Abstract
Background
To assess relationships between pediatric lipids and subsequent cardiovascular disease (CVD) in the 4th-5th decades, we conducted 22-31 year follow-up studies (1998-2003) in former schoolchildren first studied in 1973-1976. The follow-up included 53% of eligible former subjects.
Methods
We compared pediatric and adult body mass (kg/m2) and lipids in 19 cases with ≥1 CVD event and in 789 CVD event-free subjects.
Results
Mean ± SD age was 12.3 ± 3.3 at entry and 38.5 ± 3.8 years at follow-up. Mean age at the first CVD event was 37.1 ± 4.9 years. The major novel finding of our study was that childhood triglycerides (TG) were consistently and independently associated with young adult CVD. The distributions of both childhood and adult TG were shifted to higher levels in the cases than controls. Of the 19 cases, 7 (37%) had childhood TG > the pediatric 95th percentile (153 mg/dl), and 6 of these 7 had high TG (≥ 150 mg/dl) at adult follow-up. Overall, 61% of cases had high TG as adults. After adjusting for age, sex, and race, by analysis of variance, cases had higher TG levels both in childhood and in young adulthood. A bootstrapping method and Cox’s proportional hazard analysis was used to predict CVD in the cohort with explanatory variables sex, race, and childhood BMI, LDL, log HDL cholesterol, and log TG, and adult cigarette smoking and type 2 diabetes. Childhood TG was a significant, independent, explanatory variable for young adult CVD hazard (hazard ratio 5.35, 95% CI 1.69-20.0 for each 1 unit increasing in natural logarithm scale) along with adult type 2 diabetes (hazard ratio 19.4, 95% CI 4.24-114.2).
Conclusions
Pediatric hypertriglyceridemia appears to be a significant, independent, potentially reversible correlate of young adult CVD.
INTRODUCTION
The role of triglycerides (TG) in the constellation of cardiovascular disease (CVD) risk factors has been somewhat controversial due to its strong, inverse correlation with high-density lipoprotein cholesterol (HDLC), a major CVD risk factor [1, 2]. In univariate analyses, high adult TG predict CVD, but the triglyceride-CVD relationship weakens after adjustment for plasma HDLC [1]. Nevertheless, even after adjustment for HDLC, a significant, independent relationship between TG and CVD events remains [2]. In addition, non-fasting [3-5] and fasting hypertriglyceridemia (HTG) [2, 6] are independent risk factors for CVD, particularly for women. HTG is often concurrent with centripetal obesity, hyperglycemia, hypertension, and low HDLC, metabolic syndrome [4] components associated with increased CVD events [7]. Heterogeneity in lipoprotein lipase, with reduced degradation of TG, is associated with increased CVD [8]. The question remains, however, whether HTG alone or CVD risk factors associated with HTG (obesity, hyperglycemia, hypertension, hyperinsulinemia [9]) are most important [4] in predicting CVD.
Lowering TG and raising HDLC by fibric acids have been associated with significant reductions in cardiovascular event rates [10-14]. After acute coronary syndrome, on-treatment TG <150 mg/dl was independently associated with reduced risk of recurrent CVD events [15].
As the number of childhood CVD risk factors increases, so does the severity of asymptomatic coronary and aortic atherosclerosis in young adulthood [16]. Atherosclerosis begins to develop in early life [17]. Childhood obesity is associated with young adult carotid intimal-medial thickness (CIMT), a surrogate marker of CVD [18]. In Finnish children ages 12 to 18 years at baseline, with follow-up 23 years later [19], apoB and the apoB/apoA-I ratio were directly (p < 0.001) related and apoA-I was inversely (p = 0.01) related with adulthood CIMT. Juonala et al [20] have reported that cardiovascular risk factors identified in childhood and adolescence predict decreased carotid artery elasticity in adulthood and suggested that risk factors operating in early life may have sustained deleterious effects on arterial elasticity [20] [21]. Childhood obesity predicts adult metabolic syndrome [22], which is associated with increased CVD events [7]. In the Muscatine, Iowa study [23], total cholesterol was a significant childhood predictor of CIMT.
None of the longitudinal follow-up studies from childhood to young adulthood [18] [19] [20] [23] has focused on relationships of childhood CVD risk factors to CVD events in adults. For the current report, we conducted 22-31 year follow-up studies (1998-2003) in former schoolchildren first studied in 1973-1976. to assess relationships between pediatric BMI and lipids and subsequent CVD in the 4th-5th decades using participants in the Princeton Follow-up Study population [24].
MATERIALS AND METHODS
Princeton Follow-up study (PFS)
This study was carried out following a protocol approved by the Children’s Hospital Institutional Review Board, with signed informed consent
The Princeton Follow-up study (PFS) was a 22-31 year follow-up (1998-2003) [24] of former schoolchildren and their parents from the Cincinnati Clinic of the NIH-NHLBI Lipid Research Clinics (LRC) Prevalence Program (1973-8). The LRC [25, 26] and PFS [24] have both been described previously. Briefly, the LRC was a multistage survey of lipids and other (CVD) risk factors [25, 26] At the first stage of the LRC (Visit 1), total cholesterol and TG were measured, basic demographic data were collected, and family relationships among participating members from the same family determined. At stage 2 (Visit 2), randomly selected and hyperlipidemic visit 1 subjects were recalled, independent of other members of the family, for a second screening, at which complete lipid profiles and anthropometric data were recorded. At stage 3 (Family Study), complete lipid profiles were measured on all first-degree relatives of a random and hyperlipidemic subset of stage 2 subjects. Eighty-four percent of eligible students participated at the initial LRC study visit and 91% participated at subsequent visits; participation rates did not differ significantly between races.
