Abstract
Recent studies have suggested a possible role of insulin dysfunction in the pathogenesis of sporadic Alzheimer’s disease (AD). In AD, brain glucose metabolism is impaired, and this impairment appears to precede the pathology and clinical symptoms of the disease. However, the exact contribution of impaired insulin signaling to AD is not known. In this study, by using a nontransgenic rat model of sporadic AD generated by intracerebroventricular administration of streptozotocin, we investigated insulin signaling, glucose transporters, protein O-GlcNAcylation, and phosphorylation of tau and neurofilaments in the brain. We found impaired insulin signaling, overactivation of glycogen synthase kinase-3β, decreased levels of major brain glucose transporters, down- regulated protein O-GlcNAcylation, increased phosphorylation of tau and neurofilaments, and decreased microtubule-binding activity of tau in the brains of streptozotocin-treated rats. These results suggest that impaired brain insulin signaling may lead to overactivation of glycogen synthase kinase-3β and down-regulation of O-GlcNAcylation, which, in turn, facilitate abnormal hyperphosphorylation of tau and neurofilaments and, consequently, neurofibrillary degeneration.
Alzheimer’s disease (AD), the most devastating chronic neurodegenerative disease in adults, causes dementia in, and eventually, death of the affected individuals. In less than 1% of cases, AD is caused by autosomal dominant mutations of presenilin-1, presenilin-2, or β-amyloid precursor protein. Most AD cases are sporadic and are believed to result from multiple etiologic factors including genetic susceptibility (such as the ApoE4 allele) and environmental and metabolic factors.1 One of these factors, impaired brain glucose metabolism, can be detected many years before the appearance of clinical symptoms of AD.2 We recently found that altered O-GlcNAcylation, an O-linked post-translational modification of nucleocytoplasmic proteins by a monosaccharide β-N-acetylglucosamine (O-GlcNAc), of the microtubule-associated protein tau, links the impairment of brain glucose metabolism to hyperphosphorylation of tau.3,4 On the basis of these findings, we hypothesized that the impairment of brain glucose metabolism contributes to neurodegeneration via down-regulation of O-GlcNAcylation, which is regulated by glucose metabolism, and the resultant abnormal hyperphosphorylation of tau, which is crucial to neurodegeneration in AD.5
Peripheral glucose metabolism is mainly regulated by insulin. Recent studies have indicated that insulin signaling also regulates glucose metabolism in the brain and plays important roles in neural development and neuronal activities and affects learning and memory.6 The role of possible insulin dysfunction in AD has been suggested recently.7,8,9,10 However, how the impaired insulin signaling contributes to the pathogenesis of AD is not known.
To investigate the possible pathogenic mechanism related to the impaired brain insulin signaling and glucose metabolism in sporadic AD, we investigated insulin signaling pathways, glucose transporters (GLUT), protein O-GlcNAcylation and phosphorylation of tau and neurofilaments (NFs) in a rat model of sporadic AD, which was generated by intracerebroventricular (i.c.v.) injection of streptozotocin (STZ).11,12,13 STZ is a glucosamine-nitrosourea compound and is commonly administrated peripherally to generate animal models of diabetes because of its ability to damage insulin-producing cells and to increase insulin resistance. We found the impaired insulin signaling pathway, overactivation of glycogen synthase kinase-3β (GSK-3β), decreased major glucose transporters, down-regulation of protein O-GlcNAcylation, increased phosphorylation of tau and NFs, and decreased microtubule-binding activity of tau in the brain of the rat model of AD. Our results provide in vivo evidence showing that impaired brain insulin signaling and O-GlcNAcylation contribute to hyperphosphorylation of tau and NFs.
Materials and Methods
Antibodies
Polyclonal antibodies against insulin receptor (IR), insulin-like growth factor-1 receptor β (IGF-1Rβ), the catalytic subunit phosphatidylinositol-3 kinase (PI3K), [PI3K(p110)] and the regulatory subunit [PI3K(p85)], as well as phosphorylated PI3K(p85), mitogen-activated protein kinase (MAPK), pMAPK, GSK-3β, and pGSK3β(S9) were bought from Cell Signaling, Inc. (Beverly, MA). Polyclonal antibodies against GLUT1 and GLUT3 were bought from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). A monoclonal antibody RL2 against O-GlycNAcylated proteins was bought from Affinity BioReagents (Golden, CO). A polyclonal antibody against O-GlcNAc transferase (OGT) and monoclonal antibody DM1A against tubulin were bought from Sigma-Aldrich Co. (St. Louis, MO). The phosphorylation-independent tau antibody R134d was raised in rabbits, as previously described.14 Polyclonal antibodies against site-specific tau phosphorylation were bought from BioSource International (Camarillo, CA). Monoclonal antibody PHF-1 was a gift from Dr. P. Davies of the Albert Einstein College of Medicine (Bronx, NY). The polyclonal antibody R61d against total NF was raised in our laboratory.15 The polyclonal antibody NF160 against NF-M was bought from Abcam, Inc. (Cambridge, MA). The monoclonal antibody SMI31 against phosphorylated NF-M/H was bought from Sternberger Monoclonals, Inc. (Baltimore, MD).
Animals and I.C.V. Injection
Wistar rats (male, 6 months old, weighing 280 to 320 g) from Charles River Laboratories (Wilmington, MA) were housed in a temperature-controlled room, and fed with standard rodent food pellets and water. They were housed for 1 week before use in our institutional animal colony. The use of animals was in accordance with the guidelines of the National Institutes of Health and was approved by the Animal Welfare Committee of the New York State Institute for Basic Research in Developmental Disabilities.
Rats were first anesthetized by an i.p. injection of 0.3% pentobarbital sodium (40 mg/kg). STZ (Sigma-Aldrich Co., St. Louis, MO) dissolved in 0.9% NaCl was injected into the bilateral ventricles of the brains at a dose of 1.5 mg/kg each site. The same volume (5 μl) of 0.9% NaCl alone was injected in control rats. The stereotaxic coordinates for the i.c.v. injection were: 0.9 mm posterior, 1.8 mm lateral, and 3.8 mm ventral from the Bregma. Three weeks (21 days) after i.c.v. injection, rats were euthanized with CO2, and the brains were removed immediately. The right hemisphere of each brain was frozen immediately and stored at −80°C for Western blots and microtubule binding experiments. The left hemispheres were fixed with 4% buffer-neutralized paraformaldehyde for immunohistochemical studies.
Western Blot Analysis
Brain tissue was homogenized in pre-chilled buffer containing 50 mmol/L Tris, pH 8.0, 150 mmol/L NaCl, 0.5 mmol/L EDTA, 1 mmol/L dithiothreitol, 1% NP-40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate, 100 mmol/L sodium vanadate, 0.1 μmol/L 0-(2-acetamido-2-deoxy-d-glucopyranosylidone) amino-N-phenylcarbamate (PUGNAc), and a cocktail of protease inhibitors. Protein concentrations of the samples were measured by the modified Lowry method.16 The samples were resolved in 10% or 7.5% (for NF) SDS-polyacrylamide gel electrophoresis and electrotransferred onto Immobilon-P membrane (Millipore, Bedford, MA). The blots were then probed with primary antibodies and developed with the corresponding horseradish peroxidase–conjugated secondary antibody and enhanced chemiluminescence kit (Pierce, Rockford, IL). Densitometric quantification of protein bands in Western blots were analyzed using TINA software (Raytest IsotopenmeBgerate GmbH, Straubenhardt, Germany). Quantitative comparisons were analyzed by using student t-test, and the differences between groups were regarded to be significant when P < 0.05.
