Abstract
Hibernation results in dramatic changes in body temperature and metabolism; however, the central nervous system remains active during deep torpor. By cloning c-fos cDNA from the 13-lined ground squirrel (Spermophilus tridecemlineatus) and using squirrel c-fos mRNA probe for in situ hybridization histochemistry, we systematically analyzed and identified specific brain regions that were activated during six different phases of the hibernation bout. During entrance into torpor, we detected activation of the ventrolateral subdivision of the medial preoptic area (‘thermoregulatory center’), and the reticular thalamic nucleus, which is known to inhibit the somatomotor cortex. During torpor, c-fos expression in the cortex was suppressed while the reticular thalamic nucleus remained uniformly active. Throughout torpor the suprachiasmatic nucleus (‘biological clock’) showed increasing activity, likely participating in phase-change regulation of the hibernation bout. Interestingly, during torpor very strong c-fos activation was seen in the epithelial cells of the choroid plexus and in tanycytes at the third ventricle, both peaking near the beginning of arousal. In arousal, activity of the suprachiasmatic and reticular thalamic nuclei and choroid epithelial cells diminished, while ependymal cells in the lateral and fourth ventricles showed stronger activity. Increasing body temperature during arousal was driven by the activation of neurons in the medial part of the preoptic area. In interbout awake animals, we demonstrated the activation of hypothalamic neurons located in the arcuate nucleus and the dorsolateral hypothalamus, areas involved in food intake. Our observations indicate that the hibernation bout is closely regulated and orchestrated by specific regions of the central nervous system.
Indexing terms: hibernation bout, thermoregulation, c-fos, choroid plexus, ependyma, tanycytes, preoptic nucleus
Hibernation occurs in some mammalian species as a natural adaptation to seasonal cold and limited food supply. Hibernating mammals, like the 13-lined ground squirrel, do not reside continuously in deep torpor throughout a winter season, they intersperse brief euthermic periods and return to torpor on an intermittent basis. During entrance into torpor the heart and respiratory rate decreases (Kayser, 1961), whole body metabolism drops to less than 5% (Storey, 2003), body temperature drops dramatically to some degrees above ambient temperature, and cerebral blood flow falls to severely oligemic levels (Frerichs et al., 1994). Yet despite these profound changes, hibernators are able to arouse from torpor by means of internal heat production and return to a euthermic state without any tissue damage. Their ability to regularly interrupt the torpor and return to an active interbout state implies a precise coordination of numerous functions orchestrated by the central nervous system (CNS).
This precise neurophysiological regulation of hibernation allows ground squirrels to survive extreme environmental conditions. The potential for the hibernating state to induce tolerance against hypoxia and hypoglycemia (Frerichs and Hallenbeck, 1998) may propel studies to develop novel treatments for stroke. In order to guide future experiments that examine mechanisms regulating the hibernation bout and confer its cytoprotective properties, it is of great importance to identify the brain areas that participate in hibernation.
During deep torpor the hibernator’s brain temperature can drop close to the freezing point of water and the brain becomes electrically quiescent to surface EEG (Heller and Ruby, 2004); specific brain regions, however, still remain active (Heller, 1979). The hypothalamus has been shown to play an important role in arousal (O’Hara et al., 1999). Lesions of the preoptic thermoregulatory center resulted in abnormal torpor (Satinoff, 1967). Recent studies revealed the essential importance of the suprachiasmatic nucleus in order to maintain adequate timing of hibernation and induce arousal (Kilduff et al., 1989; Bitting et al., 1994; Ruby et al., 2002). Numerous attempts have been made to reveal the localization and characterization of brain areas that may control entrance, maintenance, or arousal during the hibernation bout (Kilduff et al., 1990); however, the participating brain areas and the mechanisms of neurophysiological regulation are still obscure. Reportedly, some of the neurotransmitters may have specific roles in the hibernation cycle. The serotoninergic raphe nuclei might be involved in inducing and regulating entrance into the hibernation cycle (Canguilhem et al., 1986; Haak et al., 1991). Injection of histamine in the hippocampus has been reported to prolong hibernation (Sallmen et al., 2003a), and indeed, upregulation of hippocampal H1 and H2 receptors during torpor suggests that histamine may have a crucial role in maintaining hibernation (Sallmen et al., 2003b).
In the present study we aimed to reveal and systematically describe the activation of brain regions throughout six phases of the hibernation bout. To detect activated neurons, we used a highly sensitive method, c-fos proto-oncogene in situ hybridization immunohistochemistry (Bullitt, 1990; Morgan and Curran, 1991; Bitting et al., 1994) with c-fos cDNA from the 13-lined ground squirrel. We identified certain areas being active during deep torpor, while others were activated at arousal or even after returning to normothermia. Our results lead us to conclude that functional aspects of the activated nuclei can guide future studies examining regulatory mechanisms of the hibernation bout.
MATERIALS AND METHODS
Animal procedures and hibernaculum
Thirteen-lined ground squirrels, Spermophilus tridecemlineatus, were captured by a United States Department of Agriculture-licensed trapper (TLS Research, Bartlett, IL). All animal procedures were approved by the Animal Care and Use Committee of the National Institute of Neurological Disorders and Stroke. We used four animals in each study group for statistical significance. Male squirrels were housed individually in a holding room with an ambient temperature of 21°C and a 12/12-hour light/dark cycle and were fed standard rodent diet and water ad libitum. Induction of hibernation was carried out as previously described (Frerichs et al., 1994). All animals were studied and sacrificed during their natural hibernation phase: during winter months (January and February) in the Northern hemisphere. Briefly, squirrels were moved to a cold chamber (hibernaculum) maintained at 4 –5°C and 60% humidity and placed separately in cages containing wood shavings. The hibernaculum was kept in constant darkness, except for a photographic red safe light (3–5 lux), and could be entered only through a darkened anteroom. Noise within the chamber was kept at a minimum level. Squirrels were implanted with Implantable Programmable Temperature Transponders IPTT-200 (Bio Medic Data Systems, Seaford, DE) for long-term monitoring of body temperature.
Animals in six different phases of the hibernation bout (cycle) were differentiated by temperature, time, and respiratory rate (Fig. 1). Phase 1: ART designates Active animals at Room Temperature (n = 4) that were placed in the holding room with an ambient temperature of 21°C (Tb = 34–37°C). Phase 2: ET designates animals (n = 4) that are in the Entrance into Torpor phase of the hibernation bout (Tb = 25–18°C) after having been in the interbout active state for more than 14 hours since full arousal from torpor. Animals were selected after they had been in the torpor phase of the hibernation bout for more than 5 days, aroused, and then reentered torpor. Phase 3: LT designates animals (n = 4) that have remained in the Late Torpor phase of the hibernation bout for more than 5 days, but have not begun a periodic arousal (Tb = 5–8°C). The next two phases designate animals that are arousing spontaneously without any stress such as touching or substantial warming. Phase 4: EA designates Early Arousal animals (n = 4) characterized by an increased respiratory rate of more than 60 breaths per minute accompanied by a persistent low body temperature (Tb = 9–12°C). Phase 5: LA designates Late Arousal animals (n = 4) that reached an even higher respiratory rate and high body temperature (Tb = 28–32°C). Phase 6: IBA designates InterBout Active animals (n = 4) that were naturally aroused after the torpor phase of the hibernation bout and reached the respiratory rate and temperature of fully awake, active animals at room temperature. These animals were active at least for 6 hours in the hibernaculum without reentering torpor.
Fig. 1.
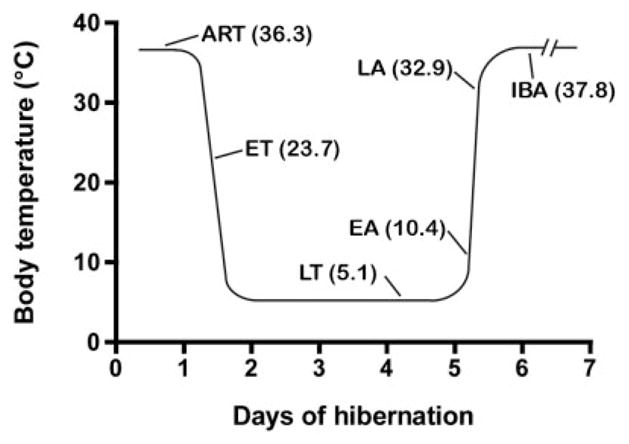
Schematic depiction of the hibernation bout indicating time-points and body temperatures when animals were sacrificed. ART: Animal at Room Temperature; ET: Entrance into Torpor; LT: Late Torpor; EA: Early Arousal; LA: Late Arousal; IBA: InterBout Awake animal.
