Abstract
Activation of G protein-gated inwardly-rectifying K+ (GIRK or Kir3) channels by metabotropic gamma-aminobutyric acid (B) (GABAB) receptors is an essential signalling pathway controlling neuronal excitability and synaptic transmission in the brain. To investigate the relationship between GIRK channel subunits and GABAB receptors in cerebellar Purkinje cells at post- and pre-synaptic sites, we used biochemical, functional and immunohistochemical techniques. Co-immunoprecipitation analysis demonstrated that GIRK subunits are co-assembled with GABAB receptors in the cerebellum. Immunoelectron microscopy showed that the subunit composition of GIRK channels in Purkinje cell spines is compartment-dependent. Thus, at extrasynaptic sites GIRK channels are formed by GIRK1/GIRK2/GIRK3, postsynaptic densities contain GIRK2/GIRK3 and dendritic shafts contain GIRK1/GIRK3. The postsynaptic association of GIRK subunits with GABAB receptors in Purkinje cells is supported by the subcellular regulation of the ion channel and the receptor in mutant mice. At presynaptic sites, GIRK channels localized to parallel fibre terminals are formed by GIRK1/GIRK2/GIRK3 and co-localize with GABAB receptors. Consistent with this morphological evidence we demonstrate their functional interaction at axon terminals in the cerebellum by showing that GIRK channels play a role in the inhibition of glutamate release by GABAB receptors. The association of GIRK channels and GABAB receptors with excitatory synapses at both post- and presynaptic sites indicates their intimate involvement in the modulation of glutamatergic neurotransmission in the cerebellum.
Keywords: potassium channels, immunohistochemistry, subunit composition, electron microscopy, cerebellum, glutamate release
Introduction
G protein-coupled signalling is a major cellular mechanism for controlling excitability in central neurons. One of the main targets for this control at postsynaptic membranes are G protein-gated inwardly rectifying K+ (GIRK or Kir3) channels, which generate slow inhibitory postsynaptic potentials following the activation of Gi/o-protein-coupled receptors (North, 1989). Therefore, a major role of the GIRK channels is to mediate inhibitory responses in the nervous system by decreasing neuronal excitability. While the effect of postsynaptic GIRK channels has been clearly demonstrated, they can also play a role at presynaptic sites (Yum et al. 2008), which is supported by their consistent localization in axon terminals (Morishige et al. 1996; Ponce et al. 1996; Kulik et al. 2006; Koyrakh et al. 2005; Marker et al. 2005; Aguado et al. 2008). A major G protein-coupled receptor activating postsynaptic GIRK channels is the metabotropic γ-aminobutyric acid (B) (GABAB) receptor (Lüscher et al. 1997). Although electrophysiological studies do not support a role for a presynaptic GIRK activation as a primary mechanism by which GABAB receptors modulate neurotransmitter release (Lüscher et al. 1997), we recently reported that GIRK channel-mediated inhibition of glutamate release occurs through GABAB receptors in the cerebral cortex (Ladera et al. 2008).
Four different cDNAs encode GIRK channel subunits in mammals (GIRK1–4) and they combine to form functional homomeric as well as heteromeric channels (Dascal, 1997; Wickman et al. 2002). In central neurons most neuronal GIRK channels are heteromeric complexes containing both GIRK1 and GIRK2 (Liao et al. 1996; Luscher et al. 1997; Koyrakh et al. 2005; Marker et al. 2005). However, recent studies suggest that many GIRK channel subtypes with different subunit combinations exist in the CNS (Labouèbe et al. 2007; Aguado et al. 2008; Perry et al. 2008). This large molecular diversity is best illustrated in the cerebellum, among the brain regions with the highest density of GIRK channels (Karschin et al. 1996; Liao et al. 1996; Aguado et al. 2008), where there are seven distinct GIRK subunit patterns that are expressed in a cell type specific manner (Aguado et al. 2008).
GIRK1, GIRK2 and GIRK3 are all expressed in granule cells and Purkinje cells (PC) but at very different levels (Aguado et al. 2008). Cerebellar PCs also express a high density of GABAB receptors, which are mainly localized at postsynaptic sites, but a small fraction is found presynaptically at parallel fibre terminals (Kulik et al. 2003; Luján & Shigemoto, 2006). Although postsynaptic GABAB-activated GIRK currents can be recorded in PCs (Tabata et al. 2005), the GIRK subunit combinations underlying these current are unknown. We now used molecular, functional and morphological techniques to show that GIRK channels subtypes are distributed in a compartment-dependent manner in PCs and that presynaptic GABAB-activated GIRK channels influence transmitter release from parallel fibre terminals.
