Abstract
The role of the vitronectin receptor (αvβ3-integrin) as a tumor promoter seems well established, and, consequently, therapies that block this integrin are currently in clinical testing. We undertook the current study to determine whether αvβ3-integrin is an appropriate target in ovarian cancer treatment. Expression of β3-integrin in SKOV3ip1 ovarian cancer cells led to the overexpression of αvβ3-integrin on the cell surface and increased adhesion. However, αvβ3-integrin-overexpressing cells showed impaired invasion, protease expression, and colony formation. These results were recapitulated in xenograft studies: αvβ3-integrin-expressing cells showed increased adhesion to mouse peritoneum, but the overall number of metastatic nodules (105 versus 68 tumors) and tumor weight were significantly lower than those in the parental SKOV3ip1 cells. The αvβ3-integrin-overexpressing cells had a decreased proliferation rate mediated by inhibition of cyclin B1 and induction of phospho-Cdc2 and p53 expression, consistent with a G2M cell cycle arrest. Confirming the above results, inhibition of β3-integrin in cultured or primary OvCa cells decreased adhesion but increased invasion and proliferation. Patients with tumors expressing high β3-integrin had significantly better disease-free and overall survival (52 months versus 27 months, P < 0.05). This study shows that αvβ3-integrin expression on tumor cells actually slows tumor progression and acts as a tumor suppressor. Therefore, the vitronectin receptor might not be an appropriate therapeutic target in ovarian cancer.
Ovarian cancer is the leading cause of death from pelvic gynecologic cancer in women living in industrialized countries.1 It generally begins with the malignant transformation of the ovarian surface epithelium, a single continuous layer of epithelial cells surrounding the ovary, although recently an origin in the distal fallopian tube has been emphasized.2,3 The metastasis of ovarian tumors differs from that of other epithelial malignancies in that ovarian cancer spreads predominantly within the abdominal cavity and rarely to distant sites. The most common metastatic sites of ovarian cancer are the infracolic omentum, the peritoneal surfaces (e.g., diaphragm and the small bowel mesentery), and the surface of the abdominal organs inside the peritoneal cavity (e.g., sigmoid and bladder serosa). These sites are all covered by mesothelium and therefore possess a microenvironment similar to that of the ovary.4 It is worthy of note that the mesothelial and ovarian epithelial cells share a common embryologic origin in the coelomic mesothelium.
An early step in peritoneal metastasis is the binding of ovarian cancer cells to mesothelial cells and exposed proteins of the extracellular matrix (ECM) via cell surface-based integrins.5 Integrins are a group of transmembrane glycoproteins consisting of α- and β-subunits, which are integrated across the plasma membrane and provide a link between the ECM and the cytoskeletal molecules within the cells. On interaction of the cell with its extracellular environment, integrins mediate cell adhesion and migration and activate intracellular signaling pathways modulating cell survival and apoptosis.5 Altered expression of integrins, in the form of down- or up-regulated expression, has been detected in the majority of malignant tumors but varies considerably according to the origin of the neoplasm. One of the most diversely functional integrins that is consistently overexpressed in epithelial tumors is αvβ3-integrin, the classic vitronectin receptor. High expression of αvβ3-integrin and β3-integrin correlates with metastasis to the bone in breast cancer,6 defines the conversion from radial to vertical growth in melanoma,7 and is clearly involved in tumor angiogenesis because it is highly expressed on endothelial cells.8
However, the tumor-promoting role of αvβ3-integrins has been challenged by studies in which αvβ3-integrin expression leads to suppression,9,10 rather than enhancement of tumor growth. Expression of β3-integrin leading to overexpression of αvβ3-integrin in human ras transformed astrocytes impairs the colonization of the brain by tumor cells and slows tumor growth. The αvβ3-integrin-overexpressing tumors have a defective vasculature with fewer pericytes, leading to hypoxia.11 β3-Integrin has been shown to be consistently overexpressed in some ovarian cancer cell lines and primary ovarian cancer cells.12,13,14 Carreiras et al15 evaluated the expression of β3-integrin in 19 human ovarian tumors and found that it is more highly expressed in well differentiated cancers than it is in high-grade cancers. This finding was confirmed by the same group in a larger study of 38 patients.16 The expression of β3-integrin is clearly not limited to ovarian cancer. Borderline tumors17 and normal ovarian epithelial cells also express β3-integrin and, indeed, require it for migration18 and adhesion to fibronectin.14
Although these studies have characterized the expression pattern of αvβ3-integrin on tumor and endothelial cells in vitro and defined its function in adhesion and in angiogenesis,14,19 it is not clear how its expression contributes to ovarian tumor biology. However, because adhesion is the first step of ovarian cancer metastasis,3 clinical trials in ovarian cancer using the αvβ3-integrin inhibitors20 are being considered. Therefore, our aim was to gain a broader understanding of the role of αvβ3-integrin overexpression in ovarian cancer both in vitro and in vivo. Although our results confirm that β3-integrin increases adhesion of ovarian tumor cells, it also shows that it significantly suppresses tumor growth and metastasis. These effects are mediated by a reduced proliferation rate caused by an arrest in the G2M phase of the cell cycle and increased apoptosis. Consistent with the data obtained from cell lines, we found that patients with high β3-integrin expression have an improved prognosis.
Materials and Methods
Reagents and Cell Lines
The human ovarian cancer cell line SKOV3ip1 was first described by Dr. J. Price,21 and CaOV3 was first described by Dr. T. Strobel and Dr. S. A. Cannistra.13 The human primary ovarian cancer cells, MONTY-1, were established from an omental metastasis of a patient with ovarian cancer, and passages 4 to 7 were used for experiments. The β3-integrin antibody (N-20) for immunoblotting was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). The αvβ3-integrin functional blocking antibody (LM 609) and the β3-integrin antibodies for immunoblotting (B3A) and immunohistochemistry (SAP) were from Chemicon International (Billerica, MA). The control and the β3-integrin small interfering RNAs (siRNAs) were purchased from Ambion (Austin, TX). Phospho-Cdc2 antibodies (catalog no. 4539) were from Cell Signaling Technology, Inc. (Danvers, MA). Fibronectin and vitronectin were purchased from BD Biosciences (Bedford, MA). Matrigel and the β-actin (CP01) and p53 (Ab-7) antibodies were purchased from EMD Biosciences (San Diego, CA). The Ki-67 (Sp6) antibody was from LabVision (Fremont, CA). The urokinase antibody (rabbit polyclonal) was from Abcam Inc. (Cambridge, MA).
Transfections
SKOV3ip1 cells were stably transfected with empty vector, pBabe-puro, and the pBabe-puro-β3-integrin plasmid22 using the SuperFect transfection reagent (Qiagen, Valencia, CA). Twenty-four hours after transfection, the medium was changed, and cells were selected with 0.5 μg/ml puromycin for 14 days. Resistant clones were picked for expansion and characterization. β3-Integrin expression was determined by Western blot, flow cytometry, confocal microscopy, and RT-PCR and confirmed periodically. CAOV3 and MONTY-1 cells were transfected with a validated sequence of siRNAs targeting β3-integrin. A nonspecific (scrambled sequence) siRNA was used as negative control. CAOV3 (5 × 105) and MONTY-1 (2 × 105) cells were transfected in a six-well plate using the siPORT NeoFX transfection reagent (Ambion), and knockdown of β3-integrin was confirmed by immunoblotting.
Adhesion Assays of Ovarian Cancer Cells to Fibroblasts, the Three-Dimensional Omentum Culture, Human Omentum, and In Vivo to Mouse Abdominal Organs
Specimens of human omentum were obtained from patients undergoing surgery for benign conditions by S.D.Y. or E.L. Informed consent was obtained before surgery and the study was approved by the institutional review board of the University of Chicago. Primary human mesothelial cells (HPMCs) and human primary fibroblasts (HPFs) were isolated from the omentum, and purification was verified by vimentin, keratin 8 (CAM5.2), and prolyl-hydroxylase immunohistochemistry as described previously.4,23 Primary mesothelial and fibroblast cells at early passages (1 to 3) were used for the experiments to minimize dedifferentiation and modification of the original phenotype. In brief, the three-dimensional (3D) omentum culture was assembled by plating 2000 primary fibroblast cells/well along with collagen in a 96-well culture plate. On polymerization, 10,000 primary mesothelial cells were added to the culture and incubated at 37°C until a confluent layer of mesothelial cells formed. For the adhesion assay to the 3D omentum culture, SKOV3ip1 and SKOV3ip1 β3-integrin cells were fluorescently labeled with 5-chloromethylfluoroscein diacetate (green) (Invitrogen, Carlsbad, CA) at 37°C. Then 50,000 cancer cells were added to the 3D culture and incubated at 37°C for 2 hours. Cells were then washed in PBS and fixed in 10% formalin. The number of adherent cells was quantified by measuring the fluorescent intensity (excitation 488 nm, emission 528 nm) with a fluorescence spectrophotometer (Synergy HT) by using a standard curve of cells in each experiment. Samples were run in triplicate.
