Abstract
Background
In murine embryonic stem cells (ESCs), the onset of vascular endothelial growth factor receptor 2 (VEGFR-2) expression identifies endothelial precursors. Undifferentiated human ESCs express VEGFR2, and VEGFR2 expression persists upon differentiation. The objective of our study was to identify a single population of endothelial precursors with common identifying features from both human and murine ESCs.
Methods and Results
We report expression of the VEGF co-receptor NRP-1 coincides with expression of Brachyury and VEGFR-2, and identifies endothelial precursors in murine and human ESCs prior to CD31 or CD34 expression. When sorted and differentiated, VEGFR-2+NRP-1+ cells form endothelial-like colonies that express CD31, CD34 seven fold more efficiently than NRP-1− cells. Finally, antagonism of both the VEGF and Semaphorin-binding functions of NRP-1 impairs the differentiation of vascular precursors to endothelial cells.
Conclusions
The onset of NRP-1 expression identifies endothelial precursors in murine and human stem cells. The findings define the origin of a single population of endothelial precursors from human and murine stem cells to endothelial cells. Additionally, the function of both the VEGF and Semaphorin binding activities of NRP1 have important roles in the differentiation of stem cells to endothelial cells, providing novel insights into the role of NRP1 in a model of vasculogenesis.
Keywords: Vasculogenesis, endothelium, embryonic stem cells, vascular biology
Introduction
In development and in adults, blood vessel formation occurs via two mechanisms: vasculogenesis (de novo blood vessel formation), or angiogenesis (proliferation and extension of existing vascular tissues). While there are many experimental models to study the impact of therapies on angiogenesis, very few model systems are available to study vasculogenesis. Despite similarities in vasculogenesis between species (1), often pre-clinical testing of new therapies relies on animal studies, and may fail when translated to humans (2). A human model of vasculogenesis is required to identify the mechanisms controlling differentiation of human stem cells to endothelial cells, and to understand the mechanisms that control vessel formation.
In murine ESCs, the onset of expression of VEGFR-2 identifies endothelial precursors that form functional vessels in vivo (3), and VEGFR-2 expression coincides with the mesoderm defining transcription factor Brachyury (Bry) (4). However, undifferentiated human ESCs express VEGFR-2, and its expression persists after differentiation (5), indicating additional insights are required to identify human endothelial precursors. Several authors have demonstrated isolation of functional endothelial cells from human ESCs via differentiation on stroma or as embroid bodies in serum containing medium using endothelial cell markers including CD31 and CD34 (6). The origin and pathway for derivation of endothelial cells from human ESCs remains unknown.
The finding that VEGFR-2 identifies vascular precursors in murine ESCs suggests an important role for VEGF in vasculogenesis. VEGFR-2 null mice die in utero after 8.5–9.5d with an absence of organized yolk sac vessels (7). Neuropilins (NRP-1 and 2) are multifunctional proteins that bind Semphorins as well as function as co-receptors for VEGF (8). In adults both Neuropilins 1 and 2 are expressed in multiple tissue types including endothelial and vascular smooth muscle cells, as well as lymphocytes (8), however the role of NRP1 in vasculogenesis is unknown. Knockout of both NRP-1 and 2 has a similar phenotype as the VEGFR-2 knockout, resulting in embryonic lethality at E8.5 with an avascular yolk sac (9). The NRP-1/2 double knockout mouse phenotype suggests the onset of Npl 1 expression occurs very early in development, during mesoderm formation, and suggests that Neuropilins have an essential function in vasculogenesis.
We hypothesized that the VEGF co-receptor NRP-1 would display similar timing of expression in differentiating human and murine ESCs, and facilitate identification of endothelial precursor cells. In murine ESCs we find that NRP-1 is co-expressed with Bry and VEGFR-2, and VEGFR-2+ versus NRP-1+ cells form endothelium with equivalent efficiency. In human ESCs, the onset of NRP-1 expression identifies endothelial precursors prior to expression of the endothelial markers CD31 and CD34. Finally, antagonism of both the VEGF binding and the Semaphorin binding functions of NRP1 impairs the differentiation of vascular precursors to endothelial cells in vitro, indicating a functional requirement for NRP1 in vasculogenesis. Our findings highlight the utility of the stem cell model to understand the mechanisms controlling vasculogenesis in mice and in humans.
Methods and Materials
Murine Embryonic Stem Cell Lines
Murine embryonic stem cell lines were maintained on embryonic fibroblast feeders using standard methods. Murine ESC lines were adapted to serum free, feeder free conditions as described (10). Brachyury GFP murine ESCs were provided as a gift from Gordon Keller (Ontario Cancer Institute, Toronto ON Canada). Rosa 26 murine ESCs were a gift from Phillipe Soriano (Fred Hutchinson Cancer Institute, Seattle WA).
Human Embryonic Stem Cell Lines
Human ESCs were cultured under conditions defined previously (11). See Supplemental Methods and materials for additional detail.
Differentiation of Human and Murine Embryonic Stem Cell Lines As Embryoid Bodies
Both human and murine embryonic stem cells were differentiated as embryoid bodies using methods previously described (12 and 13). See supplemental methods for additional detail.
Differentiation of Sorted Cells to Endothelium
Murine and human ESCs were differentiated as embryoid bodies, and stained for flow cytometry. Cells were sorted based on expression of VEGFR-2 or NRP-1 (murine ESCs) or VEGFR-2+NRP-1+/− cells (human ESCs). In some cases human ESC derived cells were sorted as NRP-1+CD34− cells. Murine ESCs were plated to 1% gelatin coated dishes in Alpha MEM medium with 10% FCS and 10ng/mL VEGF as described (2). Human ESC derived cells were plated to 1% gelatin coated culture dishes at a density of 1 × 105 cells/cm2 in EBM2 medium (Lonza) supplemented with 50ng/mL human VEGF and cultured for 5–10 days.
Matrigel In Vitro Angiogenesis Assays
Undiluted Matrigel (BD Bioscience) was added to each well of a 24 well tissue culture dish and allowed to solidify at 37°C for one hour. Seventy-five thousand cells were plated onto Matrigel coated culture dishes, and grown in EGM2 with 50 ng/mL VEGF for 24 hours to 7 days. Cultures were examined for cord formation using a phase contrast microscope.