Material and Methods
Animals
All animal use was approved by the Institutional Animal Care and Use Committee of the University of Castilla-La Mancha and followed Spanish and European Union regulations (86/609/CEE). Efforts were made to minimize the pain and discomfort of the animals throughout the study. The generation of GIRK knockout (KO) mice was described previously (Signorini et al. 1997; Wickman et al. 1998; Bettahi et al. 2002; Torrecilla et al. 2002); the null Girk mutations (created using 129/Sv ES cells) have all been backcrossed for more than 17 rounds against the C57BL6/J strain. The generation of GABAB1 KO mice was described previously (Schuler et al. 2001); GABAB mutant mice are kept on a pure Balb/c genetic background. Age- and sex-matched wild-type (WT) mice were obtained from Charles River Laboratories (Barcelona, Spain) and they were housed on a 12 h light/dark cycle, with food and water available ad libitum.
Synaptosomal Preparation and glutamate release
Synaptosomes from mice cerebella were purified on discontinuous Percoll gradients and glutamate release was assayed by on-line fluorimetry as described previously (Millán et al. 2002) (Supplementary information).
Antibodies
A complete list of the primary and secondary antibodies, including their source, dilution and combined use, used for this study is given in Table 1 (Supplementary information).
Immunoblotting and immunoprecipitation
Protein expression of GIRK subunits and GABAB receptors was analyzed by immunoblotting analysis of cerebellum homogenates. Immunoblotting and densitometric analysis were performed as described (Bettahi et al. 2002) (Supplementary information). For immunoprecipitation, membranes from cerebellum were obtained as described (Ciruela et al. 2001; Burgueño et al. 2003) (Supplementary information).
Immunohistochemistry for electron microscopy
Electron microscopic examination of immunoreactivity for GIRK subunits and GABAB receptors in the cerebellum of WT and GIRK KO mice was performed as described using the pre-embedding immunoperoxidase and immunogold method, and the post-embedding immunogold method (Luján et al. 1996) (Supplementary information).
Quantitative analysis at EM level
To establish the relative abundance of GIRK1, GIRK2 and GIRK3 immunoreactivity in PC dendritic spines, quantification of immunolabeling was performed in the molecular layer of the cerebellum from 60 µm coronal slices as described (Luján et al. 1996) (Supplementary information).
Controls
To test method specificity in the procedures for both light and electron microscopy primary antibodies were either omitted or replaced with 5% (v/v) normal serum of the species of the primary antibody. Under these conditions, no selective labelling was observed. When double labelling for electron microscopy was carried out, some sections were always incubated with only one primary antibody and the full complement of secondary antibodies to test for any cross-reactivity. Other sections were incubated with two primary antibodies and one secondary antibody, followed by the full sequence of signal detection. No cross-labelling was detected that would influence the results.
Results
Interaction of GABAB receptors and GIRK subunits in cerebellar membranes
To determine whether GABAB receptors and GIRK channels are co-assembled in the cerebellum immunoprecipitation experiments were performed. Using GIRK1, GIRK2 or GIRK3 specific antibodies for immunoprecipitations from cerebellar extracts, followed by Western blotting, demonstrated that all three subunits were co-immunoprecipitated (Fig. 1A). In addition, all three anti-GIRK antibodies co-immunoprecipitated the GABAB2 receptor (∼ 100 kDa) from the same extracts (Fig. 1A). An anti-GABAB2 antibody immunoprecipitated all three GIRK proteins (not shown). These results indicate that GIRK channels containing all GIRK1, GIRK2, and GIRK3 are expressed in cerebellum where they associate with GABAB receptors.
Figure 1. Co-immunoprecipitation of GABAB receptors and GIRK and impact of their genetic ablation in the cerebellum.
