Abstract
Inflammatory processes, including the episodic and/ or chronic elaboration of cytokines, have been identified as playing key roles in a number of neurological disorders. Whether these activities impart a disease-resolving and/or contributory outcome depends at least in part on the disease context, stage of pathogenesis, and cellular milieu in which these factors are released. Interferon-γ (IFNγ) is one such cytokine that produces pleiotropic effects in the brain. It is protective by ensuring maintenance of virus latency after infection, yet deleterious by recruiting and activating microglia that secrete potentially damaging factors at sites of brain injury. Using the triple-transgenic mouse model of Alzheimer’s disease (3×Tg-AD), which develops amyloid and tau pathologies in a pattern reminiscent of human Alzheimer’s disease, we initiated chronic intrahippocampal expression of IFNγ through delivery of a serotype-1 recombinant adeno-associated virus vector (rAAV1-IFNγ). Ten months of IFNγ expression led to an increase in microglial activation, steady-state levels of proinflammatory cytokine and chemokine transcripts, and severity of amyloid-related pathology. In contrast, these rAAV1-IFNγ-treated 3×Tg-AD mice also exhibited diminished phospho-tau pathology and evidence of increased neurogenesis. Overall, IFNγ mediates what seem to be diametrically opposed functions in the setting of AD-related neurodegeneration. Gaining an understanding as to how these apparently divergent functions are interrelated and controlled could elucidate new therapeutic strategies designed to harness the neuroprotective activity of IFNγ.
Dysregulated inflammatory processes have emerged as clear contributors to the progression of many neurodegenerative diseases affecting the aged, including Alzheimer’s disease (AD). Environmentally derived insults combined with underlying genetic susceptibilities seem to incite inflammatory reactions, which promote the elaboration of proinflammatory cytokines, including interferon-γ (IFNγ), tumor necrosis factor-α (TNF-α), interleukin (IL) 1β, and IL-6. This complex neuroimmune interaction, when chronically active and insufficiently controlled, ultimately leads to alterations in neural transmission, behavior, and disease-related pathogenesis (reviewed in Ref. 1).
IFNγ, a potent molecule normally expressed by natural killer cells, T cells, glia, and neurons, exhibits a multitude of immunoregulatory functions within the central nervous system (CNS) compartment, including activation of macrophages/microglia and the stimulation of macrophages to release toxic oxygen radicals (reviewed in Ref. 2). The expression of IFNγ and target CD8+ T cells is enhanced as a function of age and neurodegeneration.3,4 Moreover, IFNγ enhances vascular permeability to T cells and natural killer cells and promotes the expression of TNF-α and IL-1β by microglia (reviewed in Ref. 5), suggesting that IFNγ is a key player in the elaboration of immune and inflammatory responses within the CNS and is involved in neurodegeneration.
Human studies have linked dysregulated IFNγ expression to neurodegenerative processes associated with AD. Blasko et al.6 demonstrated a correlation between IFNγ and the induction of both the amyloid plaque-associated fibrillogenic peptides, amyloid-β 1-40 (Aβ1-40) and 1-42 (Aβ1-42), and Meda et al7 reported that IFNγ enhances the production of TNF-α by microglia once stimulated with Aβ peptides in vitro.7 Using immunocytochemical and in situ hybridization analyses, Abbas et al8 demonstrated that IFNγ expression is significantly augmented in amyloidogenic Tg2576 mice at 3 months of age (pre-amyloid pathology) and levels of this cytokine progressively increased as a function of age compared with those in wild-type mice.8 Interestingly, the AD mouse cohort exhibited a concomitant decrease in the anti-inflammatory cytokine IL-4, whereas an opposite cytokine expression profile was observed in the brains of wild-type mice. Others have shown that there exists a synergistic AD pathology-aggravating effect when IFNγ and TNF-α are coexpressed in the brain, leading to exacerbated Aβ peptide production and reduced pathogenic peptide clearance in a mouse model of AD.9
Based on these and other observations, we sought to further dissect the role of IFNγ in AD pathophysiology in a complex AD mouse model. Triple transgenic mice (3×Tg-AD), which express human presenilin-1 mutant M146V (PS1M146V), the Swedish mutation of human amyloid precursor protein (APPswe), and the P301L mutation of human tau (tauP301L), develop both plaques and neurofibrillary tangles in a progressive and age-dependent pattern.10 In this model, intracellular Aβ and soluble Aβ oligomers are first observed at 3 to 5 months of age, extracellular plaque formations appear at approximately 15 to 18 months, and neurofibrillary tangles arise at ages greater than 18 months.11 These mice also exhibit early deficits in synaptic function, including long-term potentiation, which are manifested in an age-dependent manner. To assess the pathological impact of IFNγ on the patterns and severity of AD-related pathologies in 3×Tg-AD mice but avoid developmental compensation effects that can arise in use of traditional transgenic approaches, we created a recombinant adeno-associated virus (rAAV) vector that could constitutively express human IFNγ within the CNS of adult mice after stereotactic delivery. Chronic overexpression of IFNγ by a serotype-1 recombinant rAAV vector in the 3×Tg-AD mouse over a 10-month period revealed both potential positive and negative activities of this cytokine. Accompanying increases in proinflammatory marker transcript levels was a concomitant enhancement in the number of microglia/macrophages and intracellular Aβ42 accumulation in the hippocampi of rAAV1-IFNγ-injected 3×Tg-AD mice. Surprisingly, however, we also observed that rAAV1-IFNγ-treated 3×Tg-AD mice exhibited evidence of reduced phospho-tau levels, enhanced synaptic marker transcript expression, and neurogenesis, suggesting that IFNγ serves seemingly juxtaposed roles in the setting of the diseased CNS.
Materials and Methods
Generation of IFNγ-Expressing rAAV Vector
The pFBGR plasmid, herein referred to as pAAV-eGFP, harbors a cytomegalovirus promoter-driven enhanced green fluorescent protein (eGFP) gene flanked by inverted terminal repeats (kindly provided by Dr. Robert Kotin). Human IFNγ cDNA from pHIIF-SV-γ1 (American Type Culture Collection, Manassas, VA) was cloned into the pBSFBRmcs shuttle vector and subsequently into a modified pFBGR plasmid backbone devoid of the eGFP gene. The resultant plasmid was designated pAAV-IFNγ. The pAAV-IFNγ plasmid or a β-galactosidase reporter gene-expressing plasmid (pHSVlac) was transiently transfected into baby hamster kidney cells, and human IFNγ transgene expression was subsequently confirmed in cell lysates and culture supernatants by enzyme-linked immunosorbent assay (ELISA) (eBioscience, San Diego, CA) before viral packaging.
rAAV Serotype-1 Vector Packaging
The pAAV-eGFP and pAAV-IFNγ plasmids were separately transposed into DH10-BAC Escherichia coli (Invitrogen, Carlsbad, CA), and DNA was purified before transfection into SF9 cells to produce baculovirus. Plasmids encoding AAV Rep and serotype 1 Cap genes were separately transposed into DH10-BAC cells. Resultant baculovirus stocks harboring pAAV-eGFP or pAAV-IFNγ were co-introduced into SF9 insect cells with baculovirus preparations expressing Rep and Cap to produce serotype 1 rAAV vectors according to methodology published previously.12 Recombinant AAV1-IFNγ and AAV1-eGFP virions were purified over a cesium chloride gradient and collected at a refractive index of 1.374 to 1.370. 293A cells were transduced with either rAAV1-IFNγ or rAAV1-eGFP to obtain titer information via flow cytometry and quantitative real-time PCR, and titers were expressed as transducing units per ml.13
Transgenic Mice
The 3×Tg-AD mice were kindly provided by Frank LaFerla (University of California, Irvine, Irvine, CA). All animal were housed and procedures were performed in compliance with guidelines established by the University Committee of Animal Resources at the University of Rochester.
