Abstract
Vascular endothelial growth factor receptor (VEGFR)-2 is a major stimulator of hemangiogenesis (HA), whereas VEGFR-3 stimulates lymphangiogenesis (LA). Contrary to this understanding, we demonstrate that implantation of pellets containing VEGFR-3-specific ligands (VEGF-C156S and recombinant murine VEGF-D) into the corneal stroma induce not only LA but also robust HA characterized by blood vessels that are positive for VEGFR-3 expression. The implantation of pellets containing VEGFR-3-specific ligands also leads to the recruitment of VEGF-A-secreting macrophages. Depletion of these infiltrating macrophages using clodronate-liposome administration shows a significant reduction in HA as well as LA. Blockade of either VEGFR-2 or VEGFR-3 signaling reduces both HA and LA; however, the percent reduction of HA is greater in the VEGFR-2 blockade group. In addition, in the VEGFR-3 blockade group, the percent reduction of HA is significantly greater with VEGFR-3-specific ligands than that by VEGF-A or VEGF-C. Collectively, our data suggest that VEGFR-3-specific signaling can induce new blood vessels, to which macrophages contribute a major role, and signify its potential as an additional therapeutic target to the existing VEGF-A/VEGFR-2 signaling-based antiangiogenesis strategies.
Vascular endothelial growth factors (VEGFs), the key regulators of vasculogenesis, exert their effect via specific transmembrane tyrosine kinases, which include VEGF receptor (VEGFR)-1 (Flt-1), VEGFR-2 (KDR or Flk-1), and VEGFR-3 (Flt-4).1,2,3 VEGF-A binds to both VEGFR-1 and VEGFR-2 and regulates hemangiogenesis (HA), whereas VEGF-C and VEGF-D bind to VEGFR-3 and regulate lymphangiogenesis (LA).2,4 VEGF-C can also directly bind to VEGFR-2 and induce HA.5 Furthermore, VEGF-D and VEGF-C can be proteolytically processed and bind VEGFR-2 in addition to VEGFR-3.4
The major function of VEGFR-2 is the stimulation of blood vascular endothelial cell survival/growth and promotion of HA.2,4 VEGFR-2 is highly expressed in vascular endothelial progenitors in early embryogenesis.6 During later stages of vascular development, VEGFR-2 expression declines but can be up-regulated under conditions of pathological angiogenesis such as in tumors and in inflammation. During early embryogenesis, VEGFR-3 mRNA is expressed by most of the endothelial cells, and VEGFR-3 gene inactivation results in embryonic death because of abnormal remodeling of the primary vascular plexus.7 In the later stages of development, VEGFR-3 expression becomes gradually restricted to lymphatic vessels (LVs),8 although fenestrated blood capillaries of some adult organs continue to express low levels of VEGFR-3.9 VEGFR-3 is also expressed by some subsets of bone marrow-derived cells, including monocytes, macrophages, and dendritic cells.10,11,12 The major functional role of VEGFR-3 during the postnatal period is thought to be limited to the induction of LVs.3,4,13 It is reported that signaling via VEGFR-3 alone is sufficient for lymphangiogenic signals, because mutant VEGF-C156S, which only activates VEGFR-314,15 but not VEGFR-2, induces a similar phenotype to nonmutant VEGF-C-transgenic mice.16,17
On the basis of our current understanding, VEGFR-3-specific ligands, VEGF-C156S, and recombinant murine VEGF-D (rmVEGF-D)16,17,18 should principally induce new LVs without significant concurrent blood vessel (BV) formation. Herein, we present data demonstrating that contrary to our expectations, VEGF-C156S and rmVEGF-D induce not only LVs but also significant BVs. Moreover, these newly formed blood vascular endothelial cells express copious VEGFR-3. Previously, VEGFR-3 expression has been shown only in neovascularization related to vascular tumors and some nonvascular tumors.19,20,21 Induction of new BVs by VEGFR-3-mediated signaling alone has not yet been reported, even though one study previously reported the de novo expression of VEGFR-3 in pre-existing iris BVs injected with VEGF-A.22 This is important, because the contribution of VEGFR-3 to HA sheds light on potential limitations to most current antiangiogenic strategies that rely solely on blockade of VEGF-A or VEGFR-2. Our study also demonstrates that innate immune cells, particularly macrophages, are recruited in response to VEGFR-3 stimulation and contribute significantly to angiogenesis. Herein, we delineate the mechanisms of VEGFR-3+ newly formed BVs through VEGFR-3-specific signaling and demonstrate the effects of macrophage depletion as well as selective blockade of VEGFR-2 and VEGFR-3 on angiogenesis induced by VEGFR-3-specific ligands VEGF-C156S and rmVEGF-D.
Materials and Methods
Animals
Male 6- to 8-week-old BALB/c mice were used as recipients for VEGF pellets. All animals were anesthetized before any surgery and treated in accordance with the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research. All experiments described herein were conducted under Institutional Animal Care and Use Committee approval.
VEGF Pellet Implantation
Mutant recombinant human VEGF-C156S and rmVEGF-D, which have specific affinity for VEGFR-3 but not for VEGFR-2 in the mouse, were used to test VEGFR-3 specific stimulation.14,15,18 VEGF-A and VEGF-C were used as controls to compare with VEGFR-3-specific ligands. Pellets containing 80 ng of VEGF-A (a gift from BRB Preclinical Repository, National Cancer Institute, Bethesda, MD), VEGF-C, VEGF-C156S, or -D (R&D Systems, Minneapolis, MN) were prepared as described previously.23 Briefly, an initial half thickness linear incision was made at the center of cornea using a disposable ophthalmic microknife. A lamellar pocket incision was then made parallel to the corneal plane using a Von Graefe knife and advanced to the temporal limbus at lateral canthal area. The pellets were positioned into the pocket 1.0 mm apart from the limbal vascular arcade, and tetracycline ophthalmic ointment was applied to the eye after pellet implantation.
