading was assessed by probing membranes with actin antibody (lower panel). This experiments were repeated twice (biological replicates) and results were similar (N = 3 per treatment group).
Polypodium Leucotomos Decreases UV-Induced Cox-2 Expression in Mouse Skin
To evaluate Cox-2 expression total protein from homogenized wild-type and Xpc+/− mouse skin was harvested at 0, 24, 48, and 72 hours after UVB irradiation and was processed for Western blot analysis. While constitutive Cox-2 levels varied significantly in different animals, PL treatment did not affect constitutive Cox-2 levels neither in wild-type (see Supplemental Figure S1, A and B at http://ajp.amjpathol.org), nor in Xpc+/− mice (Figure 2A and B). In Xpc+/− mice UV-induced Cox-2 levels increased comparably in vehicle- and PL-fed mice up to 24 hours after irradiation (Figure 2, A and B). However, between 48 and 72 hours, Cox-2 levels were significantly down-regulated in PL-fed mice, suggesting that PL inhibits UV-induced Cox-2 expression in mouse skin in vivo (Figure 2, A and B). We quantified Cox-2 expression by performing densitometric analysis of Cox-2 and actin bands. After loading adjustment (by actin expression) densitometric analysis showed that compared with vehicle-fed mice, UV-induced Cox-2 levels were decreased four- to fivefold in PL-fed mice (P < 0.04 and P < 0.05, 48 and 72 hours, respectively) (Figure 2, A and B).
Figure 2.
Cox-2 expression in homogenized tissue of mice fed with vehicle or PL for 10 days then UV irradiated once (25 mJ/cm2). A: Western blot analysis show the expression of Cox-2 (upper panel) and actin (lower panel, loading control) in the skin of three mice per treatment group at 0, 24, 48, and 72 hours after UV radiation. Please note that homogenates for 0 and 24 hours were run separately from 48 and 72 hours samples, and due to different exposure times of each membrane 0-hour and 24-hour samples should not be compared with 48-hour and 72-hour samples; however, comparisons within one membrane are acceptable. B: Densitometric analysis of Cox-2 Western blots (adjusted by actin expression) showed that compared with vehicle-fed mice, UV-induced Cox-2 levels were decreased four- to fivefold in PL-fed mice at 48 and 72 hours (N = 3 per treatment group).
Interestingly, in wild-type mice already by 6 hours Cox-2 levels were decreased (compared with vehicle-fed) more than fourfold (P < 0.001) in PL-fed mice and remained 70% lower (P < 0.03) than in vehicle-fed mice up to 24 hours (see Supplemental Figure S1, A and B, at http://ajp.amjpathol.org), suggesting that similar to Xpc+/− mice PL inhibits UV-induced Cox-2 expression in wild-type mice, however in difference with Xpc+/− mice significantly earlier (as early as 6 hours vs 48 hours, in wild-type versus Xpc+/− mice) (Figure 2, A and B, and see Supplemental Figure S1, A and B, at http://ajp.amjpathol.org). Please note that by 48 and 72 hours in wild-type mice Cox-2 levels returned to pre-UV irradiated levels in both vehicle-fed and PL-fed mice (data not shown).
Polypodium Leucotomos Reduces UV-Induced Acute Inflammatory Responses in Mouse Skin
To quantify inflammatory infiltrate a single dermatopathologist blindfolded to treatment conditions examined all slides and assigned inflammatory score using a scale from 0 to 3. Representative images of H&E-stained UV-irradiated mouse skin fed with vehicle or PL showed ∼two- to threefold decreases in UV-induced inflammatory infiltrate in PL-fed mice at 24 and 48 hours (P < 0.01 and P < 0.05, respectively) (see Supplemental Figure S2, A and B, at http://ajp.amjpathol.org).
Polypodium Leucotomos Reduces UV-Induced Neutrophil Infiltration
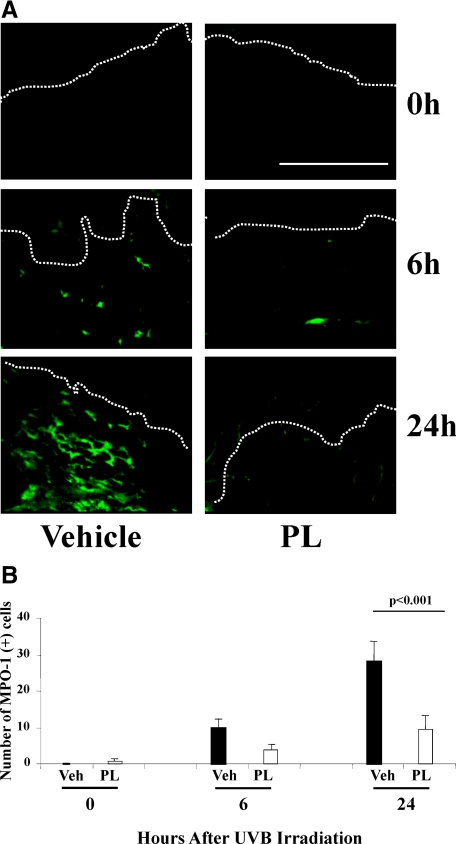
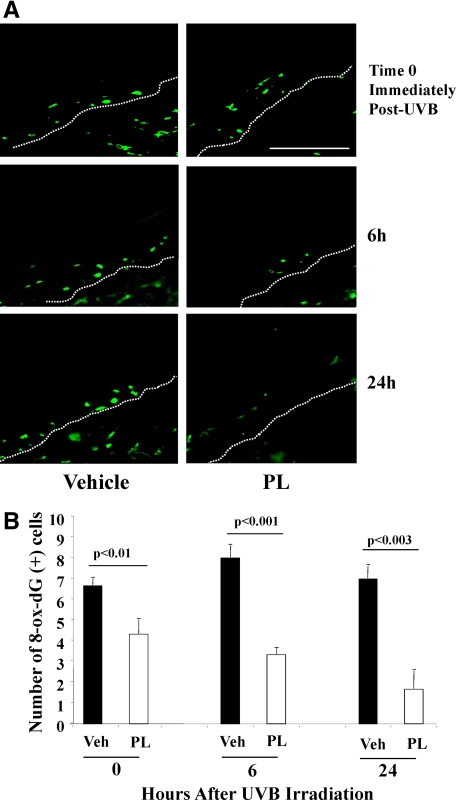
We evaluated the expression of MPO-1, a neutrophil marker, in PL-fed and vehicle-fed mice. Of note, MPO is an antimicrobial enzyme located in the primary granule of neutrophils and MPO-1 is the main MPO isozyme.22 Representative images of immunofluorescence of PL-fed or vehicle-fed mouse skin harvested at 0, 6, and 24 hours after UV irradiation are provided for MPO-1 immunostaining (Figure 3A) and for propidium iodide (PI) nuclear stain (see Supplemental Figure S3 at http://ajp.amjpathol.org). Quantification of MPO-1 positive cells showed no difference immediately post-UVB (time 0), a 60% decrease (p = NS) in number of cells with MPO-1 positive staining in PL-fed mice at 6 hours and a 68% decrease of MPO-1 positivity in PL-fed mice at 24 hours (P < 0.001) (Figure 3B) after UV irradiation, suggesting that PL treatment significantly reduces UV-induced neutrophil infiltration.