All animals were anesthetized with isoflurane and decapitated within 2 minutes from their removal from the hibernation chamber. This procedure minimized their stress and our study’s bias in interpreting acute neuronal activation.
Subcloning of c-fos cDNA fragment of 13-lined ground squirrel
To obtain the 13-lined ground squirrel c-fos cDNA fragment, primers were designed as follows to produce an 884 basepair (bp)-long fragment; 5′-actacgaggcgtcatcctc-3′ and 5′-ggtcagacgacgtatcttcc-3′. Reverse transcriptase polymerase chain reaction (RT-PCR) amplification using a 13-lined ground squirrel brain cDNA library was performed with GeneAmpTM DNA amplification reagent kit, the amplified cDNA fragments were subcloned into pGEM-T vector (Promega, Madison, WI), and sequenced (GenBank database Access. No. DQ104400).
In situ hybridization
During the predefined six phases of the hibernation cycle (Fig. 1), ground squirrels were anesthetized with isoflurane and decapitated. Brains were removed, frozen on dry ice, and stored at −80°C until sectioned. Serial coronal sections of 12 μm thickness were cut throughout the entire brain axis at −20°C in a Leica CS 3050 S cryostat (Leica Microsystems, Wetzlar, Germany), thaw-mounted on positively charged microscope slides, and stored at −80°C until hybridization. [S35]UTP-labeled sense RNA probe was employed corresponding to nucleotides 1– 884 of the squirrel c-fos cDNA sequence (described previously). A PCR-amplified fragment was prepared using the cloned squirrel cDNA as template with the following antisense primers: 5′actacgaggcgtcatcctcccg-3′ and 5′ccagtctgctgcatagaaggaacc-3′. Radioactive labeled riboprobes were generated from T7 primed DNA fragments using T7 RNA polymerase with a Maxiscript transcription kit (Ambion, Austin, TX). In situ hybridization was carried out according to the detailed description in the Protocols section of the following Website: http://intramural.nimh.nih.gov/lcmr/snge/.
After hybridization the slides were exposed to Kodak BioMax MR film (Eastman Kodak, Rochester, NY) for 3 days at room temperature. Subsequently, slides were dipped in Kodak NTB3 nuclear track emulsion, stored at 4°C for 12 days, then developed and fixed with Kodak Dektol and Kodak fixer (Eastman Kodak), respectively, and counterstained with Giemsa. The pictures of c-fos-expressing cells were taken from emulsion-dipped and counterstained slides using a Zeiss Axiophot upright microscope (Carl Zeiss MicroImaging, Thornwood, NY) with a Photometrics CoolSnap HQ digital camera (Roper Scientific, Ottobrunn, Germany).
Basal c-fos expression due to handling of the animals was minimized by anesthetizing and decapitating them within 2 minutes from their removal from the hibernation chamber. Since c-fos mRNA expression is detectable earliest at 5 minutes after stressful stimulus and peaks at about 30 minutes (Sharp et al., 1991), our results represent a time period at least 5 minutes prior to the decapitation and thus do not include the stress of handling and anesthesia.
Statistical analysis
Quantitative analysis of c-fos expression was carried out on coronal sections of the brains. Digital images were taken of the areas of interest with a Nikon micro-Nikkor 55 mm lens (Nikon, Tokyo, Japan) and a MS4030 CCD camera (Sierra Scientific, Los Angeles, CA), and analyzed using NIH ImageJ 1.31v software. An image was thresholded to background signal level, the subdivision of interest was outlined, and the signal intensity was automatically calculated with respect to the surface area of the outlined section. The pixel density data of c-fos expression/μm2 value was calculated in identical coronal brain sections of four squirrels sacrificed at the same phase of the hibernation bout. These four identical values of every single phase of the hibernation bout were expressed as mean ± SEM (Table 1).
TABLE 1.
Quantitative Statistical Analysis of c-fos Expression in Definite Brain Areas during the Hibernation Bout
| Phase of Hibernation Bout | ART | ET | LT | EA | LA | IBA |
|---|---|---|---|---|---|---|
| Temperature (average, n = 4) | 36.4°C | 23.1°C | 5.4°C | 10.9°C | 32.1°C | 37.3°C |
| Brain regions | ||||||
| Medial preoptic area (MPA) | 78 ± 9 | 95 ± 19 P > 0.05 |
72 ± 12 P > 0.05 |
68 ± 9 P > 0.05 |
149 ± 12 P < 0.001 |
164 ± 17 P < 0.001 |
| Ventromedial subdivision of MPA | 88 ± 9 | 54 ± 7 P < 0.01 |
50 ± 8 P < 0.001 |
111 ± 14 P > 0.05 |
167 ± 12 P < 0.001 |
175 ± 19 P < 0.001 |
| Ventrolateral subdivision of MPA | 59 ± 8 | 121 ± 12 P < 0.001 |
43 ± 6 P > 0.05 |
48 ± 10 P > 0.05 |
59 ± 8 P > 0.05 |
121 ± 20 P < 0.001 |
| Choroid plexus of lateral ventricle | 51 ± 5 | 111 ± 13 P < 0.001 |
239 ± 16 P < 0.001 |
232 ± 14 P < 0.001 |
75 ± 14 P < 0.05 |
41 ± 7 P > 0.05 |
| Ependyma of lateral ventricle | 30 ± 8 | 51 ± 9 P > 0.05 |
139 ± 18 P < 0.001 |
176 ± 17 P < 0.001 |
200 ± 21 P < 0.001 |
31 ± 7 P > 0.05 |
| Suprachiasmatic nucleus | 61 ± 6 | 125 ± 16 P < 0.001 |
195 ± 19 P < 0.001 |
220 ± 20 P < 0.001 |
174 ± 19 P < 0.001 |
144 ± 17 P < 0.001 |
| Reticular thalamic nucleus | 29 ± 6 | 158 ± 10 P < 0.001 |
137 ± 19 P < 0.001 |
190 ± 16 P < 0.001 |
175 ± 13 P < 0.001 |
82 ± 17 P < 0.001 |
| Somatomotor cortex | 128 ± 9 | 54 ± 8 P < 0.001 |
31 ± 11 P < 0.001 |
53 ± 12 P < 0.001 |
60 ± 10 P < 0.001 |
120 ± 12 P > 0.05 |
| Paraventicular nucleus | 65 ± 5 | 81 ± 13 P > 0.05 |
88 ± 14 P < 0.05 |
97 ± 21 P < 0.05 |
141 ± 15 P < 0.001 |
212 ± 25 P < 0.001 |
| Arcuate nucleus | 54 ± 4 | 32 ± 6 P < 0.01 |
34 ± 3 P < 0.01 |
41 ± 8 P > 0.05 |
58 ± 6 P > 0.05 |
145 ± 11 P < 0.001 |
| Dorsolateral hypothalamic area | 71 ± 9 | 56 ± 8 P > 0.05 |
43 ± 9 P < 0.05 |
49 ± 14 P > 0.05 |
69 ± 6 P > 0.05 |
171 ± 19 P < 0.001 |
| Ependyma of 3rd ventricle | 47 ± 9 | 70 ± 14 P > 0.05 |
191 ± 19 P < 0.001 |
230 ± 13 P < 0.001 |
196 ± 18 P < 0.001 |
46 ± 8 P > 0.05 |
| Tanycytes | 45 ± 4 | 169 ± 14 P < 0.001 |
242 ± 26 P < 0.001 |
247 ± 19 P < 0.001 |
169 ± 25 P < 0.001 |
42 ± 9 P > 0.05 |
| Hippocampus | 49 ± 7 | 31 ± 3 P > 0.05 |
44 ± 8 P > 0.05 |
40 ± 9 P > 0.05 |
38 ± 10 P > 0.05 |
43 ± 8 P > 0.05 |
| Choroid plexus of 4th ventricle | 47 ± 8 | 71 ± 13 P < 0.05 |
248 ± 24 P < 0.001 |
205 ± 29 P < 0.001 |
156 ± 23 P < 0.001 |
93 ± 13 P < 0.01 |
| Ependyma of 4th ventricle | 34 ± 3 | 48 ± 9 P > 0.05 |
69 ± 13 P < 0.01 |
160 ± 14 P < 0.001 |
235 ± 19 P < 0.001 |
42 ± 12 P > 0.05 |
| Raphe nuclei | 53 ± 4 | 46 ± 7 P > 0.05 |
41 ± 9 P > 0.05 |
48 ± 11 P > 0.05 |
53 ± 6 P > 0.05 |
61 ± 13 P > 0.05 |
Values are quantitative density data generated by ImageJ software, calculated by identical areas of four different animals and expressed as mean with standard error of mean. Statistical significance among the different phases of hibernation bout is calculated by one-way ANOVA with Bonferroni post-hoc tests and P-values expressed for every studied brain region in comparison with measurement values in the ART group.