Stereotactic Infusion of rAAV1-IFNγ and rAAV1-eGFP
Two-month-old 3×Tg-AD mice (N = 4 to 5 per experimental group) were stereotactically injected intrahippocampally with either rAAV1-IFNγ or rAAV1-eGFP in accordance with approved University of Rochester animal use guidelines. Mice were anesthetized with Avertin (300 mg/kg) and monitored throughout the stereotactic procedure for maintenance of anesthesia. The mouse was positioned in a stereotactic apparatus (ASI Instruments, Warren, MI), the skull was exposed via a sagittal incision, and two burr holes were drilled bilaterally over the designated hippocampal coordinates: (i) bregma −2.7 mm, lateral 2.0 mm, and ventral 1.3 mm; and (ii) bregma −2.9 mm, lateral 2.5 mm, and ventral 1.7 mm. A 33-gauge needle was gradually advanced to the desired depth over a 1.5-minute period. All injections were performed using a microprocessor controlled pump (UltraMicro-Pump, WPI Instruments, Sarasota, FL). A total of 2 μl (3 × 109 transducing units) per injection was delivered at a constant rate of 200 nl/min. After each injection, the needle was extracted over a 3-minute period. After the final injection, the incision was closed using a Vicryl suture, a topical 5% lidocaine ointment (Fougera, Melville, NY) was applied, and the mice were allowed to recuperate in a heated recovery chamber before returning to their housing cage and the vivarium. Most mice were housed until 12 months of age, at which time they were sacrificed via pentobarbital overdose and transcardiac perfusion with saline (0.9% sodium chloride).14 The brains were removed and bisected with the right hemisphere being submerged in 4% paraformaldehyde in 0.1 mol/L phosphate buffer (PB) and the left hemisphere being microdissected and frozen at −80°C for subsequent RNA isolation. A subset of mice received daily i.p. injections of 5-bromo-2′-deoxyuridine (BrdU) (50 mg/kg, Sigma-Aldrich, St. Louis, MO) for 3 days at 35 days after rAAV vector injection and were sacrificed 2 days after the final BrdU injection as described above.
IFNγ ELISA
Mouse hippocampal tissue was harvested and snap-frozen on dry ice followed by storage at 80°C. Frozen tissue was homogenized using a motorized pestle in 10 μl of lysis solution per milligram of tissue. Lysis solution consisted of 10 mmol/L Tris, pH 8.0, 150 mmol/L NaCl, 1 mmol/L EDTA, 1 mmol/L phenylmethylsulfonyl fluoride prepared in ethanol added just before use, 1% Triton X-100, and 5 μl/ml protease inhibitor (all chemicals obtained from Sigma-Aldrich). Tissue was incubated in lysis solution for 1 hour at 4°C, followed by centrifugation at 10,000 × g for 10 minutes to remove cellular debris. The supernatant was removed and diluted 1:10 or 1:50 for analysis of total protein content and for IFNγ concentrations by capture ELISA (R&D Systems, Minneapolis, MN). The assay was performed according to the manufacturer’s instructions. In brief, capture antibody was reconstituted and diluted to 4 mg/ml, and 100 μl was added to each well of 96-well microplate flat-bottom plates (Immulux HB Dynex Technologies, Chantilly, VA) and incubated overnight at 27°C. Plates were washed three times with wash buffer (PBS + 0.05% Tween 20), followed by blocking for 1 hour at 27°C with PBS + 0.05% Tween 20 + 1% bovine serum albumin and 0.05% NaN3. Standards and samples were diluted with Reagent Diluent (DY995, R&D Systems) and diluted 1:100 with Tris-buffered saline + 0.05% Tween 20, pH 7.2 to 7.4.). Plates were washed three times with wash buffer, samples were added, and the plates were incubated for 2 hours at 27°C. Detection antibody was reconstituted with 1 ml of Reagent Diluent and diluted to 50 ng/ml. Plates were washed three times with wash buffer, detection antibody was added to each well, and the plates were allowed to incubate for 2 hours at 27°C. Plates were washed 3 times with wash buffer, 100 ml of streptavidin horseradish peroxidase diluted 1:200 was added, and plates were allowed to incubate for 20 minutes at 27°C. Plates were washed three times with wash buffer and developed with tetramethybenzidine in phosphate citrate buffer (Sigma-Aldrich) for 15 minutes at 27°C and quenched with 2 N H2SO4. Absorbance readings were taken at 450 nm using a plate reader (BioRad, Hercules, CA).
Antibodies
The following antibodies were used at the designated working dilutions: anti-amyloid precursor protein A4, amino acids 66 to 81 corresponding to the NPXY motif of human APP (Clone Y188, 1:750, Abcam, Cambridge, MA); anti-amyloid β 1-42 clone 12F4 reactive to the C terminus of β-amyloid and specific for the isoform ending at amino acid 42 (1:1000, Covance, Berkeley, CA); anti-amyloid β 1-42 polyclonal antibody for intracellular amyloid-β staining (1:1000, Invitrogen [formerly Biosource, Hopkinton, MA]); HT7 antibody, reactive to the epitope corresponding to human tau amino acid residues 159 to 163 (1:200, Pierce, Rockford, IL); AT180 antibody, specific for human tau phosphorylated at threonine 231 and serine 235 (1:200, Pierce); CP-13 antibody, specific for human tau phosphorylated at serine 202 (1:200, kindly provided by Dr. P. Davies); anti-human anti-glial fibrillary acidic protein (GFAP) (1:1000, Dako Cytomation, Glostrup, Denmark); anti-IBA-1 (1:750, Wako Chemicals USA, Inc., Richmond, VA) for detection of mouse microglia and monocytes; anti-human IFNγ (5 μg/ml, R&D Systems); anti-GFP (1:2000, Invitrogen, Eugene, OR); anti- NeuN (1:200, Millipore, Billerica, MA) for mature neurons; anti-doublecortin (DCX) (1:750, Abcam, Cambridge, MA) for immature neurons; and anti-BrdU (1:750, BD Pharmingen, San Jose, CA).
Immunohistochemistry
The right hemispheres from surgically manipulated mice were postfixed for 24 hours in 4% paraformaldehyde in 0.1 mol/L PB, followed by equilibration in 20% sucrose in 0.1 mol/L PBS and then 30% sucrose in 0.1 mol/L PBS. Brains were coronally sectioned on the freezing stage of a sliding microtome (Microm, Walldorf, Germany) at 30 μm and stored in cryoprotectant at −20°C until immunohistochemical processing. Brain sections were washed with 0.15 mol/L PB for 2 hours to remove the cryoprotectant and then were incubated with 3% H2O2 in 0.15 mol/L PB for 20 minutes to quench endogenous peroxidase activity. For Aβ peptide-specific stains, the sections were treated with 70% formic acid for 15 minutes for epitope retrieval. For the intracellular Aβ 1-42 in place of formic acid the sections were mounted onto slides and allowed to dry. The target buffer was heated to 98°C in a microwave oven (GE, Louisville, KY), and the slides were submerged into the buffer and placed in the microwave twice for 3 minutes at 450 W, and allowed to rest for 5 minutes between microwave steps. Brain sections were processed similarly for immunohistochemical analysis as detailed below. The sections were washed and permeabilized in 0.15 mol/L PB and 0.4% Triton X-100, followed by blocking in 0.15 mol/L PB with 10% normal goat serum and 0.4% Triton X-100. After blocking, the sections were incubated in 0.15 mol/L PB with 1% normal goat serum and 0.4% Triton X-100, with the designated primary antibody. The sections were washed with 0.15 mol/L PB, followed by an incubation with the appropriate biotin-conjugated secondary antibodies (1:1000, Vector Laboratories, Burlingame, CA) in 0.15 mol/L PB with 1% normal goat serum and 0.4% Triton X-100. The sections were washed with 0.15 mol/L PB with 1% normal goat serum and 0.4%Triton X-100, and incubated in the avidin-biotin complex per the manufacturer’s protocol (Vectastain ABC System, Vector Laboratories). Sections were washed in 0.15 mol/L PB followed by rinses in distilled water. The sections were developed with nickel-enhanced diaminobenzidine (Vector Laboratories). Sections were mounted on Superfrost Plus slides (VWR International, West Chester, PA), coverslipped, and viewed using an Olympus AX-70 microscope and motorized stage (Olympus, Center Valley, PA), and the MCID 6.0 Imaging software (Interfocus Imaging/GE Healthcare, Cambridge, UK) was used for quantifying all stains.
eGFP and human IFNγ immunocytochemical analyses were performed using fluorescently labeled secondary antibodies. Tissue sections were washed with PBS to remove cyroprotectant as described above. Sections were permeabilized with PBS + 0.1% Triton X-100 for 5 minutes and then incubated with blocking serum (PBS + 0.1% Triton X-100 and 10% donkey serum) for 1 hour at 22°C. Primary antibody was diluted into PBS + 0.1% Triton X-100 + 0.1% donkey serum overnight at 4°C. Sections were washed three times for 10 minutes each and then incubated in secondary antibody for 2 hours at 22°C. Anti-goat Alexa 568 (1:500, Invitrogen) was used for human IFNγ immunohistochemistry and anti-rabbit Alexa 488 (1:500, Invitrogen) was used for eGFP immunohistochemistry. Sections were mounted and visualized using a Leica TCS SP1 confocal scanning microscope (Leica Microsystems, Bannockburn, IL) using LCS software (Leica Microsystems).