Figure 3.
A: Representative images of immunostained (green fluorescence) MPO-1 positive (+) cells (neutrophils) in mice treated with PL or vehicle at 0, 6, and 24 hours after UV radiation. Please note that by 48 and 72 hours after UV irradiation the number of MPO-1+ cells were negligible in PL-fed and vehicle-fed mice (data not shown), which did not permit quantification of MPO-1+ cells to allow comparison of the effects. B: Graphic representation of the number of MPO-1+ cells per 200 linear μm of epidermal length showed threefold significant decrease (N = 5 per treatment group, of six to eight visual fields/mouse) of MPO-1+ cells in PL-fed mice 24 hours after UV irradiation. Dermo-epidermal junctions (D-E) were identified by PI nuclear staining (see Supplemental Figure S3 at http://ajp.amjpathol.org) and are outlined by white dotted lines. Scale bar = 200 μm.
Polypodium Leucotomos Reduces UV-Induced Macrophage Infiltration
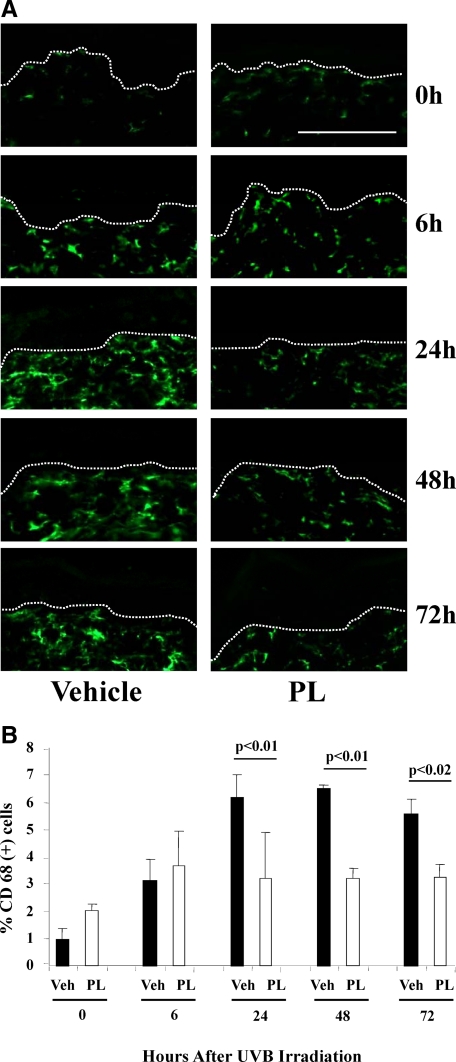
We evaluated the expression of CD-68, a glycoprotein normally expressed on macrophages, also known in mice as macrosialin,23 in PL-fed or vehicle-fed mice. Representative images of CD-68 immunostained PL or vehicle-fed mouse skin harvested at 0, 6, 24, 48, and 72 hours after UV irradiation (Figure 4A) and for PI nuclear stain (see Supplemental Figure S4 at http://ajp.amjpathol.org). Quantitative evaluation of the percentage of CD-68 positive cells showed a 48% decrease in CD-68 positive cells in PL-fed versus vehicle-fed mice 24 hours post-UVB (P < 0.01). This twofold decrease in PL-fed versus vehicle-fed mice had persisted throughout 48 and 72 hours (51% and 41%, P < 0.01 and P < 0.02, respectively) (Figure 4, A and B).
Figure 4.
A: Representative images of immunostained (green fluorescence) CD-68+ cells (macrophages) in mice treated with PL or vehicle at 0, 6, 24, 48, and 72 hours after UV irradiation. B: Graphic representation of the number of CD-68+ cells per 200 linear μm of epidermal length showed a marked decrease of these cells in PL-fed mice at 24, 48, and 72 hours after UV irradiation (N = 3 to 5 per treatment group, of six to eight visual fields per mouse). D-E junctions were identified by PI nuclear staining (see Supplemental Figure S4 at http://ajp.amjpathol.org) and are outlined by dotted lines. Scale bar = 200 μm.