For adhesion assays to full human omentum, tissue was cut into 6-mm circular disks and placed in full growth media, and 100,000 fluorescently labeled SKOV3ip1 and SKOV3ip1 β3-integrin cells were added on top of the omentum. After incubation at 37°C for 2 hours, omental disks were washed in PBS and digested in 5% nonyl phenoxylpolyethoxylethanol (NP-40) for 30 minutes at 37°C. Cells removed during digestion were placed in a 24-well plate, and the total fluorescent intensity per well was quantified. Adhesion assays were run in quadruplicate.
To study early adhesion to mouse peritoneum in vivo, SKOV3ip1 and SKOV3ip1 β3-integrin cells were fluorescently labeled and 4 × 106 cells/ml were injected into the peritoneal cavity of female athymic nude mice (Harlan, Indianapolis, IN). After 4 hours, mice were sacrificed, and full-thickness peritoneum and omentum were excised. After the specimens were gently washed in PBS to remove nonadherent cells, the tissue was lysed with 1 ml of 1% NP-40 and the fluorescence was measured.23
Invasion Assay through Matrigel and 3D Omentum Culture
Fifty thousand cells (unlabeled for Matrigel and fluorescently labeled for 3D omentum culture) were plated on each well of a 24-well transwell plate (8-μm pore size) precoated with 50 μg of Matrigel or the 3D culture assembled with 4000 HPFs and 20,000 HPMCs.24 The bottom of the transwell was filled with 400 μl of full growth media. After incubation at 37°C, membranes were washed in PBS, and the cells and matrix were scraped off using a Q-tip. For the Matrigel invasion assay, the membrane was fixed in methanol, stained in Giemsa, and dried. For the 3D culture, the membrane was fixed in formalin. The number of invading cells was quantified in five high power fields by bright-field and fluorescent microscopy using a Leica Axiovert 200 microscope equipped with a digital camera. Each experiment was conducted in triplicate.
Colony Formation Assay
To study anchorage-independent growth, six-well plates were layered with 1.5 ml of 0.5% agarose in Dulbecco’s modified Eagle’s medium with 10% fetal bovine serum, and penicillin-streptomycin. Subsequently, SKOV3ip1, SKOV3ip1 pBabe-puro, or SKOV3ip1 β3-integrin cells (5000 cells/well) were mixed in 0.35% agarose in complete medium and plated over the base agar and incubated at 37°C at 95% humidity and 5% CO2. Colonies were stained with 0.005% crystal violet and counted after incubation for 5 weeks.
Western Blot Analysis
SKOV3ip1, SKOV3ip1 pBabe-puro, and SKOV3ip1 β3-integrin cells were plated in six-well plates. At 70% confluence, cells were centrifuged and lysed in ice-cold RIPA buffer.25 An equal amount (20 μg) of cell extract was separated by 10% SDS-polyacrylamide gel electrophoresis, transferred to nitrocellulose membranes, and blocked with 5% milk. Membranes were incubated with the respective antibodies: goat anti β3-integrin (1:2000), mouse anti-cyclin B1 (1:1000), and mouse anti-actin (1:10,000) for 1 hour at room temperature or overnight at 4°C. The blots were incubated with secondary horseradish peroxidase-conjugated IgG or IgM and visualized with enhanced chemiluminescence reagents.
Proliferation Assay
The proliferation of ovarian cancer cells was measured using the CyQuant cell proliferation assay kit (Molecular Probes, Eugene, OR) as described.26
Cell Cycle Analysis and Annexin V/Propidium Iodide Binding Assay
Apoptosis was determined in cells by staining them with both annexin V-fluorescein isothiocyanate antibody and propidium iodide followed by flow cytometry. In brief, medium was removed, and cells were washed twice with PBS, removed from the plate with a cell scraper, and incubated in binding buffer (10 mmol/L HEPES, 140 mmol/L NaCl, 5 mmol/L KCl, 1 mmol/L MgCl2, and 1.8 mmol/L CaCl2, pH 7.4) containing annexin V-fluorescein isothiocyanate (25 μg/ml) and propidium iodide (25 μg/ml) for 30 minutes. Cells were washed three times in binding buffer, and staining was quantified using a FACSCalibur flow cytometer (BD Biosciences).
Cell cycle analysis was performed after the cells were scraped off the plate, washed twice with PBS, and fixed in 70% ethanol for 30 minutes. Cells were then washed again with PBS, and aliquots of 100,000 cells were resuspended in 0.5 ml of propidium iodide/RNase Staining Buffer (catalog number 550825, BD Biosciences) and incubated for 30 minutes at room temperature. Samples were analyzed by flow cytometry using a FACSCalibur flow cytometer.
Animal Experiments
Female athymic nude mice of at least 20 g were housed in filtered-air laminar-flow cabinets. Procedures involving animals and their care were approved by the Institutional Committee on Animal Care, University of Chicago. SKOV3ip1 cells (1 × 106) were suspended in a volume of 500 μl of PBS and injected intraperitoneally (i.p.). Thirty-two days after injection, mice were sacrificed, and the tumor colonies were counted, dissected, collected, and weighed together as described previously.24,26
For the treatment study, SKOV3ip1 and SKOV3ip1 β3-integrin cells (1 × 106) were injected into the peritoneal cavity of female athymic nude mice and 10 days after injection, paclitaxel (3 mg/kg/mouse) was injected once a week for 3 weeks (10 mice/group). The mice were sacrificed after 32 days. Thirty-two days has been established in this model as the time when there are a significant number of i.p. metastases.24 Tumor tissue was fixed in 10% PBS-buffered formalin for Ki-67 immunohistochemistry.
Animal data were analyzed by an unpaired, two-tailed Student’s t-test of significance, assuming equal variance of the test and the control populations at the P < 0.05 level of significance.
Quantitative Real-Time PCR
Quantitative real-time RT-PCR was performed using the Prism7500 TaqMan PCR detector with the following probes from Applied Biosystems (Foster City, CA): αv-integrin (Hs00233808_m1), β3-integrin (Hs01001475_m1), and glyceraldehyde-3-phosphate dehydrogenase (Hs00266705_ gl). RNA was extracted using TRIzol and was transcribed into cDNA using a High Capacity cDNA Kit (Applied Biosystems). Relative levels of mRNA gene expression were calculated using the 2−ΔΔCT method as described previously.27
Patients, Tissue Microarray, and Immunohistochemistry
Tissue blocks from 62 patients with International Federation of Gynecology and Obstetrics (FIGO) stage I to IV advanced ovarian cancer who had undergone surgery performed by a gynecologic oncologist at the University of Chicago were selected for the study after institutional review board approval was obtained. Samples of tissue sections were stained with H&E, and 1.5-mm cores were punched from donor blocks, inserted into a recipient block, and stained with H&E. The presence of tumor was confirmed by a gynecologic pathologist (A.M.). Demographic and histopathologic data were collected as previously reported.24,26
Tissue microarray slides were deparaffinized in xylene and hydrated with alcohol before being placed for 2 minutes in 0.4 N hydrochloric acid at 45°C followed by 5 minutes in pepsin at 50°C. Incubation with the antibody against β3-integrin was done with a 1:100 dilution. The slides were stained using the Envision avidin-biotin-free detection system and counterstained with hematoxylin. Immunoscoring was done using the cellular image analysis system ACIS (Clarient, Aliso Viejo, CA). Within the tissue core the most representative tumor area of standardized size was selected at ×100 magnification, and the fraction of positively stained cells and the intensity of the staining were read with the ACIS. The staining intensity in this area was measured if the area contained more than 2% epithelial cells. Staining intensity was used as a continuous variable, and samples were categorized as being positive or negative split on the median of the dataset. Overall and progression-free survival estimates were computed using the Kaplan-Meier method, and comparisons between groups were analyzed using the log-rank test.
Mouse tumors were formalin-fixed and paraffin sections were stained with H&E and counterstained with eosin. Immunohistochemical analysis was performed with antibodies against Ki-67 (1:100) and CD31 (1:50) (Sigma-Aldrich, St. Louis, MO).