The PFS was conducted to assess changes in the family lipoprotein cholesterol correlations from the period of shared households to that of separate households [24]. Hence, eligibility for PFS required participation in stage 3 (LRC Family Study), or stage 2 (Visit 2) along with either a sibling or parent. The student population from which participants came was 73% white and 27% black, 52% male and 48% female [26]. The interval between LRC study and PFS visits ranged between 22 and 31 years, depending on when the subjects attended their LRC study and PFS visits. In 1998, eligible former schoolchildren who had participated in the LRC family study or at Visit 2, along with either a sibling or a parent, were invited by mail and by follow-up phone call to participate in the PFS, 22-31 years after their initial LRC sampling. There had been no contacts with these eligible former schoolchildren for the 22-31 year interval from their LRC studies.
In the LRC, data were collected according to the collaborative NIH-NHLBI protocol [27]. In the PFS, data were collected using standard protocols [25, 28]. In the LRC and PFS, fasting blood was drawn into vacutainers containing EDTA, kept on ice (LRC) or cold packs (PFS), and delivered to the laboratory within three hours for processing. A detailed medical history was obtained at PFS, including information on angioplasty, coronary artery bypass grafts, myocardial infarction, other vascular surgery (carotid or femoral bypass), and ischemic stroke, diabetes, and cigarette smoking.
Statistical Methods
All analysis was done using SAS 9.1.
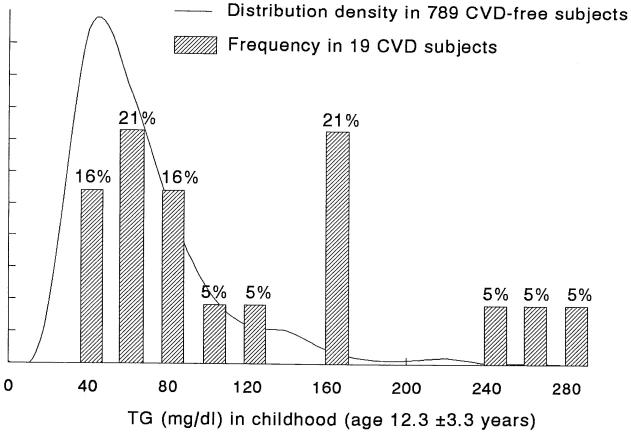
If PFS participants had data for these analyses from more than one stage of the LRC (Visits 2 and/or 3) data from the last LRC examination were used. Percentile distributions of TG in the analysis cohort of 808 children were calculated. The distribution of TG in the 19 CVD cases and 789 CVD-free controls were plotted for both childhood (Figure 1) and adulthood (Figure 2).
Figure 1.
The distribution density functions of triglyceride (TG) in subsequent CVD cases and subsequent CVD- free subjects during childhood (mean age 12.3 ± 3.3 years)
Figure 2.
The distribution density functions of triglyceride (TG) in CVD cases and CVD-free subjects during young adulthood (mean age 38.5 ± 3.7 years)
Childhood entry and young adult follow-up characteristics of the 19 former schoolchildren who sustained CVD events are displayed in Table 1. Table 2 summarizes the number of cardiovascular events (one, two, or three per subject), cross tabulated by the nature of the cardiovascular events.
Table 1.
Nineteen former schoolchildren who sustained cardiovascular events during young adulthood. Childhood and young adulthood lipids, BMI.
| Age (yr) |
BMI(kg/m2) |
TG (mg/dl) |
__ HDLC (mg/dl) |
LDLC (mg/dl)___ |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ID | Gender | Race | initial | Event | initial | follow-up | initial | follow-up | initial | follow-up | initial | follow-up |
| 1 | F | B | 11.3 | 25 | 16.5 | 30.6 | 41 | 38 | 107 | 72 | 83 | 73 |
| 2 | M | B | 17.8 | 41 | 19.2 | 31.8 | 46 | --- | 59 | --- | 135 | --- |
| 3 | M | W | 13.4 | 32 | 16.2 | 29.2 | 55 | 175 | 67 | 43 | 102 | 129 |
| 4 | F | W | 14.1 | 39 | 21.9 | 30.0 | 62 | 217 | 69 | 46 | 97 | 130 |
| 5 | F | B | 12.4 | 38 | 21.5 | 23.7 | 70 | 43 | 62 | 64 | 107 | 98 |
| 6 | F | B | 15.1 | 40 | 31.2 | 41.4 | 75 | 165 | 50 | 46 | 125 | 132 |
| 7 | M | W | 17.3 | 42 | 20.7 | 29.7 | 76 | 91 | 45 | 30 | 94 | 137 |
| 8 | M | W | 12.7 | 38 | 18.6 | 30.8 | 90 | 242 | 49 | 24 | 109 | 150 |
| 9 | F | B | 17.5 | 40 | 24.9 | 39.5 | 92 | 73 | 63 | 50 | 102 | 128 |
| 10 | M | W | 17.2 | 41 | 26.1 | 43.8 | 92 | 350 | 49 | 25 | 108 | 95 |
| 11 | M | W | 13.7 | 34 | --- | 36.8 | 113 | 231 | 44 | 42 | 103 | 157 |
| 12 | M | W | 12.3 | 37 | 24.1 | 28.8 | 120 | 237 | 32 | 37 | 102 | 111 |
| 13 | M | W | 11.1 | 32 | 22.3 | 32.9 | 163 | 279 | 38 | 24 | 155 | 132 |
| 14 | F | W | 16.2 | 41 | 34.8 | 38.3 | 168 | 632 | 41 | 18 | 122 | 67 |
| 15 | F | W | 16.7 | 39 | 27.3 | 37.5 | 168 | 739 | 59 | 58 | 74 | 40 |
| 16 | M | W | 18.6 | 31 | 20.7 | 23.2 | 172 | 198 | 47 | 35 | 109 | 115 |
| 17 | M | W | 16.5 | 41 | 29.6 | 43.5 | 251 | 379 | 45 | 29 | 105 | 101 |
| 18 | M | W | 18.1 | 30 | 35.1 | 31.2 | 265 | 290 | 37 | 28 | 184 | 107 |
| 19 | M | W | 17.0 | 43 | 26.5 | 27.1 | 285 | 147 | 46 | 40 | 104 | 82 |
Table 2.