Immunofluorescence Staining
To detect the tau phosphorylation, paraffin sections (6-μm thickness) of the rat brains were developed with monoclonal antibody PHF-1, followed by anti-mouse IgG conjugated with Alexa488 (Molecular Probes, Eugene, OR). For NF staining, double-immunofluorescence staining was performed by using monoclonal antibody SMI31 and polyclonal antibody NF160. The sections were then developed by using Alexa488-conjugated anti-mouse IgG and Alexa543-conjugated anti-rabbit IgG (Molecular Probes, Eugene, OR). In some experiments, the tissue sections were also counterstained with TO-PRO3, a nucleic acid-specific marker, to visualize the nuclei at a 633-nm excitation wavelength. Stained sections were examined by using a confocal microscope (PCM2000, Nikon, Melville, NY). The negative control staining was performed simultaneously, in which the primary antibody was omitted.
Microtubule Binding Assay
Brain tissue was homogenized in a buffer (80 mmol/L PIPES, 0.5 mmol/L MgCl2, 1 mmol/L EGTA) containing a protease inhibitor cocktail. The debris was removed by centrifugation at 16,000 × g at 4°C for 10 minutes. The resulting supernatants were heated and then centrifuged again at 10,000 × g at 4°C for 10 minutes to enrich heat-stable tau protein. The resulting supernatants were divided into several aliquots and mixed with taxol-stabilized microtubules prepared as described previously.17 After incubation at 32°C for 3 hours, the samples were centrifuged at 50,000 × g at 32°C for 30 minutes to separate the microtubules-bound tau from the unbound tau. The amounts of tau and tubulin (protein subunit of microtubules) in both fractions, as well as before centrifugation, were analyzed by quantitative Western blots developed with R134d and DM1A, respectively.
Results
Insulin Signaling Is Impaired in STZ-Treated Rat Brain
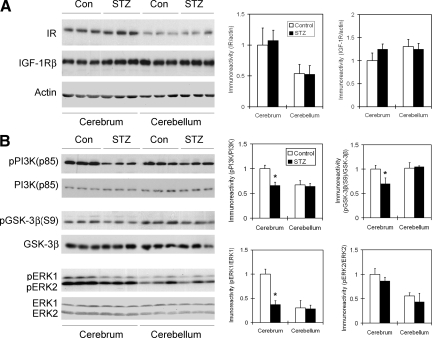
To study the insulin signaling pathway in STZ-treated rat brain, we first determined the level of IR and IGF-1Rβ in brain homogenates. We did not find any difference in the levels of these two insulin receptors between the STZ-injected and control saline-injected rat brains (Figure 1A). However, the level of IR, but not of IGF-1Rβ, in the cerebrum was twofold that in the cerebellum. These results suggest that i.c.v. injection of STZ does not alter the expression of IR nor IGF-1Rβ in the brain.
Figure 1.
Western blot analysis of IR, IGF-1Rβ, PI3k, GSK-3β, and MAPK in rat brain homogenates after STZ treatment. Homogenates of cerebrum or cerebellum, from rats 21 days after i.c.v. injection of STZ or saline (in control rats), were analyzed by Western blots developed with the antibodies indicated at the left side of each blot (A). Actin blots were included as loading controls. In B, the upper panel of each kinase was developed with the corresponding phosphorylation-dependent antibody that detected only the activated (in case of PI3K and ERK1/2) or inactivated (in case of GSK3) form, whereas the lower panel was developed with antibody against the total kinase. Densitometric quantifications (mean ± SE) of the blots are shown on the right side of the blots. *P < 0.05 vs. controls.
To investigate whether STZ i.c.v. injection modulates the brain insulin signaling pathway via altering its activation, we determined the activation of the major downstream components of the insulin signaling pathway, including PI3K, GSK-3β, and MAPK, by measuring their site-specific phosphorylation, which is known to determine their activation. When IR is activated, it activates PI3K via phosphorylation of PI3K’s regulatory subunit, p85, at Tyr458.18 The activated PI3K then further activates its downstream kinase MAPK via phosphorylation at Thr202/Tyr204 (ERK1) or Thr183/Tyr185 (ERK2)19 and inactivates GSK-3β via phosphorylation at serine 9.18 Quantitative Western blot analyses indicated that while STZ injection did not alter the levels of these kinases in the brain, it markedly decreased the phosphorylation levels of these kinases in the cerebrum, but not in the cerebellum (Figure 1B). These results suggest that STZ treatment resulted in impaired insulin signaling pathway in the rat cerebrum. There are two major MAPKs in the brain: ERK1 (p44) and ERK2 (p42). It is interesting that only the cerebral level of phosphorylated/activated ERK1 was markedly decreased in the STZ-treated rat brains, as compared with controls.
Glucose Transporters Are Decreased in STZ-Treated Rat Brain
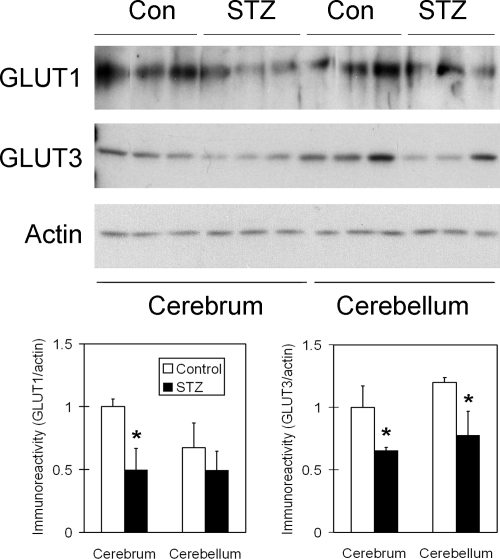
We recently demonstrated that the major brain glucose transporters, GLUT1 and GLUT3, are decreased in AD brain, and this decrease is correlated to hyperphosphorylation of tau.20 Thus, we investigated whether i.c.v. administration of STZ affects these two GLUTs. We found that the levels of both GLUT1 and GLUT3 were markedly decreased in the STZ-treated rat brains, as compared with the control-injected rat brains, but the decrease of GLUT1 did not reach a statistical significance in the cerebellum (Figure 2).
Figure 2.
Western blot analysis of GLUT1 and GLUT3 in rat brain homogenates after STZ treatment. Homogenates of cerebrum or cerebellum from rats 21 days after i.c.v. injection of STZ or saline as a control were analyzed by Western blots developed with antibody against GLUT1 or GLUT3, as indicated at the left side of the blots. Actin blots were included as loading controls. Densitometric quantifications (mean ± SE) of the blots are shown below the blots. *P < 0.05 vs. controls.
STZ Causes a Decrease in Protein O-GlcNAcylation in Rat Brain
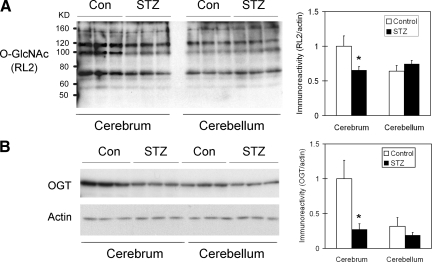
Inefficient insulin signaling activity and glucose transporters can both lead to decreased intracellular glucose metabolism that could, in turn, lead to deficient intracellular UDP-GlcNAc and thus a decrease in protein O-GlcNAcylation.21 We therefore studied the protein O-GlcNAcylation level. We found that the protein O-GlcNAcylation level was decreased markedly in the cerebrum, but not cerebellum, of STZ-injected rats, as determined by Western blots developed with antibody RL2 (Figure 3A) or CTD110.6 (data not shown), both of which recognize O-GlcNAcylated proteins. We further determined the level of OGT, which catalyzes protein O-GlcNAcylation, and found that the OGT level in the cerebrum of STZ-injected rats was ∼25% that found in control-injected rats (Figure 3B).
Figure 3.