Statistical analysis of the four identical values was performed using one-way analysis of variance (ANOVA) with Bonferroni post-hoc tests. Measurement values were compared to values of the ART group and statistical significance (P values) indicated below every measurement in Table 1.
RESULTS
Sequence analysis of the 13-lined ground squirrel c-fos cDNA fragment
Using the cDNA brain library of the 13-lined ground squirrel, a cDNA fragment of c-fos was cloned by RT-PCR. The cDNA fragment is 884 bp long and shows strong similarity with rat c-fos (88.69% identity), mouse c-fos (88.46% identity), and human c-fos (91.18% identity). The sequential identity among species confirms that the cDNA fragment is derived from the c-fos gene. The complete sequence of this c-fos cDNA fragment has been deposited in the GenBank database (Access. No. DQ104400 [13-lined ground squirrel c-fos cDNA fragment]).
Expression of c-fos mRNA in brain areas during the hibernation cycle
In situ hybridization of coronal brain slices obtained during different phases of the hibernation cycle (Fig. 1) shows differences in the distribution and the amount of c-fos expression (Table 1). The observed pattern of distribution of cells with strong c-fos expression combined with known functions of neuroanatomical areas suggests that specific activated areas functionally relate to each other; thus, below we show our data according to a functional neuroanatomical classification of brain regions identified by c-fos in situ hybridization immunohistochemistry.
We prepared serial brain sections from the olfactory bulb to the medulla oblongata and performed a systematic analysis of brain areas and nuclei. In the following list, we describe brain regions where c-fos expression was detected. Regions that are not described below did not show any c-fos expression throughout all the phases of the entire hibernation bout.
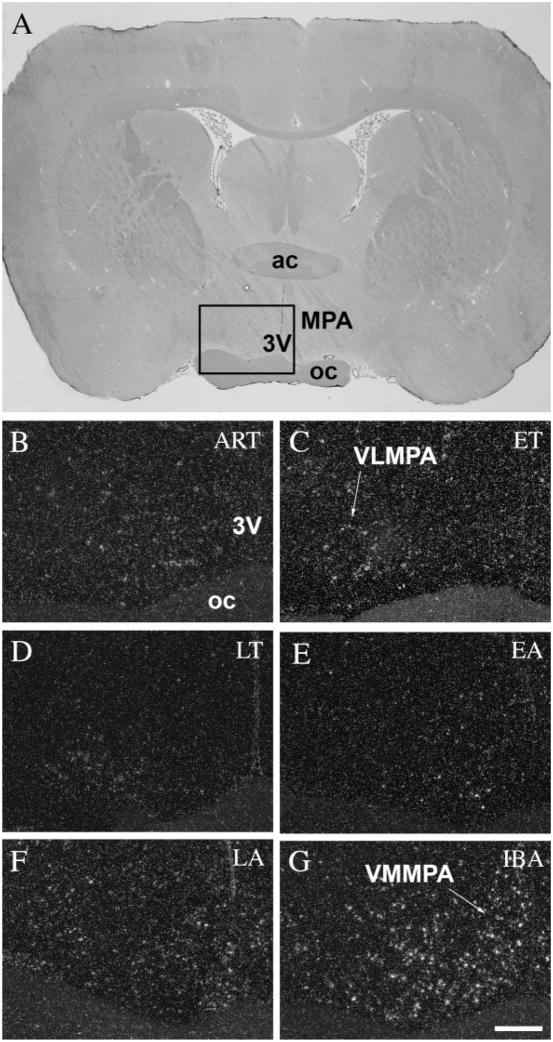
The preoptic area of the hypothalamus, known as the center of thermoregulation (Boulant, 1981), exhibited spatial and temporal differences in the distribution of c-fos expression in neuronal cells during the hibernation cycle (Table 1). In active animals at room temperature, every subdivision of the preoptic area showed only low levels of c-fos expression (Fig. 2B). During the ET phase, a higher level of c-fos expression was detected in the ventrolateral subdivision of the medial preoptic area (Fig. 2C). This activated area is located in the ventral part of the medial preoptic area (MPA), adjacent to the optic chiasm, sparing the periventricular region. The rest of the MPA remained relatively inactive in the entrance phase. During LT (Fig. 2D), a very low signal level was detected throughout the entire preoptic area. In EA phase animals showed again a higher level of c-fos expression (Fig. 2E), but at this time mainly in the ventromedial subdivision of the medial pre-optic area (Fig. 2F), right next to the third ventricle. Following hibernation, fully active animals (IBA) exhibited strong c-fos expression, especially in the ventromedial subdivision of the MPA (Fig. 2G).
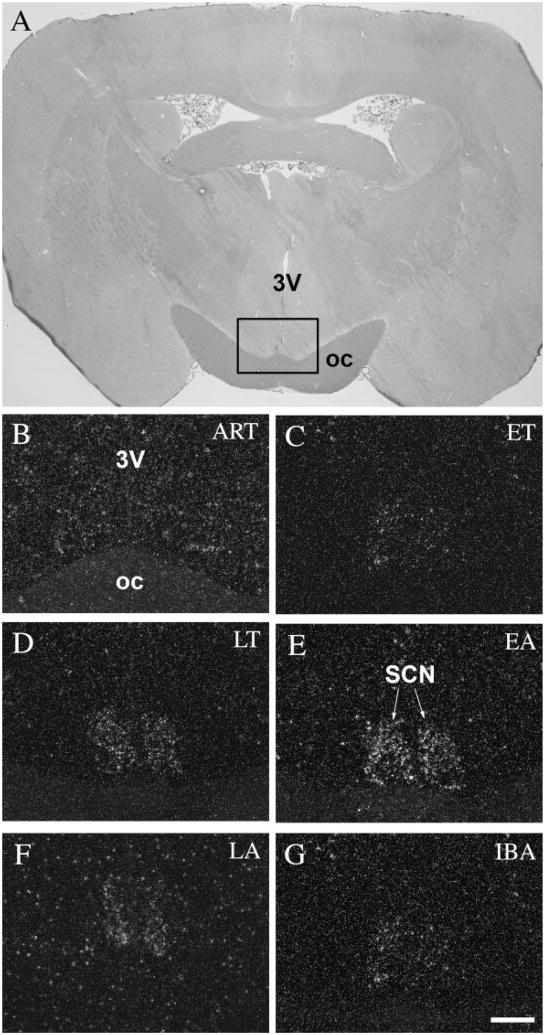
The suprachiasmatic nucleus, which functions as the biological clock in the mammalian brain and contributes to the regulation of circadian rhythm (Miller, 1993) and temperature, showed the strongest activity in the EA phase (Table 1). As expected, this nucleus, like other hypothalamic nuclei, showed low activity in ART phase animals (Fig. 3B). Light c-fos expression was detected during ET (Fig. 3C). During LT phase, c-fos expression further increased (Fig. 3D) and reached its peak in EA (Fig. 3E). Neurons both in the ventral and dorsal subdivisions of the suprachiasmatic nucleus showed the same high level of activity. The expression of c-fos significantly dropped in the LA phase (Fig. 3F), and arrived at its lowest level during the hibernation bout in fully awake animals (IBA) (Fig. 3G).