Nineteen former schoolchildren who sustained cardiovascular events during young adulthood, nature and age of the cardiovascular event.
| ID | Diabetes | Cigarette smoke* | Event age | Angioplasty | CABG | Myocardial infarction | Carotid or femoral bypass | Stroke | number of events | medication to lower cholesterol |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | No | 1 | 25 | --- | No | No | Yes | No | 1 | No |
| 2 | No | 3 | 41 | --- | --- | --- | Yes | --- | 1 | Yes |
| 3 | No | 1 | 32 | No | No | Yes | No | No | 1 | No |
| 4 | No | 3 | 39 | No | No | No | Yes | --- | 1 | No |
| 5 | No | 3 | 38 | No | No | No | Yes | No | 1 | No |
| 6 | Yes | 2 | 40 | Yes | No | No | No | No | 1 | Yes |
| 7 | No | 2 | 42 | Yes | No | Yes | No | No | 2 | No |
| 8 | No | 3 | 38 | No | No | Yes | Yes | No | 2 | No |
| 9 | Yes | 1 | 40 | No | No | No | Yes | No | 1 | No |
| 10 | No | 3 | 41 | No | No | No | No | Yes | 1 | Yes |
| 11 | No | 1 | 34 | No | No | Yes | Yes | No | 2 | No |
| 12 | Yes | 1 | 37 | Yes | Yes | No | No | No | 2 | Yes |
| 13 | No | 3 | 32 | Yes | No | Yes | No | --- | 2 | No |
| 14 | Yes | 3 | 41 | Yes | No | Yes | No | No | 2 | Yes |
| 15 | Yes | 3 | 39 | Yes | No | Yes | Yes | No | 3 | Yes |
| 16 | No | 1 | 31 | No | No | No | Yes | No | 1 | No |
| 17 | No | 3 | 41 | No | No | No | Yes | No | 1 | No |
| 18 | Yes | 1 | 30 | No | No | No | Yes | Yes | 2 | No |
| 19 | No | 2 | 43 | Yes | No | Yes | No | No | 2 | Yes |
| ---------------------------------------------------------------------------------------- | ||||||||||
| total | 7 | 1 | 8 | 11 | 2 | 29 | ||||
cigarette smoke code: 1=never, 2=quit, 3=current smoking
Differences between CVD cases and all CVD-free subjects, both at childhood entry and at young adult follow-up, were assessed by analysis of variance (ANOVA), after adjusting for race, gender, and age (Table 3).
Table 3.
Nineteen former schoolchildren who developed cardiovascular disease as young adults (mean age 37.1 ± 4.9), compared with 789 former schoolchildren without young adult cardiovascular disease, at childhood study entry baseline, and at follow-up, p values were by analysis of variance, adjusted for race, sex and age.
| CVD cases (n=19) Event age 37.1 ±4.9 | Subjects Without CVD (n=789) | p (ANOVA) | |
|---|---|---|---|
| Baseline (Age 12.3 ±3.3) | Age 15.3 ±2.5 | Age 12.3 ±3.3 | |
| BMI (kg/m2) | 24.3 ±5.7 | 20.0 ±4.3 | .012 |
| TC (mg/dl) | 186 ±25 | 174 ±33 | .094 |
| TG (mg/dl) | 127 ±75 | 76 ±45 | <.0001 |
| HDLC (mg/dl) | 53 ±17 | 55 ±12 | .93 |
| LDLC (mg/dl) | 112 ±25 | 107 ±30 | .40 |
| Diastolic BP(mmHg) | 68 ±11 | 62 ±12 | .75 |
| Systolic BP (mmHg) | 114 ±13 | 104 ±13 | .27 |
| Glucose (mg/dl) | 87 ±9 | 86 ±8 | .60 |
| Follow-up (Age 38.5 ±3.8) | Age 40.7 ±2.8 | Age 38.5 ±3.8 | |
|---|---|---|---|
| BMI (kg/m2) | 33.2 ±6.2 | 28.6 ±6.8 | .0078 |
| TC (mg/dl) | 196 ±24 | 194 ±42 | .79 |
| TG (mg/dl) | 251 ±186 | 135 ±133 | .0016 |
| HDLC (mg/dl) | 40 ±15 | 46 ±15 | .16 |
| LDLC (mg/dl) | 110 ±31 | 121 ±36 | .098 |
| Diastolic BP(mmHg) | 85 ±11 | 79 ±11 | .080 |
| Systolic BP (mmHg) | 128 ±16 | 120 ±15 | .062 |
| Glucose (mg/dl) | 122 ±53 | 90 ±26 | <.0001 |
| Cigarette smoking, n (%) | 7 (37%) never 3 (16%) quit 9 (47%)current smoke |
482 (61%) never 118 (15%) quit 189 (24%) current smoke |
Mantel-Haenszel Χ2=5.84, p=.016 |
| Type 2 diabetes, n (%) | 6 (32%) | 31 (4%) | .0001 (Fisher) |
To improve upon the estimates given by the Cox proportional hazard model with backward selection (alpha=0.5), we followed the guidelines of Austin [29], using a zero-corrected bootstrap model selection method. In the 808 subjects, the bootstrap method samples, with replacement, 1000 times with the full analysis size= 808 each time [29]. The parameter estimate was set to zero if the candidate explanatory variable was removed from the selection [29]. Then the mean of all the parameter estimations from the 1000 resultant models was used for the parameter estimate, while the 2.5th and 97.5th percentiles were used for the 95 percent confidence interval. Childhood explanatory variables included race, gender, BMI, LDL cholesterol, log high density lipoprotein cholesterol (HDL-C), and log triglyceride (TG). Adult explanatory variables included type 2 diabetes and cigarette smoking. Analyses of cigarette smoking included 3 levels: 1=never, 2=quit, 3=current smoking, and thus, two dummy variables for cigarette smoking (never smoke vs rest; current smoking vs rest) were used.