Western blot analysis of protein O-GlcNAcylation and OGT in rat brain homogenates after STZ treatment. Homogenates of cerebrum or cerebellum from rats 21 days after i.c.v. injection of STZ or saline a control were analyzed by Western blots developed with monoclonal antibody RL2 against O-GlcNAc (A) or anti-OGT (B), as indicated at the left side of the blots. Actin blots were included as loading controls. Densitometric quantifications (mean ± SE) of all positive O-GlcNAcylated protein bands of the RL2 blot and of the OGT band of the OGT blot are shown at the right side of each blot. *P < 0.05 vs. controls.
STZ Induces Changes of Tau Phosphorylation and Decreases Its Microtubule-Binding Activity in Rat Brain
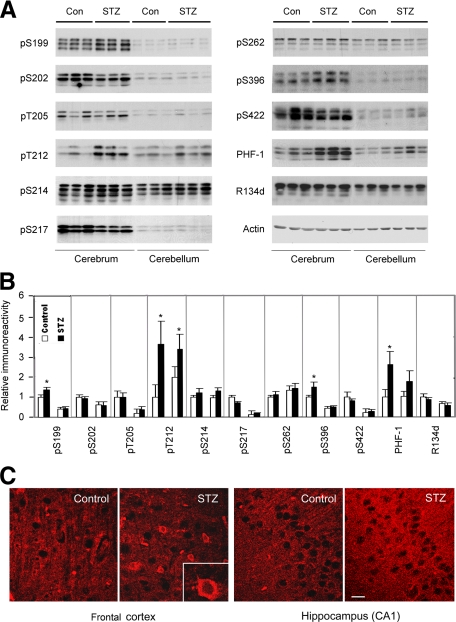
Abnormal hyperphosphorylation and decreased activity of tau is one of the most important biochemical changes in AD-affected brain. Decreased brain glucose metabolism and O-GlcNAcylation can lead to hyperphosphorylation of tau in various systems.3,4 Hence, we studied phosphorylation of tau in STZ-treated rat brain. Western blots developed with a phosphorylation-independent tau antibody (R134d) indicated that the total tau level was not altered in STZ-treated rats. Tau phosphorylation was determined by Western blots developed with several phosphorylation-dependent and site-specific tau antibodies. Among nine different phosphorylation sites of tau we studied, STZ induced a significant increase in tau phosphorylation at Ser199, Thr212, and Ser396 in the cerebrum, but not at Ser202, Thr205, Ser214, Ser217, Ser262, or Ser422 (Figure 4, A and B). Consistent with the results observed with pS396 antibody, tau staining with the monoclonal antibody PHF-1, which recognizes tau phosphorylated at Ser396 and Ser404,22 also increased markedly in the STZ-treated rat brain (Figure 4, A and B). As expected, most antibodies detected three major tau bands that represent the major isoforms of tau in the rat brain. We also studied the immunohistochemical staining of the rat brain sections with PHF-1 antibody and observed an increased staining in the neuronal cell bodies and neurites in the cerebral cortex and the hippocampus of the STZ-treated rats (Figure 4C). The increased staining in neurites was more marked in the hippocampus. The nuclei of neurons were not stained by PHF-1.
Figure 4.
Alterations of tau phosphorylation in STZ-treated rat brains. A: Homogenates of cerebrum or cerebellum from rats 21 days after i.c.v. injection of STZ or saline a control were analyzed by Western blots developed with antibody R134d against total tau and several phosphorylation-dependent tau antibodies that recognize tau phosphorylation at specific sites, as indicated at the left side of each blot. Antibody PHF-1 recognizes tau phosphorylation at Ser396/404. Actin blots were included for loading controls. B: Tau protein bands in the blots, as shown in panel A, were quantified, and the relative immunoreactivities (mean ± SE), where those of cerebral control samples were defined as 1, are shown. In each subpanel, the left two bars are data from cerebrum, and the right two bars are data from cerebellum. Data of total tau (R134d blot) had been normalized by the actin blots, and those of tau phosphorylation by total tau (the R134d blots). *P < 0.05 vs. controls. C: Immunofluorescence staining of rat brain tissue sections with monoclonal antibody PHF-1. Scale bar = 12.5 μm.
Figure 4 also demonstrates the differences in tau level and tau phosphorylation between cerebrum and cerebellum. Cerebrum contained approximately 30% more tau protein than cerebellum, whereas the basic tau phosphorylation level in the cerebrum was much higher at several phosphorylation sites, including Ser199, Ser202, Thr205, Ser217, Ser396, and Ser422, than that in the cerebellum (Figure 4, A and B). The cerebellum was also found to be less affected by the STZ-induced alterations of tau phosphorylation.
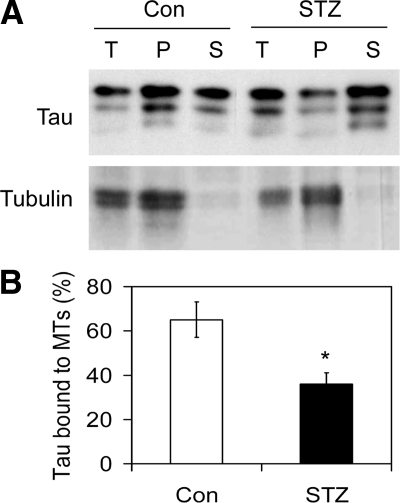
To investigate if STZ treatment affected tau’s biological activity, we enriched tau protein from rat brain extract and then incubated it with taxol-stabilized microtubules. The microtubule-bound tau was then separated from the unbound tau by sedimentation of microtubules by centrifugation. As expected, tubulin (protein subunit of microtubules) was found mostly in the pellet (P) fraction (Figure 5A). The amount of tau bound to microtubules (in the pellet) was dramatically less in samples from STZ-treated rat brains than from control-treated rat brains (Figure 5B). As a control experiment, all tau was found to be in the supernatant fraction when not incubated with microtubules (data not shown).
Figure 5.
Microtubule-binding of tau. A: Tau enriched from rat brain extracts was incubated with taxol-stabilized microtubules (MTs) at 32°C for 3 hours, followed by centrifugation to separate the bound tau from the unbound tau. The samples before centrifugation (total, T), the pellets (P) and the supernatants (S) were then analyzed by Western blots developed with antibody R134d toward tau or antibody DM1A toward tubulin. For T samples, only half of the amounts equivalent to those of the pellet and the supernatant fractions was loaded. B: Tau bands in both pellets and supernatants were then quantified, and the percentages of tau in the pellet fractions were calculated and are presented as mean ± SE. *P < 0.05 vs. controls.
STZ Increases Phosphorylation of NFs in Rat Brain
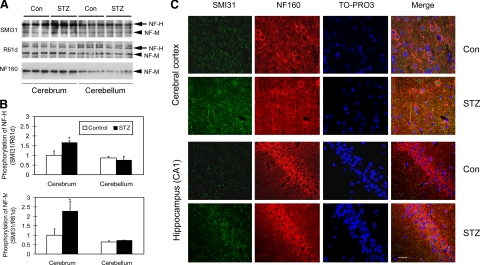
Like tau, phosphorylation of NFs is also increased in AD-affected brain.23,24,25 Thus, we also determined phosphorylation level of NF-H and NF-M in STZ-treated rat brain by Western blots developed with monoclonal antibody SMI31, which recognizes only the phosphorylated form of NF-H and NF-M. We found that the STZ treatment led to increased phosphorylation of NF-H/M as compared with controls in the cerebrum, but not in the cerebellum (Figure 6, A and B). We also observed that the level of NF-M, but not of NF-H, was lower in rat cerebellum than cerebrum, as detected by both phosphorylation-independent antibodies R61d, which recognizes both NF-H and NF-M, and NF160, which is a NF-M–specific antibody (Figure 6A). However, the level of neither NF-H nor NF-M was affected by STZ treatment.