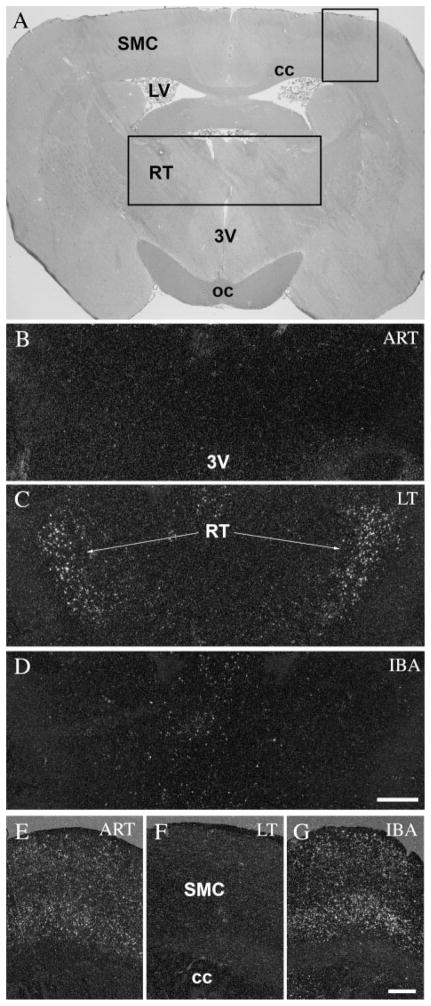
The reticular thalamic nucleus showed an opposite pattern of c-fos activation to that of the somatomotor cortex (Table 1). Neurons in the reticular thalamic nucleus, which gate information ascending to the cortex, exhibited strong activation throughout the hibernation bout, including the entrance (ET) and early arousal (EA) periods (Fig. 4C), while in active animals, both before and after hibernation, neurons in the nucleus remained relatively inactive (Fig. 4B,D). It should be noted that no activity was detected in any other thalamic nuclei during the six investigated phases of the hibernation bout. In the somato-motor cortex of active animals (ART and IBA) (Fig. 4E,G), c-fos in situ hybridization showed strong activation, especially in the first, fourth, and fifth layers; during torpor (LT), none of the cells in any layers of somatomotor cortex showed c-fos expression (Fig. 4F).
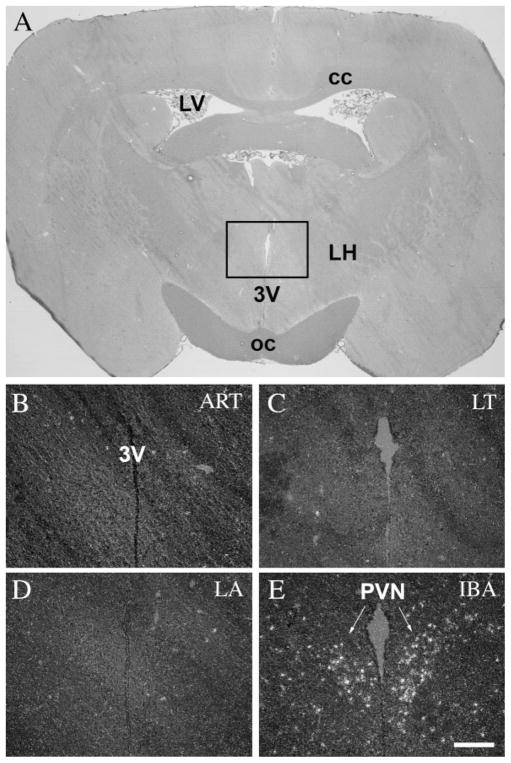
The paraventricular nucleus of the hypothalamus (PVN), a nucleus known to activate due to stress, showed no c-fos activity in entrance (ET) (Fig. 5B), deep torpor (LT) (Fig. 5C), and arousal periods (EA and LA) (Fig. 5D). Expression of c-fos mRNA was detected only in awake animals (IBA), mainly in the parvocellular subdivisions of the nucleus (Fig. 5E).
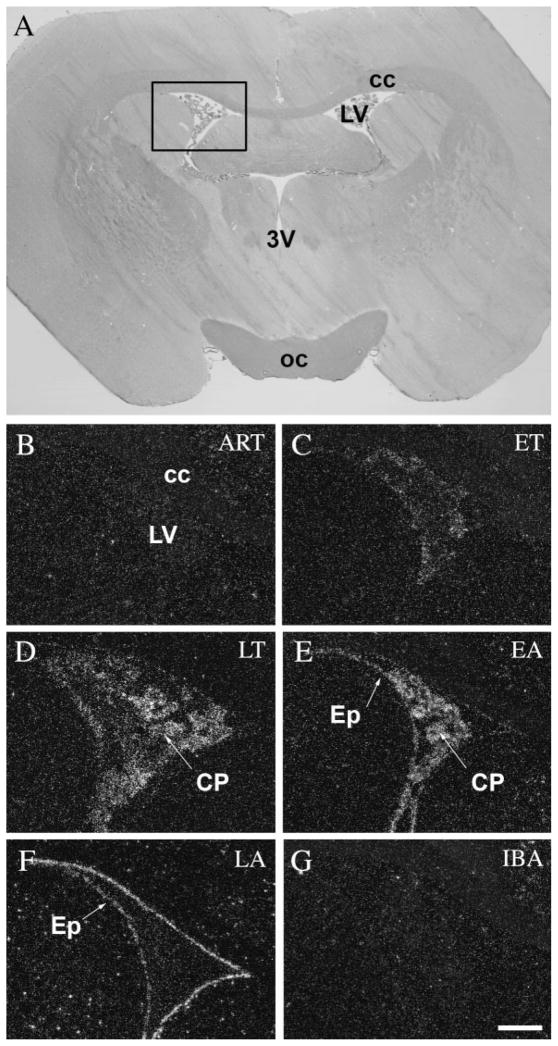
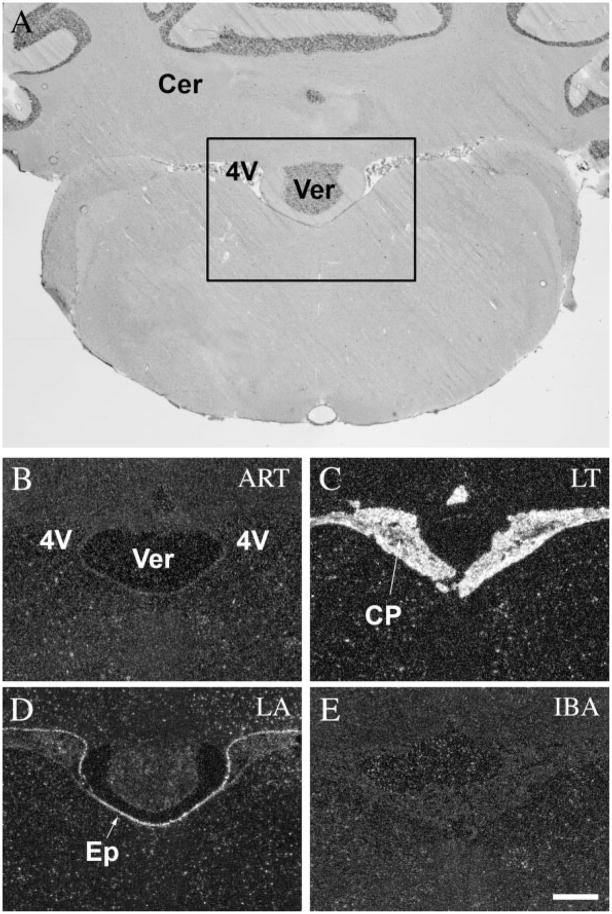
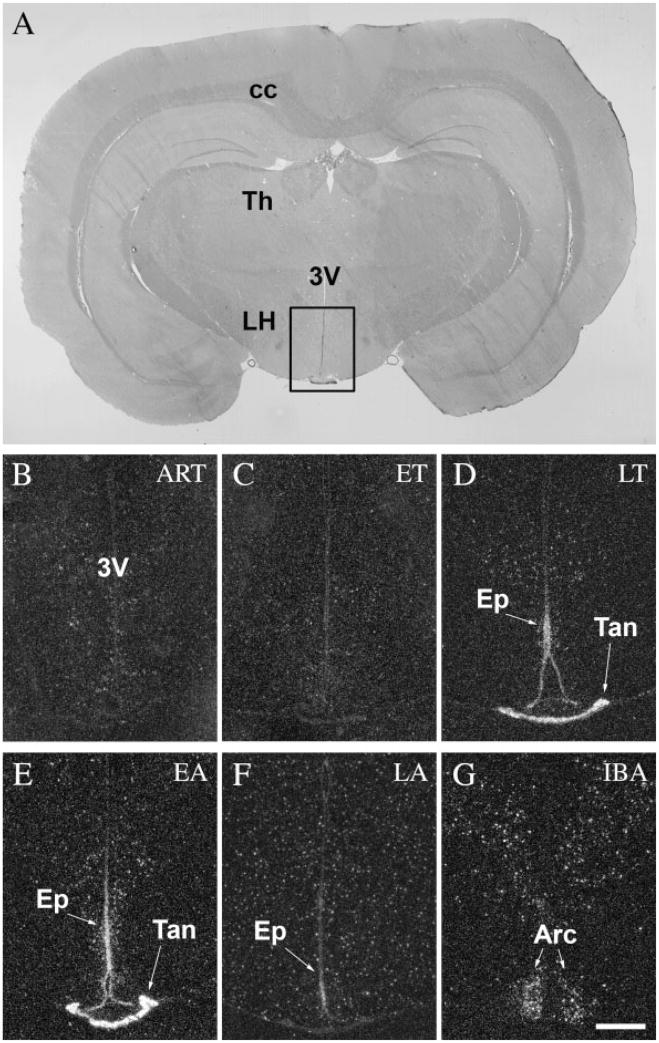
The choroid plexus of the lateral and fourth ventricles showed no activity in active animals (ART and IBA) (Figs. 6B,G, 7B,E, respectively). A low level of c-fos expression was detected in the ET phase (Fig. 6C), and extremely high levels during LT phase (Figs. 6D, 7C). In EA the level of c-fos expression started to decrease rapidly (Fig. 6E) and it diminished completely in the LA phase (Figs. 6F, 7D). Interestingly, the ependymal cells surrounding the ventricles were silent in active animals (ART and IBA) (Figs. 6B,G, 7B,E), in ET (Fig. 6C), and LT phases (Figs. 6D, 7C), but became active in the EA phase (Fig. 6E). It reached a very high level of c-fos expression in LA (Figs. 6F, 7D), just as the activation detected in the choroid plexus decreased. It should be noted that the specialized ependymal cells of the ventral portion of the third ventricle, where no choroid plexus is present, did not show alterations in c-fos activity like that of the ependymal cells in the lateral or fourth ventricles (Fig. 8).