Western Blot Analysis
Cells were harvested in SDS-PAGE sample buffer in the absence of reducing agents and total protein content was equalized between samples. Samples were separated on 4–12% SDS-PAGE gels under non-reducing conditions, and transferred to PVDF membranes. Blots were probed with primary antibodies (see supplemental methods and materials), followed by species appropriate horseradish peroxidase conjugated secondary antibodies (WestPico kit, Pierce Biotechnology). Protein bands were visualized by exposure to X-ray film.
PCR Analysis
Total RNA was extracted using a commercial kit (RNeasy mini kit-Qiagen). Total mRNA was amplified using Superscript reverse transcriptase (Gibco) and oligo dT primers. RT-PCR was performed using Taq DNA polymerase (Gibco) using primers indicated in the Supplemental Methods and Materials. RT-PCR products were analyzed on Ethidium-bromide stained agarose gels.
Inhibition of NRP1 With Function Blocking Antibodies
NRP1A, NRP1B and Mouse IgG isotype control antisera were generously provided by Genentech (14). Murine ESCs were differentiated as embryoid bodies, and Bry+VEGFR2+ cells were sorted and differentiated to endothelial cells as described above. Sorted cells were incubated in VEGF (10ng/mL) with no additional treatment, mouse IgG (10ng/mL), NRP1A antibody (10ng/mL) or NRP1B antibody (10ng/mL) for seven days. The quantity of VE-Cadherin and CD31+ cells as a percentage of total cells were enumerated using immunoflurescence microscopy.
Statistics
Data are expressed as means ± SEM unless specified otherwise. Statistical comparisons between two groups were performed usinga t test. Comparisons between more than two groups at one time point were performed using a one-way ANOVA with a Holm-Sidak post-hoc test. Comparisons between two groups over a time course were compared using a two-way ANOVA with a Holm-Sidak post-hoc test.
Statement of Responsibility
The authors had full access to and take full responsibility for the integrity of the data. All authors have read and agree to the manuscript as written.
Results
VEGFR-2 and NRP-1 Are Expressed in Bry+ Differentiating Murine Embryonic Cells Prior to CD34 Expression
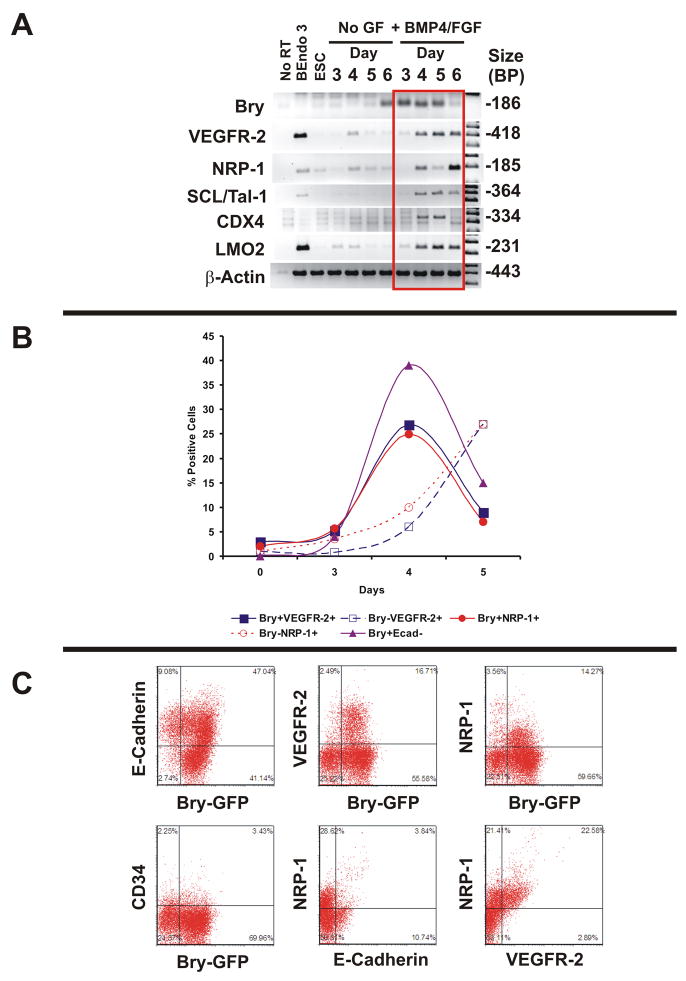
Human ESCs are different from murine ESCs in both their marker expression and growth factor requirements. Undifferentiated human ESCs express the endothelial and progenitor cell surface proteins VEGFR-2, CD133 and CD146, but not NRP-1 (Supplemental Figure 1). To test the hypothesis that the onset of NRP-1 expression identified VEGFR-2+ embryonic vascular precursors, murine embryonic stem cells expressing GFP under control of the Bry locus (4) were differentiated as embryoid bodies (EBs) under serum-free conditions. To determine if the differentiation conditions used were adequate to stimulate differentiation to mesoderm, we surveyed differentiating Bry-GFP mESCs for the expression transcripts encoding cell surface molecules and transcription factors that are required for vasculogenesis by PCR. This included the transcription factors SCL/Tal-1 (15), CDX4 (16) and LMO2 (17) because of their roles in formation of endothelial cells. Bry mRNA was expressed at EB day 3 when treated with BMP4 and bFGF, and decreased by day 6 (Figure 1A). In the absence of GF, the onset of Bry expression occurs on EB day 6. VEGFR-2, NRP-1, Tie2, SCL/Tal-1, CDX4, LMO2 all were expressed at low levels in untreated EBs, and were robustly increased when differentiated in the presence of BMP4 and bFGF. Time course experiments revealed that Bry-GFP+ cells emerged on EB day 3, and peaked on day 4. Bry+VEGFR-2+ and NRP-1+ cells represented a subpopulation of Bry+ E-Cadherin− cells, consistent with observations of VEGFR-2+ cells derived from murine ESCs in the literature (3). We found that NRP-1 was expressed in Bry+ cells from Day 4 EBs, and correlated quantitatively with Bry+ VEGFR-2+ cells (Figure 1B). However, other markers of endothelial cells were not present in the Bry+ cell population, including CD34 (Figure 1C) and VE-Cadherin (not shown). In separate experiments with Rosa 26 mESCs, we observed cells that were double positive for VEGFR-2 and NRP-1, and NRP-1+ were E-Cadherin− in day 4 EBs (Figure 1C), but did not express CD34 or VE-Cadherin (not shown). Collectively, the findings indicate that NRP-1 is co-expressed in Bry+, VEGFR-2+, E-Cadherin−cells.