Results
Overexpression of β3-Integrin Increases Cancer Cell Adhesion
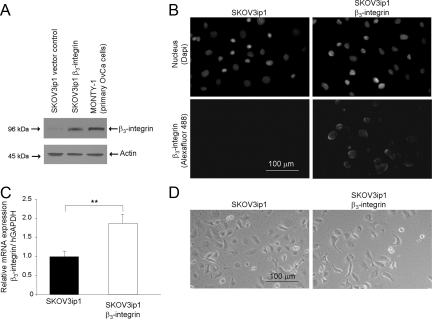
Previous studies have shown that αvβ3-integrin expression can either suppress or enhance the growth of human tumors, but its role in ovarian cancer has not been elucidated. To clarify the role of β3-integrin overexpression in ovarian cancer metastasis, SKOV3ip1 cells were stably transfected either with the empty pBabe-puro vector (control) or the pBabe-puro β3-integrin expression plasmid, and after puromycin selection, were examined for expression of β3-integrin. Immunoblotting for β3-integrin showed higher expression in the β3-integrin transfected cell clones compared with pBabe-puro transfected SKOV3ip1 cells (Figure 1A). In addition, primary ovarian cancer cells (MONTY-1) expressed high levels of β3-integrin. We know that β3-integrin heterodimerizes only with αv-integrin on the epithelial cell surface,5 but there is currently no antibody that effectively detects the αvβ3-integrin heterodimer through immunoblotting. With the most commonly used antibody against αvβ3-integrin, LM-609, for immunofluorescence (Figure 1B) and fluorescence-activated cell sorting (data not shown), we found that the expression of β3-integrin led to the surface expression of αvβ3-integrin heterodimers, whereas minimal αvβ3-integrin could be detected in the parental and vector control SKOV3ip1 cells. The high expression of β3-integrin protein was mirrored by an increase in steady-state β3-integrin mRNA (Figure 1C). Of note, the microscopic appearance of the stable β3-integrin-overexpressing cells did not differ from that of the parental cell line (Figure 1D). Because β3-integrin only heterodimerizes with αv-integrin on epithelial cells, the biologic effects of β3-integrin overexpression were treated as if they were a consequence of αvβ3-integrin overexpression as reported previously.6,7,11,28,29 CaOV3 ovarian cancer cells and a primary ovarian cancer cell line (MONTY-1), which express β3-integrin constitutively, were used to study the effects of its inhibition (Figures 1A and 2A).
Figure 1.
Overexpression of αVβ3-integrin in an ovarian cancer cell line. A: Western blot. SKOV3ip1 cells transfected with pBabe-puro-β3-integrin or with empty pBabe-puro (vector control) and primary ovarian cancer (OvCa, MONTY-1) cells were harvested. Cell lysates were resolved by SDS-polyacrylamide gel electrophoresis and immunoblotted with anti-β3 antibody. The membrane was stripped and reprobed with β-actin. B: Immunofluorescence. Cells were plated on chamber slides and stained by indirect immunofluorescence using a monoclonal antibody against αvβ3-integrin followed by an Alexa 488-conjugated goat anti-mouse secondary antibody. The nucleus was counterstained with 4,6-diamidino-2-phenylindole (Dapi). The experiment was repeated twice. C: Quantitative real-time RT-PCR. Total RNA was extracted from cells and the relative expression of β3-integrin normalized to glyceraldehyde-3-phosphate dehydrogenase was measured using TaqMan quantitative real-time RT-PCR. D: Phase contrast image of SKOV3ip1 cells. Significant differences: **P < 0.01.
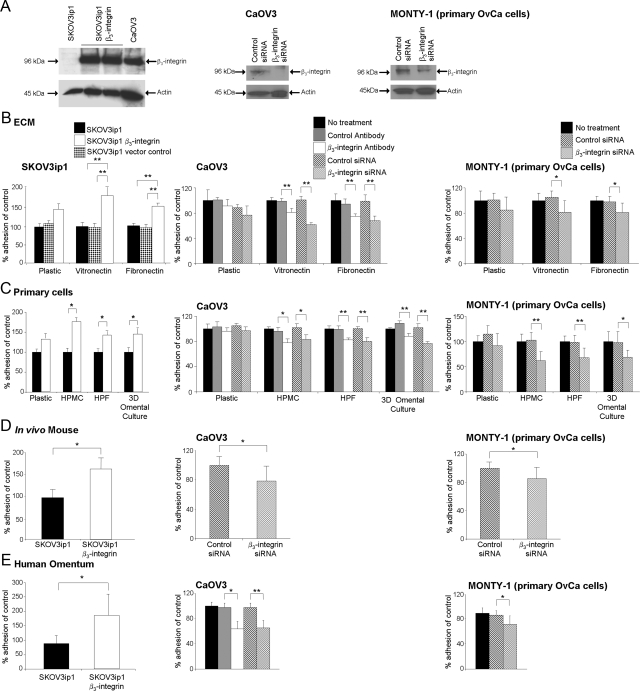
Figure 2.
Overexpression of β3-integrin increases adhesion of SKOV3ip1 cells in vitro and in vivo. A: Western blot. Parental SKOV3ip1, SKOV3ip1 transfected with pBabe-puro-β3-integrin or CaOV3 were harvested. CaOV3 and primary OvCa (MONTY-1) cells were transfected with control or β3-integrin siRNA and harvested 72 hours later. Cell lysates were resolved by SDS-polyacrylamide gel electrophoresis and immunoblotted with anti-β3 antibody. In vitro (B, C, and E) and in vivo (D) adhesion assays. Three sets of cells were tested: first, parental SKOV3ip1, vector control SKOV3ip1, or β3-integrin overexpressing SKOV3ip1 cells; second, CaOV3 and MONTY-1 cells treated with control or β3-integrin functional blocking antibodies; and third, CaOV3 and MONTY-1 cells treated with control or β3-integrin siRNA. B: Ovarian cancer cells (50,000) were fluorescently labeled and added to the indicated ECM components. Adhesion was measured by washing the plates and the number of adherent cells was quantified by measuring fluorescence intensity and use of a standard curve of appropriate cells. Each bar represents the mean of three wells and SD. Each graph is representative of at least three independent experiments. C: Ovarian cancer cells (50,000) were labeled fluorescently and plated onto 96-well plates coated with HPMCs, HPFs, or a 3D culture of HPFs grown in collagen and covered by a confluent layer of mesothelial cells. Each bar represents the average and SD of the means of three independent experiments. D: SKOV3ip1 cells were fluorescently labeled and 4 × 106 cells were injected i.p. into nude mice (n = 3). The mice were sacrificed after 4 hours, omentum and peritoneum were lysed with NP-40 and fluorescence was measured. CAOV3 and MONTY-1 cells were incubated with blocking antibody (LM 609) and 100 nmol/L of siRNA for 72 hours. Cells were injected i.p. into nude mice (n = 3) for the in vivo adhesion assay. Each bar represents the mean and SD of three wells. Each graph is representative of at least three independent experiments. E: Ovarian cancer cells (100,000) were added to a piece of human omentum. After incubation for 2 hours, the human omentum was washed with PBS, adherent cells on the omentum were lysed with NP-40, and total fluorescence intensity was measured with a fluorescence spectrophotometer. Each bar represents the average and SD of three independent experiments. Statistical significance was determined using an unpaired, two-tailed Student’s t-test. Significant differences: *P < 0.05; **P < 0.01.
Ovarian tumor cells detach from the primary tumor and metastasize to the peritoneal surfaces and to organs within the abdominal cavity. These organs are covered by mesothelium, which is composed of a monolayer of mesothelial cells, resting on a collagen- and vitronectin-rich basement membrane that is interspersed with fibroblasts.30,31 Because cancer cell adhesion is the first step in ovarian cancer metastasis, we investigated the effects of αvβ3-integrin overexpression on the adhesion (Figure 2) and invasion (Figure 3) of ovarian cancer cells. To strengthen our results, we investigated the effects of inhibiting β3-integrin using a functional blocking antibody or siRNA (Figure 2A). β3-Integrin overexpression increased the adhesion of cancer cells to the αvβ3-integrin ligands, fibronectin (P < 0.001) and vitronectin (P < 0.001) (Figure 2B). Blocking β3-integrin expression in cancer cells significantly reduced adhesion to the ECM components (Figure 2B).
