Abstract
Mucopolysaccharidoses are a group of lysosomal storage diseases characterized by the build-up of glycosaminoglycans (GAGs) and severe skeletal abnormalities. As GAGs can regulate the collagenolytic activity of the major osteoclastic protease cathepsin K, we investigated the presence and activity of cathepsin K and its co-localization with GAGs in mucopolysaccharidosis (MPS) type I bone. The most dramatic difference between MPS I and wild-type mice was an increase in the amount of cartilage in the growth plates in MPS I bones. Though the number of cathepsin K-expressing osteoclasts was increased in MPS I mice, these mice revealed a significant reduction in cathepsin K-mediated cartilage degradation. As excess heparan and dermatan sulfates inhibit type II collagen degradation by cathepsin K and the spatial overlap between cathepsin K and heparan sulfate strongly increased in MPS I mice, the build up of subepiphyseal cartilage is speculated to be a direct consequence of cathepsin K inhibition by MPS I-associated GAGs. Moreover, isolated MPS I and Ctsk−/− osteoclasts displayed fewer actin rings and formed fewer resorption pits on dentine disks, as compared with wild-type cells. These results suggest that the accumulation of GAGs in murine MPS I bone has an inhibitory effect on cathepsin K activity, resulting in impaired osteoclast activity and decreased cartilage resorption, which may contribute to the bone pathology seen in MPS diseases.
Mucopolysaccharidoses are a group of inherited lysosomal storage diseases. They result from the diminished activity of certain lysosomal enzymes responsible for glycosaminoglycan degradation. The subsequent accumulation of glycosaminoglycans (GAGs) in tissues triggers various pathological features including mental retardation, short stature, dysostosis multiplex, and corneal clouding.1 The extent of these pathological features depends on the severity of the disease, which can range from mild to a severe form resulting in death in early childhood. Mucopolysaccharidosis type I (MPS I) is caused from an accumulation of heparan sulfate (HS) and dermatan sulfate (DS) due to the inactivity of the lysosomal enzyme α-iduronidase (Idua). Patients with MPS I display the typical characteristics of MPS diseases, including skeletal abnormalities such as dysostosis multiplex and spinal misalignment. Although much work is being done on enzyme replacement therapy to treat the disease,2 the cellular and molecular mechanisms remain largely unknown.
Cathepsin K, a lysosomal cysteine protease highly expressed by osteoclasts, is responsible for a significant part of total bone resorption.3 The lack of functioning enzyme results in a condition known as pycnodysostosis in humans.4 Osteoclasts are not able to degrade the organic matrix and so undegraded collagen accumulates in the resorption lacuna and in cellular lysosomes.5 Patients with pycnodysostosis typically have decreased bone resorption accompanied by enhanced bone density and are dwarfs.6,7 Although the cathepsin K-deficient murine model does not display the build-up of collagen in osteoclast lysosomes and the dwarfism phenotype,5 it mimics the human disorder in most other ways.
Recently we have shown that GAGs play an important role in the collagenolytic activity of cathepsin K.8,9 Chondroitin sulfate (CS) was shown to form a complex with cathepsin K, which enabled the enzyme to fully degrade type I collagen.10 However other GAGs, like DS and HS, were shown to inhibit the collagenolytic activity of cathepsin K.11 As both DS and HS are present in high concentrations in MPS I tissue, we investigated the effect these concentrations would have on cathepsin K activity. During endochondral ossification, cathepsin K degrades type II collagen (cartilage) below the growth plate area to provide room for new bone to be laid down by osteoblasts. This process is essential for the normal development of long bones.12 As patients with MPS I have short stature with severe problems in bone growth and development, it is tempting to speculate that an impairment of cathepsin K activity may contribute to the disease phenotype. Therefore, we investigated the colocalization of HS and cathepsin K at the growth plate, the subepiphyseal cartilage structure, and the degradation of type II collagen in MPS I murine bone, as compared with wild-type bone. We also looked at the effect of GAGs in MPS I murine osteoclast activity. We hypothesize that high concentrations of HS and DS in MPS I may inhibit the activity of cathepsin K in osteoclasts and that this coupled with lysosomal dysfunction in osteoclasts contributes to MPS I bone pathology.
Materials and Methods
Mouse Models
In these studies, the mouse models used were wild-type C57BL/6 (The Jackson Laboratories, Maine), cathepsin K-deficient mice (Ctsk−/−) 129:C57BL/613 and ∝-l-idurono-hydrolase–deficient (Idua−/−) 129:C57BL/6 mice.14 Euthanasia of mice was performed using carbon dioxide inhalation. All animal experiments followed the animal care provisions of the University of British Columbia.
Immunohistochemistry
Long bones, including shoulder and knee joint, and spine, were dissected from eight Idua−/− mice and nine wild-type mice; skin and muscles were discarded. Limbs were fixed in 10% neutral buffered formalin at 4°C overnight and decalcified in 20% sodium citrate and 50% formic acid; solution was changed every day for 2 weeks. The bone tissues were then dehydrated in ethanol and embedded in paraffin. Decalcified bone sections (5 μm) were cut with a power microtome (Leica Microtom, Nussloch, Germany) and mounted on slides (Fisher Scientific, Pittsburgh, PA) for staining. Bone sections were incubated at 60°C for 1 hour, and deparaffinized in Safeclear (Fisher Scientific, Kalamazoo, MI) before rehydration in graded ethanol. Antigen retrieval was performed by steaming slides in citrate buffer for 30 minutes. For protein localization sections were incubated with polyclonal rabbit anti-mouse cathepsin K antibody M4 (1:50 dilution) or monoclonal anti-HS (1:100 dilution) (Seikagaku Corporation, Tokyo Japan) overnight at 4°C. The specificity of the M4 antibody has been previously described.15 For the detection of a specific cathepsin K cleavage site in type II collagen, the polyclonal rabbit antibody C2K was used as previously described.16 Detection was achieved using the ImmunoPure Ultra-Sensitive ABC Peroxides Staining kit (Pierce, Rockford, IL). The following secondary antibodies were used: CY2 conjugated affinity purified goat anti-mouse IgG for HS detection (Rockland, Gilbertville, PA) and CY3 conjugated affinity purified goat anti-rabbit IgG for cathepsin K and the type II collagen fragment (Rockland, Gilbertville, PA) for 2 hours each. After washing, sections were allowed to air dry before mounting with SlowFade Gold antifade reagent containing 4,6-diamidino-2-phenylindole (Invitrogen-Gibco, Burlington, ON). Sections were visualized fluorescently using a Leica DMI 6000B microscope (Leica Microsystems, Inc. Richmond Hill, ON). For quantification, the growth plate area was identified in wild-type and Idua−/− mice. The area of cleaved type II collagen, cathepsin K and HS staining, as well as cathepsin K and HS overlap staining, was measured using Openlab 4.0.3 software (Improvision-Deutschland, Tübingen, Germany). Area of positive staining was described as a percentage of total area (about 700,000 μm2) beneath the growth plate. Sections stained for cathepsin K using the antibody M4 and the diaminobenzidine method were treated with 1% hydrogen peroxide to inhibit endogenous peroxidase activity. After incubation with goat anti-rabbit, horseradish peroxidase-conjugated antibody (1:200, Southern Biotech, Birmingham, AL) and diaminobenzidine treatment (Vector Laboratories, Inc. Burlingame, CA), sections were counterstained with toluidine blue.