Polypodium Leucotomos Accelerates the Removal of UV-Induced Photoproducts (CPDs) in Mouse Skin
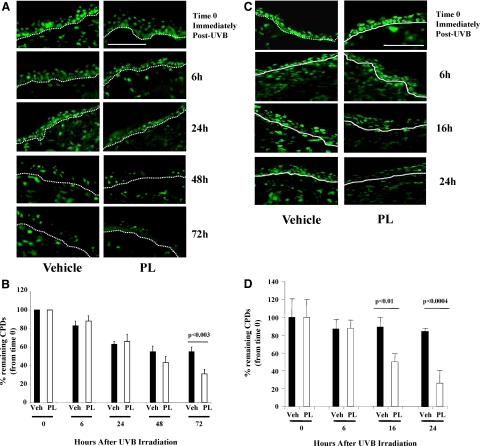
Adjacent sections of tissue samples from the same mice shown in Figure 4A were reacted with fluorescently tagged antibodies to CPDs. The abundant CPD positivity persisted in the skin of vehicle–fed Xpc+/− mice up to 72 hours post-UVB, whereas by 72 hours there were fewer CPD+ nuclei in PL-fed mice, suggesting improved CPD removal in PL-fed then UV-irradiated Xpc+/− mice (Figure 5A). Graphic representation of CPD+ nuclei per 200 linear μm of epidermal length (Figure 5B). For each treatment condition at each time point percent remaining CPDs were determined as the ratio of the CPD+ nuclei at that time, as compared with that immediately after irradiation (time 0). Maximum CPDs were detected immediately post-UV and initial CPD+ nuclei were comparable in two treatment groups. The removal of UV-induced photoproducts was not different in vehicle-fed versus PL-fed mice, up to 48 hours post-UV. However, by 72 hours (the last time point examined) there was a nearly twofold decrease (P < 0.05) in CPD+ cells in PL-fed versus vehicle-fed mice (Figure 5B), indicating substantial reduction of detectable UV-induced photoproducts in PL-fed then UV irradiated partially DNA repair-deficient Xpc+/− mice.
Figure 5.
A: Representative images of immunostained (green fluorescence) CPDs+ cells in Xpc+/− mice treated with PL or vehicle at 0, 6, 24, 48, and 72 hours after UV radiation. In vehicle-fed Xpc+/− mice, CPD positivity persisted up to 72 hours post-UVB, whereas by 72 hours there was noticeably less detectable CPDs remained in PL-fed Xpc+/− mouse skin (in epidermis as well as dermis). Percentage of remaining detectable CPDs were determined as the ratio of the CPD+ nuclei at that time compared with that immediately after irradiation (time 0). D-E junctions are outlined by dotted lines. Scale bar = 200 μm. B: Graphic representation of CPD+ nuclei per 200 linear μm of epidermal length showed a statistically significant decrease of percent remaining CPDs in PL-fed Xpc+/− mice at 72 hours. C: Representative images of immunostained (green fluorescence) CPDs+ cells in wild-type mice treated with PL or vehicle at 0, 6, 16, and 24 hours after UV irradiation. In vehicle-fed wild-type mice more than 80% CPD positivity persisted up to 24 hours post-UVB, whereas by 16 and 24 hours there was noticeably less detectable CPDs remained in PL-fed wild-type mouse skin (in epidermis as well as dermis). Percentage of remaining detectable CPDs were determined as the ratio of the CPD+ nuclei at that time compared with that immediately after irradiation (time 0). D-E junctions are outlined by dotted lines. Scale bar = 200 μm. D: Graphic representation of CPD+ nuclei per 200 linear μm of epidermal length showed statistically significant decrease of percent remaining CPDs in PL-fed wild-type mice (clear bars) at 16 and 24 hours.
To determine the effect of Xpc gene heterozygosity on removal of UV-induced photoproducts we evaluated removal of CPDs after a single irradiation in hairless wild-type mice, also. The abundant CPD positivity persisted in the skin of vehicle-fed wild-type mice up to 6 hours post-UVB, whereas there were 43% (P < 0.01) and 69% (P < 0.0004) fewer CPD+ nuclei in PL-fed mice already by 16 and 24 hours, respectively (Figure 5, C and D), suggesting that similar to Xpc+/− mice PL accelerates the removal of UV-induced CPDs in wild-type mice, however in difference with Xpc+/− mice significantly earlier (as early as 16 hours vs. 72 hours, in wild-type vs. Xpc+/− mice) (Figure 5, D, C vs. Figure 5, A, B).
Polypodium Leucotomos Decreases UV-Induced Oxidative DNA Damage
We evaluated the oxidative DNA damage using antibodies against 8-ox-dG, in PL-fed or vehicle-fed mouse skin 0, 6, and 24 hours after UVB irradiation. Representative images of 8-ox-dG positive cells are provided (Figure 6A). Representative images of nuclear (PI) staining and control samples were also taken (see Supplemental Figure S5, A and B, at http://ajp.amjpathol.org). Quantification of the number of 8-ox-dG+ cells showed a statistically significant 37% (P < 0.01) decrease of 8-ox-dG+ cells in PL-fed versus vehicle-fed mice already by 0 hours (presumably constitutive levels). Further 67% and 78% decreases in PL-fed versus vehicle-fed mice was observed at 6 and 24 hours after UV irradiation (P < 0.001, P < 0.003, respectively) (Figure 6, A and B).
Figure 6.
A: Representative images of immunostained (green fluorescence) 8-ox-dG (marker of oxidative damage) positive cells in mice treated with PL or vehicle at 0, 6, and 24 hours after UV radiation. As for MPO-1+ cells, the number (N = 5/treatment group of 6 to 8 visual fields/mouse) of 8-ox-dG+ cells were negligible at 48 and 72 hours post-UVB. We used isotope-specific IgG staining as negative control for 8-ox-dG (see Supplemental Figure S5A at http://ajp.amjpathol.org). D-E junctions were identified by PI staining (see Supplemental Figure S5B at http://ajp.amjpathol.org) and are outlined by white dotted lines. Scale bar = 200 μm. B: Graphic representation of the number of 8-ox-dG+ cells per 200 linear μm of epidermal length showed a marked decrease of these cells in PL-fed mice at 0, 6, and 24 hours after UV radiation (N = 5 per treatment group, of six to eight visual fields per mouse).