(A) Membranes from cerebellum were solubilised and processed for immunoprecipitation using rabbit anti-FLAG polyclonal antibody as a control IgG (4 µg/ml), rabbit anti-GIRK1 polyclonal antibody (4 µg/ml), rabbit anti-GIRK2 polyclonal antibody (4 µg/ml), rabbit anti-GIRK3 polyclonal antibody (4 µg/ml) or guinea-pig anti-GABAB2 polyclonal antibody (4 µg/ml). Immunoprecipitates were analyzed by SDS-PAGE and immunoblotted using rabbit anti-GIRK1 (1/1000), rabbit anti-GIRK2 whole serum (1/1000), rabbit anti-GIRK3 whole serum (1/1000) or guinea-pig anti-GABAB2 polyclonal antibody (1/1000) and HRP-conjugated anti-rabbit IgG TrueBlot™ (1/1000) or goat anti-guinea-pig IgG (1/10000) as a secondary antibody. These blots are representative of four different experiments with similar qualitative results. (B) Representative immunoblots of cerebellar membrane protein samples from WT and GABAB1 (GB1) KO mice. For all the blots, 20 µg of membrane protein was loaded. Blots were probed with antibodies (Abs) for GIRK1, GIRK2, GIRK3 and GABAB1. Immunoreactivity for GABAB1 was absent in the samples from GABAB1 KO. (C) Densitometric analysis of the impact of GABAB1 ablation on GIRK protein levels in the cerebellum. There was no significant effect of GABAB1 ablation on GIRK1 or GIRK2 expression levels. However, we observed a significant up-regulation of GIRK3 after ablation of GABAB1 (*p<0.01). (D) Representative immunoblots of cerebellar membrane protein samples from WT, GIRK1 (G1), GIRK2 (G2), GIRK3 (G3), and GIRK2/GIRK3 (G2/3) KO mice. Blots were probed with antibodies (Abs) for GABAB1. GABAB immunoreactivity was observed as two prominent bands at ∼140 and 100 kDA, corresponding to 1a and 1b splice variants of GABAB1 subunit, respectively. The level of GABAB1 was similar in samples from GIRK KO mice. (E) Densitometric analysis of the impact of GIRK subunit ablation on GABAB1 protein levels in the cerebellum. There was no significant effect of GIRK subunit ablation GABAB1 receptor levels. However, we detected a tendency in the up-regulation of GABAB1 receptor levels in GIRK3 KO mice as compared to WT.
Regulation of GIRK and GABAB in the cerebellum of mutant mice
We next examined if the expression of GIRK channel subunits and GABAB receptors is altered in the GABAB1 and GIRK KO mice, respectively, reasoning that the loss of one protein would affect the expression and/or turnover of the interacting proteins because of the lack of cross-stabilization. The levels of GIRK1 and GIRK2 subunits were not altered in the cerebellum of GABAB1 KO mice (Fig. 1B–C), as assessed by quantitative immunoblotting. In contrast, GIRK3 subunit expression was significantly increased (24.4% ± 6.7% increase, p<0.01) (Fig. 1C). While the level of GABAB receptors in the cerebellum was not significantly altered by the loss of any GIRK subunit (Fig. 1D–E), we did detect a tendency toward up-regulation, which was particularly prominent in the GIRK3 KO mice (Fig. 1E).
