Abstract
Sandy mice have a deletion mutation in the gene encoding dysbindin-1, Dtnbp1, with consequent reduction of the protein in heterozygotes and its loss in homozygotes. The sandy mouse thus serves as an animal model of dysbindin-1 function. Since this protein is concentrated in synaptic tissue and affects transmitter release, it may affect neuronal processes that mediate behavior. To investigate the neurobehavioral effects of the Dtnbp1 mutation, we studied littermate sandy and wild-type controls on a C57BL/6J genetic background. The three animal groups were indistinguishable in their external physical characteristics, sensorimotor skills, and indices of anxiety-like behaviors. In the open field, however, homozygous animals were hyperactive and appeared to show less habituation to the initially novel environment. In the Morris water maze, homozygous animals displayed clear deficits in spatial learning and memory with marginal deficits in visual association learning. Apart from the last mention deficits, these abnormalities are consistent with hippocampal dysfunction and in some cases with elevated dopaminergic transmission via D2 dopamine receptors. Since similar deficits in spatial learning and memory have been found in schizophrenia, where decreased dysbindin-1 has been found in the hippocampus, the sandy mouse may also model certain aspects of cognition and behavior relevant to schizophrenia.
Keywords: anxiety, DTNBP1, dysbindin, habituation, hippocampus, locomotor activity, motor coordination, learning, memory, schizophrenia, water maze
INTRODUCTION
The sandy or sdy mouse is named for an autosomal recessive coat color mutation leading to sandy-colored fur in homozygous (i.e., sdy/sdy) animals (Swank et al., 1991). The mutation occurred spontaneously in the DBA/2J mouse strain at the Jackson Laboratory in 1983, but its specific locus remained unknown until Li et al. (2003) reported that sdy mice have a 38,129 nucleotide deletion in the dystrobrevin binding protein 1 (Dtnbp1) gene and that transgenic addition of the complete gene largely restores normal DBA/2J coat color and corrects other phenotypic abnormalities. Dtnbp1 encodes the protein dysbindin-1, which is reduced in heterozygous (i.e., sdy/+) mice and is absent in sdy/sdy mice (Li et al., 2003).The sdy mouse thus serves as an animal model of dysbindin-1 functions.
Discovered by Benson et al. (2001), dysbindin-1 is the largest and most ubiquitously expressed member of the dysbindin protein family (Talbot et al., 2008). It is a highly conserved protein expressed by neuronal cell bodies throughout the central nervous system (Talbot et al., 2008). As shown by immunohistochemistry at the electron microscopic level and by western blotting of tissue fractions, dysbindin-1 is also highly concentrated in synaptic tissue, including synaptic vesicles and postsynaptic densities (Talbot et al., 2006). Since this applies to both the cerebral cortex and the hippocampal formation, where loss of dysbindin-1 impairs glutamatergic transmission (Chen et al., 2008; Numakawa et al., 2004), there is reason to believe dysbindin-1 plays a role in neuronal processes mediating animal behavior. Preliminary reports support that view (Askari et al., 2007; Jentsch et al., 2007). These reports, however, are based on studies of sdy mice on the original DBA/2J background. Unlike more robust C57BL/6J (BL6) mice, DBA/2J mice are homozygous for four mutations that can affect behavior: (1) cadherin 23ahl (Cdh23ahl = Cdh753A) associated with an age-related hearing loss (Johnson et al., 2000, 2006, (2–3) glycoprotein (transmembrane) nmbR150X (GpnmbR150X) and tyrosinase-related protein 1isa (Tyrp1isa) both associated with pigmentary glaucoma (Howell et al., 2007), and finally (4) hemolytic complement0 (Hc0) associated with loss of immune complement component 5 (Wetsel et al., 1990) which impairs inflammatory responses to infection (Allegretti et al. (2005), as well as neuronal and astrocytic responses to excitotoxicity (Pasinetti et al., 2006).
In the present study, we provide the first characterization of the sdy behavioral phenotype in animals on a BL6 background for six generations using a battery of neurobehavioral tests. The results revealed that loss of dysbindin-1 is not associated with abnormalities in sensorimotor functions, but is associated with hyperactivity and with deficits in spatial learning and memory ability that are indicative of disrupted hippocampal function (Morris, 2007). Analogous deficits have been found in schizophrenia (Hanlon et al. (2006) with which genetic variation in DTNBP1 has often been associated (Allen et al., 2008; Duan et al., 2007; Williams et al., 2005) and in which reduced synaptic dysbindin-1 has been reported (Talbot et al., 2004). Sdy mice may thus model certain features of schizophrenia.
METHODS & MATERIALS
Animals
Breeding
As noted earlier, the sdy autosomal recessive mutation arose spontaneously in DBA/2J mice at the Jackson Laboratory and were maintained as a closed breeding colony using obligate sdy/+ mice. The Jackson Laboratory later backcrossed sdy/sdy mice on the original DBA background with pure C57BL/6J mice for 5 generations and were then intercrossed to obtain homozygous sdy/BL6 mice. We again mated males of these sixth generation homozygotes from the Jackson Laboratory with female BL6 mice from the same source. Female and male sdy/+ offspring of this mating were then intercrossed to produce all the wild-type, sdy/+, and sdy/sdy mice tested in this study.
Genotyping & PCR
While sdy/sdy mice are readily identified by their sand-colored fur, the coat color of sdy/+ mice is not sufficiently different from that of wild type animals to allow reliable identification. All animals were thus genotyped using a duplex polymerase chain reaction (PCR) procedure designed to yield PCR products across the segment of Dtnbp1 deleted in sdy mice (Li et al., 2003). The primers for the wild-type gene, yielding a PCR product of 472 base pairs, were SE3R (5’-AGCTCCACCTGCTGAACATT-3’) and SE3F (5’-TGAGCCATTAGGAGATAAGAGCA-3’). The primers for the sdy gene, yielding a product of 274 base pairs, were SF (5’-TCCTTGCTTCGTTCTCTGCT-3’) and SR (5’ –CTTGCCAGCCTTCGTATTGT -3’). The 472 base pair product is detected only in wild-type and sdy/+ mice, while the 274 base pair product is detected only in the sdy/+ and sdy/sdy mice. The 472 base pair product is not detected in sdy/sdy mice.
Husbandry
The present study was conducted on 3 genotypes×2 sexes = 6 groups of 10–11 mice each. Mice were housed in same sex cages, 5 per cage and maintained in a temperature/humidity controlled room under circadian light cycle of 12 hrs light/dark with the light cycle beginning at 7:00 am. Mice had access to food and water ad libitum. Mice were weaned at postnatal day 21, and all testing began when mice where 3–4 month of age. Animals were allowed to acclimate to testing rooms for one hour prior to testing. All behavioral tests were performed between 10:30 a.m. - 6:00 p.m. The animals had never experienced any form of behavioral testing prior to the studies described here, and the order of the testing was designed to proceed from the least stressful to the most stressful tests. While behavioral testing was conducted blind to genotype for the wild-type versus heterozygous sdy/+ mice, the pale coat color of homozygous sdy/sdy mice precluded completely blind testing. All procedures related to animals were performed in accordance with University of Pennsylvania Institutional Animal Care and Use Committee and University Laboratory Animal Resources policies and guidelines.
Experimental Behavioral Procedures
Neurological Tests
The neurological screen was adapted from the Irwin screen (1968), which has been widely used for testing neurological toxicity of drug candidates by pharmaceutical companies. This neurological screen is similar to phase 1 of the SHIRPA screen (Rogers et al., 2001). The mouse was weighed, then placed into an empty cage and observed for 3 min. A number of physical characteristics, including poor grooming, bald patches, absence of whiskers, labored breathing and blood around the nostrils, were characterized and recorded. Several behavioral responses were assessed (i.e., jumping, sniffing, rearing, approaching an object, movement throughout the cage, and urination and defecation). Visual acuity was assessed by placing the animal on a visual cliff platform (28 cm from the ground) for 2 min. Behavioral responses were recorded (i.e. approach to edge and poking nose over the edge). Lastly, sensorimotor reflexes (stabilization, righting, eye blink, ear twitch and whisker touch) were evaluated and recorded. The animals’ behaviors during the neurological test were scored qualitatively in real time by the same investigator.
