Abstract
Cripto-1 is a membrane-bound protein that is highly expressed in embryonic stem cells and in human tumors. In the present study, we investigated the effect of low levels of oxygen, which occurs naturally in rapidly growing tissues, on Cripto-1 expression in mouse embryonic stem (mES) cells and in human embryonal carcinoma cells. During hypoxia, Cripto-1 expression levels were significantly elevated in mES cells and in Ntera-2 or NCCIT human embryonal carcinoma cells, as compared with cells growing with normal oxygen levels. The transcription factor hypoxia-inducible factor-1α directly regulated Cripto-1 expression by binding to hypoxia-responsive elements within the promoter of mouse and human Cripto-1 genes in mES and NCCIT cells, respectively. Furthermore, hypoxia modulated differentiation of mES cells by enhancing formation of beating cardiomyocytes as compared with mES cells that were differentiated under normoxia. However, hypoxia failed to induce differentiation of mES cells into cardiomyocytes in the absence of Cripto-1 expression, demonstrating that Cripto-1 is required for hypoxia to fully differentiate mES cells into cardiomyocytes. Finally, cardiac tissue samples derived from patients who had suffered ischemic heart disease showed a dramatic increase in Cripto-1 expression as compared with nonischemic heart tissue samples, suggesting that hypoxia may also regulate Cripto-1 in vivo.
Human and mouse Cripto-1 (CR-1/Cr-1), also known as teratocarcinoma-derived growth factor-1, are multifunctional signaling proteins that perform pivotal roles during embryonic development and tumorigenesis.1 In the developing vertebrate embryo, Cripto-1 functions as an obligatory co-receptor for the transforming growth factor-β-related ligands, including Nodal and growth and differentiation factor 1 and 3.2 Cripto-1 is essential for Nodal to signal through the serine-threonine kinase activin type I (ALK4)/activin type II receptor complex, leading to activation of the cytoplasmic receptor-activated Smad proteins, Smad-2 and Smad-3, which in a complex with the common Smad, Smad-4, translocate into the nucleus to regulate transcription of target genes.1,2 During mouse embryonic development, Cripto-1 is expressed in the inner cell mass of 4-day mouse blastocysts and in the primitive streak at day 6.5, whereas in later stages Cripto-1 expression is restricted to the developing heart, specifically in the myocardium and the truncus arteriosus.3 Mouse embryos that are deficient for Cripto-1 expression die around day 6.5 of embryogenesis as a result of defects in mesoderm formation, axial organization, and cardiac development, as evidenced by the absence of terminal myocardial differentiation genes such as α-myosin heavy chain (α-MHC) and myosin light chain 2v (MLC2v).4 In addition, experiments with embryoid bodies (EBs) derived from Cripto-1−/− mES cells have implicated Cripto-1 in cardiomyocyte induction and differentiation.5,6 Critpo-1 has also been identified as a marker for undifferentiated embryonic stem cells in vitro and is involved in maintaining the pluripotential and self-renewal capacity of mouse and human embryonic stem cells.7,8,9 In adult tissues, Cripto-1 is expressed at low levels, while levels of Cripto-1 mRNA and/or protein are elevated in several different types of human tumors, including breast and colon cancer, which can result in enhanced cell proliferation, epithelial to mesenchymal transition and tumor angiogenesis.10,11,12,13 Several molecular mechanisms that might contribute to the ability of Cripto-1 to function as an oncogenic protein have been proposed, including activation of Glypican-1/ras/c-src/mitogen-activated protein kinase and phosphatidylinositol 3-kinase/AKT pathways as well as inhibition of transforming growth factor-β1 and activin A and B signaling.14,15,16,17,18
Hypoxia, a reduction in the normal levels of oxygen tension, occurs naturally in rapidly growing tissues, such as developing embryos or solid tumors.19,20 Low oxygen tension triggers transcription of a large number of genes involved in glycolysis and angiogenesis, and this hypoxic transcriptional response is mediated primarily by hypoxia-inducible factor-1 (HIF-1), which consists of a common β subunit (HIF-1β) (also known as aryl hydrocarbon receptor nuclear translocator), and an oxygen-sensitive α-subunit (HIF-1α).21 Under normoxia, HIF-1α protein is rapidly degraded by the von-Hippel-Lindau tumor suppressor gene-mediated ubiquitin proteosome pathway.22 HIF is required for normal embryonic development, and targeted deletion of HIF-1α and aryl hydrocarbon receptor nuclear translocator genes leads to embryonic lethality.23,24 In addition, several reports have demonstrated that hypoxia has a profound effect on embryonic stem cells.21 These effects include the activation of pathways that affect maintenance of stem cell self-renewal and undifferentiated state, such as Notch-1, Oct3/4, c-Myc, and β-catenin.25 On the other hand, an increased hypoxic response in cancers promotes tumor progression by altering cellular metabolism and stimulating angiogenesis.20,26
Because Cripto-1 performs an essential role during tumor formation and angiogenesis as well as during embryonic development and these processes are also regulated by hypoxia, we investigated the effects of low oxygen levels on Cripto-1 expression in mouse embryonic stem cells and in human cancer cell lines. We further investigated the effect of hypoxia on the differentiation of embryonic stem cells, demonstrating a key role for hypoxia and Cripto-1 in the specification of embryonic stem cells toward a cardiac lineage. Finally, we evaluated whether ischemia in vivo induces Cripto-1 expression in cardiac myocytes using an animal model of cardiac ischemia-reperfusion in pigs and in human subjects who had suffered myocardial infarction (MI).
Materials and Methods
Cell Culture
Murine embryonic Rosa 26,27 Cripto-1−/−,5 and 7AC5/EYFP stem cells (American Type Culture Collection, Manassas, VA) were maintained in the undifferentiated state by culture on irradiated mouse embryonic fibroblast feeder layers in high-glucose DMEM supplemented with 15% fetal bovine serum, 0.1 mmol/L 2-mercaptoethanol, 1 mmol/L sodium pyruvate, 1× nonessential amino acids, 2 mmol/L glutamine, 100 U/ml penicillin/streptomycin, and 1000 U/ml murine leukemia inhibitor factor (LIF). Human Ntera-2 and NCCIT embryonal carcinoma cell lines were grown in McCoy’s 5A medium containing 15% fetal bovine serum or DMEM containing 10% fetal bovine serum, respectively. COS7 cells were grown as described previously.15 For hypoxic treatment of mES cells, undifferentiated mES cells growing on mitotically inactivated embryonic fibroblasts were detached with ES medium containing 1.5 mg/ml collagenase IV. The collected mES cells were then incubated with 0.25% trypsin solution and seeded at 2 × 105 cells in 60-mm plates that had been precoated with 0.1% gelatin. Experiments were performed in an incubator at 37°C under normoxia by maintaining the cells in 95% air and 5% CO2 or under hypoxia (0.5% O2) by incubating the cells in a sealed chamber (C chamber and proox model C21 gas O2/CO2 controller; BioSpherix, Redfield, NY) gassed with 0.5% O2, 5% CO2, and 94.5% N2 for 24 hours. After incubation in normoxia or hypoxia for 24 hours, cells were counted with a hemocytometer for the cell proliferation assay and analyzed by real-time PCR or Western blot. Ntera-2 or NCCIT cells were seeded in 60-mm plates at 5 × 105 cell/plate. The following day, the cells were grown under normoxic or hypoxic conditions for an additional 24 hours. All of the experiments with mES cells were performed with Rosa 26 or 7AC5/EYFP stem cells using duplicate samples.
Luciferase Assay
COS7 cells (1 × 105 cell/well in 12-well plates) were transfected with 0.5 μg of 2.5-kb CR-1 promoter in pGL3-luciferase enhancer vector (Promega, Madison, WI) and 50 ng of renilla luciferase control reporter vector (pRL-EF2) (Promega) using Fugene 6 (Roche, Nutley, NJ), as described previously.28 Six hours after transfection, medium was changed, and cells were treated with the hypoxic mimetics CoCl2 (Sigma-Aldrich, St. Louis, MO) (100 or 200 μmol/L) or desferrioxamine (Sigma-Aldrich) (65, 130, or 260 μmol/L) or incubated in a hypoxic chamber. Twenty-four hours later, the cells were lysed, and luciferase activity was measured using the Dual-Luciferase Reporter Assay Kit (Promega) according to the manufacturer’s instruction. All experiments were repeated four times with duplicate samples.