Biomicroscopic Examination
Eyes were examined by slit lamp biomicroscopy on postoperative day 7, and photographs were taken at the same time point. Slit lamp photographs were taken using retroillumination technique after dilating the pupil with 2.5% phenylephrine hydrochloride (AK-dilate, Akorn, NJ) and 1% tropicamide (Tropicacyl, Akorn, NJ) ophthalmic solutions.
Immunohistochemistry and Morphometry of HA and LA
After taking pictures under the slit lamp, five mice per group were sacrificed at day 7 postimplantation. Corneal flat mounts were prepared for immunohistochemical staining with FITC-conjugated CD31 (Santa Cruz Biotechnology, Santa Cruz, CA) and lymphatic vessel endothelial hyaluronan receptor-1 (LYVE-1) (abcam, Cambridge, MA), and rhodamine-conjugated secondary antibody to LYVE-1. Low magnification power (×2) digital pictures of immunostained corneal flat mounts were taken with the fluorescent microscope and the area covered by CD31high/LYVE-1− BVs and CD31low/LYVE-1high lymph vessels were calculated using NIH Image software (Image J 1.34). HA and LA were evaluated by measuring the percentage of the total corneal area covered by BVs and LVs. To compare the inhibitory effect of macrophage depletion, VEGFR-2 blockade, or VEGFR-3 blockade on HA and LA, the percent reduction of BV and LV was calculated by comparing to mean values of BV and LV of control groups.
Immunohistochemical Study for Detection of Innate Immune Cells
Seven days after pellet implantation, five mice per group were sacrificed, and corneal flat mounts were prepared for immunohistochemical staining of macrophages with rat anti-mouse F4/80 (GeneTex, San Antonio, TX) and neutrophils with rat anti-mouse NIMP-R14 (abcam). The F4/80 antigen is expressed on a wide range of mature tissue macrophages and a subpopulation of dendritic cells.24 The monoclonal antibody NIMP-R14 has a high specificity for murine neutrophils.25 Digital pictures of immunostained whole-mount corneas were taken with a confocal microscope, and the numbers of recruited F4/80+ and NIMP-R14+ cells (macrophages and neutrophils, respectively) per high magnification power field (×400) were counted manually. To compare the inhibitory effect of macrophage depletion, VEGFR-2 blockade or VEGFR-3 blockade in innate immune cell recruitment, the percent reduction of enumerated cell number was calculated by comparing to mean values of control groups.
Depletion of Macrophages with Clodronate Liposome
Depletion of macrophages in vivo was achieved with dichloromethylene diphosphonate-liposome (CL2MDP-lip) as described previously.26,27,28 Clodronate (Roche Diagnostics, Mannheim, Germany) was encapsulated in liposomes as described earlier.25 Briefly, phosphatidylcholine (Lipoid, Ludwigshafen, Germany) and cholesterol (Sigma Chemical, St. Louis, MO) were dissolved in a mixture of methanol and chloroform. The lipids were mixed with clodronic acid dissolved in PBS. Resuspension of liposomes was achieved by water sonication at room temperature, and resultant liposomes were washed in an ultracentrifuge. Systemic macrophage depletion was achieved by CL2MDP-lip (200 μl = 1 mg) via i.p. administration at 4 days and 24 hours before pellet implantation and then at every 24 hours after implantation for 1 week. Subconjunctival (SCj) injections of 50 μg (10 μl) CL2MDP-lip were performed to deplete local macrophages. Control groups received i.p. and subconjunctival administration of PBS liposomes.
Flow cytometric analysis was performed to evaluate the efficacy of systemic macrophage depletion. Briefly, white blood cells were separated from EDTA-anticoagulated whole blood by gradient separation technique. The white blood cells were labeled with a rat anti-mouse F4/80 antigen-FITC conjugate (eBioscience, San Diego, CA) and rat anti-mouse CD 45 antigen-rhodamine conjugate (eBioscience). CD45+F4/80+ cells in peripheral blood decreased from 10 to 1% by repeated i.p. injection of clodronate liposome. Local corneal depletion of macrophages was evaluated by counting F4/80+ cells under ×400 high-power field confocal photomicrographs of flat mount corneas. The enumerated numbers of F4/80+ cells in cornea showed significant reduction up to 70% in clodronate liposome-treated mice. The inhibitory effect of clodronate liposome on HA and LA was evaluated by calculating the percent reduction of BV and LV by comparing to mean values of control groups.
VEGFR Neutralization with Blocking Antibodies
DC101, a monoclonal anti-VEGFR-2 blocking antibody,29,30 and mF4-31C1 (31C1), an antibody to murine VEGFR-3,31,32,33 were used to block each receptor. Both of these blocking antibodies were supplied by Imclone Systems (New York, NY) based on our signed material transfer agreement. One milligram (0.5 ml) of anti-mouse VEGFR-2 (DC101) and/or anti-mouse VEGFR-3 (mF4-31C1) was injected via the i.p. route at day 0 (between anesthesia and pellet implantation) and days 2, 4, 7, 10, and 13 to neutralize the function of each receptor. The same amount of rat IgG was injected as an isotype control in control mice. Mice were grouped according to the VEGF ligand used; each group was further subdivided based on the treatment received (N = 5 per subgroup): control group, DC101 treatment group, mF4-31C1 treatment group, and DC101 plus mF4-31C1 treatment group. The inhibitory effect of DC101 and/or mF4-31C1 on HA and LA was evaluated by calculating the precent reduction of BV and LV in each treated eye.
Statistical Analysis
The two-tailed Student’s t-test was used to compare the blood/LVs area and the numbers of infiltrating innate immune cells in VEGF implanted eyes between the groups. Each experiment consisted of five mice per group and was performed at least twice. A P value <0.05 was considered as significant.