GIRK subunits are preferentially localized to extrasynaptic membranes of PC spines
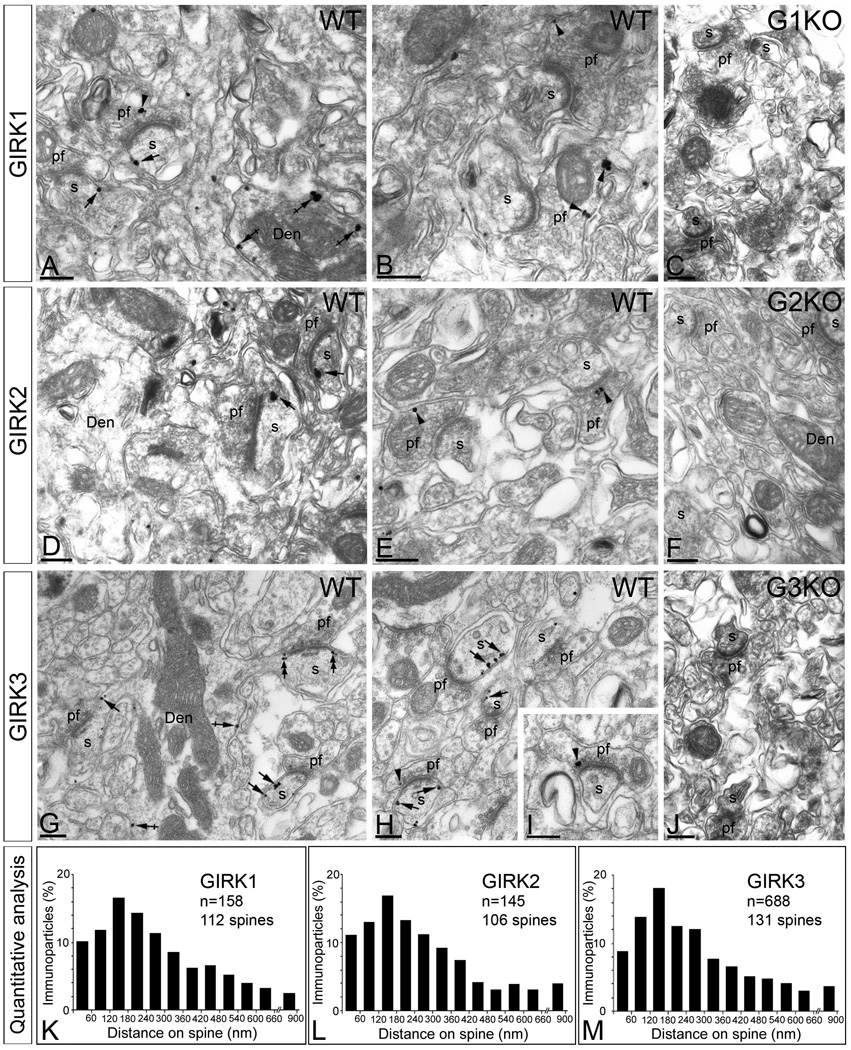
To investigate the subcellular localization of GIRK subunits in PCs, we used pre-embedding immunoperoxidase and immunogold methods for electron microscopy (Fig. 2 and Suppl. Fig. 1). Immunoreactivity for GIRK1, GIRK2, and GIRK3 was found along the plasma membrane (64% of GIRK1, n=216; 67% of GIRK2, n=178; and 58% of GIRK3, n=1013) and also associated with the endoplasmic reticulum (ER) cisterna of dendritic shafts and the spine apparatus (intracellular labelling: 36% of GIRK1, n=121; 33% of GIRK2, n=88; and 42% of GIRK3, n=738) (Fig. 2). Of the immunoparticles found in the plasma membrane, most (83% of GIRK1, n=179; 81% of GIRK2, n=145; and 72% of GIRK3, n=730) were found in postsynaptic compartments, mainly in dendritic spines of PCs where they were distributed along the extrasynaptic plasma membrane or at the edge of asymmetrical synapses established by parallel fibre terminals with PC spines (Fig. 2A, D, G and F).
Figure 2. Subcellular localization of GIRK subunits in Purkinje cells.
Electron micrographs show immunolabelling for GIRK1, GIRK2 and GIRK3 in the molecular layer of the cerebellum of WT mice. (A–B) Few immunoparticles for GIRK1 were detected along the extrasynaptic plasma membrane of PC spines (s) (arrows) and dendritic shafts (Den) (crossed arrows), as well as at presynaptic sites in parallel fibre terminals (pf) (arrowheads). (D–E) Few immunoparticles for GIRK2 were detected along the extrasynaptic plasma membrane of PC spines (s) (arrows) but not in dendritic shafts (Den), as well as at presynaptic sites in parallel fibre terminals (pf) (arrowheads). (G–H) A large density of immunoparticles for GIRK3 was detected along the extrasynaptic plasma membrane of PC spines (s) (arrows) and dendritic shafts (Den) (crossed arrows), as well as at presynaptic sites in parallel fibre terminals (pf) (arrowheads). (C,F,J) Immunoreactivity for GIRK1, GIRK2 and GIRK3 was totally absent in the corresponding GIRK KO mice. (K,L,M) Distribution of immunoreactive GIRK1, GIRK2 and GIRK3 in relation to glutamate release sites in PC dendritic spines, as assessed from immunogold reactions. Immunoparticles were recorded in 60-nm-wide bins along the extrasynaptic plasma membrane of PC spines. Data are expressed as the proportion of immunoparticles at a given distance from the edge of the synaptic specialization. The measurements show that the three GIRK subunits are distributed in virtually the same way along the extrasynaptic plasma membrane of PC spines. Scale bars, 0.2 µm.