Open Field Exploration
Spontaneous locomotor activity was evaluated using the open field exploration test 24 h after neurological tests. The square opaque white plexiglass apparatus (40×40×32 cm [Everything Plastic, Philadelphia, PA]) was cleaned prior to testing and between animals with 95% ethanol. A video camera was mounted directly above the apparatus. The field was divided virtually into two regions of interest: a center area (15×15 cm) and a peripheral area (Viewpoint VideoTrack version 2.0, Champagne Au Mont D’or, France; see Behavioral Analysis). A lamp with a 60W bulb approximately 1.92 m away and 1.40 m above the apparatus was the sole source of illumination, providing an illumination intensity of 7 lux in the center of the field. The animals were placed in the center of the open field and given two five-minute sessions, 35 min apart. Distance traveled and time spent in the brighter center (which tends to be aversive to mice) vs. peripheral areas during the two 5-min sessions were recorded automatically using the Viewpoint tracking system.
Rotarod
Motor coordination and balance were assessed using an accelerating rotarod (San Diego Instruments, Inc.) 24 h after the open field test. Mice were first habituated to the rotating rod at a constant speed of 4 rpm for 300 s (5 min), during which time latency to fall was not recorded. The habituation trial was only performed on the first day. If a mouse fell off, it was quickly placed back on top of the rod until the 5-min trial was completed. Mice were subsequently exposed to a rotating rod starting at 4 rpm and linearly accelerated to 40 rpm over a 5-min period. Three trials were administered per day with a maximum time of 300 s (5 min) and a 30 min inter-trial rest interval for 5 days consecutively. Performances in the daily trials were averaged for data analysis. Animals were tested at approximately the same time each day.
Elevated Zero Maze
Anxiety-like behavior was assessed using the elevated zero maze 24 h after the rotarod. The maze is a circular platform (62 cm wide) consisting of two opposing open and closed quadrants. A lamp with a 60W bulb approximately 1.92 m away and 1.4 m above the maze was the sole source of illumination, providing an illumination intensity of 7 lux from the center of the maze. The maze was cleaned prior to testing and between animal tests with 95% ethanol. A video camera was mounted directly above the maze. Mice were placed in one of the closed quadrants and activity was recorded automatically for 5 min using the Viewpoint VideoTrack version 2.0 (see Behavioral Analysis).
Morris Water Maze
24 h after the elevated zero maze, spatial learning and memory were assessed with the water maze as described by Morris (1984) and adapted for mice. The mouse water maze consisted of a circular pool (1.2 m diameter and 36 cm high), filled to a depth of 17 cm. The pool circumference was divided into four virtual quadrants and arbitrarily marked with the start positions: north (N), south (S), east (E) and west (W). The water was opacified with white non-toxic Crayola paint. The water temperature was between 22°C and 24°C during testing. The platform was made of transparent Plexiglas (10 cm×10 cm), and its placement in the N quadrant of the pool remained fixed throughout training. The top of the platform was 1 cm below the surface of the water. The pool was surrounded by various distal spatial cues and illuminated by two lamps. All trials were recorded with a video camera (see Behavioral Analysis) situated above the pool. To prevent hypothermia, trials were separated from each other by a period of 20 min. Each animal rested on warming mats between trials.
Pretraining
Before the first trial, each animal was placed on the visible platform whose submerged location was made visible by an attached flag as a proximal cue for 10 s. The animals were then placed in the water and allowed to swim for 10 s before being guided back to the platform, where they were allowed to rest for 10 s.
Visible Training
Each trial began by placing the animal in the water at the edge of the pool facing the wall from different quadrants. During the trial, each mouse was allowed 60 s to locate the platform. If the mouse failed to reach the fixed platform in the allotted time, it was guided to the platform. The start locations sequence was S, W and E. Each mouse was given two blocks of three trials for two consecutive days.
Hidden Training
The flag was removed from the platform. Each mouse was placed in the water and allowed 60 s to locate the platforms. It was given two blocks of three trials daily until the wild-type animals were able to locate the fixed platform in 15 s or less. The start locations sequence was S, W and E. Hidden platform training was performed for 5 days with two blocks of three trials.
Probe Trial
On the final day of hidden training, the platform was removed from the pool, and the mouse was placed in the quadrant directly across from where the platform had been. Each animal was allowed 60 s to search the pool. At the end of the trial, the mice were removed by hand.
During the platform trials, latencies (the time to reach the platform from the start location) were measured. Data from trials/day were averaged. In the probe trials, the performance measures were the mean swim speed, percentage of time spent in the each quadrant and the number of annulus crossings (number of times an animal crossed the exact place where the platform had been located during training).
Behavioral Analysis
Open field exploration and elevated zero maze tests were video tracked using Viewpoint VideoTrack Version 2.0. The system recorded distance traveled by the animals and the time spent in defined areas. The Morris water maze test was videotaped using a Sony DCR-TRV 280 video camera mounted above the pool. The raw data were analyzed by the Smart software version 2.0 (San Diego Instruments, Inc.).
Statistical Analysis
Data were analyzed with a statistical software package, GraphPad Prism 5.0 for Windows, (San Diego, CA) and JMP6 (SAS, Cary, NC)). Normality of the data for each variable was determined using the D’Agostino & Pearson (1973) test. One way analysis of variance (ANOVA) was used for analysis of weight. For analysis of the open field data, the distance variable could not be considered normal and was not transformable to be sufficiently close to normally distributed to allow application of normal based statistical approaches. Therefore, to compare the open field distance across genotype and sex, a nonparametric version of a repeated measures ANOVA was used, as described in Brunner et al. (2002) The model used (denoted F2_LD_F1 in the book) allowed for three factors, two non-repeated (sex and genotype) and one repeated (time). For analysis of the data from rotarod, elevated zero maze, and Morris water maze, repeated measures ANOVAs and one-sample t-tests were used. All analyses compared group differences by genotype, sex, and genotype by sex interactions. All figures display the mean ± SE. For all analyses, significance was defined as p ≤0.05.
RESULTS
Physical and Neurological Tests
There were no qualitative differences in physical characteristics, visual acuity or sensorimotor reflexes among genotypes or sex. Body weight of same sex animals did not differ among genotypes, but all females as expected had significantly lower weight than males for all genotypes (F[1,57] = 0.62, p < 0.0001; data not shown) and a marginally significant genotype by sex interaction with females weighing less than their male counterparts (F[2,57] = 3.2, p<0.05; data not shown).
Open Field Exploration
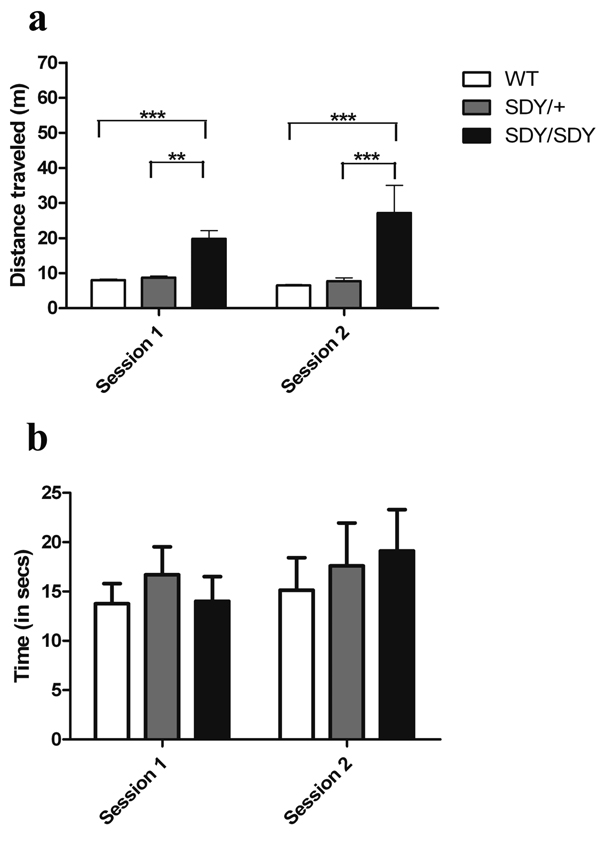
Non-parametric repeated measures ANOVA found a significant effect of genotype on locomotor activity in the two open field sessions (Figure 1a; Box-Approximation with Chi-square “B”[1.8,47.4] = 81.99, p = 0.000). Post hoc individual group comparisons showed that the sdy/sdy mice were significantly more active than the littermate wild-type (F [1,40] = 217.96; p < 0.000) and sdy/+ mice during both sessions; F [1,40] = 92.24; p < 0.000, respectively). Sdy/+ mice did not differ from wild-type littermates in locomotor activity (F [1,40] = 0.003; p = 0.96).