Figure 6.
Alteration of phosphorylation of NF-H and NF-M in STZ-treated rat brains. A: Homogenates of cerebrum or cerebellum from rats after i.c.v. injection of STZ or control saline were analyzed by Western blots developed with either antibody SMI31 against the phosphorylated NF-H and NF-M or phosphorylation-independent antibodies R61d to NF-H/M or NF160 to NF-M. B: The respective NF bands in the blots, as shown in panel A, were quantified, and the relative SMI31 immunoreactivities (mean ± SE), after normalization with the respective R61d immunoreactive bands, are shown. *P < 0.05 vs. controls. C: Triple immunofluorescence staining of rat brain tissue sections with SMI31, NF160, and a nuclear marker TO-PRO3. Scale bar = 12.5 μm.
Immunohistochemical studies also demonstrated the increased NF phosphorylation in the tissue sections of STZ-treated rat cerebrum, as evidenced by triple immunofluorescence staining with antibodies SMI31 and NF160, as well as a nuclear marker TO-PRO3 (Figure 6C). Increased SMI31 staining was seen in the STZ-treated rat brains as compared with the control brains, whereas the phosphorylation-independent antibody NF160 did not show any significant difference in immunoreactivity between the two groups.
Discussion
The insulin signaling pathway, including IR/IGF-1R and the downstream PI3K pathway, plays a critical role in the regulation of peripheral carbohydrate, lipid, and protein metabolism. Recent studies have indicated that insulin signaling also regulates glucose metabolism in the brain and plays important roles in neural development and neuronal activities and affect learning and memory.6 Although neurons also express insulin,26,27 the majority of the brain insulin is originated from the periphery through the blood brain barrier via a saturable transport mechanism.28 A role of possible insulin dysfunction in AD has been suggested recently.7,8 Binding of insulin to IR leads to its rapid autophosphorylation and activation of its tyrosine kinase activity, which recruits and phosphorylates different substrates, such as insulin receptor substrate-1. Tyrosine-phosphorylated insulin receptor substrate-1 then displays binding sites for various downstream signaling partners, of which PI3K is the major one. When PI3K is activated by phosphorylation at Tyr458 of the regulatory subunit p85, it leads to activation of the downstream pathways including the protein kinase B (AKT) and MAPK cascades.18 Recent studies suggest that insulin signaling is impaired in AD-affected brain.9,10 Some diabetic changes resulted from insulin resistance, such as the Maillard-reaction-related modifications and protein glycation, have also been observed in AD-affected brain.29,30,31 Because of the potentially significant role of impaired brain insulin signaling in AD, de la Monte and her colleagues9 recently proposed that AD is a type 3 diabetes. In this study, we found that in the brain of STZ rat model of AD, the activity of the insulin signaling is decreased, as evidenced by the decreased phosphorylation of PI3K and the downstream GSK-3β and MAPK. In a recent study, Grunblatt et al32 reported that the expression of IR is also decreased in rat brains 3 months after a single i.c.v. injection of a lower dose of STZ (1 mg/kg). Thus, the disturbance of insulin signaling may last for a long period of time in the STZ rat model of sporadic AD.
Because GSK-3β activity is negatively regulated by its phosphorylation at Ser9 with AKT, the impaired PI3K-AKT signaling leads to overactivation of GSK-3β due to the decreased phosphorylation at Ser9, which was indeed observed in the cerebrum of the STZ-injected rats. GSK-3β is known to be the most important kinase involved in regulation of tau phosphorylation and abnormal hyperphosphorylation of tau in AD-affected brain.33,34 Thus, brain insulin resistance can cause tau phosphorylation via activation of GSK-3β (Figure 7), which might be an early event in tau pathology. The positive immunostaining of early neurofibrillary tangles with anti–GSK-3β has been reported in AD-affected brain.35,36 In consistent with this notion, Ser199, Thr212, Ser396, and PHF-1 sites (Ser396 and Ser404) of tau, the phosphorylation of which was marked increased in the STZ-injected rat brains, are the GSK-3β sites.37,38
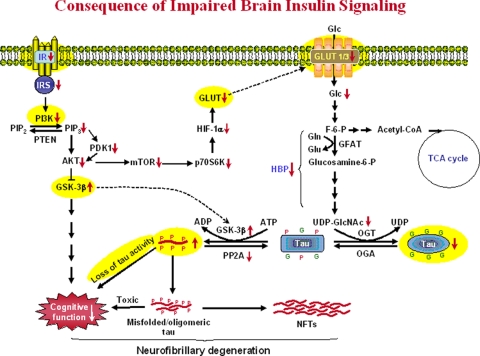
Figure 7.
Proposed consequences of impaired insulin signaling in AD brain and in the STZ-injected rat brain. Insulin signaling impairment first leads to decreased PI3K-AKT signaling activity via decreased IR activation, resulting in overactivation of GSK-3β. Over-activation of GSK-3β not only leads to hyperphosphorylation of tau directly, but also causes cognitive impairments via other pathways. Insulin resistance also leads to decreased GLUT1/3 expression and thus glucose uptake/metabolism in the brain. Decreased intraneuronal glucose metabolism results in decreased level of UDP-GlcNAc via the hexosamine biosynthetic pathway (HBP) and, consequently, decreased tau O-GlcNAcylation. Because the latter regulates tau phosphorylation inversely, decreased O-GlcNAcylation could facilitate hyperphosphorylation of tau, which, in turn, forms toxic tau oligomers and eventually leads to neurofibrillary degeneration. In this figure, G on tau protein represents the O-GlcNAc group, and P represents the phosphate group. Alterations observed in the STZ-injected rat brains in the present study are highlighted yellow color. Abbreviations used in this figure: ADP, adenosine diphosphate; AKT, protein kinase B; ATP, adenosine triphosphate; F-6-P, fructose-6-phosphate; Glc, glucose; GlcNAc, β-N-acetylglucosamine; Gln, glutamine; Glu, glutamate; GLUT, glucose transporter; GSK-3β, glycogen synthase kinese-3β; HIF-1α, hypoxia inducible factor-1α; IR, insulin receptor; IRS, insulin receptor substrate; mTOR, mammalian target of rapamycin; NFTs, neurofibrillary tangles; OGA, O-GlcNAcase; OGT, O-GlcNAc transferase; p70S6K, ribosomal protein S6 kinase with a molecular weight of 70kDa; PDK1, phosphoinositide dependent kinase 1; PI3K, Phosphoinositide 3-kinase; PIP2, phosphatidylinositol (3,4)-bisphosphate; PIP3, phosphatidylinositol (3,4,5)-trisphosphate; PP2A, protein phosphatase 2A; PTEN, phosphatase and tensin homolog; TCA, tricarboxylic acid cycle; UDP, uridine diphosphate.
MAPK (ERK1 and ERK2) can be activated by PI3K via Rac and PAK, which is consistent with our observations of down-regulation of both PI3K and MAPK in the STZ-injected rat brains. However, MAPK can also be activated by many other factors and under various conditions. Besides the impairment of insulin signaling and glucose metabolism, inflammation has been well documented in AD-affected brain, which could stimulate activation of MAPK.39 Activated MAPK (pERK1/2) has been reported to associate with neurofibrillary tangles in AD.40,41 The net activation of MAPK in AD-affected brain is probably because the activation had overridden the down-regulation induced by the impairment of insulin signaling and glucose metabolism. In the present study, we observed that the activation level of only ERK1, but not of ERK2, was decreased significantly in the STZ-injected rat cerebrum, suggesting that ERK1 activation is regulated by the insulin-PI3K pathway. Whether ERK1 and ERK2 play any different roles in AD pathogenesis is currently not known.
