Abstract
Sphingosine kinase (SK)-1 promotes endothelial cell (EC) survival through the cell junction molecule CD31 (platelet endothelial cell adhesion molecule-1). The integrin αvβ3 is also essential for EC survival; inhibition of αvβ3 ligation promotes apoptosis. Herein we demonstrate that under basal conditions, SK-1, αvβ3, and CD31 exist as a heterotrimeric complex. Under conditions that affect EC survival such as loss of contact with the extracellular matrix or growth factor activation, more of this heterotrimeric complex forms. Overexpression studies demonstrate a requirement for SK-1 phosphorylation at serine 225 for increased heterotrimeric complex formation, activation of αvβ3, and EC survival signals, including Bcl-X and nuclear factor-κB pathways. Moreover, β3 integrin depletion confirmed the requirement for this heterotrimeric complex in SK-1-mediated EC survival. Thus, with αvβ3 integrin being identifiable primarily on angiogenic ECs and SK-1 being highly expressed in tumors, targeting SK-1 may affect multiple survival pathways, and its inhibition may be highly efficacious in controlling pathological EC survival.
Survival of vascular endothelial cells (ECs) demands three specific conditions: attachment to an extracellular matrix (ECM),1 cell-cell interactions (reviewed in Ref. 2), and exposure to growth factors (GF) (for example, vascular endothelial growth factor [VEGF]) and fibroblast growth factor [FGF]).3 ECM and GF-induced survival signals involve integrins, a family of transmembrane heterodimeric cell surface proteins that are formed by two noncovalently associated subunits, α and β. Integrins exist in two distinct functional states, an inactive nonadhesion-promoting state and a ligand-bound, active, and adhesion-promoting conformation.4 On ligand binding, integrins are aggregated in transmembrane complexes (focal contacts) that are enriched in cytoskeletal proteins (eg, talin and vinculin) as well as signaling proteins (eg, focal adhesion kinase [FAK]) (reviewed in Refs. 5,6). Integrin αvβ3 is key to EC survival because disruption of αvβ3 ligation inhibits blood vessel formation and initiates apoptosis7 and is also found at its highest levels on angiogenic ECs in wounds and tumors.8 GF receptors also collaborate with integrins by partitioning into common complexes within which they become activated and signal more efficiently.9,10
Sphingosine kinase (SK) is a lipid kinase that catalyzes the phosphorylation of sphingosine to form sphingosine-1-phosphate (S1P) (reviewed in Ref. 11). SK has two isoforms (SK-1 and -2), and although most cells can synthesize S1P, large amounts are present in platelets12 and recent reports have identified erythrocytes as well as vascular endothelium as major contributors of S1P in circulation.13,14,15 S1P can act extracellularly through the G protein-coupled S1P receptors (S1P1-5). Mature ECs express S1P receptors S1P1-3, and these ligand/receptor interactions promote EC survival, migration, proliferation, adherens junction assembly, increased revascularization, and wound healing both in vitro and in vivo (reviewed in Ref. 16). SK-1 can also act intracellularly through as yet unknown binding partners in which the ablation of S1P receptor signaling through both chemical or genetic mechanisms does not abrogate SK-1 effects on cell proliferation, Ca2+ mobilization, or the survival and motility of ECs (reviewed in Ref. 11). SK-1 seems to have two functional states: an intrinsic or basal state and an agonist-induced activation state that requires its phosphorylation and translocation to the plasma membrane and is responsible for its oncogenic properties.17
Recently, we demonstrated that SK-1 can promote EC survival through the cell junction molecule CD31 (platelet endothelial cell adhesion molecule-1) acting specifically through phosphatidylinositol 3-kinase/Akt and not the mitogen-activated protein kinase pathway.18 With previous reports that CD31 and SK-1 can interact, at least when overexpressed in cells19 and that there is cross talk between CD31 and integrins in ECs,20 we investigated whether SK-1 and integrins may be linked in their capacities to regulate EC survival. The results presented here show that enforced overexpression of SK-1 increases the levels of αvβ3 integrin as well as its activation state, resulting in regulation of the Bcl-XL/Bim and nuclear factor-κB (NF-κB) survival pathways. Moreover, we demonstrate that under basal conditions, a complex of αvβ3, SK-1, and CD31 exists, and, on conditions of cell stress, enhanced levels of this heterotrimeric complex form. Complex formation and αvβ3 integrin activation requires SK-1 to be phosphorylated, thus suggesting that agonist-induced activation of SK-1 is essential for cell survival. These results, combined with our previous work showing that CD31 is an important intermediary in SK-1-induced survival, demonstrates that SK-1 is a fulcrum for the activities of these two well known key survival proteins in ECs, the integrin αvβ3 and CD31.
Materials and Methods
Cells and Cell Culture
Primary human umbilical vein endothelial cells (HUVECs) were grown as described.21 Cells were used at two or fewer passages. Unless otherwise stated, all HUVECs used underwent extensive washing and 120 minutes of culture in media without serum and GF (endothelial growth factor supplement, BD BioSciences, North Ryde, NSW, Australia). Cells were then trypsin-digested (Invitrogen, Carlsbad, CA) to dislodge them from their tissue culture plates and resuspended in media (without serum and GF) for 30 minutes in a 37°C water bath with intermittent mixing.
SK-1 Adenoviral Constructs
Wild-type human SK-1 (SK-1), FLAG, and green fluorescent protein tagged versions were generated and a mutant possessing an alanine mutation at serine 225 (S225A) were made as described previously.18,22 For infection of adenoviral constructs, HUVECs were exposed to 1 plaque forming unit/cell for 2 hours in M119 medium (Sigma-Aldrich, St. Louis, MO) with 2% fetal calf serum (Hyclone, Waltham, MA) and a further 94 hours with medium containing 20% fetal calf serum. Cells were infected with a dose of virus demonstrated to lead to a 5- to 10-fold increase in SK-1 protein and activity (Supplemental Figure S1, see http://ajp.amjpathol.org). The same dose of control empty vector (EV) adenovirus was used.
SK-1 Activity Assay
SK-1 activity was determined in HUVECs, after serum/GF/ECM deprivation, as described previously23 using d-erythro sphingosine (Biomol Industries, Plymouth Meeting, PA) solubilized by Triton X-100 (0.05%) and [γ-32P]ATP (PerkinElmer, Melbourne, VIC, Australia) as substrates. The results were quantified by PhosphorImaging Typhoon 9410 system (Beckman Coulter, Fullerton, CA) and the ImageQuant 5.2 program.
Immunoprecipitation and Immunoblotting
HUVECs were serum/GF/ECM-deprived (as above) and lysed in 0.5 to 1 ml of lysis buffer (50 mmol/L HEPES, 150 mmol/L NaCl, 5 mmol/L EDTA, 0.1% Nonidet P-40/Triton X-100, 20 mmol/L NaF, 1 mmol/L sodium orthovanadate, and 10 μg/ml leupeptin and aprotinin). The lysates equalized with the same amount of proteins (Bradford assay, Bio-Rad Laboratories, Hercules, CA) were used in immunoblots or immunoprecipitated with the M2 anti-FLAG (Sigma-Aldrich), anti-SK-1,22 anti-αvβ3 (Chemicon, North Ryde, NSW, Australia), or control mouse IgG1 (Sigma-Aldrich) monoclonal antibodies before immune complexes were precipitated by incubation with protein A magnetic beads (Miltenyi Biotech, Gladbach, Germany) and immunoprecipitates were separated by SDS-polyacrylamide gel electrophoresis and transferred to Hybond-P (Amersham Biosciences, Piscataway, NJ.).