Polypodium Leucotomos Reduces Mutations in Murine Skin after a Single UV Exposure
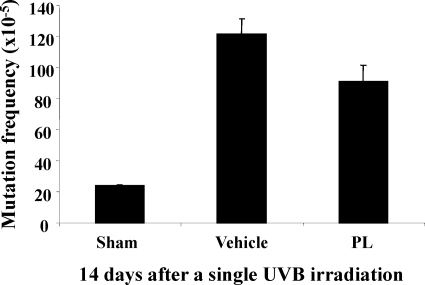
Because PL accelerates the removal of UV-induced photoproducts (CPDs) (Figure 5, A–D) and decreases the UV-induced oxidative DNA damage (Figure 6, A and B) in mouse skin, we wanted to determine whether feeding mice with PL for 2 weeks, before a single UV exposure may also reduce mutations in mouse skin in vivo, as anticipated from the known reciprocal relationship between decrease in DNA repair capacity and increase in mutations.24 Sham-irradiated mice had only a background level of mutations (24 ± 0 × 10−5) as reported previously for this assay.19,25 And this level increased nearly fivefold (121.5 ± 10 × 10−5) after a single UV exposure (Figure 7). Compared with vehicle control, UV-induced mutations in PL-fed-mice were decreased by ∼25% (121.5 ± 10.05 × 10−5 vs. 90.9 ± 10.6 × 10−5, vehicle vs. PL, P < 0.058) when assessed 2 weeks after the single UV exposure (Figure 7).
Figure 7.
Hairless Xpc+/− mice also transgenic for lacZ mutation-indicator gene were fed with vehicle or PL for 10 days and then were irradiated with a single dose of UVB (25 mJ/cm2). Two weeks after UVB, we evaluated mutation frequency (MF) in the lacZ mutation-indicator gene (seven to eight mice per treatment condition). Plotted values are MF per transgene in the harvested and homogenized epidermis (N = 7 to 8 per treatment group).
Discussion
UV light is known to be the principal causative agent in most skin cancers.26 Exposure of the skin to UV has been shown to cause inflammation, accumulation of reactive oxygen species, and photoproducts, such as CPDs.6,26,27 Each of these UV and other environmental carcinogen-induced biological responses taken separately or in combination are known etiological factors in processes of mutagenesis and carcinogenesis.6,18,27,28 In addition, compared with young skin, there is an age-dependent increase in constitutive and UV irradiation-induced prostaglandin E2 production and Cox-2 expression in adult human skin.29 This may result in chronic low grade inflammation that may have crucial pathophysiologic implications for many age-associated inflammatory processes, including but not limited to development of cutaneous malignancies,29,30 as well as tumors of colon, lung, prostate, breast, urinary bladder, pancreas, and liver.7,31,32,33
In the current work, we examined the photoprotective and anti-inflammatory role of a hydrophilic extract of PL in the prevention of UV-induced photocarcinogenesis.
Previous findings have indicated that PL has significant antioxidant activity when administered orally in humans (in limited number of subjects).34 PL has also been shown to reduce phototoxicity by decreasing inflammation (acute sunburn and depletion of Langerhans cells) and photoaging in both humans and an animal model.35,36 In addition, PL has been reported to prevent the loss of cell viability (apoptosis) and proliferation induced by UVA.37 Moreover, PL has also been proven to reduce inflammation through down-regulation of tumor necrosis factor-α.38 Our present findings support and significantly extend the current knowledge of PL’s photoprotective effects.
UV-induced p53 mutations are known to play a key role in the development of skin cancers.26 Conversely, when p53 is activated through post-translational modifications such as phosphorylation, tumor suppressive activities are shown.39 UV-induced photoproducts (CPDs), reactive oxygen species, and inflammation have been implicated not only with initiation, but also with promotion and progression of mutagenesis and carcinogenesis.40 Interestingly, p53 upregulation and activation has been reported to play direct role in modulation of key regulatory genes in DNA damage repair and inflammation.41,42 Indeed, upregulation of active p53 has been reported to play a role in the removal of oxidative DNA damage thereby reducing UV-induced carcinogenesis.28 In addition, activation of p53 is known to accelerate the removal of UVB-induced photoproducts—most importantly CPDs, which are considered to be the most significant mutagenic photoproducts based on their abundance and slow repair.43
Activation of p53 has been shown to decrease the expression of Cox-2, thereby reducing the inflammatory response.4,44,45 We4 and others46,47 have previously demonstrated that several biologically active substances,14 including p53 tumor suppresser gene, can induce transcriptional repression of Cox-2, as well as decrease Cox-2 protein levels. In this study we found that levels of phospho-p53ser15, presumably active form of p53, were increased in the skin of PL-fed mice, which inversely correlated with decreased Cox-2 levels, suggesting that orally administered PL reduces UV-induced Cox-2 levels in mouse skin through, at least in part, by activating tumor suppressor protein p53. In addition, we also found significant decrease in UV-induced inflammatory infiltrate in the skin of PL-fed mice strongly implying PL-mediated p53 activation and decrease in Cox-2 levels in reduction of UV-induced inflammatory responses in PL-fed mice.