Tanycytes of the ventral portion of the third ventricle compose a unique group of modified ependymal cells that bridge the ventricle to the outer surface of the hypothalamus as well as to some hypothalamic vessels (Millhouse, 1971) and are involved in transport functions. These tanycytes showed a very high level of c-fos expression in LT and EA phases (Fig. 8D,E), while they remained silent during the rest of the hibernation bout (Fig. 8, Table 1).
In IBA animals c-fos expression appeared in cells of the arcuate nucleus and the dorsolateral hypothalamic area (Fig. 8G). Interestingly, these cells were otherwise silent through the hibernation bout (Table 1).
Fig. 2.
c-Fos expression in the preoptic area during the hibernation bout. A: Coronal section of the brain of a 13-lined ground squirrel 0.5 mm anterior to the level of the bregma. Insert area is shown enlarged in B–G. In ART, low level of c-fos expression was detected in neurons throughout the entire preoptic area (B), while in ET the ventrolateral subdivision of the preoptic area exhibited stronger c-fos expression (C). In animals during torpor (LT) the area remained silent (D). During arousal (EA and LA) we found activity in the ventromedial subdivision of the preoptic area (E,F), and in IBA animals, strong c-fos activity was detected all throughout the area (G). 3V, third ventricle; ac, anterior commissure; oc, optic chiasm; MPA, medial preoptic area; VLMPA, ventrolateral subdivision of MPA; VMMPA, ventromedial subdivision of MPA. Scale bar = 50 μm.
Fig. 3.
c-Fos expression in the suprachiasmatic nucleus during the hibernation bout. A: Coronal section of the brain of a 13-lined ground squirrel 1.5 mm posterior to the level of the bregma. Insert area is shown enlarged in B–G. The SCN showed low activity in ART (B), while during torpor, c-fos expression in the neurons of the SCN became progressively stronger (C–E) peaking in EA (E) and returning to lower levels of expression in LA (F) and IBA (G). 3V, third ventricle; oc, optic chiasm; SCN, suprachiasmatic nucleus. Scale bar = 50 μm.
Fig. 4.
c-Fos expression in the reticular thalamic nucleus and the somatomotor cortex during the hibernation bout. A: Coronal section of the brain of a 13-lined ground squirrel 1.5 mm posterior to the level of the bregma. Insert areas (1, 2) are shown enlarged in B–D and E–G, respectively. The reticular thalamic nucleus showed no activity in awake animals (ART and IBA) (B,D), meanwhile during the torpor phase of the hibernation bout (LT), strong c-fos expression was detected in the nucleus (C). In contrast, activated neurons were found in the somatomotor cortex in only awake animals (ART and IBA) (E,G), but not during torpor (LT) (F). 3V, third ventricle; cc, corpus callosum; LV, lateral ventricle; oc, optic chiasm; RT, reticular thalamic nucleus; SMC, somatomotor cortex. Scale bars = 100 μm in B–D; 50 μm in E–G.
Fig. 5.
c-Fos expression in the paraventricular nucleus during the hibernation bout. A: Coronal section of the brain of a 13-lined ground squirrel 1.5 mm posterior to the level of the bregma. Insert area is shown enlarged in B–D. The PVN showed no c-fos activity in ART (B), in ET (C), and in LT (D); however, in IBA strong c-fos expression was detected mainly in the parvocellular neurons of the nucleus (E). 3V, third ventricle; cc, corpus callosum; LH, lateral hypothalamus; LV, lateral ventricle; oc, optic chiasm; PVN, paraventricular nucleus. Scale bar = 50 μm.
Fig. 6.
c-Fos expression in the choroid plexus and the ependymal cells of the lateral ventricle during the hibernation bout. A: Coronal section of the brain of a 13-lined ground squirrel 0.5 mm posterior to the level of the bregma. Insert area is shown enlarged in B–G. Neither the epithelial cells of the choroid plexus nor the ependymal cells of the lateral ventricle showed any c-fos expression in awake animals (ART and IBA) (B,G). During torpor, we detected increasing c-fos expression in the choroid plexus, starting in ET (C), peaking in EA (E), and diminishing in LA (F), while the ependymal cells of the lateral ventricle exhibited activity only in the arousal phases (EA and LA) (E,F). 3V, third ventricle; cc, corpus callosum; CP, choroids plexus; Ep, ependymal cells of the lateral ventricle; LV, lateral ventricle; oc, optic chiasm. Scale bar = 50 μm.
Fig. 7.
c-Fos expression in the choroid plexus and the ependymal cells of the fourth ventricle during the hibernation bout. A: Coronal section of the brain of a 13-lined ground squirrel 10.5 mm posterior to the level of the bregma. Insert area is shown enlarged in B–E. Strongest expression of c-fos in the choroid plexus was detected during LT (C) and EA (not shown), while the ependymal cells of the fourth ventricle showed activity in LA phase (D). In awake animals both areas remained silent (B,E). 4V, fourth ventricle; Cer, cerebellum; CP, choroids plexus; Ep, ependymal cells of the fourth ventricle; Ver, vermis. Scale bar = 50 μm.
Fig. 8.
c-Fos expression in tanycytes and the ependymal cells of the third ventricle during the hibernation bout. A: Coronal section of the brain of a 13-lined ground squirrel 3.5 mm posterior to the level of the bregma. Insert area is shown enlarged in B–G. No c-fos activity was detected in ART (B) and ET (C) phases. Strong c-fos expression was observed in both tanycytes and third ventricular ependymal cells during LT (D) and EA (E), while this activity diminished in LA (F). Interestingly, neurons in the arcuate nucleus and in the dorsomedial hypothalamus exhibited strong activity in IBA (G). 3V, third ventricle; Arc, arcuate nucleus; cc, corpus callosum; Ep, ependymal cells of the third ventricle; LH, lateral hypothalamus; Tan, tanycytes; Th, thalamus. Scale bar = 50 μm.
The hippocampus did not show c-fos expression throughout the entire hibernation bout.
We observed only a minimal level of c-fos expression in the raphe nuclei of active animals. The c-fos expression of the raphe nuclei did not change during the different phases of torpor and the hibernation cycle.