Antibodies against FLAG-epitope (M2), β3 (Cell Signaling Technology, Danvers, MA), CD31 (Santa Cruz Biotechnology, Inc., Santa Cruz, CA), SK-1, talin (Cell Signaling Technology), α6 (Cell Signaling Technology), β1 (Santa Cruz Biotechnology, Inc.), Tyr576-FAK (Santa Cruz Biotechnology, Inc.), Bcl-XL (Cell Signaling Technology), Bim (BD BioSciences), inhibitor of nuclear factor-κB (IκBα) (Santa Cruz), and actin (Boehringer Mannheim, Mannheim, Germany) were used before washing and incubation with horseradish peroxidase-conjugated secondary antibody. Reactive bands were detected by chemiluminescence (ECL-Plus Western Blotting Detection Reagents, Amersham Pharmacia Biotech, Little Chalfont, Buckinghamshire, UK) using an LAS-4000 (Fuji Photo Film Co. Ltd., Valhalla, NY.) and quantified using the ImageQuant 5.2 program.
Immunofluorescence and Confocal Microscopy
HUVECs were replated into fibronectin-coated glass LabTek chamber slides (Nalg Nunc, Rochester, NY) 48 hours after adenovirus infection. Twelve hours later adherent cells were washed, serum/GF-deprived for 150 minutes, and then fixed with 4% paraformaldehyde and blocked with 5% bovine serum albumin/PBS and, if required, 0.1% Triton X-100 in PBS was used to permeabilize cells before 1 μg of antibody to WOW-1 (gift of S Shattil, University of California, San Diego, La Jolla, CA), Tyr576-FAK, talin, vinculin (Chemicon), or isotype control was administered followed by Alexa 594 (Invitrogen). Cell nuclei were counterstained with 0.5 μg/ml 4,6-diamidino-2-phenylindole (Sigma). Cells were viewed and captured using a ×60 water-immersion objective on an Olympus IX70 inverted microscope linked to a Bio-Rad Radiance 2100 confocal microscope (Bio-Rad, Gladesville, NSW, Australia). Fluorescence intensity was quantified using AnalySIS Life Sciences software (Olympus); an average of 15 cells were captured per experiment and six separate experiments were executed.
Attachment Assay
HUVECs (3 × 104 cells) were plated in quadruplicate into vitronectin (1 μg/ml, Promega, Madison, WI) or laminin (0.5 μg/ml, Roche Diagnostics, Indianapolis, IN)-precoated 96-well plates and allowed to adhere for 30 minutes at 37°C before aspiration of nonadherent cells and extensive washing. MTS reagent (Invitrogen) was used to determine the percentage of cells remaining (as per the manufacturer’s instructions) and compare with control wells still containing 3 × 104 cells.
Small Interfering RNA Transfection
As adapted from methods described previously,24 small interfering RNA (siRNA) targeted to human β3 (5′-CCACUGUAUAAAGAGGCCACGUCUA-3′ and 5′-UAGACGUGGCCUCUUUAUACAGUGG-3′) and control “Validated Stealth” siRNA were synthesized by Invitrogen before transfection into HUVECs using HiPerfect (Qiagen, Gaithersburg, MD) in EGMII medium (Clonetics, Lonza, Basel, Switzerland) when cells were at 60% confluent. After 24 hours, culture media were changed to complete Medium 199-20% fetal calf serum until cells were harvested at 48 hours post-transfection.
Caspase 3 Activity
Activity of the enzyme caspase 3 was measured using a colorimetric assay kit (R&D Systems, Lille, France) according to the manufacturer’s instructions.
Statistical Analysis
Data are expressed as the mean ± SEM from at least four experiments, unless stated otherwise. Statistical analysis was performed by Student’s t-test or two-way analysis of variance for multiple comparisons, and P < 0.05 was considered significant.
Results
Sphingosine Kinase-1 Enhances αVβ3 Expression and Activity by ECs
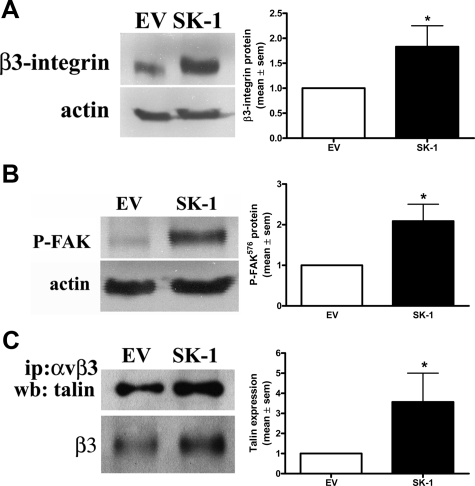
To investigate a role for SK-1 in αvβ3 function we used an adenovirus delivery system to overexpress SK-1 cDNA in HUVECs. The dose of virus was adjusted to give a 4- to 8-fold increase in SK-1 protein and activity above EV control (Supplemental Figure S1, A–C, see http://ajp.amjpathol.org). This level has previously been shown to enhance EC survival18 and is similar to the levels achieved for endogenous increases in SK activity when stimulated with known activators of ECs (eg, tumor necrosis factor-α).25 On exposure of the cells to pro-apoptotic conditions, namely removal of serum, GFs, and the ECM, the β3-integrin protein levels in the SK-1-overexpressing ECs demonstrated a significant increase compared with EV (Figure 1A). Notably, this 150-minute serum/GF/ECM deprivation was the minimum time required for significant differences to be seen between EV and SK-1-overexpressing ECs. To determine whether overexpression of SK-1 promotes integrin activity we investigated the phosphorylation status of FAK with the catalytic site of tyrosine 576 being indicative of integrin activation.26 Figure 1B shows that ECs overexpressing SK-1 and depleted of serum and GF exhibit an increase in Tyr576 phosphorylation of FAK. In addition, immunoprecipitation of αvβ3 from SK-1-overexpressing ECs not only showed increased β3 protein levels but also showed a 3.5 ± 1.4-fold increase in αvβ3-associated talin in these cells (Figure 1C), a further indication of integrin activation. This SK-1-mediated increase in αvβ3 integrin expression and activation of ECs seems restricted to β3 as no change was observed for β1 integrin (Supplemental Figure S2, see http://ajp.amjpathol.org).
Figure 1.
SK-1 promotes αvβ3 expression and activation on ECs in response to serum/GF/ECM deprivation. A: Levels of β3 protein in HUVECs infected with adenovirus containing SK-1 cDNA (SK-1) or empty vector control (EV) and deprived of serum/GF/ECM for 150 minutes were examined for β3 integrin (A) and FAK phosphorylation (B) at tyrosine 576 by immunoblotting. Actin levels were used to confirm equal loading; results are normalized to actin and EV control and expressed as the mean ± SEM of three experiments; *P < 0.05 versus EV control. C: Equal lysate amounts from factor-deprived HUVECs overexpressing empty vector control or SK-1 were immunoprecipitated (ip) with an anti-αvβ3 antibody. Precipitates were examined for talin co-association via immunoblotting (wb) and quantified with results being normalized to EV control and expressed as the mean ± SEM of three experiments; *P < 0.05 versus EV control. Integrin β3 levels were used to confirm pulldowns.