Figure 1. Open Field.
(a) Total distance traveled during the two 5 minute sessions in meters (m) and (b) total time spent in the center of the open field during the two 5 minute sessions for wild-type (WT) (□), sdy/+ ( ), and sdy/sdy (■) mice. ** p < 0.001, *** p < 0.0001. The amount of time expected in the center field by chance (42s) is larger than the observed times.
), and sdy/sdy (■) mice. ** p < 0.001, *** p < 0.0001. The amount of time expected in the center field by chance (42s) is larger than the observed times.
There was also a significant effect of session on locomotor activity (B(1) = 20.1; p = 0.00001) and an interaction between genotype and session (B[1.8] = 6.26; p < 0.003). Within mouse genotype comparisons of session 1 and session 2 showed less activity in the second session relative to the first for the wild-type mice (Wilcoxon sign-rank Z[20] =9 9.5, p = 0.000) and sdy/+ mice (Z[20] = 67.5, p < 0.02), suggesting habituation to the open field whereas the sdy/sdy mice showed no difference in distance traveled in the open field between sessions (Z[20] = 2.5, p = 0.39), suggesting a lack of habituation (Figure 1a). There were no significant effects of sex.
All genotypes exhibited a clear preference for the periphery of the open-field box as indicated by the amount of time spent there (Figure 1b). There was no significant difference in amount of time spent in the center of the open field between wild type and sdy/sdy mice in either session. Group data from the first session showed that wild-type mice spent an average of 13.75 ± 2.05 seconds and sdy/sdy mice 14.00 ± 2.50 s in the center of the field. In the second session, wild-type mice spent an average time of 15.13 ± 3.29 and sdy/sdy 19.12 ± 4.16 seconds in the center of the field. Similarly, there were no differences for the sdy/+ mice compared to either of the other genotypes, nor between males and females for any group. Since preference for the periphery is generally considered an index of anxiety, these results provided no evidence for increased anxiety in the sdy/sdy mice.
Rotarod
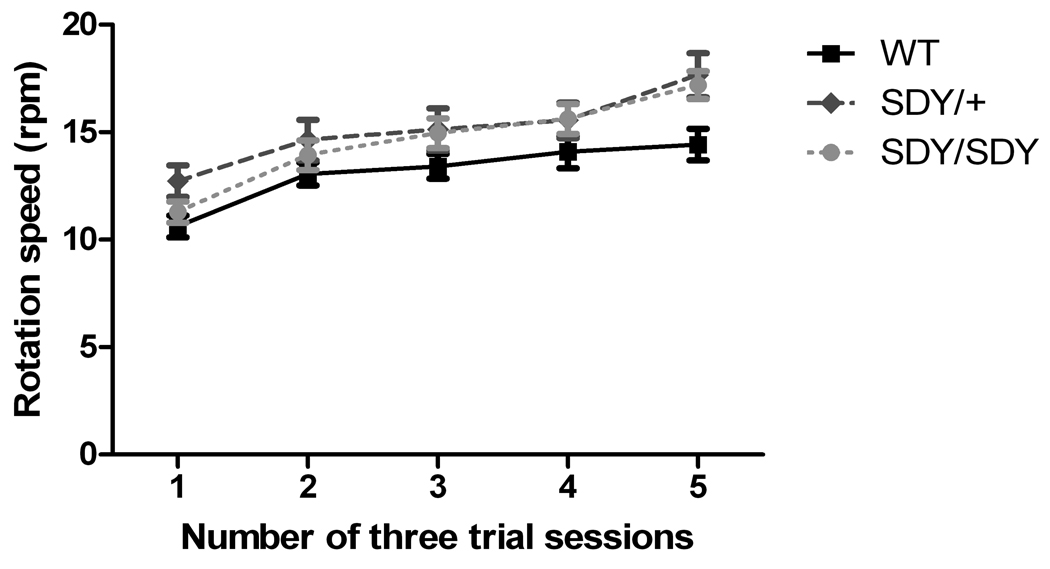
The performance of all three groups of mice improved over the five days of training; however, there was a marginally significant main effect of genotype (Figure 2; repeated measures ANOVA F[2,57] = 3.17, p <0.05). Posthoc group comparisons showed that both sdy/sdy and sdy/+ mice had better motor balance skills than wild-type mice (F[1,40] = 5.1, p < 0.03; F[1,40] = 4.7, p = 0.03 respectively). No performance difference were found between sdy/sdy and sdy/+ mice (F[1,40] = 0.4, p = 0.53). Sex had a significant effect (F[1,57] = 14.1, p = 0.0004), with females performing better than males in the sdy/sdy (F[1,19] = 13.5, p < 0.002) and sdy/+ (F ([1,19] = 9.45, p < 0.007) groups, but not the wild-type group (F([1,19] = 0.49, p = 0.49). There was a trend towards an interaction between genotype and sex observed (Figure 2; F[2,57[ = 2.63, p = 0.08).
Figure 2. Rotarod Performance.
Improvement in the time animals managed to stay atop accelerating rotarod on the 5 sessions with 3 trials per session. Symbols indicate WT (■), sdy/+ ( ), and sdy/sdy (
), and sdy/sdy ( ) mice. No significant differences among groups were found.
) mice. No significant differences among groups were found.
Elevated Zero Maze
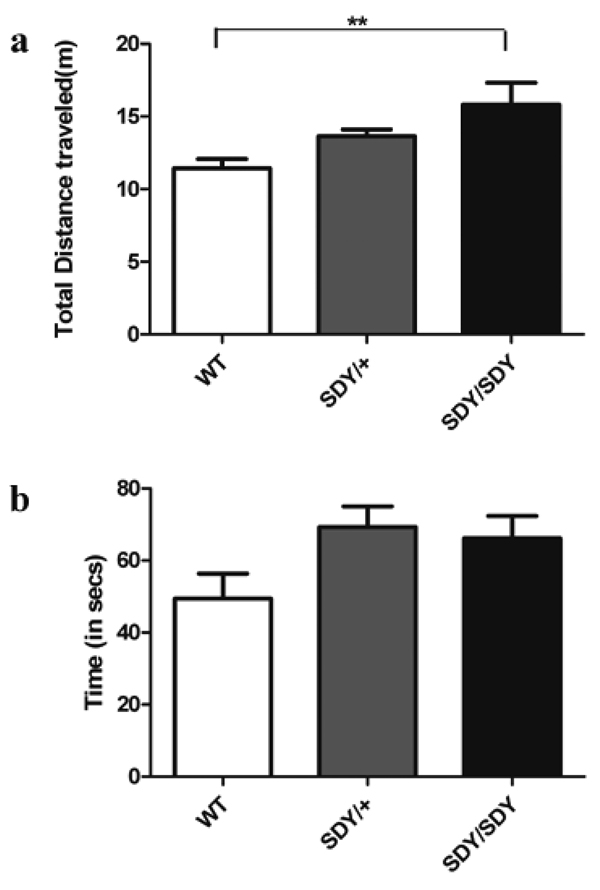
Consistent with the open field test findings of hyperactivity, there were significant differences between groups for total distance traveled in the elevated zero maze (Figure 3a; F[2,57] = 7.08; p < 0.002). Posthoc Student’s t-tests found that both sdy/sdy (p < 0.0005) and sdy/+ (p < 0.04) traveled greater distances in the open quadrants than wild-type mice. There was also a significant effect of sex (F[1,57] = 5.43; p < 0.03) with female mice traveling a greater distance than males in the allotted time. There was also a significant genotype by sex interaction (F[2,57] = 3.9; p < 0.03). Sdy/sdy females traveled greater distances than other females of both genotypes while for the males, both sdy/sdy and sdy/+ genotypes traveled greater distances than their male wild-type counterparts There was a trend towards an effect of genotype on time spent in the open quadrants (Figure 3b; F[2,57] = 2.88; p = 0.06) with post hoc analyses showing more time spent in the open arms for sdy/sdy (p = 0.06) and sdy/+ (p = 0.03). There was no effect of sex nor interaction between sex and genotype on time spent in the open arms (F[1,57] = 0.48; p = 0.48 and F[2,57] = 0.62; p = 0.54, respectively).