Chromatin Immunoprecipitation Assay
Chromatin immunoprecipitation (ChIP) assays were performed with the ChIP kit (Upstate Biotechnology, Lake Placid, NY) according to the manufacturer’s instructions. Briefly, NCCIT or mES cells (1 × 106 cells in 100-mm diameter plates) were incubated under normoxia or hypoxia for 24 hours, as above described. Following cross-linking using 1% formaldeyde at 37°C for 10 minutes, NCCIT and mES cells were resuspended in SDS lysis buffer (Upstate Biotechnology), and DNA was sheared to small fragments of 200 to 900 bp by sonication. The supernatant was recovered, diluted, and precleared using salmon sperm DNA/protein A agorose slurry. The recovered supernatant was incubated with either anti-HIF-1α monoclonal antibody (MAB5382; Chemicon International, Temecula, CA) or an isotype control IgG overnight at 4°C. After a 1-hour incubation in the presence of salmon sperm DNA/protein A agorose beads, the immunoprecipitated DNA/protein complexes were then washed and eluted from the beads with 1% SDS and 0.1 M NaHCO3 solution. Protein/DNA cross-links were reversed by adding 5 M NaCl at 65°C for 4 hours, and DNA was recovered by phenol/chloroform extraction and ethanol precipitation. PCR was done on the extracted DNA using human CR-1 promoter-specific primers (HRE1): forward (5′-ACTCCCACTGGAGAGTCCCA-3′) and reverse (5′-CTGAGATAGAAGTTCAGGGTCAGG-3′) for NCCIT cells; and mouse Cr-1 promoter primers (HRE1) forward: (5′-CTGGCCCAGACAGAATGCTGTATA-3′) and reverse (5′-CAAACACCCGAGTCTCTTTTCTGC-3′) for mES cells.
Quantitative Real-Time PCR
Following incubation of mES cells in normoxia or hypoxia for 24 hours, total RNA was isolated using RNeasy mini kit (Qiagen, Valencia, CA) according to manufacturer’s instruction. One microgram of total RNA was used for cDNA synthesis using the RETROscript kit (Ambion; Applied Biosystems, Foster City, CA) following the manufacturer’s protocol. Quantitative real-time PCR was performed on Startagene MX300P using Brilliant II SYBR Green PCR master mix (Stratagene, Cedar Creek, TX). Quantification of mRNA expression was performed by using the ΔΔct method (Δctsample − Δctcalibrator). Primers for mouse HIF-1α, atrial natriuretic factor, and troponin T2 were purchased from Qiagen (QuantiTech Primer assay); primers for β3-tubulin were purchased from SABiosciences (Frederick, MD). Sequences of the other primers are shown in Table 1.
Table 1.
Sequence of the Primers Used in Real-Time PCR
| Primer | Forward sequences | Reverse sequences |
|---|---|---|
| mCripto-1* | 5′-TGTTCGCAAAGAGCACTGTGG-3′ | 5′-TGAGGTCCTGGTCCATCACTTGAC-3′ |
| hCripto-1† | 5′-CACGATGTGCGCAAAGAGAA-3′ | 5′-TGACCGTGCCAGCATTTACA-3′ |
| mNodal | 5′-TGCTGAAACGATACCAACCCC-3′ | 5′-CCTGCCATTGTCCACATAAAGC-3′ |
| mMespin 1 | 5′-GCGACATGCTGGCTCTTCTA-3′ | 5′-TGGTATCACTGCCGCCTCTTCC-3′ |
| mNFM | 5′-CACATCACGGTAGAGCGCAA-3′ | 5′-CGCCGTGGAGATGTCTGTCT-3′ |
| m/h 28S | 5′-TTGAAAATCCGGGGGAGAG-3′ | 5′-ACATTGTTCCAACATGCCAG-3′ |
| mTbx5 | 5′-GGAGCCTGATTCCAAAGACA-3′ | 5′-TTCAGCCACAGTTCACGTTC-3′ |
| mMef2c | 5′-AGATACCCACAACACACCACGCGCC-3′ | 5′-CATTATCCTTCAGAGAGTCGCATGCGCTT-3′ |
| mAFP | 5′-CCACGTTAGATTCCTCCCAGTGCGT-3′ | 5′-CATACTTGTTAGAGAGTTCCGTCTC-3′ |
| mBrachyury | 5′-GACTTCGTGACGGCTGACAA-3′ | 5′-CGAGTCTGGGTGGATGTAG-3′ |
| mNkx2.5 | 5′-CAGTGGAGCTGGACAAAGCC-3′ | 5-TAGCGACGGTTGTGGAACCA-3′ |
| mGATA4 | 5′-CTGTCATCTCACTATGGGCA-3′ | 5′-CCAAGTCCGAGCAGGAATTT-3′ |
| mVEGF | 5′-TTACTGCTGTACCTCCACC-3′ | 5′-ACAGGACGGCTTGAAGATG −3′ |
| mOct3/4 | 5′-GGCGTTCTCTTTGGAAGGTGTTC-3′ | 5′-CTCGAACCACATTCCTTCTCT-3′ |
| mα-MHC | 5′-TGAAAACGGAAAGACGGTGA-3′ | 5′-TCCTTGAGGTTGTACAGCACA-3′ |
| mβ-MHC | 5′-CTACAGGCCTGGGCTTACCT-3′ | 5′-TCTCCTTCTAGACTTCCGC-3′ |
| mMLC2v | 5′-AAAGAGGCTCCAGGTCCAAT-3′ | 5′-CCTCTCTGCTTGTGTGGTCA-3′ |
m indicates mouse species.
h indicates human species.
Western Blot Analysis
Following 24 hours of incubation in normoxia or hypoxia, mES cells, Ntera-2, or NCCIT embryonal carcinoma cells were lysed and analyzed by Western blot, as described previously.14 In some experiments, mES were also incubated with the HIF-1α inhibitors emetine (0.25 or 0.5 μmol/L) (Sigma-Aldrich) or echinomycin (2.5 or 5 nmol/L) (Alexis Biochemicals, San Diego, CA) in hypoxic conditions for 24 hours. The following primary antibodies were used: anti-mouse Cr-1 goat polyclonal antibody (D-19, 1/1,000; Santa Cruz Biotechnology, Santa Cruz, CA), anti-β-actin mouse monoclonal antibody (1/10,000; Sigma-Aldrich), and anti-human-CR-1 (B3F6) mouse monoclonal antibody (1/2000; Biogen-Idec, Cambridge, MA). Densitometric analysis of the bands on the Western blots was performed with the NIH Image program (http://rbs.info.nih.gov/nih-image). Density of the bands was normalized to β-actin protein expression.
Transfection of mES Cells with Small Interfering RNA
mES cells were seeded in complete ES medium containing LIF on gelatin coated 60 mm plates (5 × 105 cell/plate). The following day the cells were transfected with 100 nmol/L ON-TARGETplus smartpool small-interfering RNA (siRNA) anti-mouse HIF-1α (Dharmacon, Chicago, IL) or with 100 nmol/L ON-TARGETplus nontargeting pool siRNA (Dharmacon) using the DharmaFECT 1 (Dharmacon) transfection reagent, as suggested by the supplier. Twenty-four hours after transfection, plates were incubated in a hypoxic chamber for an additional 24 hours. Control nontransfected mES cells were either left in regular incubator or transferred to the hypoxic chamber for 24 hours. Cells were then lysed for RNA or protein extraction and analyzed by real-time PCR or Western blot, as described above. Experiments were repeated three times with duplicate samples.
Differentiation of mES Cells into EBs
mES and Cripto-1−/− mES cells were differentiated with the hanging drop method, as described previously.8 Twenty-microliter drops, containing 400 cells, were placed on the undersurface of the lid of a 100-mm culture dish in ES medium without LIF and incubated at 37°C (day 1). The following day (day 2) of hanging drop culture, half of the plates were incubated in a hypoxic chamber for 24 hours. At day 3, cell aggregates contained in the drops were collected in 10 ml of ES medium without LIF and transferred to a bacterial petri dish, where the aggregates were grown in suspension for 3 additional days. At day 6, the aggregates were transferred onto a 60-mm tissue culture dish coated with gelatin, where they spread and differentiated. Starting from day 9, plates were scored for beating areas, which were counted. For immunofluorescent staining, the EBs were plated onto gelatin-coated chamber slides. During the differentiation process, RNA samples were collected for further analysis at days 3, 6, 9, and 13 (Rosa 26 or YEFP EBs) or at days 3, 9, and 13 for Cr-1−/− EBs. For Western blot analysis, EBs at day 13 were lysed and analyzed for sarcomeric-myosin expression (MF-20, 1/500; mouse monoclonal supernatant obtained from the Developmental Studies Hybridoma Bank, University of Iowa).