Results
Induction of Both BVs and LVs by VEGF-C156S and rmVEGF-D
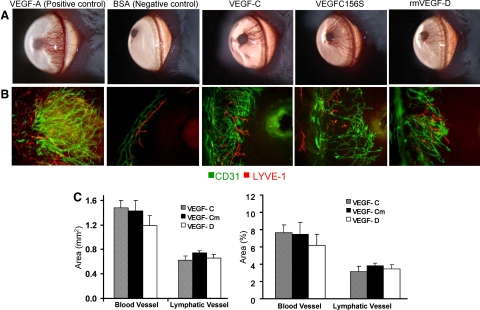
Pellets of VEGF-C, VEGF-C156S, and rmVEGF-D were surgically implanted into the corneas of BALB/c mice. The angiogenic response of corneas stimulated by 80 ng of VEGF-C156S and rmVEGF-D reached levels that approximated those of VEGF-C (Figure 1, A and B). Quantitatively no significant difference was observed in the densities of HA or LA among the three different VEGFs (Figure 1C).
Figure 1.
Corneal blood and lymph vessel growth by VEGFR-3-specific stimulation via VEGF-C156S or rmVEGF-D pellet implants. A: Pellets containing 80 ng of VEGF-C156S or rmVEGF-D induced comparable neovascularization to VEGF-C as detected by biomicroscopy. Pellets containing VEGF-A and BSA were implanted as positive and negative controls, respectively. B: Immunohistochemical staining of flat mount corneas (×100 magnification) revealed similar densities of CD31highLYVE-1− blood vessels (green) and CD31lowLYVE-1high (red) lymph vessels in VEGF-C, VEGF-C156S (VEGF-Cm), and rmVEGF-D pellet-implanted corneas. The growth of the vessels can be seen from the left toward the pellets implanted in the avascular cornea, toward the right of the figures. C: For quantitative comparison of HA and LA induced by different ligands, fluorescent micrographs under low magnification power (×2) were analyzed with NIH software (Image J 1.34). The area of vessels was measured in mm2 and then analyzed as percentage of the total corneal area covered by vessels. Graphs represent mean values (±SEM) of five mice in each group. No significant difference was observed in the area covered by either blood vessels or lymphatic vessels among the different VEGF pellet-implanted groups.
VEGF-C156S-Induced Newly Formed Blood Vascular Endothelial Cells Express VEGFR-3
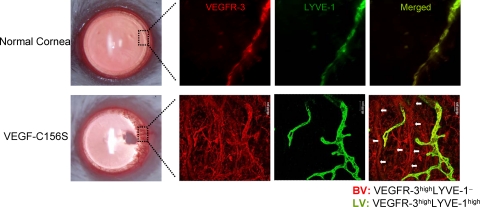
To determine whether the endothelial cells of the newly formed BVs induced by VEGFR-3-specific ligands express VEGFR-3, we double-stained the flat-mount corneas for LYVE-1 and VEGFR-3 1 week after VEGF-C156S pellet implantation. VEGFR-3highLYVE-1− vascular structures could be easily identified (Figure 2). These vascular structures had a more linear architecture than VEGFR-3high/LYVE-1high LVs and expressed CD31 (data not shown), confirming their BV identity.
Figure 2.
Expression of VEGFR-3 by VEGF-C156S induced new blood vessels. A representative portion (boxed area) of newly formed vessels invading the cornea after 1 week post-VEGF-C156S pellet implantation, and the same area from the normal cornea without pellet implantation was photographed after double immunostaining of the whole-mount cornea with VEGFR-3 (red) and LYVE-1 (green). Merged images of LYVE-1 and VEGFR-3 represent lymphatic vessels (VEGFR-3highLYVE-1high) in yellow-green color, whereas LYVE-1− tubular structures in red color (marked with white arrows) represent VEGFR-3-positive blood vessels. Negative controls stained with isotype-matched antibodies showed no staining.
Recruitment of VEGF-A-Secreting Macrophages to the Corneal Stroma after Implanting VEGF-C156S and rmVEGF-D Pellets
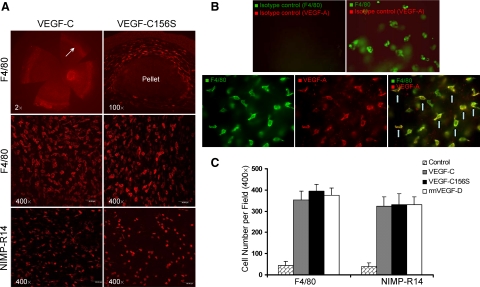
To determine whether innate immune cells infiltrate the corneal stroma after implantation of VEGF-containing pellets, we harvested corneas 1 week after VEGF-C, -C156S, and rmVEGF-D pellet implantation and prepared flat mounts to stain for macrophage-specific F4/80 and neutrophil-specific NIMP-R14 expression. Abundant numbers of cells were recruited to the corneal stroma, especially around the pellet site and in the corneal periphery, near the limbus (Figure 3A). To verify whether these F4/80+ cells and NIMP-R14+ cells secrete VEGF-A, flat-mount corneas were prepared and double-stained with VEGF-A and F4/80 or NIMP-R14. A majority (∼80%) of F4/80-labeled cells were found positive for VEGF-A (Figure 3B), but none of the NIMP-R14 cells was costained with VEGF-A (data not shown). For comparison of cell recruitment as a result of the different VEGF implants, cells were manually counted under high-magnification confocal scanning. There were no significant differences in the numbers of macrophages and neutrophils infiltrating the cornea among the VEGF-C, -C156S, and rmVEGF-D groups (Figure 3C).
Figure 3.