To assess for differences between eligible former schoolchildren studied in the PFS and those not studied in the PFS, Wilcoxon tests were first done of childhood age, and childhood BMI, total cholesterol, LDL cholesterol, HDL cholesterol, and TG, followed by analysis of variance adjusting for age, sex, race and BMI.
RESULTS
Follow-up of the schoolchild cohort
There were 1756 LRC student-participants who met eligibility criteria for the PFS, and 22-31 year follow-up data were obtained on 933 (53%) in PFS, including 18 who had died prior to PFS. By review of autopsy reports, physicians’ records, and interviews with family members, 1 subject (with 2 generation familial hypertriglyceridemia) had had a lethal myocardial infarction, 6 subjects had deaths unrelated to CVD, and 11 subjects could not be classified with regard to cause of death. After excluding 18 former schoolchildren who had died and 107 subjects due to missing data on one or more childhood explanatory variables, the analysis sample included 808 former schoolchildren, 19 with morbid CVD events (Tables 1,2), and 789 CVD event-free (Table 3, Figures 1,2). These 808 participants came from 556 families as follows; there were 384 families (69%) with one student-participant, 118 families with 2 student-participants, 38 families with 3 student-participants, 11 families with 4 student-participants, 2 families with 5 student-participants, 1 family with 6 student-participants, and 2 families with 7 student-participants. Each of the 19 CVD cases came from separate families, 8 of the 19 cases had 1 sibling as a non-case. There were 438 girls (54%) and 370 boys (46%), 585 white (72%) and 223 black (28%). Thus, the racial make-up of the cohort was similar at the LRC and PFS (73-27% and 72-28%, respectively). Comparisons of LRC summary data on the 933 follow-up subjects and the 823 eligible LRC subjects not in the PFS indicated that the ages of the two groups were similar, but PFS subjects had higher BMI than non-participants (19.4 vs 18.7 kg/m2, p = 0.0006). There were no differences (p>0.2) between PFS participants and non-participants for LRC total, HDL, and LDL cholesterol and TG non-participants, after adjustment for age, sex, race, and BMI.
Characteristics of the CVD Case Population
The mean (±SD) age of the analysis cohort was 12.3 ± 3.3 in the LRC and 38.5 ± 3.8 years at PFS, Table 3. At the time of the PFS, 19 participants (cases) had sustained ≥ 1 CVD event; 7 were women, 12 men, 5 were black, 14 white, Table 1. Event ages ranged from 25 to 43, with mean ± SD age 37 ± 5 years, Table 1. Of the 19 subjects, 7 (37%) had childhood TG > the schoolchild 95th percentile (153 mg/dl), Table 1. Of these 7 hypertriglyceridemic children (2 girls, 5 boys), follow-up TG were high (≥ 150 mg/dl) in 6 (86%), Table 1. Of the 18 cases with adult lipids (venipuncture was not successful for one case subject), 11 of 18 (61%) had high TG (≥ 150 mg/dl) as adults, Table 1.
The 19 cases had had 29 CVD events including 7 angioplasties, 1 coronary artery bypass grafting, 8 myocardial infarctions, 11 carotid or femoral bypasses, and 2 ischemic strokes, Table 2. Eight of 19 subjects had 2 CVD events, and 1 had 3 events (Table 2). In the 7 subjects where carotid-femoral artery events were the sole CVD event, 4 of the 7 cases were current smokers (Table 2).
At PFS follow-up, the 19 cases had higher TG than former schoolchildren without CVD events (251 vs 135 mg/dl, p =.0016). LDL cholesterol was 110 mg/dl in cases vs 121 mg/dl in non-cases, p =.098, but 7 of 19 cases (37%) reported taking lipid lowering drugs vs 3% of non-case (24 of 789), p<.05.
CVD Cases versus CVD-free subjects: Risk Factor Comparisons
The TG distributions of cases and CVD-free comparison subjects in both childhood (Figure 1), and adulthood (Figure 2) were skewed to the right (higher values), but the shift was markedly greater in the cases in both childhood and adulthood. Of the 19 cases, 7 (37%) had childhood TG > the schoolchild population 95th percentile TG (153 mg/dl), Table 1. Comparing CVD risk factors in cases and CVD-free subjects after adjusting for age, sex, and race, cases had higher least square mean TG (p<.0001) and higher BMI (p =.012) in both childhood and adulthood (p =.0016 and .0078, respectively), Table 3. In addition, plasma glucose was higher in adult CVD cases than CVD-free subjects (p < .0001), Table 3. At adult follow-up, cigarette smoking was more prevalent in cases than CVD-free subjects: 9 of the 19 cases (47%) reported smoking cigarettes and 3 (16%) reported being ex-smokers vs 24% of CVD-free subjects reporting smoking (189 of 789) and 15% reporting being ex-smokers (n=118), Mantel-Haenszel χ2=5.84, p=.016, Table 3. Type 2 diabetes was more prevalent in cases, 6 of 19 cases (32%) vs 31 of 789 CVD-free subjects (4%), Fisher’s p=.0001, Table 3.
Pediatric determinants of CVD events
Using Austin’s bootstrapping method [29] and Cox’s proportional hazard model, significant independent explanatory variables for CVD included adult type 2 diabetes mellitus (hazard ratio 19.4, 95% CI 4.24-114.2) and childhood TG (hazard ratio 5.35, 95% CI 1.69-20.0 for each 1 unit increasing in natural logarithm scale).