Glucose is the primary source of the energy required for brain activity, although it cannot enter the brain freely. The transport of glucose from the bloodstream into the brain is mediated by GLUTs. To date, 14 GLUTs have been reported in the human tissue.42 In mammalian brain, GLUT1 and GLUT3 are the predominant GLUTs responsible for glucose transport.43 Decreased GLUT1 and GLUT3 have been observed in AD-affected brain,44,45,46 and this decrease associates with decreased O-GlcNAcylation and abnormal hyperphosphorylation of tau in AD-affected brain,20 suggesting that the decrease may contribute to tau pathology and neurodegeneration in AD. In the present study, we found that the GLUT1 and GLUT3 levels were also decreased in the icv STZ-injected rat brains. This decrease might be resulted from the STZ-induced impaired insulin signaling, because the GLUT expression is mainly regulated by hypoxia-inducible factor-1α that, in turn, regulated by the PI3K pathway via AKT, mammalian target of rapamycin, and p70S6K (Figure 7). The observations of the decrease in GLUT1 and GLUT3 in the STZ-treated rat brains further support the relevance of this rat model for sporadic AD.
Protein O-GlcNAcylation is a common dynamic post-translational modification of nucleocytoplasmic proteins.47 This modification is mainly regulated by intracellular glucose metabolism via hexosamine biosynthetic pathway and thus is also regarded as a sensor of glucose metabolism.48 A reciprocal relationship between O-GlcNAcylation and phosphorylation has been observed in many proteins including tau and NF.3,4,25,49 Our recent studies suggest that in AD-affected brain, the impaired glucose metabolism may have led to down-regulation of O-GlcNAcylation that, in turn, facilitates abnormal hyperphosphorylation of tau and neurodegeneration.3,4,5 These alterations seen in AD-affected brain were also observed in the present study in the STZ rat model of sporadic AD, further suggesting the role of down-regulation of O-GlcNAcylation in AD pathogenesis. Consistent with our conclusions, a recent study has demonstrated that elevation of brain O-GlcNAcylation by a pharmacological method inhibit tau phosphorylation in vivo.50 In the STZ rat brain, the down-regulation of O-GlcNAcylation may be caused not only by STZ-induced decrease in glucose metabolism and GLUT1/3, but also by down-regulation of OGT, the enzyme that catalyzes protein O-GlcNAcylation. It is interesting to note that the significant decrease in O-GlcNAcylation and OGT was seen only in the cerebrum, but not in the cerebellum, of the STZ rat model. This phenomenon coincides with the fact that only the cerebrum is affected in AD, and cerebellum is almost intact in AD.
Tau and NF are hyperphosphorylated and accumulated in AD-affected brain. The abnormal hyperphosphorylation of tau is the most characteristic biochemical change in AD brain and is critical to the formation of neurofibrillary tangles, the hallmark brain lesion of AD.51,52 Therefore, we investigated tau phosphorylation at ten individual phosphorylation sites, which are hyperphosphorylated in AD-affected brain, in the STZ rat model in this study. Among these phosphorylation sites, we found a marked increase in tau phosphorylation at Ser199, Thr212, Ser396, and PHF-1 sites (Ser396 and Ser404) in the STZ-injected rat brain. These sites of tau are also hyperphosphorylated in PC12 cells cultured under the glucose deficient condition and in the brains of fasting mice.3,4 Thus, it is likely that the increased tau phosphorylation we observed in the rat AD model results from STZ-induced decrease in brain glucose metabolism in addition to GSK-3β activation. Furthermore, we found that the microtubule-binding activity of tau enriched from the STZ-treated rats was reduced. These observations suggest that, in addition to previously recognized phosphorylation sites at the microtubule-binding domains, tau phosphorylation at Ser199, Thr212, and/or Ser396/404 may also affect tau’s biological activity to bind to microtubules. These findings are consistent with our recent studies showing decreased biological activity of tau after phosphorylation by Dyrk1A (dual-specificity tyrosine-phosphorylated and regulated kinase 1A) that phosphorylates tau mainly at Thr212.53 Thr212 and Ser396/404 are also among those phosphorylation sites whose phosphorylation levels differ dramatically between tau in the fetal brain and the pathological tau in AD-affected brain.54 It is worth noting that Ser199, Ser202, Thr205, Thr212, Ser396, and Ser404 of tau can also be phosphorylated by ERK in vitro.52 Despite the decrease in ERK1 activation, tau phosphorylation levels at these sites were increased, rather than decreased, in the STZ-injected rat brains, suggesting that ERK1 might not play a significant role in phosphorylating tau directly in the brain. Except for Thr212, increased phosphorylation of tau was only seen in the cerebrum, but not cerebellum of the STZ-treated rats. This finding is consistent with the involvement of the cerebrum, among the central nervous system, in AD.
Neurofibrillary degeneration is a chronic neurodegenerative process characterized by filamentous aggregation of tau in the affected neurons. Despite of many studies focused on tau abnormalities and neurofibrillary degeneration, the exact sequential changes in tau and neurofibrillary formation during the development of AD remain to be elucidated. It has been proposed that abnormal hyperphosphorylation of tau is an initial critical alteration that induces the subsequent conformational changes,55 a loss of its biological activity,56 and a gain of a toxic activity,57,58 and leads to its polymerization into neurofibrillary tangles.59 Our findings of increased phosphorylation and decreased microtubule-binding activity of tau in the STZ-injected rat brains support the above notion. Tau isolated from AD is found to be phosphorylated at as many as 40 sites.52 Not all these phosphorylation sites are equally important to the pathological changes of tau,38,54 or are hyperphosphorylated at the same time.60,61 Among the ten phosphorylation sites studied, we found a marked increase in tau phosphorylation at Ser199, Thr212, Ser396, and PHF-1 sites (Ser396 and Ser404) in the STZ-injected rat brains, suggesting that phosphorylation of tau at these sites may be more affected by insulin signaling and glucose metabolism. Because a relatively short time (21 days) after i.c.v. injection of STZ was investigated in the present study, the hyperphosphorylation of tau at these sites may represent an early change of tau abnormalities. The increased tau phosphorylation appears to last long, because a dramatic increase in tau phosphorylation at PHF-1 sites was observed 3 months after STZ treatment in a previous study.32 It is worth noting that the most marked increase in tau phosphorylation in the STZ-injected rat brains was at Thr212. Phosphorylation of tau at this site has been shown to be critical for generating the Alzheimer-specific epitope of antibody AT100.62 It will be interesting to see if tau also undergoes Alzheimer-like conformational changes in the STZ-treated rat brains.
STZ is a drug selectively toxic for insulin producing/secreting cells. In moderate to low dosages, STZ caused insulin resistance63 by a decreased autophosphorylation of the IR.64 The molecular mechanism of STZ action in the brain is currently not known. It could be similar to the mechanism of decreasing insulin sensitivity in the periphery. Our observations of decreased insulin signaling activity in the cerebrum of STZ-treated rat brain are consistent with this hypothesis. Decreased glucose utilization, cholinergic deficiency, increased oxidative stress and glial activation in the brain, as well as impairments in learning and memory have been reported previously in the STZ rat model of sporadic AD [for review, see 65]. In the present study, we further demonstrated the impaired insulin signaling pathway, increased GSK-3β activation, decreased major glucose transporters, down-regulation of protein O-GlcNAcylation, increased phosphorylation of tau and NF, and decreased microtubule-binding activity of tau in the brain. All these abnormalities observed in the STZ rat model of AD have been documented in patients with sporadic AD. Taken together, we propose that brain insulin resistance, which occurs in AD-affected brain and in the STZ-injected rat brain, could lead to neurofibrillary degeneration via two additive, or synergistic, pathways (Figure 7). First, brain insulin resistance leads to decreased PI3K-AKT signaling activity, resulting in overactivation of GSK-3β. Overactivation of GSK-3β not only leads to hyperphosphorylation of tau directly, but also causes cognitive impairments via other pathways.66,67 Second, insulin resistance also leads to decreased GLUT1/3 expression and glucose uptake/metabolism in the brain. Decreased intraneuronal glucose metabolism results in decreased level of UDP-GlcNAc and, consequently, decreased tau O-GlcNAcylation. Because the latter regulates tau phosphorylation inversely,3,4,5 decreased O-GlcNAcylation could lead to hyperphosphorylation of tau, which, in turn, turns tau protein into toxic oligomers and eventually leads to neurodegeneration in AD and the learning and memory deficits in the STZ-injected rats.