Abundant numbers of F4/80+ macrophages and NIMP-R14+ neutrophils are recruited to the corneal stroma. A: Fluorescent micrographs of immunohistochemically stained (F4/80 or NIMP-R14) flat-mount corneas showed that abundant numbers of innate immune cells are recruited to the corneal stroma, especially near the limbus (marked with arrows) and around the pellet. Two weeks after implantation of VEGF-C and VEGF-C156S pellets, F4/80+ macrophages reached peak levels of infiltration. Neutrophils identified with the specific marker NIMP-R14 were relatively smaller in size and showed maximal infiltration at week 1 postimplantation of VEGF-C and VEGF-C156S pellets. B: Double staining of F4/80 with VEGF-A after VEGF-C156S pellet implantation showed that a majority of the F4/80+ cells (green) are costained with VEGF-A (red) as shown in merged images (yellow; marked with arrows). C: F4/80+ and NIMP-R14+ cells were counted manually under high magnification power (×400) confocal fluorescein micrographs at 2 and 1 week, respectively. Graphs represent mean values (±SEM) of five mice in each group, and no significant difference in the numbers of infiltrating cells was found among different VEGF pellet-implanted groups.
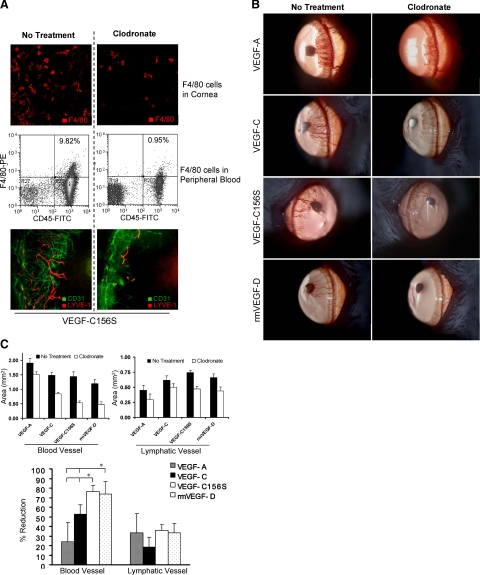
Diminution of HA by Depletion of Macrophages in VEGF-C156S and rmVEGF-D-Implanted Corneas
Since recruited F4/80+ cells were verified to secrete VEGF-A, we investigated whether the HA observed with VEGFR-3 stimulation was mediated by macrophages. Systemic and local depletion of macrophages by clodronate administration (Figure 4A) inhibited both HA and LA in all VEGF-A, -C, -C156S, and rmVEGF-D pellet-implanted corneas (Figure 4, B and C), although the reduction of BVs did not reach a statistically significant level in VEGF-A-mediated HA. The inhibition of HA as a result of macrophage depletion was significantly greater in VEGF-C156S- and rmVEGF-D-induced groups compared with the inhibition observed in the VEGF-A and VEGF-C groups (Figure 4, B and C). There was no significant difference in the percent reduction of LA among different VEGF-implanted groups.
Figure 4.
Antiangiogenic effects of macrophage depletion. A: Intraperitoneal injection of clodronate effectively depleted the F4/80+ cells both locally in cornea (IHC, panel 1) and systemically in peripheral blood (FACS plots, panel 2). Representative micrographs (panel 3) taken 1 week after VEGF-C156S pellet implantation showed significant reduction of both blood vessels (CD31highLYVE-1−, green) and lymphatic vessels (CD31lowLYVE-1high, red) in clodronate liposome-treated eyes as compared with untreated eyes. B: Slit lamp photographs taken 1 week after pellet implantation showed that systemic macrophage depletion by CL2MDP-lip has an inhibitory effect on corneal vasculogenesis induced by all four proangiogenic growth factors: VEGF-A, VEGF-C, VEGF-C156S, and rmVEGF-D. C: The area of blood vessels and lymphatic vessels was measured in mm2 and then analyzed as the percent reduction in vessel area by comparing to the mean values of vessel area in control groups (no treatment). The percent reduction of blood vessels was significantly higher in the VEGF-C156S and rmVEGF-D groups as compared with those in the VEGF-A and VEGF-C. The difference in percent reduction of lymphatic vessels was not statistically significant among the different VEGF-implanted groups (*P < 0.05). Graphs represent mean values (±SEM) of five mice in each group.
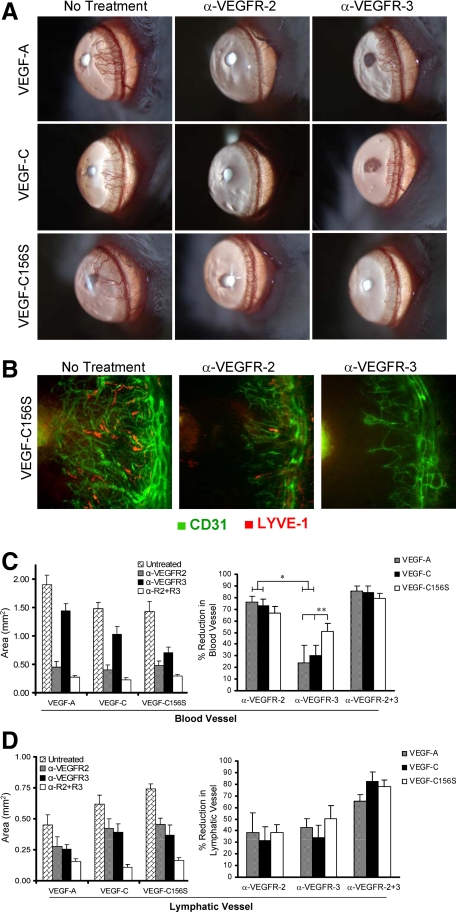
Functional Relevance of VEGFR-2 versus VEGFR-3 in Angiogenesis Induced by VEGFR-3-Specific Ligands
To compare the relative contribution of VEGFR-2 versus VEGFR-3 to HA and LA, we evaluated the effects of selective blockade of VEGFR-2 and/or VEGFR-3 on HA and LA in VEGF-A, VEGF-C, and VEGF-C156S pellet-implanted corneas. We observed that both VEGFR-2 and VEGFR-3 blockade suppress HA as well as LA (Figure 5, A and B). The percent reduction of BVs was significantly greater in the VEGFR-2 blockade group than in the VEGFR-3 blockade group in VEGF-A and VEGF-C pellet-implanted corneas. However, no significant difference was observed in the case of VEGF-C156S pellet-implanted corneas (Figure 5C), confirming the functional relevance of VEGFR-3-specific signaling in HA induction. Indeed, the inhibition of HA by VEGFR-3 blockade was significantly greater in VEGF-C156S pellet-implanted corneas as compared with those in the VEGF-A or VEGF-C implanted corneas (Figure 5C). There was no significant difference in the inhibition of LA between VEGFR-2 and VEGFR-3 blockade among the different VEGF implants (Figure 5D).
