Abstract
Soluble oligomeric amyloid β (oAβ) 1-42 causes synaptic dysfunction and neuronal injury in Alzheimer’s disease (AD). Although accumulation of microglia around senile plaques is a hallmark of AD pathology, the role of microglia in oAβ1-42 neurotoxicity is not fully understood. Here, we showed that oAβ but not fibrillar Aβ was neurotoxic, and microglia activated with unmethylated DNA CpG motif (CpG), a ligand for Toll-like receptor 9, attenuated oAβ1-42 neurotoxicity in primary neuron-microglia co-cultures. CpG enhanced microglial clearance of oAβ1-42 and induced higher levels of the antioxidant enzyme heme oxygenase-1 in microglia without producing neurotoxic molecules such as nitric oxide and glutamate. Among subclasses of CpGs, class B and class C activated microglia to promote neuroprotection. Moreover, intracerebroventricular administration of CpG ameliorated both the cognitive impairments induced by oAβ1-42 and the impairment of associative learning in Tg2576 mouse model of AD. We propose that CpG may be an effective therapeutic strategy for limiting oAβ1-42 neurotoxicity in AD.
The senile plaque is a pathological hallmark of Alzheimer’s disease (AD). Fibrillar amyloid β (fAβ), a major component of senile plaques, induces tau hyperphosphorylation and neuronal dystrophy.1,2 Soluble oligomeric Aβ (oAβ) has been reported to exhibit higher neurotoxicity than fAβ. Naturally secreted oAβ inhibits hippocampal long-term potentiation and disrupts synaptic plasticity in rats in vivo3. In addition, oAβ induces neuronal reactive oxygen species (ROS) through a mechanism requiring N-methyl-d-aspartate receptor activation.4 Exposure to oAβ induces rapid and massive neuronal death, while fAβ is required at higher concentrations and for longer incubations to cause neuronal dystrophy.5
Microglia, macrophage-like cells in the central nervous system, cluster both in and around senile plaques and have been proposed to have pivotal roles in the pathogenesis of AD. Microglia activated with Aβ may be involved in the inflammatory component of AD.6 Both fAβ and oAβ stimulate microglial secretion of proinflammatory cytokines, chemokines, complement components, and free radicals.7 However, microglia also perform neuroprotective functions such as releasing neurotrophic factors8 and phagocytosing and degrading Aβ.9,10
Toll-like receptor (TLR) ligands enhance microglial phagocytosis of Aβ. Peptidoglycan, a TLR2 ligand, and unmethylated DNA CpG motifs (CpG), a TLR9 ligand, increase Aβ phagocytosis through the G protein-coupled formyl peptide receptor-like 2.11,12 Similarly, the TLR4 ligand lipopolysaccharide increases phagocytosis through the CD14 receptor.13 However, microglia activated with TLR ligands also produce neurotoxic molecules such as proinflammatory cytokines, nitric oxide (NO), ROS, and peroxynitrite.14 In particular, lipopolysaccharide-activated microglia produce a large amount of glutamate, a neurotransmitter but also potent neurotoxin.15 Thus, factors that increase microglial clearance of oAβ without producing inflammatory mediators are candidates for the treatment of AD.
Here, we investigated the role of microglia in neurotoxicity mediated by oAβ1-42. We found that microglia activated with a low dose of CpG attenuated the neurotoxic effects of oAβ1-42 without producing other neurotoxic molecules in vitro. Moreover, intracerebroventricular (ICV) administration of CpG ameliorated the cognitive impairment induced by ICV injection of oAβ1-42 and the impairment of associative learning in Tg2576 mouse model of AD.
Materials and Methods
Cell Culture
The protocols for animal experiments were approved by the Animal Experiment Committee of Nagoya University. Primary neuronal cultures were prepared from the cortices of embryonic day 17 (E17) C57BL/6 mice embryos as described previously.8 Briefly, cortical fragments were dissociated into single cells in dissociation solution (Sumitomo Bakelite, Akita, Japan) and resuspended in Nerve Culture Medium (Sumitomo Bakelite). Neurons were plated onto 12-mm-polyethyleneimine-coated glass coverslips (Asahi Techno Glass, Chiba, Japan) at a density of 5 × 104 cells/well in 24-well multidishes and incubated at 37°C in a humidified atmosphere containing 5% CO2. The purity of the cultures was >95% as determined by NeuN-specific immunostaining. Microglia were isolated on 14 days in vitro with the “shaking off” method previously described from primary mixed glial cell cultures prepared from newborn C57BL/6 mice.16 Cultures were 97 to 100% pure as determined by Fc receptor-specific immunostaining and were maintained in Dulbecco’s modified Eagle medium supplemented with 10% fetal calf serum, 5 μg/ml bovine insulin, and 0.2% glucose. Microglia were plated at a density of 7 × 104 cells/well in 8-well glass slides or at a density of 7 × 104 cells/well in 48-well multidishes. Neuron-microglia co-cultures were prepared as follows: 1 × 105 microglia in 100 μl of neuronal medium were added to neuronal cultures (5 × 104 neuronal cells) on 13 day in vitro in 24-well multidishes.
Preparations of Aβ Solutions
fAβ1-42 was prepared as described previously.17 Briefly, synthetic human Aβ1-42 (Peptide Institute, Osaka, Japan) was dissolved in 0.02% ammonia solution at a concentration of 250 μmol/L, diluted to 25 μmol/L in PBS, and incubated at 37°C for 24 hours. oAβ1-42 was prepared as described previously.18 Briefly, Aβ1-42 was dissolved to 1 mmol/L in 100% 1,1,1,3,3,3-hexafluoro-2-propanol. 1,1,1,3,3,3-Hexafluoro-2-propanol was dried by the vacuum desiccator and resuspended to 5 mmol/L in DMSO. To form oligomers, amyloid peptide was diluted to a final concentration of 100 μmol/L with Ham’s F-12 and incubated at 4°C for 24 hours and then immediately added to cultures at a final concentration 5 μmol/L.
Transmission Electron Microscopy
To assess quaternary structures of Aβ, oAβ1-42 and fAβ1-42 solutions were spread on carbon-coated grids. Negative staining was performed with 3% phosphotungstic acid (pH 7.0). Proteins were then examined with a JEM-2000ExII Electron Microscope with an acceleration voltage of 160 kV.
Thioflavin T Assay
Optimum fluorescence measurements of amyloid fibrils were obtained at excitation and emission wavelength of 446 and 490 nm, respectively, with a reaction mixture containing 5 μmol/L thioflavin T (Nakalai tesque, Kyoto, Japan) and 50 mmol/L glycine-NaOH buffer (pH 8.5). Ten microliters of oAβ1-42 or fAβ1-42 solution was mixed with 100 μl of the reaction mixture, respectively.
Measurement of Heme Oxygenase-1, Interleukin-10, Matrix Metalloproteinase-9, Tumor Necrosis Factor-α, NO, and Glutamate
To measure factors produced by microglia treated with CpG and oAβ1-42, microglia were plated at a density of 7 × 104 cells/well (300 μl) in 48-well multidishes and then treated with 100 nmol/L CpG-DNA (HyCult Biotechnology, Uden, Netherlands), 100 nmol/L class A CpG (synthetic oligodeoxynucleotides (ODNs) 1585), class B CpG (ODN 1668), and class C CpG (ODN 2395). These CpG subtypes were from Alexis Biochemicals (San Diego, CA). After 3 hours of treatment with CpG, 5 μmol/L oAβ1-42 was added for 24 hours. Supernatants from microglia were assessed by ELISA kits for tumor necrosis factor-α (TNF-α) and interleukin (IL)-10 (BD Pharmingen, Franklin Lakes, NJ) and matrix metalloproteinase (MMP)-9 (R&D Systems, Minneapolis, MN). Cell extracts from microglia in extraction buffer (1% Nonidet P-40 in PBS) were measured for heme oxygenase-1 (HO-1) with an ELISA kit (Takara Bio, Mie, Japan). Measurement of NO was determined using the Griess reaction.19 To measure glutamate, Glutamate Assay Kit colorimetric assay (Yamasa, Tokyo, Japan) was used as described previously.20
The mRNA expression of HO-1 was assessed by RT-PCR. Briefly, total RNA was extracted using the guanidinium thiocyanate method (RNeasy Mini Kit; Qiagen, Valencia, CA). cDNAs were generated by RT-PCR using SuperScript II (Invitrogen, Carlsbad, CA) and Ampli TaqDNA polymerase (Applied Biosystems, Branchburg, NJ) in the presence of the specific primers. HO-1 sense, 5′-CTATGTAAAGCGTCTCCA-3′; and HO-1 antisense, 5′-GTCTTTGTGTTCCTCTGTC-3′.