Figure 1. NRP-1 Expression Coincides With Bry and VEGFR-2 in Differentiating Murine ESCs.
Panel A: RT-PCR Time course analysis of embryoid bodies from Bry GFP murine ESCs in the absence and presence of BMP4 and bFGF. Results are representative of 3 experiments. The murine endothelialioma cell line bEnd.3: positive control for VEGFR-2, NRP-1, SCL/Tal-1, and LMO 2.
Panel B: Time course analysis of murine ESC differentiation as embryoid bodies in serum free conditions. Percent positive cells determined by flow cytometry analysis
Panel C: Representative flow cytometry plots of Bry GFP murine ESCs stained with antibodies to E-Cadherin, VEGFR-2, NRP-1, and CD34. Double staining experiments with NRP-1/E-cadherin and NRP-1/VEGFR-2 were performed in day 4 embryoid bodies in Rosa 26 mESCs. Quadrants set with IgG control. Data are representative of 5 experiments.
Bry+ VEGFR-2+ and Bry+ NRP-1+ Cells Derived from Murine Embryonic Stem Cells Differentiate to Endothelial-like Cells with Equal Efficiency
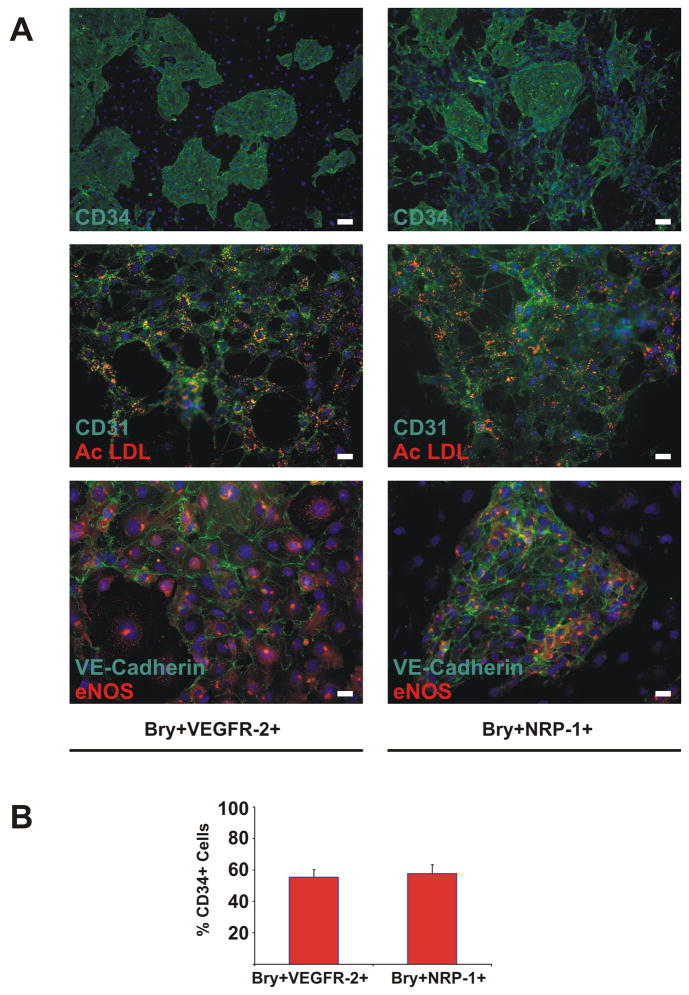
We found that VEGFR-2 and NRP-1 were induced by identical growth conditions (Supplemental Figure 2), and that VEGFR-2 and NRP-1 were co-expressed in differentiating EBs To determine whether NRP-1 expression identifies endothelial precursors derived from murine ESCs, we performed flow cytometry based sorting of Bry+VEGFR-2+ and Bry+NRP-1+ cells from day 4 murine ESCs EBs. Sorted cells were then grown under conditions that support differentiation to endothelial cells. Both Bry+VEGFR-2+ and Bry+NRP-1+ cells differentiated to endothelial cells. The differentiated cells grew as colonies and expressed CD31, CD34, VE-Cadherin, endothelial nitric oxide synthase (eNOS), and absorbed acetylated LDL (Figure 2). To determine if Bry+VEGFR-2+ and Bry+NRP-1+ cells formed endothelium with equivalent efficiency, the quantity of CD34+ cells was determined after differentiation. In Bry+VEGFR-2+ cells, 55.5±4.6% of total cells cultured were CD34+ versus 57.7±5.7% of total Bry+NRP-1+ cells (Figure 2) Collectively, these findings indicate that NRP-1+ cells differentiate to endothelial-like cells with equivalent efficiency as VEGFR-2+ cells.
Figure 2. Bry+VEGFR-2+ and Bry+NRP-1+ Murine ESC derived cells differentiate to endothelium in vitro.
Panel A: Differentiation of Bry+VEGFR-2+ and Bry+NRP-1+ murine ESC derived cells to endothelium. Specific antibodies indicated on each photo. Nuclei were counterstained with bis-benzamide in all photos. CD34 images shown at 4× magnification (scale bar=100 microns), CD31/AcLDL and VE-Cadherin/eNOS shown at 20× magnification (scale bar=20 microns). Panel B: Quantification of CD34+ cells derived from Bry+VEGFR-2+ and Bry+NRP-1+ cells. Means ± SEM of three independent experiments are shown.