DISCUSSION
Prospective studies of the relationships of childhood CVD risk factors to CVD in young adulthood have relied on the CVD surrogate, carotid artery intimal-medial thickening (CIMT). The following childhood CVD risk factors have been associated with CIMT and other vascular surrogates (carotid artery compliance, Young’s elastic modulus, and stiffness index) in young adulthood: total cholesterol [23], obesity [18] [20], and the ratio of ApoB to ApoA1 [19] [21]. Subjects with type IIB hyperlipidemia in childhood had increased CIMT 21 years later in young adulthood [21].
None of the longitudinal follow-up studies from childhood to young adulthood to date [18] [19] [20] [21] has focused on relationships of childhood CVD risk factors to CVD events in adults. By virtue of having a longer follow-up (22-31 years) than the previous childhood risk factor→adult CVD studies [18] [19] [20] [21], our study allowed direct examination of the relationship of childhood BMI and lipids to young adult CVD events including angioplasty, coronary, carotid, and femoral artery bypass surgery, myocardial infarction, and ischemic stroke. The major novel and original finding in the current study was that childhood TG was consistently and independently associated with young adult CVD. The distribution of both childhood and adult TG shifted to higher levels in the 19 CVD cases than in CVD-free subjects. Of the 19 subjects, 7 (37%) had childhood TG > 153, the schoolchild 95th percentile. Of the 7 hypertriglyceridemic children, 6 (86%) had high TG (≥ 150 mg/dl) as adults. Overall, 13 of the 18 CVD cases (72%%) who had TG measured as young adults had high TG (≥ 150 mg/dl). Mean adult TG in the CVD cases was 251 mg/dl as adults compared to 135 mg/dl in CVD-free subjects. After adjusting for sex, race, and age by analysis of variance, children who subsequently sustained CVD events had higher TG levels both in childhood and in young adulthood than CVD-free subjects. Moreover, by Cox’s proportional hazard analysis, childhood TG was a significant, independent, explanatory variable for young adult CVD hazard (hazard ratio 5.35, 95% CI 1.69-20.0 for each 1 unit increasing in natural logarithm scale). An additional significant explanatory variable in the Cox model included adult type 2 diabetes (hazard ratio 19.4, 95% CI 4.24-114.2).
Our finding that 7 of the 19 CVD events in former schoolchildren were either femoral and/or carotid bypass as the only CVD event raised the question of TG as an etiology for femoral and or carotid disease. Four of these 7 carotid-femoral event cases were current smokers, a known association with carotid [30, 31] and peripheral vascular disease [32, 33]. Beyond cigarette smoking, Genoud et al [34] reported that TG and HDLC were major contributors to peripheral atherosclerosis in 120 overweight cases. In 21-year follow-up in the young Finns study which started in 1980 in children then ages 3 to 18 years, type IIB dyslipidemia was related to CIMT [21]. Mori et al [35] have reported a significant association between postprandial TG levels and CIMT in Japanese patients with type 2 diabetes mellitus. Shoji et al [36] reported that small dense LDL was the best marker of carotid IMT, along with TG. Since small dense LDL is commonly high in hypertrigyceridemic subjects [36], the findings of Shoji et al may also point to high TG associated with CIMT. In subjects with carotid atherosclerosis, reduction in TG on Crestor 10 mg was a significant predictor of change in CIMT after adjustment for established CVD risk factors [37]. Rizzo et al [38] has reported that the concomitant presence of high TG, low HDLC, and high small dense LDL was 26% in patients with peripheral arterial disease vs 0% in controls (p=.0024). Vaudo et al has reported that femoral IMT was associated with TG, among other CVD risk factors [39].
To the best of our knowledge, previous prospective studies of CVD risk factors in childhood and expression of surrogates for CVD in young adulthood [19] [20] have not identified TG as a significant, independent explanatory variable. Our current study is congruent with studies in adults where non-fasting TG [3-5] and fasting TG [2, 6] are independent risk factors for CVD.
The major limitation of the current study is the small number of CVD cases (n=19). A general recommendation for proportional hazards regression is that the number of cases (if this is the smaller outcome group) should be at least 10 times larger than the number of “potential” predictors [40, 41]. The initial Cox proportional hazards model had 8 explanatory variables and 19 cases. The conventional backward elimination method may filter out explanatory variable “winners,” and may result in effect estimates that are too high and p-values that are too small because of the numerous statistical tests that are performed [29, 42-44]. To deal with this issue in our data set, we used Austin’s bootstrapping method [29] with the Cox model. However, in a simulation study, Austin [45] noted that “...bootstrap model selection tended to result in an approximately equal proportion of selected models being equal to the true regression model compared with the use of conventional backward variable elimination.” In the final model, there were 2 explanatory variables (log childhood TG and adult type 2 diabetes) that were significantly associated with CVD. LDL cholesterol was not higher in cases than controls at follow-up in the PFS; but lipid-lowering drugs were reported by 37% of cases vs 3% of controls. The interaction of BMI, diabetes, and both TG and HDL cholesterol may be difficult to dissect in 19 cases with CVD and in 789 subjects free of CVD. An additional limitation of our study was that we used self-identified and interviewer recorded history of CVD, without direct validation by review of physician-hospital records. Family history of CVD has, however, been shown by Murabito et al [46] to provide accurate data.
We are able to re-study 53% of our childhood cohort as young adults, 22-31 years after their initial assessment in the LRC-Family studies. Comparing eligible former schoolchildren who were and were not studied as young adults, the participants had higher pediatric BMI, but there were no differences in childhood LDL -and HDL-cholesterol or TG after covariance adjusting for age, sex, race, and BMI. Hence, the PFS participants might reflect some bias towards somewhat higher BMI as children.
Triglyceride-rich lipoproteins and their associated atherogenic remnants [47] produce a pro-inflammatory and oxidative state which may enhance adhesion molecule and foam cell production, and adversely affect smooth muscles [48]. High TG are associated with an increased population of small dense LDL particles that are highly atherogenic, and can be oxidatively modified [49, 50].