Measurement of ROS
To measure ROS in neuron-microglia co-cultures, we used the acetate ester form of 2′,7′-dichlorofluorescein diacetate (H2DCFDA-AM) probe (Invitrogen). After neuron-microglia co-cultures were treated with or without 100 nmol/L CpG for 3 hours, cells were loaded with dye by replacing media with fresh nerve culture medium containing 5 μmol/L H2DCFDA-AM for 30 minutes. After washing, culture medium containing 5 μmol/L oAβ1-42 was added and the fluorescence of the wells was measured. Fluorescent measurements were made using a Wallac 1420 ARVOMX (PerkinElmer Japan, Yokohama, Japan).
Immunocytochemistry
Neuronal, microglial, and neuron-microglia co-cultures were fixed with 4% paraformaldehyde for 30 minutes at room temperature, then blocked with 5% normal goat serum in PBS and permeabilized with 0.3% Triton X-100. Neurons were stained with rabbit polyclonal anti-microtubule-associated protein (MAP)-2 antibody (1/500; Chemicon, Temecula, CA) and secondary antibodies conjugated to Alexa 488 (1/1000; Invitrogen). Synthetic Aβ was stained with a mouse monoclonal anti-Aβ antibody (4G8) (1/1000; Chemicon) and secondary antibodies conjugated to Alexa 568 or 647. Microglia were stained with phycoerythrin-conjugated rat anti-mouse CD11b monoclonal antibody (1/300; BD Pharmingen) before fixation. Images were analyzed with a deconvolution fluorescent microscope system (BZ-8000; Keyence, Osaka, Japan). To assess neuronal death induced by Aβ, purified neurons (5 × 104 cells/well) were plated in 24-well multidishes. A total of 5 μM oAβ1-42 or fAβ1-42 was added to the cultures on 13 days in vitro for 24 hours. To assess neuronal death in neuron-microglia co-cultures, 3 hours after treatment with or without TLR ligands, 5 μmol/L oAβ1-42 was added to cultures for 24 hours. Surviving neurons were identified by cytoskeletal morphology of neurons as described previously.8 Viable neurons stained strongly with an anti-MAP-2 antibody, whereas damaged neurons stained more weakly. The number of MAP-2-positive neurons was counted in representative areas per well. More than 200 neurons were examined in each of five independent trials by a scorer blind to the experimental condition. The number of untreated viable neurons was normalized to 100%.
Western Blotting
Neuronal cultures were treated with 5 μmol/L oAβ1-42 for 24 hours. Neuron-microglia co-cultures were pre-treated with CpG for 3 hours before addition of 5 μmol/L oAβ1-42 for 24 hours. The supernatants of these cultures were collected. oAβ in 10-month-old-Tg 2576 mouse brain was extracted from the soluble, extracellular-enriched fraction as described previously.21 Hemi-forebrains were harvested in 500 μl of solution containing 50 mmol/L Tris-HCl (pH 7.6), 0.01% Nonidet P-40, 150 mmol/L NaCl, 2 mmol/L EDTA, 0.1% SDS, and protease inhibitor mixture (Sigma-Aldrich, St. Louis, MO). Soluble, extracellular-enriched proteins were collected from mechanically homogenized lysates following centrifugation for 5 minutes at 3000 rpm.
Collected samples were mixed with sample buffer (200 mmol/L Tris-HCl, 8% SDS, and 1% glycerol). Proteins were separated on a 5 to 20% Tris-glycine SDS-polyacrylamide gel and transferred to Hybond-P polyvinylidene difluoride membrane (GE Healthcare UK, Buckinghamshire, UK). Membranes were blocked with 1% skim milk in Tris-buffered saline (TBS) containing 0.05% Tween20 (TBS-T). Blots were incubated in mouse anti-Aβ monoclonal antibody (6E10) (1/1000; Chemicon) diluted in 1% skim milk overnight at 4°C. Subsequently, membranes were washed in TBS-T 3 × 5 minutes and incubated with a horseradish peroxidase-conjugated anti-mouse IgG (1/5000; GE Healthcare) diluted in 1% skim milk for 1 hour. After washing in TBS-T for 1 × 15 minutes, 2 × 5 minutes, and TBS for 1 × 5 minutes, signals were visualized with SuperSignal West Pico Chemiluminescent Substrate (Thermo Fisher Scientific, Rockford, IL). The intensity of the bands was calculated by using CS Analyzer 1.0 (Atto, Tokyo, Japan).
Novel-Object Recognition Test in oAβ1-42-Induced Cognitive Impairment Mouse Model
oAβ (300 pmol/3 μl), CpG (100 nmol/L), or both oAβ and CpG were ICV injected as described previously.22,23 The vehicle (PBS) was injected as the control. Briefly, a microsyringe with a 28-gauge stainless-steel needle 3.0 mm long was used for these experiments. C57/BL6 mice were anesthetized lightly with ether, and the needle was inserted unilaterally 1 mm to the right of the midline point equidistant from each eye, at an equal distance between the eyes and the ears and perpendicular to the plane of the skull. A single injection of 3 μl of peptide or vehicle was delivered gradually over 3 minutes. The injection site was confirmed in preliminary experiments. Neither insertion of the needle nor the volume of injection had a significant influence on survival and behavioral responses or cognitive functions.
The novel-object recognition test (NORT) was performed 7 to 8 days after ICV injection of oAβ1-42 or CpG as described previously.24,25 The experimental apparatus consisted of a plexiglas open-field box (30 × 30 × 35 high cm), with a sawdust-covered floor. The apparatus was located in a sound-attenuated room and was illuminated with a 60 lux light source. The NORT procedure consisted of three sessions: habituation, training, and retention. Each mouse was individually habituated to the box with 10 minutes of exploration in the absence of objects for 3 consecutive days (habituation session, days 4 to 6). During the training session, two novel objects were symmetrically fixed to the floor of the box, 8 cm from the walls, and each animal was allowed to explore in the box for 10 minutes (day 7). The objects were constructed from a golf ball, wooden column, and wooden triangular pyramid. They were different in shape and color but similar in size. An animal was considered to be exploring the object when its head was facing the object or it was touching or sniffing the object. The time spent exploring each object was recorded. After training, mice were immediately returned to their home cages. During the retention sessions (day 8), the animals were placed back into the same box 24 hours after the training session, but one of the familiar objects used during training had been replaced with a novel object. The animals were then allowed to explore freely for 5 minutes, and the time spent exploring each object was recorded. Throughout the experiments, the objects were used in a counterbalanced manner. A preference index in the retention session, a ratio of the amount of time spent exploring the novel object over the total time spent exploring both objects, was used to measure cognitive function. In the training session, the preference index was calculated as a ratio of the time spent exploring the object that was replaced by the novel object in the retention session over the total time exploring.
Cued and Contextual Fear-Conditioning Tests in Tg 2576 Mouse Model of AD
Cued and contextual fear conditioning tests were performed at 10 months of age according to previous report,26 with minor modifications. For measuring basal levels of freezing response (preconditioning phase), mice were individually placed in a neutral cage (a block plexiglas box with abundant wood tips,30 × 30 × 40 high cm) for 1 minute, then in the conditioning cage (a transparent plexiglas box, 30 × 30 × 40 high cm) for 2 minutes. For training (conditioning phase), mice were placed in the conditioning cage, then a 15-second tone (80 dB) was delivered as a conditioned stimulus. During the last 5 seconds of the tone stimulus, a foot shock of 0.6 mA was delivered as an unconditioned stimulus through a shock generator (Neuroscience Idea). This procedure was repeated four times with 15-second intervals. Cued and contextual tests were performed 1 day after fear conditioning. For the contextual test, mice were placed in the conditioning cage, and the freezing response was measured for 2 minutes in the absence of the conditioned stimulus. For the cued test, the freezing response was measured in the neutral cage for 1 minute in the presence of a continuous-tone stimulus identical to the conditioned stimulus.
Stereotaxic injection was used for these experiments. Mice were anesthetized with sodium pentobarbital (50 mg/kg, i.p.) before stereotaxic implantation of a microinjection cannula into the right lateral ventricle (anteroposterior, −0.3 mm, and mediolateral, +1.0 mm, from the bregma, and dorsoventral, +2.5, from the skull according to the atlas of Franklin and Paxinos).27 CpG was dissolved in PBS at a concentration of 10 or 100 nmol/L and was injected at a volume of 3 μl for 3 minutes. Same volume of PBS was injected to vehicle mouse. A week after injection, behavioral experiment was performed.