Immunofluorescent Staining of EBs
Immunofluorescent analysis of EBs at day 13 for sarcomeric-myosin and βIII-tubulin was performed as previously described8 using MF-20 monoclonal supernatant (1/50) or anti-β-tubulin subunit III (1/400; Sigma-Aldrich). EBs were counterstained with 4′,6′-diamidino-2-phenylindole, visualized with a Zeiss Axioplan 2 microscope, and images were captured using a Perkin/Elmer Ultra View camera and analyzed with IP Lab 3.9 software.
Porcine Model of MI
To induce MI, we used a porcine model of reperfused MI induced by sustained intracoronary balloon inflation (90 minutes) followed by reperfusion29 Animals were subsequently euthanized at different time points after coronary occlusion (2 hours, 3 days, 10 days, and 2 months), and hearts were harvested and fixed in formaldehyde and paraffin-embedded. As control, heart was also isolated from a control healthy animal. All animal care research protocols were approved by the Animal Care and Use Committee at the National Heart, Lung and Blood Institute.
Immunohistochemistry and Immunofluorescence of Human Cardiovascular and Porcine Heart Tissue Sections
Paraffin and frozen tissue sections from patients who suffered acute MI (AMI) were purchased from Biochain Institute (Hayward, CA): eight AMI serial human heart tissue sections and six non-AMI (congenital heart disease or hypertension) serial human heart tissue slides. Immunohistochemistry and confocal immunofluoresce analysis were performed as previously described30,31 using an anti-CR-1 rabbit polyclonal antibody (Rockland, Gilbertsville, PA) diluted at 1/100 and anti-α-sarcomeric actin mouse monoclonal antibody (1/500; Abcam). Negative controls were obtained by incubating the slides with secondary antibodies only. Slides for immunohistochemistry analysis were counterstained with hematoxylin and slides for immunofluorescence analysis were stained with 4′,6′-diamidino-2-phenylindole.
Statistical Analysis
Statistical calculations to assess significance of the differences between groups were done with the nonparametric Wilcoxon rank-sum test using the Statistical Package for Social Sciences software package, version 11.0 (SPSS, Chicago, IL). The statistical tests were two-sided, and data were considered statistically significant at P < 0.05. All experiments were repeated at least twice with duplicate or triplicate samples.
Results
Hypoxia Enhances Cr-1 mRNA and Protein Expression in mES Cells
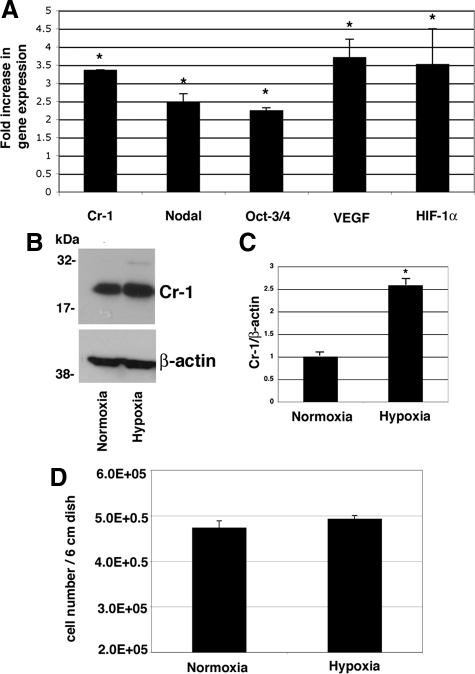
To evaluate if low oxygen levels might modulate expression of Cr-1 in mES cells, wild-type mES cells were grown under normoxic or hypoxic conditions for 24 hours. mES cells showed a statistically significant threefold increase in the levels of Cr-1 mRNA following hypoxia stimulation, as compared with mES cells grown under normoxic conditions (Figure 1A). The Cr-1 mRNA increase was simultaneously accompanied by an up-regulation in the expression of the Cr-1 ligand, Nodal, the stem cell marker Oct3/4, and the angiogenic molecule vascular endothelial growth factor (VEGF), used as control for the hypoxic stimulus (Figure 1A).32 As expected, HIF-1α mRNA expression was also increased in mES cells growing in low oxygen conditions (Figure 1A), suggesting that hypoxia induced stabilization and activation of this transcription factor. To confirm that increased Cr-1 mRNA expression in mES cells stimulated by hypoxia is also translated into an increase in Cr-1 protein expression, mES cells growing in normoxia or hypoxia for 24 hours were analyzed by Western blot using a specific anti-mouse Cr-1 antibody. As shown in Figure 1B, Cr-1 protein was highly expressed in cell lysates of mES cells, and hypoxia stimulation strongly increased the levels of Cr-1 (∼2.5-fold increase) (Figure 1C). Western blot analysis for β-actin confirmed that equal amounts of proteins were loaded (Figure 1B). No differences in cell proliferation were observed between mES cells grown in normoxia or hypoxia for 24 hours (Figure 1D). An increase in the levels of CR-1 mRNA and protein expression in response to hypoxia after 24 hours was also observed in Ntera-2 or NCCIT human embryonal carcinoma cells (Supplemental Fig. S1, see http://ajp.amjpathol.org).
Figure 1.
Hypoxia up-regulates Cr-1 mRNA and protein expression in mES cells. A: mES cells were grown in a hypoxic chamber for 24 hours, and RNA was extracted and analyzed by real-time PCR. Data were normalized to 28S expression and presented as fold increase versus the corresponding mRNA level in mES growing with normal oxygen levels. Data are representative of five experiments with triplicate samples. ∗P < 0.05. B: Western blot analysis for Cr-1 in mES cells growing in normoxia or hypoxia for 24 hours. C: Densitometric analysis of Cr-1 in mES cell lysates normalized to β-actin content. Densitometric analysis is representative of three Western blot experiments. ∗P < 0.05. D: Proliferation assay of mES cells growing in normoxia or hypoxia for 24 hours. Data are representative of three experiments with duplicate samples.
Hypoxia Enhances Cr-1 mRNA and Protein Expression through HIF-1α in mES Cells
To further define the mechanism by which hypoxia increases Cripto-1 mRNA and protein expression in stem cells and embryonal carcinoma cells, mES cells were treated under normoxic or hypoxic conditions in the absence or presence of various concentrations of two HIF-1α inhibitors, echinomycin or emetine, for 24 hours.33,34 As shown in Figure 2, A and B, hypoxia induced an increase in Cr-1 protein expression, which was inhibited by the addition of echinomycin or emetine in a dose-dependent manner. No inhibition of β-actin protein expression was observed in the presence of echinomycin or emetine (Figure 2A). To further validate the involvement of HIF-1α in the transcription of Cr-1 in mES cells, HIF-1α expression was knocked down by siRNA. Transfection of a pool of specific HIF-1α siRNAs in mES cells growing under hypoxic conditions strongly decreased HIF-1α mRNA expression as compared with mES cells stimulated by hypoxia in the presence or absence of a control nonsilencing siRNA (Figure 3A). Interestingly, down-regulation of hypoxia driven HIF-1α expression in mES cells was accompanied by an approximately 60% reduction in Cr-1 mRNA and protein expression, as compared with hypoxia-stimulated mES cells or cells transfected with control nonsilencing siRNA (Figure 3, B–D). Therefore, these results support a role of the transcription factor HIF-1α in the regulation of Cr-1 mRNA and protein expression under hypoxic conditions in mES cells.
Figure 2.
The HIF-1α inhibitors, echinomycin and emetine, interfere with induction of Cr-1 protein expression by hypoxia in mES cells. A: mES cells were incubated in a hypoxic chamber in the presence or absence of various concentrations of echinomycin (5 or 2.5 nmol/L) or emetine (0.25 or 0.5 μmol/L) for 24 hours and analyzed by Western blot using anti-Cr-1 or anti-β-actin antibodies. B: Fold difference in Cr-1 protein expression by desitometric analysis of Cr-1 in mES cell lysates normalized to β-actin content. Densitometric analysis is representative of three Western blot experiments. ∗P < 0.05, as compared with hypoxia-stimulated nontreated cells.
Figure 3.
Hypoxia up-regulates Cr-1 mRNA and protein expression through HIF-1α. Real-time PCR for HIF-1α (A) and Cr-1 (B) in mES cells transfected with HIF-1α or control siRNAs. mRNA expression was normalized to 28S expression. Data are representative of three experiments with duplicate samples. ∗P < 0.05, as compared with hypoxia-stimulated nontransfected cells. C: Western blot analysis for Cr-1 and β-actin in siRNA transfected mES cells. D: Fold difference in Cr-1 protein expression by desitometric analysis of Cr-1 in mES cell lysates normalized to β-actin content. Densitometric analysis is representative of three Western blot experiments. ∗P < 0.05, as compared with hypoxia-stimulated nontransfected cells.