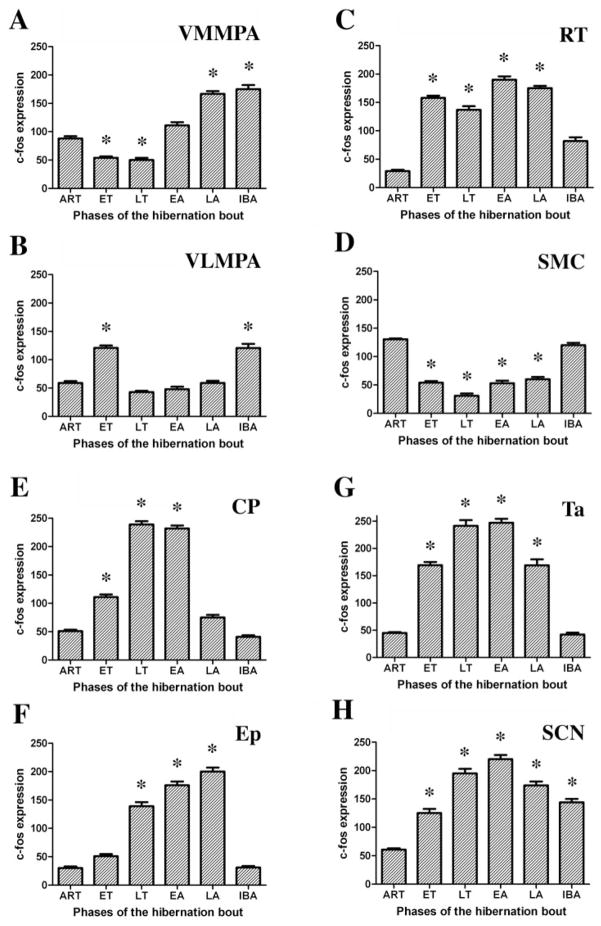
A summary of c-fos expression in the most important brain nuclei showing statistically significant changes during the studied phases of the hibernation bout is shown in Figure 9.
Fig. 9.
Histogram of the statistically significant changes in c-fos expression occurring throughout the hibernation bout in the following nuclei: the ventromedial (A) and ventrolateral (B) subdivisions of the medial preoptic area (VMMPA and VLMPA, subsequently), the reticular thalamic nucleus (C) (RT), the somatomotor cortex (D) (SMC), the choroid plexus (E) (CP) and the ependymal cells (F) (Ep) of the lateral ventricle, tanycytes of the third ventricle (G) (Ta), and the suprachiasmatic nucleus (H) (SCN). c-Fos expression was calculated by ImageJ software (NIH, Bethesda, MD) and values are expressed as mean and SEM of pixel density/μm2. Statistical significance of the measurement values was calculated by one-way ANOVA with Bonferroni post-hoc tests, and phases of the hibernation bout significantly different (P > 0.01) compared to the ART group are indicated with an asterisk (*).
DISCUSSION
As an amazing adaptation in animals, hibernation has evolved to survive periods of harsh environmental circumstances. During deep torpor, despite the dramatic changes in body temperature and metabolism, the CNS remains active and appears to precisely regulate the hibernation cycle. Several studies have been conducted to identify activated brain regions in hibernation (Kilduff et al., 1990; Nurnberger, 1995; O’Hara et al., 1999; Ruby, 2003), but participating brain areas and the possible role of these areas in the hibernation bout remains incompletely known. By cloning c-fos cDNA from the 13-lined ground squirrel and using a squirrel c-fos mRNA probe for in situ hybridization histochemistry, we applied a highly accurate and sensitive method to localize activated neuronal, glial, ependymal, and choroid epithelial cells in brain areas through the major phases of the hibernation bout. We identified brain regions that activate specifically at certain phases of the hibernation cycle and remain silent at other timepoints. Based on the known functions of these areas, this systematic temporal analysis of brain regions allows us to infer their roles in the regulation of hibernation.
The essential role of the suprachiasmatic nucleus (SCN) in hibernation has already been shown by various techniques (Kilduff et al., 1989; Dark et al., 1990; Sutin and Kilduff, 1992; Bitting et al., 1994; Ruby et al., 1996, 2002; Yu et al., 2002). Since this nucleus has been proposed to control the rhythm of hibernation (Heller and Ruby, 2004), as it does in circadian cycling (Miller, 1993), the pattern of activation suggests that the SCN may be responsible for initiating the arousal of hibernators. We confirmed these prior findings by detecting phase-dependent alterations in the c-fos expression of the nucleus during hibernation. Our findings reinforce the results of previous studies focusing on phase-dependent 2-DG uptake of the SCN (Kilduff et al., 1990; O’Hara et al., 1999). Cells in the SCN exhibited very low signal levels in active animals, then increasingly activated through the entrance and deep torpor, peaking at the beginning of arousal and returning again to lower levels of activation in awake interbout animals. Thus, the ‘biological clock’ appears to be working very precisely during hibernation. The SCN does not seem to play a role in the induction of the hibernation bout; however, it may be responsible for the arousal of the animals. By timing the hibernation cycle, the SCN may be the nucleus that initiates all the changes at the arousal period, just like the increase of body temperature.
Decreasing and increasing the body temperature of hibernators from 37°C to 5°C and back requires activation of thermoregulatory areas of the brain (Satinoff, 1967; Kilduff et al., 1990). As expected, the preoptic thermoregulatory center showed the strongest activation in entrance into torpor and in late arousal; however, it showed different patterns in the distribution of c-fos-expressing cells in these two phases. In entrance into torpor, neurons in the ventrolateral part of the MPA exhibited stronger c-fos expression. These cells are distributed in the ventral part of the MPA, dorsal to the optic chiasm, laterally displaced from the third ventricle, but not extending until the lateral edge of the optic chiasm. In rats, the ventrolateral subdivision of the MPA is known to become activated if the animal is exposed to a hyperthermic environment (Bratincsak and Palkovits, 2004). This preoptic subdivision drives thermoregulatory mechanisms to reduce body temperature, just as needed during entrance into torpor. However, this area in rats is adjacent to the ventrolateral subdivision of the preoptic area (VLPO) that is involved in sleep regulation (Steininger et al., 2001). Without chemical characterization of the activated region of the MPA during entrance into torpor, we cannot distinguish between the two above-mentioned functional subdivisions in squirrels based on their neuroanatomical localization. In contrast, the ventromedial part of the medial preoptic area, located ventrally and adjacent to the third ventricle, where neurons are activated to increase body temperature by cold stress (Bratincsak and Palkovits, 2004), showed strong signal during arousal. These specific preoptic areas, in which activity fluctuations were observed, may induce mechanisms that result in the change of body temperature. The SCN may be triggering the activation of the ventromedial part at the time of arousal. Which area triggers the ventrolateral part at the beginning of the hibernation bout is still unknown.
With the change of body temperature, the animal sinks into a profoundly reduced responsiveness as the torpor phase of the hibernation bout is entered. Cortical activities diminish and the animal does not respond to the usual external stimuli. Neurons also in the somatomotor cortex are generally deactivated during torpor (Mihailovic et al., 1968), but the mechanism of this deactivation is still unknown. Here we demonstrated that cortical neurons were active before and after torpor, but their c-fos expression was suppressed during deep torpor. The somatomotor cortex is reciprocally interconnected by the reticular thalamic nucleus that serves as a gate for ascending information to the cortex. The reticular thalamic cells showed strong c-fos activation first in the entrance phase, then through deep torpor, followed by a decline in activity in the arousal phase that coincided with the return of activity of the somatomotor cortex. The reticular thalamic nucleus is known to project to the midbrain reticular formation (Steriade et al., 1984) besides its direct cortical projections, and it has been shown to participate in the process of arousal from sleep (Steriade and Contreras, 1995). Our observations suggest that the activation of the reticular thalamic nucleus may be responsible for depressing cortical arousal mechanisms by filtering information to the cortex, hence keeping the cortex ‘asleep.’ The activation of the SCN at the beginning of arousal may block the depressing effect of the reticular thalamic nucleus and, thus, activate the cortex resulting in the arousal of the animal.
After deep torpor, hibernators return to their euthermic state, experiencing the same physiological needs as other animals. It was proposed that the return to normothermia might occur in order to allow the animal to sleep (Daan et al., 1991) and to satisfy other basic internal needs. Strong expression of c-fos in neurons of hypothalamic areas that are involved in food intake besides other physiological regulations (Sakurai et al., 1998; Palkovits, 2003), i.e., in the arcuate nucleus and the dorsolateral hypothalamus as well as in the PVN, suggests that hibernating squirrels experience some kind of stress in the interbout active phase. Although animals in the interbout phase do not eat even though food is present, the activation of these areas may indicate that they experience hunger.