Footnotes
Address reprint requests to Cheng-Xin Gong, Department of Neurochemistry, New York State Institute for Basic Research in Developmental Disabilities, 1050 Forest Hill Rd., Staten Island, New York, NY, 10314. E-mail: cxgong@mail.csi.cuny.edu.
Supported in part by the New York State Office of Mental Retardation and Developmental Disabilities and grants from the U.S. National Institute of Health (R01 AG027429 and R01 AG019158), the U.S. Alzheimer’s Association (IIRG-05-13095), the Education Ministry of China (Scientific Research Foundation for Returned Overseas Chinese Scholars), the Applied Basic Research Program Foundation of Tianjin City, China (No. 08JCYBJC27500), and the Science and Technique Development Projects for Higher Educational Institutions of Tianjin City, China (No. 20070203).
References
- Iqbal K, Grundke-Iqbal I. Metabolic/signal transduction hypothesis of Alzheimer’s disease and other tauopathies. Acta Neuropathol (Berl) 2005;109:25–31. doi: 10.1007/s00401-004-0951-y. [DOI] [PubMed] [Google Scholar]
- Hoyer S. Causes and consequences of disturbances of cerebral glucose metabolism in sporadic Alzheimer disease: therapeutic implications. Adv Exp Med Biol. 2004;541:135–152. doi: 10.1007/978-1-4419-8969-7_8. [DOI] [PubMed] [Google Scholar]
- Li X, Lu F, Wang JZ, Gong CX. Concurrent alterations of O-GlcNAcylation and phosphorylation of tau in mouse brains during fasting. Eur J Neurosci. 2006;23:2078–2086. doi: 10.1111/j.1460-9568.2006.04735.x. [DOI] [PubMed] [Google Scholar]
- Liu F, Iqbal K, Grundke-Iqbal I, Hart GW, Gong CX. O-GlcNAcylation regulates phosphorylation of tau: a mechanism involved in Alzheimer’s disease. Proc Natl Acad Sci USA. 2004;101:10804–10809. doi: 10.1073/pnas.0400348101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong CX, Liu F, Grundke-Iqbal I, Iqbal K. Impaired brain glucose metabolism leads to Alzheimer neurofibrillary degeneration through a decrease in tau O-GlcNAcylation. J Alzheimers Dis. 2006;9:1–12. doi: 10.3233/jad-2006-9101. [DOI] [PubMed] [Google Scholar]
- Gerozissis K. Brain insulin, energy and glucose homeostasis: genes, environment and metabolic pathologies. Eur J Pharmacol. 2008;585:38–49. doi: 10.1016/j.ejphar.2008.01.050. [DOI] [PubMed] [Google Scholar]
- Gasparini L, Netzer WJ, Greengard P, Xu H. Does insulin dysfunction play a role in Alzheimer’s disease? Trends Pharmacol Sci. 2002;23:288–293. doi: 10.1016/s0165-6147(02)02037-0. [DOI] [PubMed] [Google Scholar]
- Baki L, Neve RL, Shao Z, Shioi J, Georgakopoulos A, Robakis NK. Wild-type but not FAD mutant presenilin-1 prevents neuronal degeneration by promoting phosphatidylinositol 3-kinase neuroprotective signaling. J Neurosci. 2008;28:483–490. doi: 10.1523/JNEUROSCI.4067-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steen E, Terry BM, Rivera EJ, Cannon JL, Neely TR, Tavares R, Xu XJ, Wands JR, de la Monte SM. Impaired insulin and insulin-like growth factor expression and signaling mechanisms in Alzheimer’s disease–is this type 3 diabetes? J Alzheimers Dis. 2005;7:63–80. doi: 10.3233/jad-2005-7107. [DOI] [PubMed] [Google Scholar]
- Zhu X, Perry G, Smith MA. Insulin signaling, diabetes mellitus and risk of Alzheimer disease. J Alzheimers Dis. 2005;7:81–84. doi: 10.3233/jad-2005-7108. [DOI] [PubMed] [Google Scholar]
- Nitsch R, Hoyer S. Local action of the diabetogenic drug, streptozotocin, on glucose and energy metabolism in rat brain cortex. Neurosci Lett. 1991;128:199–202. doi: 10.1016/0304-3940(91)90260-z. [DOI] [PubMed] [Google Scholar]
- Duelli R, Schrock H, Kuschinsky W, Hoyer S. Intracerebroventricular injection of streptozotocin induces discrete local changes in cerebral glucose utilization in rats. Int J Dev Neurosci. 1994;12:737–743. doi: 10.1016/0736-5748(94)90053-1. [DOI] [PubMed] [Google Scholar]
- Lannert H, Hoyer S. Intracerebroventricular administration of streptozotocin causes long-term diminutions in learning and memory abilities and in cerebral energy metabolism in adult rats. Behav Neurosci. 1998;112:1199–1208. doi: 10.1037//0735-7044.112.5.1199. [DOI] [PubMed] [Google Scholar]
- Tatebayashi Y, Iqbal K, Grundke-Iqbal I. Dynamic regulation of expression and phosphorylation of tau by fibroblast growth factor-2 in neural progenitor cells from adult rat hippocampus. J Neurosci. 1999;19:5245–5254. doi: 10.1523/JNEUROSCI.19-13-05245.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong CX, Wang JZ, Iqbal K, Grundke-Iqbal I. Inhibition of protein phosphatase 2A induces phosphorylation and accumulation of neurofilaments in metabolically active rat brain slices. Neurosci Lett. 2003;340:107–110. doi: 10.1016/s0304-3940(03)00096-x. [DOI] [PubMed] [Google Scholar]
- Bensadoun A, Weinstein D. Assay of proteins in the presence of interfering materials. Anal Biochem. 1976;70:241–250. doi: 10.1016/s0003-2697(76)80064-4. [DOI] [PubMed] [Google Scholar]
- Gong CX, Lidsky T, Wegiel J, Zuck L, Grundke-Iqbal I, Iqbal K. Phosphorylation of microtubule-associated protein tau is regulated by protein phosphatase 2A in mammalian brain. Implications for neurofibrillary degeneration in Alzheimer’s disease. J Biol Chem. 2000;275:5535–5544. doi: 10.1074/jbc.275.8.5535. [DOI] [PubMed] [Google Scholar]
- Carnero A, Blanco-Aparicio C, Renner O, Link W, Leal JF. The PTEN/PI3K/AKT signalling pathway in cancer: therapeutic implications. Curr Cancer Drug Targets. 2008;8:187–198. doi: 10.2174/156800908784293659. [DOI] [PubMed] [Google Scholar]
- McKay MM, Morrison DK. Integrating signals from RTKs to ERK/MAPK. Oncogene. 2007;26:3113–3121. doi: 10.1038/sj.onc.1210394. [DOI] [PubMed] [Google Scholar]
- Liu Y, Liu F, Iqbal K, Grundke-Iqbal I, Gong CX. Decreased glucose transporters correlate to abnormal hyperphosphorylation of tau in Alzheimer disease. FEBS Lett. 2008;582:359–364. doi: 10.1016/j.febslet.2007.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreppel LK, Hart GW. Regulation of a cytosolic and nuclear O-GlcNAc transferase. Role of the tetratricopeptide repeats. J Biol Chem. 1999;274:32015–32022. doi: 10.1074/jbc.274.45.32015. [DOI] [PubMed] [Google Scholar]
- Otvos L, Jr, Feiner L, Lang E, Szendrei GI, Goedert M, Lee VM. Monoclonal antibody PHF-1 recognizes tau protein phosphorylated at serine residues 396 and 404. J Neurosci Res. 1994;39:669–673. doi: 10.1002/jnr.490390607. [DOI] [PubMed] [Google Scholar]