For BrdU immunohistochemistry, tissue sections were washed with PBS to remove cyroprotectant, as above, then incubated with 3% H2O2 in 0.15 mol/L PB for 20 minutes to quench endogenous peroxidase activity. The sections were permeabilized using 0.15 mol/L PB with 0.4% Triton X-100 (Sigma-Aldrich), followed by a denaturing step using 2 N HCl with 0.5% Triton X-100 for 30 minutes. The tissue was neutralized using 0.07 N NaOH for 2 minutes, followed by blocking in 0.15 mol/L PB + 0.4% Triton X-100 + 10% normal goat serum for 2 hours. BrdU-positive cells were detected using a mouse monoclonal anti-BrdU antibody (1:750, BD Pharmingen). The sections were washed with 0.15 mol/L PB, followed by an incubation with goat anti-mouse, biotin-conjugated secondary antibodies (1:1000, Vector Laboratories) in 0.15 mol/L PB with 1% normal goat serum and 0.4% Triton X-100. The sections were washed with 0.15 mol/L PB with 1% normal goat serum and 0.4% Triton X-100 and incubated in the avidin-biotin complex per the manufacturer’s protocol (Vectastain ABC System). Sections were washed in 0.15 mol/L PB followed by rinses in distilled water. The sections were developed with nickel- enhanced diaminobenzidine. Sections were imaged as described above.
For fluorescent co-immunocytochemical detection of NeuN and BrdU, brain sections were processed as described above with the following modifications. Tissue was initially incubated with mouse monoclonal anti-BrdU antibody overnight at 4°C. The sections were washed with 0.15 mol/L PB, followed by incubation with a goat anti-mouse Alexa 568 secondary antibody (1:1000). Sections were then washed and incubated for 4 hours with a mouse monoclonal anti-NeuN antibody conjugated with Alexa 405 (1:500, Millipore, Bedford, MA). Sections colabeled for DCX or GFAP and BrdU were permeablized and blocked as described above. Tissue was initially incubated with mouse monoclonal anti-BrdU antibody and rabbit polyclonal anti-DCX or rabbit polyclonal anti-GFAP overnight at 4°C. The sections were washed with 0.15 mol/L PB, followed by incubation with goat anti-mouse Alexa 568 secondary antibodies and goat anti-rabbit Alexa 405 (1:1000, Invitrogen). Sections were washed, mounted, and imaged as described above.
Quantitation of Staining Intensities
Quantification of positively stained targets was done with the image analysis tools in the MCID program (Interfocus Imaging/GE Healthcare) allowing for a target intensity threshold for staining to be set, and a target pixel count to be assessed and compared with the total scanned area. In brief, ×20 images in the CA1 hippocampal regions, corresponding to 2.5 to 3.0 mm posterior from bregma, the area encompassing the sites of injection, were captured along the length of the pyramidal layer of the CA1 using an Olympus AX-70 microscope equipped with a motorized stage (Olympus, Melville, NY). All images were digitally analyzed based on a target threshold for pixel intensity. Fifteen ×20 images from five hippocampal sections per rAAV vector-injected mouse were analyzed. Counts were averaged across all images and mice within a group, and all statistics were analyzed using Prism GraphPad software (GraphPad Software, San Diego CA).
Quantitative Real-Time RT-PCR Analysis
RNA was isolated from microdissected hippocampi of rAAV1-IFNγ- or rAAV1-eGFP-injected 3×Tg-AD mice with TRIzol solution (Invitrogen), as described previously.15 RNA was treated with RQ1 DNase (Promega, Madison, WI) to selectively degrade any contaminating genomic DNA, followed by phenol-chloroform extraction and ethanol precipitation. One microgram of total RNA was reverse transcribed using a High-Capacity cDNA Archive Kit (Applied Biosystems, Foster City, CA). An aliquot of cDNA (100 ng) was used to assess the levels of 17 targets per mouse and was analyzed in a standard PE7900HT quantitative RT-PCR reaction using TaqMan Assay-on-Demand primer probe sets in Microfluidic cards (Applied Biosystems) and 100 μl of MasterMix containing HotStart DNA polymerase (Eurogentec, Seraing, Belgium). 18S RNA served as the control to which all samples were normalized (Applied Biosystems). The resultant data were analyzed using the ΔΔCT method, normalizing the rAAV1-IFNγ values to samples from rAAV1-eGFP-injected 3×Tg-AD mice. To determine statistical significance, Student’s t-test was used.
Results
Creation and Initial Testing of an IFNγ-Expressing Recombinant Adeno-Associated Virus Vector
To assess the potential effects that chronic expression of IFNγ may exact on the progression and severity of AD-related pathologies in 3×Tg-AD mice, two rAAV vectors were constructed: one expressing human IFNγ (rAAV1-IFNγ) and a second expressing the reporter gene product, enhanced green fluorescent protein (rAAV1-eGFP). The human homolog of IFNγ was used in this study to facilitate immunological differentiation of the rAAV-vectored transgene from endogenous mouse IFNγ. The individual transgenes were placed under the transcriptional control of the human cytomegalovirus promoter (Figure 1A). An ELISA performed on lysates and culture supernatants derived from transiently transfected baby hamster kidney cells confirmed that human IFNγ was robustly expressed from the rAAV1-IFNγ plasmid construct (Figure 1B). Both rAAV1-IFNγ and rAAV1-eGFP vectors were subsequently packaged into serotype 1 virions using a previously described baculovirus-based rAAV vector production method.12
Figure 1.
Generation and initial testing of a serotype-1 rAAV vector expressing human IFNγ. A: The cDNA encoding human IFNγ (hIFNγ) or eGFP reporter protein was cloned into a rAAV shuttle vector under the transcriptional control of the cytomegalovirus immediate-early promoter to create pAAV-IFNγ and pAAV-eGFP, respectively. B: Baby hamster kidney cells were transiently transfected with either pAAV-IFNγ or pAAV-eGFP, and expression of human IFNγ in cellular lysates or supernatant samples was determined by ELISA. A nontransfection control (NTC) was used to determine assay background. Error bars indicate SD. C–F: pAAV-IFNγ and pAAV-eGFP were subsequently packaged into serotype-1 rAAV particles. Two-month-old 3×Tg-AD mice (N = 4 to 5 per experimental group) were bilaterally infused with 2 μl (∼3 × 109 transducing particles) of rAAV1-IFNγ or 2 μl of rAAV1-eGFP, and sacrificed 10 months later. Brains were sectioned and processed for immunocytochemistry for human IFNγ (red; C and E) or eGFP (green; D and F). Scale bar in F = 10 μm. Subsets of sections from rAAV1-eGFP injected mice were processed for co-immunocytochemistry for the neuronal markers NeuN and eGFP (H), astrocytic marker GFAP and eGFP (I), and microglial marker IBA-1 and eGFP (J) to determine the cell type(s) transduced by the serotype-1 rAAV vector particles. Scale bar in J = 10 μm. G: Nine-month-old 3×Tg-AD mice (N = 4 per experimental group) were stereotactically infused with 2 μl (∼3 × 109 transducing particles) of rAAV1-IFNγ into the ipsilateral hippocampus and 2 μl of rAAV1-eGFP into the contralateral hippocampus, and sacrificed 3 months later. Injection sites were microdissected, and lysates were generated and analyzed by ELISA to assess IFNγ levels. Error bars indicate SEM. *P < 0.05.
The rAAV1-IFNγ and rAAV1-eGFP control vectors were stereotactically delivered to the CA1 layer of the 3×Tg-AD at 2 months of age. At this early age, 3×Tg-AD mice do not harbor detectable extracellular human APP/Aβ or hyperphosphorylated tau as determined by immunohistochemical methods.10,11 Mice were sacrificed 10 months after injection to assess vector-mediated expression. Respective transgene expression from both rAAV1-eGFP and rAAV1-IFNγ was detectable within the hippocampus 10 months after the stereotactic infusion (Figure 1, C–F). Vector-mediated transduction was confined to the hippocampal formation, as examination of more distal brain regions did not reveal any rAAV vector transgene-expressing cells (data not shown). Co-immunocytochemical analyses of rAAV1-eGFP injected 3×Tg-AD mice to determine the major cell type(s) initially transduced showed that eGFP positivity was localized primarily to cells expressing the mature neuronal marker NeuN (Figure 1H), although rarely detectable within astrocytes or microglia (Figure 1, I and J, respectively). Stereotactic infusion of a separate cohort of 3×Tg-AD mice at 9 months of age and subsequent ELISA-based analysis of microdissected tissue at 12 months of age indicated that rAAV1-IFNγ injected mice produced significantly higher levels of human IFNγ in hippocampal tissue than rAAV1-eGFP injected mice (Figure 1G).