Figure 3. Elevated Zero Maze.
(a) Total distance in meters traveled during the 5 minute session and (b) total time spent in the open arms during the 5 minute session for WT (□), sdy/+ ( ) and sdy/sdy mice (■). **Difference at p = 0.002
) and sdy/sdy mice (■). **Difference at p = 0.002
Morris Water Maze
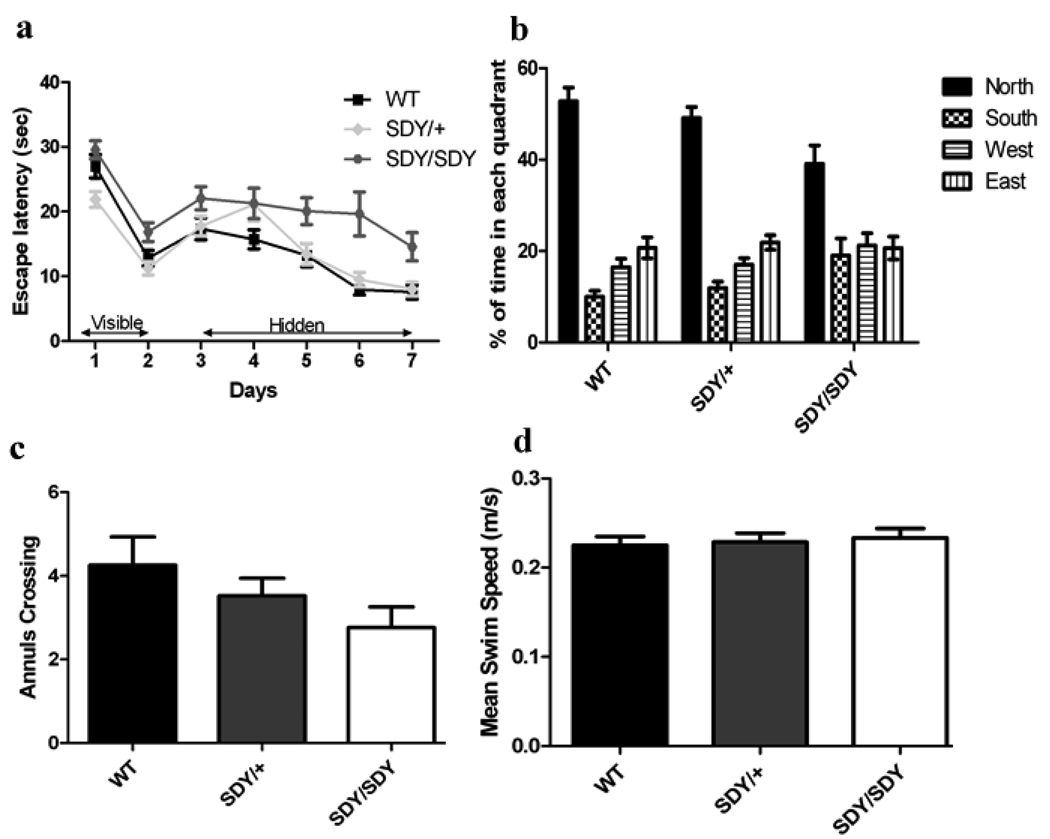
Repeated measures ANOVA revealed significant differences among genotype groups in the latencies of acquisition in the visible platform (Figure 4a; F[2,56] = 8.97, p = 0.0004), with sdy/sdy mice exhibiting a longer latency to reach the platform than both the WT (p = 0.04) and sdy/+ (p= 0.004) groups in post-hoc analyses. There were no effects of sex (F1,56 = 2.13, p-=0.15) and no genotype x sex interaction (F2,56 = 0.04, p = 0.95).
Figure 4. Morris Water Maze.
(a) Escape latency (s) during visible and hidden platform for WT (■), sdy/+ ( ), and sdy/sdy (
), and sdy/sdy ( ) mice, (b) percentage of time spent in all quadrants during probe trial, (c) annulus crossings during probe trial soon after final hidden platform trial for WT (■), sdy/+ (
) mice, (b) percentage of time spent in all quadrants during probe trial, (c) annulus crossings during probe trial soon after final hidden platform trial for WT (■), sdy/+ ( ), and sdy/sdy (□) mice, and (d) average swim speed during the probe trial for WT mice (■), sdy/+ (
), and sdy/sdy (□) mice, and (d) average swim speed during the probe trial for WT mice (■), sdy/+ ( ), and sdy/sdy mice (□).
), and sdy/sdy mice (□).
Highly significant differences in escape latencies were observed among groups in the hidden platform trials (Figure 4a; F[2,56] = 9.09, p = 0.0004).This was driven by the sdy/sdy group of mice, most of which never reached criterion performance. Post hoc individual group comparisons showed significant differences between the sdy/sdy mice and wild-type (F[1,39] = 16.04, p = 0.0003) and sdy/+ mice (F[1,40] = 6.68, p < 0.02) while there was no difference in escape latencies between wild-type and sdy/+ mice (F[1,39] = 2.30, p = 0.14). On the first day of hidden platform training, wild-type mice found the hidden platform after an average of 20.4 ± 2.6 seconds compared to 22.3 ± 2.0 seconds for the sdy/sdy mice (p = 0.74). After meeting test criterion on the final day of hidden platform training, wild-type mice had an average escape latency of 6.8 ± 1.9 seconds while sdy/sdy mice had an average escape latency of 17.6 ± 11.6 seconds (p < 0.00002). As in the visible platform training, there were no significant effects of sex (F[1,56] = 0.39, p = 0.53) nor were there any significant interactions between genotype and sex (F[2,56] = 0.82, p = 0.44) on the hidden platform trials.
Probe trial measurements of time spent in the correct quadrant demonstrated significant effects of genotype on preference for the target quadrant (Figure 4b, F[2,56] = 4.78, p = 0.01). Preference for the target quadrant was attenuated in the sdy/sdy mice compared to the other two groups with post hoc individual comparison showing sdy/sdy spent significantly less time in the target quadrant than wild-type (p = 0.004) or sdy/+ (p = 0.03). In kind, time spent in the opposite quadrant also differed (F[2,56] = 3.73, p < 0.03), with post-hoc individual comparison showing sdy/sdy mice spending significantly more time in the opposite quadrant than wild-type (p = 0.01) or sdy/+ (p < 0.05). Sdy/sdy mice had fewer annulus crossings than wild-type or sdy/+ (Figure 4c), though differences among groups were not significant (F[2,56] = 1.9, p = 0.16). While impaired relative to the other two groups, the sdy/sdy mice did still exhibit spatial learning as evidenced by significantly greater time spent in the target quadrant than would be predicted by chance (i.e., 25%; t20 = 3.5, p < 0.003). Finally, there were no significant differences among groups for swim speed (F[2,56] = 0.8, p = 0.43; Figure 4d).