Cartilage Staining and Measurement
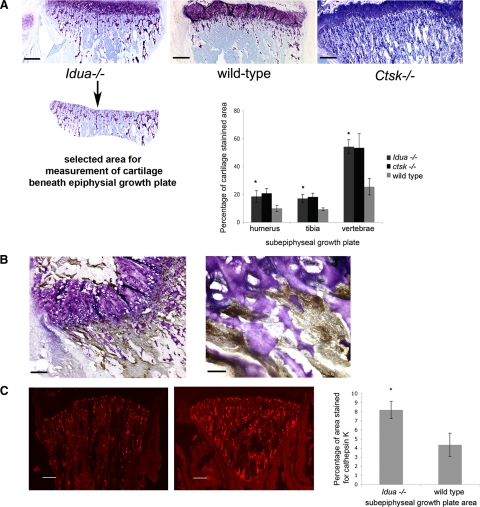
Consecutive sections of legs, forearms, and vertebra were stained with toluidine blue solution for 30 minutes. Images were captured with a Leica DMI 6000B microscope and the cartilage area was measured with Openlab 4.0.3 software. A defined area of about 700,000 μm2 underneath the epiphyseal growth plate was selected for the measurement of cartilage to compare the amount of toluidine blue-positive cartilage in wild-type, Idua−/− and Ctsk−/− bones (see Figure 1A).
Figure 1.
A: Toluidine blue staining of the tibial growth plate area in Idua−/−, wild-type (WT), and Ctsk−/− mice. Graph showing percentage of cartilage area in the subepiphyseal growth plate of shoulder and knee joints, as well as vertebra bones. *P < 0.05 vs wild-type. The panel below the Idua−/− growth plate represents an example for the measured cartilage-containing area (in all sections a constant area was analyzed: about 700,00 μm2 in tibia). Scale bars = 260 μm. B: Diaminobenzidine staining of cathepsin K as an osteoclast marker in subepyphysial growth plate area. The asterisk in the left panel represents the area in the right panel at a higher magnification (magnification bars are 130 μm and 40 μm, respectively). Cathepsin K-expressing cells are clearly seen adjacent to toluidine-blue stained cartilage. C: Cathepsin K staining of the subepiphyseal growth plate area in tibia of wild-type and Idua−/− mice. Graph showing the average percentage of subepiphyseal area stained positive for cathepsin K in shoulder, knee joints, and vertebra bones for wild-type and Idua−/−. *P < 0.05 vs. wild-type. Scale bars = 260 μm.
Osteoclast Isolation from Neonatal Murine Long Bones
Mature osteoclasts from wild-type, Ctsk−/− and Idua−/− mice were isolated from 6-day-old mouse long bones.17 Long bones were isolated and collected in α-modified minimal essential medium (Invitrogen-Gibco, Burlington, ON) supplemented with 10% fetal bovine serum, 2 mmol/L l-glutamate (Invitrogen-Gibco, Burlington, ON); they were then diced into small pieces and bone cells were released by gentle pipetting. The resulting cell suspension (without bone pieces) was then plated onto collagen-coated slides in a 24 well plate and incubated at 37°C (95% air and 5% CO2). After 2 hours, nonattached cells were washed away, and cells were treated with 10 μg/ml lipopolysaccharide (Sigma-Aldrich, St-Louis, MO) and additional treatments as specified below. Cells were then cultured at 37°C (5% CO2) for 24 hours. To confirm the presence of osteoclasts, cells were stained for tartrate-resistant acid phosphatase (TRAP), an osteoclast marker, according to the manufacturers’ instructions (Sigma-Aldrich, St-Louis, MO). The number of positive TRAP-stained cells was about 50% of the total number of cells.
Collagen-Coated Coverslips
Thin collagen coatings were generated as described by R&D systems (Minneapolis, MN) on glass coverslips. Soluble type I collagen (USB, Cleveland, OH) was diluted in 500 μl 0.02 mmol/L acetic acid to a final concentration of 50 μg/ml and 200 μl added per coverslip. Coverslips were incubated at room temperature for 1 hour, residual volume was removed, and coverslips were washed with PBS, before cells were added. Type I collagen was also pre-degraded with 400 nmol/L recombinant human cathepsin K in 100 mmol/L sodium acetate buffer, pH 5.5 at 28°C for 4 hours. Digested collagen was then added at the same concentration as nondigested collagen to coverslips. The expression and purification of recombinant human cathepsin K has been previously described.18
Cell Treatments
The potent and irreversible vinyl sulfone cathepsin inhibitor, LHVS (Mu-hPhe-Len-VS-Ph),19,20 was used at a final concentration of 5 μmol/L. 0.15% DS or CS (percentage based on total volume) was dissolved in osteoclast media and incorporated into type I collagen substrate. All cell treatments were for 24 hours unless otherwise stated.
Fluorescent Staining
To detect actin ring formation in osteoclasts, the cells were washed with PBS, fixed with 3.7% formaldehyde in PBS, and permeabilized with 0.2% Triton X-100 for 10 minutes. Osteoclast actin rings were visualized using fluorescein isothiocyanate-phalloidin (Sigma-Aldrich, St-Louis, MO) (1:50 dilution) staining as previously described.21 After staining, cells were washed with PBS and the nuclei were stained with bisbenzimide (Sigma- Aldrich, St-Louis, MO) (2 μg/ml) for 5 minutes and then rinsed with water. Cells were mounted with Fluoromount (Sigma-Aldrich, St-Louis, MO). Actin rings were visualized fluorescently using a Leica DMI 6000B microscope (Leica Microsystems, Inc, Richmond Hill, ON) and their total number per slide was counted as previously described.22 Osteoclasts were identified by the presence of at least two nuclei. Typically osteoclasts contained between three and seven nuclei, no distinction was made between large and small osteoclasts. If an osteoclast displayed one or more actin rings, it was denoted as actin ring–positive (AR+); osteoclasts without or with disrupted actin rings were actin ring–negative (AR−). The ratio of normal versus disrupted actin rings was calculated. An actin ring was considered disrupted if less than half of it exhibited typical actin ring morphology.23
Resorption Assay
Cells were plated out onto dentine disks (Osteosite Dentine Disks, Immunodiagnostic Systems Inc, Fountain Hills, AZ) in a 96-well plates as previously described.17 After 2 hours, disks were removed and placed in 6-well plates containing α-modified minimal essential medium (∼pH 7.0) and test substance. There were typically four dentine disks per group. After 48 hours, slices were then stained for TRAP, which allowed the identification of osteoclasts (TRAP+ with more than two nuclei). Once osteoclasts were counted, cells were removed by 5% sodium hypochlorite treatment for 10 minutes. Disks were rinsed with water and stained with 1% (w/v) toluidine blue in 0.5% sodium borate for 30 seconds and then washed with water. The number and the area of resorption pits were then measured by light microscopy using Openlab 4.0.3 software. Results are expressed as the number of resorption pits and total area resorbed per dentine disk.