NRP-1 is Co-Expressed with VEGFR-2 in Differentiating Human ESCs Grown In Serum Free Differentiation Conditions
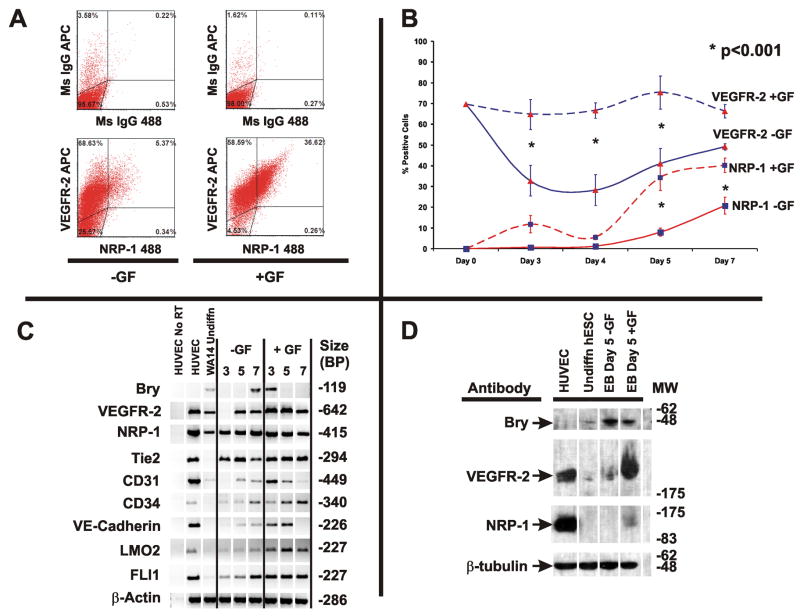
Undifferentiated human ESCs express VEGFR-2 protein, but not NRP-1 protein (Supplemental Figure 1). We hypothesized that NRP-1 would identify endothelial precursors derived from human ESCs prior to expression of other endothelial cell markers (CD31 and CD34). Additionally, we hypothesized that endothelial precursors could be obtained from human ESC EB cultures differentiated under serum free conditions. We performed experiments to determine if VEGFR-2 expression persisted upon differentiation in mesoderm forming conditions, and to determine the time dependence of NRP-1 cell surface protein expression in human ESCs. EBs were cultured in suspension in the absence (−GF) or presence of BMP4 (10ng/mL), bFGF (10ng/mL) and VEGF165 (10ng/mL) (+GF). The quantity of VEGFR-2+ and NRP-1+ cells was determined on days 3, 4, 5 and 7 after differentiation by flow cytometry analysis. Representative flow cytometry plots of human ESC EBs at day 5 −/+ GF are shown (Figure 3A). The number of VEGFR-2+ cells decreases to 32.8±7.3% of total cells grown -GF at day 3 (Figure 3B). NRP-1+ cells were not detectable -GF until day 5 (8±2.0% of total cells), and increased further on day 7 (20.7±4.1% of total cells). In the presence of GFs, VEGFR-2 expression persisted in 74% of total cells throughout the time course. Npl1 expression appeared to be biphasic, with an initial peak of expression at day 3. However, the quantity of NRP-1+ cells obtained from day 3 EBs was not significantly different from untreated cells (11.8±4.2% +GF vs. 0.63±0.17% No GF, p=0.21). A second peak of NRP-1 expression is found in day 5 EBs in the presence of growth factors (34.2±6.2% of total cells), and NRP-1 expression persists to day 7.
Figure 3. Time Dependence of VEGFR-2 and NRP-1 Expression in Human ESCs Differentiated in Serum Free Conditions.
Panel A: Representative flow cytometry analysis of day 5 human ESC EBs differentiated in the absence or presence of GF (BMP4 (10ng/mL), VEGF (10ng/mL), and bFGF (10ng/mL)). Quadrants are set to mouse IgG controls.
Panel B: Time course analysis of VEGFR-2 and NRP-1 cell surface protein expression measured by flow cytometry analysis. Human ESCs (NIH Code UC06) were differentiated as embryoid bodies in the presence or absence of GF as described in the Methods and Materials. Quantity of VEGFR-2+ and NRP-1+ cells was determined by flow cytometry and presented as % positive cells. Significant differences between untreated and GF treated cells were assessed by a two-way ANOVA with Holm-Sidak post hock test, and indicated with * for each time point. Means ± SEM of four independent experiments are shown.
Panel C: PCR time course analysis of mesoderm and vascular markers in differentiating human ESCs in the absence or presence of GF. HUVEC used as a positive control for vascular markers. Results are representative of >3 experiments.
Panel D: Western blot analysis of VEGFR-2 and NRP-1 expression in day 5 embryoid bodies derived from human ESCs. Results are representative of four experiments.
To determine if the growth factor conditions used in the EBs resulted in induction of mesoderm and endothelial cell markers, we performed RT-PCR analysis (Figure 3C). Undifferentiated human ESCs express low levels of Bry transcripts, and Bry expression peaks in day 7 EBs grown in the absence of GFs. Bry transcript expression was augmented in the presence of GFs, resulting in earlier expression in day 3 and 5 EBs. Transcripts, but not protein (Supplemental Figure 1B and D), encoding NRP-1 are found in undifferentiated human ESCs, and showed rapid induction in the presence of growth factors. We detected expression of CD31, LMO2 and FLI1 transcripts at low levels in undifferentiated human ESCs. Upon differentiation, human ESCs treated with GFs expressed CD31, CD34 and VE-Cadherin earlier than in the absence of GFs.
To verify that Bry, VEGFR-2, and NRP-1 protein expression was induced in EBs grown in the absence or presence of GFs, western blot analysis was performed on day 5 EBs (Figure 3D). Bry protein was expressed at low levels in undifferentiated human ESCs, and Bry protein levels increased upon differentiation, consistent with our PCR results. VEGFR-2 protein expression was observed at low levels in undifferentiated human ESCs, and EBs without GFs, but augmented in day 5 EBs grown in the presence of GFs. NRP-1 protein expression was detected only in human ESC derived EBs in the presence of GFs,. Importantly, the protein bands detected with Bry, VEGFR-2 and NRP-1 antibodies migrated at the appropriate molecular weight for each protein (Bry: 49 kDa, VEGFR-2: 200 kDa, NRP-1: 130 kDa).
Collectively, these findings indicate that VEGFR-2 expression persists in the majority of human ESC derived EBs grown in serum-free mesoderm inducing conditions. Additionally, the VEGF co-receptor NRP-1 protein displays a distinct time course of expression, peaking in day 5 EBs grown under mesoderm inducing conditions. In the presence of GFs, expression of Bry transcript and protein, transcripts encoding the endothelial markers CD31, CD34, VE-Cadherin, and the transcription factors LMO2 and FLI1 are induced earlier than in cells grown in the absence of GFs.