Figure 5.
Antiangiogenic effects of VEGFR blockade. Systemic administration of either anti-VEGFR-2 (α-VEGFR-2)- or anti-VEGFR-3 (α-VEGFR-3)-blocking antibodies inhibit formation of both blood vessels and lymphatic vessels. A: Slit lamp photographs taken 1 week after corneal pellet implantation in control and treatment groups show that neutralization of either VEGFR-2 or VEGFR-3 causes suppression of neovascularization, although the inhibitory effects were more evident in eyes treated with VEGFR-2 blockade. B: Representative immunofluorescence photomicrographs (×100 magnification) taken 1 week after VEGF-C156S pellet implantation with or without treatment confirm that both VEGFR-2 and VEGFR-3 have an inhibitory effect on both blood vessels (green) and lymphatic vessels (red). C: The area of blood vessels was measured in mm2 and then analyzed as the percent reduction in vessel area by comparing to the mean values of vessel area in untreated group. Suppression of HA in VEGFR-2 blockade-treated eyes was significantly greater in VEGF-A and VEGF-C induced neovascularization as confirmed by morphometric analysis. The difference in the inhibition of VEGF-C156S induced HA between VEGFR-2 blockade- and VEGFR-3 blockade-treated eye was not statistically significant. D: The area of lymphatic vessels was measured in mm2 and then analyzed as the percent reduction in vessel area by comparing to the mean values of vessel area in untreated group. Inhibition of LA was not significantly different between the VEGFR-2 and VEGFR-3 blockade-treated groups (*P < 0.007; **P < 0.05). Graphs represent mean values (±SEM) of five mice in each group.
Discussion
In the present study, we show that the VEGFR-3-specific ligands VEGF-C156S and rmVEGF-D induce not only LVs but also BVs. Indeed, VEGF-C156S and rmVEGF-D almost rival VEGF-C in the degree of HA and LA. To explain this, we hypothesized that direct ligation and downstream signaling of VEGFR-3 can induce BVs in addition to LVs. Verification of VEGFR-3 expression by new BVs induced by VEGFR-3-specific ligands is supportive of this hypothesis. To our knowledge, this is the first report showing that VEGFR-3-specific signaling induces growth of VEGFR-3+ newly formed BVs in a nontumor setting, although other studies have reported the expression VEGFR-3 in tumor vessels.19,20,21
Our study also confirms data by previous studies regarding the function of VEGFs as chemoattractants for immune cells.12,34,35 Indeed, it is known that certain bone marrow-derived cells, including macrophages, monocytes, and dendritic cells, express VEGFR-3.10,11,12 VEGFR-3 has also been shown to modulate adaptive immunity in an experimental model of transplantation via mediating chemotaxis of antigen-presenting cells.12 Macrophages are known to express several VEGFRs, including constitutive expression of VEGFR-1, -3, and inducible expression of VEGFR-2. All of these VEGFRs have been implicated in the recruitment of macrophages in various inflammatory settings.10,11,12,36,37,38,39 Depletion of macrophages through administration of clodronate liposome26,27,28 clearly inhibits both HA and LA regardless of the specific VEGF species mediating the response, although the inhibitory effect in VEGF-A-mediated HA does not reach statistically significant levels. Interestingly, the inhibitory effect of HA, calculated as the percent reduction of BVs, is more dramatic in VEGFR-3-specific ligands than that in VEGF-A. This signifies that the contribution of macrophages is definitely more crucial in HA induced by VEGFR-3-specific ligands. Macrophages are known to be a rich source of VEGF-A, which can induce and amplify the angiogenic (HA) response.38
Although inhibition of HA via macrophage depletion significantly suppresses the angiogenic response, it is unable to completely prevent angiogenesis. Thus, to determine whether direct VEGFR-3 stimulation can function in a hemangiogenic pathway independent of VEGFR-2, we selectively blocked VEGFR-2 and/or VEGFR-3. By analyzing the inhibitory effects of VEGFR-2 and/or VEGFR-3 blockade on HA and LA, we determined the relative contribution of VEGFR-2 and VEGFR-3 to HA and LA. Morphometric analysis of BVs and LVs in control and treatment groups showed that VEGFR-2 blockade is significantly a stronger inhibitor of HA than VEGFR-3 blockade regardless of the specific VEGF species mediating the response.