To date, attention has been mostly directed toward developing therapies that can inhibit Cox-2 enzyme activity, non-selectively using NSAIDs (aspirin, ibuprofen, naproxen)48 and selectively using Cox-2 inhibitors (celecoxib and other coxibs),32,49 and minimal attention has been given to modulation of Cox-2 protein levels, known to be constitutively increased in many tumors.48 Indeed, recent studies suggest that regular oral administration of NSAIDs has preventative effect against colon, breast, prostate, and melanoma.14,50 In addition, in murine models of skin carcinogenesis, it has been shown that administration of NSAIDs, especially selective Cox-2 inhibitors, reduces the prevalence and multiplicity of UV light-induced neoplasms9,51,52 strongly implying direct involvement of Cox-2 in cutaneous carcinogenesis. Furthermore, specific inhibitors of Cox-2 such as celecoxib have been shown not only to decrease tumorigenesis and increase tumor latency in hairless mice models,53 but also to decrease tumor growth in hairless mice with pre-existing UVB-induced tumors.54 However, chronic long-term use of these medications has been associated with a number, in some cases life-threatening, side effects including decreased gastric protection that may lead to gastrointestinal bleeding, impairment of renal function, and inhibition of platelet aggregation55 and most recently, increased incidence of myocardial infarction in elderly.56,57
These negative findings of long-term use of NSAIDs and selective Cox-2 inhibitors underscores significantly the necessity to identify safer natural products capable of affording protections after UV damage to reduce UV-induced inflammation, improve DNA damage repair and ultimately prevent mutagenesis and carcinogenesis. In this regard, UV protective effects of PL can be compared with other widely used natural product such as green tea. In many studies it has been shown that administration of green tea polyphenols in drinking water or topical application of their major and most chemopreventative compound, (−)-epigallocatechin-3-gallate, inhibits UV-induced immunosuppression and macrophage/neutrophil infiltration, hence inhibiting generation of reactive oxygen species and generation of prostaglandin metabolites (eg, Cox-2) thereby preventing UV-induced skin carcinogenesis.58,59 Moreover, similar to PL (−)-epigallocatechin-3-gallate has been shown to improve DNA repair capacity after UV radiation through improvement of repair or removal of CPDs, major UV-induced photoproducts.60 However in difference with PL (p53 activation) UV-protective effects of (−)-epigallocatechin-3-gallate are mediated mainly through activation of interleukin-12.58,60 Despite of different photoprotective mechanisms afforded by (−)-epigallocatechin-3-gallate or PL both natural substances demonstrate numerous biologically relevant UV damage protective and anti-cancer effect including: (a) enhanced repair of UV-induced major photoproducts, CPDs; (b) prevention of generation of reactive oxygen species and free radicals,61,62 and improved repair of oxidative DNA damage; and (c) inhibition of generation/production of various mediators of inflammation such as prostaglandins, histamines, leukotrienes, and other cytoxines.63 Due to these significant UV damage-preventative effects on several well-recognized harmful UV-damaging mechanisms, it is strongly indicative that these natural substances may be of great use and importance in our efforts to minimize acute UV irradiation mediated harmful genotoxic responses.
In summary, oral supplementation of the natural antioxidant Polypodium Leucotomos extract supplementation affords the following photoprotective effects: (a) increase in the expression of active p53; (b) inhibition of UV-induced Cox-2 enzyme levels; (c) reduction UV-induced acute inflammatory responses; (d) acceleration of the removal of UV-induced photoproducts (CPDs); (e) decrease of UV-induced oxidative DNA damage; and (f) decrease of UV-induced mutations. The results of our study strongly suggest that oral administration of Polypodium leucotomos extract could offer significant photoprotective effects essential to the treatment and prevention of UV-induced skin cancer. Furthermore, we propose that PL supplementation may also be useful for prevention and treatment of low-grade inflammation that accompanies aging process.29
Supplementary Material
Footnotes
Address reprint requests to David A. Goukassian, M.D., Ph.D., Department of Dermatology, Boston University School of Medicine, 609 Albany Street. Boston, MA 02118. E-mail: dgoukass@bu.edu.
Supported by Industrial Farmacéutica Cantabria, Spain.
Conflict of interest: Dr. Salvador Gonzalez serves as a medical consultant for Industrial Farmacéutica Cantabria.
Supplemental material for this article can be found on http://ajp.amjpathol.org.
Current address for E.Z., Clinica Dermatologica di Padova, Via Cesare Battisti 206, Padova, Italy, Departments of Dermatology.
References
- Lewis KG, Weinstock MA. Trends in nonmelanoma skin cancer mortality rates in the United States, 1969 through 2000. J Invest Dermatol. 2007;127:2323–2327. doi: 10.1038/sj.jid.5700897. [DOI] [PubMed] [Google Scholar]