The Onset of NRP-1 Protein Expression in Differentiating Human ESCs Occurs Prior to CD31 and CD34 Expression in Multiple Human ESC Lines
Our time course analyses indicated that VEGFR-2 expression was persistent in human ESC derived EBs grown in the presence of GFs. Our PCR analysis indicated that induction of the endothelial transcripts CD31, CD34 and VE-Cadherin occurred prior to or during the peak of NRP-1 cell surface protein expression. We hypothesize that NRP-1 identifies endothelial precursors in human ESCs. Therefore, we wished to determine if NRP-1+ cells expressed the endothelial cell proteins CD31 and CD34 in day 5 embryoid bodies. Additionally, we wanted to test whether the temporal pattern of NRP-1 expression could be observed in multiple different human ESC lines.
We differentiated human ESCs under serum free conditions in the absence or presence of GFs. We performed flow cytometry analysis on live cells derived from day 5 human ESC EBs to determine the quantity of VEGFR-2, NRP-1, CD31 and CD34+ cells in seven different human ESC lines, and the data were pooled (Figure 4). VEGFR-2+ cells are present in 57.6±10.5% of human ESC derived EBs grown in the presence of GFs, while 19.8±7.3% of cells express VEGFR-2 in the absence of GFs. NRP-1 was expressed in 27.2±7.6% of human ESCs +GFs versus 6.1±1.9% −GFs. Both CD31 (7.5±1.6% with GF vs. 2.8±0.8% without GF) and CD34+ (5.9±1% with GF vs. 3±0.9% without GF) cells were increased by differentiation with GFs, however the overall quantity of these markers were significantly less than that number of NRP-1+ cells obtained. These findings indicate that NRP-1 is expressed prior to the onset of CD31 and CD34 expression in human ESCs, and is consistent with the onset of NRP-1 expression as an identifying feature of endothelial precursors from human ESCs. Additionally, the findings indicate that NRP-1 expression is reproducible in seven different human ESC lines, indicating the findings apply to human ESCs from multiple providers.
Figure 4. VEGFR-2+NRP-1+ Cells Are CD31− and CD34−.
Panel A: Representative flow cytometry plots of human ESC derived embryoid bodies grown in the absence or presence of GF for 5 days. Mouse IgG controls shown in the left-most panels. Quadrants set by mouse IgG control for each secondary combination.
Panel B: Quantitative analysis of VEGFR-2, NRP-1, CD31+ and CD34+ cells in day 5 human ESC EBs. Means ± SEM of seven different human ESC lines (NIH Codes: WA07, WA13, WA14, UC01, UC06, BG02, TE06) are shown.
VEGFR-2+NRP-1+ Cells Derived from Human ESCs are Endothelial Precursors In Vitro
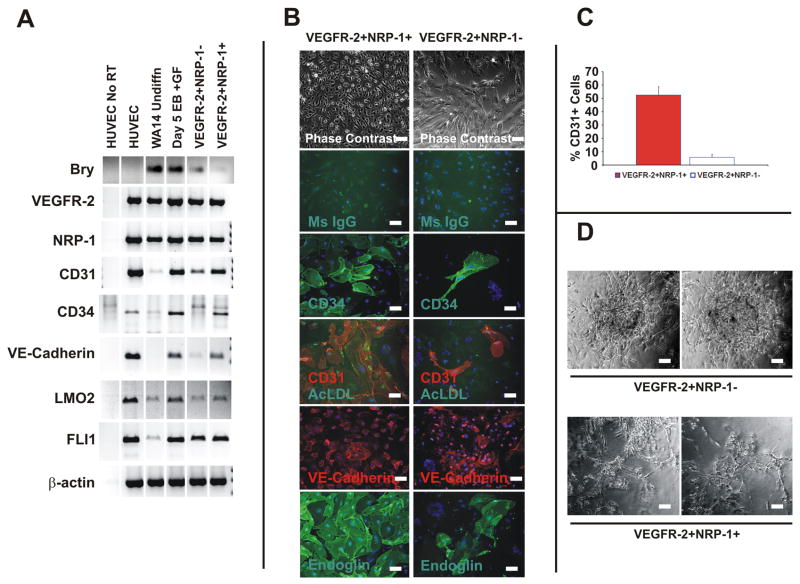
We hypothesized that onset of expression of NRP-1 identifies a population of endothelial precursors derived from human ESCs. To test this hypothesis, we differentiated human ESCs as EBs under serum free conditions in the presence of GFs. We sorted VEGFR-2+NRP-1+ and VEGFR-2+NRP-1 − cells from day 5 EBs by flow cytometry methods. PCR analysis of the sorted cell populations revealed that VEGFR-2+NRP-1+ cells were enriched for transcripts encoding VE-Cadherin, CD31, CD34, LMO2 and FLI1 versus VEGFR-2+NRP-1 −cells (Figure 5A). VEGFR-2+NRP-1+ and VEGFR-2+NRP-1− cells were differentiated in endothelial growth conditions. Upon differentiation there was a significant difference in the morphology of VEGFR-2+NRP-1+ versus VEGFR-2+NRP-1−cells (Figure 5B). Both VEGFR-2+NRP-1+ and VEGFR-2+NRP-1− cells differentiated to CD31, CD34, VE-Cadherin and Endoglin expressing cells that also absorbed acetylated LDL (Figure 5B). The quantity of CD31+ or CD34+ cells derived from VEGFR-2+NRP-1+ versus VEGFR-2+NRP-1− cells was determined by quantitative microscopy. VEGFR-2+NRP-1+ cells versus VEGFR-2+ NRP-1−cells differentiate to CD31+ cells (59.2±8% vs. 5.2±1.7% of total cells) and CD34+ cells (48.7±6.5 vs. 7±1.2 % total cells) more efficiently than VEGFR-2+NRP-1− cells (Figure 5C). As a model of in vitro angiogenesis, VEGFR-2+NRP-1+ and VEGFR-2+NRP-1− cells were plated to Matrigel and cultured for up to 14 days to determine if either cell type formed vessel like structures. VEGFR-2+NRP-1+ cells formed elongated cord-like structures in Matrigel, while VEGFR-2+NRP-1− cells remained tightly clustered, and did not appear to elongate or form networks with each other (Figure 5D). To exclude the possibility that CD31+ or CD34+ cells were included in our VEGFR-2+NRP-1+ population, we performed flow cytometry based sorting of NRP-1+CD34− cells versus NRP-1 −CD34− cells differentiated with GF. We find that NRP-1+CD34− cells differentiate to endothelial-like cells, while NRP-1-CD34− cells do not (not shown). Collectively the findings indicate the onset of NRP-1 expression identifies endothelial precursors derived from human ESCs.