DISCUSSION
While the sdy mutation arose in DBA/2J mice (Swank et al., 1991), we have investigated its behavioral phenotype in sdy/BL6 mice to avoid effects on behavioral performance due to other mutations in DBA/2J mice (see Introduction). We found that sdy/+ and sdy/sdy mice are normal in physical appearance and in sensorimotor abilities, but that the sdy/sdy are hyperactive in the open field and elevated zero maze, do not habituate in exploratory behavior, and display spatial learning and memory deficits in the Morris water maze. As expanded upon below, these abnormalities are consistent with dysfunction of the hippocampal formation, where synaptic dysbindin-1 is normally abundant (Talbot et al., 2004, 2006) and where evoked excitatory responses in hippocampal field CA1 are diminished in sdy/sdy mice (Chen et al., (2008).
The sdy/sdy mice traveled significantly greater distances than their wild-type littermates in the open field and elevated zero maze tests. They showed no differences compared to wild-type mice in their preference for the periphery of the open field or for the closed quadrants in the elevated zero maze. Consequently, their hyperactivity is not readily attributable to increased anxiety. Since they showed no impairments in motor coordination on the rotarod, their hyperactivity was also not attributable to loss of motor control. These results differ from conflicting reports on homozygous sdy/DBA mice. Feng et al. (2008) found no locomotor abnormalities in such mice. Hattori et al. (2008) reported that such animals were hypoactive over the first fifteen minutes in the open field and showed evidence of anxiety in that field and in the elevated plus maze. Yet Askari et al. (2007) found that sdy/DBA mice – like homozygous sdy/BL6 mice - are hyperactive in the open field and show no evidence of anxiety on the elevated plus maze.
The observed hyperactivity of homozygous sdy/BL6 mice may reflect an abnormality in dopaminergic activity. Such an abnormality is expected given that dysbindin-1 loss in sdy/sdy mice is associated with elevated rates of dopamine turnover in both the hippocampal formation and in corticolimbic regions suggestive of increased dopamine release in those brain areas (Murotani et al. (2007) and (that in vitro knockdown of dysbindin-1 in cultured cerebrocortical neurons (as occurs naturally in sdy mice) leads to increased cell surface expression of the dopamine D2 receptor (D2R), but not of the D1 receptor Iizuka et al. (2007). The opposite condition in D2R knockout mice leads to decreased locomotion (hypoactivity or bradykinesis: see Glickstein & Schmauss, 2001).
Increased cell surface expression of D2R in the hippocampus combined with increased dopamine release in that structure could promote hyperactivity for three related reasons. First, dopamine activation of D2R in hippocampal field CA1 depolarizes the resting membrane potential in 50% of the pyramidal cells tested and reduces the after-hyperpolarization in 67% of those cells (Berretta et al., 1990). Second, CA1 pyramidal cells in the ventral hippocampus innervate nucleus accumbens (Friedman et al., 2002; van Groen & Wyss, 1990), an area modulating motor activity along with other striatal structures (David et al., 2005; Taepavarapruk et al., 2000). Third, activation of ventral hippocampal output to nucleus accumbens is known to induce hyperactivity (Bast et al., 2001), an effect dependent on ventral hippocampal dopamine receptors as shown by its blockage with the D1R antagonist SCH 23390 and its initial attenuation with the D2R antagonist raclopride (Zornoza et al., 2005). Hyperactivity may also result from altered dopaminergic activity in the nucleus accumbens itself. Loss of dysbindin-1 in sdy/sdy mice is expected to elevate cell-surface expression of D2R not only in the hippocampus, but also in nucleus accumbens. This could promote hyperlocomotion, because the ventral hippocampus exerts a tonic facilitation of locomotor activity via D2-like postsynaptic receptors within nucleus accumbens (Rouillon et al., 2007).
The performance of sdy/sdy mice in the Morris water maze is also suggestive of hippocampal dysfunction. Performance of sdy/sdy mice in the hidden platform trials compared to the other two groups indicated a spatial learning deficit, while in the probe trial, sdy/sdy displayed less preference for the location where the hidden platform had been. Probe trial performance is dependent on both the strength of the acquired information, as well as recollection of that information Thus, the impairment in the sdy/sdy mice could reflect impaired spatial memory as well as poorer initial learning and acquisition. Such a pattern of deficits in water maze performance is characteristic of rodents suffering hippocampal lesions (D’Hooge & De Deyn, 2001; Morris, 2007).
In the visible platform stage of the Morris water maze, the sdy/sdy mice exhibited a marginally longer average escape latency compared to the wild-type and sdy/+ animals. This is consistent with impairment in visual associative learning and suggests dysfunction of brain regions outside the hippocampus as well. Unlike the case for hyperactivity, the impaired water maze performance of sdy/sdy mice is not consistent with increased hippocampal dopaminergic activity, which should facilitate spatial learning and memory (Stuchlik et al., 2007; Wilkerson & Levin, 1999). The impaired performance in the water maze may instead be due to decreased responsiveness of hippocampal field CA1 to excitatory input from CA3 reported in sdy/sdy mice (Chen et al., 2008). Normal responsiveness to such input is important for spatial memory, but not for visual association memory as indicated by the finding that CA1-specific knockout of NMDA glutamate receptors impairs location of the hidden, but not the visible, platform in the Morris water maze (Tsien et al., 1996).
Since dysbindin-1 is reduced in the brains of sdy mice (see Li et al., 2003) and of schizophrenia cases (Straub et al., 2004; Talbot et al., 2004), it can be asked whether these mice display phenotypical features of schizophrenia. That appears to be true for most behavioral abnormalities of sdy/sdy mice that have been reported to date. Their hyperactivity in the open field is shared by diverse mouse models of schizophrenia (Powell and Miyakawa, 2006; see also Mohn et al., 1999, Hikidia et al., 2007, Powell et al., 2007), which may have the same causes as the psychomotor agitation estimated to frequently occur in schizophrenia patients admitted for emergency psychiatric care in the U.S. (Marco and Vaughan, 2005). Impaired habituation of sdy/sdy mice in the open field likewise resembles the decreased habituation to diverse stimuli reported in schizophrenia (e.g., Taiminen et al., 2000; Meincke et al., 2004; Holt et al., 2005). The learning and memory deficits shown by sdy/sdy mice in the Morris water maze also resemble those shown by schizophrenia cases in a virtual Morris water maze task (Hanlon et al., 2006). Finally, while not yet tested in sdy/BL6 mice, the decreased social interactions of homozygous sdy/DBA mice (Feng et al., 2008; Hattori et al., 2008) is consistent with impaired social functions, especially impaired social cognition, in schizophrenia (see Couture et al., 2006 and Yager and Ehmann, 2006).
We cannot expect, however, that a mouse strain with just one of the biological anomalies found in schizophrenia will model all aspects of that disorder. That limitation is suggested by studies on sdy/DBA mice. These animals show no deficits in prepulse inhibition (Hattori et al., 2008; see also Li et al., 2003), which is a frequently cited abnormality in schizophrenia (Quednow et al., 2008; Turetsky et al., 2007) and in diverse mouse models of that disorder (Clapcote et al., 2007, Erbel-Sieler et al., 2004; Fradley et al., 2005; Miyakawa et al., 2003; Stefansson et al., 2002). Homozygous sdy/DBA mice also do not react in a consistent manner to psychotomimetic drugs. While they display increased locomotor activation after an acute dose of the NMDA receptor antagonist MK-801, they display decreased locomotor activation after an acute dose of D-amphetamine (Askari et al., 2007).
The present results on Morris water maze performance nevertheless suggest that the sdy mouse may model at least some important dysbindin-1 related cognitive deficits of schizophrenia. This is consistent with an increasing number of studies showing that single nucleotide polymorphisms in DTNBP1 associated with schizophrenia are also associated in that disorder with lower general cognitive ability (Burdick et al., 2006, 2007), lower scores on verbal, performance, and full-scale IQ tests (Zinstock et al., 2007), and deficits in several tasks of attentional response control and/or working memory (Donohoe et al., 2007). Since the dysbindin-1 reductions in sdy mice are not dependent on these SNPs, but rather upon a deletion mutation partially overlapping the SNP locations, such mice cannot be said to model cognitive endophenotypes of schizophrenia. But they may model cognitive deficits related to altered DTNBP1 gene expression in general.