HIF-1α Binds to Specific Hypoxia-Responsive Element Regions within the Mouse and Human CR-1 Promoter of mES and NCCIT Cells
HIF-1α is a major transcription factor that activates transcription of several genes involved in oxygen homeostasis, by binding to specific hypoxia-responsive elements (HREs) within the promoter of target genes.35 Using the Genomatix software application program, potential HREs were identified in both the mouse Cr-1 (HRE1 from +71 to +80 in the first intronic region of the Cr-1 gene; HRE2 from −1396 to −1388) and human CR-1 promoter (HRE1 from −776 to −788; HRE2 from −1057 to −1067). CHIP assays were then performed to examine binding of HIF-1α to the mouse and human Cripto-1 promoter HRE1 regions. NCCIT and mES cells were stimulated under hypoxic conditions for 24 hours, and a CHIP assay was then performed to measure recruitment of HIF-1α to HREs of human and mouse Cripto-1 promoter. As shown in Figure 4A, the anti-HIF-1α antibody, but not the control IgG antibody, precipitated the human and mouse Cripto-1 promoter spanning the specific HRE1 in both NCCIT and mES cells, and a significant increase in HIF-1α binding to DNA was detected in both NCCIT and mES cells stimulated by hypoxia as compared with control unstimulated cells. Next, we determined the effects of HIF-1α on the transcriptional activity of the Cripto-1 promoter by luciferase reporter gene assay.28 The hypoxic mimetics desferrioxamine or CoCl2 induced a dose-dependent increase in CR-1 promoter luciferase activity in COS7 cells (Figure 4B). Hypoxia also enhanced the CR-1 promoter activity in these cells, supporting the involvement of HIF-1α in enhancing CR-1 promoter activity through binding to a specific HRE (Figure 4B).
Figure 4.
HIF-1α directly binds to the mouse and human Cripto-1 promoter in mES and NCCIT cells and enhances CR-1 promoter luciferase activity in COS7 cells. A: CHIP assays in mES and NCCIT cells following 24 hours of incubation in a hypoxic chamber. The cross-linked protein-DNA complexes were immunoprecipitated with anti-HIF-1α antibody or with an isotype control IgG as negative control, and the purified DNA was amplified by PCR using specific primer sets that amplified the HRE region (HRE1) within the mouse and human Cripto-1 promoters. The input control and hypoxia samples are DNA samples before the immunoprecipitation that were used as loading control for the PCR. M, DNA 100 bp marker. B: Dual-luciferase assay of transiently transfected COS7 cells with a full-length 2.5-kb human CR-1-luciferase promoter. Cells were subsequently treated with the hypoxic mimetics desferrioxamine (DFX) (65, 130, or 260 μmol/L) or CoCl2 (100 or 200 μmol/L) or incubated in a hypoxic chamber for an additional 24 hours. Data are representative of four experiments with duplicate samples. ∗P < 0.001. RLU, relative luciferase units.
Hypoxia Enhances Differentiation of EBs toward Cardiomyocytes
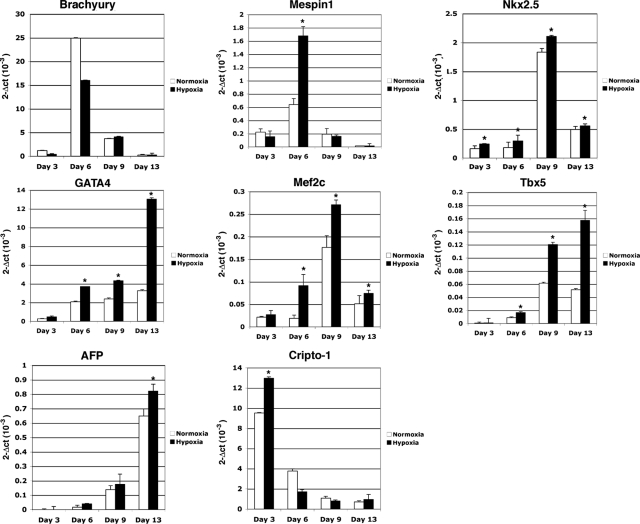
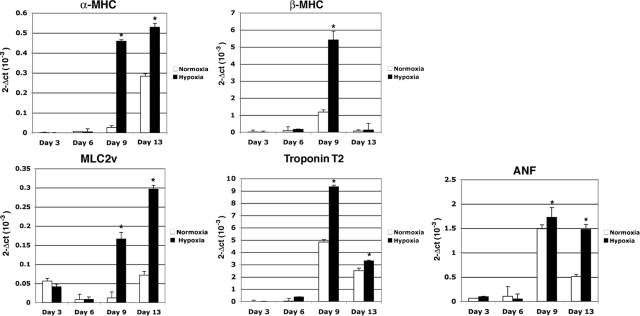
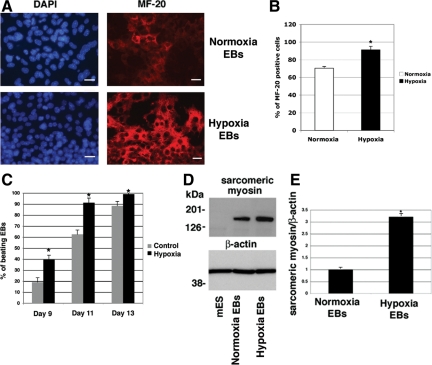
We next ascertained whether hypoxia had any significant effect on the in vitro differentiation of mES cells. We therefore established differentiation cultures by removing mES from the mouse fibroblast feeder layer in hanging drop cultures to facilitate the formation of size-controlled aggregates of EBs and cultured the cells in the absence of LIF.8 After 1 day in hanging drop culture, part of the mES cells was stimulated under hypoxic conditions for 24 hours and subsequently exposed again to normal oxygen levels until the end of the experiment. Normoxic and hypoxic growth of the mES cells gave rise to EBs of similar size and with similar morphology (data not shown). To further delineate the functional role of hypoxia in cardiogenic induction and differentiation, we examined the expression of several mesodermal and cardiac specific markers and major contractile proteins of cardiomyocytes such as MHC and MLC2v using real-time PCR. In the early phases of differentiation (days 3 and 6), commitment of ES cells toward mesodermal progenitors was documented by a robust up-regulation of the mesodermal marker Brachyury and Mespin1, one of the earliest markers of cardiac mesoderm36 (Figure 5). Specific cardiac transcription factors were detected starting approximately from day 6 with a significant increase in GATA4, Mef2c, Nkx2.5, and Tbx5 mRNA expression in both normoxia- and hypoxia-grown EBs (Figure 5). Interestingly, hypoxia treatment significantly enhanced their expression as compared with EBs formed under normoxic conditions (Figure 5). Cripto-1 expression was found to be up-regulated by hypoxia at day 3, whereas by day 6 Cr-1 mRNA levels started to decline, which is in agreement with the transient accumulation of Cr-1 during early phases of cardiomyocyte differentiation6 (Figure 5). A similar mRNA expression pattern was shown by HIF-1α (data not shown). The neuroectodermal marker NFM (neurofilament protein M) was not expressed or induced by hypoxia in EBs (data not shown), whereas α-feto protein mRNA was gradually increased during cardiomyocyte differentiation and enhanced by hypoxia (Figure 5). EBs that were stimulated by hypoxia exhibited a large increase in the mRNA expression of terminal myocardial differentiation genes such as α-MHC, β-MHC, MLC2v, troponin T2, and atrial natriuretic factor as compared with EBs grown under normoxia, suggesting that a transient hypoxic stimulation in the early phases of differentiation can significantly improve the terminal differentiation of ES cells toward cardiomyocytes (Figure 6). In fact, an approximately twofold increase in the number of contracting EBs was detected by day 9 in hypoxia-stimulated EBs as compared with EBs grown in normoxia (Figure 7C). To demonstrate that EBs express functional cardiac contractile proteins, Western blot and immunofluorescent analysis were performed after 13 days of differentiation using MF-20 mouse monoclonal antibody, which binds sarcomeric myosin. As shown in Figure 7A, a specific immunostaining was detected in both normoxia- and hypoxia-stimulated EBs at day 13 with an increase in MF-20-positive cells in hypoxia-stimulated EBs as compared with normoxia-grown EBs (Figure 7B). Western blot analysis with the MF-20 antibody confirmed an increase (approximately threefold) in the levels of sarcomeric myosin in hypoxia-stimulated EBs as compared with normoxia-stimulated EBs (Figure 7, D and E).