Quantitative Real-Time RT-PCR Analysis of mRNA from 3×Tg-AD Mice Injected with rAAV1-IFNγ and rAAV1-eGFP Revealed a Number of Differences in Proinflammatory Molecule Expression Profiles
The rAAV1-IFNγ and rAAV1-eGFP control vectors were bilaterally delivered to the CA1 layer of a new cohort of 3×Tg-AD mice at 2 months of age (N = 4 to 5 per experimental group). Ten months later, the right hemisphere of each brain was postfixed in 4% paraformaldehyde, equilibrated in sucrose, and sectioned for immunohistochemical analyses. Left hemisphere tissue was microdissected to remove the vector-infused hippocampi, mRNA was isolated, and quantitative real-time RT-PCR subsequently performed for several proinflammatory mediators, whose transcript expression levels modulate in response to IFNγ. Ten months of INFγ expression activated a broad spectrum of factors associated with proinflammatory processes, including the chemokine (C-C motif) ligand 2 (CCL2), CCL3, CCL6, TNF-α, and its cognate receptors, TNFrsf1a and TNFrsf1b, as well as transcription factors c-Jun and NFκB2 (Table 1). In contrast to what our group has previously observed after induction of TNF-α responses in 3×Tg-AD and nontransgenic mice,13 chronic expression of IFNγ led to a small but significant down-regulation of the C3 component of complement (−1.53 ± 0.1436-fold, P < 0.05).
Table 1.
Fold Change in Steady-State Proinflammatory Transcript Expression in 3×Tg-AD Mice Injected with rAAV1-IFNγ versus rAAV1-eGFP at 10 Months Post-Transduction
| Target Gene | Fold-change rAAV1-IFNγ- versus rAAV1-eGFP-infused 3×Tg-AD mice | SEM | P values for significance (Student t-test) |
|---|---|---|---|
| CCL2 (MCP-1) | 2.1399 | 0.3565 | <0.01 |
| CCL3 (MIP-1α) | 1.8079 | 0.1982 | <0.01 |
| CCL6 (MRP-1) | 1.8787 | 0.0937 | <0.001 |
| TNF-α | 2.9663 | 0.6033 | <0.01 |
| TNFrsf1a | 1.4606 | 0.0754 | <0.001 |
| TNFrsf1b | 1.4793 | 0.1261 | <0.01 |
| C3 | −1.5346 | 0.1436 | <0.05 |
| Jun | 1.8071 | 0.0673 | <0.001 |
| NFκb2 | 2.2097 | 0.1907 | <0.001 |
Transcriptional analysis of 3×Tg-AD mice injected with rAAV1-eGFP or rAAV1-IFNγ for additional proinflammatory markers of interest. Total RNA was purified from microdissected hippocampus from 12-month-old 3×Tg-AD mice that had been previously stereotactically infused with rAAV1-IFNγ or rAAV1-eGFP. cDNA pools were generated and subjected to Applied Biosystems Microfluidic Card analysis of additional inflammation-related transcriptional targets. Fold changes in steady-state transcript levels in rAAV1-IFNγ- versus rAAV1-eGFP-injected animals were calculated with SEM and subsequently tested for significance by Student’s t-test.
Chronic IFNγ Expression Leads to Marked Activation of Microglia but Seems to Minimally Affect Astrocyte Status
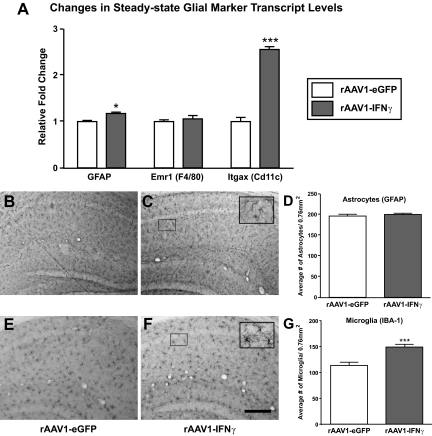
Mice infused with rAAV1-IFNγ showed a minimal, but significant, 1.175-fold enhancement of astrocyte marker GFAP transcript levels compared with 3×Tg-AD mice injected with rAAV1-eGFP (Figure 2A). This slight increase in GFAP transcript level was not substantiated by immunohistochemical/stereological analyses of astrocyte numbers in the identically treated contralateral hemisphere, where rAAV1-IFNγ and rAAV1-eGFP injected animals exhibited no difference in numbers of GFAP-positive astrocytes (Figure 2, B–D). Analyses of steady-state transcript levels for two markers, egf-like module containing, mucin-like, hormone receptor-like 1 (Emr1; also known as F4/80) for microglia/macrophages, and integrin alpha X (Itgax; also known as CD11c) for all dendritic-like cells, including microglia, revealed interesting disparities. Although transcript levels for F4/80 remained comparable, CD11c was significantly enhanced in the hippocampi of mice receiving rAAV1-IFNγ (Figure 2A). Moreover, immunohistochemical staining for the macrophage/microglia marker IBA-1 revealed a significant enhancement in IBA-1+ cells within the rAAV1-IFNγ-transduced hippocampus compared with rAAV1-eGFP-infused control animals, indicating that unlike astrocytes, microglia are markedly affected by the prolonged presence of IFNγ in 3×Tg-AD mouse brain (Figure 2, E–G).
Figure 2.
Differential activation of microglia and astrocytes after chronic IFNγ expression. Two-month-old 3×Tg-AD mice (N = 4 to 5 per experimental group) were stereotactically infused bilaterally twice with 2 μl (∼3 × 109 transducing particles) of rAAV1-IFNγ or rAAV1-eGFP and sacrificed 10 months later. A: The left hemisphere was processed for total RNA isolation and transcriptional marker levels for astrocytes and microglia were analyzed via a PE7900HT quantitative real-time RT-PCR reaction. Relative fold changes in transcript levels for the astrocyte marker GFAP and macrophage/microglial markers F4/80 and Cd11c are reported. Error bars indicate SEM. *P < 0.05; ***P < 0.001. B–G: The right hemisphere was processed for immunohistochemistry to detect astrocytes (anti-GFAP; B and C) or macrophages/microglia (anti-IBA-1; E and F). Insets in C and F represent digitally magnified images of the respective outlined region in each photomicrograph for better visualization of stained cell morphology. Scale bar in F = 200 μm. Quantitative image analyses for cells staining positive for GFAP (D) and IBA-1 (G) were performed on each brain and are presented in histogram format. Error bars indicate SEM. ***P < 0.001.
Intracellular Aβ-Related Pathology Is Significantly Exacerbated by Chronic IFNγ Expression
Quantitative RT-PCR was subsequently performed to assess the extent to which IFNγ expression influenced human APPswe transgene expression and its endogenous mouse wild-type counterpart. No statistically significant changes were detected in either APP homolog when rAAV1-IFNγ mice were compared with rAAV1-eGFP control animals (Figure 3A), although a slight trending increase in human APPswe steady-state RNA sequences could be observed in IFNγ-expressing mice (1.449 ± 0.2396-fold, P = 0.0839). This human APPswe transcript level difference did not correlate to immunohistochemical analysis of human APP protein using the Y188 antibody, in that both cohorts stained to similar intensities (Figure 3, B–D). Immunostaining with the human APP (hAPP)/Aβ-specific monoclonal antibody 6E10 revealed that rAAV1-IFNγ-infused 3×Tg-AD mice stain significantly more intensely than rAAV1-eGFP controls (Figure 3, E–G), suggesting that the former may harbor higher levels of intracellular Aβ peptides, which have been proposed to play an early triggering role in AD-related neurodegeneration.16,17,18,19 To further detail the evolution of intracellular Aβ pathology in rAAV1 vector-injected 3×Tg-AD mice, we used an immunohistochemical staining method optimized for visualization of intracellular Aβ42 peptide,20,21 which more readily unmasks intracellular Aβ peptide epitopes than standard formic acid epitope retrieval methods used for extracellular plaque immunohistochemistry. Using this methodology, we have been able to reproducibly detect intracellular Aβ42 in 3×Tg-AD mice.11 In the present study, intracellular Aβ42 staining intensity was markedly enhanced in rAAV1-IFNγ injected 3×Tg-AD mice compared with control mice (Figure 3, H–J), further substantiating the hypothesis that IFNγ exacerbates AD-related pathogenic processes.
Figure 3.