Sphingosine Kinase-1 Activates αvβ3 Integrin and Mediates Focal Adhesion Development
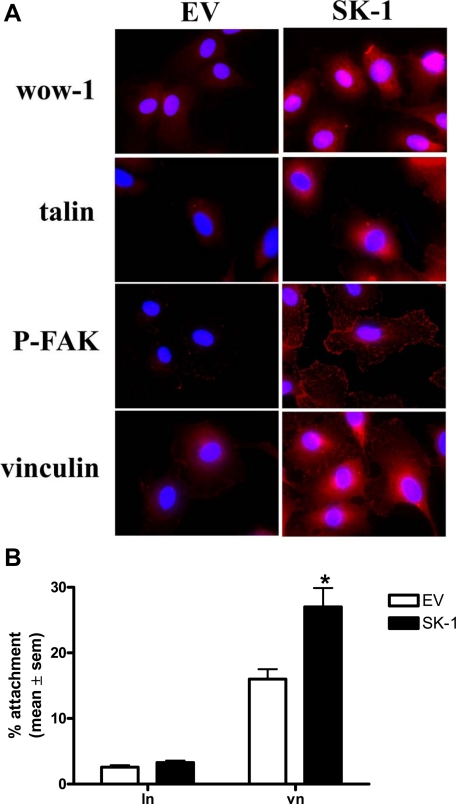
To determine whether the affinity of αvβ3 was also modulated by SK-1 we used the monovalent ligand-mimetic antibody, WOW-1, which selectively binds to activated αvβ3 with high affinity.27 As shown in Figure 2A, very little WOW-1 binds to EV cells deprived of serum/GF. In contrast, the SK-1 overexpressing ECs exhibit distinct WOW-1-positivity as reported previously.28
Figure 2.
SK-1 promotes WOW-1 binding and focal adhesion formation in ECs. A: HUVECs overexpressing SK-1 or EV control after serum/GF deprivation for 120 minutes were assessed for localization of activated αvβ3 (WOW-1), talin, P-FAK, and vinculin (red) with cells identified by nuclei staining with 4,6-diamidino-2-phenylindole (DAPI; blue), immunofluorescence, and confocal microscopy. Results are representative of six experiments. B: EV and SK-1 cells were serum/GF-deprived for 120 minutes and plated on laminin (ln) or vitronectin (vn) for 30 minutes before extensive washing. Attached cell numbers were determined by microscopy. Results are the mean ± SEM of six experiments; *P < 0.05 versus EV control.
Activated integrins relocate and cluster into focal adhesions in conjunction with talin and vinculin for contribution to cell survival signaling.28,29 ECs were seeded onto chamber slides at subconfluent numbers such that the serum/GF-deprived cells could be assessed independent of cell-cell contact interactions. As shown in Figure 2A, an increase in talin clustering was observed in ECs overexpressing SK-1. An increase in clustering of phosphorylated FAK and vinculin was also observed in ECs overexpressing SK-1 (Figure 2A) further supporting integrin activation. AnalySIS Life Sciences software confirmed differences in fluorescence intensity between groups, and liposome-mediated transfection of pcDNA3-SK-1 recapitulated these results (not shown). To investigate whether αvβ3 activation translated to functional differences, we assessed the ability of SK-1 to alter EC attachment. As shown in Figure 2B, ECs overexpressing SK-1 exhibited significantly greater attachment to vitronectin compared their EV controls. This difference was not observed on laminin to which multiple integrins bind. Thus, enhanced SK-1 expression up-regulates and activates αvβ3 in EC after survival factor deprivation.
Sphingosine Kinase-1, β3, and CD31 Form a Complex in ECs
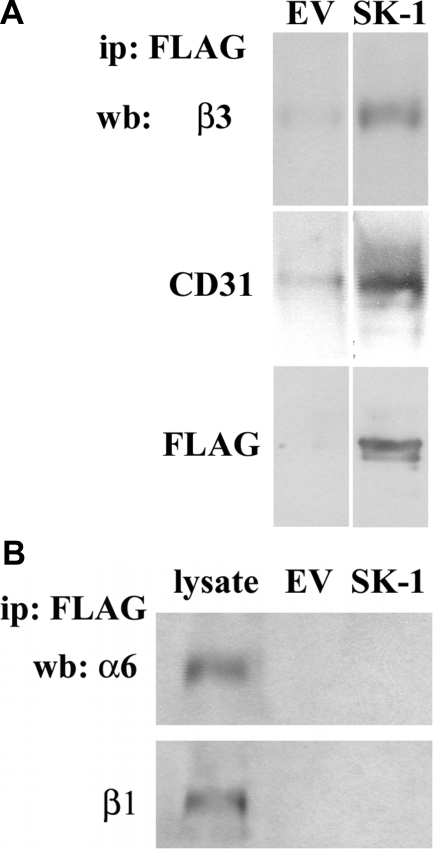
Because SK-1 has previously been identified to associate with membrane proteins19 we next used immunoprecipitation assays to investigate whether SK-1 was associated with αvβ3 in HUVECs. As shown in Figure 3A (top panel), immunoprecipitations targeting overexpressed SK-1 (using anti-FLAG) in serum/GF/ECM-deprived ECs together with subsequent immunoblots identified β3 integrin in a complex with SK-1. Immunoblotting with anti-CD31 also identified its association with SK-1 and β3 (Figure 3A, middle panels). Reciprocal immunoprecipitations with antibodies to either αvβ3 or CD31 confirmed these associations. Importantly, this association was not seen with two other EC-expressed integrin subunits, α6 and β1 (Figure 3B).
Figure 3.
SK-1 overexpression induces co-association of SK-1, αvβ3 and CD31. A: HUVECs overexpressing SK-1 or EV control were serum/GF/ECM-deprived for 150 minutes before immunoprecipitation (ip) of equal lysate amounts with an anti-FLAG antibody to pulldown FLAG-tagged SK-1. Precipitates were examined for β3 and CD31 co-association as well as FLAG pulldown via immunoblotting (wb). Results are representative of five experiments. B: Precipitates were examined for α6 and β1 association with FLAG-SK-1 via immunoblotting. Results are representative of three experiments.