In the blinded, placebo-controlled Helsinki Heart Study [10] in asymptomatic middle-aged men, there was a 34% reduction in the incidence of CVD in the gemfibrozil treatment group. In the blinded, placebo-controlled VA-HIT secondary prevention study [51], CVD events were reduced by 11% with gemfibrozil for every 5-mg/dl increase in HDLC (p = .02). Since high TG track from childhood to young adulthood [52-54], and since TG are an independent risk factor for CVD [2], recognition of high TG in childhood and treatment may offer a pediatric approach to primary prevention of CVD in adults.
Acknowledgments
This research was supported in part by American Heart Association (National)-9750129N and NIH-HL62394 (Dr Morrison), by the Taft Research Fund (Dr. Horn), and by the Lipoprotein Research Fund of the Jewish Hospital of Cincinnati (Dr Glueck).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no conflicts of interest or financial links to this manuscript
REFERENCES
- [1].Criqui MH, Heiss G, Cohn R, et al. Plasma triglyceride level and mortality from coronary heart disease. N Engl J Med. 1993;328:1220–5. doi: 10.1056/NEJM199304293281702. [DOI] [PubMed] [Google Scholar]
- [2].Sarwar N, Danesh J, Eiriksdottir G, et al. Triglycerides and the risk of coronary heart disease: 10,158 incident cases among 262,525 participants in 29 Western prospective studies. Circulation. 2007;115:450–8. doi: 10.1161/CIRCULATIONAHA.106.637793. [DOI] [PubMed] [Google Scholar]
- [3].Bansal S, Buring JE, Rifai N, et al. Fasting compared with nonfasting triglycerides and risk of cardiovascular events in women. Jama. 2007;298:309–16. doi: 10.1001/jama.298.3.309. [DOI] [PubMed] [Google Scholar]
- [4].McBride PE. Triglycerides and risk for coronary heart disease. Jama. 2007;298:336–8. doi: 10.1001/jama.298.3.336. [DOI] [PubMed] [Google Scholar]
- [5].Nordestgaard BG, Benn M, Schnohr P, et al. Nonfasting triglycerides and risk of myocardial infarction, ischemic heart disease, and death in men and women. Jama. 2007;298:299–308. doi: 10.1001/jama.298.3.299. [DOI] [PubMed] [Google Scholar]
- [6].Austin MA, McKnight B, Edwards KL, et al. Cardiovascular disease mortality in familial forms of hypertriglyceridemia: A 20-year prospective study. Circulation. 2000;101:2777–82. doi: 10.1161/01.cir.101.24.2777. [DOI] [PubMed] [Google Scholar]
- [7].Espinola-Klein C, Rupprecht HJ, Bickel C, et al. Impact of metabolic syndrome on atherosclerotic burden and cardiovascular prognosis. Am J Cardiol. 2007;99:1623–8. doi: 10.1016/j.amjcard.2007.01.049. [DOI] [PubMed] [Google Scholar]
- [8].Wittrup HH, Tybjaerg-Hansen A, Nordestgaard BG. Lipoprotein lipase mutations, plasma lipids and lipoproteins, and risk of ischemic heart disease. A meta-analysis. Circulation. 1999;99:2901–7. doi: 10.1161/01.cir.99.22.2901. [DOI] [PubMed] [Google Scholar]
- [9].Bonora E, Kiechl S, Willeit J, et al. Insulin resistance as estimated by homeostasis model assessment predicts incident symptomatic cardiovascular disease in caucasian subjects from the general population: the Bruneck study. Diabetes Care. 2007;30:318–24. doi: 10.2337/dc06-0919. [DOI] [PubMed] [Google Scholar]
- [10].Frick MH, Elo O, Haapa K, et al. Helsinki Heart Study: primary-prevention trial with gemfibrozil in middle-aged men with dyslipidemia. Safety of treatment, changes in risk factors, and incidence of coronary heart disease. N Engl J Med. 1987;317:1237–45. doi: 10.1056/NEJM198711123172001. [DOI] [PubMed] [Google Scholar]
- [11].Rubins HB, Robins SJ, Collins D, et al. Veterans Affairs High-Density Lipoprotein Cholesterol Intervention Trial Study Group Gemfibrozil for the secondary prevention of coronary heart disease in men with low levels of high-density lipoprotein cholesterol. N Engl J Med. 1999;341:410–8. doi: 10.1056/NEJM199908053410604. [DOI] [PubMed] [Google Scholar]
- [12].Rubins HB, Robins SJ, Collins D, et al. Diabetes, plasma insulin, and cardiovascular disease: subgroup analysis from the Department of Veterans Affairs high-density lipoprotein intervention trial (VA-HIT) Arch Intern Med. 2002;162:2597–604. doi: 10.1001/archinte.162.22.2597. [DOI] [PubMed] [Google Scholar]
- [13].Keech A, Simes RJ, Barter P, et al. Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomised controlled trial. Lancet. 2005;366:1849–61. doi: 10.1016/S0140-6736(05)67667-2. [DOI] [PubMed] [Google Scholar]
- [14].Bloomfield Rubins H, Davenport J, Babikian V, et al. Reduction in stroke with gemfibrozil in men with coronary heart disease and low HDL cholesterol: The Veterans Affairs HDL Intervention Trial (VA-HIT) Circulation. 2001;103:2828–33. doi: 10.1161/01.cir.103.23.2828. [DOI] [PubMed] [Google Scholar]
- [15].Miller M, Cannon CP, Murphy SA, et al. Impact of triglyceride levels beyond low-density lipoprotein cholesterol after acute coronary syndrome in the PROVE IT-TIMI 22 trial. J Am Coll Cardiol. 2008;51:724–30. doi: 10.1016/j.jacc.2007.10.038. [DOI] [PubMed] [Google Scholar]