Collagen Digests
Soluble type II collagen (0.6 mg/ml calf skin) (USB, Cleveland, OH) was incubated with human cathepsin K (800 nmol/L) in sodium acetate buffer, pH 5.5, containing 2.5 mmol/L dithiothreitol, and EDTA. Recombinant human cathepsin K was expressed using the Pichia pastoris expression system as previously described.18 Total volume of each reaction was 100 μl, digestions were performed at 28°C for 4 hours. Cathepsin K collagen digestions were performed in the presence of 800 nmol/L chondroitin 4-sulfate (Sigma-Aldrich, St-Louis, MO) (chondroitin 4-sulfate concentration is based on an average molecular weight of 30 kDa). Digests were also performed in the additional presence of 800 nmol/L, 6.4 μmol/L and 16 μmol/L DS and HS (concentration is based on an average molecular weight of 30 kDa for DS and 15 kDa for HS). The collagen digests were inactivated by 5 μmol/L cysteine protease inhibitor LHVS. Collagen degradation was analyzed by SDS polyacrylamide gel electrophoresis using 4% to 20% gradient gels (1.5 hours at 125V) (Invitrogen, Carlsbad, CA). Bands were visualized using Coomassie Brilliant Blue R 250.
Bone Digests
Long bones and vertebrae from 4- to 6-week-old wild-type or Idua−/− mice were isolated and cleaned, and bone marrow was removed. Lipids were removed by incubating long bones overnight in xylene; bones were then frozen and crushed with a pestle and mortar to obtain bone powder. Bone powder was washed three times with sodium acetate buffer before enzyme was added. Human cathepsin K was added to bone powder (15 mg with 1 ml reaction volume) at a concentration of 800 nmol/L and incubated at 28°C for 24 hours. Digestion by cathepsin K was inactivated by the addition of 5 μmol/L cysteine protease inhibitor LHVS. Samples were then spun down and the soluble fraction was removed. 100 μl of supernatants from bone digests were hydrolyzed in 100 μl HCl in a sealed hydrolysis vial at 110°C overnight. Samples were assayed for hydroxyproline content as described previously.8
Statistical Analysis
Experiments were performed in duplicate three times using osteoclast cultures from three different mice. Data are expressed as mean ± SD. The statistical significance of the difference between the control and the experimental group was determined by student t-test. In bone resorption assays experiments were performed four times and comparisons between control and each treatment group was made using the Mann Whitney U-test. Effects were considered statistically significant when P ≤ 0.05.
Results
Cartilage Content and Cathepsin K Expression in MPS I Bone
To assess the cartilage content in MPS I and wild-type mice, bone sections were stained with toluidine blue (Figure 1A). Knee and shoulder contained similar amounts of cartilage staining in the growth plate: around 20% of the area for cartilage in Idua−/− bone and around 10% in wild-type bone. The growth plate area of the vertebrae was found to contain cartilage staining up to 60% in MPS I bone, as compared with 27% in wild-type bone. Overall, the amount of cartilage in Idua−/− murine specimens of shoulder, knee, and vertebrae was found to be about twice as high as in wild-type bones. Similar to Idua−/− bones, cathepsin K-deficient specimens showed about twice as much cartilage in the subepiphyseal zones as the wild-type litters. Then, we evaluated the presence of cathepsin K below the Idua−/− and wild-type growth plates. Measurement of the area staining for cathepsin K showed cathepsin K expression in MPS I bone was surprisingly about twofold increased over that in wild-type bone (Figure 1C). These findings indicate that despite the increased occurrence of cathepsin K-expressing osteoclasts in MPS I specimens, cartilage in the subepiphyseal growth plate area of MPS I murine bone appears to be less degraded during the ossification period. Cathepsin K-expressing osteoclasts were found in high numbers just below the growth plate and frequently adjacent to toluidine blue-stained cartilage (Figure 1B). As cathepsin K is the major bone- and cartilage-degrading protease in osteoclasts, we next evaluated whether cathepsin K is active in Idua−/− specimens.
Presence of Type II Collagen Cleaved by Cathepsin K
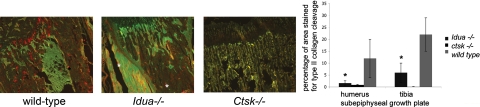
To assess the activity of cathepsin K in the MPS I growth plate, an anti-neoepitope antibody, which detects a cathepsin K-specific cleavage site in type II collagen, was used. The specificity of this antibody has been previously characterized.16 The growth plate area of the shoulder and knee were labeled for cleaved type II collagen and assessed by immunofluorescence. The area of cleaved type II collagen was measured in relation to the subepiphyseal growth plate area used for the measurements of cathepsin K expression and cartilage presence. In the MPS I growth plate areas of the humerus and tibia there was a three- to sixfold decrease in cathepsin K mediated neoepitope exposure in type II collagen when compared with wild-type growth plates (Figure 2). This reduction was observed despite the higher presence of cathepsin K-positive cells in this area of MPS I joints. As expected, Ctsk−/− mice displayed no or negligible staining by the neoepitope antibody (Figure 2). This finding suggests that the increase in cartilage content in Idua−/− and Ctsk−/− murine tissue samples correlates with a decrease or the absence of cathepsin K- mediated type II cartilage degradation. Next, we determined whether Idua−/−-associated increased tissue levels of GAGs affect cathepsin K activity.
Figure 2.
Neoepitope staining of cathepsin K catalyzed type II collagen cleavage in subepiphyseal growth plate area of wild-type (WT) and Idua−/− tibia (red or yellow for neoepitopes; green is enhanced background using the green filter to visualize the joint structure). Asterisks indicate sporadic artifact fluorescent precipitations). Graph showing percentage of area beneath growth plate containing cleaved type II collagen staining in humerus (shoulder) and tibia (knee) joints of wild-type, idua−/−, and ctsk−/− mice. *P < 0.05 vs. wild-type.