ACKNOWLEDGMENTS
The authors thank Drs. Darrick Balu, Julie Blendy, Tamar Gur, Irwin Lucki, David Oslin, and Dr. Roland Tallarida for their assistance and helpful comments with this work as well as Dr. Warren Bilker and Colleen M. Brensinger for their assistance with biostatistics. This work was supported by grants from the National Institute of Mental Health (MH64045, MH072880, MH 019931), and the National Alliance for Research in Schizophrenia and Depression.
REFERENCES
- Allegretti M, Moriconi A, Beccari AR, Di Bitondo R, Bizzarri C, Bertini R, Colotta F. Targeting C5a: recent advances in drug discovery. Curr Med Chem. 2005;12:217–236. doi: 10.2174/0929867053363379. [DOI] [PubMed] [Google Scholar]
- Allen NC, Bagade S, McQueen MB, Ioannidis JPA, Kavvoura FK, Khoury MJ, Tanzi RE, Bertram L. Systematic meta-analyses and field synopsis of genetic association studies in schizophrenia: the SzGene database. Nat Genet. 2008;40:827–834. doi: 10.1038/ng.171. [DOI] [PubMed] [Google Scholar]
- Arnold SE, Carlson GC, Talbot K, Novak EK, Swank RT, Coulter DA. Failure of inhibition in the hippocampus of the dysbindin mutant “sandy” mouse; Society for Neuroscience Annual Meeting; Washington, DC. 2005. Abstract 936.13 from 2005. [Google Scholar]
- Askari BS, Bhardwaj SK, Srivastava LK. Behavioral impairments in dysbindin-deficient sandy mice; Society for Neuroscience Annual Meeting; San Diego, CA. 2007. (Abstract 59.1 from 2007) [Google Scholar]
- Bast T, Zhang W-N, Heidbreder C, Feldon J. Hyperactivity and disruption of prepulse inhibition induced by N-methyl-D-aspartate stimulation of the ventral hippocampus and the effects of pretreatment with haloperidol and clozapine. Neuroscience. 2001;103:325–335. doi: 10.1016/s0306-4522(00)00589-3. [DOI] [PubMed] [Google Scholar]
- Benson MA, Newey SE, Martin-Rendon E, Hawkes R, Blake DJ. Dysbindin, a novel coiled-coil-containing protein that interacts with the dystrobrevins in muscle and brain. J Biol Chem. 2001;276:24232–24241. doi: 10.1074/jbc.M010418200. [DOI] [PubMed] [Google Scholar]
- Berretta N, Berton F, Bianchi R, Capogna M, Francesconi W, Brunelli M. Effects of dopamine, D-1 and D-2 dopaminergic agonists on the excitability of hippocampal pyramidal cells in guinea pig. Exp Brain Res. 1990;83:124–130. doi: 10.1007/BF00232200. [DOI] [PubMed] [Google Scholar]
- Brunner E, Domhof S, Langer F. Nonparametric Analysis of Longitudinal Data in Factorial Experiments. Malden, MA: Wiley-Interscience; 2001. [Google Scholar]
- Burdick KE, Lencz T, Funke B, Finn CT, Szeszko PR, Kane JM, Kucherlapati R, Malhotra AK. Genetic variation in DTNBP1 influences general cognitive ability. Hum Mol Genet. 2006;15:1563–1568. doi: 10.1093/hmg/ddi481. [DOI] [PubMed] [Google Scholar]
- Burdick KE, Goldberg TE, Funke B, Bates JA, Lencz T, Kucherlapati R, Malhotra AK. DTNBP1 genotype influences cognitive decline in schizophrenia. Schizophr Res. 2007;89:169–172. doi: 10.1016/j.schres.2006.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X-W, Feng Y-Q, Hoo C-J, Guo X-L, He X, Zhou Z-Y, Guo N, Huang H-P, Xiong W, Zheng H, Zuo P-L, Zhang CX, Li W, Zhou Z. DTNBP1, a schizophrenia susceptibility gene, affects kinetics of transmitter release. J Cell Biol. 2008;181:791–801. doi: 10.1083/jcb.200711021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapcote SJ, Lipina TV, Millar JK, Mackie S, Christie S, Ogawa F, Lerch JP, Trimble K, Uchiyama M, Sakuraba Y, Kaneda H, Shiroishi T, Houslay MD, Henkelman RM, Sled JG, Gondo Y, Porteous DJ, Roder JC. Behavioral phenotypes of Disc1 missense mutations in mice. Neuron. 2007;54:387–402. doi: 10.1016/j.neuron.2007.04.015. [DOI] [PubMed] [Google Scholar]
- Couture SM, Penn DL, Roberts DL. The functional significance of social cognition in schizophrenia: a review. Schizophr Bull. 2006;32:S44–S63. doi: 10.1093/schbul/sbl029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox DR, Oakes D. Analysis of Survival Data. London: Chapman and Hall; 1984. [Google Scholar]
- D’Agostino R, Pearson ES. Empirical results for the distribution of b2 and √b1. Biometrika. 1973;60:613–622. [Google Scholar]
- David HN, Ansseau M, Abraini JH. Dopamine-glutamate reciprocal modulation of release and motor responses in the rat caudate-putamen and nucleus accumbens of “intact” animals. Brain Res Rev. 2005;50:336–360. doi: 10.1016/j.brainresrev.2005.09.002. [DOI] [PubMed] [Google Scholar]
- D’Hooge R, De Deyn PP. Applications of the Morris water maze in the study of learning and memory. Brain Res Rev. 2001;36:60–90. doi: 10.1016/s0165-0173(01)00067-4. [DOI] [PubMed] [Google Scholar]
- Donohoe G, Morris DW, Clarke S, McGhee KA, Schwaiger S, Nangle J-M, Garavan H, Robertson IH, Gill M, Corvin A. Variance in neurocognitive performance is associated with dysbindin-1 in schizophrenia: a preliminary study. Neuropsychologia. 2007;45:454–458. doi: 10.1016/j.neuropsychologia.2006.06.016. [DOI] [PubMed] [Google Scholar]
- Duan J, Martinez M, Sanders AR, Hou C, Burrell GJ, Krasner AJ, Schwartz DB, Gejman PV. DTNBP1 (Dystrobrevin Binding Protein 1) and schizophrenia: association evidence in the 3’ end of the gene. Human Hered. 2007;64:97–106. doi: 10.1159/000101961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erbel-Sieler C, Dudley C, Zhou Y, Wu X, Estill SJ, Han T, Diaz-Arrastia R, Brunskill EW, Potter SS, McKnight SL. Behavioral and regulatory abnormalities in mice deficient in the NPAS1 and NPAS3 transcription factors. Proc Natl Acad Sci U S A. 2004;101:13648–13653. doi: 10.1073/pnas.0405310101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y-Q, Zhou Z-Y, He X, Wang H, Guo X-L, Hao C-J, Guo Y, Zhen X-C, Li W. Dysbindin deficiency in sandy mice causes reduction of snapin and displays behaviors related to schizophrenia. Schizophr Res. 2008 doi: 10.1016/j.schres.2008.07.018. (in press) [DOI] [PubMed] [Google Scholar]
- Fradley RL, O'Meara GF, Newman RJ, Andrieux A, Job D, Reynolds DS. STOP knockout and NMDA NR1 hypomorphic mice exhibit deficits in sensorimotor gating. Behav Brain Res. 2005;163:257–264. doi: 10.1016/j.bbr.2005.05.012. [DOI] [PubMed] [Google Scholar]
- Friedman DP, Aggleton JP, Saunders RC. Comparison of hippocampal, amygdala, and perirhinal projections to the nucleus accumbens: combined anterograde and retrograde tracing study in the macaque brain. J Comp Neurol. 2002;450:345–365. doi: 10.1002/cne.10336. [DOI] [PubMed] [Google Scholar]
- Glickstein SB, Schmauss C. Dopamine receptor functions: lessons from knockout mice. Pharmacol Ther. 2001;91:63–83. doi: 10.1016/s0163-7258(01)00145-0. [DOI] [PubMed] [Google Scholar]