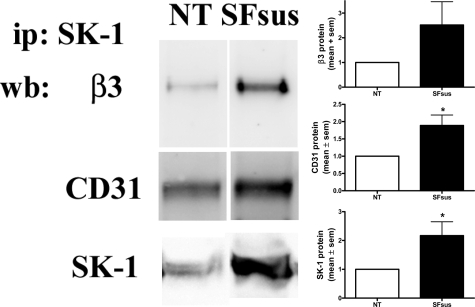
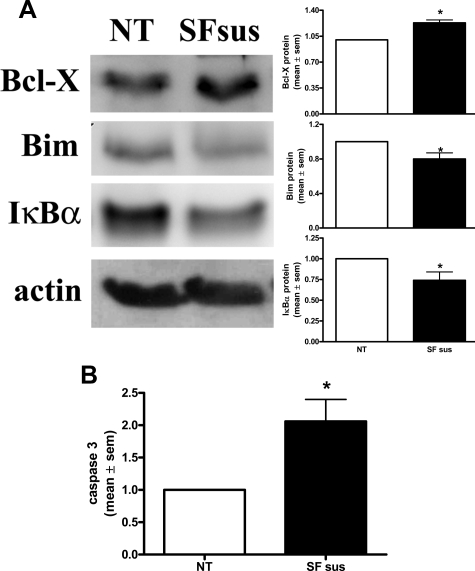
The SK-1, αvβ3, and CD31 complex is not just an artifact of SK-1 overexpression but occurs with endogenous proteins in ECs. In untreated HUVECs endogenous SK-1 forms a complex with β3 and CD31 (Figure 4, NT), and the amount of this complex is significantly increased after serum/GF/ECM deprivation (Figure 4, SFsus). EC deprivation of serum/GF/ECM also caused a significant increase in the expression of the prosurvival factor Bcl-XL and a decrease the anti-apoptotic factor Bim (Figure 5A). NF-κB activation has been linked to αvβ3 integrin-induced EC survival30 and in response to factor deprivation ECs also exhibit reduced IκBα levels (Figure 5A), thus suggesting an attempt by ECs to activate a repertoire of cell survival signals under these conditions. However, the increased caspase 3 levels suggest that the cells are challenged in their survival and ultimately undergo apoptosis (Figure 5B). We assume that this failure to rescue the cells is a result of the extent and duration of the apoptotic challenge.
Figure 4.
HUVECs deprived of serum/GF/ECM exhibit increased endogenous co-association of SK-1, αvβ3 and CD31. Equal lysate amounts from HUVECs either untreated (NT) or serum/GF/ECM-deprived (SFsus) were immunoprecipitated (ip) with an anti-SK-1 antibody to pull down endogenous SK-1. Precipitates were examined for β3 and CD31 co-association via immunoblotting (wb). Quantified results shown are the mean ± SEM of five experiments and normalized untreated (NT) controls. *P < 0.05 versus NT.
Figure 5.
Activation of Bcl-XL/Bim and NF-κB survival pathways after serum/GF/ECM deprivation of ECs. A: Equal lysates from HUVECs either untreated (NT) or serum/GF/ECM-deprived (SFsus) were assessed for Bcl-XL, Bim and IκBα protein expression by immunoblotting. Membranes were counterblotted with antibody to actin to confirm equal loading. All results are normalized to NT control and expressed as the mean ± SEM of at least nine, nine, and five experiments, respectively. *P < 0.05 versus NT control. B: Equal lysate amounts were assessed for caspase 3 activity. Results depict the mean ± SEM of six experiments and normalized to NT controls. *P < 0.05 versus NT controls.
Sphingosine Kinase-1 Phosphorylation Is Required for Heterotrimeric Complex Formation and EC Survival
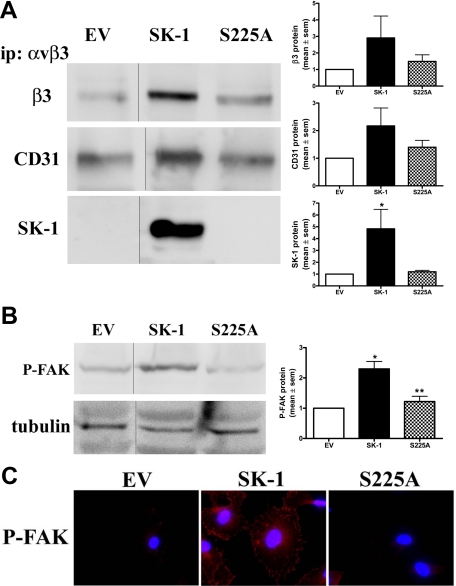
To test whether the activation of SK-1 is critical for SK-1/β3/CD31 complex formation during serum/GF/ECM deprivation we overexpressed wild-type SK-1 or the SK-1 mutant (S225A), which retains intrinsic catalytic activity (Supplemental Figure S3A, see http://ajp.amjpathol.org) but cannot be further activated and therefore does not translocate to the plasma membrane.17,22 Figure 6A shows increased association of β3, CD31 and SK-1 in SK-1 wild-type-overexpressing ECs compared with EV controls. In cells overexpressing the S225 mutant there was no increase in this heterotrimeric complex (Figure 6A) (at times, below the level of detection for endogenous SK-1 in EV and S225A ECs). Furthermore, the S225A mutant failed to induce the phosphorylation of FAK (Figure 6B) and failed to induce clustering of phosphorylated FAK (Figure 6C), both of which were changed with wild-type SK-1 overexpression. Taken together, these results suggest that the recruitment of more SK-1, β3 and CD31 into a complex and subsequent integrin activation is, in part, dependent on SK-1 activation via serine 225 phosphorylation.
Figure 6.
SK-1 phosphorylation promotes co-association of SK-1, αvβ3 and CD31 in factor-deprived HUVECs. A: Equal lysate amounts from HUVECs overexpressing empty vector control (EV), SK-1 (SK-1), or SK-1 mutant (S225A) after serum/GF/ECM deprivation were immunoprecipitated (ip) for αvβ3. Precipitates were examined for β3, CD31 and SK-1 co-association via immunoblotting. Gel lanes from the same experiment have been cut and re-orientated to better illustrate the changes from EV to SK and S225A. Results are quantified and normalized to EV controls and show the mean ± SEM of three experiments. *P < 0.05 versus EV. B: Immunoblotting of EV-, SK-1-, and S225A-overexpressing HUVECs deprived of serum/GF/ECM were examined for protein levels of P-FAK. Membranes were counterblotted with antibody to tubulin to confirm equal loading and gel lanes from the same experiment have been cut and re-orientated to better illustrate the changes from EV to SK-1 and S225A. Results are quantified, normalized to EV control, and expressed as the mean ± SEM of three experiments. *P < 0.05 versus EV control; **P < 0.05 versus SK-1. C: Immunofluorescence and confocal microscopy determined localization of P-FAK in HUVEC overexpressing EV, SK-1, or S225A (red) with nuclei staining by 4,6-diamidino-2-phenylindole (DAPI; blue). Results are representative of four experiments.