Immunohistochemistry
Immunohistochemistry was performed on brain tissue of mice after cued and contextual fear-conditioning test. Mice were transcardially perfused with ice-cold borate-buffered 4% paraformaldehyde under deep anesthesia. The brains were rapidly removed after decapitation. Brains were then postfixed overnight in periodate lysine paraformaldehyde, equilibrated in phosphate buffered 20% sucrose for 48 hours, and embedded into Tissue-Tek OCT compound (Sakura Finetechnical, Tokyo, Japan) and frozen at −80°C overnight. Coronal brain sections (20 μm) were cut with a cryostat. The sections were permeabilized with 1% Triton X-100 after blocking with 10% normal goat serum for 30 minutes. The cell nucleus was stained with Hoechst 33342 (1 μg/ml; Invitrogen). Aβ was stained with mouse monoclonal anti-Aβ antibody (4G8) (1/1000; Chemicon) and secondary antibodies conjugated to Alexa 488. Microglia were stained with a rat anti-mouse CD11b monoclonal antibody (1/1000; AbD Serotec, Oxford, UK) and secondary antibodies conjugated to Alexa 568. Images were collected and analyzed with a deconvolution fluorescent microscope system. Aβ load in immunostained tissue sections were quantified using BZ-analyzer (Keyence) as reported previously.28 Seven sections were analyzed per animal. Total Aβ burden was quantified for the cortex and for the hippocampus on coronal plane sections stained with the monoclonal antibody 4G8. The cortical area was dorsomedial from the cingulate cortex and extended ventrolaterally to the rhinal fissure within the right hemisphere. Test areas (640 μm × 480 μm) were randomly selected. Total Aβ burden was calculated as the percentage of test area occupied by Aβ. Hippocampal measurements (600 × 600 μm) were performed similarly to the cortical analysis.
Statistical Analysis
Statistical significance of the biochemical experiments and the behavioral data were assessed with one-way analysis of variance, followed by post hoc Tukey test or Newman-Keuls test using GraphPad Prism version 5.0 (GraphPad Software, La Jolla, CA).
Results
Neurotoxicity of oAβ1-42
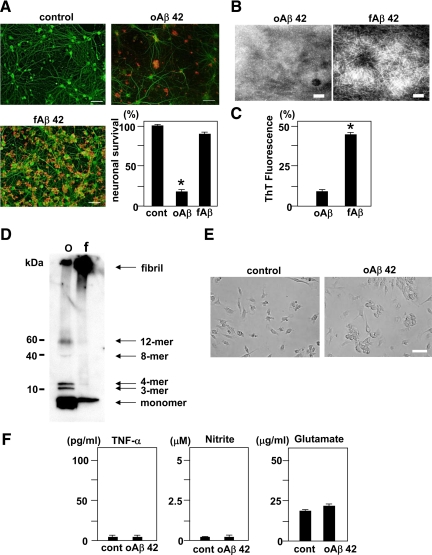
First, we investigated the toxic effects of oAβ and fAβ on primary cortical neurons. Administration of 5 μmol/L oAβ1-42 to cortical cultures on DIV 13 for 24 hours resulted in significant neuronal death. The network of MAP-2-positive dendrites collapsed and neuronal survival decreased to 20% (Figure 1A). In contrast, administration of fAβ1-42 did not induce neuronal cell death, although Aβ deposition was observed on dendrites (Figure 1A). Thus, oAβ1-42 exhibits a more potent neurotoxicity than fAβ1-42. Both oAβ1-40 and fAβ1-40 did not induce neuronal cell death (Supplemental Figure S1, see http://ajp.amjpathol.org). We evaluated the morphology of oAβ1-42 and fAβ1-42 by transmission electron microscopy. We observed fine spherical particles of oAβ1-42 and fibril formation by fAβ1-42 (Figure 1B). The fluorescence of Thioflavin T, a marker for amyloid fibril formation, was associated with fAβ1-42 (Figure 1C). Western blotting with an antibody directed against Aβ (6E10) revealed that a solution of oAβ1-42 contained monomers, trimers (3-mer), tetramers (4-mer), and larger oligomers (octamers (8-mer) and dodecamers (12-mer)). In contrast, a solution of fAβ1-42 contained monomers and fibrils, but not oligomers (Figure 1D).
Figure 1.
Neurotoxicity and morphologies of oAβ1-42 and fAβ1-42, and microglial response to oAβ1-42. A: The evaluation of neurotoxicity induced by oAβ1-42 and fAβ1-42. Neuronal cultures were treated with 5 μmol/L oAβ1-42 or fAβ1-42 for 24 hours. Neurons were stained with an anti-MAP-2 antibody (green). Aβ was stained with a mouse anti-amyloid β protein monoclonal antibody (4G8) (red). oAβ1-42 exhibited more striking neurotoxicity than fAβ1-42. Scale bar, 50 μm. Neuronal survival rate in oAβ1-42 treatment significantly decreased. ∗P < 0.05 as compared with the rate in control cultures. Each column indicates the mean ± SEM (n = 5). B: Images of oAβ1-42 (left) and fAβ1-42 (right) collected with an electron microscope. Scale bar, 100 nm. C: Thioflavin T assay for oAβ1-42 and fAβ1-42. ∗P < 0.05 as compared with the value of oAβ1-42. D: Western blot analysis of oAβ1-42 and fAβ1-42. oAβ1-42 (o) contained monomers, small oligomeric trimers (3-mer) and tetramers (4-mer), and the larger oligomers (octamers (8-mer) and dodecamers (12-mer)), whereas fAβ1-42 (f) contained monomers and fibrils. E: Microglial cultures were treated with or without 5 μmol/L oAβ1-42 for 24 hours. In a phase contrast, oAβ1-42 induced microglial adhesion. Scale bar, 50 μm. F: The measurement of TNF-α (left), nitrite (middle), and glutamate (right) produced by microglia activated with oAβ1-42. Microglial cultures were treated with 5 μmol/L oAβ1-42 for 24 hours. Each column indicates the mean ± SEM (n = 7).
In primary microglial culture, administration of 5 μmol/L oAβ1-42 for 24 hours induced microglial adhesion (Figure 1E), but did not induce the production of neurotoxic mediators such as TNF-α, glutamate, or nitrite, a stable breakdown product of NO (Figure 1F).
Microglia Activated with CpG Attenuate the Neurotoxicity Induced by oAβ1-42
To define the role of microglia in the neurotoxicity of oAβ1-42, we evaluated neuronal survival in neuron-microglia co-cultures. Neurons stained with anti-MAP-2 antibody exhibited no detectable morphological abnormalities and possessed intact cell bodies and dendrites, and microglia stained with anti-CD11b antibody were also intact in unstimulated co-cultures (Figure 2A). After treatment of neuronal cultures with 5 μmol/L oAβ1-42 for 24 hours, the neuronal cells were severely damaged, and the survival rate decreased to 18% (Figure 2, B and G). Similarly, the neuronal survival rate was not improved in neuron-microglia co-cultures treated with oAβ1-42 (Figure 2, C and G), which implies that unstimulated microglia have not protective effect against oAβ1-42 neurotoxicity. Administration of CpG to neuronal cultures or neuron microglia co-cultures induced no toxic change (Supplemental Figure S2A, see http://ajp.amjpathol.org). After 3 hours of treatment with 1 nmol/L, 10 nmol/L, or 100 nmol/L CpG, 5 μmol/L oAβ1-42 was added to neuron-microglia co-cultures for 24 hours. The neuroprotective effect was not evident in culture with 1 nmol/L CpG (Figure 2, D and G). However, microglia treated with 10 or 100 nmol/L CpG prevented neuronal cell death, and the neuronal survival rate was significantly improved reaching 53 and 62%, respectively (Figure 2, E–G). In neuronal cultures, CpG did not attenuate the neurotoxicity induced by oAβ1-42 (Figure 2G). Moreover, 100 nmol/L CpG attenuated oAβ1-42-induced neurotoxicity for 48 hours, whereas other TLR ligands such as peptidoglycan and lipopolysaccharide did not (Supplemental Figure S2B, see http://ajp.amjpathol.org). We conclude from these findings that CpG-activated microglia have neuroprotective effect against oAβ1-42 neurotoxicity in vitro.
Figure 2.