Immunolabelling for GIRK1 and GIRK2 was significantly lower than immunolabelling for GIRK3, suggesting that GIRK3 is the predominant GIRK subunit in PC spines. Much less GIRK immunolabelling was seen in PC dendritic shafts (Fig. 2A, D and G). Interestingly, immunoparticles for GIRK1 and GIRK3 (GIRK1, n = 21; GIRK3, n = 42) (Fig. 2), but not GIRK2 (n = 0) (Fig. 2), were found in PC dendritic shafts (n = 25 dendritic shafts analysed; GIRK1, 15 out of 25; GIRK3, 21 out of 25), suggesting that spine GIRK channels may contain GIRK1, 2, and 3 while GIRK channels in dendritic shafts of PCs contain only GIRK1 and GIRK3. Furthermore, we found significant labelling for GIRK1 (17%; n=37), GIRK2 (19%; n=34), and GIRK3 (28%; n=283) in presynaptic terminals of parallel fibres (Fig. 2A, B, E, H and I). In these terminals, immunoparticles for the three GIRK subunits were localized to the extrasynaptic plasma membrane as well as the active zone (Fig. 2A, B, E, H and I). The specificity of the immunolabelling for GIRK1, GIRK2, and GIRK3 in pre-embedding material at EM level was confirmed by the absence of labelling in the corresponding GIRK KO mice (Fig. 2C, F and J).
Given the similar subcellular localization observed for the three neuronal GIRK subunits in PCs, we investigated whether the loss of one GIRK subunit alters the subcellular localizations of other subunits. We found that immunoreactivity for GIRK subunits were reduced in sections from GIRK KO mice (Suppl. Fig. 2).
Enrichment of GIRK subunits around excitatory synapses
The subcellular distribution patterns for GIRK1, GIRK2 and GIRK3 in PCs, particularly the strong labelling for GIRK3 on dendritic spines, is very similar to that of GABAB receptors (Kulik et al. 2003; Luján & Shigemoto, 2006). To compare the distribution of the GIRK subunits and GABAB1 on PC spines in relation to glutamate release sites, we analyzed the position of immunoparticles (GIRK1, n = 158 on 112 spines; GIRK2, n=145 on 106 spines; and GIRK3, n=688 on 131 spines) in relation to the closest edge of the postsynaptic membrane specialization. This analysis revealed that GIRK1, GIRK2, GIRK3 and GABAB1 share the same subcellular distribution in PC spines (Fig. 2K, L and M), peaking between 0 and 300 nm from PC spine-parallel fibre synapses (Fig. 2K, L and M). Altogether, we found that nearly 85% of the immunoparticles for GIRK channel subunits and GABAB receptors were located within a distance of 300 nm from the edge of parallel fibre synapses indicating an enrichment of the metabotropic receptor and the effector K+ channel in the vicinity of parallel fibre synapses on PC spines.