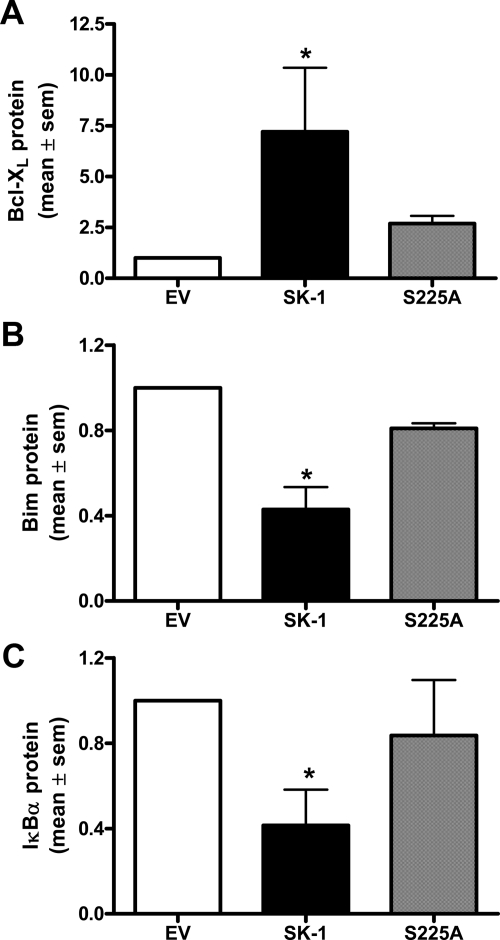
In ECs deprived of serum/GF/ECM, overexpression of wild-type SK-1 significantly increased the expression of the prosurvival factor Bcl-XL and decreased the anti-apoptotic factor Bim. In contrast, ECs overexpressing the S225A mutant did not significantly alter their levels of Bcl-XL, Bim, or IκBα compared with EV controls (Figure 7, A–C; immunoblots shown in Supplemental Figure S3B, see http://ajp.amjpathol.org). The levels of IκBα and Bim, under the conditions of analysis, are low and although the decreases seen are small (Supplemental Figure S3B, see http://ajp.amjpathol.org), they are the consistent as seen with the quantification of three to five lines (Figure 7, B and C).
Figure 7.
SK-1 phosphorylation is required for the activation of Bcl-XL/Bim and NF-κB survival pathways. Quantification of immunoblots of equal lysates from HUVECs overexpressing empty vector control (EV), SK-1 (SK-1), or SK-1 mutant (S225A) and serum/GF/ECM-deprived were examined for protein levels of Bcl-XL (A), Bim (B) and IκBα (C). Membranes (exemplified in Supplemental Figure S3, see http://ajp.amjpathol.org) were counterblotted with antibody to actin to confirm equal loading before quantification. All results are normalized to EV control and expressed as the mean ± SEM of three to five experiments; One-way analysis of variance with Bonferroni correction. *P < 0.05 versus EV control.
β3 Integrin Association with SK-1 Promotes EC Survival
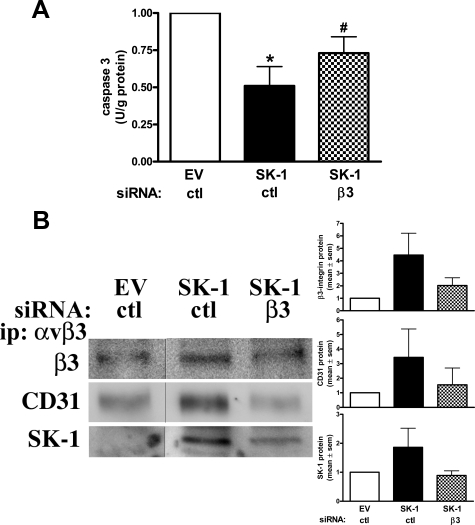
Finally, to confirm a role for β3 integrin with SK-1 in EC survival after factor deprivation we depleted β3 using siRNA when β3 protein levels were reduced to 0.5 ± 0.1 of control levels (Supplemental Figure S4, see http://ajp.amjpathol.org). In these cells the increased survival capabilities gained by overexpression of SK-1 are attenuated when β3 is knocked down as evidenced by the reduced caspase 3 activity levels (Figure 8A). Knockdown of β3 also resulted in a significant loss of β3/SK-1/CD31 complex formation (Figure 8B). Together with our previous results linking CD31 in SK-1-mediated survival, these results suggest that the complex formation of β3, CD31 and SK-1 is important in SK-1-mediated EC survival during times of EC stress.
Figure 8.
SK-1-induced SK-1/αvβ3/CD31 complex formation and rescue of EC apoptosis is attenuated with β3 knockdown. HUVECs overexpressing SK-1 or control EV were transfected with siRNA against β3 or control (ctl) and serum/GF/ECM deprived for 150 minutes. A: Equal lysate amounts were assessed for caspase 3 activity. Results depict the mean ± SEM of three experiments and normalized to EV controls. *P < 0.05 versus EV controls; #P < 0.05 versus SK-1 ctl controls. B: Equal lysate amounts were immunoprecipitated (ip) for αvβ3 and immunoblotted for β3, CD31 or SK-1. Results depict the mean ± SEM of four experiments.
Discussion
Our studies here demonstrate a physical association between three major signaling molecules involved in EC survival: SK-1, αvβ3, and CD31. Because depletion of any of these proteins result in cell death,18,30,31,32 we postulate that this heterotrimeric complex is essential for maintenance of EC survival. Our results also suggest that SK-1 is a key signaling molecule linking the integrin and CD31 survival pathways in EC.
Herein we demonstrate that SK-1 overexpression, to levels of activity equivalent to that seen after VEGF or GF stimulation, leads to increased αvβ3 integrin activation and increased SK-1/αvβ3/CD31 complex formation. Evidence for this observation comes from the phosphorylation of FAK at tyrosine 576 (P-FAK) when SK-1 is overexpressed. Because FAK is an early modulator of the activated integrin signaling cascade, phosphorylation at this site is known to increase its catalytic activity and is an important step in formation of an active signaling complex.26 In addition, SK-1 overexpression results in enhanced binding of the WOW-1 antibody, which contains an RGD tract in its variable region and binds only to unoccupied, high-affinity αvβ3 and αvβ5 integrins.27 Finally, P-FAK, talin and vinculin are reorganized into clusters consistent with integrin activation.33,34,35 We demonstrate that SK-1, αvβ3 and CD31 are closely associated in ECs, and modification of this heterotrimeric complex is biologically important with increased complex formation during a stress response (such as proapoptotic conditions of serum, GF, and ECM deprivation). On ECs, αVβ3 integrin is intimately linked with the receptor for VEGF (VEGFR-2) with its ligation also promoting αvβ3 integrin expression and activation.36 This is a reciprocal relationship with αvβ3 being able to enhance the phosphorylation of VEGFR-2 in response to VEGF and subsequent activation of stress-activated protein kinase 2/p38 and FAK. More recently, a role for SK/S1P in VEGFR-2 phosphorylation has been identified.37,38 Together with our own observations of SK-1 promoting EC survival through CD3118 there is now increasing evidence for a complex and convoluted relationship between SK-1/S1P, αvβ3, CD31 and VEGF signaling.