Chronic IFNγ expression enhances APP/Aβ-related pathology. Two-month-old 3×Tg-AD mice (N = 4 to 5 per experimental group) were stereotactically infused bilaterally twice with 2 μl (∼3 × 109 transducing particles) of rAAV1-IFNγ or rAAV1-eGFP and sacrificed 10 months later. A: The left hemisphere was processed for total RNA isolation and transcriptional marker levels for human and mouse APP were analyzed via a PE7900HT quantitative real-time RT-PCR reaction. Relative fold changes in transcript levels are reported. Error bars indicate SEM. B–J: The right hemisphere was processed for immunohistochemistry to detect human APPswe transgene product (Y188; B and C), human APP/Aβ (6E10; E and F), or intracellular Aβ1-42 (anti-Aβ42; H and I). Insets in C, F, and I represent digitally magnified images of the respective outlined region in each photomicrograph for better visualization of stained cell morphology. Scale bar: 200 μm (E); 100 μm (H). Quantitative image analyses for cells staining positive for hAPP (D), hAPP/Aβ (G), and intracellular Aβ1-42 (J) were performed on each brain and are presented in histogram format. Error bars indicate SEM. *P < 0.05; ***P < 0.001.
rAAV1-IFNγ-Infused 3×Tg-AD Mice Exhibit an Unexpected Reduction in Immunohistochemically Detectable Phospho-Tau Pathology
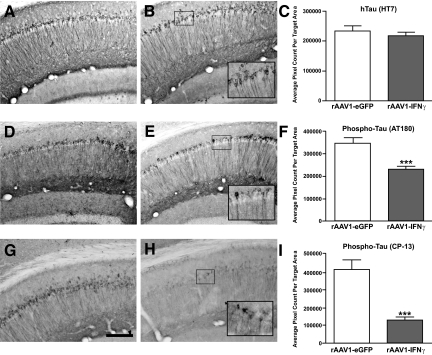
In addition to a human APPswe transgene and presenilin-1 knock-in mutation, 3×Tg-AD mice harbor a human tauP301L mutant transgene, which results in progressive elaboration of pathogenic hyperphosphorylated forms of tau beginning at 9 months of age and eventually neurofibrillary tangle-like pathology by 18 months.10,11 Immunohistochemical analysis of human tau transgene expression using the HT7 antibody and subsequent quantitation indicated that rAAV1-IFNγ transduction did not overtly affect tau expression compared with rAAV1-eGFP-injected control animals (Figure 4, A–C). We then incubated brain sections with the anti-human phosphorylated tau AT180 antibody, specific for human tau phosphorylated at the Thr231Ser235 residues, or the CP-13 antibody, which is specific for human tau phosphorylated at Ser202. Quantitation of AT180 and CP-13 staining intensities revealed marked reductions specifically within rAAV1-IFNγ-infused 3×Tg-AD mice, indicating that chronic IFNγ expression differentially affects amyloid- and tau-related pathologies in this mouse model (Figure 4, D–F and G–I).
Figure 4.
Tau pathology in 3×Tg-AD mice is reduced by chronic IFNγ expression. Two-month-old 3×Tg-AD mice (N = 4 to 5 per experimental group) were stereotactically infused bilaterally twice with 2 μl (∼3 × 109 transducing particles) of rAAV1-IFNγ or rAAV1-eGFP, and sacrificed 10 months later. The right hemisphere was processed for immunohistochemistry to detect human tau transgene product (HT7; A and B) or phospho-threonine 231 and phospho-serine 235 (AT180; D and E) or phospho-serine 202 (CP-13; G and H) of human tau. Insets in B, E, and H represent digitally magnified images of the respective outlined region in each photomicrograph for better visualization of stained cell morphology. Scale bar in G = 200 μm. Quantitative image analyses for cells staining positive for HT7 (C), AT180 (F), and CP-13 (I) were performed on each brain and are presented in histogram format. Error bars indicate SEM. ***P < 0.001.
IFNγ Expression Enhances Neurogenesis within the Hippocampi of rAAV1-IFNγ-Transduced 3×Tg-AD Mice
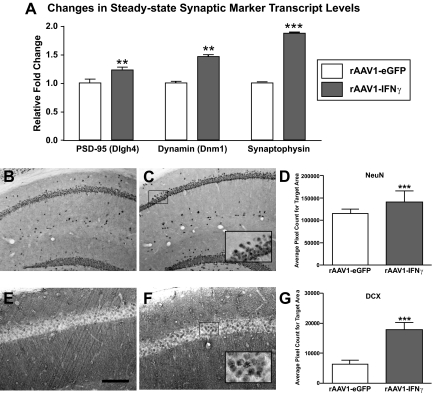
The unexpected “protective” or “pathology-suppressing” effect that chronic IFNγ expression produced on phospho-tau staining patterns within 3×Tg-AD mice led us to monitor neuronal status in these animals at 10 months after transduction. 3×Tg-AD mice that received rAAV1-IFNγ exhibited increases in mRNA expression of a number of synaptic markers. PSD-95, a specialized scaffold protein in the postsynaptic protein complex, was 1.228 ± 0.0556-fold (P < 0.01) greater in rAAV1-eGFP injected 3×Tg-AD mice (Figure 5A). Dynamin-1, a GTPase expressed exclusively within the CNS, which is important in the formation and recycling of synaptic vesicles,22 was enhanced by 1.463 ± 0.077-fold (P < 0.01) in the rAAV1-IFNγ cohort. The transcript encoding synaptophysin, an integral membrane protein found in synaptic vesicles of neurons,23 was up-regulated 1.873 ± 0.0722-fold (P < 0.001) in 3×Tg-AD mice injected with rAAV1-human IFNγ. In aggregate, the enhancement of these transcripts suggested that chronic IFNγ expression was promoting a process suggestive of synaptogenesis in the 3×Tg-AD brain.
Figure 5.
Markers for enhanced neurogenesis increase in response to chronic IFNγ expression. Two-month-old 3×Tg-AD mice (N = 4 to 5 per experimental group) were stereotactically infused bilaterally twice with 2 μl (∼3 × 109 transducing particles) of rAAV1-IFNγ or rAAV1-eGFP and sacrificed 10 months later. A: The left hemisphere was processed for total RNA isolation, and transcriptional marker levels for PSD-95 (Dlgh4), dynamin (Dnm1), and synaptophysin were analyzed via a PE7900HT quantitative real-time RT-PCR reaction. Relative fold changes in transcript levels are reported. Error bars indicate SEM. B–G: The right hemisphere was processed for immunohistochemistry to detect the mouse neuronal marker NeuN (B and C) or the neuroprogenitor marker DCX (E and F). Insets in C and F represent digitally magnified images of the respective outlined region in each photomicrograph for better visualization of stained cell morphology. Scale bar in E = 100 μm. Quantitative image analyses for cells staining positive for NeuN (D) and DCX (G) were performed on each brain and are presented in histogram format. Error bars indicate SEM. **P < 0.01; ***P < 0.001.
To determine the IFNγ effects on markers for mature and progenitor neurons, we initially analyzed brain sections for NeuN, a marker of mature neuronal nuclei. Mice injected with rAAV1-eGFP (Figure 5B) showed qualitatively reduced staining intensity compared with the mice injected with rAAV1-IFNγ (Figure 5C). Quantitation of staining intensities across the treatment groups confirmed higher NeuN signal in rAAV1-IFNγ-injected 3×Tg-AD mice (Figure 5D). An antibody specific for DCX was used to identify immature neurons in the process of neural migration, as this marker is found in the soma of migrating neurons and the axons of differentiating neurons.24 Mice injected with rAAV1-IFNγ exhibit an increase in DCX nuclear staining in cells residing in the CA1 of the hippocampus (Figure 5F) compared with mice injected with rAAV1-eGFP (Figure 5E). When quantified there was a significant increase in DCX+ staining intensities in mice injected with rAAV1-IFNγ for within the CA1 hippocampus (Figure 5G), suggesting that IFNγ imparts a positive influence on neurogenesis.
Additional subsets of mice were intrahippocampally injected with either rAAV1-eGFP or rAAV1-IFNγ, and 35 days postinjection received daily intraperitoneal injections of BrdU for 3 days and were sacrificed 2 days after the final BrdU injection. Brains were postfixed with paraformaldehyde, sectioned, and incubated with antibodies specific for BrdU alone with diaminobenzidine development to facilitate quantitation. Cells with rounded cell bodies staining robustly for BrdU, reminiscent of progenitor morphology, were subsequently quantified within the CA1 region of the hippocampus and dentate gyrus using MCID software, and post hoc statistical analyses were performed. Mice injected with rAAV1-eGFP harbored qualitatively few BrdU+ cells that met the established morphological criteria for migrating progenitor cells within the CA1 subfield of the hippocampus (Figure 6A). In contrast, mice injected with rAAV1-IFNγ possessed a number of rounded darkly stained bodies slightly dorsal to the pyramidal layer of the CA1 subfield (Figure 6B). The rAAV1-IFNγ-injected animals exhibited a statistically significant enhancement in BrdU+ cell numbers within the injected CA1, whereas the differences in numbers of BrdU+ cells within the more distal dentate gyrus did not quite reach significance compared with rAAV1-eGFP control mice (Figure 6C).