Figure 5. VEGFR-2+NRP-1+ Human ESC Derived Cells Differentiate to Endothelial-like Cells In Vitro.
Panel A: RT-PCR analysis of sorted VEGFR-2+ NRP-1− and VEGFR-2+ NRP-1+ cells prior to endothelial differentiation. HUVECs used as a positive control.
Panel B: Differentiation of VEGFR-2+NRP-1+ and VEGFR-2+NRP-1− cells in endothelial growth conditions. Phase contrast images shown at 10× magnification (scale bar=40 microns). Fluorescence images shown at 20× (scale bar=20 microns). Results are representative of four independent experiments.
Panel C: Quantification of CD31+ and CD34+ cells derived from VEGFR-2+NRP-1+ and VEGFR-2+NRP-1− cells. Means ± SEM from four independent experiments are shown.
Panel D: Matrigel in vitro angiogenesis assays in VEGFR-2+NRP-1+ and VEGFR-2+NRP-1− cells. Images shown are representative of three independent experiments. Scale bar=20 microns.
The VEGF and Semaphorin Binding Activities of NRP1 are Required for Differentiation of Stem Cells to Endothelial Cells
We hypothesized that the function of NRP1 in the differenation of stem cells to endothelial cells was to facilitate activation of VEGF signaling, promoting endothelial cell differentiation and growth in our model of vasculogenesis. However, NRP1 has multiple functions including roles in binding of Semaphorins in neuronal guidance as well as binding additional growth factors including hepatocyte growth factor. Therefore, an alternative hypothesis would be that NRP1 has additional activities that are unrelated to VEGF signaling. To determine if the non-VEGF function of NRP1 is involved in the differentiation of stem cells to endothelial cells we used two function blocking antibodies previously shown to bind and inhibit the binding of NRP1 ligands VEGF (NRP1B Ab) and Semaphorins (NRP1A). In this experiment we differentiated murine embryonic stem cells, and sorted VEGFR2+ vascular precursors 3.5 days after differentiation as embryoid bodies. VEGFR2+ vascular precursors were then isolated from other cells using flow cytometry based cell sorting, and differentiated to endothelial cells for 7 days in the presence of VEGF-A, and either mouse IgG (10ng/mL), NRP1A Ab (10ng/mL) or NRP1B (10ng/mL). After 7 days, cultures were assessed for the number of VE-Cadherin and CD31+ cells as a percentage of total cells in culture using immunoflurescence microscopy. We find that in the presence of VEGF-A alone or in combination with mouse IgG that 39±2% express VE-Cadherin, consistent with differentiation to endothelial cells. The addition of either NRP1A or NRP1B function blocking antibodies inhibited the differentiation of VE-Cadherin + endothelial cells by 57±8% and 49±7%, respectively, and CD31+ endothelial cells by 62±17% and 54±3% for NRP1A and NRP1B blocking antibodies (Figure 6). The findings are consistent with a role for both the VEGF and Semaphorin binding functions of NRP1 as an essential component of the differentiation of stem cells to endothelial cells.
Figure 6. Effect of NRP1 Function Blocking Antibodies on Differentiation of Stem Cells to Endothelial Cells.
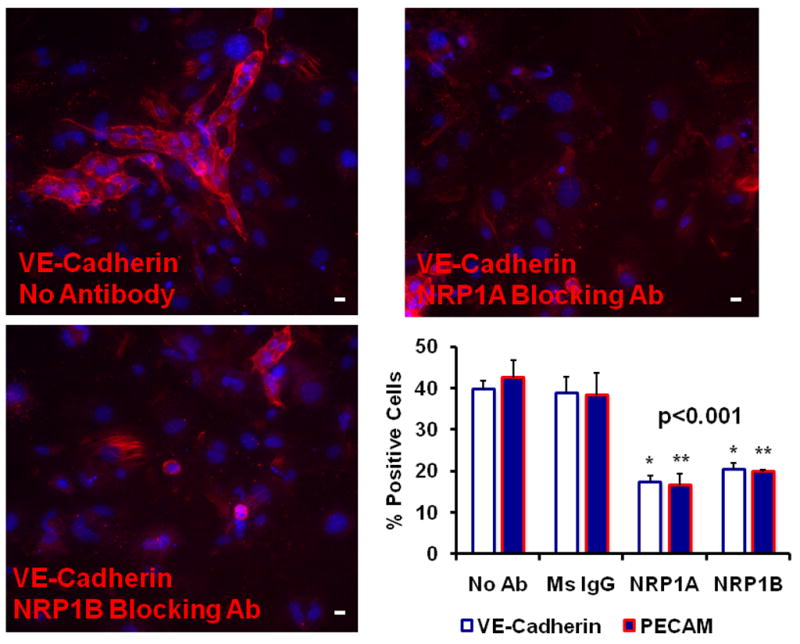
Panel A–C: Photomicrographs of representative photomicrographs of murine stem cells differentiated to endothelial cells for seven days in the presence of mouse IgG (Panel A), NRP1A Ab (Panel B), NRP1B Ab (Panel C). Results are representative of three independent experiments. Scale bar=20 microns.
Panel D: Summary of three independent experiments, means ± SEM are shown. Significant differences in a one-way ANOVA with Holm-Sidak post-hoc test for VE-Cadherin (*) and CD31 (**) at p>0.001.
Discussion
Our work establishes two major findings: 1) NRP1 expression occurs early in the differentiation of both murine and human stem cells to endothelial cells, and 2) Both the Semaphorin and VEGF binding functions of NRP1 are required for the differentiation of stem cells to ECs in the vasculogenesis model system. The findings illustrate the utility of the stem cell model for testing effects on vasculogenesis, and indicate a surprising role for both the Semaphorin and VEGF binding activities of NRP1 in vasculogenesis.