- Serrano H, Scotto J, Shornick G, Fears TR, Greenberg ER. Incidence of non melanoma skin cancer in New Hampshire and Vermont. Am Acad Dermatol. 1991;4:574–579. doi: 10.1016/0190-9622(91)70086-h. [DOI] [PubMed] [Google Scholar]
- Goukassian DA, Gilchrest BA. The interdependence of skin aging, skin cancer, and DNA repair capacity: a novel perspective with therapeutic implications. Rejuv Res. 2004;7:175–185. doi: 10.1089/rej.2004.7.175. [DOI] [PubMed] [Google Scholar]
- Marwaha V, Chen YH, Helms E, Arad S, Inoue H, Bord E, Kishore R, Der Sarkissian R, Gilchrest BA, Goukassian DA. T-oligo treatment decreases constitutive and UVB-induced COX-2 levels through P53-and NFkappa B-dependent repression of the COX-2 promoter. J Biol Chem. 2005;280:32379–32388. doi: 10.1074/jbc.M503245200. [DOI] [PubMed] [Google Scholar]
- Middelkamp-Hup MA, Pathak MA, Parrado C, Goukassian D, Rius-Diaz F, Mihm MC, Fitzpatrick TB, Gonzalez S. Oral Polypodium leucotomos extract decreases ultraviolet-induced damage of human skin. J Am Acad Dermatol. 2004;51:910–918. doi: 10.1016/j.jaad.2004.06.027. [DOI] [PubMed] [Google Scholar]
- Rundhaug JE, Mikulec C, Pavone A, Fischer SM. A role for cyclooxygenase-2 in ultraviolet light-induced skin carcinogenesis. Mol Carcinogen. 2007;46:692–698. doi: 10.1002/mc.20329. [DOI] [PubMed] [Google Scholar]
- Buckman SY, Gresham A, Hale P, Hruza G, Anast J, Masferrer J, Pentland AP. COX-2 expression is induced by UVB exposure in human skin: implications for the development of skin cancer. Carcinogenesis. 1998;19:723–729. doi: 10.1093/carcin/19.5.723. [DOI] [PubMed] [Google Scholar]
- Tripp CS, Blomme EA, Chinn KS, Hardy MM, LaCelle P, Pentland AP. Epidermal COX-2 induction following ultraviolet irradiation: suggested mechanism for the role of COX-2 inhibition in photoprotection. J Invest Dermatol. 2003;121:853–861. doi: 10.1046/j.1523-1747.2003.12495.x. [DOI] [PubMed] [Google Scholar]
- Fischer SM, Lo HH, Gordon GB, Seibert K, Kelloff G, Lubet RA, Conti CJ. Chemopreventive activity of celecoxib, a specific cyclooxygenase-2 inhibitor, and indomethacin against ultraviolet light-induced skin carcinogenesis. Mol Carcinogen. 1999;25:231–240. [PubMed] [Google Scholar]
- Pentland AP, Schoggins JW, Scott GA, Khan KN, Han R. Reduction of UV-induced skin tumors in hairless mice by selective COX-2 inhibition. Carcinogenesis. 1999;20:1939–1944. doi: 10.1093/carcin/20.10.1939. [DOI] [PubMed] [Google Scholar]
- Cheuk BL, Leung PS, Lo AC, Wong PY. Androgen control of cyclooxygenase expression in the rat epididymis. Biol Reprod. 2000;63:775–780. doi: 10.1093/biolreprod/63.3.775. [DOI] [PubMed] [Google Scholar]
- Subbarayan V, Sabichi AL, Llansa N, Lippman SM, Menter DG. Differential expression of cyclooxygenase-2 and its regulation by tumor necrosis factor-alpha in normal and malignant prostate cells. Cancer Res. 2001;61:2720–2726. [PubMed] [Google Scholar]
- Evans JF, Kargman SL. Cancer and cyclooxygenase-2 (COX-2) inhibition. Curr Pharm Des. 2004;10:627–634. doi: 10.2174/1381612043453126. [DOI] [PubMed] [Google Scholar]
- Fosslien E. Review: molecular pathology of cyclooxygenase-2 in cancer-induced angiogenesis. Ann Clin Lab Sci. 2001;31:325–348. [PubMed] [Google Scholar]
- Gonzalez S, Alonso-Lebrero JL, Del Rio R, Jaen P. Polypodium leucotomos extract: a nutraceutical with photoprotective properties. Drugs Today (Barc) 2007;43:475–485. doi: 10.1358/dot.2007.43.7.1062667. [DOI] [PubMed] [Google Scholar]
- Sands AT, Abuin A, Sanchez A, Conti CJ, Bradley A. High susceptibility to ultraviolet-induced carcinogenesis in mice lacking XPC. Nature. 1995;377:162–165. doi: 10.1038/377162a0. [DOI] [PubMed] [Google Scholar]
- Cheo DL, Ruven HJ, Meira LB, Hammer RE, Burns DK, Tappe NJ, van Zeeland AA, Mullenders LH, Friedberg EC. Characterization of defective nucleotide excision repair in XPC mutant mice. Mutat Res. 1997;374:1–9. doi: 10.1016/s0027-5107(97)00046-8. [DOI] [PubMed] [Google Scholar]
- Tober KL, Wilgus TA, Kusewitt DF, Thomas-Ahner JM, Maruyama T, Oberyszyn TM. Importance of the EP(1) receptor in cutaneous UVB-induced inflammation and tumor development. J Invest Dermatol. 2006;126:205–211. doi: 10.1038/sj.jid.5700014. [DOI] [PubMed] [Google Scholar]
- Goukassian DA, Helms E, van Steeg H, van Oostrom C, Bhawan J, Gilchrest BA. Topical DNA oligonucleotide therapy reduces UV-induced mutations and photocarcinogenesis in hairless mice. Proc Natl Acad Sci USA. 2004;101:3933–3938. doi: 10.1073/pnas.0306389101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allan AE, Archambault M, Messana E, Gilchrest BA. Topically applied diacylglycerols increase pigmentation in guinea pig skin. J Invest Dermatol. 1995;105:687–692. doi: 10.1111/1523-1747.ep12324466. [DOI] [PubMed] [Google Scholar]
- Boerrigter ME, Dolle ME, Martus HJ, Gossen JA, Vijg J. Plasmid-based transgenic mouse model for studying in vivo mutations. Nature. 1995;377:657–659. doi: 10.1038/377657a0. [DOI] [PubMed] [Google Scholar]
- Park YJ, Lee SH, Jang SJ, Moon DS, Park CY, Chung CH, Cha JH, Lee BR, Choi YH. Myeloperoxidase (MPO) isozymes of normal neutrophils from normal persons and reactive neutrophils from patients with neutrophilia; using 10% native PAGE and activity stain for MPO. Korean J Hematol. 2003;38:48–54. [Google Scholar]