Figure 6.
rAAV1-IFNγ-injected 3×Tg-AD mice exhibit evidence of heightened neurogenesis within the transduced CA1 subregion of the hippocampus. Two-month-old 3×Tg-AD mice (N = 4 to 5 per experimental group) were stereotactically infused bilaterally twice with 2 μl (∼3 × 109 transducing particles) of rAAV1-eGFP (A, D, G, J, and L) or rAAV1-IFNγ (B, E, H, K, and M). Mice received daily intraperitoneal injections of BrdU (50 mg/kg) for 3 days at 35 days after rAAV vector injection and were sacrificed 2 days after the final BrdU injection. A and B: diaminobenzidine immunohistochemical analysis for cells staining positive for BrdU incorporation was performed. C: The average numbers of BrdU-positive cells per microscopic field were quantified in the CA1 and dentate gyrus (DG) of the hippocampus and are presented in histogram format. Error bars indicate SEM. ***P < 0.001. Sections were co-incubated with a primary antibody specific for cell markers (red signal) for mature neurons (NeuN; D and E), neuronal progenitors (DCX; G and H), astrocytes (GFAP; J and K), or microglia (IBA-1; L and M), and a primary antibody for BrdU (blue signal). After incubation with a designated set of secondary antibodies, photomicrographs of the transduced CA1 subregion of the hippocampal formation were captured by two-color confocal microscopy. Overlapping signals appear pink. Insets in B, E, H, K, and M represent digitally magnified images of the respective outlined region in each photomicrograph for better visualization of stained cell morphology. Scale bars in A and L = 200 μm. Quantitative image analyses for cells costaining positive for NeuN and BrdU (F) and DCX and BrdU (I) were performed on each brain and presented in histogram format. Error bars indicate SEM. **P < 0.01.
Brain sections from rAAV vector-injected, BrdU-pulsed mice were subsequently co-incubated with a BrdU-specific antibody and antibodies specific for various cellular markers to determine the identity of the enhanced proliferating population in the rAAV1-IFNγ cohort. We found that BrdU+ cells within the CA1 subfield of mice injected with rAAV1-eGFP did not stain for NeuN, a mature neuronal marker (Figure 6D), whereas a subset of BrdU+ cells in mice injected with rAAV1-IFNγ did stain positive for NeuN (Figure 6E) and exhibited a quantitative increase in numbers of NeuN+/BrdU+ cells (Figure 6F). Further examination of neuroprogenitor cells using an antibody specific for DCX revealed that rAAV1-IFNγ-injected mice harbored a nearly significant increase in BrdU+/DCX+ cells compared with rAAV1-eGFP control mice (Figure 6, G–I). In addition, we assessed whether any BrdU+ cell subsets expressed GFAP, a marker for astrocytes, or the microglial marker IBA-1. Rarely did we observe colocalization of BrdU and GFAP or BrdU and IBA-1 signals in either rAAV1-eGFP controls (Figure 6, J and L, respectively) or rAAV1-IFNγ-injected mice (Figure 6, K and M, respectively), indicating that a majority of the proliferating population induced by chronic IFNγ expression belonged to the neuronal lineage.
Discussion
For more than a decade, it was widely accepted that microglia were the sole cell type responsible for expressing IFNγ within the brain, but evidence has since revealed that many CNS-resident cells, including neurons and astrocytes, as well as microglia, can produce this proinflammatory cytokine in response to infection or other pathological stimuli (reviewed in Ref. 25). These more recent studies have shown that brain IFNγ expression is significantly induced in response to infections by herpes simplex virus-1,26,27 human herpervirus 6,26,28 and Chlamydia pneumoniae.29,30,31,32 Moreover, increased pathogen burdens of each of these infectious agents have been associated, albeit controversially so, with heightened risk for development of sporadic AD.31,33 Such provocative findings raise the possibility that pathogen-induced IFNγ expression, which could feasibly occur at subclinical levels over a lifetime due to intermittent waves of latency and re-activation phases of infection, may serve as an active participant in disease-exacerbating and/or disease-ameliorating cellular processes.
The purported roles of IFNγ in the CNS are diverse, and our group is not the first to examine the effects of IFNγ overexpression in the context of the healthy or diseased mouse CNS. LaFerla et al34 generated IFNγ transgenic mice and observed altered development of neuronally rich regions, such as the hippocampus and cerebellum. These mice, which express IFNγ from a GFAP promoter-driven transcription unit, present with reduced developmental weights, death before sexual maturity, and a number of gross alterations in the hindbrain. Moreover, transgenic mice containing multiple copies of the IFNγ gene under the transcriptional control of the immunoglobulin A chain enhancer exhibit a number of skeletal developmental aberrations.35 These prior studies, although they may provide insight into IFNγ activity/function within the CNS, make it evident that overexpression of IFNγ throughout embryogenesis results in aberrant development and potential induction of compensatory mechanisms that undoubtedly complicate interpretation of the action of this proinflammatory cytokine within the adult brain.
Perhaps more pertinent to our present study, Baron et al36 used a similar transgenic approach for which they recently reported that mice arising from a cross between a transgenic line expressing IFNγ from an oligodendrocyte-specific promoter and an human APP-overexpressing mouse model of AD resulted in marked enhancement of neurogenesis within the dentate gyrus and improved spatial learning and memory. Moreover, these compound transgenic mice showed evidence of modest inflammatory marker transcript increases, neuroprotection as measured by enhanced synaptophysin staining densities within the highly innervated CA1 and CA3 regions of the hippocampus, and suppression of oligodendrogenesis. Effects of transgenic IFNγ expression on amyloid pathology severity in those mice were not reported. Despite the potential complications related to developmental compensation and effects on cellular composition within the brains of these compound transgenic mice during development, the report from Baron et al provides an informative data set derived via independent means that strongly substantiates our findings that within the context of an AD-affected CNS, IFNγ conveys potent neuroprotective activity.
To circumvent the developmental impact of IFNγ on murine brain architecture and cell type composition, we used rAAV vectors to stereotactically and stably deliver IFNγ to an adult mouse brain genetically predisposed to developing AD-like amyloid and tau pathologies. As stated previously, brains of human AD patients and mouse models elicit a number of reactive changes, a subset of which includes inflammatory processes, as neurodegeneration progresses. The gene transfer vector chosen for studying IFNγ effects in the context of AD pathogenesis should therefore ideally be devoid of inflammatory or immune stimulatory activity. rAAV vectors have been shown to be well tolerated in the human and rodent CNS, and, whenever they are introduced free of helper adenovirus, which was the case in the present study, measures of host inflammatory reaction are minimal or undetectable.37,38,39,40
We had predicted that rAAV vector-mediated IFNγ expression in the 3×Tg-AD mouse model would exacerbate both amyloid and tau pathologies, based on the marked signs of inflammation that were illustrated by increased transcript expression of cytokines, chemokines, and the microglia/macrophage marker CD11c, as well as the increase in numbers of IBA-1+ microglia (Figure 2). Consistent with that hypothesis, intracellular Aβ42 staining intensity was increased by 10 months of IFNγ expression within the hippocampi of 3×Tg-AD mice (Figure 3). The increase in Aβ accumulation was not a consequence of altered expression of either the APP transgene or the endogenous mouse APP gene but possibly was due to modulated APP processing and/or Aβ secretion. In contrast, IFNγ overexpression exhibited a number of “neuroprotective” activities in this compound transgenic mouse model. For example, AT180 and CP-13 staining intensities for hyperphosphorylated tau were significantly depressed in IFNγ-overexpressing 3×Tg-AD mice compared with those in rAAV-eGFP-infused control mice (Figure 4).
Hence, it seems that IFNγ represents a proinflammatory mediator with unique properties compared with those of other cytokines implicated in AD-related neuroinflammation. For example, whereas TNF-α promotes intracellular accumulation of Aβ42 and eventual neuronal death when overexpressed in 3×Tg-AD mouse brain,13 the proinflammatory cytokine IL-1β seems to serve a neuroprotective role by enhancing the ability of microglia to prevent amyloid plaque pathology in a conditional mouse model of AD.41 Cytokines such as IL-1 and IL-6 have been shown to act through p38-mitogen-activated protein kinase and cdk5/p35-related signaling pathways, respectively, to enhance the pathogenic phosphorylation state of neuronal tau.42,43,44 Although untested, there is a possibility that IFNγ antagonizes these latter pathways or acts via a completely unrelated mechanism to suppress tau hyperphosphorylation. Signs of marked neurogenesis were also evident in IFNγ-expressing 3×Tg-AD mice, a finding confirmed in a different murine AD model in the recent report from Baron et al.36 IFNγ has been shown to promote neuronal differentiation in vitro,45,46 neuronal plasticity, and receptor clustering.47 Moreover, human neural stem/progenitor cell cultures express the cognate receptors for IFNγ, termed IFN-γ-Rα and IFN-γ-Rβ, and are driven toward a neuronal fate when cultured in the presence of IFNγ.48 At present, we do not know whether the mechanisms underlying IFNγ-mediated tau hyperphosphorylation suppression and enhanced neurogenesis are related or are driven by disparate signaling cascades.