SK-1 can exist in a basal, intrinsic state, which we have shown inhibits EC permeability.24 SK-1 also has an agonist-induced activation state, which occurs, at least in response to tumor necrosis factor-α and phorbol esters, as a direct consequence of phosphorylation at serine 225 by extracellular signal-regulated kinase 1/2.22 The effects of this single phosphorylation are twofold: it is required for agonist-induced increases in the catalytic activity of SK-1 and is necessary for translocation of this protein from the cytosol to the plasma membrane.17 In the current study, we report that the co-association of SK-1 with αvβ3 and CD31 requires SK-1 phosphorylation at serine 225. Mutation at serine 225 (S225A) retained an equivalent increase in SK protein levels as the wild-type SK-1 but was not available for recruitment into a complex with αvβ3 nor did it enhance the recruitment of β3 and CD31 into the complex. Furthermore, S225A did not activate integrins as judged by its failure to induce FAK phosphorylation or FAK cluster formation nor did it induce changes in Bcl-XL, Bim, or NFκB activation. With previous reports identifying a role for SK-1 phosphorylation to induce translocation of SK-1 to the plasma membrane for oncogenic signaling17 but not required at the phagosomal membrane for phagocytosis,39 we now extend these findings to suggest that phosphorylation of SK-1 is integral for αvβ3 integrin activation, SK-1/αvβ3/CD31 complex formation, and subsequent downstream signaling for EC survival. Although factor-deprived ECs overexpressing SK-1 exhibit a reduction in caspase 3 (Figure 8), this is not seen in normal ECs under factor-free conditions despite the pro-survival Bcl-X and NF-κB being up-regulated (Figure 5). These results may suggest that under the experimental system used the levels of endogenous SK-1 are insufficient to overcome the major stress stimulant of GF removal. Although we have not yet fully elucidated these seemingly opposing results, increasing evidence suggests that these survival pathways are intimately linked. Serum-deprived ECs overexpressing SK-1 have been shown to exhibit increased Akt phosphorylation18 and prolonged activation of Akt is likely to delay caspase 3 activity.40 In addition, studies suggest that caspase 3, NF-κB and the Bcl family may act in both directly competing and noncompeting pathways to control cell survival.41,42 Thus, the recovery effect of ECs to factor deprivation is likely to involve an intricate network of survival pathways and further investigations are required to unravel their relationship.
Sphingosine, the substrate of SK-1, is concentrated in the plasma membrane43 and one explanation for the need to localize SK-1 is to place it in the appropriate sphingosine microenvironment. Indeed, the generation of S1P at the plasma membrane may result in S1P secretion and an extracellular mode of action via its surface receptors (S1P1-5), a pathway previously identified to promote cell survival (reviewed in Ref. 11). S1P also acts intracellularly and independent of S1P receptors to enhance EC proliferation and suppress apoptosis.44 Increasing evidence for S1P as a bone fide second messenger11 includes our own studies showing that S1P receptor inhibitors VPC23019 and JTE-013, although efficient for inhibiting agonist-induced SK-1 activity,24,45 failed to have any effect on survival in the SK-1-overexpressing EC24 or on the differentiation of endothelial progenitor cells.46 Paik et al47 previously described S1P1- and S1P3-mediated Rho signaling in αvβ3 activation for EC migration. This current study suggests a mode of action via membrane-localized components that is key to survival signaling pathways. Whether this involves one or more of the S1P family of receptors is yet to be fully elucidated.
Fukuda et al19 previously showed a physical interaction, at least in transfected cells, between CD31 and SK-1 with SK-1 binding at residues 594 to 643 in the cytoplasmic domain of CD31, and the association strengthened with CD31 de-phosphorylation. Our previous study identified increased EC survival with SK-1 overexpression and was congruent with increased CD31 protein levels and decreased CD31 dephosphorylation.18 Formation of a heterotrimeric complex also supports Wong et al20 for cis-interaction between αvβ3 with CD31 on pro-T cells. Indeed, in vivo studies of CD31-, αv-, and SK-1-deficient mice all demonstrate vascular abnormalities either during their development or angiogenic responses to foreign body challenges.24,48,49,50 Although these vascular defects have not been attributed to a functional deficiency of the SK-1/αvβ3/CD31 complex, it is tantalizing to speculate on its involvement. Evidence for SK-1 having multiple associations and binding partners comes from recent data identifying its association with filamin A, an actin-binding protein for regulation of cell migration and lamellipodia formation51 as well as the eukaryotic elongation factor 1A, an association that regulates the catalytic activity of SK-1.52 Herein we have identified the formation of a heterotrimeric complex between SK-1, αvβ3 and CD31 in ECs that is integral for their survival.
Angiogenic vessels in wounds and in tumors express high levels of SK (reviewed in Ref. 53) and αvβ38 and indeed therapeutic targeting of this integrin is currently being evaluated. Our results presented here establish the link between endogenous SK-1 and two signaling cascades deemed essential for EC survival (integrins and CD31). Thus, targeting of SK-1 may serve to inhibit tumor cell growth by controlling the aberrant tumor-associated angiogenesis through action on the integrin αvβ3 as well as CD31, both of which mediate EC survival.
Supplementary Material
Acknowledgments
We thank Jen Drew and Anna Sapa for preparing the endothelial cells and the staff at Women’s and Children’s Hospital and Burnside Memorial Hospital for collection of the umbilical cords.
Footnotes
Address reprint requests to Claudine S. Bonder, Ph.D., Division of Human Immunology, Centre for Cancer Biology, Hanson Institute, PO Box 14, Rundle Mall, Adelaide, SA 5000, Australia. E-mail: claudine.bonder@health.sa.gov.au; or Jennifer R. Gamble, Ph.D., Centenary Institute for Cancer Medicine and Cell Biology, Locked Bag No 6, Newtown 2042, NSW, Australia. E-mail: j.gamble@centenary.org.au.
Supported by the Australian National Health and Medical Research Council (NHMRC) (Project Grant 430907 to C.S.B.), an NHMRC Peter Doherty Fellowship (278806 to C.S.B.), a Royal Adelaide Hospital Florey Fellowship (to C.S.B.), a Medical Foundation Fellowship, University of Sydney to J.R.G., an NHMRC Program Grant (349332 to J.R.G. and M.A.V.), and the National Heart Foundation of Australia (Project Grant GO6A2493).
Supplemental material for this article can be found on http://ajp.amjpathol.org.