Our results show that NRP1 is expressed early in the differentiation of stem cells, in cells that have begun the process of forming vascular, hematopoietic and cardiac precursors (mesodermal cell types). Prior findings in the literature suggested that NRP1 expression occurs early in development, as the NRP1 and 2 double knockout mouse is lethal after 8.5 days of development due to failure of blood vessel formation (9). Since mesoderm formation occurs during 6–7 days of development, and prior work has shown that VEGFR2 expression begins during this period in a population of cells that form both blood vessels and the earliest hematopoietic cells (4), it seemed plausible that NRP1 expression would begin due to its function as a co-receptor for VEGFR2. Studies in Zebrafish have also shown that NRP1 was expressed at the end of gastrulation and during somitogenesis, stages of development where vasculogenesis occurs, and is a comparable time point to our ES cell model (18). Additionally, our experiments show that NRP1 expression occurs early in both murine and human stem cells, illustrating that this event is conserved across species.
A secondary finding of our results was that the growth factors BMP4 and basic fibroblast growth factor are sufficient for expression of NRP1, while the BMP antagonist Noggin inhibits NRP1 expression. These findings are not surprising in that the same conditions are sufficient to drive expression of VEGFR2 from murine and human stem cells. However, the stem cell model of vasculogenesis may facilitate further investigation of the mechanisms controlling NRP1 expression. This is of clinical relevance as NRP1 expression has been documented in several types of cancer, is associated with worse outcomes with cancer, and the regulation of NRP1 expression in cancer may represent a therapeutic target for cancer therapy (19, 20).
NRP1 function blocking antibodies significantly decreased the number of endothelial cells derived from differentiating stem cells (Figure 6) and antagonism of both the Semaphorin and VEGF binding activities resulted in decreased endothelial cell formation, a novel finding. Prior work in several developmental and tumor angiogenesis models indicated that antagonism of the VEGF binding function of NRP1 primarily blocked vessel formation in these contexts (21). Our results are novel in that they indicate that both the VEGF and Semaphorin binding domains of NRP1 appear to be required for differentiation of endothelial cells from stem cells. Our findings are somewhat limited by the fact that the NRP1 function blocking antibodies did not completely inhibit differentiation of endothelial cells. The reason for this is not known, however it is possible that NRP2 expression is also present in a portion of the cells and that NRP2 may have an overlapping function with NRP1 in the differentiation of stem cells to endothelial cells. The NRP1 function blocking antibodies used in these experiments have no effect on NRP2 function (21)
Prior results have indicated that antagonism of the VEGF binding activity of NRP1 only modestly impaired VEGFR2 activation. Work by Pan and coworkers indicate that the Semaphorin and VEGF domain binding antibodies disrupt formation of the VEGF/NRP1/VEGFR2 signaling complex (21). Therefore, the effect of both VEGF and Semaphorin binding function blocking NRP1 antibodies are likely acting to inhibit signaling events that are coupled to NRP1 and VEGFR2 complex formation, or events downstream of this complex, but not simply by impairing VEGFR2 activity alone. These findings suggest an important function of the VEGFR2/NRP1 molecular complex in the initial differentiation of stem cells to endothelial cells, and is a novel mechanism of this pathway, as known components of VEGF signaling downstream of VEGFR2 (ERK, p38 and AKT) are unaffected by either of the blocking antibodies in proliferating endothelial cell cultures. One likely candidate for the downstream effects of the VEGF/VEGFR2/NRP1 signaling complex is Neuropilin Interacting Protein (NIP), also known as GIPC, a PDZ domain containing protein that interacts directly with NRP1 (22). However, the interaction of GIPC with the VEGF/VEGFR2/NRP1 complex have not been extensively explored to identify a precise mechanism, and the role of this signaling complex in vasculogenesis remains open to further investigation. Greater understanding of the VEGF/VEGFR2/NRP1 signaling complex and events outside of canonical VEGF signaling represents the next advance in our understanding of VEGF signaling and further development of therapeutics in this field. Additionally, extension of our finding that NRP1 expression and function as a critical event in de novo endothelial cell formation to adult stem cell models including tissue specific cancer stem cells and cardiac stem cells may represent a therapeutic target in ischemic and malignant vasculogenesis.
In conclusion, our results indicate that NRP1 is expressed early on in the differentiation of stem cells to endothelial cells, and that NRP1 function is required for the differentiation of ECs from stem cells. Surprisingly, both the Semaphorin and VEGF binding functions of NRP1 are required for EC formation suggesting that events downstream of VEGF/VEGFR2/NRP1 complex formation and not canonical VEGF signaling alone are required for this process.
Supplementary Material
Acknowledgments
We thank the staff of the NIH Stem Cell Facility for assistance in human ESC experiments.
Funding Sources: This research was supportedby the Intramural Research Program of the National Human GenomeResearch Institute, National Heart Lung and Blood Institute, and National Institute of Neurologic Disorders and Stroke, National Institutes of Health.