- Wang J, Tung YC, Wang Y, Li XT, Iqbal K, Grundke-Iqbal I. Hyperphosphorylation and accumulation of neurofilament proteins in Alzheimer disease brain and in okadaic acid-treated SY5Y cells. FEBS Lett. 2001;507:81–87. doi: 10.1016/s0014-5793(01)02944-1. [DOI] [PubMed] [Google Scholar]
- Liu Q, Xie F, Siedlak SL, Nunomura A, Honda K, Moreira PI, Zhua X, Smith MA, Perry G. Neurofilament proteins in neurodegenerative diseases. Cell Mol Life Sci. 2004;61:3057–3075. doi: 10.1007/s00018-004-4268-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Y, Li B, Liu F, Iqbal K, Grundke-Iqbal I, Brandt R, Gong CX. Regulation between O-GlcNAcylation and phosphorylation of neurofilament-M and their dysregulation in Alzheimer disease. FASEB J. 2008;22:138–145. doi: 10.1096/fj.07-8309com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schechter R, Abboud M. Neuronal synthesized insulin roles on neural differentiation within fetal rat neuron cell cultures. Brain Res Dev Brain Res. 2001;127:41–49. doi: 10.1016/s0165-3806(01)00110-9. [DOI] [PubMed] [Google Scholar]
- Schechter R, Beju D, Gaffney T, Schaefer F, Whetsell L. Preproinsulin I and II mRNAs and insulin electron microscopic immunoreaction are present within the rat fetal nervous system. Brain Res. 1996;736:16–27. doi: 10.1016/0006-8993(96)00664-6. [DOI] [PubMed] [Google Scholar]
- Banks WA. The source of cerebral insulin. Eur J Pharmacol. 2004;490:5–12. doi: 10.1016/j.ejphar.2004.02.040. [DOI] [PubMed] [Google Scholar]
- Smith MA, Taneda S, Richey PL, Miyata S, Yan SD, Stern D, Sayre LM, Monnier VM, Perry G. Advanced Maillard reaction end products are associated with Alzheimer disease pathology. Proc Natl Acad Sci USA. 1994;91:5710–5714. doi: 10.1073/pnas.91.12.5710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan SD, Yan SF, Chen X, Fu J, Chen M, Kuppusamy P, Smith MA, Perry G, Godman GC, Nawroth P, Zweier JL, Stern D. Non-enzymatically glycated tau in Alzheimer’s disease induces neuronal oxidant stress resulting in cytokine gene expression and release of amyloid beta-peptide. Nat Med. 1995;1:693–699. doi: 10.1038/nm0795-693. [DOI] [PubMed] [Google Scholar]
- Smith MA, Sayre LM, Perry G. Diabetes mellitus and Alzheimer’s disease: glycation as a biochemical link. Diabetologia. 1996;39:247. doi: 10.1007/BF00403972. [DOI] [PubMed] [Google Scholar]
- Grunblatt E, Salkovic-Petrisic M, Osmanovic J, Riederer P, Hoyer S. Brain insulin system dysfunction in streptozotocin intracerebroventricularly treated rats generates hyperphosphorylated tau protein. J Neurochem. 2007;101:757–770. doi: 10.1111/j.1471-4159.2006.04368.x. [DOI] [PubMed] [Google Scholar]
- Ferrer I, Gomez-Isla T, Puig B, Freixes M, Ribe E, Dalfo E, Avila J. Current advances on different kinases involved in tau phosphorylation, and implications in Alzheimer’s disease and tauopathies. Curr Alzheimer Res. 2005;2:3–18. doi: 10.2174/1567205052772713. [DOI] [PubMed] [Google Scholar]
- Takashima A. GSK-3 is essential in the pathogenesis of Alzheimer’s disease. J Alzheimers Dis. 2006;9:309–317. doi: 10.3233/jad-2006-9s335. [DOI] [PubMed] [Google Scholar]
- Yamaguchi H, Ishiguro K, Uchida T, Takashima A, Lemere CA, Imahori K. Preferential labeling of Alzheimer neurofibrillary tangles with antisera for tau protein kinase (TPK) I/glycogen synthase kinase-3 beta and cyclin-dependent kinase 5, a component of TPK II. Acta Neuropathol. 1996;92:232–241. doi: 10.1007/s004010050513. [DOI] [PubMed] [Google Scholar]
- Pei JJ, Braak E, Braak H, Grundke-Iqbal I, Iqbal K, Winblad B, Cowburn RF. Distribution of active glycogen synthase kinase 3beta (GSK-3beta) in brains staged for Alzheimer disease neurofibrillary changes. J Neuropathol Exp Neurol. 1999;58:1010–1019. doi: 10.1097/00005072-199909000-00011. [DOI] [PubMed] [Google Scholar]
- Liu F, Liang Z, Shi J, Yin D, El-Akkad E, Grundke-Iqbal I, Iqbal K, Gong CX. PKA modulates GSK-3beta- and cdk5-catalyzed phosphorylation of tau in site- and kinase-specific manners. FEBS Lett. 2006;580:6269–6274. doi: 10.1016/j.febslet.2006.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Li B, Tung EJ, Grundke-Iqbal I, Iqbal K, Gong CX. Site-specific effects of tau phosphorylation on its microtubule assembly activity and self-aggregation. Eur J Neurosci. 2007;26:3429–3436. doi: 10.1111/j.1460-9568.2007.05955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho GJ, Drego R, Hakimian E, Masliah E. Mechanisms of cell signaling and inflammation in Alzheimer’s disease. Curr Drug Targets Inflamm Allergy. 2005;4:247–256. doi: 10.2174/1568010053586237. [DOI] [PubMed] [Google Scholar]
- Ferrer I, Blanco R, Carmona M, Ribera R, Goutan E, Puig B, Rey MJ, Cardozo A, Vinals F, Ribalta T. Phosphorylated map kinase (ERK1. ERK2) expression is associated with early tau deposition in neurones and glial cells, but not with increased nuclear DNA vulnerability and cell death, in Alzheimer disease, Pick’s disease, progressive supranuclear palsy and corticobasal degeneration. Brain Pathol. 2001;11:144–158. doi: 10.1111/j.1750-3639.2001.tb00387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei JJ, Braak H, An WL, Winblad B, Cowburn RF, Iqbal K, Grundke-Iqbal I. Up-regulation of mitogen-activated protein kinases ERK1/2 and MEK1/2 is associated with the progression of neurofibrillary degeneration in Alzheimer’s disease. Brain Res Mol Brain Res. 2002;109:45–55. doi: 10.1016/s0169-328x(02)00488-6. [DOI] [PubMed] [Google Scholar]
- Scheepers A, Joost HG, Schurmann A. The glucose transporter families SGLT and GLUT: molecular basis of normal and aberrant function. JPEN J Parenter Enteral Nutr. 2004;28:364–371. doi: 10.1177/0148607104028005364. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Reagan LP. Glucose transporter expression in the central nervous system: relationship to synaptic function. Eur J Pharmacol. 2004;490:13–24. doi: 10.1016/j.ejphar.2004.02.041. [DOI] [PubMed] [Google Scholar]
- Kalaria RN, Harik SI. Reduced glucose transporter at the blood-brain barrier and in cerebral cortex in Alzheimer disease. J Neurochem. 1989;53:1083–1088. doi: 10.1111/j.1471-4159.1989.tb07399.x. [DOI] [PubMed] [Google Scholar]