References
- Meredith JE, Jr, Fazeli B, Schwartz MA. The extracellular matrix as a cell survival factor. Mol Biol Cell. 1993;4:953–961. doi: 10.1091/mbc.4.9.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain RK. Molecular regulation of vessel maturation. Nat Med. 2003;9:685–693. doi: 10.1038/nm0603-685. [DOI] [PubMed] [Google Scholar]
- Araki S, Shimada Y, Kaji K, Hayashi H. Apoptosis of vascular endothelial cells by fibroblast growth factor deprivation. Biochem Biophys Res Commun. 1990;168:1194–1200. doi: 10.1016/0006-291x(90)91155-l. [DOI] [PubMed] [Google Scholar]
- Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- Parsons JT, Martin KH, Slack JK, Taylor JM, Weed SA. Focal adhesion kinase: a regulator of focal adhesion dynamics and cell movement. Oncogene. 2000;19:5606–5613. doi: 10.1038/sj.onc.1203877. [DOI] [PubMed] [Google Scholar]
- Katsumi A, Orr AW, Tzima E, Schwartz MA. Integrins in mechanotransduction. J Biol Chem. 2004;279:12001–12004. doi: 10.1074/jbc.R300038200. [DOI] [PubMed] [Google Scholar]
- Brooks PC, Montgomery AM, Rosenfeld M, Reisfeld RA, Hu T, Klier G, Cheresh DA. Integrin αvβ3 antagonists promote tumor regression by inducing apoptosis of angiogenic blood vessels. Cell. 1994;79:1157–1164. doi: 10.1016/0092-8674(94)90007-8. [DOI] [PubMed] [Google Scholar]
- Brooks PC, Clark RA, Cheresh DA. Requirement of vascular integrin αvβ3 for angiogenesis. Science. 1994;264:569–571. doi: 10.1126/science.7512751. [DOI] [PubMed] [Google Scholar]
- Sundberg C, Rubin K. Stimulation of β1 integrins on fibroblasts induces PDGF independent tyrosine phosphorylation of PDGF β-receptors. J Cell Biol. 1996;132:741–752. doi: 10.1083/jcb.132.4.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soldi R, Mitola S, Strasly M, Defilippi P, Tarone G, Bussolino F. Role of αvβ3 integrin in the activation of vascular endothelial growth factor receptor-2. EMBO J. 1999;18:882–892. doi: 10.1093/emboj/18.4.882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alemany R, van Koppen CJ, Danneberg K, Ter BM, Meyer Zu HD. Regulation and functional roles of sphingosine kinases. Naunyn Schmiedebergs Arch Pharmacol. 2007;374:413–428. doi: 10.1007/s00210-007-0132-3. [DOI] [PubMed] [Google Scholar]
- Yatomi Y, Ozaki Y, Ohmori T, Igarashi Y. Sphingosine 1-phosphate: synthesis and release. Prostaglandins Other Lipid Mediat. 2001;64:107–122. doi: 10.1016/s0090-6980(01)00103-4. [DOI] [PubMed] [Google Scholar]
- Pappu R, Schwab SR, Cornelissen I, Pereira JP, Regard JB, Xu Y, Camerer E, Zheng YW, Huang Y, Cyster JG, Coughlin SR. Promotion of lymphocyte egress into blood and lymph by distinct sources of sphingosine-1-phosphate. Science. 2007;316:295–298. doi: 10.1126/science.1139221. [DOI] [PubMed] [Google Scholar]
- Hanel P, Andreani P, Graler MH. Erythrocytes store and release sphingosine 1-phosphate in blood. FASEB J. 2007;21:1202–1209. doi: 10.1096/fj.06-7433com. [DOI] [PubMed] [Google Scholar]
- Venkataraman K, Lee YM, Michaud J, Thangada S, Ai Y, Bonkovsky HL, Parikh NS, Habrukowich C, Hla T. Vascular endothelium as a contributor of plasma sphingosine 1-phosphate. Circ Res. 2008;102:669–676. doi: 10.1161/CIRCRESAHA.107.165845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez T, Hla T. Structural and functional characteristics of S1P receptors. J Cell Biochem. 2004;92:913–922. doi: 10.1002/jcb.20127. [DOI] [PubMed] [Google Scholar]
- Pitson SM, Xia P, Leclercq TM, Moretti PA, Zebol JR, Lynn HE, Wattenberg BW, Vadas MA. Phosphorylation-dependent translocation of sphingosine kinase to the plasma membrane drives its oncogenic signalling. J Exp Med. 2005;201:49–54. doi: 10.1084/jem.20040559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limaye V, Li X, Hahn C, Xia P, Berndt MC, Vadas MA, Gamble JR. Sphingosine kinase-1 enhances endothelial cell survival through a PECAM-1-dependent activation of PI-3K/Akt and regulation of Bcl-2 family members. Blood. 2005;105:3169–3177. doi: 10.1182/blood-2004-02-0452. [DOI] [PubMed] [Google Scholar]
- Fukuda Y, Aoyama Y, Wada A, Igarashi Y. Identification of PECAM-1 association with sphingosine kinase 1 and its regulation by agonist-induced phosphorylation. Biochim Biophys Acta. 2004;1636:12–21. doi: 10.1016/j.bbalip.2003.11.006. [DOI] [PubMed] [Google Scholar]
- Wong CW, Wiedle G, Ballestrem C, Wehrle-Haller B, Etteldorf S, Bruckner M, Engelhardt B, Gisler RH, Imhof BA. PECAM-1/CD31 trans-homophilic binding at the intercellular junctions is independent of its cytoplasmic domain; evidence for heterophilic interaction with integrin αvβ3 in Cis. Mol Biol Cell. 2000;11:3109–3121. doi: 10.1091/mbc.11.9.3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litwin M, Clark K, Noack L, Furze J, Berndt M, Albelda S, Vadas M, Gamble J. Novel cytokine-independent induction of endothelial adhesion molecules regulated by platelet/endothelial cell adhesion molecule (CD31). J Cell Biol. 1997;139:219–228. doi: 10.1083/jcb.139.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitson SM, Moretti PA, Zebol JR, Lynn HE, Xia P, Vadas MA, Wattenberg BW. Activation of sphingosine kinase 1 by ERK1/2-mediated phosphorylation. EMBO J. 2003;22:5491–500. doi: 10.1093/emboj/cdg540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitson SM, D'Andrea RJ, Vandeleur L, Moretti PA, Xia P, Gamble JR, Vadas MA, Wattenberg BW. Human sphingosine kinase: purification, molecular cloning and characterization of the native and recombinant enzymes. Biochem J. 2000;350 Pt 2:429–441. [PMC free article] [PubMed] [Google Scholar]
- Li X, Stankovic M, Bonder CS, Hahn CN, Parsons M, Pitson SM, Xia P, Proia RL, Vadas MA, Gamble JR. Basal and angiopoietin-1-mediated endothelial permeability is regulated by sphingosine kinase-1. Blood. 2008;111:3489–3497. doi: 10.1182/blood-2007-05-092148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia P, Gamble JR, Rye KA, Wang L, Hii CS, Cockerill P, Khew-Goodall Y, Bert AG, Barter PJ, Vadas MA. Tumor necrosis factor-α induces adhesion molecule expression through the sphingosine kinase pathway. Proc Natl Acad Sci USA. 1998;95:14196–14201. doi: 10.1073/pnas.95.24.14196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calalb MB, Polte TR, Hanks SK. Tyrosine phosphorylation of focal adhesion kinase at sites in the catalytic domain regulates kinase activity: a role for Src family kinases. Mol Cell Biol. 1005;15:954–963. doi: 10.1128/mcb.15.2.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pampori N, Hato T, Stupack DG, Aidoudi S, Cheresh DA, Nemerow GR, Shattil SJ. Mechanisms and consequences of affinity modulation of integrin αvβ. J Biol Chem. 1999;274:21609–21616. doi: 10.1074/jbc.274.31.21609. [DOI] [PubMed] [Google Scholar]
- Kiosses WB, Shattil SJ, Pampori N, Schwartz MA. Rac recruits high-affinity integrin αvβ3 to lamellipodia in endothelial cell migration. Nat Cell Biol. 2001;3:316–320. doi: 10.1038/35060120. [DOI] [PubMed] [Google Scholar]