- Kurushima H, Ramprasad M, Kondratenko N, Foster DM, Quehenberger O, Steinberg D. Surface expression and rapid internalization of macrosialin (mouse CD68) on elicited mouse peritoneal macrophages. J Leukoc Biol. 2000;67:104–108. doi: 10.1002/jlb.67.1.104. [DOI] [PubMed] [Google Scholar]
- Moriwaki S, Ray S, Tarone RE, Kraemer KH, Grossman L. The effect of donor age on the processing of UV-damaged DNA by cultured human cells: reduced DNA repair capacity and increased DNA mutability. Mutat Res. 1996;364:117–123. doi: 10.1016/0921-8777(96)00029-8. [DOI] [PubMed] [Google Scholar]
- van Steeg H, Mullenders LH, Vijg J. Mutagenesis and carcinogenesis in nucleotide excision repair-deficient XPA knock out mice. Mutat Res. 2000;450:167–180. doi: 10.1016/s0027-5107(00)00023-3. [DOI] [PubMed] [Google Scholar]
- Ichihashi M, Ueda M, Budiyanto A, Bito T, Oka M, Fukunaga M, Tsuru K, Horikawa T. UV-induced skin damage. Toxicology. 2003;189:21–39. doi: 10.1016/s0300-483x(03)00150-1. [DOI] [PubMed] [Google Scholar]
- Nishigori C, Hattori Y, Toyokuni S. Role of reactive oxygen species in skin carcinogenesis. Antioxid Redox Signal. 2004;6:561–570. doi: 10.1089/152308604773934314. [DOI] [PubMed] [Google Scholar]
- Achanta G, Huang P. Role of p53 in sensing oxidative DNA damage in response to reactive oxygen species-generating agents. Cancer Res. 2004;64:6233–6239. doi: 10.1158/0008-5472.CAN-04-0494. [DOI] [PubMed] [Google Scholar]
- Seo JY, Kim EK, Lee SH, Park KC, Kim KH, Eun HC, Chung JH. Enhanced expression of cylooxygenase-2 by UV in aged human skin in vivo. Mech Ageing Dev. 2003;124:903–910. doi: 10.1016/s0047-6374(03)00150-7. [DOI] [PubMed] [Google Scholar]
- An KP, Athar M, Tang X, Katiyar SK, Russo J, Beech J, Aszterbaum M, Kopelovich L, Epstein EH, Jr, Mukhtar H, Bickers DR. Cyclooxygenase-2 expression in murine and human nonmelanoma skin cancers: implications for therapeutic approaches. Photochem Photobiol. 2002;76:73–80. doi: 10.1562/0031-8655(2002)076<0073:ceimah>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Shiota G, Okubo M, Noumi T, Noguchi N, Oyama K, Takano Y, Yashima K, Kishimoto Y, Kawasaki H. Cyclooxygenase-2 expression in hepatocellular carcinoma. Hepatogastroenterology. 1999;46:407–412. [PubMed] [Google Scholar]
- Soslow RA, Dannenberg AJ, Rush D, Woerner BM, Khan KN, Masferrer J, Koki AT. COX-2 is expressed in human pulmonary, colonic, and mammary tumors. Cancer. 2000;89:2637–2645. doi: 10.1002/1097-0142(20001215)89:12<2637::aid-cncr17>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Tucker ON, Dannenberg AJ, Yang EK, Zhang F, Teng L, Daly JM, Soslow RA, Masferrer JL, Woerner BM, Koki AT, Fahey TJ., 3rd Cyclooxygenase-2 expression is up-regulated in human pancreatic cancer. Cancer Res. 1999;59:987–990. [PubMed] [Google Scholar]
- Gombau L, Garcia F, Lahoz A, Fabre M, Roda-Navarro P, Majano P, Alonso-Lebrero JL, Pivel JP, Castell JV, Gomez-Lechon MJ, Gonzalez S. Polypodium leucotomos extract: antioxidant activity and disposition. Toxicol In Vitro. 2006;20:464–471. doi: 10.1016/j.tiv.2005.09.008. [DOI] [PubMed] [Google Scholar]
- Gonzalez S, Pathak MA, Cuevas J, Villarrubia VG, Fitzpatrick TB. Topical or oral administration with an extract of Polypodium leucotomos prevents acute sunburn and psoralen-induced phototoxic reactions as well as depletion of Langerhans cells in human skin. Photodermatol Photoimmunol Photomed. 1997;13:50–60. doi: 10.1111/j.1600-0781.1997.tb00108.x. [DOI] [PubMed] [Google Scholar]
- Middelkamp-Hup M, Pathak M, Parrado C, Garcia-Caballero T, Rius-Diaz F, Fitzpatrick T, Gonzalez S. Orally administered Polypodium leucotomos extract decreases psoralen-UVA-induced phototoxicity, pigmentation, and damage of human skin. J Am Acad Dermatol. 2003;49:1–9. doi: 10.1016/s0190-9622(03)02732-4. [DOI] [PubMed] [Google Scholar]
- Alonso-Lebrero JL, Dominguez-Jimenez C, Tejedor R, Brieva A, Pivel JP. Photoprotective properties of a hydrophilic extract of the fern Polypodium leucotomos on human skin cells. J Photochem Photobiol B. 2003;70:31–37. doi: 10.1016/s1011-1344(03)00051-4. [DOI] [PubMed] [Google Scholar]
- Janczyk A, Garcia-Lopez MA, Fernandez-Penas P, Alonso-Lebrero JL, Benedicto I, Lopez-Cabrera M, Gonzalez S. A Polypodium leucotomos extract inhibits solar-simulated radiation-induced TNF-alpha and iNOS expression, transcriptional activation and apoptosis. Exp Dermatol. 2007;16:823–829. doi: 10.1111/j.1600-0625.2007.00603.x. [DOI] [PubMed] [Google Scholar]
- Kastan MB. Wild-type p53: tumors can’t stand it. Cell. 2007;128:837–840. doi: 10.1016/j.cell.2007.02.022. [DOI] [PubMed] [Google Scholar]
- Nishigori C. Cellular aspects of photocarcinogenesis. Photochem Photobiol Sci. 2006;5:208–214. doi: 10.1039/b507471a. [DOI] [PubMed] [Google Scholar]
- Komarova EA, Krivokrysenko V, Wang K, Neznanov N, Chernov MV, Komarov PG, Brennan ML, Golovkina TV, Rokhlin OW, Kuprash DV, Nedospasov SA, Hazen SL, Feinstein E, Gudkov AV. p53 is a suppressor of inflammatory response in mice. FASEB J. 2005;19:1030–1032. doi: 10.1096/fj.04-3213fje. [DOI] [PubMed] [Google Scholar]