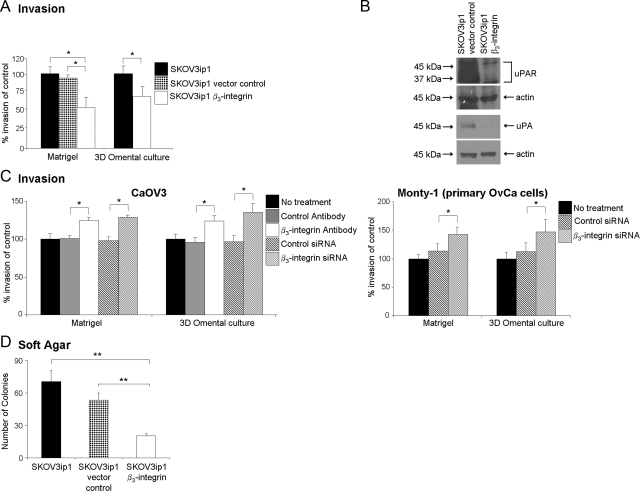
Figure 3.
β3-Integrin overexpression reduces invasion of SKOV3ip1 cells and colony formation. A: Invasion assays were performed using a Matrigel or 3D culture-coated Boyden chamber. Equal numbers (5 × 104) of fluorescently-labeled cells were placed on the top chamber and allowed to invade for 24 hours. Invading cells were photographed and counted. Columns are the mean of three experiments. B: Western blot. Lysates from vector control and β3-integrin-overexpressing SKOV3ip1 cells were resolved by SDS-polyacrylamide gel electrophoresis and immunoblotted with an antibody against urokinase or the urokinase receptor. The blots were stripped and reprobed for β-actin. C: CaOV3 and MONTY-1 cells were silenced for β3-integrin expression using siRNA and blocking antibody. Invasion assays were performed using Matrigel or 3D culture-coated Boyden chamber as described above. Each bar represents the average and SD of the means of three independent experiments. D: Anchorage-independent malignant growth was determined using a soft agar assay. Cells (20,000) were plated onto agar in six-well plates, and colonies were counted under the microscope after 21 days. The data are representative of three independent experiments. The columns represent the mean from six different wells. Statistical significance was determined using an unpaired, two-tailed Student’s t-test. Significant differences: *P < 0.05; **P < 0.01.
To investigate the contribution of the cellular components of the mesothelium to the adhesion of β3-integrin stably transfected cells, we extracted the cellular components of the mesothelium—HPMCs and HPFs. We then tested them individually as well as in an organotypic 3D culture,23,31 in which the two cell types are combined within a collagen ECM that mimics the histologic appearance of human omentum. Overexpression of β3-integrin increased cancer cell adhesion to mesothelial cells (P = 0.002), fibroblasts (P = 0.001), and the 3D omentum culture (P = 0.002) (Figure 2C). Functionally blocking β3-integrin decreased adhesion of CaOV3 cells to HPMCs (P = 0.010), HPFs (P < 0.001), and the 3D culture (P < 0.001), respectively. In addition, knocking down β3-integrin expression significantly decreased adhesion of CaOV3 and MONTY-1 cells to HPMCs, HPFs, and the 3D culture, respectively (Figure 2C).
To verify the applicability of these in vitro results in vivo, SKOV3ip1 and SKOV3ip1 αvβ3-integrin cells were injected i.p. into nude mice. Adhesion was evaluated after 4 hours,24 the period of time required for ovarian cancer cells to attach to the peritoneum and the omentum, as described previously.23 The results (Figure 2D) show that the β3-integrin-overexpressing SKOV3ip1 cells attach more efficiently to mouse omentum and peritoneum than the parental SKOV3ip1 cells (41,661 SKOV3ip1 αvβ3-integrin cells versus 23,653 SKOV3ip1 cells, P = 0.008). Next, in vivo studies were done with CaOV3 and MONTY-1 cells silenced for β3-integrin expression. Adhesion was significantly reduced in β3-integrin siRNA transfected cells compared with control siRNA transfected cells (P < 0.036 and P < 0.050).
To mimic the human in vivo situation as closely as possible, normal full-thickness human omentum was obtained from patients undergoing surgery for benign conditions. Addition of fluorescently labeled SKOV3ip1 αvβ3-integrin cells to human omentum (Figure 2E) showed 2.4-fold higher tumor cell adhesion compared with that for the parental cells (P < 0.005). As expected, inhibition (siRNA and antibody) of β3-integrin in CaOV3 and MONTY-1 cells significantly decreased ovarian cancer cell adhesion to the omentum (Figure 2E).
αvβ3-Integrin Overexpression Decreases Cancer Cell Invasion and In Vivo Tumor Growth
Given the increased adhesive ability of ovarian cancer cells overexpressing αvβ3-integrin, we sought to determine whether these cells are also more invasive. Therefore, invasion into Matrigel or the 3D-culture was compared between αvβ3-integrin-overexpressing SKOV3ip1, parental, and vector control cells. Cells were added onto the Matrigel-coated wells or the 3D culture and incubated for 24 hours, and the number of invading cells was counted. Contrary to our expectations, αvβ3-integrin overexpression inhibited cell invasion compared with parental and vector control cells (P < 0.038 and P < 0.050) respectively (Figure 3A). This decrease was paralleled by a decrease in the expression of the serine protease urokinase and its receptor, urokinase plasminogen activator receptor (Figure 3B), but not of matrix metalloproteinase-2/9 (data not shown). Conversely, inhibition (siRNA and antibody) of β3-integrin in CaOV3 and MONTY-1 cells increased invasion (Figure 3C). We further studied the effect of αvβ3-integrin expression on anchorage-independent growth of SKOV3ip1 cells in soft agar, as a marker of their transformation and tumorigenic potential. As shown in Figure 3D, SKOV3ip1 β3-integrin-overexpressing cells showed a 72% and 61% reduction in colony number compared with the parental and vector control SKOV3ip1 cells (P < 0.008 and P = 0.012) respectively. Taken together, these results suggest that the expression of αvβ3-integrin promotes cell adhesion but decreases invasion and the anchorage-independent growth of ovarian cancer cells.
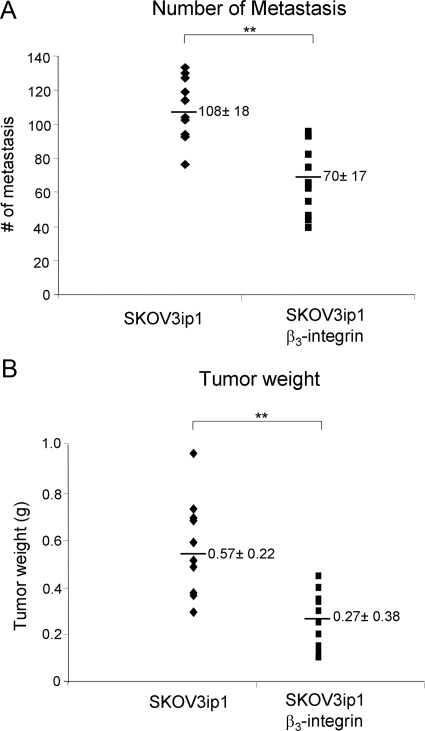
We next investigated whether the effect of αvβ3-integrin on in vitro invasion translated into impaired tumor growth and metastasis in vivo. αvβ3-Integrin-overexpressing SKOV3ip1 and SKOV3ip1 parental cells were injected i.p. into nude mice, which were sacrificed 32 days later. The numbers of visible metastases were counted, and the tumors were weighed. The tumor distribution within the abdominal cavities of mice injected with either of the cell lines resembled the clinical appearance of human ovarian cancer with multiple tumors on the peritoneal surface, a large omental tumor, and involvement of the small bowel mesentery and the ovaries. However, across two independent studies, injection of the αvβ3-integrin-overexpressing cells resulted in 35% fewer intra-abdominal metastases and tumors that weighed on average 53% less than those with injection of the parental SKOV3ip1 cells (Figure 4, A and B) These results confirm the soft agar findings (Figure 3D), which had indicated that cells expressing αvβ3-integrin are less tumorigenic.
Figure 4.
Effect of β3-integrin overexpression on tumor weight and metastasis in vivo. A and B: SKOV3ip1 β3-integrin and control cells (1.0 × 106) were suspended in 500 μl of PBS and injected i.p. into nude mice (n = 10). After 32 days, the animals were sacrificed, and the tumors counted, excised, and weighed. The number of tumor metastases (A) and tumor weight (B) was determined. Each point represents an individual animal and the horizontal bar is the mean ± SD. Statistical significance was determined using an unpaired, two-tailed Student’s t-test. Significant differences: **P < 0.005. Each graph is representative of two independent experiments with five mice in each group (n = 10).