Recently, there have been several reports that support the possible involvement of VEGFR-3 in HA in tumors. Alitalo et al40 showed that VEGFR-3 is involved in angiogenesis and growth of some tumors. Similarly, Kubo et al41 have also reported that VEGFR-3 monoclonal antibody inhibits tumor xenograft growth in mice. Although it is not clear whether direct ligation and downstream signaling of VEGFR-3 can stimulate HA completely independent of VEGFR-2, it is clear through our studies that VEGFR-3 can directly functionally contribute to HA. Taken together, our data suggest that HA induced by VEGFR-3-specific signaling results in part from cooperative functioning of VEGFR-2 and VEGFR-3. For example, VEGFR-3 signaling could possibly induce nascent BVs, and these immature vessels could mature with the aid of VEGFR-2 signaling induced by VEGF-A produced by macrophages, which are recruited through chemoattraction to VEGFR-3-specific ligands. The findings of Veikkola et al42 also lend support to this notion. They have shown that human umbilical vein endothelial cells when grown in cell culture express VEGFR-3, which is down-regulated when grown in co-culture with perivascular smooth muscle cells, retaining their in vivo phenotype, suggesting VEGFR-3 signaling might be important in the generation of nascent BVs and its role becomes redundant as the vessels mature.42 Tammela et al43 have recently demonstrated that VEGFR-3 is highly expressed in angiogenic sprouts, and genetic targeting of VEGFR-3 or blocking of VEGFR-3 signaling with monoclonal antibodies results in decreased sprouting, vascular density, vessel branching, and endothelial cell proliferation in mouse model of retinal HA.43 These findings are in accord with the conclusion derived from our data.
Taken together, our data suggest that signaling through VEGFR-3-specific ligands can induce HA and that macrophages contribute a significant role. Whereas macrophages are also present in VEGF-A-mediated HA, their depletion has the greatest inhibitory effect on VEGFR-3-mediated HA. Although, we have not investigated the direct role of VEGFR-3 expression on BVs in HA, the recent report by Tammela et al43 has demonstrated that angiogenic sprouting is impaired without VEGFR-3 signals, and VEGFR-3 can drive angiogenesis even in conditions of therapeutic targeting of VEGFR-2.43 These findings have potentially significant indications for antiangiogenesis strategies in that they highlight the relevance of non-VEGF-A-mediated mechanisms in HA.
Acknowledgments
We thank to Dr. Bronislaw Pytowski, Imclone Systems, for providing anti-VEGFR-2 and anti-VEGFR-3 antibodies. We also acknowledge Roche Diagnostics for their gift of clodronate liposomes, and BRB Preclinical Repository, National Cancer Institute, for providing VEGF-A.
Footnotes
Address reprint requests to Reza Dana, Professor and Senior Scientist, Department of Ophthalmology, Harvard Medical School, Schepens Eye Research Institute, 20 Staniford Street, Boston, MA 02114. E-mail: reza.dana@schepens.harvard.edu.
Supported in part by National Institutes of Health grant NEI-RO1-12963, U.S. Department of Defense grant W81XWH-07-2-0038, and New England Transplant Research Fund (to R.D.).
E-S.C. and S.K.C. contributed equally to this work.
References
- Ferrara N, Davis-Smyth T. The biology of vascular endothelial growth factor. Endocr Rev. 1997;18:4–25. doi: 10.1210/edrv.18.1.0287. [DOI] [PubMed] [Google Scholar]
- Shibuya M, Ito N, Claesson-Welsh L. Structure and function of vascular endothelial growth factor receptor-1 and -2. Curr Top Microbiol Immunol. 1999;237:59–83. doi: 10.1007/978-3-642-59953-8_4. [DOI] [PubMed] [Google Scholar]
- Alitalo K, Carmeliset P. Molecular mechanism of lymphangiogenesis in health and disease. Cancer Cell. 2002;1:219–227. doi: 10.1016/s1535-6108(02)00051-x. [DOI] [PubMed] [Google Scholar]
- Shibuya M, Claesson-Welsh L. Signal transduction by VEGF receptors in regulation of angiogenesis and lymphangiogenesis. Exp Cell Res. 2005;312:549–560. doi: 10.1016/j.yexcr.2005.11.012. [DOI] [PubMed] [Google Scholar]
- Cao Y, Linden P, Farnebo J, Cao R, Eriksson A, Kumar V, Qi JH, Claesson-Welsh L, Alitalo K. Vascular endothelial growth factor C induces angiogenesis in vivo. Proc Natl Acad Sci USA. 1998;95:14389–14394. doi: 10.1073/pnas.95.24.14389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalaby F, Rossant J, Yamaguchi TP, Gertsenstein M, Wu XF, Breitman ML, Schuh AC. Failure of blood-island formation and vasculogenesis in Flk-1-defecient mice. Nature. 1995;376:62–66. doi: 10.1038/376062a0. [DOI] [PubMed] [Google Scholar]
- Dumont DJ, Jussila L, Taipale J, Lymboussaki A, Mustonen T, Pajusola K, Breitman M, Alitalo K. Cardiovascular failure in mouse embryos deficient in VEGF receptor-3. Science. 1998;282:946–949. doi: 10.1126/science.282.5390.946. [DOI] [PubMed] [Google Scholar]