Figure 5.
Hypoxia enhances mesoderm and cardiac differentiation of EBs. mES cells were differentiated into cardiomyocytes using the hanging drop technique, and RNA samples were collected at days 3, 6, 9, and 13 of differentiation. Hypoxia stimulation of EBs occurred at day 2 of differentiation for 24 hours. Markers were analyzed by real-time PCR, and data were normalized to 28S expression. Data are representative of four experiments with duplicate samples. ∗P < 0.001.
Figure 6.
Hypoxia promotes cardiomyocyte differentiation of ES cells enhancing expression of terminal myocardial differentiation genes. Real-time PCR for α-MHC, β-MHC, MLC2v, troponin T2, and atrial natriuretic factor (ANF) in differentiating normoxia or hypoxia stimulated EBs. Data are representative of four experiments with duplicate samples. ∗P < 0.001.
Figure 7.
Hypoxia increases sarcomeric myosin expression in EBs. A: Immunofluorescence staining for sarcomeric-myosin using anti-MF-20 monoclonal antibody (red) of normoxia or hypoxia-stimulated EBs at 13 days of differentiation. Nuclear staining is shown in blue (4′,6′-diamidino-2-phenylindole). Scale bar = 20 μm. Magnification is ×40. B: Percentage of MF-20-positive cells/field. Values are averages of number of positive cells counted in several fields (between 5 and 8). ∗P < 0.05. C: Percentage of beating EBs counted from days 9 to 13 of differentiation. For each time point the number of EBs counted were between 35 and 50. Data are representative of three experiments. D: Western blot analysis for sarcomeric-myosin in normoxia or hypoxia stimulated EBs at day 13 of differentiation using the MF-20 antibody. Membranes were reprobed with an anti-β-actin antibody and densitometrically analyzed (E) by normalization of sarcomeric-myosin levels to β-actin content. Densitometric analysis is representative of two Western blot experiments. ∗P < 0.05.
Hypoxia Blocks Neuronal Differentiation of Cr-1−/− EBs
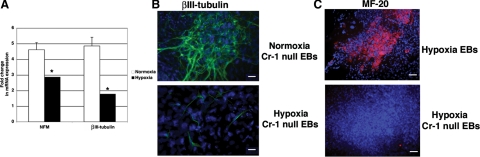
We next examined the effect of hypoxia stimulation on the differentiation of Cr-1−/− mES cells, which were generated by knockout of both copies of the wild-type Cr-1 alleles by homologous recombination.5 Previous reports have shown that Cr-1−/− mES cells lose their ability to form beating cardiomyocytes while spontaneous differentiation into neurons preferentially occurs.5,6 Using the hanging drop differentiation assay, we analyzed the effect of hypoxia stimulation on Cr-1−/−-derived EBs for their ability to differentiate into cardiomyocytes or neuroectodermal cells. The neuronal markers NFM and βIII-tubulin were both expressed in Cr-1−/− EBs grown under normoxia at day 13, whereas these neuroectodermal proteins were down-regulated in Cr-1−/− EBs grown under hypoxia at day 13, as assessed by real-time PCR (Figure 8A). Furthermore, immunofluorescence staining for βIII-tubulin in Cr-1−/−-derived EBs at day 13 showed that hypoxia reduced the number of neuronal-like cells, which failed to organize characteristic neuronal networks that were observed in EBs grown under normoxia (Figure 8B). In contrast, mesodermal and cardiac specific genes were expressed in Cr-1−/−-derived EBs and were further increased under hypoxic conditions at day 3 and/or 9 of differentiation, whereas the expression of these genes declined by day 13 (Figure 9), although the expression levels of these markers in Cr-1−/−-derived EBs were significantly lower than the levels observed in wild-type mES-derived EBs. However, beating cardiomyocytes were never observed in hypoxia-stimulated Cr-1−/− EBs, and no positive staining for MF-20 was detected in hypoxia-stimulated Cr-1−/− EBs (Figure 8C).
Figure 8.
Hypoxia interferes with neuronal differentiation of Cr-1−/− EBs. A: Real-time PCR for neuronal markers NFM and βIII-tubulin in normoxia or hypoxia-stimulated Cr-1−/− EBs at 13 days of differentiation. Data were normalized to 28S content. Data are representative of two experiments with triplicate samples. ∗P < 0.001. B: Immunofluorescence staining of βIII-tubulin (green) in normoxia or hypoxia stimulated Cr-1−/− EBs at 13 days of differentiation. 4′,6′-Diamidino-2-phenylindole staining of nuclei is shown in blue. Scale bar = 20 μm. Magnification is ×40. C: Immunofluorescence staining for MF-20 in hypoxia-stimulated wild-type and Cr-1−/− EBs. Magnification is ×20.
Figure 9.
Hypoxia enhances mesodermal and cardiac gene expression in Cr-1−/− EBs but it is not sufficient to induce cardiomyocyte differentiation. Real-time PCR for mesodermal and cardiac markers in normoxia or hypoxia stimulated Cr-1−/− EBs at different time points during differentiation. Data were normalized to 28S expression. Data are representative of two experiments with triplicate samples. ∗P < 0.001.
Expression of CR-1 in AMI
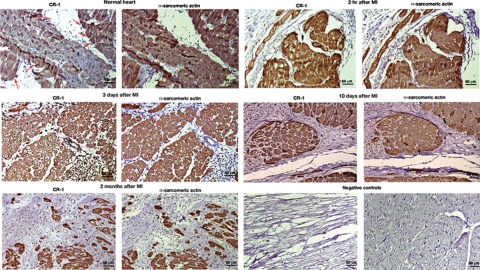
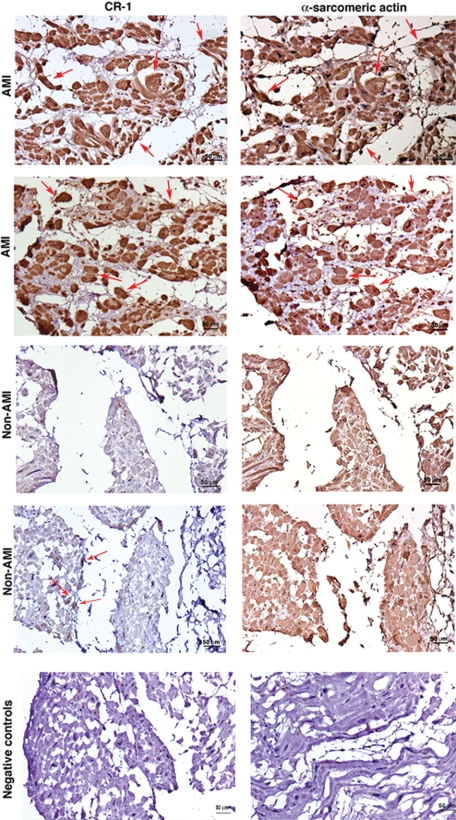
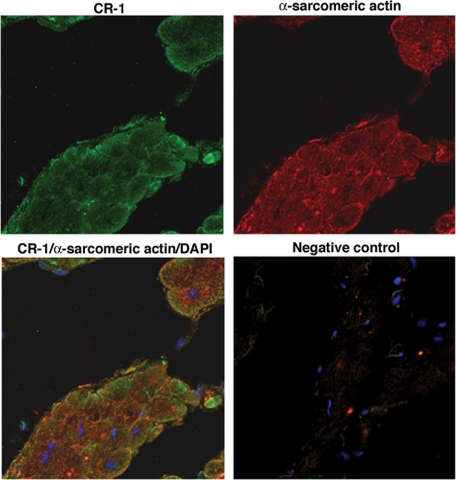
We finally evaluated whether hypoxia modulation of Cripto-1 expression in mES cells and in human embryonal carcinoma cells might also be relevant in a clinical setting. We therefore analyzed the effects of in vivo ischemia on CR-1 expression in cardiac myocytes in a porcine model of reperfused MI. Immunohistochemical analysis of porcine heart serial tissue sections for CR-1 and α-sarcomeric actin at different time points after induction of MI revealed strong CR-1 expression in cardiomyocytes as early as 2 hours after MI induction. CR-1 was also found to be expressed in cardiomyocytes in later stages of MI infarction (3 and 10 days) and in chronic MI (2 months) (Figure 10). In contrast, immunostaining of normal porcine heart tissue sections revealed a weak staining for CR-1 only in a subpopulation of cardiomyocytes (Figure 10). Finally, we analyzed CR-1 expression in human ischemic and nonischemic heart disease tissue samples. Results from immunohistochemical staining of serial heart tissue sections with CR-1 and α-sarcomeric actin showed strong expression of CR-1 in cardiomyocytes of all heart tissue sections of analyzed AMI (two different patients are shown in Figure 11; the other patients are not shown). On the contrary, heart tissue sections from patients with nonischemic related heart diseases showed very weak staining for CR-1 in a subpopulation of cardiomyocytes (Figure 11). Immunofluorescent labeling with CR-1 and α-sarcomeric actin in frozen heart tissue sections from patients with ischemic heart disease showed a punctate membrane staining pattern for CR-1 and a cytoplasmic staining pattern for α-sarcomeric actin in cardiomyocytes (Figure 12). Co-staining with CR-1 and α-sarcomeric actin showed co-expression of CR-1 and α-sarcomeric actin by cardiac cells (Figure 12). In conclusion, heart ischemia in vivo can enhance CR-1 expression in cardiac myocytes.