- Smith ML, Fornace AJ., Jr p53-mediated protective responses to UV irradiation. Proc Natl Acad Sci USA. 1997;94:12255–12257. doi: 10.1073/pnas.94.23.12255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You YH, Szabo PE, Pfeifer GP. Cyclobutane pyrimidine dimers form preferentially at the major p53 mutational hotspot in UVB-induced mouse skin tumors. Carcinogenesis. 2000;21:2113–2117. doi: 10.1093/carcin/21.11.2113. [DOI] [PubMed] [Google Scholar]
- Gallo O, Schiavone N, Papucci L, Sardi I, Magnelli L, Franchi A, Masini E, Capaccioli S. Down-regulation of nitric oxide synthase-2 and cyclooxygenase-2 pathways by p53 in squamous cell carcinoma. Am J Pathol. 2003;163:723–732. doi: 10.1016/S0002-9440(10)63699-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subbaramaiah K, Altorki N, Chung WJ, Mestre JR, Sampat A, Dannenberg AJ. Inhibition of cyclooxygenase-2 gene expression by p53. J Biol Chem. 1999;274:10911–10915. doi: 10.1074/jbc.274.16.10911. [DOI] [PubMed] [Google Scholar]
- Brecher AR. The role of cyclooxygenase-2 in the pathogenesis of skin cancer. J Drugs Dermatol. 2002;1:44–47. [PubMed] [Google Scholar]
- Dannenberg AJ, Subbaramaiah K. Targeting cyclooxygenase-2 in human neoplasia: rationale and promise. Cancer Cell. 2003;4:431–436. doi: 10.1016/s1535-6108(03)00310-6. [DOI] [PubMed] [Google Scholar]
- Koki AT, Masferrer JL. Celecoxib: a specific COX-2 inhibitor with anticancer properties. Cancer Control. 2002;9:28–35. doi: 10.1177/107327480200902S04. [DOI] [PubMed] [Google Scholar]
- Kulkarni S, Rader JS, Zhang F, Liapis H, Koki AT, Masferrer JL, Subbaramaiah K, Dannenberg AJ. Cyclooxygenase-2 is overexpressed in human cervical cancer. Clin Cancer Res. 2001;7:429–434. [PubMed] [Google Scholar]
- Harris RE, Beebe-Donk J, Namboodiri KK. Inverse association of non-steroidal anti-inflammatory drugs and malignant melanoma among women. Oncol Rep. 2001;8:655–657. doi: 10.3892/or.8.3.655. [DOI] [PubMed] [Google Scholar]
- Fischer SM. Is cyclooxygenase-2 important in skin carcinogenesis? J Environ Pathol Toxicol Oncol. 2002;21:183–191. [PubMed] [Google Scholar]
- Pentland AP. Cyclooxygenase inhibitors for skin cancer prevention: are they beneficial enough? Arch Dermatol. 2002;138:823–824. doi: 10.1001/archderm.138.6.823. [DOI] [PubMed] [Google Scholar]
- Orengo IF, Gerguis J, Phillips R, Guevara A, Lewis AT, Black HS. Celecoxib, a cyclooxygenase 2 inhibitor as a potential chemopreventive to UV-induced skin cancer: a study in the hairless mouse model. Arch Dermatol. 2002;138:751–755. doi: 10.1001/archderm.138.6.751. [DOI] [PubMed] [Google Scholar]
- Fischer SM, Conti CJ, Viner J, Aldaz CM, Lubet RA. Celecoxib and difluoromethylornithine in combination have strong therapeutic activity against UV-induced skin tumors in mice. Carcinogenesis. 2003;24:945–952. doi: 10.1093/carcin/bgg046. [DOI] [PubMed] [Google Scholar]
- Lapane KL, Spooner JJ, Pettitt D. The effect of nonsteroidal anti-inflammatory drugs on the use of gastroprotective medication in people with arthritis. Am J Manag Care. 2001;7:402–408. [PubMed] [Google Scholar]
- Dajani EZ, Islam K. Cardiovascular and gastrointestinal toxicity of selective cyclo-oxygenase-2 inhibitors in man. J Physiol Pharmacol. 2008;59 Suppl 2:117–133. [PubMed] [Google Scholar]
- Fosbol EL, Gislason GH, Jacobsen S, Folke F, Hansen ML, Schramm TK, Sorensen R, Rasmussen JN, Andersen SS, Abildstrom SZ, Traerup J, Poulsen HE, Rasmussen S, Kober L, Torp-Pedersen C. Risk of myocardial infarction and death associated with the use of nonsteroidal anti-inflammatory drugs (NSAIDs) among healthy individuals: a nationwide cohort study. Clin Pharmacol Ther. 2009;85:190–197. doi: 10.1038/clpt.2008.204. [DOI] [PubMed] [Google Scholar]
- Katiyar SK, Challa A, McCormick TS, Cooper KD, Mukhtar H. Prevention of UVB-induced immunosuppression in mice by the green tea polyphenol (−)-epigallocatechin-3-gallate may be associated with alterations in IL-10 and IL-12 production. Carcinogenesis. 1999;20:2117–2124. doi: 10.1093/carcin/20.11.2117. [DOI] [PubMed] [Google Scholar]
- Mantena SK, Meeran SM, Elmets CA, Katiyar SK. Orally administered green tea polyphenols prevent ultraviolet radiation-induced skin cancer in mice through activation of cytotoxic T cells and inhibition of angiogenesis in tumors. J Nutr. 2005;135:2871–2877. doi: 10.1093/jn/135.12.2871. [DOI] [PubMed] [Google Scholar]
- Meeran SM, Mantena SK, Elmets CA, Katiyar SK. (−)-Epigallocatechin-3-gallate prevents photocarcinogenesis in mice through interleukin-12-dependent DNA repair. Cancer Res. 2006;66:5512–5520. doi: 10.1158/0008-5472.CAN-06-0218. [DOI] [PubMed] [Google Scholar]
- Athar M, Agarwal R, Bickers DR, Mukhtar H. Role of reactive oxygen species. Mukhtar H, editor. Boca Raton: CRC Press Inc.,; 1992:pp 269–279. [Google Scholar]
- Dalle Carbonare M, Pathak MA. Skin photosensitizing agents and the role of reactive oxygen species in photoaging. J Photochem Photobiol B. 1992;14:105–124. doi: 10.1016/1011-1344(92)85086-a. [DOI] [PubMed] [Google Scholar]
- Norris PG, Gange RW, Hawk JLM. Acute effects of ultraviolet radiation on the skin. Fitzpatrick TB, Eisen AZ, Wolff K, Freedberg IM, Austen KF, editors. New York,: McGraw-Hill, Inc.,; 1993:pp 1651–1658. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.