HS and Cathepsin K Colocalization
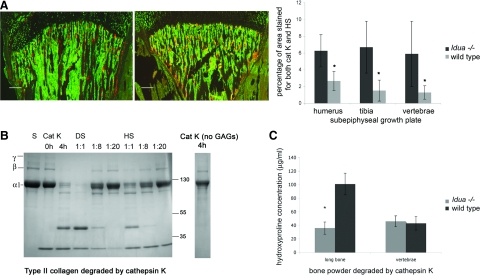
To investigate whether the strong bone phenotype of MPS I could partly be explained by deregulation of cathepsin K activity, we determined the colocalization of cathepsin K and the glycosaminoglycan HS. HS and DS both accumulate during the pathology of MPS I disease1 and have both been shown to inhibit the type I collagenolytic activity of cathepsin K.11 However, due to the lack of an appropriate antibody for DS, we have only described HS staining. The growth plate of the femur at the shoulder joint, the tibia at the knee joint, and the spine, were stained for cathepsin K and HS, and the overlapping area resulting in orange fluorescence was measured (Figure 3A). We found that the percentage of area, which overlapped for cathepsin K and HS was significantly higher in Idua−/− murine bone than wild-type. The greatest overlap was observed within the spine and knee. Overall, the spatial overlap of cathepsin K staining and HS staining was three to five times higher in Idua−/− than in wild-type mice.
Figure 3.
A: Co-localization (orange) of heparan sulfate (green) and cathepsin K (red) in the growth plate of tibia in wild-type (WT) and Idua−/− mice. Graph of percentage of overlap in total subgrowth plate area of humerus, tibia, and vertebrae. *P < 0.05 vs wild-type. Scale bars = 260 μm. B: SDS-polyacrylamide gel electrophoresis of type II collagen degradation at a 1:1 ratio of cathepsin K (800 nmol/L) and chondroitin sulfate (800 nmol/L) (CS) and in the presence of additional and increasing amounts of MPS1-related GAGs (DS or HS) added at final ratios of cathepsin K to DS or HS at 1:1, 1:8, and 1:20. Reactions were in sodium acetate buffer for 4 hours at 28°C. C: Hydroxyproline assay: long bone and vertebrae bone powder from Idua−/− and wild-type bone were crushed and subject to digestion by 800 nmol/L cathepsin K in sodium acetate buffer overnight at 28°C. The soluble fragments were assayed for hydroxyproline against known standards. *P < 0.05 vs. wild-type.
Type II Collagen Degradation by Cathepsin K in Presence of GAGs
We have previously demonstrated that the GAGs chondroitin 4-sulfate, chondroitin 6-sulfate, and keratin sulfate enabled cathepsin K to degrade type I collagen, but DS, HS, and heparin strongly inhibited the degradation.11 Here, we investigated the ability of DS and HS to inhibit cathepsin K degradation of cartilage constituting type II collagen. Type II collagen was incubated with 800 nmol/L human recombinant cathepsin K in the presence of CS and increasing amounts of HS and DS. The quantities of accumulated GAGs in Idua−/− tissues have been determined in tissues such as liver and lung, but not for bone or joint. In liver, lungs, and ovaries, the concentration of GAGs can reach up to 20 times the normal physiological concentration24,25; therefore in our degradation assay, we added the equivalent concentration to mimic in vivo conditions. The extent of degradation was visualized by Coomassie staining of type II collagen cleavage fragments after gel electrophoresis. In the absence of any GAGs, cathepsin K is unable to degrade triple helical type I collagen, whereas in the presence of CS, cathepsin K is able to effectively degrade type II collagen, as shown by the loss of the α 1 band in Figure 3B. However, the addition of increasing amounts of both HS and DS completely inhibited the degradation of type II collagen by cathepsin K. The extent of collagen degradation in bone was investigated using a hydroxyproline assay, which measures the amount of degraded collagen released from insoluble bone powder after incubation with cathepsin K. The amount of collagen degradation by cathepsin K was significantly higher in wild-type long murine bone, as compared with Idua−/− samples (Figure 2). Taken together, both the collagen and bone digestion studies suggest that the presence of high levels of DS and HS in Idua−/− murine bone have an inhibitory effect on the collagenolytic potential of cathepsin K. Next we asked how MPS I-related GAGs affect the functionality of bone and cartilage-degrading osteoclasts.
Osteoclast Activity in the Presence of Glycosaminoglycans
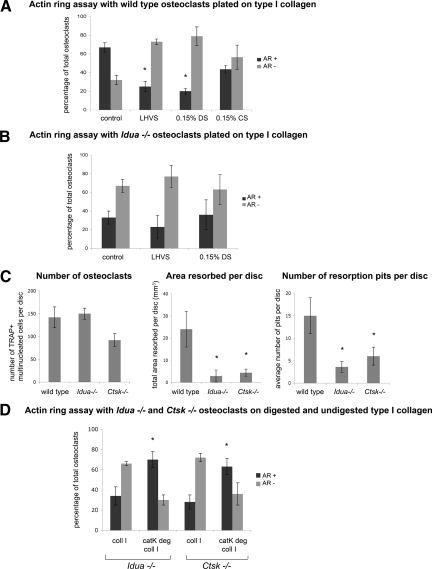
Neonatal osteoclasts were cultured in vitro from Idua−/−, Ctsk−/− and wild-type murine long bones. After incubation with test compounds (GAGs, protease inhibitors), the cultures were stained for actin. Osteoclast activity requires the formation of the sealing zone, within which solubilization and degradation of the matrix occurs. The sealing zone is composed in part by an actin ring, after the cytoskeleton of the osteoclast is reorganized during a sequence of signaling events initiated by integrin binding to components of the bone matrix. In non-resorbing osteoclasts, actin is found in clumps around the cell or in podosomes. To investigate the activity of osteoclasts in a culture, we measured the percentage of actin ring−positive osteoclasts, as compared with actin ring−negative osteoclasts. Cultures of wild-type osteoclasts typically contain around 60% cells with actin rings (Figure 4A), images of which we have previously shown.22 The presence of the broad specificity cysteine protease inhibitor, LHVS, resulted in a reduction of the amount of osteoclasts with an actin ring. When wild-type osteoclasts were cultured with 0.15% DS incorporated into the matrix and media the number of osteoclasts displaying an actin ring decreased by almost 70% (Figure 4A). The incorporation of C4S, which does not inhibit cathepsin K activity, did not cause a significant decrease in actin ring presence (Figure 4A). However, the presence of neither LHVS nor dermatan sulfate completely eliminated the presence of actin rings indicating that other enzymes such as matrix metalloproteinases or other cathepsins may partly contribute to actin ring formation. We have previously shown that GM6001, a general MMP inhibitor, decreases the percentage of actin rings in osteoclasts albeit to a lesser degree than a cysteine protease inhibitor.22
Figure 4.