Figure 10.
Cripto-1 cardiac expression in an animal model of MI. Immunohistochemistry for CR-1 and α-sarcomeric actin in serial heart tissue sections of a porcine model of myocardial ischemia-reperfusion. Heart tissue samples were collected after 2 hours, 3 days, 10 days, and 2 months of induced MI. A normal heart sample was harvested from a healthy pig. Red arrows in the normal heart are pointing to some of the cells that show positive staining for CR-1. Negative controls were obtained by omitting the primary antibodies only. Scale bar = 50 μm. Original magnifications, ×20.
Figure 11.
CR-1 expression is increased in human AMI. Immunohistochemistry for CR-1 and α-sarcomeric actin in AMI and non-AMI human heart serial tissue sections. The figure shows two different patients with AMI and two different patients with non-AMI. Red arrows in AMI samples are pointing to some of the cells that are positive for both CR-1 and α-sarcomeric actin. Negative controls were obtained by omitting the primary antibodies only. Scale bar = 50 μm. Original magnification, ×20.
Figure 12.
Immunofluorescent analysis for CR-1 and α-sarcomeric actin in heart frozen tissue section from patients with ischemic heart disease. Negative control was obtained by incubating the slides with secondary antibodies only. Fluorescence images were acquired by laser scanning confocal microscopy.
Discussion
The Activin/Nodal/Cripto-1 signaling performs a critical role in maintaining ES cells in an undifferentiated state and regulates their self-renewal and pluripotency by directly controlling expression of a key pluripotency factor Nanog in human ES cells and in mouse epiblast stem cells.7,9,37 However, Nodal/Cripto-1 signaling can also drive differentiation of mES cells toward functional beating cardiomyocytes, suggesting that Nodal/Cripto-1 signaling can both maintain pluripotency of mES cells through Nanog and induce their differentiation toward mesoendoderm while preventing neuroectoderm differentiation.6,37,38 The microenviroment in which stem cells reside might play a role in converting Nodal/Cripto-1 signaling into an inductive signal of differentiation and low levels of oxygen present in stem cell “niches” might influence stem cell function and differentiation.19 Hypoxia also occurs in a number of pathological conditions, particularly when rapid tissue growth exceeds blood supply, such as during tumorigenesis.35 Similar to hypoxia, Cripto-1 is also involved in cancer progression by stimulating cell proliferation, motility, invasion, epithelial mesenchymal transition, tumor angiogenesis, and mammary gland tumorigenesis.1 The present study was designed to ascertain if there is a potential functional interaction between Cripto-1 and hypoxia during mES cells differentiation and in carcinoma cells. We found that hypoxia, through HIF-1α activation and stabilization, directly upregulates Cripto-1 expression in mES cells and in two human embryonal carcinoma (EC) cell lines. Similar to Cr-1, the stem cell marker Oct3/4 was also found to be increased under hypoxic conditions in undifferentiated mES cells, in agreement with previous reports demonstrating regulation of Oct3/4 expression by HIF-2α.39 Notably, transient Oct3/4 upregulation is essential for mesodermal and cardiac commitment of ES cells.40
In addition, we further demonstrated that HIF-1α is directly involved in regulating Cripto-1 expression in undifferentiated mES cells, by binding to a hypoxia responsive element within the promoter sequences of the mouse and human Cripto-1 genes. Interestingly, the mouse HRE1 was found to be located in the first intronic region of the mouse Cr-1 gene, where TCF/LEF binding elements (TBE) that are regulated by canonical Wnt signaling have also been identified,41 suggesting clustering of regulatory elements within the first intronic region of the mouse Cripto-1 gene.
Furthermore, a short and transient hypoxic stimulation of mES cells at an early time point after the initiation of EB differentiation, when Cripto-1 is highly expressed in mesoendoderm cells,6 significantly enhanced cardiomyocyte formation, as demonstrated by an increase in the number of beating cardiomyocytes that were expressing contractile cardiac proteins in comparison with EBs grown under normoxic conditions. In the early phases of EB differentiation, hypoxia preferentially increased expression of Mespin1, suggesting a more restricted effect of hypoxia on specific cardiac mesodermal markers. Similarly, the cardiac specific transcription factors, such as GATA4, Mef2c, Nkx2.5, and Tbx5 were all expressed in differentiating EBs, and they were significantly enhanced by hypoxic stimulation. GATA4, Mef2c, Nkx2.5, and Tbx5 are involved in the transcriptional control of cardiac contractile proteins, such as MHC and MLC.42 Their preferential increase in hypoxia-treated EBs might explain the enhanced differentiation of EBs exposed to hypoxia toward a terminal cardiac differentiation program, as evidenced by a twofold increase in the number of hypoxia-treated beating EBs in the early phases of contractile cardiomyocyte appearance. From days 11 to 13, the difference in the number of beating EBs between hypoxia and normoxia EBs declined, suggesting that hypoxia accelerates formation of contracting EBs (Figure 7C). Notably, although hypoxia stimulation of wild-type ES cells enhanced cardiomyocyte differentiation, in Cr-1−/− EBs, hypoxia inhibited neuronal differentiation. Interestingly, mesodermal and cardiac-specific genes were also expressed in Cr-1−/− EBs but at levels significantly lower than levels detected in wild-type derived EBs (compare 2-Δct(10−3) values in Figures 5 and 6 with 2-Δct(10−3) values in Figure 9). This observation is in agreement with previous reports demonstrating that Cr-1−/− EBs, although preferentially differentiating into neuroectodermal cell types in vitro, can still express mesoendoderm markers.5,43 Hypoxia was also found to enhance expression of mesoderm cardiac markers in Cr-1−/− EBs, in agreement with studies demonstrating a direct regulation of cardiac transcription factors, such as Nkx2.5, Mef2c, and Tbx5, by hypoxia.44,45 Therefore, hypoxia might, at least partially, redirect Cr-1−/− EBs toward a mesodermal fate. However, hypoxia in the absence of Cr-1 expression in Cr-1−/− EBs cannot completely rescue cardiac myogenesis, as evidenced by the lack of MF-20-positive cells (Figure 8C) and the lack of beating cardiomyocytes.
We also have demonstrated for the first time that Cripto-1 is induced in cardiomyocytes by ischemia in vivo in an animal model of myocardial ischemia-reperfusion injury and in human ischemic heart failure. In contrast, Cripto-1 is expressed at very low levels in normal pig heart and nonischemic human heart, suggesting that ischemic injury can trigger Cripto-1 expression. However, since Cripto-1 can be either cell-membrane associated or secreted,31 it is difficult to discern by immunohistochemistry the precise cell types that are expressing Cripto-1 in cardiac tissues. Confocal microscopy analysis of human infarct heart frozen tissue sections showed co-expression of CR-1 and α-sarcomeric actin proteins in the same subset of cardiac cells (Figure 12). A more refined examination of this issue by in situ hybridization would determine whether Cripto-1 is expressed in a subpopulation of differentiated or undifferentiated cardiomyocytes, smooth muscle cells, or endothelial cells. Nevertheless, sustained expression for Cripto-1 was also detected in later stages of MI (chronic MI; Figure 10), suggesting a role for Cripto-1 in both acute and chronic ischemic heart injury. It is possible that Cripto-1 in the ischemic heart might have either a local function and/or might be released into the circulation, thereby regulating cardiovascular functions aimed at maintaining circulatory homeostasis. In fact, in agreement with our findings, Cripto-1 has been recently shown to stimulate cardiac cellular proliferation and enhance ventricular function in ischemic cardiomyopathy, suggesting a role for Cripto-1 in myocardial repair.46 Furthermore, we have previously shown that soluble Cripto-1 can be detected in the plasma of colon and breast cancer patients functioning as an early detection marker for cancer.47 Therefore, assessment of Cripto-1 plasma levels might prove also useful in detecting cardiac ischemia and provide a method for monitoring the progression of this condition in patients.