αvβ3-Integrin Overexpression Induces G2M Phase Cell Cycle Arrest
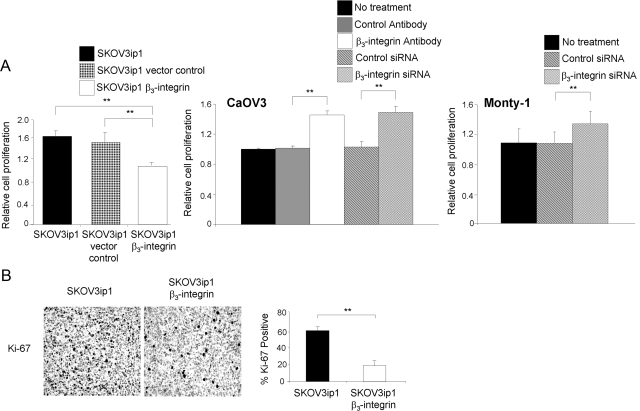
To further understand the mechanism by which β3-integrin affects tumor growth, proliferation was studied. The proliferation of SKOV3ip1 αvβ3-integrin-overexpressing cells was 32% less than that of vector control cells (P < 0.0078) (Figure 5A). Inhibition of β3-integrin (siRNA, antibody) in CaOV3 and MONTY-1 cell lines increased their proliferation rate (Figure 5A).
Figure 5.
β3-Integrin overexpression reduces proliferation. A: Proliferation (in vitro). An equal number of cells were seeded and grown on a 96-well plate, followed by washing and freezing. The frozen cells were lysed and stained with a fluorescent dye that intercalates into DNA, and fluorescence was measured using a spectrophotometer. CaOV3 and MONTY-1 cells were silenced for β3 expression using siRNA and functional blocking antibody, and the proliferation assay was performed. Statistical significance was determined using an unpaired, two-tailed Student’s t-test. Significant differences: *P < 0.05; **P < 0.01. Each bar represents the mean ± SD and is representative of three independent experiments. B: Proliferation (in vivo). SKOV3ip1 tumors collected at the conclusion of the animal study in Figure 4 were stained with a Ki-67 antibody. Representative sections at ×100 final magnification are shown. The mean percentage of Ki-67-positive cells was analyzed from at least 3 slides per group.
These findings were validated in vivo with tumors from the xenograft experiment (Figure 4). Tumors from control mice showed twice as many Ki-67-positive cells (P < 0.002) (Figure 5B), a finding that is in line with the observation that the parental SKOV3ip1 cells produced larger tumors. These findings suggest that the expression of β3-integrin suppresses cell proliferation in vivo and in vitro, thus explaining, at least in part, the reduced tumor growth of SKOV3ip1 αvβ3-integrin cells.
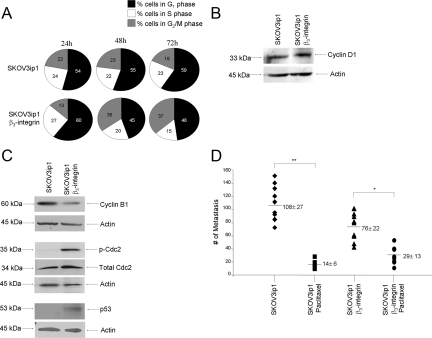
To understand the mechanism by which αvβ3-integrin impairs cell proliferation, the distribution of cells in different cell cycle phases was analyzed. The G1 and S phases of the cell cycle were not affected by αvβ3-integrin overexpression, because the proportions of cells in G1/S phases were similar (Figure 6A) and cyclin D1 protein expression was comparable in the two cell clones (Figure 6B). However, overexpression of αvβ3-integrin in SKOV3ip1 cells resulted in a marked accumulation of cells in the G2/M phase compared with that in the parental line (37% versus 18%, P < 0.005) (Figure 6A). Transition into mitosis at the G2/M checkpoint is regulated by association of cyclin B1 with cdc2.26 Phosphorylation of cdc2 at Tyr-15 leads to its inactivation and degradation of cyclin B1, resulting in a G2/M block. To understand how αvβ3-integrin overexpression leads to the G2/M phase cell cycle arrest, cyclin B1 and cdc2 levels were compared in both cell lines. SKOV3ip1 αvβ3-integrin cells show increased cdc2 Tyr-15 phosphorylation but decreased levels of cyclin B1 compared with the parental cells (Figure 6C). The G2/M checkpoint is regulated by p53 through cyclin B1.32 Immunoblotting for p53 shows that p53 was up-regulated in αvβ3-integrin-overexpressing cells (Figure 6C),23 a finding consistent with a block in the G2 phase of the cell cycle.
Figure 6.
β3-Integrin overexpression induces a G2 phase cell cycle arrest. A: Fluorescence-activated cell sorter analysis. Cell cycle phases were determined by staining of cells with propidium iodide, followed by flow cytometry. Expression of β3-integrin caused a significant increase in the number of cells in the G2/M phase of the cell cycle. B and C: Western blot. SKOV3ip1 or SKOV3ip1 β3-integrin was lysed and 30 μl of lysates were subjected to immunoblotting with antibodies against cyclin D1, cyclin B1, phospho (p)-Cdc2, total Cdc2, or p53. β-Actin was used as an internal loading control. D: Effect of paclitaxel on tumor growth in β3-integrin-overexpressing cells. Mice (n = 10) were injected with β3-integrin SKOV3ip1 or control cells. Ten days after the injection, paclitaxel (3 mg/kg) was injected once a week for 3 weeks. At autopsy, tumors were counted, excised, and weighed. Each point represents an individual animal and the horizontal bar is the mean ± SD. Statistical significance was determined using an unpaired, two-tailed Student’s t-test. Significant differences: *P < 0.05; **P < 0.005. Each bar represents the mean ± SD (n = 10).
Paclitaxel chemotherapy, which is the standard of care for first-line treatment of ovarian cancer, acts by stabilizing microtubules forming in the M phase of the cell cycle.33 Therefore, given the G2 arrest on αvβ3-integrin overexpression, we hypothesized that the tumor inhibitory effect of paclitaxel as a mitotic inhibitor would be reduced in cells locked in the G2 phase. Indeed, paclitaxel treatment 10 days after injection of the tumor cells reduced the mean number of metastases by 87.2% in mice injected with SKOV3ip1 cells (P < 0.005), whereas in mice injected with β3-integrin-overexpressing SKOV3ip1 cells the reduction was only 61.7% (P < 0.05) (Figure 6D). The difference in paclitaxel response between αvβ3-integrin-overexpressing cells and parental cells was significant (P < 0.05), suggesting that αvβ3-integrin expression makes cells partially resistant to the effect of paclitaxel.
β3-Integrin Overexpression Is a Marker for a Favorable Progression in Ovarian Cancer
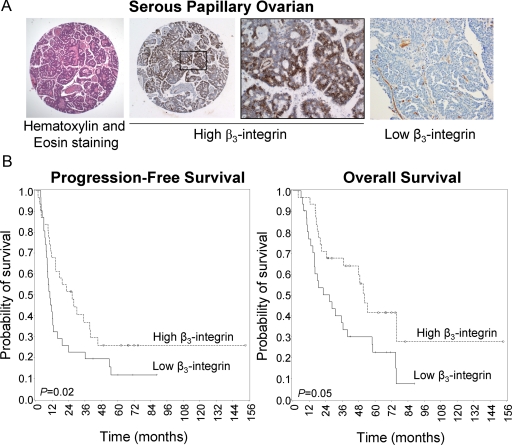
The above in vitro and in vivo experiments show that β3-integrin overexpression increases adhesion but reduces invasion and tumor dissemination. To determine the net result of these effects in patients, we evaluated β3-integrin expression by immunohistochemistry, using a tissue microarray assembled from ovarian tissue of patients with invasive ovarian cancer (FIGO stage I to IV) who underwent tumor cytoreductive surgery at the University of Chicago. The tissue microarray was stained with an antibody against β3-integrin, and the slides were read with an automatic imaging system. The staining intensity was used to dichotomize the results at the median, and survival curves were calculated. Patients with high (n = 32) β3-integrin expression had a significantly better prognosis than those with low (n = 32) β3-integrin expression (P = 0.021). The median overall survival with high β3-integrin expression was 52 months, whereas it was 27 months in patients with low β3-integrin expression (Figure 7A). There was also a statistically significant difference in progression-free survival between patients whose tumors had high or low β3-integrin expression (P = 0.05) (Figure 7B). In addition, the size of the residual tumor left at the end of surgery was a statistically significant predictor of overall and progression-free survival, as has been reported previously (Table 1).34
Figure 7.