- Cluzel C, Saltel F, Lussi J, Paulhe F, Imhof BA, Wehrle-Haller B. The mechanisms and dynamics of αvβ3 integrin clustering in living cells. J Cell Biol. 2005;171:383–392. doi: 10.1083/jcb.200503017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rüegg C, Mariotti A. Vascular integrins: pleiotropic adhesion and signaling molecules in vascular homeostasis and angiogenesis. Cell Mol Life Sci. 2003;60:1135–1157. doi: 10.1007/s00018-003-2297-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao C, Sun W, Christofidou-Solomidou M, Sawada M, Newman DK, Bergom C, Albelda SM, Matsuyama S, Newman PJ. PECAM-1 functions as a specific and potent inhibitor of mitochondrial-dependent apoptosis. Blood. 2003;102:169–179. doi: 10.1182/blood-2003-01-0003. [DOI] [PubMed] [Google Scholar]
- Erdreich-Epstein A, Tran LB, Cox OT, Huang EY, Laug WE, Shimada H, Millard M. Endothelial apoptosis induced by inhibition of integrins αvβ3 and αvβ5 involves ceramide metabolic pathways. Blood. 2005;105:4353–4361. doi: 10.1182/blood-2004-08-3098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin KH, Boerner SA, Parsons JT. Regulation of focal adhesion targeting and inhibitory functions of the FAK related protein FRNK using a novel estrogen receptor “switch.”. Cell Motil Cytoskeleton. 2002;51:76–88. doi: 10.1002/cm.10018. [DOI] [PubMed] [Google Scholar]
- Arold ST, Hoellerer MK, Noble ME. The structural basis of localization and signaling by the focal adhesion targeting domain. Structure. 2002;10:319–327. doi: 10.1016/s0969-2126(02)00717-7. [DOI] [PubMed] [Google Scholar]
- Hayashi I, Vuori K, Liddington RC. The focal adhesion targeting (FAT) region of focal adhesion kinase is a four-helix bundle that binds paxillin. Nat Struct Biol. 2002;9:101–106. doi: 10.1038/nsb755. [DOI] [PubMed] [Google Scholar]
- Eliceiri BP, Cheresh DA. The role of αv integrins during angiogenesis: insights into potential mechanisms of action and clinical development. J Clin Invest. 1999;103:1227–1230. doi: 10.1172/JCI6869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusnati M, Tanghetti E, Dell'Era P, Gualandris A, Presta M. αvβ3 integrin mediates the cell-adhesive capacity and biological activity of basic fibroblast growth factor (FGF-2) in cultured endothelial cells. Mol Biol Cell. 1997;8:2449–2461. doi: 10.1091/mbc.8.12.2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanimoto T, Jin ZG, Berk BC. Transactivation of vascular endothelial growth factor (VEGF) receptor Flk-1/KDR is involved in sphingosine 1-phosphate-stimulated phosphorylation of Akt and endothelial nitric-oxide synthase (eNOS). J Biol Chem. 2002;277:42997–43001. doi: 10.1074/jbc.M204764200. [DOI] [PubMed] [Google Scholar]
- Kusner DJ, Thompson CR, Melrose NA, Pitson SM, Obeid LM, Iyer SS. The localization and activity of sphingosine kinase 1 are coordinately regulated with actin cytoskeletal dynamics in macrophages. J Biol Chem. 2007;282:23147–23162. doi: 10.1074/jbc.M700193200. [DOI] [PubMed] [Google Scholar]
- Rusiñol AE, Thewke D, Liu J, Freeman N, Panini SR, Sinensky MS. AKT/protein kinase B regulation of BCL family members during oxysterol-induced apoptosis. J Biol Chem. 2004;279:1392–1399. doi: 10.1074/jbc.M308619200. [DOI] [PubMed] [Google Scholar]
- Kaufmann T, Schlipf S, Sanz J, Neubert K, Stein R, Borner C. Characterization of the signal that directs Bcl-xL, but not Bcl-2, to the mitochondrial outer membrane. J Cell Biol. 2003;160:53–64. doi: 10.1083/jcb.200210084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karam SM, Leblond CP. Dynamics of epithelial cells in the corpus of the mouse stomach. I. Identification of proliferative cell types and pinpointing of the stem cell. Anat Rec. 1993;236:259–279. doi: 10.1002/ar.1092360202. [DOI] [PubMed] [Google Scholar]
- Slife CW, Wang E, Hunter R, Wang S, Burgess C, Liotta DC, Merrill AH., Jr Free sphingosine formation from endogenous substrates by a liver plasma membrane system with a divalent cation dependence and a neutral pH optimum. J Biol Chem. 1989;264:10371–10377. [PubMed] [Google Scholar]
- Van Brocklyn JR, Lee MJ, Menzeleev R, Olivera A, Edsall L, Cuvillier O, Thomas DM, Coopman PJ, Thangada S, Liu CH, Hla T, Spiegel S. Dual actions of sphingosine-1-phosphate: extracellular through the Gi-coupled receptor Edg-1 and intracellular to regulate proliferation and survival. J Cell Biol. 1998;142:229–240. doi: 10.1083/jcb.142.1.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MD, Clemens JJ, Macdonald TL, Lynch KR. Sphingosine 1-phosphate analogs as receptor antagonists. J Biol Chem. 2005;280:9833–9841. doi: 10.1074/jbc.M412356200. [DOI] [PubMed] [Google Scholar]
- Bonder CS, Sun WY, Matthews T, Cassano C, Li X, Ramshaw HS, Pitson SM, Lopez AF, Coates PTH, Proia RL, Vadas MA, Gamble JR. Sphingosine kinase regulates the rate of endothelial progenitor cell differentiation. Blood. 2009;13:2108–2111. doi: 10.1182/blood-2008-07-166942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paik JH, Chae S, Lee MJ, Thangada S, Hla T. Sphingosine 1-phosphate-induced endothelial cell migration requires the expression of EDG-1 and EDG-3 receptors and Rho-dependent activation of αvβ3- and β1-containing integrins. J Biol Chem. 2001;276:11830–11837. doi: 10.1074/jbc.M009422200. [DOI] [PubMed] [Google Scholar]
- Duncan GS, Andrew DP, Takimoto H, Kaufman SA, Yoshida H, Spellberg J, Luis de la PJ, Elia A, Wakeham A, Karan-Tamir B, Muller WA, Senaldi G, Zukowski MM, Mak TW. Genetic evidence for functional redundancy of platelet/endothelial cell adhesion molecule-1 (PECAM-1): CD31-deficient mice reveal PECAM-1-dependent and PECAM-1-independent functions. J Immunol. 1999;162:3022–3030. [PubMed] [Google Scholar]
- Bader BL, Rayburn H, Crowley D, Hynes RO. Extensive vasculogenesis, angiogenesis, and organogenesis precede lethality in mice lacking all αv integrins. Cell. 1998;95:507–519. doi: 10.1016/s0092-8674(00)81618-9. [DOI] [PubMed] [Google Scholar]
- Kohno M, Momoi M, Oo ML, Paik JH, Lee YM, Venkataraman K, Ai Y, Ristimaki AP, Fyrst H, Sano H, Rosenberg D, Saba JD, Proia RL, Hla T. Intracellular role for sphingosine kinase 1 in intestinal adenoma cell proliferation. Mol Cell Biol. 2006;26:7211–7223. doi: 10.1128/MCB.02341-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maceyka M, Alvarez SE, Milstien S, Spiegel S. Filamin A links sphingosine kinase 1 and S1P1 receptor at lamellipodia to orchestrate cell migration. Mol Cell Biol. 2008;28:5687–5697. doi: 10.1128/MCB.00465-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leclercq TM, Moretti PA, Vadas MA, Pitson SM. Eukaryotic elongation factor 1A interacts with sphingosine kinase and directly enhances its catalytic activity. J Biol Chem. 2008;283:9606–9614. doi: 10.1074/jbc.M708782200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vadas M, Xia P, McCaughan G, Gamble J. The role of sphingosine kinase 1 in cancer: oncogene or non-oncogene addiction? Biochim Biophys Acta. 2008;1781:442–447. doi: 10.1016/j.bbalip.2008.06.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.