In conclusion, we have demonstrated that hypoxia through HIF-1α directly regulates Cr-1 expression in mES cells, accelerating and enhancing cardiomyocyte differentiation of embryonic stem cells. Although hypoxia is able to promote cardiac mesoderm specification of Cr-1-deficient mES cells, it is not sufficient to fully differentiate these cells into beating cardiomyocytes. Finally, the possibility to dramatically enhance cardiomyocyte formation from undifferentiated embryonic stem cells by locally lowering oxygen levels might have a therapeutic potential for degenerative cardiovascular diseases or ischemic heart failure.
Supplementary Material
Footnotes
Address reprint requests to David S. Salomon, Ph.D., National Cancer Institute, 37 Convent Drive, Building 37, Room 1118, Bethesda, MD 20892. E-mail: Salomond@mail.nih.gov.
Supported by Intramural Research program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research, by a grant from the Associazione Italiana per la Ricerca sul Cancro (to G.M.), and by a grant of the Faculty of Medicine, University of Glaskow (to C.B.).
Supplemental material for this article can be found on http://ajp.amjpathol.org.
Current address of L.S.: Children’s Memorial Research Center, Robert H. Lurie Comprehensive Cancer Center, Northwestern University Feinberg School of Medicine, Chicago, IL.
References
- Bianco C, Strizzi L, Normanno N, Khan N, Salomon DS. Cripto-1: an oncofetal gene with many faces. Curr Top Dev Biol. 2005;67:85–133. doi: 10.1016/S0070-2153(05)67003-2. [DOI] [PubMed] [Google Scholar]
- Schier AF. Nodal signaling in vertebrate development. Annu Rev Cell Dev Biol. 2003;19:589–621. doi: 10.1146/annurev.cellbio.19.041603.094522. [DOI] [PubMed] [Google Scholar]
- Dono R, Scalera L, Pacifico F, Acampora D, Persico MG, Simeone A. The murine cripto gene: expression during mesoderm induction and early heart morphogenesis. Development. 1993;118:1157–1168. doi: 10.1242/dev.118.4.1157. [DOI] [PubMed] [Google Scholar]
- Xu C, Liguori G, Persico MG, Adamson ED. Abrogation of the Cripto gene in mouse leads to failure of postgastrulation morphogenesis and lack of differentiation of cardiomyocytes. Development. 1999;126:483–494. doi: 10.1242/dev.126.3.483. [DOI] [PubMed] [Google Scholar]
- Xu C, Liguori G, Adamson ED, Persico MG. Specific arrest of cardiogenesis in cultured embryonic stem cells lacking Cripto-1. Dev Biol. 1998;196:237–247. doi: 10.1006/dbio.1998.8862. [DOI] [PubMed] [Google Scholar]
- Parisi S, D'Andrea D, Lago CT, Adamson ED, Persico MG, Minchiotti G. Nodal-dependent Cripto signaling promotes cardiomyogenesis and redirects the neural fate of embryonic stem cells. J Cell Biol. 2003;163:303–314. doi: 10.1083/jcb.200303010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minchiotti G. Nodal-dependant Cripto signaling in ES cells: from stem cells to tumor biology. Oncogene. 2005;24:5668–5675. doi: 10.1038/sj.onc.1208917. [DOI] [PubMed] [Google Scholar]
- Minchiotti G, Parisi S, Persico MG. Cripto signaling in differentiating embryonic stem cells. Methods Mol Biol. 2006;329:151–169. doi: 10.1385/1-59745-037-5:151. [DOI] [PubMed] [Google Scholar]
- Assou S, Lecarrour T, Tondeur S, Strom S, Gabelle A, Marty S, Nadal L, Pantesco V, Reme T, Hugnot JP, Gasca S, Hovatta O, Hamamah S, Klein B, De Vos J. A meta-analysis of human embryonic stem cells transcriptome integrated into a web-based expression atlas. Stem Cells. 2007;25:961–973. doi: 10.1634/stemcells.2006-0352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianco C, Strizzi L, Ebert A, Chang C, Rehman A, Normanno N, Guedez L, Salloum R, Ginsburg E, Sun Y, Khan N, Hirota M, Wallace-Jones B, Wechselberger C, Vonderhaar BK, Tosato G, Stetler-Stevenson WG, Sanicola M, Salomon DS. Role of human cripto-1 in tumor angiogenesis. J Natl Cancer Inst. 2005;97:132–141. doi: 10.1093/jnci/dji011. [DOI] [PubMed] [Google Scholar]
- Normanno N, Bianco C, De Luca A, Salomon DS. The role of EGF-related peptides in tumor growth. Front Biosci. 2001;6:D685–D707. doi: 10.2741/normano. [DOI] [PubMed] [Google Scholar]
- Bianco C, Normanno N, Salomon DS, Ciardiello F. Role of the cripto (EGF-CFC) family in embryogenesis and cancer. Growth Factors. 2004;22:133–139. doi: 10.1080/08977190410001723290. [DOI] [PubMed] [Google Scholar]
- Strizzi L, Bianco C, Normanno N, Seno M, Wechselberger C, Wallace-Jones B, Khan NI, Hirota M, Sun Y, Sanicola M, Salomon DS. Epithelial mesenchymal transition is a characteristic of hyperplasias and tumors in mammary gland from MMTV-Cripto-1 transgenic mice. J Cell Physiol. 2004;201:266–276. doi: 10.1002/jcp.20062. [DOI] [PubMed] [Google Scholar]
- Bianco C, Strizzi L, Rehman A, Normanno N, Wechselberger C, Sun Y, Khan N, Hirota M, Adkins H, Williams K, Margolis RU, Sanicola M, Salomon DS. A Nodal- and ALK4-independent signaling pathway activated by Cripto-1 through Glypican-1 and c-Src. Cancer Res. 2003;63:1192–1197. [PubMed] [Google Scholar]
- Bianco C, Adkins HB, Wechselberger C, Seno M, Normanno N, De Luca A, Sun Y, Khan N, Kenney N, Ebert A, Williams KP, Sanicola M, Salomon DS. Cripto-1 activates nodal- and ALK4-dependent and -independent signaling pathways in mammary epithelial Cells. Mol Cell Biol. 2002;22:2586–2597. doi: 10.1128/MCB.22.8.2586-2597.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray PC, Shani G, Aung K, Kelber J, Vale W. Cripto binds transforming growth factor β (TGF-β) and inhibits TGF-β signaling. Mol Cell Biol. 2006;26:9268–9278. doi: 10.1128/MCB.01168-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray PC, Harrison CA, Vale W. Cripto forms a complex with activin and type II activin receptors and can block activin signaling. Proc Natl Acad Sci USA. 2003;100:5193–5198. doi: 10.1073/pnas.0531290100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adkins HB, Bianco C, Schiffer SG, Rayhorn P, Zafari M, Cheung AE, Orozco O, Olson D, De Luca A, Chen LL, Miatkowski K, Benjamin C, Normanno N, Williams KP, Jarpe M, LePage D, Salomon D, Sanicola M. Antibody blockade of the Cripto CFC domain suppresses tumor cell growth in vivo. J Clin Invest. 2003;112:575–587. doi: 10.1172/JCI17788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon MC, Keith B. The role of oxygen availability in embryonic development and stem cell function. Nat Rev Mol Cell Biol. 2008;9:285–296. doi: 10.1038/nrm2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao D, Johnson RS. Hypoxia: a key regulator of angiogenesis in cancer. Cancer Metastasis Rev. 2007;26:281–290. doi: 10.1007/s10555-007-9066-y. [DOI] [PubMed] [Google Scholar]
- Keith B, Simon MC. Hypoxia-inducible factors, stem cells, and cancer. Cell. 2007;129:465–472. doi: 10.1016/j.cell.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenza GL. Hypoxia-inducible factor 1 (HIF-1) pathway. Sci STKE. 2007;2007:cm8. doi: 10.1126/stke.4072007cm8. [DOI] [PubMed] [Google Scholar]