β3-Integrin overexpression is a prognostic marker in ovarian cancer. A: Representative immunohistochemical pictures of two different ovarian cancers stained using an anti-human β3-integrin antibody. B: β3-Integrin overexpression correlates with a good prognosis in patients with ovarian cancer. Kaplan-Meier curves of progression-free survival (left) and overall survival (right).
Table 1.
Clinical Data of Patients with FIGO Stage I to IV Ovarian Tumors
| n (%) | *P value (overall survival) | |
|---|---|---|
| FIGO stage | ||
| I | 9 (14.5) | 0.0316 |
| IIC | 3 (4.8) | |
| III | 37 (59.7) | |
| IV | 13 (21.0) | |
| Histology | ||
| Serous-papillary | 43 (69.4) | 0.0356 |
| Endometrioid | 5 (8.1) | |
| Clear cell | 4 (6.4) | |
| Mucinous | 10 (16.1) | |
| Disease | ||
| Ovarian | 60 (96.8) | |
| Primary peritoneal | 2 (3.2) | |
| Grading | ||
| G1 + G2 | 17 (27.4) | 0.6946 |
| G3 | 45 (72.6) | |
| Ascites volume | ||
| ≤500 ml | 34 (54.8) | 0.0645 |
| >500 ml | 20 (32.3) | |
| Unknown | 8 (12.9) | |
| Residual tumor | ||
| ≤1 cm | 10 (16.1) | 0.0002 |
| >1 cm | 45 (72.6) | |
| Unknown | 7 (11.3) | |
| Chemotherapy type | ||
| Neoadjuvant | 42 (67.7) | 0.7978 |
| Primary | 19 (30.7) | |
| None | 1 (1.6) | |
| Chemotherapy | ||
| Taxane/platinum | 51 (82.3) | |
| Platinum only | 2 (3.2) | |
| Other/platinum | 1 (1.6) | |
| Taxane | 1 (1.6) | |
| None | 7 (11.3) |
n = 62. Median age (range) at diagnosis: 59 (36–87) years. Median (range) follow-up: 68 (4–154) months.
P values were calculated with the log-rank test to determine whether the clinicopathologic factors are predictors of overall survival.
Discussion
Although the role of αvβ3-integrin as a tumor promoter seemed well established,6,7,8,35,36,37 recent studies suggested that β3-integrin may actually inhibit tumor progression.11,28,38 In our current study, we analyzed the effect of αvβ3-integrin overexpression in an ovarian cancer cell line and human ovarian cancer tumors to understand the role of αvβ3-integrin in ovarian cancer. SKOV3ip1 αvβ3-integrin cells, CaOv3, and a primary ovarian cancer cell line adhered efficiently to both full human omentum and peritoneum, which is consistent with the role of αvβ3-integrin as an adhesion receptor.5 Adhesion was mediated by an overall improved ability of the cells to attach to both the cellular components (ie, mesothelial cells and fibroblasts) and the ECM components (ie, fibronectin and vitronectin) of the human omentum and peritoneum. These data could be seen as indicating that αvβ3-integrin-expressing cells are more aggressive and metastatic. However, αvβ3-integrin-overexpressing cells also showed impaired collagen and Matrigel invasion and colony formation. These findings were recapitulated in xenograft studies: αvβ3-integrin-overexpressing cells showed increased adhesion to mouse peritoneum in vivo within 1 day (Figure 2E), but after 32 days the overall number of metastatic nodules and tumor weight were significantly lower in these mice (Figure 4), suggesting that tumors expressing αvβ3-integrin tend to be less aggressive.
The reduced tumor load of SKOV3ip1 β3-integrin-injected mice is, at least in part, caused by a reduction in serine protease expression, an increase in apoptosis (data not shown), and a reduction in the proliferation rate. The reduced DNA content is caused by an arrest in the G2 phase of the cell cycle on αvβ3-integrin overexpression. This conclusion is supported by (i) the fluorescence-activated cell sorter analysis showing a higher proportion of αvβ3-integrin cells in the G2/M phase compared with that in parental cells, (ii) a low expression of cyclin B1, and (iii) a high expression of both p53- and tyrosine 15-phosphorylated cdc215 in αvβ3-integrin-expressing cells, and (iv) the observation that αvβ3-integrin-overexpressing cells are less susceptible to the inhibitory effect of paclitaxel. Two known p53-dependent pathways can contribute to a G2 cell cycle arrest. The p53 transcription factor can inhibit cyclin B1 promoter activity and decrease the intracellular level of cyclin B1, thereby regulating the G2 checkpoint.32 Alternatively, p53 inhibits G2/M progression through the 14-3-3 protein family.39 The 14-3-3σ isoform can prevent entry into mitosis through inactivation of cdc25C, a phosphatase that activates cdc2 through dephosphorylation on tyrosine 15.40 In αvβ3-integrin-overexpressing cells both mechanisms could mediate the G2 arrest, because p53 up-regulation is paralleled by cdc2 Tyr-15 phosphorylation and a loss of cyclin B1. In addition to the effect of p53 on the cell cycle, induction of p53 by αvβ3-integrin could also be responsible for the higher apoptosis rate in the αvβ3-integrin clones (data not shown), because p53 is a known apoptosis regulator.41
The tumor inhibitory effect of αvβ3-integrin expression is mediated not only by a reduction in proliferation and increased apoptosis but also through inhibition of urokinase and its receptor, which are important for ECM cleavage42 and tumor cell invasion and are up-regulated in ovarian cancers.43 αvβ3-Integrin probably regulates urokinase transcriptionally, because we showed previously that in Chinese hamster ovary cells αvβ3-integrin expression can inhibit urokinase44 and its receptor22 through a PEA3 transcription factor binding site. We did not find an induction of matrix metalloproteinase-2/9 activity by αvβ3-integrin, which is counter to the results of a previous publication in which ligation of αvβ3-integrin was found to induce matrix metalloproteinase-2 activity in melanoma cells.35
Initial studies on the role of αvβ3-integrin in tumor biology suggested a tumor-promoting role, but recently several reports have shown that β3-integrin actually decreases tumorigenicity and angiogenesis.11,28,38 These findings require a re-evaluation of the function of β3-integrin in epithelial tumors. Convincing evidence that αvβ3-integrin promotes tumor progression is limited to studies of melanoma progression and bone metastasis from epithelial tumors (e.g., breast and prostate cancers). Several investigators have shown that αvβ3-integrin participates in the bone tropism of epithelial cancer cells.6,36,37 For example, overexpression of αvβ3-integrin in MDA-MB-231 breast cancer cells promoted bone metastases and bone destruction, which could be inhibited with an αvβ3-integrin antagonist.36 A tumorigenic role of αvβ3-integrin in melanomas is supported by two studies showing that ligation of αvβ3-integrin stimulates invasion35 and that β3-integrin overexpression mediates the transition from the radial growth phase to the more aggressive vertical growth phase of primary human cutaneous melanomas.7 However, Danen et al38 found that transfection of β3-integrin inhibited the invasion and metastasis of melanoma cells. This study was the first to suggest a tumor inhibitory role for β3-integrin. Later, a growth-suppressive effect of β3-integrin was described in a study of rat glioblastoma cells overexpressing ras, in which cotransfection of β3-integrin inhibited tumor formation and tumor growth.11 The decreased tumor load was explained by the ability of αvβ3-integrin-expressing cells to recruit tumor necrosis factor-α-secreting, apoptosis-inducing macrophages, thus suggesting a mechanism by which αvβ3-integrin-expressing cells promote macrophage-mediated tumor clearance.29 αvβ3-Integrin was also, at first, thought to enhance angiogenesis,8 but then a genetic study clearly showed that mice lacking β3-integrin show enhanced angiogenesis and tumor growth.28 While this article was under review, Reynolds et al10 showed that nanomolar concentrations of RGD-mimetic αvβ3/αvβ5-integrin inhibitors enhance tumor growth and tumor angiogenesis in preclinical xenograft models. Together with our data, their results suggest that, in some cancers, αvβ3-integrin is actually a tumor suppressor receptor. This suggestion is strongly supported by our finding that patients with β3-integrin-overexpressing tumors have significantly better disease-free and overall survival. These data may have important implications, because αvβ3-integrin inhibitors are now being considered for the treatment of solid tumors, including ovarian cancer. Currently, there are three classes of integrin inhibitors in preclinical and clinical development: a monoclonal antibody targeting the extracellular domain of the αvβ3-integrin heterodimer (etaracizumab [Abegrin, previously named Vitaxin or MEDI-522],45 synthetic peptides containing an RGD sequence [EMD121974, cilengitide],20,46 and peptidomimetics [S247, Pfizer, New York, NY]).47 Based on our data examining the role of αvβ3-integrin on ovarian tumor cells and our review of the current literature, we are concerned that blocking β3-integrin could be detrimental and actually accelerate the growth of some cancer cell types.