Osteoclast cultures obtained from murine long bones were seeded on type I collagen substrate, after 24 hours incubation cultures were stained for actin with FITC-phalloidin. Osteoclasts displaying a full actin ring or disrupted actin rings with more than 50% intact were identified as active. Osteoclasts displaying a disrupted actin ring (diffuse ring or less than 50% intact ring), or complete lack of actin structure, were identified as inactive. After 24 hours incubation, cultures were stained for actin and the total number of osteoclasts with actin rings were counted and compared with the total number of osteoclasts present (determined by cells containing two or more nuclei). Bar chart shows comparison between percentage of osteoclasts displaying an actin ring (positive for Actin Ring +) or without (negative for Actin Ring −) under different experimental conditions. Data are expressed as percentage. A: Osteoclasts were incubated with or without 5 μmol/L of the broad spectrum cathepsin inhibitor, LHVS. Osteoclasts were also incubated in the presence of 0.15% dermatan sulfate (DS) or chondroitin sulfate (CS), GAGs were incorporated in the type I collagen matrix and the cell media. After 24 hours incubation, cultures were stained for actin and the total number of osteoclasts with actin rings were counted and compared with the total number of osteoclasts. *P < 0.05 vs wild-type untreated control. B: Idua−/− osteoclasts were incubated in the presence and absence of 5 μmol/L of the broad spectrum cathepsin inhibitor, LHVS, and 0.15% dermatan sulfate in the matrix and media. C: Wild-type, Idua−/− and Ctsk−/− osteoclasts were cultured on dentine disks for 48 hours. The number of TRAP+ osteoclasts and the number and area of resorption pits were analyzed. Values are the means plus SD of four dentine slices. *P < 0.05 vs. wild-type. D: Long bone osteoclasts obtained from Idua−/− and Ctsk−/− mice were seeded onto intact type I collagen matrix (coll 1) or type I collagen pre-degraded by cathepsin K (catK deg coll 1) and analyzed for actin ring presence. Collagen type I was pre-degraded by 400 nmol/L cathepsin K in the presence of chondroitin 4-sulfate for 4 hours at 28°C. After 24 hours, cultures were stained for actin and the percentage of osteoclasts displaying an actin ring (AR+) is shown compared with the percentage without (AR−). *P ≤ 0.05 when compared with same osteoclasts cultured on intact collagen matrix.
Idua−/− Osteoclast Activity and Bone Resorption
The presence of actin rings in primary Idua−/− murine cell cultures was much more reduced than in wild-type murine osteoclasts, with only around 30% displaying an actin ring (Figure 4B). The presence of the cysteine protease inhibitor, LHVS, had no significant effect on the presence of actin rings. The levels of actin rings did not change in the presence of DS either (Figure 4B). Bone resorption studies also displayed an inhibition of resorbed area and pit number compared with wild-type osteoclasts despite similar osteoclast numbers in each culture, as confirmed by TRAP staining. The number and size of the pits formed by Idua−/− murine osteoclasts were significantly lower than wild-type osteoclasts but similar to those observed in Ctsk−/− cells (Figure 4C).
In previous experiments, we demonstrated that the ability of Ctsk−/− murine osteoclasts to form actin rings could be increased to wild-type levels by plating them onto cathepsin K-, but not cathepsin L-predigested collagen.22 As seen in Figure 4D, the ratio between the number of cells displaying actin rings or lacking them reversed under these condition for both Idua−/− and Ctsk−/− osteoclasts. Similar to wild-type osteoclasts (Figure 4A), the number of osteoclasts exhibiting actin rings was about twice as high as those missing actin rings. This implies that the decrease in actin ring formation in Idua−/− murine osteoclasts, ie, the reduction in matrix-resorbing activity, is linked to the inhibition of cathepsin K.
Discussion
MPS I is thought to be the archetypal form of mucopolysaccharidosis, as it displays most of the clinical manifestations of this group of diseases. Lack of the enzyme iduronidase (Idua−/−) results in lysosomal dysfunction due to the build-up of HS and DS within cells causing vacuolation. In bones and joints, this results in the skeletal phenotype known as dysostosis multiplex. In our studies we used a murine MPS I model in which the Idua gene is disrupted.14
The murine model of MPS I was first identified by Clarke and co-workers14 as pertaining several features of the human phenotype of the disease. Noted pathological features of the murine MPS I skeleton included course facial features, dysostosis multiplex (with widening and thickening of the ribs and zygomatic arches), and thoracic vertebral kyphosis, as well as broadened and thickened long bones. Further studies revealed aberrant bone remodeling and growth plate development, and maturation with abnormalities in the early cortical bone structure.26 In young mice, the growth plate appeared thickened with a high number of chondrocytes, and a disorganized structure was observed in the hypertrophic zone. Arrangement of the primary trabecular bone was abnormal and contained an increased presence of cartilage within the woven bone, suggesting difficulties in cartilage resorption during endochondral ossification. This condition worsened with age, with obvious retained cartilage within the ossified bone. Similar late growth plate abnormalities have been found in cat MPS I and MPS VI, mouse MPS VI, mouse MPS VII.26,27,28,29,30 Clarke et al14 reported that the skeletal dysmorphia in murine MPS I was a more severe condition than that observed in feline or canine MPS I, and of similar severity to MPS VII and VI murine models. However, other studies on murine and canine MPS I models have failed to identify significant shortening of long bones or severe growth plate abnormalities, although they did report bone thickening.31,32,33
In this study, we investigated the subepiphyseal growth plate area of Idua−/− mice and compared it to wild-type and Ctsk.−/− As mentioned, it is thought that some of the skeletal abnormalities are a result of insufficient endochondral ossification. Endochondral ossification is the process by which long bone is formed by replacement of cartilage. During this process cathepsin K, as the main osteoclastic protease,34,35 is thought to degrade calcified cartilage. Histological analysis showed that the Idua−/− bones had more cartilage in their growth plates, when compared with specimens from wild-type litters, thus, suggesting decreased cartilage resorption activity. The increased cartilage content in Idua−/− mice was observed despite a simultaneous increase in cathepsin K-expressing osteoclasts in these mice.
In other studies, DS and HS proteoglycans have been implicated in promoting osteoclastogenesis.36,37,38 Moreover, Simonaro et al39 described a dramatic up-regulation of tumor necrosis factor-α and an osteoclast survival factor leading to an increase in osteoclasts or related multinucleated cells. Tumor necrosis factor-α and other pro-inflammatory cytokines have been demonstrated to increase cathepsin K expression.40 Interestingly, increased osteoclast numbers (though mostly dysfunctional) also have been observed as a response to anti-resorptive bisphosphonates in osteoporotic patients.41
However, how can we explain that Idua-deficiency leads to an insufficient degradation of epiphyseal cartilage, despite an overall increase in cathepsin K expressing osteoclasts? This could be due in part to a general lysosomal dysfunction caused by GAG accumulation in MPS I osteoclasts. Alternatively, it is tempting to speculate that the major collagenase activity of osteoclasts, cathepsin K, is directly affected by GAG accumulation. We have previously demonstrated that GAGs are important regulators of cathepsin K activity.8,9,11 The normal growth plate area contains mostly CS, and our previous studies have shown that bone-residing CS is capable of forming a complex with cathepsin K, enabling the enzyme to degrade type I collagen.9,10 Other GAGs, such as DS and HS are also able to form complexes with the enzyme; however, these result in an inhibition of collagenolytic activity.11 In diseases like MPS I, where the amount of GAGs like DS and HS is dramatically increased, the likelihood of them forming an inhibitory complex with cathepsin K over C4S is high.