- Iyer NV, Kotch LE, Agani F, Leung SW, Laughner E, Wenger RH, Gassmann M, Gearhart JD, Lawler AM, Yu AY, Semenza GL. Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1α. Genes Dev. 1998;12:149–162. doi: 10.1101/gad.12.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maltepe E, Schmidt JV, Baunoch D, Bradfield CA, Simon MC. Abnormal angiogenesis and responses to glucose and oxygen deprivation in mice lacking the protein ARNT. Nature. 1997;386:403–407. doi: 10.1038/386403a0. [DOI] [PubMed] [Google Scholar]
- Barnhart BC, Simon MC. Metastasis and stem cell pathways. Cancer Metastasis Rev. 2007;26:261–271. doi: 10.1007/s10555-007-9053-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulda S, Debatin KM. HIF-1-regulated glucose metabolism: a key to apoptosis resistance? Cell Cycle. 2007;6:790–792. doi: 10.4161/cc.6.7.4084. [DOI] [PubMed] [Google Scholar]
- Zambrowicz BP, Imamoto A, Fiering S, Herzenberg LA, Kerr WG, Soriano P. Disruption of overlapping transcripts in the ROSA β geo 26 gene trap strain leads to widespread expression of β-galactosidase in mouse embryos and hematopoietic cells. Proc Natl Acad Sci USA. 1997;94:3789–3794. doi: 10.1073/pnas.94.8.3789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancino M, Strizzi L, Wechselberger C, Watanabe K, Gonzales M, Hamada S, Normanno N, Salomon DS, Bianco C. Regulation of human Cripto-1 gene expression by TGF-β1 and BMP-4 in embryonal and colon cancer cells. J Cell Physiol. 2008;215:192–203. doi: 10.1002/jcp.21301. [DOI] [PubMed] [Google Scholar]
- Arai AE, Kasserra CE, Territo PR, Gandjbakhche AH, Balaban RS. Myocardial oxygenation in vivo: optical spectroscopy of cytoplasmic myoglobin and mitochondrial cytochromes. Am J Physiol. 1999;277:H683–H697. doi: 10.1152/ajpheart.1999.277.2.H683. [DOI] [PubMed] [Google Scholar]
- Wechselberger C, Strizzi L, Kenney N, Hirota M, Sun Y, Ebert A, Orozco O, Bianco C, Khan NI, Wallace-Jones B, Normanno N, Adkins H, Sanicola M, Salomon DS. Human Cripto-1 overexpression in the mouse mammary gland results in the development of hyperplasia and adenocarcinoma. Oncogene. 2005;24:4094–4105. doi: 10.1038/sj.onc.1208417. [DOI] [PubMed] [Google Scholar]
- Watanabe K, Bianco C, Strizzi L, Hamada S, Mancino M, Bailly V, Mo W, Wen D, Miatkowski K, Gonzales M, Sanicola M, Seno M, Salomon DS. Growth factor induction of cripto-1 shedding by GPI-phospholipase D and enhancement of endothelial cell migration. J Biol Chem. 2007;282:31643–31655. doi: 10.1074/jbc.M702713200. [DOI] [PubMed] [Google Scholar]
- Brusselmans K, Bono F, Collen D, Herbert JM, Carmeliet P, Dewerchin M. A novel role for vascular endothelial growth factor as an autocrine survival factor for embryonic stem cells during hypoxia. J Biol Chem. 2005;280:3493–3499. doi: 10.1074/jbc.M406613200. [DOI] [PubMed] [Google Scholar]
- Zhou YD, Kim YP, Mohammed KA, Jones DK, Muhammad I, Dunbar DC, Nagle DG. Terpenoid tetrahydroisoquinoline alkaloids emetine, klugine, and isocephaeline inhibit the activation of hypoxia-inducible factor-1 in breast tumor cells. J Nat Prod. 2005;68:947–950. doi: 10.1021/np050029m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong D, Park EJ, Stephen AG, Calvani M, Cardellina JH, Monks A, Fisher RJ, Shoemaker RH, Melillo G. Echinomycin, a small-molecule inhibitor of hypoxia-inducible factor-1 DNA-binding activity. Cancer Res. 2005;65:9047–9055. doi: 10.1158/0008-5472.CAN-05-1235. [DOI] [PubMed] [Google Scholar]
- Mabjeesh NJ, Amir S. Hypoxia-inducible factor (HIF) in human tumorigenesis. Histol Histopathol. 2007;22:559–572. doi: 10.14670/HH-22.559. [DOI] [PubMed] [Google Scholar]
- David R, Brenner C, Stieber J, Schwarz F, Brunner S, Vollmer M, Mentele E, Muller-Hocker J, Kitajima S, Lickert H, Rupp R, Franz WM. MesP1 drives vertebrate cardiovascular differentiation through Dkk-1-mediated blockade of Wnt-signalling. Nat Cell Biol. 2008;10:338–345. doi: 10.1038/ncb1696. [DOI] [PubMed] [Google Scholar]
- Vallier L, Mendjan S, Brown S, Chng Z, Teo A, Smithers LE, Trotter MW, Cho CH, Martinez A, Rugg-Gunn P, Brons G, Pedersen RA. Activin/Nodal signalling maintains pluripotency by controlling Nanog expression. Development. 2009;136:1339–1349. doi: 10.1242/dev.033951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallier L, Reynolds D, Pedersen RA. Nodal inhibits differentiation of human embryonic stem cells along the neuroectodermal default pathway. Dev Biol. 2004;275:403–421. doi: 10.1016/j.ydbio.2004.08.031. [DOI] [PubMed] [Google Scholar]
- Covello KL, Kehler J, Yu H, Gordan JD, Arsham AM, Hu CJ, Labosky PA, Simon MC, Keith B. HIF-2α regulates Oct-4: effects of hypoxia on stem cell function, embryonic development, and tumor growth. Genes Dev. 2006;20:557–570. doi: 10.1101/gad.1399906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeineddine D, Papadimou E, Chebli K, Gineste M, Liu J, Grey C, Thurig S, Behfar A, Wallace VA, Skerjanc IS, Puceat M. Oct-3/4 dose dependently regulates specification of embryonic stem cells toward a cardiac lineage and early heart development. Dev Cell. 2006;11:535–546. doi: 10.1016/j.devcel.2006.07.013. [DOI] [PubMed] [Google Scholar]
- Hamada S, Watanabe K, Hirota M, Bianco C, Strizzi L, Mancino M, Gonzales M, Salomon DS. β-Catenin/TCF/LEF regulate expression of the short form human Cripto-1. Biochem Biophys Res Commun. 2007;355:240–244. doi: 10.1016/j.bbrc.2007.01.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunwoodie SL. Combinatorial signaling in the heart orchestrates cardiac induction, lineage specification and chamber formation. Semin Cell Dev Biol. 2007;18:54–66. doi: 10.1016/j.semcdb.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Sonntag KC, Simantov R, Bjorklund L, Cooper O, Pruszak J, Kowalke F, Gilmartin J, Ding J, Hu YP, Shen MM, Isacson O. Context-dependent neuronal differentiation and germ layer induction of Smad4−/− and Cripto−/− embryonic stem cells. Mol Cell Neurosci. 2005;28:417–429. doi: 10.1016/j.mcn.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Nagao K, Taniyama Y, Kietzmann T, Doi T, Komuro I, Morishita R. HIF-1α signaling upstream of NKX2.5 is required for cardiac development in Xenopus. J Biol Chem. 2008;283:11841–11849. doi: 10.1074/jbc.M702563200. [DOI] [PubMed] [Google Scholar]
- Krishnan J, Ahuja P, Bodenmann S, Knapik D, Perriard E, Krek W, Perriard JC. Essential role of developmentally activated hypoxia-inducible factor 1α for cardiac morphogenesis and function. Circ Res. 2008;103:1139–1146. doi: 10.1161/01.RES.0000338613.89841.c1. [DOI] [PubMed] [Google Scholar]
- Cohen JE, Atluri P, Heisinger W, McCormick R, Laporte C, Smith MJ, Hsu VM, Patel AB, Woo YJ. Abstract 4302: Cripto stimulates myocardial proliferation and enhances ventricular function in ischemic cardiomyopathy. Circulation. 2008;118:S_862–S_863. [Google Scholar]
- Bianco C, Strizzi L, Mancino M, Rehman A, Hamada S, Watanabe K, De Luca A, Jones B, Balogh G, Russo J, Mailo D, Palaia R, D'Aiuto G, Botti G, Perrone F, Salomon DS, Normanno N. Identification of cripto-1 as a novel serologic marker for breast and colon cancer. Clin Cancer Res. 2006;12:5158–5164. doi: 10.1158/1078-0432.CCR-06-0274. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.