In summary, the view of αvβ3-integrin as a tumor-promoting adhesion receptor and the therapeutic implications of this view, should be reassessed. Several studies, including ours, now show that, when expressed on tumor cells, αvβ3-integrin can be a marker for a less aggressive cancer cell population and therefore, may not be an appropriate target for inhibition.
Acknowledgments
We greatly appreciate Gail Isenberg (Department of Obstetrics and Gynecology, University of Chicago) for editing the manuscript. We sincerely thank Kay McLeod, Ph.D. (Ben May Department for Cancer Research, University of Chicago) for help with the cell cycle analysis. We thank Lorna Rodriguez-Rodriguez, M.D. Ph.D. (The Cancer Institute of New Jersey, Division of Gynecologic Oncology) for her support of this work.
Footnotes
Address reprint requests to Ernst Lengyel, M.D., Ph.D., University of Chicago, Department of Obstetrics and Gynecology/Section of Gynecologic Oncology, 5841 South Maryland Ave., Chicago, IL 60637. E-mail: elengyel@uchicago.edu.
Supported by the Ovarian Cancer Research Fund (Liz-Tilberis Scholars Program) and by a National Cancer Institute grant (R01-CA111882 to E.L.). E.L. holds a Clinical Scientist Award in Translational Research from the Burroughs Wellcome Fund.
References
- Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C, Thun M. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- Medeiros F, Muto MG, Lee Y, Elvin J, Callahan M, Felmate C, Garber J, Cramer D, Crum CP. The tubal fimbria is a preferred site for early adenocarcinoma in women with familial ovarian cancer syndrome. Am J Surg Pathol. 2006;30:230–236. doi: 10.1097/01.pas.0000180854.28831.77. [DOI] [PubMed] [Google Scholar]
- Auersperg N, Wong AST, Choi K, Kang S, Leung P. Ovarian surface epithelium: biology, endocrinology and pathology. Endocr Rev. 2001;22:255–288. doi: 10.1210/edrv.22.2.0422. [DOI] [PubMed] [Google Scholar]
- Fidler IJ. The pathogenesis of cancer metastasis: the “seed and soil” hypothesis revisited. Nat Rev Cancer. 2003;5:355–366. doi: 10.1038/nrc1098. [DOI] [PubMed] [Google Scholar]
- Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- Sloan EK, Pouliot N, Stanley KL, Chia J, Moseley JM, Hards DK, Anderson RL. Tumor-specific expression of αVβ3 integrin promotes spontaneous metastasis of breast cancer to bone. Breast Cancer Res. 2006;8:1–14. doi: 10.1186/bcr1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu M, Shih D, Meier FE, van Belle PA, Hse J, Elder DE, Buck C, Herlyn M. Adenoviral gene transfer of β3 integrin subunit induces conversion from radial to vertical growth phase in primary human melanoma. Am J Pathol. 1998;153:1435–1441. doi: 10.1016/s0002-9440(10)65730-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks PC, Clark R, Cheresh D. Requirement of vascular integrin αVβ3 for angiogenesis. Science. 1994;264:569–571. doi: 10.1126/science.7512751. [DOI] [PubMed] [Google Scholar]
- Wu Y, Zuo J, Ji G, Saiyin H, Liu X, Yin F, Cao N, Wen Y, Li J, Yu L. Proapoptotic function of integrin beta3 in human hepatocellular carcinoma cells. Clin Cancer Res. 2009;15:60–69. doi: 10.1158/1078-0432.CCR-08-1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds A, Hart IR, Watson A, Welti JC, Silva RG, Robinson SD, Da Violante G, Gourlaouen M, Salih M, Jones MC, Jones DT, Saunders G, Kostourou V, Perron-Sierra F, Norman JC, Tucker GC, Hodivala-Dilke KM. Stimulation of tumor growth and angiogenesis by low concentrations of RGD-mimetic integrin inhibitors. Nat Med. 2009;15:392–400. doi: 10.1038/nm.1941. [DOI] [PubMed] [Google Scholar]
- Kanamori M, Vanden Berg S, Bergers G, Bergers M, Pieper R. Integrin β3 overexpression suppresses tumor growth in a human model of gliomagenesis: implications for the role of β3 overexpression in glioblastoma multiforme. Cancer Res. 2004;64:2751–2758. doi: 10.1158/0008-5472.can-03-3354. [DOI] [PubMed] [Google Scholar]
- Ahmed N, Pansino F, Clyde R, Murthi P, Quinn MA, Rice GE, Agrez MV, Mok SC, Baker MS. Overexpression of αvβ6 integrin in serous epithelial ovarian cancer regulates extracellular matrix degradation via the plasminogen activation cascade. Carcinogenesis. 2002;23:237–244. doi: 10.1093/carcin/23.2.237. [DOI] [PubMed] [Google Scholar]
- Strobel T, Cannistra SA. β1-integrins partly mediate binding of ovarian cancer cells to peritoneal mesothelium in vitro. Gynecol Oncol. 1999;73:362–367. doi: 10.1006/gyno.1999.5388. [DOI] [PubMed] [Google Scholar]
- Cannistra SA, Ottensmeier C, Niloff J, Orta B, DiCarlo J. Expression and function of β1 and αvβ3 integrins in ovarian cancer. Gynecol Oncol. 1995;58:216–225. doi: 10.1006/gyno.1995.1214. [DOI] [PubMed] [Google Scholar]
- Carreiras F, Denoux Y, Staedel C, Lehmann M, Sichel F, Gauduchon P. Expression and localisation of αv integrins and their ligand vitronectin in normal ovarian epithelium and in ovarian carcinoma. Gynecol Oncol. 1996;62:260–267. doi: 10.1006/gyno.1996.0225. [DOI] [PubMed] [Google Scholar]
- Maubant S, Cruet-Hennequart S, Dutoit S, Denoux Y, Crouet H, Henry-Amar M, Gauduchon P. Expression of αV-associated integrin β subunits in epithelial ovarian cancer and its relation to prognosis in patients treated with platinum-based regimens. Journal of molecular histology J Med Histol. 2005;36:119–129. doi: 10.1007/s10735-004-4273-0. [DOI] [PubMed] [Google Scholar]
- Liapis H, Adler L, Wick MR, Rader JS. Expression of αvβ3 integrin is less frequent in ovarian epithelial tumors of low malignant potential in contrast to ovarian carcinomas. Hum Pathol. 1997;28:443–449. doi: 10.1016/s0046-8177(97)90033-2. [DOI] [PubMed] [Google Scholar]
- Carreiras F, Rigot V, Cruet S, Andre F, Gauduchon P, Marvaldi J. Migration properties of the human ovarian adenocarcinoma cell line IGROV1: importance of αvβ3 integrins and vitronectin. Int J Cancer. 1999;80:285–294. doi: 10.1002/(sici)1097-0215(19990118)80:2<285::aid-ijc19>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Cruet S, Salamanca C, Mitchell GW, Auersperg N. αvβ3 and vitronectin expression by normal ovarian surface epithelial cells: role in cell adhesion and cell proliferation. Gynecol Oncol. 1999;75:254–260. doi: 10.1006/gyno.1999.5572. [DOI] [PubMed] [Google Scholar]
- Nabors B, Mikkelsen T, Rosenfield SS, Hochberg F, Akella NS, Fisher J, Cloud GA, Zhang Y, Carson K, Wittemer SM, Colevas AD, Grossman SA. Phase I and correlative biology study of cilengitide in patients with recurrent malignant glioma. J Clin Oncol. 2007;25:1651–1657. doi: 10.1200/JCO.2006.06.6514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu D, Wolf JK, Scanlon M, Price JE, Hung M-C. Enhanced c-erb B-2/neu expression in human ovarian cancer cells correlates with more severe malignancy that can be suppressed by E1A. Cancer Res. 1993;53:891–898. [PubMed] [Google Scholar]