- [16].Berenson GS, Srinivasan SR, Bao W, et al. Association between multiple cardiovascular risk factors and atherosclerosis in children and young adults. The Bogalusa Heart Study. N Engl J Med. 1998;338:1650–6. doi: 10.1056/NEJM199806043382302. [DOI] [PubMed] [Google Scholar]
- [17].McGill HC, Jr., McMahan CA, Gidding SS. Preventing heart disease in the 21st century: implications of the Pathobiological Determinants of Atherosclerosis in Youth (PDAY) study. Circulation. 2008;117:1216–27. doi: 10.1161/CIRCULATIONAHA.107.717033. [DOI] [PubMed] [Google Scholar]
- [18].Freedman DS, Patel DA, Srinivasan SR, et al. The contribution of childhood obesity to adult carotid intima-media thickness: the Bogalusa Heart Study. Int J Obes (Lond) 2008;32:749–56. doi: 10.1038/sj.ijo.0803798. [DOI] [PubMed] [Google Scholar]
- [19].Juonala M, Viikari JS, Kahonen M, et al. Childhood levels of serum apolipoproteins B and A-I predict carotid intima-media thickness and brachial endothelial function in adulthood: the cardiovascular risk in young Finns study. J Am Coll Cardiol. 2008;52:293–9. doi: 10.1016/j.jacc.2008.03.054. [DOI] [PubMed] [Google Scholar]
- [20].Juonala M, Jarvisalo MJ, Maki-Torkko N, et al. Risk factors identified in childhood and decreased carotid artery elasticity in adulthood: the Cardiovascular Risk in Young Finns Study. Circulation. 2005;112:1486–93. doi: 10.1161/CIRCULATIONAHA.104.502161. [DOI] [PubMed] [Google Scholar]
- [21].Juonala M, Viikari JS, Ronnemaa T, et al. Associations of dyslipidemias from childhood to adulthood with carotid intima-media thickness, elasticity, and brachial flow-mediated dilatation in adulthood: the Cardiovascular Risk in Young Finns Study. Arterioscler Thromb Vasc Biol. 2008;28:1012–7. doi: 10.1161/ATVBAHA.108.163329. [DOI] [PubMed] [Google Scholar]
- [22].Sun SS, Liang R, Huang TT, et al. Childhood obesity predicts adult metabolic syndrome: the Fels Longitudinal Study. J Pediatr. 2008;152:191–200. doi: 10.1016/j.jpeds.2007.07.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Davis PH, Dawson JD, Riley WA, et al. Carotid intimal-medial thickness is related to cardiovascular risk factors measured from childhood through middle age: The Muscatine Study. Circulation. 2001;104:2815–9. doi: 10.1161/hc4601.099486. [DOI] [PubMed] [Google Scholar]
- [24].Friedman LA, Morrison JA, Daniels SR, et al. Sensitivity and specificity of pediatric lipid determinations for adult lipid status: findings from the Princeton Lipid Research Clinics Prevalence Program Follow-up Study. Pediatrics. 2006;118:165–72. doi: 10.1542/peds.2005-2968. [DOI] [PubMed] [Google Scholar]
- [25].Laskarzewski P, Morrison JA, Mellies MJ, et al. Relationships of measurements of body mass to plasma lipoproteins in schoolchildren and adults. Am J Epidemiol. 1980;111:395–406. doi: 10.1093/oxfordjournals.aje.a112914. [DOI] [PubMed] [Google Scholar]
- [26].Morrison JA, deGroot I, Edwards BK, et al. Plasma cholesterol and triglyceride levels in 6,775 school children, ages 6--17. Metabolism. 1977;26:1199–211. doi: 10.1016/0026-0495(77)90112-3. [DOI] [PubMed] [Google Scholar]
- [27].Williams OD, Stinnett S, Chambless LE, et al. Populations and methods for assessing dyslipoproteinemia and its correlates. The Lipid Research Clinics Program Prevalence Study. Circulation. 1986;73:I4–11. [PubMed] [Google Scholar]
- [28].Obesity and cardiovascular disease risk factors in black and white girls: the NHLBI Growth and Health Study. Am J Public Health. 1992;82:1613–20. doi: 10.2105/ajph.82.12.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Austin PC. Using the bootstrap to improve estimation and confidence intervals for regression coefficients selected using backwards variable elimination. Stat Med. 2008;27:3286–300. doi: 10.1002/sim.3104. [DOI] [PubMed] [Google Scholar]
- [30].Weber F. Risk factors for subclinical carotid atherosclerosis in healthy men. Neurology. 2002;59:524–8. doi: 10.1212/wnl.59.4.524. [DOI] [PubMed] [Google Scholar]
- [31].Crouse JR, 3rd, Tang R, Espeland MA, et al. Associations of extracranial carotid atherosclerosis progression with coronary status and risk factors in patients with and without coronary artery disease. Circulation. 2002;106:2061–6. doi: 10.1161/01.cir.0000033833.54884.34. [DOI] [PubMed] [Google Scholar]
- [32].Price JF, Mowbray PI, Lee AJ, et al. Relationship between smoking and cardiovascular risk factors in the development of peripheral arterial disease and coronary artery disease: Edinburgh Artery Study. Eur Heart J. 1999;20:344–53. doi: 10.1053/euhj.1998.1194. [DOI] [PubMed] [Google Scholar]
- [33].Ness J, Aronow WS, Ahn C. Risk factors for symptomatic peripheral arterial disease in older persons in an academic hospital-based geriatrics practice. J Am Geriatr Soc. 2000;48:312–4. doi: 10.1111/j.1532-5415.2000.tb02652.x. [DOI] [PubMed] [Google Scholar]
- [34].Genoud M, Wietlisbach V, Feihl F, et al. Surrogate markers for atherosclerosis in overweight subjects with atherogenic dyslipidemia: the GEMS project. Angiology. 2008;59:484–92. doi: 10.1177/0003319707307768. [DOI] [PubMed] [Google Scholar]