- Hanlon FM, Weisend MP, Hamilton DA, Jones AP, Thoma RJ, Huang M, Martin K, Yeo RA, Miller GA, Canive JM. Impairment on the hippocampal-dependent virtual Morris water task in schizophrenia. Schizophr Res. 2006;87:67–80. doi: 10.1016/j.schres.2006.05.021. [DOI] [PubMed] [Google Scholar]
- Hattori S, Murotani T, Matsuzaki S, Ishizuka T, Kumamoto N, Takeda M, Tohyama M, Yamatodani A, Kunugi H, Hashimoto R. Behavioral abnormalities and dopamine reductions in sdy mutant mice with a deletion in Dtnbp1, a susceptibility gene for schizophrenia. Biochem Biophys Res Commun. 2008;373:298–302. doi: 10.1016/j.bbrc.2008.06.016. [DOI] [PubMed] [Google Scholar]
- Hikida T, Jaaro-Peled H, Seshadri S, Oishi K, Hookway C, Kong S, Wu D, Xue R, Andrade M, Tankou S, Mori S, Gallagher M, Ishizuka K, Pletnikov M, Kida S, Sawa A. Dominant-negative DISC1 transgenic mice display schizophrenia-associated phenotypes detected by measures translatable to humans. Proc Natl Acad Sci U S A. 2007;104:14501–14506. doi: 10.1073/pnas.0704774104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt DJ, Weiss AP, Rauch SL, Wright CI, Zalesak M, Goff DC, Ditman T, Welsh RC, Heckers S. Sustained activation of the hippocampus in response to fearful faces in schizophrenia. Biol Psychiatry. 2005;57:1011–1019. doi: 10.1016/j.biopsych.2005.01.033. [DOI] [PubMed] [Google Scholar]
- Howell GR, Libby RT, Marchant JK, Wilson LA, Cosma IM, Smith RS, Anderson MG, John SWM. Absence of glaucoma in DBA/2J mice homozygous forwild type versions of Gpnmb and Tryp1. BMC Genetics. 2007;8:45. doi: 10.1186/1471-2156-8-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iizuka Y, Sei Y, Weinberger DR, Straub RE. Evidence that the BLOC-1 protein dysbindin modulates dopamine D2 receptor internalization and signaling but not D1 internalization. J Neurosci. 2007;27:12390–12395. doi: 10.1523/JNEUROSCI.1689-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin S. Comprehensive observational assessment: Ia, a systematic, quantitative procedure for assessing the behavioral and physiologic state of the mouse. Psychopharamcology. 1968;13:222–257. doi: 10.1007/BF00401402. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Tinsley M, Jairl C, Horowitz B, Seu E, Cannon T. Null mutation of the gene coding for dysbindin is associated with poor working memory and spatial learning in mice; Society for Neuroscience Annual Meeting; San Diego, CA. 2007. (Abstract 59.16 from 2007) [Google Scholar]
- Johnson KR, Zheng QY, Erway LC. A major gene affecting age-related hearing loss is common to at least ten inbred strains of mice. Genomics. 2000;70:171–180. doi: 10.1006/geno.2000.6377. [DOI] [PubMed] [Google Scholar]
- Johnson KR, Zheng QY, Noben-Trauth K. Strain background effects and genetic modifiers of hearing in mice. Brain Res. 2006;1091:79–88. doi: 10.1016/j.brainres.2006.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Zhang Q, Oiso N, Novak EK, Gautam R, O'Brien EP, Tinsley CL, Blake DJ, Spritz RA, Copeland NG, Jenkins NA, Amato D, Roe BA, Starcevic M, Dell'Angelica EC, Elliott RW, Mishra V, Kingsmore SF, Paylor RE, Swank RE. Hermansky-Pudlak syndrome type 7 (HPS-7) results from mutant dysbindin, a member of the biogenesis of lysosome-related organelles complex 1 (BLOC-1) Nat Genet. 2003;35:84–89. doi: 10.1038/ng1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marco CA, Vaughan J. Emergency management of agitation in schizophrenia. Am J Emerg Med. 2005;23:767–776. doi: 10.1016/j.ajem.2005.02.050. [DOI] [PubMed] [Google Scholar]
- Meincke U, Light GA, Geyer MA, Braff DL, Gouzoulis-Mayfrank E. Sensitization and habituation of the acoustic startle reflex in patients with schizophrenia. Psychiatry Res. 2004;126:51–61. doi: 10.1016/j.psychres.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Miyakawa T, Leiter LM, Gerber DJ, Gainetdinov RR, Sotnikova TD, Zeng H, Caron MG, Tonegawa S. Conditional calcineurin knockout mice exhibit multiple abnormal behaviors related to schizophrenia. Proc Natl Acad Sci U S A. 2003;100:8987–8992. doi: 10.1073/pnas.1432926100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohn AR, Gainetdinov RR, Caron MG, Koller BH. Mice with reduced NMDA receptor expression display behaviors related to schizophrenia. Cell. 1999;98:427–436. doi: 10.1016/s0092-8674(00)81972-8. [DOI] [PubMed] [Google Scholar]
- Morris R. Developments of a water maze procedure for studying spatial learning in the rat. J Neurosci Methods. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- Morris R. Theories of hippocampal function. In: Anderson P, Morris R, Amaral D, Bliss T, O’Keefe J, editors. The Hippocampus Book. New York: Oxford University Press; 2007. pp. 581–713. [Google Scholar]
- Murotani T, Ishizuka T, Hattori S, Hashimoto R, Matsuzaki S, Yamatodani A. High dopamine turnover in the brains of Sandy mice. Neurosci Lett. 2007;421:47–51. doi: 10.1016/j.neulet.2007.05.019. [DOI] [PubMed] [Google Scholar]
- Numakawa T, Yagasaki Y, Ishimoto T, Okada T, Suzuki T, Iwata N, Ozaki N, Taguchi T, Tatsumi M, Kamijima K, Straub RE, Weinberger DR, Kunungi H, Hashimoto R. Evidence of novel neuronal functions of dysbindin, a susceptibility gene for schizophrenia. Hum Mol Genet. 2004;13:2699–2708. doi: 10.1093/hmg/ddh280. [DOI] [PubMed] [Google Scholar]
- Pasinetti GM, Tocco G, Sakhi S, Musleh WD, DeSimoni MG, Mascarucci P, Schreiber S, Baudry M, Finch CE. Hereditary deficiencies in complement C5 are associated with intensified neurodegenerative responses that implicate new roles for the C-system in neuronal and astrocytic functions. Neurobiol Dis. 1996;3:197–204. doi: 10.1006/nbdi.1996.0020. [DOI] [PubMed] [Google Scholar]
- Powell CM, Miyakawa T. Schizophrenia-relevant behavioral testing in rodent models: a uniquely human disorder? Biol Psychiatry. 2006;59:1198–1207. doi: 10.1016/j.biopsych.2006.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell KJ, Hori SE, Leslie R, Andrieux A, Schellinck H, Thorne M, Robertson GS. Cognitive impairments in the STOP null mouse model of schizophrenia. Behav Neurosci. 2007;121:826–835. doi: 10.1037/0735-7044.121.5.826. [DOI] [PubMed] [Google Scholar]
- Quednow BB, Frommann I, Berning J, Kühn K-U, Maier W, Wagner M. Impaired sensorimotor gating of the acoustic startle response in the prodrome of schizophrenia. Biol Psychiatry. 2008;64 doi: 10.1016/j.biopsych.2008.04.019. (in press) [DOI] [PubMed] [Google Scholar]