These observations, in aggregate, indicate that IFNγ evokes a number of potentially contradicting effects in the context of a brain undergoing AD-related degeneration, by apparently driving both disease-promoting and disease-ameliorating functions. Future experimentation will be centered on dissecting how these activities are mechanistically regulated and on whether functional outcomes are dependent on co-expressed cytokine/chemokine profiles, disease stage, and cellular milieu in which IFNγ is elaborated. A more complete understanding of the seemingly dichotomous activities of INFγ may facilitate the development of therapeutic strategies designed to exploit the neuroprotective activity of this cytokine for degenerative conditions afflicting the brain.
Acknowledgments
We thank Dr. Howard Federoff (Georgetown University) for helpful discussions, Dr. Frank LaFerla (University of California, Irvine) for providing breeding pairs of 3×Tg-AD mice, Maria Frazer (University of Rochester) for animal husbandry and care, Dr. Linda Callahan (University of Rochester) for immunohistochemistry and microscopy advice, and Dr. Michelle Janelsins (University of Rochester) for assistance with RNA isolation and transcription.
Footnotes
Address reprint requests to William J. Bowers, Ph.D., Department of Neurology, Center for Neural Development and Disease, University of Rochester Medical Center, 601 Elmwood Ave., Box 645, Rochester, NY 14642. E-mail: william_bowers@urmc.rochester.edu.
Supported by the National Institutes of Health (grants R01-AG023593 and R01-AG026328 to W.J.B.).
References
- Rojo LE, Fernandez JA, Maccioni AA, Jimenez JM, Maccioni RB. Neuroinflammation: implications for the pathogenesis and molecular diagnosis of Alzheimer’s disease. Arch Med Res. 2008;39:1–16. doi: 10.1016/j.arcmed.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Blasko I, Ransmayr G, Veerhuis R, Eikelenboom P, Grubeck-Loebenstein B. Does IFNγ play a role in neurodegeneration? J Neuroimmunol. 2001;116:1–4. doi: 10.1016/s0165-5728(01)00279-x. [DOI] [PubMed] [Google Scholar]
- Saurwein-Teissl M, Blasko I, Zisterer K, Neuman B, Lang B, Grubeck-Loebenstein B. An imbalance between pro- and anti-inflammatory cytokines, a characteristic feature of old age. Cytokine. 2000;12:1160–1161. doi: 10.1006/cyto.2000.0679. [DOI] [PubMed] [Google Scholar]
- Maes M, DeVos N, Wauters A, Demedts P, Maurits VW, Neels H, Bosmans E, Altamura C, Lin A, Song C, Vandenbroucke M, Scharpe S. Inflammatory markers in younger vs elderly normal volunteers and in patients with Alzheimer’s disease. J Psychiatr Res. 1999;33:397–405. doi: 10.1016/s0022-3956(99)00016-3. [DOI] [PubMed] [Google Scholar]
- Marx F, Blasko I, Zisterer K, Grubeck-Loebenstein B. Transfected human B cells: a new model to study the functional and immunostimulatory consequences of APP production. Exp Gerontol. 1999;34:783–795. doi: 10.1016/s0531-5565(99)00049-2. [DOI] [PubMed] [Google Scholar]
- Blasko I, Veerhuis R, Stampfer-Kountchev M, Saurwein-Teissl M, Eikelenboom P, Grubeck-Loebenstein B. Costimulatory effects of interferon-gamma and interleukin-1β or tumor necrosis factor α on the synthesis of Aβ1–40 and Aβ1-42 by human astrocytes. Neurobiol Dis. 2000;7:682–689. doi: 10.1006/nbdi.2000.0321. [DOI] [PubMed] [Google Scholar]
- Meda L, Cassatella MA, Szendrei GI, Otvos L, Jr, Baron P, Villalba M, Ferrari D, Rossi F. Activation of microglial cells by β-amyloid protein and interferon-γ. Nature. 1995;374:647–650. doi: 10.1038/374647a0. [DOI] [PubMed] [Google Scholar]
- Abbas N, Bednar I, Mix E, Marie S, Paterson D, Ljungberg A, Morris C, Winblad B, Nordberg A, Zhu J. Up-regulation of the inflammatory cytokines IFN-γ and IL-12 and down-regulation of IL-4 in cerebral cortex regions of APPSWE transgenic mice. J Neuroimmunol. 2002;126:50–57. doi: 10.1016/s0165-5728(02)00050-4. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Kiyota T, Horiba M, Buescher JL, Walsh SM, Gendelman HE, Ikezu T. Interferon-γ and tumor necrosis factor-α regulate amyloid-β plaque deposition and β-secretase expression in Swedish mutant APP transgenic mice. Am J Pathol. 2007;170:680–692. doi: 10.2353/ajpath.2007.060378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oddo S, Caccamo A, Kitazawa M, Tseng BP, LaFerla FM. Amyloid deposition precedes tangle formation in a triple transgenic model of Alzheimer’s disease. Neurobiol Aging. 2003;24:1063–1070. doi: 10.1016/j.neurobiolaging.2003.08.012. [DOI] [PubMed] [Google Scholar]
- Mastrangelo MA, Bowers WJ. Detailed immunohistochemical characterization of temporal and spatial progression of Alzheimer’s disease-related pathologies in male triple-transgenic mice. BMC Neurosci. 2008;9:81. doi: 10.1186/1471-2202-9-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urabe M, Ding C, Kotin RM. Insect cells as a factory to produce adeno-associated virus type 2 vectors. Hum Gene Ther. 2002;13:1935–1943. doi: 10.1089/10430340260355347. [DOI] [PubMed] [Google Scholar]
- Janelsins MC, Mastrangelo MA, Park KM, Sudol KL, Narrow WC, Oddo S, Laferla FM, Callahan LM, Federoff HJ, Bowers WJ. Chronic neuron-specific tumor necrosis factor-α expression enhances the local inflammatory environment ultimately leading to neuronal death in 3×Tg-AD Mice. Am J Pathol. 2008;173:1768–1782. doi: 10.2353/ajpath.2008.080528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincek V, Nassiri M, Knowles J, Nadji M, Morales AR. Preservation of tissue RNA in normal saline. Lab Invest. 2003;83:137–138. doi: 10.1097/01.lab.0000047490.26282.cf. [DOI] [PubMed] [Google Scholar]
- Janelsins MC, Mastrangelo MA, Oddo S, LaFerla FM, Federoff HJ, Bowers WJ. Early correlation of microglial activation with enhanced tumor necrosis factor-α and monocyte chemoattractant protein-1 expression specifically within the entorhinal cortex of triple transgenic Alzheimer’s disease mice. J Neuroinflammation. 2005;2:23. doi: 10.1186/1742-2094-2-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masters CL, Multhaup G, Simms G, Pottgiesser J, Martins RN, Beyreuther K. Neuronal origin of a cerebral amyloid: neurofibrillary tangles of Alzheimer’s disease contain the same protein as the amyloid of plaque cores and blood vessels. EMBO J. 1985;4:2757–2763. doi: 10.1002/j.1460-2075.1985.tb04000.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chui DH, Dobo E, Makifuchi T, Akiyama H, Kawakatsu S, Petit A, Checler F, Araki W, Takahashi K, Tabira T. Apoptotic neurons in Alzheimer’s disease frequently show intracellular Aβ42 labeling. J Alzheimers Dis. 2001;3:231–239. doi: 10.3233/jad-2001-3208. [DOI] [PubMed] [Google Scholar]
- Kienlen-Campard P, Miolet S, Tasiaux B, Octave JN. Intracellular amyloid-β1-42, but not extracellular soluble amyloid-β peptides, induces neuronal apoptosis. J Biol Chem. 2002;277:15666–15670. doi: 10.1074/jbc.M200887200. [DOI] [PubMed] [Google Scholar]
- LaFerla FM, Tinkle BT, Bieberich CJ, Haudenschild CC, Jay G. The Alzheimer’s A β peptide induces neurodegeneration and apoptotic cell death in transgenic mice. Nat Genet. 1995;9:21–30. doi: 10.1038/ng0195-21. [DOI] [PubMed] [Google Scholar]