Synaptic localization of GIRK subunits in PCs
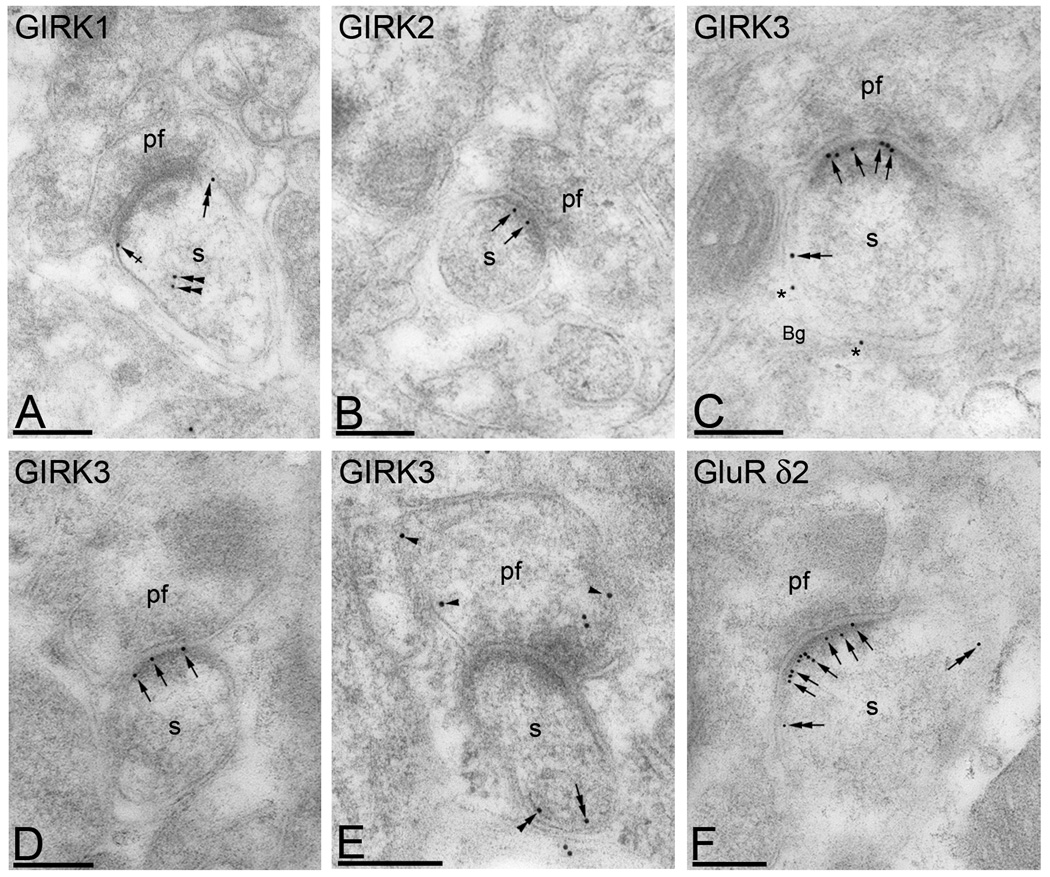
Previous immunoelectron microscopy studies (Kulik et al. 2003) showed that GABAB receptors are localized along the postsynaptic densities spines at parallel fibre-PC synapses. Therefore, we further investigated the distribution of GIRK subunits within synaptic sites using post-embedding immunogold, a method that allows resolution of synaptic versus extrasynaptic localization. We found that GIRK1 immunoparticles were never detected in the PSD (Fig. 3A), whereas GIRK2 and GIRK3 labelling was often seen in the PSD of asymmetrical synapses established by parallel fibre terminals with dendritic spines of PCs (Fig. 3B, C and D). Immunoparticles for GIRK1 were only detected at the edge of the postsynaptic membrane specialization, along the extrasynaptic plasma membrane of PC spines or inside the spine (Fig. 3A), suggesting a subsynaptic segregation of GIRK channel subunits. Consistent with the data obtained using the pre-embedding immunogold method, immunoparticles for GIRK3 were also detected in Bergmann glia cells (Fig. 3C) and at presynaptic sites in parallel fibre axon terminal (Fig. 3E).
Figure 3. Subsynaptic localization of GIRK subunits in Purkinje cells.
Electron micrographs show immunolabelling for GIRK1, GIRK2 and GIRK3 in cerebellar PCs using a post-embedding immunogold method. (A) Immunoparticles for GIRK1 were never detected in PSDs of PC spines (s), but rather at perisynaptic (crossed arrows) or extrasynaptic sites (double arrow), as well as at intracellular sites (double arrows). (C–E) Immunoparticles for GIRK2 and GIRK3 were observed in the PSD of PC spines (s) (arrows) establishing asymmetrical synapses with parallel fibre terminals (pf), although immunoreactivity in this sub-compartment was higher for GIRK3. Immunoparticles for GIRK3 were also observed along the extrasynaptic plasma membrane of PC spines (s) (double arrow), in Bergmann glia cells (bg) (asterisks), at intracellular sites (double arrowheads), and at presynaptic sites in parallel fibre terminals (pf) (arrowheads). (F) As control of the method we used anti-GluRδ2 subunit, which was predominantly located at PSD of PC spines (s) (double arrows), as well as along the extrasynaptic plasma membrane (double arrows). Scale bars, 0.2 µm.