Protective effect of microglia activated with CpG against oAβ1-42 neurotoxicity. A: Representative deconvolution fluorescent images of control neuron-microglia co-cultures (1:2 of neuron: microglia). B: Neuronal cultures treated with 5 μmol/L oAβ1-42. C: Neuron-microglia co-cultures (1:2 neuron to microglia) treated with oAβ1-42. D: Neuron-microglia co-cultures stimulated with 1 nmol/L CpG and oAβ1-42 (E) or with 10 nmol/L CpG and oAβ1-42 (F) or with 100 nmol/L CpG and oAβ1-42. After 3 hours of treatment with CpG, cultures were treated with oAβ1-42 for 24 hours. Neurons were stained with an anti-MAP-2 antibody (green). Aβ was stained with 4G8 (red) and microglia were stained with a phycoerythrin-conjugated anti-CD11b antibody (blue). Scale bar, 50 μm. G: Neuronal survival rate was quantified as the percentage of intact neurons following treatment relative to control wells. The viability of untreated neurons (control) was normalized to 100%. ∗P < 0.05 as compared with the survival rate of neuron-microglia co-cultures treated with oAβ1-42. Each column indicates the mean ± SEM (n = 7).
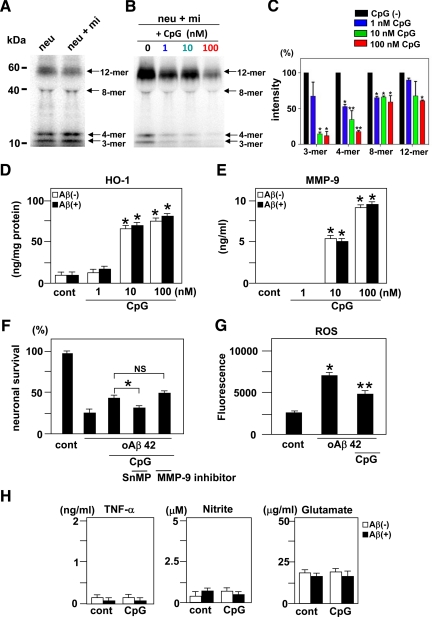
CpG-Activated Microglia Increase the Clearance of oAβ1-42, Produce the Antioxidant Enzyme HO-1 and Aβ-Degrading Enzyme MMP-9, and Release Fewer Neurotoxic Molecules
To elucidate the mechanisms of neuroprotection by microglia activated with CpG, we examined whether CpG increased the clearance of oAβ1-42. Western blot analysis revealed that there was no significant difference between the amount of oAβ1-42 present in the supernatants of neuronal cultures and in neuron-microglia co-cultures without CpG administration. (Figure 3A). However, CpG dose-dependently decreased the amount of oAβ1-42 in neuron-microglia co-cultures, especially treatment with 100 nmol/L CpG significantly decreased the amount of 3-, 4-, 8-, and 12-mer of oAβ1-42 (Figure 3, B and C). Moreover, we examined the effect of CpG on Aβ uptake by microglia alone at 1 and 24 hours time points. We found that CpG significantly enhanced microglial uptake of oAβ at both 1 and 24 hours (Supplemental Figure S3A, see http://ajp.amjpathol.org).
Figure 3.
Clearance of oAβ1-42 and the production of HO-1, MMP-9, and neurotoxic molecules by microglia activated with CpG. A: Western blot analysis of oAβ1-42 in neuronal cultures (neu) and neuron-microglia co-cultures (neu + mi). Twenty-four hours after addition of 5 μmol/L oAβ1-42, oAβ1-42 present in the supernatants of these cultures was detected by Western blotting. B: Western blot analysis of oAβ1-42 in neuron-microglia co-cultures with CpG treatment. Neuron-microglia co-cultures (neu + mi) were treated with 5 μmol/L oAβ1-42 for 24 hours following 3 hours of treatment with 1, 10, or 100 nmol/L CpG. Microglia activated with CpG dose-dependently reduced the amount of oAβ1-42 in the supernatants. C: Semiquantification of oAβ1-42 in B by densitometric analysis. The amount of oAβ1-42 in neuron-microglial co-cultures without CpG (black) was normalized to 100%. oAβ1-42 in co-cultures treated with 1 nmol/L CpG (blue), 10 nmol/L CpG (green), or 100 nmol/L CpG (red) was calculated. ∗P < 0.05 and ∗∗P < 0.01 as compared with the intensity of oAβ1-42 in neuron-microglia co-cultures without CpG. Each column indicates the mean ± SEM (n = 6). The production of HO-1 (D) and MMP-9 (E) by microglia activated with CpG in the absence or presence of oAβ1-42. After 3 hours of treatment with CpG, microglial cultures were treated with or without oAβ1-42 for 24 hours. ∗P < 0.05 as compared with untreated controls. Each column indicates the mean ± SEM (n = 3–5). F: The effect of HO-1 and MMP-9 on oAβ1-42 neurotoxicity. Neuron-microgila co-cultures were treated with 100 nmol/L CpG in the presence of 10 μmol/L tin-mesoporphyrin (SnMP) IX, a specific HO-1 inhibitor, or 50 nmol/L MMP-9 inhibitor for 3 hours, and then oAβ1-42 was added to the cultures for 24 hours. Tin-mesoporphyrin IX, but not the MMP-9 inhibitor, decreased neuronal survival rate. ∗P < 0.05 as compared with CpG-treated cultures without inhibitors. Each column indicates the mean ± SEM (n = 6–9). G: The suppressive effect of CpG on ROS production by oAβ in the neuron microglia co-cultures. After neuron-microglia co-cultures were treated with or without 100 nmol/L CpG for 3 hours, cells were loaded with fresh nerve culture medium containing 5 μmol/L H2DCFDA-AM for 30 minutes. After washing, culture medium containing 5 μmol/L oAβ1-42 was added and the increment of the fluorescence was calculated at 5 minutes. ∗P < 0.05 as compared with untreated controls. **P < 0.05 as compared with co-culture cells treated with oAβ1-42. Each column indicates the mean ± SEM (n = 4). H: The measurement of TNF-α (left), nitrite (middle), and glutamate (right) produced by microglia activated with 100 nmol/L CpG with or without oAβ1-42. After 3 hours treatment with CpG, microglial cultures were treated with or without oAβ1-42 for 24 hours. ∗P < 0.05 as compared with untreated microglia. Each column indicates the mean ± SEM (n = 7).
Because oxidative stress is a major component of oAβ1-42 neurotoxicity, we examined whether microglia activated with CpG express the antioxidant enzyme HO-1. CpG-activated microglia produced HO-1 in a dose-dependent manner. A total of 10 and 100 nmol/L CpG significantly increased the production of HO-1. The production levels were not influenced by exposure to oAβ1-42 (Figure 3D). Since the anti-inflammatory cytokine IL-10 induces HO-1 expression by macrophages,29 we also examined and confirmed that IL-10 induced HO-1 mRNA expression by microglia (Supplemental Figure S3B, see http://ajp.amjpathol.org). Although CpG induced IL-10 in microglia, the expression was suppressed by oAβ1-42 treatment (Supplemental Figure S3C, see http://ajp.amjpathol.org).
MMP-9 is also thought to play a neuroprotective role in AD because it degrades both oAβ and fAβ. A total of 10 and 100 nmol/L CpG significantly induced MMP-9 production in microglia with or without treatment of oAβ1-42 (Figure 3E). To determine whether HO-1 and MMP-9 contribute to the neuroprotective effects of CpG-activated microglia, we applied the specific HO-1 inhibitor tin-mesoporphyrin IX (Frontier Scientific, Logan, UT) and MMP-9 inhibitor (Merck, Darmstadt, Germany). The neuroprotective effect of CpG was abolished by treatment with 10 μmol/L tin-mesoporphyrin IX (Figure 3E). However, inhibition of MMP-9 with an MMP-9 inhibitor at 50 nmol/L did not influence the neuroprotective effect of CpG-activated microglia (Figure 3F). These results imply that HO-1 rather than MMP-9 may contribute to the neuroprotection of CpG. Furthermore, treatment of oAβ significantly increased ROS production in the neuron microglia co-cultures. Pretreatment of 100 nmol/L CpG significantly suppressed ROS production by oAβ (Figure 3G).
Although TLR4 ligand lipopolysaccharide generally increases microglial production of neurotoxic molecules including TNF-α, nitrite and glutamate; 100 nmol/L CpG did not induce these toxic molecules in microglia (Figure 3H).