- Kaipainen A, Korhonen J, Mustonen T, Fang GH, Dumont D, Breitman M, Alitalo K. Expression of the fms-like tyrosine kinase 4 gene becomes restricted to lymphatic endothelium during development. Proc Natl Acad Sci USA. 1995;92:3566–3570. doi: 10.1073/pnas.92.8.3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partanen TA, Arola J, Saaristo A, Jussila L, Ora A, Miettinen M, Stacker SA, Achen MG, Alitalo K. VEGF-C and VEGF-D expression in neuroendocrine cells and their receptor. VEGFR-3, in fenestrated blood vessels in human tissues. FASEB J. 2000;14:2087–2096. doi: 10.1096/fj.99-1049com. [DOI] [PubMed] [Google Scholar]
- Hamrah P, Chen L, Zhang Q, Dana R. Novel expression of vascular endothelial endothelial growth factor (VEGFR)-3 and VEGF-C on corneal dendritic cells. Am J Pathol. 2003;163:57–68. doi: 10.1016/S0002-9440(10)63630-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamrah P, Chen L, Cursiefen C, Zhang Q, Joyce NC, Dana R. Expression of vascular endothelial endothelial growth factor receptor-3 (VEGFR-3) on monocytic bone marrow-derived cells in the conjunctiva. Exp Eye Res. 2004;79:553–561. doi: 10.1016/j.exer.2004.06.028. [DOI] [PubMed] [Google Scholar]
- Chen L, Hamrah P, Cursiefen C, Zhang Q, Pytowski B, Streilein JW, Dana R. Vascular endothelial growth factor receptor-3 mediates induction of corneal alloimmunity. Nat Med. 2004;10:813–815. doi: 10.1038/nm1078. [DOI] [PubMed] [Google Scholar]
- Saharinen P, Tammelar T, Karkkainen MJ, Alitalo K. Lymphatic vasculature: development, molecular regulation and role in tumor metastasis and inflammation. Trends Immunol. 2004;25:387–395. doi: 10.1016/j.it.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Joukov V, Kumar V, Sorsa T, Arighi E, Weich H, Saksela O, Alitalo K. A recombinant mutant vascular endothelial growth factor-C that has lost vascular endothelial growth factor receptor-2 binding, activation, and vascular permeability activities. J Biol Chem. 1998;273:6599–6602. doi: 10.1074/jbc.273.12.6599. [DOI] [PubMed] [Google Scholar]
- Stacker S, Vitali A, Caesar C, Domagala T, Groenen LC, Nice E, Achen MG, Wilks AF. A mutant form of vascular endothelial growth factor (VEGF) that lacks VEGF receptor-2 activation retains the ability to induce vascular permeability. J Biol Chem. 1999;274:34884–34892. doi: 10.1074/jbc.274.49.34884. [DOI] [PubMed] [Google Scholar]
- Jeltsch M, Kaipainen A, Joukov V, Meng X, Lakso M, Rauvala H, Swartz M, Fukumura D, Jain RK, Alitalo K. Hyperplasia of lymphatic vessels in VEGF-C transgenic mice. Science. 1997;276:1423–1425. doi: 10.1126/science.276.5317.1423. [DOI] [PubMed] [Google Scholar]
- Veikkola T, Jussila L, Makinnen T, Karpanen T, Jeltsch M, Petrova TV, Kubo H, Thurston G, McDonald DM, Achen MG. Signalling via vascular endothelial growth factor receptor-3 is sufficient for lymphangiogenesis in transgenic mice. EMBO J. 2001;15:1223–1231. doi: 10.1093/emboj/20.6.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin M, Catimel B, Nice EC, Roufail S, Hall NE, Stenvers KL, Karkkainen MJ, Alitalo K, Stacker S, Achen MG. The specificity of receptor binding by vascular endothelial growth factor-D is different in mouse and man. J Biol Chem. 2001;276:19166–19171. doi: 10.1074/jbc.M100097200. [DOI] [PubMed] [Google Scholar]
- Partanen TA, Alitalo K, Miettinen M. Lack of lymphatic vascular specificity of vascular endothelial growth factor receptor 3 in 185 vascular tumors. Cancer. 1999;86:2406–2412. [PubMed] [Google Scholar]
- Niki T, Iba S, Yamada T, Matsuno Y, Enholm B, Hirohashi S. Expression of vascular endothelial growth factor receptor 3 in blood and lymphatic vessels of lung adenocarcinoma. J Pathol. 2001;193:450–457. doi: 10.1002/path.828. [DOI] [PubMed] [Google Scholar]
- Valtola R, Salven P, Heikkila P, Taipale J, Joensuu H, Rehn M, Pihlajaniemi T, Weich H, de Wall R, Alitalo K. VEGFR-3 and its ligand VEGF-C are associated with angiogenesis in breast cancer. Am J Pathol. 1999;154:1381–1390. doi: 10.1016/S0002-9440(10)65392-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witmer AN, van Blijswijk BC, Dai J, Hofman P, Partanen TA, Vrensen GFJM, Schlingemann RO. VEGFR-3 in adult angiogenesis. J Pathol. 2001;195:490–497. doi: 10.1002/path.969. [DOI] [PubMed] [Google Scholar]
- Kenyon BM, Voest EE, Chen CC, Flynn E, Folkman J, D'Amato RJ. A model of angiogenesis in the mouse cornea. Invest Ophthalmol Vis Sci. 1996;37:1625–1632. [PubMed] [Google Scholar]
- Leenen PJ, de Bruijin MF, Voerman JS, Campbell PA, van Ewijik W. Markers of macrophage development detected by monoclonal antibodies. J Immunol Methods. 1994;174:5–19. doi: 10.1016/0022-1759(94)90005-1. [DOI] [PubMed] [Google Scholar]
- Reumaux D, Hordijk PL, Duthilleul P, Roos D. Priming by tumor necrosis factor α of human neutrophil NADPH-oxidase activity induced by anti-proteinase-3 or anti-myeloperoxidase antibodies. J Leukocyte Biol. 2006;80:1424–1433. doi: 10.1189/jlb.0304144. [DOI] [PubMed] [Google Scholar]
- Rooijen NV, Sanders A. Liposome mediated depletion of macrophages: mechanism of action, preparation of liposomes and applications. J Immunol Methods. 1994;174:83–93. doi: 10.1016/0022-1759(94)90012-4. [DOI] [PubMed] [Google Scholar]
- Espinosa-Heidmann DG, Suner IJ, Hernandez EP, Monroy D, Csaky KG, Cousins SW. Macrophage depletion diminishes lesion size and severity in experimental choroidal neovascularization. Invest Ophthalmol Vis Sci. 2003;44:3586–3592. doi: 10.1167/iovs.03-0038. [DOI] [PubMed] [Google Scholar]