The subepiphyseal growth plate area stained positively for HS and cathepsin K in both Idua−/− and wild-type sections, with a higher expression of each in Idua−/− bone. HS, DS, and CS have previously been localized to the bone tissue (sites of ossification) in areas adjacent to osteoclast localization.42 The overlap between cathepsin K and HS was, as expected, significantly increased in Idua−/− bone, meaning that most of the cathepsin K protein (on average 75%) colocalized with HS in idua−/− mice, whereas in wild-type mice it was around 40%.
The activity of cathepsin K was also investigated to determine the effect of high HS concentrations. The area was also stained for type II collagen cleavage using an anti-neoepitope antibody that recognizes only cathepsin K-cleaved type II collagen. Idua−/− bone displayed a 75% to 85% reduction of cleaved collagen staining, as compared with wild-type bone. Naturally, cathepsin K-deficient mice revealed no significant staining for collagen cleavage. Taken together these results suggest that despite elevated cathepsin K expression, the increased co-localization of cathepsin K and HS (and likely DS as well) in Idua−/− mice could reduce the enzyme’s ability to degrade type II collagen.
Degradation studies demonstrated that cathepsin K digestion of type II collagen is inhibited by the presence of DS and HS. Even at concentrations equal to C4-S, the presence of DS and HS strongly inhibits the collagenolytic activity of cathepsin K. In MPS tissues, the concentration of GAGs can reach 7 to 20 times the concentration normally found in liver and bone.24,25 Therefore, cathepsin K expressed by osteoclasts would likely have a reduced ability to degrade the cartilage during endochondral ossification in such an environment. Moreover, general bone remodeling might also be affected, as shown by the decreased ability of recombinant human cathepsin K to degrade MPS I bone powder, demonstrating the importance of regulated GAG expression in bone.
It should be noted that in other MPS disease types such as MPS VI, where primarily DS accumulates, there is a decrease in bone formation resulting in osteopenia of tibial trabecular bone and rearrangement of trabecular bone architecture.43 The osteopenia described in MPS VI and MPS VII is thought to be due to early chondrocyte death and increased expression and activity of matrix metalloproteases-2 and -9, as well as decreased activity of osteoblasts.44,45,46 This could result in incomplete endochondral ossification and disorganization of the growth plate. Retained calcified cartilage can also be found in metaphyseal bone in MPS VI,29 suggesting that cathepsin K may also be involved in the apparent abnormal ossification.
We also investigated the effect of a GAG-rich environment on in vitro osteoclast activity by monitoring the presence of actin rings and bone resorption on dentine disks. By observing the presence of actin rings, which only occurs when an osteoclast is actively resorbing, we could monitor the effect of inhibitors and GAGs on osteoclast activity. Cytoskeletal rearrangement to form an actin ring is initiated by osteoclast attachment factors and is a requirement for the formation of the sealing zone and resorption lacunae. As expected, the addition of the cysteine protease inhibitor LHVS decreased the actin ring presence in wild-type osteoclasts. We have previously shown that cathepsin K activity is required for the exposure of ‘cryptic’ RGD sequences in the collagen matrix to allow the activation of osteoclasts.22 Our studies also showed that high concentrations of DS, but not C4S, were able to inhibit the actin ring presence in wild-type osteoclasts. This suggests that the presence of high concentrations of dermatan sulfate has an inhibitory effect on osteoclast activity. Human cathepsin K-deficient osteoclasts have previously been shown to form poorly developed ruffled borders and accumulate nondigested collagen within their lysosomes.5 Similarly, studies on the morphology of MPS VII osteoclasts from newborn mice revealed that they are typically detached from the matrix and do not form ruffled borders.47
When osteoclasts isolated from Idua−/− mice were cultured on type I collagen, we found they displayed a low level of actin ring presence, similar to wild-type mice under cysteine protease inhibition. In fact, the addition of the cysteine protease inhibitor had little additive effect on actin ring presence, suggesting that cathepsin activity is already limited. The addition of DS also had no effect on actin ring presence, presumably because there was already a build-up of GAGs within the Idua−/− osteoclasts. Abreu et al29 have shown the presence of swollen cells with large vacuoles (containing GAGs) in the ossification zone in MPS bone. However, when idua−/− osteoclasts were plated on cathepsin K-predigested type I collagen, with RGD units exposed, the number of actin rings present increased to wild-type levels. The same was observed for Ctsk−/− cells. This clearly suggests that primarily a lack of cathepsin K activity is responsible for the inability of Idua−/− osteoclasts to transform into an activated cell status. Idua−/− osteoclasts had not only a diminished ability to form actin rings but were, similar to Ctsk−/− osteoclasts, much less capable to degrade bone, as shown by the reduced number and size of Idua−/− osteoclast excavations, when compared with wild-type osteoclasts.
In conclusion, this report describes the effect of MPS-associated GAGs on the cartilage/bone resorbing activity of cathepsin K. DS and HS, which accumulate in MPS I disease, directly inhibits the collagenolytic activity of cathepsin K. This is reflected in the increase of cartilage beneath the subepiphyseal growth plate area, the inhibition of type II collagen degradation in this area, and the reduced capability of osteoclast activation in Idua−/− mice. The decrease in the collagenolytic activity of cathepsin K, due to the expression of GAGs, will greatly reduce osteoclast function and will thus likely contribute to the skeletal abnormalities observed in MPS I bone.
Footnotes
Address reprint requests to Dieter Brömme, 2350 Health Sciences Mall, Life Sciences Institute, Rm 4559, University of British Columbia, Vancouver V6T 1Z3, Canada. E-mail: dbromme@interchange.ubc.ca.
Supported in part by National Institutes of Health Grants AR 48669 and DK 072070. D.B. was supported by the Canada Research Chair award.