Microglia Activated with Class B and C, but not Class A CpG, Attenuate oAβ1-42 Neurotoxicity
CpG ODNs are divided into three classes by their ability to induce IFN-α expression in plasmacytoid dendritic cells (class A) and to promote survival, activation, and maturation of B cells and plasmacytoid dendritic cell (class B) or both (class C)30. We examined which class of CpG induces the neuroprotective effects of microglia. Class A CpG neither activated microglia nor induced neuroprotective effects against oAβ1-42 toxicity, whereas both class B and C CpGs activated microglia and significantly increased neuronal survival, to 58 and 49% following oAβ1-42 treatment, respectively (Figures 4, A and B). Western blot analysis revealed that class B CpG significantly decreased the amount of trimers and dodecamers of oAβ1-42 present in the supernatants of neuron-microglia co-cultures, and class C CpG significantly decreased dodecamers of oAβ1-42, whereas oAβ1-42 did not decrease by the administration of class A CpG (Figure 4, C and D). In addition, microglia activated with class B and C CpGs expressed HO-1 in both the absence or presence of oAβ1-42, whereas treatment with class A CpG only slightly increased HO-1 expression in the presence of oAβ1-42 (Figure 4E). Microglia activated with class B and C CpGs also produced MMP-9 (Figures 4F).
Figure 4.
Protective effect of microglia activated with each subclass of CpG against oAβ1-42 neurotoxicity. A: Class B and class C, but not class A, CpGs exhibited neuroprotective effects against oAβ1-42 neurotoxicity in neuron-microglia co-cultures. After 3 hours incubation with 100 nmol/L class A, class B, or class C CpGs, oAβ1-42 was added to neuron-microglia co-cultures for 24 hours. Neurons were stained with anti-MAP-2 antibody (green). Aβ was stained with 4G8 (red), and microglia were stained with anti-CD11b antibody (blue). Scale bar, 50 μm. B: Neuronal survival rate was quantified. The viability of neurons in untreated co-cultures (control) was normalized to 100%. ∗P < 0.05 as compared with the co-cultures treated with oAβ1-42 alone. Each column indicates the mean ± SEM (n = 7). C: Western blot analysis of oAβ1-42 in neuron-microglia co-cultures with subclassese of CpG treatment. Neuron-microglia co-cultures (neu + mi) were treated with 5 μmol/L oAβ1-42 for 24 hours following 3 hours of treatment with 100 nmol/L class A, class B, or class C CpGs. Microglia activated with class B or class C CpGs reduced the amount of oAβ1-42 in the supernatants. D: Semiquantification of oAβ1-42 in C by densitometric analysis. The amount of oAβ1-42 in neuron-microglial co-cultures without CpG (black) was normalized to 100%. oAβ1-42 in co-cultures treated with class A CpG (blue), class B CpG (green), or class C CpG (red) was calculated. ∗∗P < 0.01 compared with the intensity of oAβ1-42 in neuron-microglia co-cultures without CpG. Each column indicates the mean ± SEM (n = 7). The production of HO-1 (E) and MMP-9 (F) by microglia activated with subclasses of CpG in the absence or presence of oAβ1-42. After 3 hours incubation with 100 nmol/L class A, class B, or class C CpGs, microglial cultures were treated with or without oAβ1-42 for 24 hours. ∗P < 0.05 as compared with untreated control cultures. Each column indicates the mean ± SEM (n = 3–5).
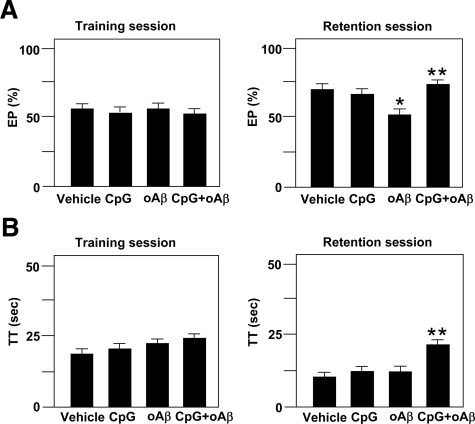
ICV Injection of CpG Ameliorates oAβ-Induced Impairment of Recognition Memory in the NORT
To examine the role of CpG-activated microglia in cognitive dysfunction induced by oAβ1-42, we investigated the effect of in vivo administration of CpG on the impairment of recognition memory in the NORT after ICV injection of oAβ1-42. Mice injected with oAβ1-42 displayed significantly reduced exploratory preference for the novel object in the retention session (F(3,21) = 3.68, P < 0.05; Figure 5A), although total exploration time in the training and retention sessions was unaffected. The result implies that oAβ1-42 induces the impairment of recognition memory. Simultaneous injection of CpG with oAβ1-42 significantly improved both exploratory preference (F(3,21) = 3.68, P < 0.05; Figure 5A) and total exploration time (F(3,21) = 4.41, P < 0.05; Figure 5B) in the retention session, although exploratory preference and total exploration time were unaffected in the training session.
Figure 5.
Effect of ICV injection of CpG on oAβ1-42-induced cognitive impairment in the NORT. A: Exploratory preference (EP) and total exploring time (TT) (B) in the training session (left) and the retention session (right). NORT was performed 7 to 8 days after ICV injection of oAβ1-42. Each column indicates the mean ± SEM (n = 6–7). ∗P < 0.05 as compared with controls. **P < 0.05 as compared with oAβ1-42-injected mice.
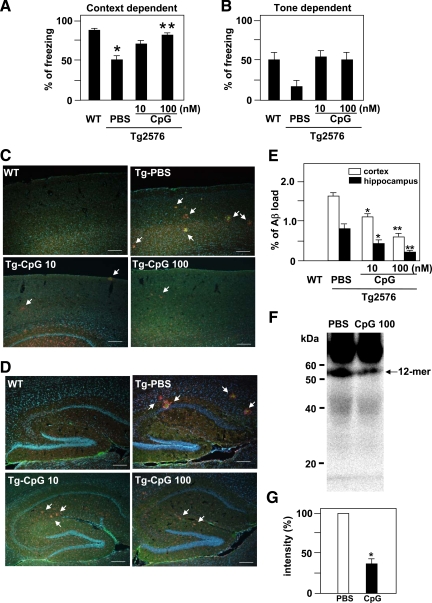
ICV Injection of CPG Ameliorates the Impairment of Associative Learning in the Cued and Contextual Fear-Conditioning Tests in Tg2576 Mice
Next, we examined the effect of CpG on the cognitive function of Tg2576 mouse model of AD. We evaluated associative learning at the age of 10 months in a conditioned fear learning test. In the preconditioning phase (training), the mice hardly showed any freezing response. There were no differences in basal levels of freezing response between the groups (data not shown). In the contextual learning test, wild-type mice showed a marked contextual freezing response 24 hours after fear conditioning (Figure 6A). However, vehicle-injected Tg2576 mice exhibited less of a freezing response in the contextual tests (Figure 6A), indicating an impairment of associative learning. The CpG (10 or 100 nmol/L)-injected Tg2576 mice were indistinguishable from wild-type mice, and the CpG treatment dose-dependently and significantly reversed the contextual freezing response as compared with vehicle-injected Tg2576 mice (F(3,35) = 9.54, P < 0.05; Figure 6A). In the cued (tone) learning test, although there was no significant difference in the cued freezing response at 24 hours after fear conditioning between wild-type and vehicle-injected Tg2576 mice, both injection of 10 and 100 nmol/L CpG showed a tendency to reverse the cued freezing response (Figure 6B). No alterations of nociceptive response were found in any of the mutant mice: there was no difference in the minimal current required to elicit flinching/running, jumping, or vocalization among the mice (data not shown). Then, we examined whether CpG decreased Aβ deposits in the cortex (Figure 6C) and the hippocampus (Figure 6D) of Tg 2576 mice. No Aβ deposits were seen in wild-type mice. Although Aβ deposits (green) were abundant in the cortex and the hippocampus of vehicle-injected Tg2576 mice, ICV injection of CpG significantly decreased Aβ deposits in both areas. Microglia (red) clustered around Aβ deposits (Figure 6, C and D). CpG decreased Aβ load in a significant, dose-dependent manner in both areas (Figure 6E). We examined oAβ in the soluble, extracellular-enriched fractions of the hemi-forebrains of mice and detected 12-mer oAβ in vehicle-injected Tg2576 mice by Western blotting. The 12-mer oAβ was strikingly and significantly decreased in 100 nmol/L CpG-injected Tg2576 mice (Figure 6, F and G).
Figure 6.