- Cheng H, Tumpey TM, Staats HF, Rooijen NV, Oakes JE, Lausch RN. Role of macrophages in restricting herpes simplex virus type 1 growth after ocular infection. Invest Ophthalmol Vis Sci. 2004;41:1402–1409. [PubMed] [Google Scholar]
- Bruns C, Shrader M, Harrison M, Portera C, Solorzano CC, Jauch KW, Hicklin DJ, Radinsky R, Ellis LM. Effect of the vascular endothelial growth factor receptor-2 antibody DC101 plus gemcitabine on growth, metastasis and angiogenesis of human pancreatic cancer growing orthotopically in nude mice. Int J Cancer. 2002;102:101–108. doi: 10.1002/ijc.10681. [DOI] [PubMed] [Google Scholar]
- Pauli SA, Tang H, Wang J, Bohlen P, Posser R, Hartman T, Sauer MV, Kitajewski J, Zimmermann RC. The vascular endothelial growth factor (VEGF)/VEGF receptor 2 pathway is critical for blood vessel survival in corpora lutea of pregnancy in the rodent. Endocrinology. 2005;146:1301–1311. doi: 10.1210/en.2004-0765. [DOI] [PubMed] [Google Scholar]
- Aoki Y, Tosato G. Lymphatic regeneration: new insights from VEGFR-3 blockade. J Natl Cancer Inst. 2005;97:2–3. doi: 10.1093/jnci/dji015. [DOI] [PubMed] [Google Scholar]
- Pytowski B, Goldman J, Persaud K, Wu Y, Witte L, Hicklin DJ, Skobe M, Boardman KC, Swartz MA. Complete and specific inhibition of adult lymphatic regeneration by a novel VEGFR-3 neutralizing antibody. J Natl Cancer Inst. 2005;97:14–21. doi: 10.1093/jnci/dji003. [DOI] [PubMed] [Google Scholar]
- Roberts N, Kloos B, Cassella M, Podgrabinska S, Persaud K, Wu Y, Pytowski B, Skobe M. Inhibition of VEGFR-3 activation with the antagonistic antibody more potently suppresses lymph node and distant metastases than inactivation of VEGFR-2. Cancer Res. 2006;66:2650–2657. doi: 10.1158/0008-5472.CAN-05-1843. [DOI] [PubMed] [Google Scholar]
- Yang ZF, Poon RT, Cheung CK, Ho DW, Lo CM, Fan ST. Up-regulation of vascular endothelial growth factor (VEGF) in small-for-size liver grafts enhances macrophages activities through VEGFR receptor 2-dependant pathway. J Immunol. 2004;173:507–2515. doi: 10.4049/jimmunol.173.4.2507. [DOI] [PubMed] [Google Scholar]
- Sawano A, Iwai S, Sakurai Y, Ito M, Nakahata T, Shibuya M. Flt-1, vascular endothelial growth factor receptor 1, is a novel cell surface marker for the lineage of monocyte-macrophages in humans. Blood. 2001;97:785–791. doi: 10.1182/blood.v97.3.785. [DOI] [PubMed] [Google Scholar]
- Xiong M, Elson G, Legarda D, Leivovich SJ. Production of vascular endothelial growth factor by murine macrophages: regulation by hypoxia, lactate, and the inducible nitric oxide synthase pathway. Am J Pathol. 1998;153:587–598. doi: 10.1016/S0002-9440(10)65601-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke B, Giannoudis A, Corke KP, Gill D, Wells M, Ziegler-Heitbroke L, Lewis CE. Hypoxia-induced gene expression in human macrophages: implications for ischemic tissues and hypoxia-regulated gene therapy. Am J Pathol. 2003;163:1233–1243. doi: 10.1016/S0002-9440(10)63483-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cursiefen C, Chen L, Borges LP, Jackson D, Cao J, Radziejewski C, D'Amore P, Dana R, Wiegand SJ, Streinlein JW. VEGF-A stimulates lymphangiogenesis and hemangiogenesis in inflammatory neovascularization via macrophage recruitment. J Clin Invest. 2004;113:1040–1050. doi: 10.1172/JCI20465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dineen SP, Lynn KD, Holloway SE, Miller AF, Sullivan JP, Shames DS, Beck AW, Barnett CC, Fleming JB, Brekken RA. Vascular endothelial growth factor receptor 2 mediates macrophage infiltration into orthotopic pancreatic tumors in mice. Cancer Res. 2008;68:4340–4346. doi: 10.1158/0008-5472.CAN-07-6705. [DOI] [PubMed] [Google Scholar]
- Laakkonen P, Waltari M, Holopainen T, Takahashi T, Pytowski B, Steiner P, Hicklin D, Persaud K, Tonra JR, Witte L. Vascular endothelial growth factor receptor 3 is involved in tumor angiogenesis and growth. Cancer Res. 2007;67:593–599. doi: 10.1158/0008-5472.CAN-06-3567. [DOI] [PubMed] [Google Scholar]
- Kubo H, Fujiwara T, Jussila L, Hashi H, Ogawa M, Shimizu K, Awane M, Sakai Y, Takabayashi A, Alitalo K. Involvement of VEGFR-3 in maintenance of integrity of endothelial cell lining during tumor angiogenesis. Blood. 2000;96:546–553. [PubMed] [Google Scholar]
- Veikkola T, Lohela M, Ikenberg K, Mäkinen T, Korff T, Saaristo A, Petrova T, Jeltsch M, Augustin HG, Alitalo K. Intrinsic versus microenvironmental regulation of lymphatic endothelial cell phenotype and function. FASEB J. 2003;17:2006–2013. doi: 10.1096/fj.03-0179com. [DOI] [PubMed] [Google Scholar]
- Tammela T, Zarkada G, Wallgard E, Murtomäki A, Suchting S, Wirzenius M, Waltari M, Hellström M, Schomber T, Peltonen R, Freitas C, Duarte A, Isoniemi H, Laakkonen P, Christofori G, Ylä-Herttuala S, Shibuya M, Pytowski B, Eichmann A, Betsholtz C, Alitalo K. Blocking VEGFR-3 suppresses angiogenic sprouting and vascular network formation. Nature. 2008;454:656–660. doi: 10.1038/nature07083. [DOI] [PubMed] [Google Scholar]