- Simpson IA, Chundu KR, Davies-Hill T, Honer WG, Davies P. Decreased concentrations of GLUT1 and GLUT3 glucose transporters in the brains of patients with Alzheimer’s disease. Ann Neurol. 1994;35:546–551. doi: 10.1002/ana.410350507. [DOI] [PubMed] [Google Scholar]
- Mooradian AD, Chung HC, Shah GN. GLUT-1 expression in the cerebra of patients with Alzheimer’s disease. Neurobiol Aging. 1997;18:469–474. doi: 10.1016/s0197-4580(97)00111-5. [DOI] [PubMed] [Google Scholar]
- Wells L, Hart GW. O-GlcNAc turns twenty: functional implications for post-translational modification of nuclear and cytosolic proteins with a sugar. FEBS Lett. 2003;546:154–158. doi: 10.1016/s0014-5793(03)00641-0. [DOI] [PubMed] [Google Scholar]
- Zachara NE, Hart GW. O-GlcNAc a sensor of cellular state: the role of nucleocytoplasmic glycosylation in modulating cellular function in response to nutrition and stress. Biochim Biophys Acta. 2004;1673:13–28. doi: 10.1016/j.bbagen.2004.03.016. [DOI] [PubMed] [Google Scholar]
- Slawson C, Hart GW. Dynamic interplay between O-GlcNAc and O-phosphate: the sweet side of protein regulation. Curr Opin Struct Biol. 2003;13:631–636. doi: 10.1016/j.sbi.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Yuzwa SA, Macauley MS, Heinonen JE, Shan X, Dennis RJ, He Y, Whitworth GE, Stubbs KA, McEachern EJ, Davies GJ, Vocadlo DJ. A potent mechanism-inspired O-GlcNAcase inhibitor that blocks phosphorylation of tau in vivo. Nat Chem Biol. 2008;4:483–490. doi: 10.1038/nchembio.96. [DOI] [PubMed] [Google Scholar]
- Gong CX, Liu F, Grundke-Iqbal I, Iqbal K. Dysregulation of protein phosphorylation/dephosphorylation in Alzheimer’s disease: a therapeutic target. J Biomed Biotechnol. 2006;2006:31825. doi: 10.1155/JBB/2006/31825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JZ, Liu F. Microtubule-associated protein tau in development, degeneration and protection of neurons. Prog Neurobiol. 2008;85:148–175. doi: 10.1016/j.pneurobio.2008.03.002. [DOI] [PubMed] [Google Scholar]
- Liu F, Liang Z, Wegiel J, Hwang YW, Iqbal K, Grundke-Iqbal I, Ramakrishna N, Gong CX. Overexpression of Dyrk1A contributes to neurofibrillary degeneration in Down syndrome. FASEB J. 2008;22:3224–3233. doi: 10.1096/fj.07-104539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, Run X, Liang Z, Li Y, Liu F, Liu Y, Iqbal K, Grundke-Iqbal I, Gong CX. Developmental regulation of tau phosphorylation, tau kinases, and tau phosphatases. J Neurochem. 2009;108:1480–1494. doi: 10.1111/j.1471-4159.2009.05882.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondragon-Rodriguez S, Basurto-Islas G, Santa-Maria I, Mena R, Binder LI, Avila J, Smith MA, Perry G, Garcia-Sierra F. Cleavage and conformational changes of tau protein follow phosphorylation during Alzheimer’s disease. Int J Exp Pathol. 2008;89:81–90. doi: 10.1111/j.1365-2613.2007.00568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso AD, Zaidi T, Grundke-Iqbal I, Iqbal K. Role of abnormally phosphorylated tau in the breakdown of microtubules in Alzheimer disease. Proc Natl Acad Sci USA. 1994;91:5562–5566. doi: 10.1073/pnas.91.12.5562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso AD, Grundke-Iqbal I, Iqbal K. Alzheimer’s disease hyperphosphorylated tau sequesters normal tau into tangles of filaments and disassembles microtubules. Nat Med. 1996;2:783–787. doi: 10.1038/nm0796-783. [DOI] [PubMed] [Google Scholar]
- Alonso AD, Grundke-Iqbal I, Barra HS, Iqbal K. Abnormal phosphorylation of tau and the mechanism of Alzheimer neurofibrillary degeneration: sequestration of microtubule-associated proteins 1 and 2 and the disassembly of microtubules by the abnormal tau. Proc Natl Acad Sci USA. 1997;94:298–303. doi: 10.1073/pnas.94.1.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso AD, Zaidi T, Novak M, Grundke-Iqbal I, Iqbal K. Hyperphosphorylation induces self-assembly of tau into tangles of paired helical filaments/straight filaments. Proc Natl Acad Sci USA. 2001;98:6923–6928. doi: 10.1073/pnas.121119298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura T, Ono T, Takamatsu J, Yamamoto H, Ikegami K, Kondo A, Hasegawa M, Ihara Y, Miyamoto E, Miyakawa T. Sequential changes of tau-site-specific phosphorylation during development of paired helical filaments. Dementia. 1996;7:177–181. doi: 10.1159/000106875. [DOI] [PubMed] [Google Scholar]
- Augustinack JC, Schneider A, Mandelkow EM, Hyman BT. Specific tau phosphorylation sites correlate with severity of neuronal cytopathology in Alzheimer’s disease. Acta Neuropathol. 2002;103:26–35. doi: 10.1007/s004010100423. [DOI] [PubMed] [Google Scholar]
- Zheng-Fischhofer Q, Biernat J, Mandelkow EM, Illenberger S, Godemann R, Mandelkow E. Sequential phosphorylation of Tau by glycogen synthase kinase-3beta and protein kinase A at Thr212 and Ser214 generates the Alzheimer-specific epitope of antibody AT100 and requires a paired-helical-filament-like conformation. Eur J Biochem. 1998;252:542–552. doi: 10.1046/j.1432-1327.1998.2520542.x. [DOI] [PubMed] [Google Scholar]
- Blondel O, Portha B. Early appearance of in vivo insulin resistance in adult streptozotocin-injected rats. Diabete Metab. 1989;15:382–387. [PubMed] [Google Scholar]
- Kadowaki T, Kasuga M, Akanuma Y, Ezaki O, Takaku F. Decreased autophosphorylation of the insulin receptor-kinase in streptozotocin-diabetic rats. J Biol Chem. 1984;259:14208–14216. [PubMed] [Google Scholar]
- Salkovic-Petrisic M, Hoyer S. Central insulin resistance as a trigger for sporadic Alzheimer-like pathology: an experimental approach, J Neural Transm Suppl. 2007:217–233. doi: 10.1007/978-3-211-73574-9_28. [DOI] [PubMed] [Google Scholar]
- Hernandez F, Borrell J, Guaza C, Avila J, Lucas JJ. Spatial learning deficit in transgenic mice that conditionally over-express GSK-3beta in the brain but do not form tau filaments. J Neurochem. 2002;83:1529–1533. doi: 10.1046/j.1471-4159.2002.01269.x. [DOI] [PubMed] [Google Scholar]
- Engel T, Lucas JJ, Gomez-Ramos P, Moran MA, Avila J, Hernandez F. Cooexpression of FTDP-17 tau and GSK-3beta in transgenic mice induce tau polymerization and neurodegeneration. Neurobiol Aging. 2006;27:1258–1268. doi: 10.1016/j.neurobiolaging.2005.06.010. [DOI] [PubMed] [Google Scholar]