Effect of ICV injection of CpG on associative learning in the cued and contextual conditioning tests and Aβ clearance in Tg2576 mice. The retention session was performed 24 hours after the training. Context-dependent (A) and tone-dependent (B) freezing times were measured at the age of 10 months. Each column indicates the mean ± SEM (wild-type (WT) mice, n = 14; vehicle-treated (PBS) Tg2576 mice, n = 9; CpG (10 nmol/L)-treated Tg2576, n = 8; CpG (100 nmol/L)-treated Tg2576, n = 8). ∗P < 0.05 compared with wild type. **P < 0.05 as compared with vehicle-treated Tg2576 mice. C: Aβ deposits in the cortex. Aβ was stained with 4G8 (green), microglia stained with anti-CD11b antibody (red), and cell nucleus stained with Hoechst 33342 (blue). White arrows indicate Aβ deposits surrounding by microglia. Scale bar, 200 μm. D: Aβ deposits in the hippocampus. Scale bar, 200 μm. E: Quantification of Aβ deposits in C and D. The ratio between the Aβ deposits area and the total area of the analyzed region was multiplied by 100. ∗P < 0.05 and ∗∗P < 0.01 as compared with vehicle-treated Tg2576 mice. Each column indicates the mean ± SEM (n = 3). F: Western blot analysis of oAβ extracted from the hemi-forebrains of Tg 2576 mice. A 12-mer oAβ was detected in vehicle-treated Tg2576 mice and decreased in 100 nmol/L CpG-injected Tg2576 mice. G: Semiquantification of 12-mer oAβ by densitometric analysis. The amount of 12-mer oAβ1-42 in vehicle-treated Tg2576 mice was normalized to 100%. ∗P < 0.05 as compared with vehicle-treated Tg2576 mice. Each column indicates the mean ± SEM (n = 4).
Discussion
Recent studies have proposed that oAβ1-42 contributes to the neurotoxicity associated with AD. AD begins with subtle alterations of hippocampal synaptic efficacy before frank neuronal degeneration, and this synaptic dysfunction is caused by diffusible oAβ.31 Disruption of hippocampal long-term potentiation and synaptic plasticity by oAβ1-42 appears to involve Ca2+ signaling,32 oxidative stress mediated by an N-methyl-d-aspartate receptor,4,33 and protein phosphatase 1.34 In addition, oAβ interferes with insulin receptor function in hippocampal neurons and inhibits the activation of specific kinases required for long-term potentiation.35 In the present study, we have confirmed that oAβ1-42 exhibits more potent neurotoxicity than fAβ1-42 in murine cortical cultures.5,36 Therefore, decreasing or preventing formation of oAβ1-42 is a potential therapeutic strategy against AD.
The precise role of microglia in oAβ1-42 toxicity remains unclear. Microglia stimulated with Aβ are reported to release proinflammatory cytokines via the nuclear factor κB37,38 and contribute to the pathogenesis of AD. However, in our experimental conditions, oAβ1-42 neither activated microglia nor induced the release of neurotoxic proinflammatory cytokines, NO, or glutamate. These results suggest that oAβ1-42 is not acting to trigger of microglial neurotoxicity.
TLR signaling pathways contribute to phagocytosis of Aβ. TLR2 acts as an endogenous receptor for the clearance of Aβ by bone marrow-derived microglia.9 Interestingly, a TLR4 mutation exacerbates Aβ burden in mouse models of AD.39 Thus, we investigated whether microglia activated with TLR ligands exert neuroprotective effects against oAβ1-42 toxicity. Consequently, we found that TLR9 ligand CpG enhanced microglial neuroprotection. Furthermore, CpG exerted the neuroprotective effect of BV-2 microglial cell line against oAβ toxicity (data not shown). TLR9, which detects single-stranded DNA containing unmethylated CpG, is located in intracellular endosomal-lysosomal compartment. We confirmed that microglia expressed TLR9 at a higher level, whereas astrocytes and neuronal cells expressed it at a lower level (data not shown). Thus, CpG mainly acts on microglia in the central nervous system. TLR7 and TLR8 are closely associated with TLR9. They are also located in endosomal-lysosomal compartment and detect single-stranded RNA. The ligands for TLR7 and TLR8 may also have some roles in microglial neuroprotection.
Western blot analysis revealed that microglia activated with CpG reduced the amount of oAβ present in the supernatant of treated cultures. Moreover, CpG was a potent inducer of antioxidant enzyme HO-1. The up-regulation of HO-1 in microglia by CpG treatment may lead to neuroprotection via suppression of ROS production by oAβ. HO-1, a member of the heat-shock protein family, is a microsomal enzyme that oxidatively cleaves heme to produce biliverdin, carbon monoxide, and iron.40 Aβ binds to heme to promote a functional heme deficiency, mitochondrial dysfunction, and neurotoxicity.41 Amyloid precursor protein also binds to HO, and oxidative neurotoxicity is markedly enhanced in cerebral cortical cultures from amyloid precursor protein Swedish mutant transgenic mice.42
HO-1 is reported to be induced by the anti-inflammatory cytokine IL-10.29 Although CpG induced IL-10 in microglia in the absence of oAβ, IL-10 production was inhibited in the presence of oAβ. Therefore, HO-1 may be induced by a discrete mechanism independent from IL-10 in AD.
MMP-9, a protease that degrades Aβ, is expressed at higher levels in the brains of AD patients and may play an important role in amyloid clearance by degrading both oAβ and fAβ.10 MMP-9 expression is increased by serum amyloid A through formyl peptide receptor-like-1.43 Although CpG stimulation induced MMP-9 in microglia, inhibiting MMP-9 pharmacologically did not affect neuroprotection by microglia. Thus, MMP-9 may not mainly contribute to the neuroprotection provided by CpG-activated microglia.
In the present study, CpG induced fewer neurotoxic molecules such as TNF-α, NO, and glutamate in microglia, whereas previous studies have reported that CpG-activated microglia produce TNF-α, IL-12, and NO44 and induce neuronal damage.45 The discrepancies between these studies and our experiments may be a consequence of differences in the concentrations of TLR ligands used. Higher concentration (10 μmol/L) of CpG have been used for microglial activation in previous reports, whereas here we have used lower concentrations (1 to 100 nmol/L) of CpG.
In addition, we observed that the neuroprotective effect differs among CpG ODN classes. The responses of microglia to the different classes of CpG have not been fully understood. Here, we showed for the first time that class A CpG did not activate microglia, whereas class B and C CpGs induced neuroprotection by microglia that was mediated by clearance of oAβ and induction of HO-1. Three major classes of CpG ODN are structurally distinct. The structures of class A CpG include poly-G motifs at the 5′ and/or 3′ ends that are capable of forming very stable but complex higher-ordered structures and a central phosphodiester region containing one or more CpG motifs in a self-complementary palindrome. Class B CpG has a completely phosphorothioate backbone and does not form typically higher-ordered structures. Class C CpG has a phosphorothioate backbone, and 3′ palindrome forms duplex.46 These distinct structures of CpG ODN may reflect different microglial responses.
Finally, we examined the effect of CpG on oAβ1-42 neurotoxicity in two different in vivo studies. The ICV administration of Aβ25-35 is reported to cause cognitive impairment in a NORT. Oxidative stress contributes to the onset of this cognitive dysfunction.23 We found that injection of oAβ1-42 also induced cognitive impairment as assessed by NORT. Surprisingly, one-time ICV injection of CpG improved both the cognitive impairment by oAβ1-42 in NORT. The impairment of associative learning in Tg 2576 mouse model of AD was also effectively suppressed by ICV injection of CpG. We also confirmed that CpG treatment decreased Aβ deposits and oAβ in Tg 2576 mice. Our results concur with the recent study that a total of 14 i.p. injection of CpG into Tg 2576 mice beginning at the age of 6 weeks, and once a month, ameliorates AD-related pathology.28 Aβ plaques are reported to form extraordinarily quickly, over 24 hours. Within 1 to 2 days of a new plaque’s appearance, microglia are activated and recruited to the site.47 ICV injection of CpG may directly induce microglial activation via TLR9 and enhance microglial rapid uptake of oAβ through fluid-phase macropinocytosis as reported recently.48 CpG may also enhance microglial phagocytosis of fAβ through formyl peptide receptor-like 2.11 Such mechanisms of Aβ clearance by CpG can decrease Aβ plaque formation in Tg 2576 mice.
Recently, the therapeutic potential of CpG has generated great interest.46 CpG offers a potent adjuvant activity that elicits a more effective immune response to infectious agents or tumors. A previous report49 demonstrated that CpG strongly inhibits the effector phase of inflammatory arthritis. In addition, CpG can serve as a potent preconditioning stimulus and provide protection against ischemic brain injury.50 Our findings suggest that CpG, especially class B and C, may also be effective therapeutic agents against oAβ1-42 neurotoxicity in AD.
Supplementary Material
Footnotes
Address reprint requests to Tetsuya Mizuno, Department of Neuroimmunology, Research Institute of Environmental Medicine, Nagoya University, Furo-cho, Chikusa-ku, Nagoya 464-8601, Japan. E-mail: tmizuno@riem.nagoya-u.ac.jp.