It is very interesting that these animals may experience the interbout active phase as a stressful period; however, changing their temperature from 37°C to 5°C and back does not activate stress centers of the brain. The PVN orchestrates numerous neuroendocrine regulatory mechanisms and is known to respond to stresses, like pain, immobilization, hypoglycemia, just as well as extreme environmental temperatures (Pacak and Palkovits, 2001). During the hibernation bout the temperature of these torpid animals drops to 5°C. Nevertheless, we did not observe c-fos expression in the PVN during torpor. This observation provides indirect evidence that hibernation is a physiological mechanism, rather than a stress, and thus the phases of the hibernation bout are not reactions to changing external stimuli, but are the result of a precisely regulated internal mechanism.
Although we may understand how the SCN triggers preoptic and thalamic areas to induce the arousal of the animals, we have no data about a brain region that is responsible for the coordination and induction of entrance into torpor. The raphe nuclei were suggested to play a role in the initial phase of the hibernation bout (Canguilhem et al., 1986; Haak et al., 1991); however, we did not find elevation of c-fos expression of these nuclei in the entrance into torpor phase. By performing a thorough systematic analysis of brain areas from the prefrontal cortex to the lower brainstem, we found activated regions that have not been previously reported in connection with hibernation, such as the choroid plexus of the lateral and fourth ventricles, the ependymal layer of the lateral, third and fourth ventricles, and the tanycytes.
The cerebrospinal fluid (CSF) is secreted by the choroid plexus and absorbed by the ependymal cell lining on the side of cerebral ventricles (Cutler et al., 1968). Interestingly, we observed a unique pattern of cell activation in these areas during the phases of hibernation. The epithelial cells of the choroid plexus showed c-fos expression first in early torpor, defined as the first day after entrance into torpor (Tb = 5–8°C) (data not shown), and this expression became progressively stronger, reaching its peak in early arousal. Meanwhile, the ependymal cells in the lateral and fourth ventricles became activated only in early arousal and returned to an inactive state in fully awake interbout animals. These findings suggest that the production of the cerebrospinal fluid may be increased during torpor, peaking at late torpor, exactly when its absorption would be expected to increase throughout arousal phases. Although there has not yet been shown any evidence that the composition or the amount of CSF would differ in deep torpor, our observation suggests that the extraordinary strong and phase-dependent changes in the activity of choroid epithelial and ependymal cells may have some functional importance in maintaining and terminating the hibernation bout. Furthermore, given the observation that the brain tissue levels of histamine increases during hibernation (Sallmen et al., 1999; Panula et al., 2000), and that histaminergic fibers innervate the choroid plexus (Nilsson et al., 1988), it can be hypothesized that histaminergic fibers have induced the activation of the choroid plexus observed during deep torpor. Our findings are consistent with a model proposed by the Kondo laboratory (Kondo et al., 2006), in which they describe the role of a hibernation-specific protein complex (HPc) in hibernation. The protein parts of the complex are produced in the liver and secreted into the circulation. In a circannual rhythm, the HPc is actively transported from the blood into the brain via the choroid plexus, where it is further processed to an active form that is a putative hibernation hormone.
Tanycytes, specialized ependymal cells in the ventral portion of the third ventricle (Krisch and Leonhardt, 1978), showed a pattern of activation during hibernation that was similar to that of the epithelial (and not ependymal) cells of the choroid plexus. The extremely strong signals detected in these regions gives them a particular importance. Which area triggers and mediates their activity and the chemical signals involved are yet to be discovered. Part of our ongoing studies aims to reveal these questions.
In conclusion, we identified activated brain regions during entrance, deep torpor, arousal, and the interbout awake phase of the hibernation bout (Fig. 9). Their temporal distributional pattern suggested functional characteristics. In the beginning of the hibernation bout, so-far unidentified brain areas or alternate mechanisms induce and coordinate entrance into torpor. They activate nuclei that regulate body temperature, cortical activity level, and other physiological mechanisms observed throughout torpor. The ventrolateral division of the medial preoptic area may drive the temperature change that brings animals to 5°C or may induce torpor itself. As a hibernator dives into deep torpor, the cortex falls ‘asleep,’ which may be a result of the activation of the reticular thalamic nucleus that suppresses arousal activity. During hibernation, the ‘biological clock’ (suprachiasmatic nucleus) is working and it might be responsible for the timing when hibernators shall arouse. Besides these, a profound activity of the choroid plexus, the ependymal cells of the lateral and fourth ventricles and third ventricular tanycytes in certain phases of the hibernation bout, suggests that the production and the transport of the CSF, or some currently unrecognized biological function of these cells—like the processing of a putative hibernation hormone—might also be an important factor in maintaining torpor. At the end of deep torpor, when the activity of the suprachiasmatic and choroid epithelial cells reach their peak, hibernators start to arouse, increasing body temperature that is driven by the activation of the medial part of the preoptic area. During arousal, as the reticular thalamic nucleus silences the somatomotor cortex regains its activity. Following torpor, fully awake interbout animals experience their basic biologic needs as indicated by the strong activation of hypothalamic neurons that participate in stress responses. Identification of participating brain regions in the course of the hibernation bout will guide future studies aiming to elucidate the fine regulatory mechanisms of hibernation.
Acknowledgments
Grant sponsor: intramural program of the National Institute of Neurological Disorders and Stroke; Grant sponsor: Hungarian National Science Foundation; Grant number: OTKA T043169.
The authors thank Mr. Ricardo Dreyfuss for the outstanding pictures.
LITERATURE CITED
- Bitting L, Sutin EL, Watson FL, Leard LE, O’Hara BF, Heller HC, Kilduff TS. C-fos mRNA increases in the ground squirrel suprachiasmatic nucleus during arousal from hibernation. Neurosci Lett. 1994;165:117–121. doi: 10.1016/0304-3940(94)90723-4. [DOI] [PubMed] [Google Scholar]
- Boulant JA. Hypothalamic mechanisms in thermoregulation. Fed Proc. 1981;40:2843–2850. [PubMed] [Google Scholar]
- Bratincsak A, Palkovits M. Activation of brain areas in rat following warm and cold ambient exposure. Neuroscience. 2004;127:385–397. doi: 10.1016/j.neuroscience.2004.05.016. [DOI] [PubMed] [Google Scholar]
- Bullitt E. Expression of c-fos-like protein as a marker for neuronal activity following noxious stimulation in rat. J Comp Neurol. 1990;296:517–530. doi: 10.1002/cne.902960402. [DOI] [PubMed] [Google Scholar]
- Canguilhem B, Miro JL, Kempf E, Schmitt P. Does serotonin play a role in entrance into hibernation? Am J Physiol. 1986;251:R755–761. doi: 10.1152/ajpregu.1986.251.4.R755. [DOI] [PubMed] [Google Scholar]
- Cutler RW, Page L, Galicich J, Watters GV. Formation and absorption of cerebrospinal fluid in man. Brain. 1968;91:707–720. doi: 10.1093/brain/91.4.707. [DOI] [PubMed] [Google Scholar]
- Daan S, Barnes BM, Strijkstra AM. Warming up for sleep? Ground squirrels sleep during arousals from hibernation. Neurosci Lett. 1991;128:265–268. doi: 10.1016/0304-3940(91)90276-y. [DOI] [PubMed] [Google Scholar]