References
- Neufeld EF, Muenzer J. The Mucopolysaccharidoses. New York: McGraw-Hill,; 2001:pp 3419–3428. [Google Scholar]
- Burrow TA, Hopkin RJ, Leslie ND, Tinkle BT, Grabowski GA. Enzyme reconstitution/replacement therapy for lysosomal storage diseases. Curr Opin Pediatr. 2007;19:628–635. doi: 10.1097/MOP.0b013e3282f161f2. [DOI] [PubMed] [Google Scholar]
- Yasuda Y, Kaleta J, Bromme D. The role of cathepsins in osteoporosis and arthritis: rationale for the design of new therapeutics. Adv Drug Deliv Rev. 2005;57:973–993. doi: 10.1016/j.addr.2004.12.013. [DOI] [PubMed] [Google Scholar]
- Gelb BD, Shi GP, Chapman HA, Desnick RJ. Pycnodysostosis, a lysosomal disease caused by cathepsin K deficiency. Science. 1996;273:1236–1238. doi: 10.1126/science.273.5279.1236. [DOI] [PubMed] [Google Scholar]
- Everts V, Hou WS, Rialland X, Tigchelaar W, Saftig P, Bromme D, Gelb BD, Beertsen W. Cathepsin K deficiency in pycnodysostosis results in accumulation of non-digested phagocytosed collagen in fibroblasts. Calcif Tissue Int. 2003;73:380–386. doi: 10.1007/s00223-002-2092-4. [DOI] [PubMed] [Google Scholar]
- Gelb BD, Brömme D, Desnick RJ. Pycnodysostosis: Cathepsin K deficiency. Sriver CR, Beaudet AL, Valle D, Sly W, editors. New York: McGraw-Hill, Inc.,; The Metabolic and Molecular Bases of Inherited Diseases. 2001:pp 3453–3468. [Google Scholar]
- Fratzl-Zelman N, Valenta A, Roschger P, Nader A, Gelb BD, Fratzl P, Klaushofer K. Decreased bone turnover and deterioration of bone structure in two cases of pycnodysostosis. J Clin Endocrinol Metab. 2004;89:1538–1547. doi: 10.1210/jc.2003-031055. [DOI] [PubMed] [Google Scholar]
- Li Z, Hou WS, Bromme D. Collagenolytic activity of cathepsin K is specifically modulated by cartilage-resident chondroitin sulfates. Biochemistry. 2000;39:529–536. doi: 10.1021/bi992251u. [DOI] [PubMed] [Google Scholar]
- Li Z, Hou WS, Escalante-Torres CR, Gelb BD, Bromme D. Collagenase activity of cathepsin K depends on complex formation with chondroitin sulfate. J Biol Chem. 2002;277:28669–28676. doi: 10.1074/jbc.M204004200. [DOI] [PubMed] [Google Scholar]
- Li Z, Kienetz M, Cherney MM, James MN, Bromme D. The crystal and molecular structures of a cathepsin K: chondroitin sulfate complex. J Mol Biol. 2008;383:78–91. doi: 10.1016/j.jmb.2008.07.038. [DOI] [PubMed] [Google Scholar]
- Li Z, Yasuda Y, Li W, Bogyo M, Katz N, Gordon RE, Fields GB, Bromme D. Regulation of collagenase activities of human cathepsins by glycosaminoglycans. J Biol Chem. 2004;279:5470–5479. doi: 10.1074/jbc.M310349200. [DOI] [PubMed] [Google Scholar]
- Ballock RT, O'Keefe RJ. The biology of the growth plate. J Bone Joint Surg Am. 2003;85-A:715–726. [PubMed] [Google Scholar]
- Saftig P, Wehmeyer O, Hunziker E, Jones S, Boyde A, Rommerskirch W, von Figura K. Impaired osteoclastic bone resorption leads to osteopetrosis in cathepsin K-deficient mice. Proc Natl Acad Sci USA. 1998;95:13453–13458. doi: 10.1073/pnas.95.23.13453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke LA, Russell CS, Pownall S, Warrington CL, Borowski A, Dimmick JE, Toone J, Jirik FR. Murine mucopolysaccharidosis type I: targeted disruption of the murine alpha-l-iduronidase gene. Hum Mol Genet. 1997;6:503–511. doi: 10.1093/hmg/6.4.503. [DOI] [PubMed] [Google Scholar]
- Xia L, Kilb J, Wex H, Lipyansky A, Breuil V, Stein L, Palmer JT, Dempster DW, Brömme D. Localization of rat cathepsin K in osteoclasts and resorption pits: inhibition of bone resorption cathepsin K-activity by peptidyl vinyl sulfones. Biol Chem. 1999;380:679–687. doi: 10.1515/BC.1999.084. [DOI] [PubMed] [Google Scholar]
- Dejica VM, Mort JS, Laverty S, Percival MD, Antoniou J, Zukor DJ, Poole AR. Cleavage of type II collagen by cathepsin K in human osteoarthritic cartilage. Am J Pathol. 2008;173:161–169. doi: 10.2353/ajpath.2008.070494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoebertz A, Arnett TR. Isolated osteoclast cultures. Helfrich MH, Ralston SH, editors. Totowa NJ: Humana Press,; Bone Research Protocols. 2003:pp 53–64. doi: 10.1385/1-59259-366-6:53. [DOI] [PubMed] [Google Scholar]
- Linnevers CJ, McGrath ME, Armstrong R, Mistry FR, Barnes M, Klaus JL, Palmer JT, Katz BA, Brömme D. Expression of human cathepsin K in Pichia pastoris and preliminary crystallographic studies of an inhibitor complex. Protein Science. 1997;6:919–921. doi: 10.1002/pro.5560060421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer JT, Rasnick D, Klaus JL, Brömme D. Vinyl sulfones as mechanism-based cysteine protease inhibitors. J Med Chem. 1995;38:3193–3196. doi: 10.1021/jm00017a002. [DOI] [PubMed] [Google Scholar]
- Riese RJ, Wolf P, Bromme D, Natkin LR, Villadangos JA, Ploegh HL, Chapman HA. Essential role for cathepsin S in MHC class II-associated invariant chain processing and peptide loading. Immunity. 1996;4:357–366. doi: 10.1016/s1074-7613(00)80249-6. [DOI] [PubMed] [Google Scholar]
- Graebert KS, Bauch H, Neumuller W, Brix K, Herzog V. Epithelial folding in vitro: studies on the cellular mechanism underlying evagination of thyrocyte monolayers. Exp Cell Res. 1997;231:214–225. doi: 10.1006/excr.1996.3456. [DOI] [PubMed] [Google Scholar]
- Wilson SR, Peters C, Saftig P, Bromme D. Cathepsin K activity-dependent regulation of osteoclast actin ring formation and bone resorption. J Biol Chem. 2009;284:2584–2592. doi: 10.1074/jbc.M805280200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakkakorpi PT, Vaananen HK. Kinetics of the osteoclast cytoskeleton during the resorption cycle in vitro. J Bone Miner Res. 1991;8:817–826. doi: 10.1002/jbmr.5650060806. [DOI] [PubMed] [Google Scholar]
- Haskins ME, Otis EJ, Hayden JE, Jezyk PF, Stramm L. Hepatic storage of glycosaminoglycans in feline and canine models of mucopolysaccharidoses I, VI, and VII. Vet Pathol. 1992;29:112–119. doi: 10.1177/030098589202900203. [DOI] [PubMed] [Google Scholar]