Supported in part by a grant-in-aid for scientific research (grant C), Global Centers of Excellence program “Integrated Functional Molecular Medicine for Neuronal and Neoplastic Disorders” funded by Ministry of Education, Culture, Sports, Science, and Technology of Japan, the Program for Promotion of Fundamental Studies in Health Sciences of the National Institute of Biomedical Innovation, the Hori Information Science Promotion Foundation, a grant-in-aid for Scientific Research on Priority Areas-Research on Pathomechanisms of Brain Disorders-from the Ministry of Education, Culture, Sports, Science, and Technology of Japan, and a grant from the Ministry of Health, Labor and Welfare of Japan (Comprehensive Research on Aging and Health grant H20-007).
Y.D. and T.M. contributed equally to this paper.
Supplemental material for this article can be found on http://ajp.amjpathol.org.
References
- Busciglio J, Lorenzo A, Yeh J, Yankner BA. β-Amyloid fibrils induce tau phosphorylation and loss of microtubule binding. Neuron. 1995;14:879–888. doi: 10.1016/0896-6273(95)90232-5. [DOI] [PubMed] [Google Scholar]
- Grace EA, Rabiner CA, Busciglio J. Characterization of neuronal dystrophy induced by fibrillar amyloid β: implications for Alzheimer’s disease. Neuroscience. 2002;114:265–273. doi: 10.1016/s0306-4522(02)00241-5. [DOI] [PubMed] [Google Scholar]
- Walsh DM, Klyubin I, Fadeeva JV, Cullen WK, Anwyl R, Wolfe MS, Rowan MJ, Selkoe DJ. Naturally secreted oligomers of amyloid β protein potently inhibit hippocampal long-term potentiation in vivo. Nature. 2002;416:535–539. doi: 10.1038/416535a. [DOI] [PubMed] [Google Scholar]
- De Felice FG, Velasco PT, Lambert MP, Viola K, Fernandez SJ, Ferreira ST, Klein WL. Aβ oligomers induce neuronal oxidative stress through an N-methyl-d-aspartate receptor-dependent mechanism that is blocked by the Alzheimer drug memantine. J Biol Chem. 2007;282:11590–11601. doi: 10.1074/jbc.M607483200. [DOI] [PubMed] [Google Scholar]
- Deshpande A, Mina E, Glabe C, Busciglio J. Different conformations of amyloid β induce neurotoxicity by distinct mechanisms in human cortical neurons. J Neurosci. 2006;26:6011–6018. doi: 10.1523/JNEUROSCI.1189-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meda L, Cassatella MA, Szendrei GI, Otvos L, Jr, Baron P, Villalba M, Ferrari D, Rossi F. Activation of microglial cells by β-amyloid protein and interferon γ. Nature. 1995;374:647–650. doi: 10.1038/374647a0. [DOI] [PubMed] [Google Scholar]
- McGeer PL, McGeer EG. Inflammation, autotoxicity and Alzheimer disease. Neurobiol Aging. 2001;22:799–809. doi: 10.1016/s0197-4580(01)00289-5. [DOI] [PubMed] [Google Scholar]
- Mizuno T, Kurotani T, Komatsu Y, Kawanokuchi J, Kato H, Mitsuma N, Suzumura A. Neuroprotective role of phosphodiesterase inhibitor Ibudilast on neuronal cell death induced by activated microglia. Neuropharmacology. 2004;46:404–411. doi: 10.1016/j.neuropharm.2003.09.009. [DOI] [PubMed] [Google Scholar]
- Richard KL, Filali M, Préfontaine P, Rivest S. Toll-like receptor 2 acts as a natural innate immune receptor to clear amyloid β 1-42 and delay the cognitive decline in a mouse model of Alzheimer’s disease. J Neurosci. 2008;28:5784–5793. doi: 10.1523/JNEUROSCI.1146-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan P, Hu X, Song H, Yin K, Bateman RJ, Cirrito JR, Xiao Q, Hsu FF, Turk JW, Xu J, Hsu CY, Holtzman DM, Lee JM. Matrix metalloproteinase-9 degrades amyloid-β fibrils in vitro and compact plaques in situ. J Biol Chem. 2006;281:24566–24574. doi: 10.1074/jbc.M602440200. [DOI] [PubMed] [Google Scholar]
- Iribarren P, Chen K, Hu J, Gong W, Cho EH, Lockett S, Uranchimeg B, Wang JM. CpG-containing oligodeoxynucleotide promotes microglial cell uptake of amyloid β 1-42 peptide by up-regulating the expression of the G-protein-coupled receptor mFPR2. FASEB J. 2005;19:2032–2034. doi: 10.1096/fj.05-4578fje. [DOI] [PubMed] [Google Scholar]
- Chen K, Iribarren P, Hu J, Chen J, Gong W, Cho EH, Lockett S, Dunlop NM, Wang JM. Activation of Toll-like receptor 2 on microglia promotes cell uptake of Alzheimer disease-associated amyloid β peptide. J Biol Chem. 2006;281:3651–3659. doi: 10.1074/jbc.M508125200. [DOI] [PubMed] [Google Scholar]
- Liu Y, Walter S, Stagi M, Cherny D, Letiembre M, Schulz-Schaeffer W, Heine H, Penke B, Neumann H, Fassbender K. LPS receptor (CD14): a receptor for phagocytosis of Alzheimer’s amyloid peptide. Brain. 2005;128:1778–1789. doi: 10.1093/brain/awh531. [DOI] [PubMed] [Google Scholar]
- Xie Z, Wei M, Morgan TE, Fabrizio P, Han D, Finch CE, Longo VD. Peroxynitrite mediates neurotoxicity of amyloid β-peptide1-42- and lipopolysaccharide-activated microglia. J Neurosci. 2002;22:3484–3492. doi: 10.1523/JNEUROSCI.22-09-03484.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi H, Jin S, Wang J, Zhang G, Kawanokuchi J, Kuno R, Sonobe Y, Mizuno T, Suzumura A. Tumor necrosis factor-α induces neurotoxicity via glutamate release from hemichannels of activated microglia in an autocrine manner. J Biol Chem. 2006;281:21362–21368. doi: 10.1074/jbc.M600504200. [DOI] [PubMed] [Google Scholar]
- Suzumura A, Mezitis SG, Gonatas NK, Silberberg DH. MHC antigen expression on bulk isolated macrophage-microglia from newborn mouse brain: induction of Ia antigen expression by γ-interferon. J Neuroimmunol. 1987;15:263–278. doi: 10.1016/0165-5728(87)90121-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naiki H, Nakakuki K. First-order kinetic model of Alzheimer’s β-amyloid fibril extension in vitro. Lab Invest. 1996;74:374–383. [PubMed] [Google Scholar]
- Dahlgren KN, Manelli AM, Stine WB, Jr, Baker LK, Krafft GA, LaDu MJ. Oligomeric and fibrillar species of amyloid-β peptides differentially affect neuronal viability. J Biol Chem. 2002;277:32046–32053. doi: 10.1074/jbc.M201750200. [DOI] [PubMed] [Google Scholar]
- Pollock JS, Forstermann U, Mitchell JA, Warne TD, Schmidt HHHW, Nakane M, Murad F. Purification and characterization of particulate endothelium-derived relaxing factor synthase from cultured and native bovine aortic endothelial cells. Proc Natl Acad Sci USA. 1991;88:10480–10484. doi: 10.1073/pnas.88.23.10480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi H, Mizuno T, Zhang G, Wang J, Kawanokuchi J, Kuno R, Suzumura A. Neuritic beading induced by activated microglia is an early feature of neuronal dysfunction toward neuronal death by inhibition of mitochondrial respiration and axonal transport. J Biol Chem. 2005;280:10444–10454. doi: 10.1074/jbc.M413863200. [DOI] [PubMed] [Google Scholar]
- Lesné S, Koh MT, Kotilinek L, Kayed R, Glabe CG, Yang A, Gallagher M, Ashe KH. A specific amyloid-β protein assembly in the brain impairs memory. Nature. 2006;440:352–357. doi: 10.1038/nature04533. [DOI] [PubMed] [Google Scholar]
- Maurice T, Lockhart BP, Privat A. Amnesia induced in mice by centrally administered β-amyloid peptides involves cholinergic dysfunction. Brain Res. 1996;706:181–193. doi: 10.1016/0006-8993(95)01032-7. [DOI] [PubMed] [Google Scholar]