- Dark J, Kilduff TS, Heller HC, Licht P, Zucker I. Suprachiasmatic nuclei influence hibernation rhythms of golden-mantled ground squirrels. Brain Res. 1990;509:111–118. doi: 10.1016/0006-8993(90)90316-4. [DOI] [PubMed] [Google Scholar]
- Frerichs KU, Hallenbeck JM. Hibernation in ground squirrels induces state and species-specific tolerance to hypoxia and aglycemia: an in vitro study in hippocampal slices. J Cereb Blood Flow Metab. 1998;18:168–175. doi: 10.1097/00004647-199802000-00007. [DOI] [PubMed] [Google Scholar]
- Frerichs KU, Kennedy C, Sokoloff L, Hallenbeck JM. Local cerebral blood flow during hibernation, a model of natural tolerance to “cerebral ischemia. J Cereb Blood Flow Metab. 1994;14:193–205. doi: 10.1038/jcbfm.1994.26. [DOI] [PubMed] [Google Scholar]
- Haak LL, Mignot E, Kilduff TS, Dement WC, Heller HC. Regional changes in central monoamine and metabolite levels during the hibernation cycle in the golden-mantled ground squirrel. Brain Res. 1991;563:215–220. doi: 10.1016/0006-8993(91)91536-a. [DOI] [PubMed] [Google Scholar]
- Heller HC. Hibernation: neural aspects. Annu Rev Physiol. 1979;41:305–321. doi: 10.1146/annurev.ph.41.030179.001513. [DOI] [PubMed] [Google Scholar]
- Heller HC, Ruby NF. Sleep and circadian rhythms in mammalian torpor. Annu Rev Physiol. 2004;66:275–289. doi: 10.1146/annurev.physiol.66.032102.115313. [DOI] [PubMed] [Google Scholar]
- Kayser C. The intervention of external and internal factors in the determinism of the hibernation of mammals. Arch Sci Physiol (Paris) 1961;15:377–420. [PubMed] [Google Scholar]
- Kilduff TS, Radeke CM, Randall TL, Sharp FR, Heller HC. Suprachiasmatic nucleus: phase-dependent activation during the hibernation cycle. Am J Physiol. 1989;257:R605–612. doi: 10.1152/ajpregu.1989.257.3.R605. [DOI] [PubMed] [Google Scholar]
- Kilduff TS, Miller JD, Radeke CM, Sharp FR, Heller HC. 14C-2-deoxyglucose uptake in the ground squirrel brain during entrance to and arousal from hibernation. J Neurosci. 1990;10:2463–2475. doi: 10.1523/JNEUROSCI.10-07-02463.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo N, Sekijima T, Kondo J, Takamatsu N, Tohya K, Ohtsu T. Circannual control of hibernation by HP complex in the brain. Cell. 2006;125:161–172. doi: 10.1016/j.cell.2006.03.017. [DOI] [PubMed] [Google Scholar]
- Krisch B, Leonhardt H. The functional and structural border of the neurohemal region of the median eminence. Cell Tissue Res. 1978;192:327–339. doi: 10.1007/BF00220750. [DOI] [PubMed] [Google Scholar]
- Mihailovic L, Beleslin B, Popeskovic D, Cupic D. Spontaneous and evoked electric activity of various cortical and subcortical structures of the brain of the squirrel (Citellus citellus L.) in the process of arousal from hibernation. Acta Med Iugosl. 1968;22:165–228. [PubMed] [Google Scholar]
- Miller JD. On the nature of the circadian clock in mammals. Am J Physiol. 1993;264:R821–832. doi: 10.1152/ajpregu.1993.264.5.R821. [DOI] [PubMed] [Google Scholar]
- Millhouse OE. A Golgi study of third ventricle tanycytes in the adult rodent brain. Z Zellforsch Mikrosk Anat. 1971;121:1–13. doi: 10.1007/BF00330913. [DOI] [PubMed] [Google Scholar]
- Morgan JI, Curran T. Stimulus-transcription coupling in the nervous system: involvement of the inducible proto-oncogenes fos and jun. Annu Rev Neurosci. 1991;14:421–451. doi: 10.1146/annurev.ne.14.030191.002225. [DOI] [PubMed] [Google Scholar]
- Nilsson C, Steinbusch HW, Lindvall-Axelsson M, Owman C. Histaminergic cells in the choroid plexus of rat. J Chem Neuroanat. 1988;1:53–57. [PubMed] [Google Scholar]
- Nurnberger F. The neuroendocrine system in hibernating mammals: present knowledge and open questions. Cell Tissue Res. 1995;281:391–412. doi: 10.1007/BF00417858. [DOI] [PubMed] [Google Scholar]
- O’Hara BF, Watson FL, Srere HK, Kumar H, Wiler SW, Welch SK, Bitting L, Heller HC, Kilduff TS. Gene expression in the brain across the hibernation cycle. J Neurosci. 1999;19:3781–3790. doi: 10.1523/JNEUROSCI.19-10-03781.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacak K, Palkovits M. Stressor specificity of central neuroendocrine responses: implications for stress-related disorders. Endocr Rev. 2001;22:502–548. doi: 10.1210/edrv.22.4.0436. [DOI] [PubMed] [Google Scholar]
- Palkovits M. Hypothalamic regulation of food intake. Ideggyogy Sz. 2003;56:288–302. [PubMed] [Google Scholar]
- Panula P, Karlstedt K, Sallmen T, Peitsaro N, Kaslin J, Michelsen KA, Anichtchik O, Kukko-Lukjanov T, Lintunen M. The histaminergic system in the brain: structural characteristics and changes in hibernation. J Chem Neuroanat. 2000;18:65–74. doi: 10.1016/s0891-0618(99)00052-6. [DOI] [PubMed] [Google Scholar]
- Ruby NF. Hibernation: when good clocks go cold. J Biol Rhythms. 2003;18:275–286. doi: 10.1177/0748730403254971. [DOI] [PubMed] [Google Scholar]
- Ruby NF, Dark J, Heller HC, Zucker I. Ablation of suprachiasmatic nucleus alters timing of hibernation in ground squirrels. Proc Natl Acad Sci U S A. 1996;93:9864–9868. doi: 10.1073/pnas.93.18.9864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruby NF, Dark J, Burns DE, Heller HC, Zucker I. The suprachiasmatic nucleus is essential for circadian body temperature rhythms in hibernating ground squirrels. J Neurosci. 2002;22:357–364. doi: 10.1523/JNEUROSCI.22-01-00357.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richardson JA, Kozlowski GP, Wilson S, Arch JR, Buckingham RE, Haynes AC, Carr SA, Annan RS, McNulty DE, Liu WS, Terrett JA, Elshourbagy NA, Bergsma DJ, Yanagisawa M. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–585. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- Sallmen T, Beckman AL, Stanton TL, Eriksson KS, Tarhanen J, Tuomisto L, Panula P. Major changes in the brain histamine system of the ground squirrel Citellus lateralis during hibernation. J Neurosci. 1999;19:1824–1835. doi: 10.1523/JNEUROSCI.19-05-01824.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallmen T, Lozada AF, Beckman AL, Panula P. Intrahippocampal histamine delays arousal from hibernation. Brain Res. 2003a;966:317–320. doi: 10.1016/s0006-8993(02)04235-x. [DOI] [PubMed] [Google Scholar]
- Sallmen T, Lozada AF, Anichtchik OV, Beckman AL, Leurs R, Panula P. Changes in hippocampal histamine receptors across the hibernation cycle in ground squirrels. Hippocampus. 2003b;13:745–754. doi: 10.1002/hipo.10120. [DOI] [PubMed] [Google Scholar]
- Satinoff E. Disruption of hibernation caused by hypothalamic lesions. Science. 1967;155:1031–1033. doi: 10.1126/science.155.3765.1031. [DOI] [PubMed] [Google Scholar]
- Sharp FR, Sagar SM, Hicks K, Lowenstein D, Hisanaga K. c-fos mRNA, Fos, and Fos-related antigen induction by hypertonic saline and stress. J Neurosci. 1991;11:2321–2331. doi: 10.1523/JNEUROSCI.11-08-02321.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steininger TL, Gong H, McGinty D, Szymusiak R. Subregional organization of preoptic/anterior hypothalmic projections to arousal-related monoaminoergic cell groups. J Comp Neurol. 2001;429:638–653. [PubMed] [Google Scholar]
- Steriade M, Contreras D. Relations between cortical and thalamic cellular events during transition from sleep patterns to paroxysmal activity. J Neurosci. 1995;15:623–642. doi: 10.1523/JNEUROSCI.15-01-00623.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M, Sakai K, Jouvet M. Bulbo-thalamic neurons related to thalamocortical activation processes during paradoxical sleep. Exp Brain Res. 1984;54:463–475. doi: 10.1007/BF00235472. [DOI] [PubMed] [Google Scholar]
- Storey KB. Mammalian hibernation. Transcriptional and translational controls. Adv Exp Med Biol. 2003;543:21–38. [PubMed] [Google Scholar]
- Sutin EL, Kilduff TS. Circadian and light-induced expression of immediate early gene mRNAs in the rat suprachiasmatic nucleus. Brain Res Mol Brain Res. 1992;15:281–290. doi: 10.1016/0169-328x(92)90119-v. [DOI] [PubMed] [Google Scholar]
- Yu EZ, Hallenbeck JM, Cai D, McCarron RM. Elevated arylalkylamine-N-acetyltransferase (AA-NAT) gene expression in medial habenular and suprachiasmatic nuclei of hibernating ground squirrels. Brain Res Mol Brain Res. 2002;102:9–17. doi: 10.1016/s0169-328x(02)00138-9. [DOI] [PubMed] [Google Scholar]