- Rogers DC, Peters J, Martin JE, Ball S, Nicholson SJ, Witherden AS, Hafezparast M, Latcham J, Robinson TL, Quilterz CA, Fisher EM. SHIRPA, a protocol for behavioral assessment: validation for longitudinal study of neurological dysfunction in mice. Neuroscience Letters. 2001;306:89–92. doi: 10.1016/s0304-3940(01)01885-7. [DOI] [PubMed] [Google Scholar]
- Rouillon C, Abraini JH, David HN. Hippocampal modulation of locomotor activity induced by activation of postsynaptic dopamine receptors in the nucleus accumbens. Hippocampus. 2007;17:1028–1036. doi: 10.1002/hipo.20337. [DOI] [PubMed] [Google Scholar]
- Stefansson H, Sigurdsson E, Steinthorsdottir V, Bjornsdottir S, Sigmundsson T, Ghosh S, Brynjolfsson J, Gunnarsdottir S, Ivarsson O, Chou TT, Hjaltason O, Birgisdottir B, Jonsson H, Gudnadottir VG, Gudmundsdottir E, Bjornsson A, Ingvarsson B, Ingason A, Sigfusson S, Hardardottir H, Harvey RP, Lai D, Zhou M, Brunner D, Mutel V, Gonzalo A, Lemke G, Sainz J, Johannesson G, Andresson T, Gudbjartsson D, Manolescu A, Frigge ML, Gurney ME, Kong A, Gulcher JR, Petursson H, Stefansson K. Neuregulin 1 and susceptibility to schizophrenia. Am J Hum Genet. 2002;71:877–892. doi: 10.1086/342734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straub RE, Egan MF, Hashimoto R, Matsumoto M, Weickert CS, Goldberg TE, Callicott JH, Hyde TM, Kleinman JE, Weinberger DR. The schizophrenia susceptibility gene dysbindin (DTNBP1, 6p22.3): analysis of haplotypes, intermediate phenotypes and alternative transcripts. Biol Psychiatry. 2003;53 suppl.:167S–168S. [Google Scholar]
- Straub RE, McClintock BW, Halim ND, Lipska BK, Hyde TM, Herman MM, Weinberger DR, Kleinman JE, Weickert CS. Dysbindin protein is decreased in the dorsolateral prefrontal cortex of schizophrenia patients. Biol Psychiatry. 2004;55 suppl. 1:116S. [Google Scholar]
- Strohmaier J, Georgi A, Schirmbeck F, Schmael C, Muehleisen TW, Jamra RA, Schumacher J, Maier W, Propping P, Noethen MM, Cichon S, Schulze TG, Rietschel M. Association between dysbindin (DTNBP1) and cognitive performance in schizophrenia; World Congress on Psychiatric Genetics; New York, NY. 2007. (Abstract 120 from 2007) [Google Scholar]
- Stuchlik A, Rehakova L, Telensky P, Vales K. Morris water maze learning in Long-Evans rats is differentially affected by blockade of D1-like and D2-like dopamine receptors. Neurosci Lett. 2007;422:169–174. doi: 10.1016/j.neulet.2007.06.012. [DOI] [PubMed] [Google Scholar]
- Swank RT, Sweet HO, Davisson MT, Reddington M, Novak EK. Sandy: a new mouse model for platelet storage pool deficiency. Genet Res. 1991;58:51–62. doi: 10.1017/s0016672300029608. [DOI] [PubMed] [Google Scholar]
- Taiminen T, Jääskeläinen S, Ilonen T, Meyer H, Karlsson H, Lauerma H, Leinonen K-M, Wallenius E, Kaljonen A, Salokangas RKR. Habituation of the blink reflex in first-episode schizophrenia, psychotic depression and non-psychotic depression. Schizophr Res. 2000;44:69–79. doi: 10.1016/s0920-9964(99)00140-1. [DOI] [PubMed] [Google Scholar]
- Taepavarapruk P, Floresco SB, Phillips AG. Hyperlocomotion and increased dopamine efflux in rat nucleus accumbens evoked by electrical stimulation of the ventral subiculum: role of ionotropic glutamate and dopamine D1 receptors. Psychopharamacology. 2000;151:242–251. doi: 10.1007/s002130000376. [DOI] [PubMed] [Google Scholar]
- Talbot K, Cho D-S, Ong W-Y, Benson MA, Han L-Y, Kazi HA, Kamins J, Hahn C-G, Blake DJ, Arnold SE. Dysbindin-1 is a synaptic and microtubular protein that binds brain snapin. Hum Mol Genet. 2006;15:3041–3054. doi: 10.1093/hmg/ddl246. [DOI] [PubMed] [Google Scholar]
- Talbot K, Eidem WL, Tinsley CL, Benson MA, Thompson EW, Smith RJ, Hahn C-G, Siegel SJ, Trojanowski JQ, Gur RE, Blake DJ, Arnold SE. Dysbindin-1 is reduced in intrinsic, glutamatergic terminals of the hippocampal formation in schizophrenia. J Clin Invest. 2004;113:1353–1363. doi: 10.1172/JCI20425. [see comment] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbot K, Ong W-Y, Blake DJ, Tang J, Louneva N, Carlson GC, Arnold SE. Dysbindin-1 and its protein family with special attention to the potential role of dysbindin-1 in neuronal functions and the pathophysiology of schizophrenia. In: Javitt D, Kantorowtz J, editors. Handbook of Neurochemistry and Molecular Neurobiology. 3rd ed. Vol. 27. 2008. (in press) [Google Scholar]
- Tsien JZ, Huerta PT, Tonegawa S. The essential role of hippocampal CA1 NMDA receptor-dependent synaptic plasticity in spatial memory. Cell. 1996;87:1327–1338. doi: 10.1016/s0092-8674(00)81827-9. [DOI] [PubMed] [Google Scholar]
- Turetsky BI, Calkins ME, Light GA, Olincy A, Radant AD, Swerdlow NR. Neurophysiological endophenotypes of schizophrenia: the viability of selected candidate measures. Schizophr Bull. 2007;33:69–94. doi: 10.1093/schbul/sbl060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Groen T, Wyss JM. Extrinsic projections from area CA1 of the rat hippocampus: olfactory, cortical, subcortical, and bilateral hippocampal formation projections. J Comp Neurol. 1990;302:515–528. doi: 10.1002/cne.903020308. [DOI] [PubMed] [Google Scholar]
- Wetsel RA, Fleischer DT, Haviland DL. Deficiency of the murine fifth complement component (C5), a 2-base pair gene deletion in a 5’-exon. J Biol Chem. 1990;265:2435–2440. [PubMed] [Google Scholar]
- Wilkerson A, Levin ED. Ventral hippocampal dopamine D1 and D2 systems and spatial working memory in rats. Neuroscience. 1999;89:743–749. doi: 10.1016/s0306-4522(98)00346-7. [DOI] [PubMed] [Google Scholar]
- Williams NM, O’Donovan MC, Owen MJ. Is the dysbindin gene (DTNBP1) a susceptibility gene for schizophrenia? Schizophr Bull. 2005;31:800–805. doi: 10.1093/schbul/sbi061. [DOI] [PubMed] [Google Scholar]
- Yager JA, Ehmann TS. Untangling social function and social cognition: a review of concepts and measurement. Psychiatry. 2006;69:47–68. doi: 10.1521/psyc.2006.69.1.47. [DOI] [PubMed] [Google Scholar]
- Zinkstok JR, deWilde O, van Amelsvoort TAMJ, Tanck MW, Linszen DH. Association between the DTNBP1 gene and intelligence: a case-control study in young patients with schizophrenia and related disorders and unaffected siblings. Behav Brain Funct. 2007;3:19. doi: 10.1186/1744-9081-3-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zornoza T, Cano-Cebríaznq MJ, Miquel M, Aragón C, Polache A, Granero L. Hippocampal dopamine receptors modulate the motor activation and the increase in dopamine levels in the rat nucleus accumbens evoked by chemical stimulation of the ventral hippocampus. Neuropsychopharamcology. 2005;30:843–852. doi: 10.1038/sj.npp.1300618. [DOI] [PubMed] [Google Scholar]