- Alkam T, Nitta A, Mizoguchi H, Itoh A, Nabeshima T. A natural scavenger of peroxynitrites, rosmarinic acid, protects against impairment of memory induced by Aβ (25-35). Behav Brain Res. 2007;180:139–145. doi: 10.1016/j.bbr.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Kamei H, Nagai T, Nakano H, Togan Y, Takayanagi M, Takahashi K, Kobayashi K, Yoshida S, Maeda K, Takuma K, Nabeshima T, Yamada K. Repeated methamphetamine treatment impairs recognition memory through a failure of novelty-induced ERK1/2 activation in the prefrontal cortex of mice. Biol Psychiatry. 2006;59:75–84. doi: 10.1016/j.biopsych.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Mizoguchi H, Takuma K, Fukakusa A, Ito Y, Nakatani A, Ibi D, Kim HC, Yamada K. Improvement by minocycline of methamphetamine-induced impairment of recognition memory in mice. Psychopharmacology. 2008;196:233–241. doi: 10.1007/s00213-007-0955-0. [DOI] [PubMed] [Google Scholar]
- Mouri A, Noda Y, Hara H, Mizoguchi H, Tabira T, Nabeshima T. Oral vaccination with a viral vector containing Aβ cDNA attenuates age-related Aβ accumulation and memory deficits without causing inflammation in a mouse Alzheimer model. FASEB J. 2007;21:2135–2148. doi: 10.1096/fj.06-7685com. [DOI] [PubMed] [Google Scholar]
- Franklin KBJ, Paxinos G. The mouse brain: in stereotaxic coordinates. San Diego: Academic; 1997:pp. 69–89. [Google Scholar]
- Scholtzova H, Kascsak RJ, Bates KA, Boutajangout A, Kerr DJ, Meeker HC, Mehta PD, Spinner DS, Wisniewski T. Induction of Toll-like receptor 9 signaling as a method for ameliorating Alzheimer’s disease-related pathology. J Neurosci. 2009;29:1846–1854. doi: 10.1523/JNEUROSCI.5715-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee TS, Chau LY. Heme oxygenase-1 mediates the anti-inflammatory effect of interleukin-10 in mice. Nat Med. 2002;8:240–246. doi: 10.1038/nm0302-240. [DOI] [PubMed] [Google Scholar]
- Krug A, Rothenfusser S, Hornung V, Jahrsdorfer B, Blackwell S, Ballas ZK, Endres S, Krieg AM, Hartmann G. Identification of CpG oligonucleotide sequences with high induction of IFN-α/β in plasmacytoid dendritic cells. Eur J Immunol. 2001;31:2154–2163. doi: 10.1002/1521-4141(200107)31:7<2154::aid-immu2154>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. Alzheimer’s disease is a synaptic failure. Science. 2002;298:789–791. doi: 10.1126/science.1074069. [DOI] [PubMed] [Google Scholar]
- Demuro A, Mina E, Kayed R, Milton SC, Parker I, Glabe CG. Calcium dysregulation and membrane disruption as a ubiquitous neurotoxic mechanism of soluble amyloid oligomers. J Biol Chem. 2005;280:17294–17300. doi: 10.1074/jbc.M500997200. [DOI] [PubMed] [Google Scholar]
- Shankar GM, Bloodgood BL, Townsend M, Walsh DM, Selkoe DJ, Sabatini BL. Natural oligomers of the Alzheimer amyloid-β protein induce reversible synapse loss by modulating an NMDA-type glutamate receptor-dependent signaling pathway. J Neurosci. 2007;27:2866–2875. doi: 10.1523/JNEUROSCI.4970-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knobloch M, Farinelli M, Konietzko U, Nitsch RM, Mansuy IM. Aβ oligomer-mediated long-term potentiation impairment involves protein phosphatase 1-dependent mechanisms. J Neurosci. 2007;27:7648–7653. doi: 10.1523/JNEUROSCI.0395-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend M, Mehta T, Selkoe DJ. Soluble Aβ inhibits specific signal transduction cascades common to the insulin receptor pathway. J Biol Chem. 2007;282:33305–33312. doi: 10.1074/jbc.M610390200. [DOI] [PubMed] [Google Scholar]
- Manelli AM, Bulfinch LC, Sullivan PM, LaDu MJ. Aβ42 neurotoxicity in primary co-cultures: effect of apoE isoform and Abeta conformation. Neurobiol Aging. 2007;28:1139–1147. doi: 10.1016/j.neurobiolaging.2006.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lue LF, Walker DG, Rogers J. Modeling microglial activation in Alzheimer’s disease with human postmortem microglial cultures. Neurobiol Aging. 2001;22:945–956. doi: 10.1016/s0197-4580(01)00311-6. [DOI] [PubMed] [Google Scholar]
- Combs CK, Karlo JC, Kao SC, Landreth GE. β-Amyloid stimulation of microglia and monocytes results in TNF α-dependent expression of inducible nitric oxide synthase and neuronal apoptosis. J Neurosci. 2001;21:1179–1188. doi: 10.1523/JNEUROSCI.21-04-01179.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahara K, Kim HD, Jin JJ, Maxwell JA, Li L, Fukuchi K. Role of Toll-like receptor signalling in Aβ uptake and clearance. Brain. 2006;129:3006–3019. doi: 10.1093/brain/awl249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maines MD. The heme oxygenase system: a regulator of second messenger gases. Annu Rev Pharmacol Toxicol. 1997;37:517–554. doi: 10.1146/annurev.pharmtox.37.1.517. [DOI] [PubMed] [Google Scholar]
- Atamna H, Frey WH., II A role for heme in Alzheimer’s disease: heme binds amyloid β and has altered metabolism. Proc Natl Acad Sci USA. 2004;101:11153–11158. doi: 10.1073/pnas.0404349101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi M, Doré S, Ferris CD, Tomita T, Sawa A, Wolosker H, Borchelt DR, Iwatsubo T, Kim SH, Thinakaran G, Sisodia SS, Snyder SH. Amyloid precursor proteins inhibit heme oxygenase activity and augment neurotoxicity in Alzheimer’s disease. Neuron. 2000;28:461–473. doi: 10.1016/s0896-6273(00)00125-2. [DOI] [PubMed] [Google Scholar]
- Lee HY, Kim MK, Park KS, Bae YH, Yun J, Park JI, Kwak JY, Bae YS. Serum amyloid A stimulates matrix-metalloproteinase-9 up-regulation via formyl peptide receptor like-1-mediated signaling in human monocytic cells. Biochem Biophys Res Commun. 2005;330:989–998. doi: 10.1016/j.bbrc.2005.03.069. [DOI] [PubMed] [Google Scholar]
- Dalpke AH, Schäfer MK, Frey M, Zimmermann S, Tebbe J, Weihe E, Heeg K. Immunostimulatory CpG-DNA activates murine microglia. J Immunol. 2002;168:4854–4863. doi: 10.4049/jimmunol.168.10.4854. [DOI] [PubMed] [Google Scholar]
- Iliev AI, Stringaris AK, Nau R, Neumann H. Neuronal injury mediated via stimulation of microglial Toll-like receptor-9 (TLR9). FASEB J. 2004;18:412–414. doi: 10.1096/fj.03-0670fje. [DOI] [PubMed] [Google Scholar]
- Krieg AM. Therapeutic potential of Toll-like receptor 9 activation. Nat Rev Drug Discov. 2006;5:471–484. doi: 10.1038/nrd2059. [DOI] [PubMed] [Google Scholar]
- Meyer-Luehmann M, Spires-Jones TL, Prada C, Garcia-Alloza M, de Calignon A, Rozkalne A, Koenigsknecht-Talboo J, Holtzman DM, Bacskai BJ, Hyman BT. Rapid appearance and local toxicity of amyloid-β plaques in a mouse model of Alzheimer’s disease. Nature. 2008;451:720–724. doi: 10.1038/nature06616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandrekar S, Jiang Q, Lee CY, Koenigsknecht-Talboo J, Holtzman DM, Landreth GE. Microglia mediate the clearance of soluble Aβ through fluid phase macropinocytosis. J Neurosci. 2009;29:4252–4262. doi: 10.1523/JNEUROSCI.5572-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu HJ, Sawaya H, Binstadt B, Brickelmaier M, Blasius A, Gorelik L, Mahmood U, Weissleder R, Carulli J, Benoist C, Mathis D. Inflammatory arthritis can be reined in by CpG-induced DC-NK cell cross-talk. J Exp Med. 2007;204:1911–1922. doi: 10.1084/jem.20070285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens SL, Ciesielski TM, Marsh BJ, Yang T, Homen DS, Boule JL, Lessov NS, Simon RP, Stenzel-Poore MP. Toll-like receptor 9: a new target of ischemic preconditioning in the brain. J Cereb Blood Flow Metab. 2008;28:1040–1047. doi: 10.1038/sj.jcbfm.9600606. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.