Subcellular regulation of GIRK and GABAB in mutant mice
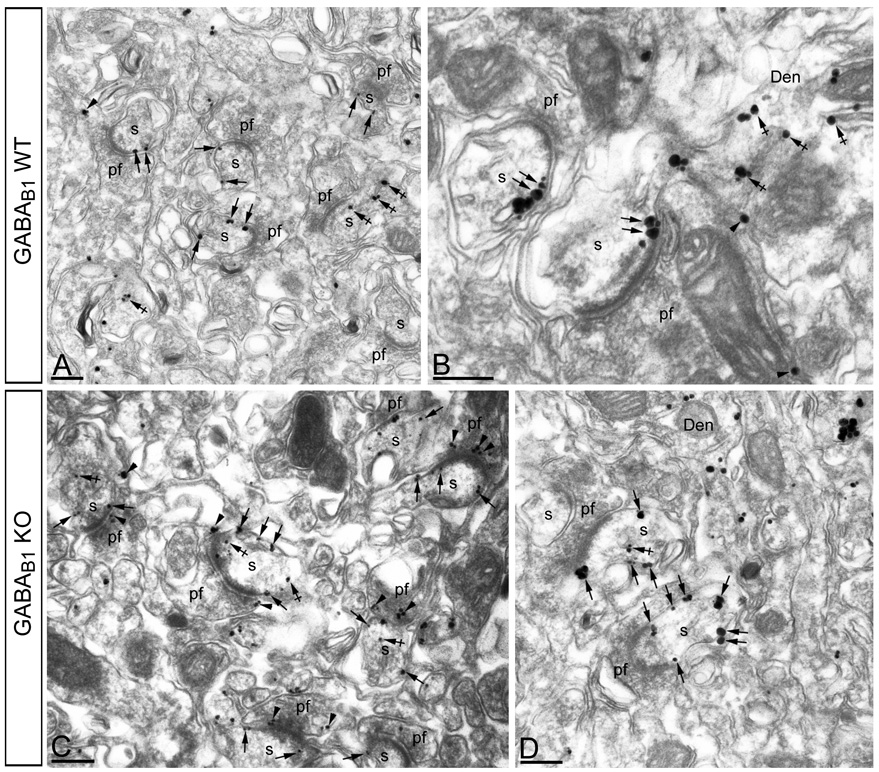
Given the association of GABAB receptors and GIRK subunits in PC spines and the up-regulation of the GIRK3 subunit in the cerebellum of GABAB1 KO, we further investigated this regulation at the subcellular level. Using pre-embedding immunogold, we found in GABAB1 KO mice, immunoparticles for GIRK3 were more frequently observed along the plasma membrane of PC spines, as well as at intracellular and presynaptic sites (Fig. 4B–C), compared to the WT (Fig. 4A–B). Quantitative analysis showed that both the mean number of GIRK3 immunoparticles at postsynaptic sites (1.8 ± 0.8 particles/spine in the WT vs. 3.1 ± 1.2 particles/spine in the GABAB1 KO; P <0,001) and presynaptic sites (1.3 ± 0.4 particles/parallel fibre terminal in the WT vs. 2.2 ± 1.0 particles/parallel fibre terminal in the GABAB1; P <0,001) were higher in the GABAB1 KO mice.
Figure 4. Subcellular regulation of GIRK3 in GABAB1 KO mice.
Electron micrographs show the subcellular localization of GIRK3 in WT GABAB1 KO mice, as revealed using a pre-embedding immunogold method. (A–B) In the WT, immunoparticles for GIRK3 were mainly localized along the perisynaptic and extrasynaptic plasma membrane of PC spines (s) (arrows), as well as at intracellular sites (crossed arrows) in spines (s) and dendrites (Den). At the presynaptic level, few immunoparticles for GIRK3 were found in parallel fibre terminals (pf) (arrowheads). (C–D) In the GABAB1 KO cerebellum, immunoparticles for GIRK3 were more frequently observed also localised along the extrasynaptic plasma membrane of PC spines (s) (arrows), as well as at presynaptic sites in parallel fibre terminals (pf) (arrowheads). Scale bars, 0.2 µm.
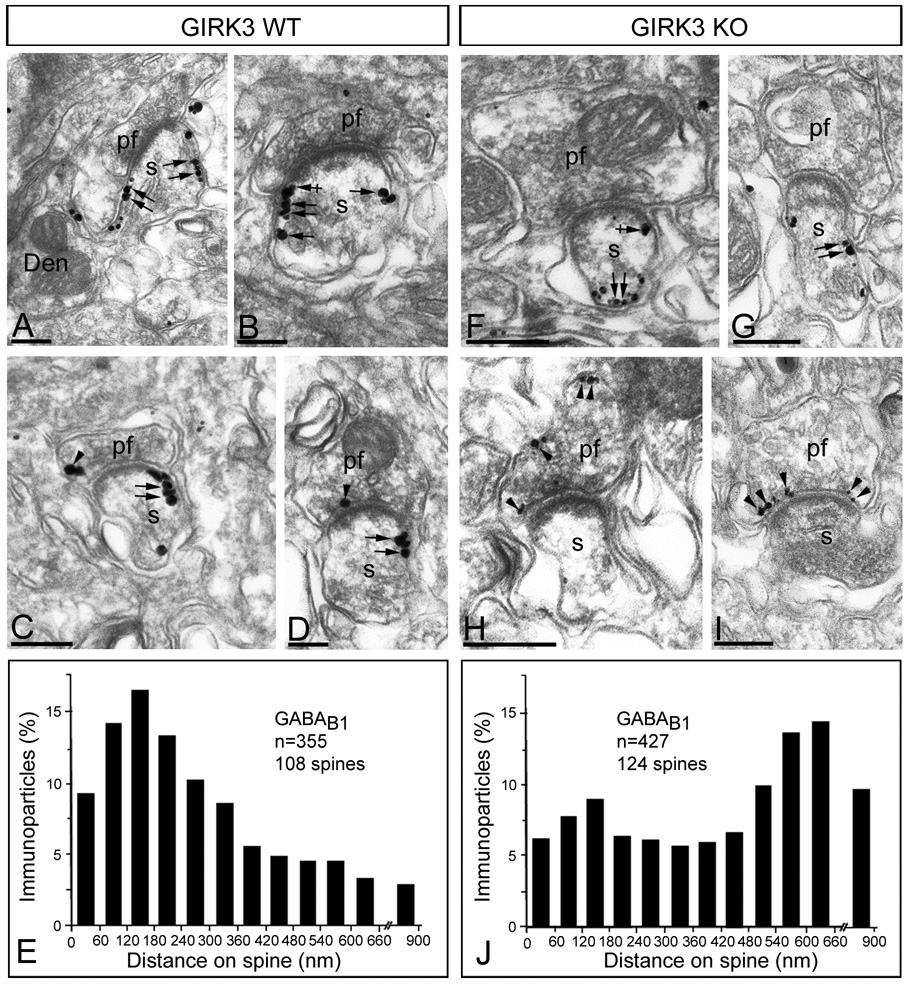
The subcellular distribution of GABAB1 seen in sections from GIRK1 KO and GIRK2 KO mice were indistinguishable from that seen in WT sections (data not shown). However, we detected significant differences in sections from GIRK3 KO mice (Fig. 5). In WT, the highest density of immunoparticles for GABAB1 was found on the perisynaptic and extrasynaptic plasma membrane of PC dendritic spines (Fig. 5A and B; see Luján & Shigemoto, 2006). At the presynaptic level, low immunolabelling for