Footnotes
Disclosures: None
References
- 1.Drake CJ. Embryonic and adult vasculogenesis. Birth Defects Res C Embryo Today. 2003;69:73–82. doi: 10.1002/bdrc.10003. [DOI] [PubMed] [Google Scholar]
- 2.Jakobsson L, Kreuger J, Claesson-Welsh L. Building blood vessels-stem cell models in vascular biology. J Cell Biol. 2007;117:751–5. doi: 10.1083/jcb.200701146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yamashita J, Itoh H, Hirashima M, Ogawa M, Nishikawa S, Yuruqi T, Naito M, Nakao K, Nishikawa S. Flk1-positive cells derived from embryonic stem cells serve as vascular progenitors. Nature. 2000;408:92–6. doi: 10.1038/35040568. [DOI] [PubMed] [Google Scholar]
- 4.Fehling HJ, Lacaud G, Kubo A, Kennedy M, Robertson S, Keller G, Kouskoff V. Tracking mesoderm induction and its specification to the hemangioblast during embryonic stem cell differentiation. Development. 2003;130:4217–27. doi: 10.1242/dev.00589. [DOI] [PubMed] [Google Scholar]
- 5.Sone M, Itoh I, Yamahara K, Yamahara K, Yamashita JK, Yurugi-Kobayashi T, Nonoguchi A, Suzuki Y, Chao T-H, Sawada N, Fukunaga Y, Miyashita K, Park K, Oyamada N, Sawada N, Taura D, Tamura N, Kondo Y, Nito S, Suemori H, Nakatsuji N, Nishikawa S-I, Nakao K. Pathway for differentiation of human embryonic stem cells to vascular cell components and their potential for vascular regeneration. Atheroscler Thromb Vasc Biol. 2007;27:2127–34. doi: 10.1161/ATVBAHA.107.143149. [DOI] [PubMed] [Google Scholar]
- 6.Levenberg S, Zoldan J, Basevitch Y, Langer R. Endothelial potential of human embryonic stem cells. Blood. 2007;110:806–14. doi: 10.1182/blood-2006-08-019190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shalaby F, Rossant J, Yamaguchi TP, Gertsenstein M, Wu XF, Breitman ML, Schuh AC. Failure of blood-island formation and vasculogenesis in Flk-1 deficient mice. Nature. 1995;376:62–6. doi: 10.1038/376062a0. [DOI] [PubMed] [Google Scholar]
- 8.Staton CA, Kumar I, Reed MWR, Brown NJ. Neuropilins in physiological and pathological angiogenesis. J Pathol. 2007;212:237–48. doi: 10.1002/path.2182. [DOI] [PubMed] [Google Scholar]
- 9.Takashima S, Kitakaze M, Asakura M, Asanuma H, Sanada S, Tashiro F, Niwa H, Miyazaki JJ, Hirota S, Kitamura Y, Kitsukawa T, Fujisawa H, Klagsbrun M, Hori M. Targeting of both mouse neuropilin-1 and neuropilin-2 genes severely impairs developmental yolk sac and embryonic angiogenesis. Proc Natl Acad Sci. 2002;99:3657–62. doi: 10.1073/pnas.022017899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ying QL, Nichols J, Chambers I, Smith A. BMP induction of Id proteins suppresses differentiation and sustains embryonic stem cell self-renewal in collaboration with STAT3. Cell. 2003;115:281–92. doi: 10.1016/s0092-8674(03)00847-x. [DOI] [PubMed] [Google Scholar]
- 11.Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–7. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 12.Kattman SJ, Huber TL, Keller GM. Multipotent flk-1+ cardiovascular progenitor cells give rise to the cardiomyocyte, endothelial, and vascular smooth muscle lineages. Dev Cell. 2006;11(5):723–32. doi: 10.1016/j.devcel.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 13.Kennedy M, D’Souza SL, Lynch-Kattman M, Schwantz S, Keller G. Development of the hemangioblast defines the onset of hematopoiesis in human ES cell differentiation cultures. Blood. 2007;109:2679–87. doi: 10.1182/blood-2006-09-047704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liang WC, Dennis MS, Stawicki S, Chanterhy Y, Pan Q, Chen Y, Eigenbrot C, Yin J, Koch AW, Wu X, Ferrara N, Bargi A, Tessier-Lavigne M, Watts RJ, Wu Y. Function blocking antibodies to neuropilin-1 generated from a designed human synthetic antibody phage library. J Mol Biol. 2007:815–29. doi: 10.1016/j.jmb.2006.11.021. [DOI] [PubMed] [Google Scholar]
- 15.Park C, Afrikanova I, Chung YS, Zhang WJ, Arentson E, Fong G, Rosendahl A, Choi K. A hierarchical order of factors in the generation of FLK1- and SCL-expressing hematopoietic and endothelial progenitors from embryonic stem cells. Development. 2004;131:2749–56. doi: 10.1242/dev.01130. [DOI] [PubMed] [Google Scholar]
- 16.Davidson AJ, Ernst P, Wang Y, Dekens MP, Kingsley PD, Palis J, Korsmeyer SJ, Daley GQ, Zon LI. Cdx4 mutants fail to specify blood progenitors and can be rescued by multiple hox genes. Nature. 2003;425:300–6. doi: 10.1038/nature01973. [DOI] [PubMed] [Google Scholar]
- 17.Gering M, Yamada Y, Rabbitts TH, Patient RK. Lmo2 and Scl/Tal1 convert non-axial mesoderm into haemangioblasts which differentiate into endothelial cells in the absence of Gata1. Development. 2003;130:6187–99. doi: 10.1242/dev.00875. [DOI] [PubMed] [Google Scholar]
- 18.Lee P, Goishi K, Davidson AJ, Mannix R, Zon L, Klagsbrun M. Neuropilin-1 is required for vascular development and is a mediator of VEGF-dependent angiogenesis in zebrafish. Proc Natl Acad Sci. 2002;99:10470–5. doi: 10.1073/pnas.162366299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miao HQ, Lee P, Lin H, Soker S, Klagsbrun M. Neuropilin-1 expression by tumor cells promotes tumor angiogenesis and progression. FASEB J. 2000;14:2532–9. doi: 10.1096/fj.00-0250com. [DOI] [PubMed] [Google Scholar]
- 20.Roskoski R. Vascular endothelial growth factor signaling in tumor progression. Crit Rev Oncol Hematol. 2007;62:179–213. doi: 10.1016/j.critrevonc.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 21.Pan Q, Chanthery Y, Lian W-C, Stawicki S, Mak J, Rathore N, Tong RK, Kowalski J, Fong Yee S, Pacheco G, Ross S, Cheng Z, Le Couter J, Plowman G, Peale F, Koch AW, Wu Y, Bagri A, Tessier-Lavigne M, Watts RJ. Blocking Neuropilin-1 Function Has an Additive Effect with Anti-VEGF to Inhibit Tumor Growth. Cancer Cell. 2007;11:53–67. doi: 10.1016/j.ccr.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 22.Chittenden TW, Claes F, Lanahan AA, Autiero M, Palac RT, Tkachenko EV, Elfenbein A, Ruiz de Almodovar C, Dedkov E, Tomanek R, Li W, Westmore M, Singh JP, Horowitz A, Mulligan-Kehoe MJ, Moodie KL, Zhuang ZW, Carmeliet P, Simons M. Selective regulation of arterial branching morphogenesis by synectin. Dev Cell. 2006;10:783–95. doi: 10.1016/j.devcel.2006.03.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.