- Hapke S, Gawaz M, Dehne K, Köhler J, Marshall JF, Graeff H, Schmitt M, Reuning U, Lengyel E. β3A-integrin downregulates the urokinase-type plasminogen activator receptor (u-PAR) through a PEA3/ets transcriptional silencing element in the u-PAR promoter. Mol Cell Biol. 2001;21:2118–2132. doi: 10.1128/MCB.21.6.2118-2132.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny HA, Kaur S, Coussens L, Lengyel E. The initial steps of ovarian cancer cell metastasis are mediated by MMP-2 cleavage of vitronectin and fibronectin. J Clin Invest. 2008;118:1367–1379. doi: 10.1172/JCI33775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawada K, Radjabi AR, Shinomiya N, Kistner E, Kenny HA, Salgia R, Yamada SD, Vande Woude GF, Tretiakova MS, Lengyel E. C-Met overexpression is a prognostic factor in ovarian cancer and an effective target for inhibition of peritoneal dissemination and invasion. Cancer Res. 2007;67:1670–1680. doi: 10.1158/0008-5472.CAN-06-1147. [DOI] [PubMed] [Google Scholar]
- Ried S, Jäger C, Jeffers M, Vande Woude GF, Graeff H, Schmitt M, Lengyel E. Activation mechanisms of the urokinase-type plasminogen activator promoter by hepatocyte growth factor/scatter factor (HGF/SF). J Biol Chem. 1999;274:16377–16386. doi: 10.1074/jbc.274.23.16377. [DOI] [PubMed] [Google Scholar]
- Sawada K, Radjabi AR, Bhaskar V, Kistner E, Tretiakova MS, Jagadeeswaran S, Montag A, Becker A, Kenny HA, Peter ME, Ramakrishnan V, Yamada SD, Lengyel E. Loss of E-cadherin promotes ovarian cancer metastasis via α5-integrin, which is a therapeutic target. Cancer Res. 2008;68:2329–2339. doi: 10.1158/0008-5472.CAN-07-5167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shell S, Park SM, Radjabi AR, Schickel R, Kistner E, Jewell DA, Feig C, Lengyel E, Peter ME. Let-7 expression defines two differentiation stages of cancer. Proc Natl Acad Sci USA. 2007;104:11400–11405. doi: 10.1073/pnas.0704372104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds LE, Wyder L, Lively JC, Taverna D, Robinson SD, Huang X, Sheppard D, Hynes RO, Hodivala-Dilke KM. Enhanced pathological angiogenesis in mice lacking β3 integrin or β3 and β5 integrins. Nat Med. 2002;8:27–34. doi: 10.1038/nm0102-27. [DOI] [PubMed] [Google Scholar]
- Kanamori M, Kawaguchi T, Berger MS, Pieper R. Intracranial microenvironment reveals independent opposing functions of host αVβ3 expression on glioma growth and angiogenesis. J Biol Chem. 2006;281:37256–37264. doi: 10.1074/jbc.M605344200. [DOI] [PubMed] [Google Scholar]
- Daya D, McCaughy WT. Pathology of the peritoneum: a review of selected topics. Semin Diagn Pathology. 1991;8:277–289. [PubMed] [Google Scholar]
- Kenny HA, Krausz T, Yamada SD, Lengyel E. Use of a novel 3D culture model to elucidate the role of mesothelial cells, fibroblasts and extra-cellular matrices on adhesion and invasion of ovarian cancer cells. Int J Cancer. 2007;121:1463–1472. doi: 10.1002/ijc.22874. [DOI] [PubMed] [Google Scholar]
- Innocente SA, Abrahamson JLA, Cogswell JP, Lee JM. p53 regulates a G2 checkpoint through cyclin B1. Proc Natl Acad Sci USA. 1999;96:2147–2152. doi: 10.1073/pnas.96.5.2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhalla K. Microtubule-targeted anticancer agents and apoptosis. Oncogene. 2003;22:9075–9086. doi: 10.1038/sj.onc.1207233. [DOI] [PubMed] [Google Scholar]
- Bristow RE, Tomacruz RS, Armstrong DK, Trimble EL, Montz FJ. Survival effect of maximal cytoreductive surgery for advanced ovarian carcinoma during the platinum era: a meta-analysis. J Clin Oncol. 2002;20:1248–1259. doi: 10.1200/JCO.2002.20.5.1248. [DOI] [PubMed] [Google Scholar]
- Seftor RE, Seftor EA, Gehlsen KR, Stetler-Stevenson WG, Brown PD, Ruoslahti E, Hendrix MJC. Role of the αvβ3 integrin in human melanoma cell invasion. Proc Natl Acad Sci USA. 1992;89:1557–1561. doi: 10.1073/pnas.89.5.1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Bachelier R, Treilleux I, Pujuguet P, Peyruchaud O, Baron R, Clément-Lacroix P, Clézardin P. Tumor αvβ3 integrin is a therapeutic target for breast cancer bone metastases. Cancer Res. 2007;67:5821–5830. doi: 10.1158/0008-5472.CAN-06-4499. [DOI] [PubMed] [Google Scholar]
- Nemeth JA, Cher ML, Zhou Z, Mullins C, Bhagat S, Trikha M. Inhibition of αvβ3 integrin reduces angiogenesis, bone turnover, and tumor proliferation in experimental prostate cancer bone metastases. Clin Exp Metastasis. 2003;20:413–420. doi: 10.1023/a:1025461507027. [DOI] [PubMed] [Google Scholar]
- Danen EHJ, van Kraats AA, Cornelissen IMHA, Ruiter DJ, van Muijen GNP. Integrin β3 cDNA transfection into a highly metastatic αvβ3-negative human melanoma cell line inhibits invasion and experimental metastasis. Biochem Biophys Res Commun. 1996;226:75–81. doi: 10.1006/bbrc.1996.1313. [DOI] [PubMed] [Google Scholar]
- Hermeking H, Lengauer C, Polyak K, He TC, Zhang L, Thiagalingam S, Kinzler KW, Vogelstein B. 14-3-3 sigma is a p53-regulated inhibitor of G2/M progression. Mol Cell. 1997;1:3–11. doi: 10.1016/s1097-2765(00)80002-7. [DOI] [PubMed] [Google Scholar]
- Hermeking H, Benzinger A. 14-3-3 proteins in cell cycle regulation. Semin Cancer Biol. 2006;16:183–192. doi: 10.1016/j.semcancer.2006.03.002. [DOI] [PubMed] [Google Scholar]
- Yonish-Rouach E, Resnitzky D, Lotem J, Sachs L, Kimchi A, Oren M. Wild-type p53 induces apoptosis of myeloid leukaemic cells that is inhibited by interleukin-6. Nature. 1991;352:345–347. doi: 10.1038/352345a0. [DOI] [PubMed] [Google Scholar]
- Edo de Bock C, Wang Y. Clinical relevance of urokinase-type plasminogen activator receptor (uPAR) expression in cancer. Med Res Rev. 2004;24:13–39. doi: 10.1002/med.10054. [DOI] [PubMed] [Google Scholar]
- Schmalfeldt B, Prechtel D, Härting K, Späthe K, Rutke S, Konik E, Fridman R, Berger U, Schmitt M, Kuhn W, Lengyel E. Increased expression of matrix metalloproteinases (MMP)-2. MMP-9, and the urokinase-type plasminogen activator is associated with progression from benign to advanced ovarian cancer. Clin Cancer Res. 2001;7:2396–2404. [PubMed] [Google Scholar]
- Hapke S, Kessler H, Arroyo de Prada N, Benge A, Schmitt M, Lengyel E, Reuning U. Integrin αvβ3/vitronectin interaction affects expression of the urokinase system in human ovarian cancer cells. J Biol Chem. 2001;276:26340–26348. doi: 10.1074/jbc.M100181200. [DOI] [PubMed] [Google Scholar]
- Delbaldo C, Raymond E, Vera K, Hammershaimb L, Kaucic K, Lozahic S, Marty M, Faivre S. Phase I and pharmacokinetic study of etaracizumab (Abegrin™), a humanized monoclonal antibody against αvβ3 integrin receptor, in patients with advanced solid tumor. Invest New Drugs. 2008;26:35–43. doi: 10.1007/s10637-007-9077-0. [DOI] [PubMed] [Google Scholar]
- Burke PA, DeNardo SJ, Miers LA, Lamborn KR, Matzku S, DeNardo GL. Cilengitide targeting of αvβ3 integrin synergizes with radioimmunotherapy to increase efficacy and apoptosis in breast cancer xenografts. Cancer Res. 2002;62:4263–4272. [PubMed] [Google Scholar]
- Abdollahi A, Griggs D, Zieher H, Roth A, Lipson K, Saffrich R, Grone H, Hallahan D, Reisfeld R, Debus J, Niethammer A, Huber P. Inhibition of αvβ3 integrin survival signaling enhances antiangiogenic and antitumor effects of radiotherapy. Clin Cancer Res. 2005;11:6270–6279. doi: 10.1158/1078-0432.CCR-04-1223. [DOI] [PubMed] [Google Scholar]