- Chung S, Ma X, Liu Y, Lee D, Tittiger M, Ponder KP. Effect of neonatal administration of a retroviral vector expressing alpha-l-iduronidase upon lysosomal storage in brain and other organs in mucopolysaccharidosis I mice. Mol Genet Metab. 2007;90:181–192. doi: 10.1016/j.ymgme.2006.08.001. [DOI] [PubMed] [Google Scholar]
- Russell C, Hendson G, Jevon G, Matlock T, Yu J, Aklujkar M, Ng KY, Clarke LA. Murine MPS I: insights into the pathogenesis of Hurler syndrome. Clin Genet. 1998;53:349–361. doi: 10.1111/j.1399-0004.1998.tb02745.x. [DOI] [PubMed] [Google Scholar]
- Birkenmeier EH, Davisson MT, Beamer WG, Ganschow RE, Vogler CA, Gwynn B, Lyford KA, Maltais LM, Wawrzyniak CJ. Murine mucopolysaccharidosis type VII. Characterization of a mouse with beta-glucuronidase deficiency. J Clin Invest. 1989;83:1258–1266. doi: 10.1172/JCI114010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haskins ME, Aguirre GD, Jezyk PF, Desnick RJ, Patterson DF. The pathology of the feline model of mucopolysaccharidosis I. Am J Pathol. 1983;112:27–36. [PMC free article] [PubMed] [Google Scholar]
- Abreu S, Hayden J, Berthold P, Shapiro IM, Decker S, Patterson D, Haskins M. Growth plate pathology in feline mucopolysaccharidosis VI. Calcif Tissue Int. 1995;57:185–190. doi: 10.1007/BF00310256. [DOI] [PubMed] [Google Scholar]
- Evers M, Saftig P, Schmidt P, Hafner A, McLoghlin DB, Schmahl W, Hess B, von Figura K, Peters C. Targeted disruption of the arylsulfatase B gene results in mice resembling the phenotype of mucopolysaccharidosis VI. Proc Natl Acad Sci USA. 1996;93:8214–8219. doi: 10.1073/pnas.93.16.8214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Wang R, Li Y, Tang L, Hu J, Xu A. Epiphyseal and physeal cartilage: normal gadolinium-enhanced MR imaging. J Huazhong Univ Sci Technolog Med Sci. 2005;25:209–211. doi: 10.1007/BF02873579. [DOI] [PubMed] [Google Scholar]
- Herati RS, Knox VW, O'Donnell P, D'Angelo M, Haskins ME, Ponder KP. Radiographic evaluation of bones and joints in mucopolysaccharidosis I and VII dogs after neonatal gene therapy. Mol Genet Metab. 2008;95:142–151. doi: 10.1016/j.ymgme.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalf JA, Zhang Y, Hilton MJ, Long F, Ponder KP. Mechanism of shortened bones in mucopolysaccharidosis VII. Mol Genet Metab. 2009 doi: 10.1016/j.ymgme.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromme D, Okamoto K. Human cathepsin O2, a novel cysteine protease highly expressed in osteoclastomas and ovary molecular cloning, sequencing, and tissue distribution. Biol Chem Hoppe Seyler. 1995;376:379–384. doi: 10.1515/bchm3.1995.376.6.379. [DOI] [PubMed] [Google Scholar]
- Drake FH, Dodds RA, James IE, Connor JR, Debouck C, Richardson S, Lee-Rykaczewski E, Coleman L, Rieman D, Barthlow R, Hastings G, Gowen M. Cathepsin K, but not cathepsins B, L, or S, is abundantly expressed in human osteoclasts. J Biol Chem. 1996;271:12511–12516. doi: 10.1074/jbc.271.21.12511. [DOI] [PubMed] [Google Scholar]
- Sanderson RD, Yang Y, Suva LJ, Kelly T. Heparan sulfate proteoglycans and heparanase–partners in osteolytic tumor growth and metastasis. Matrix Biol. 2004;23:341–352. doi: 10.1016/j.matbio.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Nakano K, Okada Y, Saito K, Tanaka Y. Induction of RANKL expression and osteoclast maturation by the binding of fibroblast growth factor 2 to heparan sulfate proteoglycan on rheumatoid synovial fibroblasts. Arthritis Rheum. 2004;50:2450–2458. doi: 10.1002/art.20367. [DOI] [PubMed] [Google Scholar]
- Dai J, Rabie AB. VEGF: an essential mediator of both angiogenesis and endochondral ossification. J Dent Res. 2007;86:937–950. doi: 10.1177/154405910708601006. [DOI] [PubMed] [Google Scholar]
- Simonaro CM, D'Angelo M, He X, Eliyahu E, Shtraizent N, Haskins ME, Schuchman EH. Mechanism of glycosaminoglycan-mediated bone and joint disease: implications for the mucopolysaccharidoses and other connective tissue diseases. Am J Pathol. 2008;172:112–122. doi: 10.2353/ajpath.2008.070564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou W-S, Li W, Keyszer G, Weber E, Levy R, Klein MJ, Gravallese EM, Goldring SR, Bromme D. Comparison of cathepsins K and S expression within the rheumatoid and osteoarthritic synovium. Arthritis Rheum. 2002;46:663–674. doi: 10.1002/art.10114. [DOI] [PubMed] [Google Scholar]
- Weinstein RS, Roberson PK, Manolagas SC. Giant osteoclast formation and long-term oral bisphosphonate therapy. N Engl J Med. 2009;360:53–62. doi: 10.1056/NEJMoa0802633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura H, Ozawa H. Immunohistochemical localization of heparan sulfate proteoglycan in rat tibiae. J Bone Miner Res. 1994;9:1289–1299. doi: 10.1002/jbmr.5650090819. [DOI] [PubMed] [Google Scholar]
- Byers RJ, Denton J, Hoyland JA, Freemont AJ. Differential patterns of altered bone formation in different bone compartments in established osteoporosis. J Clin Pathol. 1999;52:23–28. doi: 10.1136/jcp.52.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byers S, Nuttall JD, Crawley AC, Hopwood JJ, Smith K, Fazzalari NL. Effect of enzyme replacement therapy on bone formation in a feline model of mucopolysaccharidosis type VI. Bone. 1997;21:425–431. doi: 10.1016/s8756-3282(97)00175-0. [DOI] [PubMed] [Google Scholar]
- Nuttall JD, Brumfield LK, Fazzalari NL, Hopwood JJ, Byers S. Histomorphometric analysis of the tibial growth plate in a feline model of mucopolysaccharidosis type VI. Calcif Tissue Int. 1999;65:47–52. doi: 10.1007/s002239900656. [DOI] [PubMed] [Google Scholar]
- Simonaro CM, D'Angelo M, Haskins ME, Schuchman EH. Joint and bone disease in mucopolysaccharidoses VI and VII: identification of new therapeutic targets and biomarkers using animal models. Pediatr Res. 2005;57:701–707. doi: 10.1203/01.PDR.0000156510.96253.5A. [DOI] [PubMed] [Google Scholar]
- Monroy MA, Ross FP, Teitelbaum SL, Sands MS. Abnormal osteoclast morphology and bone remodeling in a murine model of a lysosomal storage disease. Bone. 2002;30:352–359. doi: 10.1016/s8756-3282(01)00679-2. [DOI] [PubMed] [Google Scholar]