- D'Andrea MR, Reiser PA, Polkovitch DA, Gumula NA, Branchide B, Hertzog BM, Schmidheiser D, Belkowski S, Gastard MC, Andrade-Gordon P. The use of formic acid to embellish amyloid plaque detection in Alzheimer’s disease tissues misguides key observations. Neurosci Lett. 2003;342:114–118. doi: 10.1016/s0304-3940(03)00252-0. [DOI] [PubMed] [Google Scholar]
- Ohyagi Y, Tsuruta Y, Motomura K, Miyoshi K, Kikuchi H, Iwaki T, Taniwaki T, Kira J. Intraneuronal amyloid β42 enhanced by heating but counteracted by formic acid. J Neurosci Methods. 2007;159:134–138. doi: 10.1016/j.jneumeth.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Ferguson SM, Brasnjo G, Hayashi M, Wolfel M, Collesi C, Giovedi S, Raimondi A, Gong LW, Ariel P, Paradise S, O'Toole E, Flavell R, Cremona O, Miesenbock G, Ryan TA, De Camilli P. A selective activity-dependent requirement for dynamin 1 in synaptic vesicle endocytosis. Science. 2007;316:570–574. doi: 10.1126/science.1140621. [DOI] [PubMed] [Google Scholar]
- Wiedenmann B, Franke WW. Identification and localization of synaptophysin, an integral membrane glycoprotein of Mr 38,000 characteristic of presynaptic vesicles. Cell. 1985;41:1017–1028. doi: 10.1016/s0092-8674(85)80082-9. [DOI] [PubMed] [Google Scholar]
- Koizumi H, Higginbotham H, Poon T, Tanaka T, Brinkman BC, Gleeson JG. Doublecortin maintains bipolar shape and nuclear translocation during migration in the adult forebrain. Nat Neurosci. 2006;9:779–786. doi: 10.1038/nn1704. [DOI] [PubMed] [Google Scholar]
- Benveniste EN. Cytokine actions in the central nervous system. Cytokine Growth Factor Rev. 1998;9:259–275. doi: 10.1016/s1359-6101(98)00015-x. [DOI] [PubMed] [Google Scholar]
- Rodriguez JD, Royall D, Daum LT, Kagan-Hallet K, Chambers JP. Amplification of herpes simplex type 1 and human herpes type 5 viral DNA from formalin-fixed Alzheimer brain tissue. Neurosci Lett. 2005;390:37–41. doi: 10.1016/j.neulet.2005.07.052. [DOI] [PubMed] [Google Scholar]
- Wozniak MA, Shipley SJ, Combrinck M, Wilcock GK, Itzhaki RF. Productive herpes simplex virus in brain of elderly normal subjects and Alzheimer’s disease patients. J Med Virol. 2005;75:300–306. doi: 10.1002/jmv.20271. [DOI] [PubMed] [Google Scholar]
- Lin WR, Wozniak MA, Cooper RJ, Wilcock GK, Itzhaki RF. Herpesviruses in brain and Alzheimer’s disease. J Pathol. 2002;197:395–402. doi: 10.1002/path.1127. [DOI] [PubMed] [Google Scholar]
- Itzhaki RF, Lin W-R, Wilcock GK, Faragher B. HSV-1 and risk of Alzheimer’s disease. Lancet. 1998;352:238. doi: 10.1016/S0140-6736(05)77844-2. [DOI] [PubMed] [Google Scholar]
- Itzhaki RF, Ling W-R, Shang D, Wilcock GK, Faragher B, Jamieson GA. Herpes simplex virus type 1 in brain and risk of Alzheimer’s disease. Lancet. 1997;349:241–244. doi: 10.1016/S0140-6736(96)10149-5. [DOI] [PubMed] [Google Scholar]
- Itzhaki RF, Wozniak MA, Appelt DM, Balin BJ. Infiltration of the brain by pathogens causes Alzheimer’s disease. Neurobiol Aging. 2004;25:619–627. doi: 10.1016/j.neurobiolaging.2003.12.021. [DOI] [PubMed] [Google Scholar]
- Balin BJ, Appelt DM. Role of infection in Alzheimer’s disease. J Am Osteopath Assoc. 2001;101:S1–6. [PubMed] [Google Scholar]
- Mori I, Kimura Y, Naiki H, Matsubara R, Takeuchi T, Yokochi T, Nishiyama Y. Reactivation of HSV-1 in the brain of patients with familial Alzheimer’s disease. J Med Virol. 2004;73:605–611. doi: 10.1002/jmv.20133. [DOI] [PubMed] [Google Scholar]
- LaFerla FM, Sugarman MC, Lane TE, Leissring MA. Regional hypomyelination and dysplasia in transgenic mice with astrocyte-directed expression of interferon-gamma. J Mol Neurosci. 2000;15:45–59. doi: 10.1385/JMN:15:1:45. [DOI] [PubMed] [Google Scholar]
- Nii A, Reynolds DA, Young HA, Ward JM. Osteochondrodysplasia occurring in transgenic mice expressing interferon-γ. Vet Pathol. 1997;34:431–441. doi: 10.1177/030098589703400507. [DOI] [PubMed] [Google Scholar]
- Baron R, Nemirovsky A, Harpaz I, Cohen H, Owens T, Monsonego A. IFN-γ enhances neurogenesis in wild-type mice and in a mouse model of Alzheimer’s disease. FASEB J. 2008;22:2843–2852. doi: 10.1096/fj.08-105866. [DOI] [PubMed] [Google Scholar]
- Büeler H. Adeno-associated viral vectors for gene transfer and gene therapy. Biol Chem. 1999;380:613–622. doi: 10.1515/BC.1999.078. [DOI] [PubMed] [Google Scholar]
- Kaplitt MG, Makimura H. Defective viral vectors as agents for gene transfer in the nervous system. J Neurosci Methods. 1997;71:125–132. doi: 10.1016/s0165-0270(96)00132-x. [DOI] [PubMed] [Google Scholar]
- Lo WD, Qu G, Sferra TJ, Clark R, Chen R, Johnson PR. Adeno-associated virus-mediated gene transfer to the brain: duration and modulation of expression. Hum Gene Ther. 1999;10:201–213. doi: 10.1089/10430349950018995. [DOI] [PubMed] [Google Scholar]
- Xiao X, Li J, McCown TJ, Samulski RJ. Gene transfer by adeno-associated virus vectors into the central nervous system. Exp Neurol. 1997;144:113–124. doi: 10.1006/exnr.1996.6396. [DOI] [PubMed] [Google Scholar]
- Shaftel SS, Kyrkanides S, Olschowka JA, Miller JN, Johnson RE, O'Banion MK. Sustained hippocampal IL-1β overexpression mediates chronic neuroinflammation and ameliorates Alzheimer plaque pathology. J Clin Invest. 2007;117:1595–1604. doi: 10.1172/JCI31450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Liu L, Barger SW, Griffin WS. Interleukin-1 mediates pathological effects of microglia on tau phosphorylation and on synaptophysin synthesis in cortical neurons through a p38-MAPK pathway. J Neurosci. 2003;23:1605–1611. doi: 10.1523/JNEUROSCI.23-05-01605.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintanilla RA, Orellana DI, Gonzalez-Billault C, Maccioni RB. Interleukin-6 induces Alzheimer-type phosphorylation of tau protein by deregulating the cdk5/p35 pathway. Exp Cell Res. 2004;295:245–257. doi: 10.1016/j.yexcr.2004.01.002. [DOI] [PubMed] [Google Scholar]
- Kitazawa M, Oddo S, Yamasaki TR, Green KN, LaFerla FM. Lipopolysaccharide-induced inflammation exacerbates tau pathology by a cyclin-dependent kinase 5-mediated pathway in a transgenic model of Alzheimer’s disease. J Neurosci. 2005;25:8843–8853. doi: 10.1523/JNEUROSCI.2868-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong G, Goldshmit Y, Turnley AM. Interferon-γ but not TNF α promotes neuronal differentiation and neurite outgrowth of murine adult neural stem cells. Exp Neurol. 2004;187:171–177. doi: 10.1016/j.expneurol.2004.01.009. [DOI] [PubMed] [Google Scholar]
- Kim SJ, Son TG, Kim K, Park HR, Mattson MP, Lee J. Interferon-gamma promotes differentiation of neural progenitor cells via the JNK pathway. Neurochem Res. 2007;32:1399–1406. doi: 10.1007/s11064-007-9323-z. [DOI] [PubMed] [Google Scholar]
- Vikman KS, Owe-Larsson B, Brask J, Kristensson KS, Hill RH. Interferon-gamma-induced changes in synaptic activity and AMPA receptor clustering in hippocampal cultures. Brain Res. 2001;896:18–29. doi: 10.1016/s0006-8993(00)03238-8. [DOI] [PubMed] [Google Scholar]
- Johansson S, Price J, Modo M. Effect of inflammatory cytokines on major histocompatibility complex expression and differentiation of human neural stem/progenitor cells. Stem Cells. 2008;26:2444–2454. doi: 10.1634/stemcells.2008-0116. [DOI] [PubMed] [Google Scholar]