- [35].Mori Y, Itoh Y, Komiya H, et al. Association between postprandial remnant-like particle triglyceride (RLP-TG) levels and carotid intima-media thickness (IMT) in Japanese patients with type 2 diabetes: assessment by meal tolerance tests (MTT) Endocrine. 2005;28:157–63. doi: 10.1385/ENDO:28:2:157. [DOI] [PubMed] [Google Scholar]
- [36].Shoji T, Hatsuda S, Tsuchikura S, et al. Small dense low-density lipoprotein cholesterol concentration and carotid atherosclerosis. Atherosclerosis. 2009;202:582–8. doi: 10.1016/j.atherosclerosis.2008.04.042. [DOI] [PubMed] [Google Scholar]
- [37].Riccioni G, Bazzano LA, Bucciarelli T, et al. Rosuvastatin reduces intima-media thickness in hypercholesterolemic subjects with asymptomatic carotid artery disease: the Asymptomatic Carotid Atherosclerotic Disease in Manfredonia (ACADIM) Study. Expert Opin Pharmacother. 2008;9:2403–8. doi: 10.1517/14656566.9.14.2403. [DOI] [PubMed] [Google Scholar]
- [38].Rizzo M, Pernice V, Frasheri A, et al. Atherogenic lipoprotein phenotype and LDL size and subclasses in patients with peripheral arterial disease. Atherosclerosis. 2008;197:237–41. doi: 10.1016/j.atherosclerosis.2007.03.034. [DOI] [PubMed] [Google Scholar]
- [39].Vaudo G, Marchesi S, Siepi D, et al. Metabolic syndrome and preclinical atherosclerosis: focus on femoral arteries. Metabolism. 2007;56:541–6. doi: 10.1016/j.metabol.2006.11.016. [DOI] [PubMed] [Google Scholar]
- [40].Harrell FE, Jr., Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15:361–87. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- [41].Altman DG, Royston P. What do we mean by validating a prognostic model? Stat Med. 2000;19:453–73. doi: 10.1002/(sici)1097-0258(20000229)19:4<453::aid-sim350>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- [42].Mundry R, Nunn CL. Stepwise model fitting and statistical inference: turning noise into signal pollution. Am Nat. 2009;173:119–23. doi: 10.1086/593303. [DOI] [PubMed] [Google Scholar]
- [43].Whittingham MJ, Stephens PA, Bradbury RB, et al. Why do we still use stepwise modelling in ecology and behaviour? J Anim Ecol. 2006;75:1182–9. doi: 10.1111/j.1365-2656.2006.01141.x. [DOI] [PubMed] [Google Scholar]
- [44].Steyerberg EW, Eijkemans MJ, Harrell FE, Jr., et al. Prognostic modelling with logistic regression analysis: a comparison of selection and estimation methods in small data sets. Stat Med. 2000;19:1059–79. doi: 10.1002/(sici)1097-0258(20000430)19:8<1059::aid-sim412>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- [45].Austin PC. Bootstrap model selection had similar performance for selecting authentic and noise variables compared to backward variable elimination: a simulation study. J Clin Epidemiol. 2008;61:1009–17. e1. doi: 10.1016/j.jclinepi.2007.11.014. [DOI] [PubMed] [Google Scholar]
- [46].Murabito JM, Nam BH, D’Agostino RB, Sr., et al. Accuracy of offspring reports of parental cardiovascular disease history: the Framingham Offspring Study. Ann Intern Med. 2004;140:434–40. doi: 10.7326/0003-4819-140-6-200403160-00010. [DOI] [PubMed] [Google Scholar]
- [47].Imke C, Rodriguez BL, Grove JS, et al. Are remnant-like particles independent predictors of coronary heart disease incidence? The Honolulu Heart study. Arterioscler Thromb Vasc Biol. 2005;25:1718–22. doi: 10.1161/01.ATV.0000173310.85845.7b. [DOI] [PubMed] [Google Scholar]
- [48].Yu KC, Cooper AD. Postprandial lipoproteins and atherosclerosis. Front Biosci. 2001;6:D332–54. doi: 10.2741/yu. [DOI] [PubMed] [Google Scholar]
- [49].Austin MA, King MC, Vranizan KM, et al. Atherogenic lipoprotein phenotype. A proposed genetic marker for coronary heart disease risk. Circulation. 1990;82:495–506. doi: 10.1161/01.cir.82.2.495. [DOI] [PubMed] [Google Scholar]
- [50].Steinberg D, Parthasarathy S, Carew TE, et al. Beyond cholesterol. Modifications of low-density lipoprotein that increase its atherogenicity. N Engl J Med. 1989;320:915–24. doi: 10.1056/NEJM198904063201407. [DOI] [PubMed] [Google Scholar]
- [51].Robins SJ, Collins D, Wittes JT, et al. Relation of gemfibrozil treatment and lipid levels with major coronary events: VA-HIT: a randomized controlled trial. Jama. 2001;285:1585–91. doi: 10.1001/jama.285.12.1585. [DOI] [PubMed] [Google Scholar]
- [52].Gardner DS, Metcalf BS, Hosking J, et al. Trends, associations and predictions of insulin resistance in prepubertal children (EarlyBird 29) Pediatr Diabetes. 2008;9:214–20. doi: 10.1111/j.1399-5448.2008.00380.x. [DOI] [PubMed] [Google Scholar]
- [53].Eisenmann JC, Welk GJ, Wickel EE, et al. Stability of variables associated with the metabolic syndrome from adolescence to adulthood: the Aerobics Center Longitudinal Study. Am J Hum Biol. 2004;16:690–6. doi: 10.1002/ajhb.20079. [DOI] [PubMed] [Google Scholar]
- [54].Laskarzewski P, Morrison JA, deGroot I, et al. Lipid and lipoprotein tracking in children. Pediatr Res. 1979;13:1082–4. doi: 10.1203/00006450-197909000-00029. [DOI] [PubMed] [Google Scholar]