GABAB1 was identifiable in parallel fibre terminals (Fig. 5C and D). In the GIRK3 KO, immunoparticles for GABAB1 were found on the extrasynaptic plasma membrane of PC dendritic spines, but most of them were mainly located away from synaptic sites (Fig. 5F and G). This redistribution along the extrasynaptic plasma membrane of PC spines was demonstrated using quantitative approaches by analysing the position of immunoparticles (GABAB1, n = 355 on 108 spines in the WT; GABAB1, n = 427 on 124 spines in the GIRK3 KO) in relation to the closest edge of the postsynaptic membrane specialization (Fig. 5E and J). Interestingly, immunoparticles for GABAB1 were more frequently observed at presynaptic sites in parallel fibre terminals (Fig. 5H and I) (mean number: 1.2 ± 0.4 particles/parallel fibre terminal in the WT vs. 2.7 ± 1.2 particles/parallel fibre terminal in the GIRK3 KO; P = <0,001). Furthermore, there was a 65% increase of parallel fibre synapses labelled for GABAB1 (20% synapses in the WT vs. 33% synapses in the GIRK3 KO).
Figure 5. Subcellular regulation of GABAB receptors in GIRK3 KO mice.
Electron micrographs show the subcellular localization of GABAB1 in WT and GIRK3 KO mice, as revealed using a pre-embedding immunogold method. (A–D) In the WT, immunoparticles for GABAB1 were mainly localized along the extrasynaptic plasma membrane of PC spines (s) (arrows), but close to the glutamate release site, as well as at the edge of PSDs (crossed arrows) of PC spines (s). At the presynaptic level, few immunoparticles for GABAB1 was found in parallel fibre terminals (pf) (arrowheads). (F–I) In the GIRK3 KO cerebellum, immunoparticles for GABAB1 were also localized along the extrasynaptic plasma membrane of PC spines (s) (arrows), although most of them far away from the glutamate release site. At presynaptic sites, an increase in the number of immunoparticles for GABAB1 was detected in parallel fibre terminals (pf) (arrowheads). (E,J) Distribution of immunoreactive GABAB1 in relation to glutamate release sites in PC dendritic spines of WT and GIRK3 KO mice, respectively, as assessed from immunogold reactions. Immunoparticles were recorded in 60-nm-wide bins along the extrasynaptic plasma membrane of PC spines. Data are expressed as the proportion of immunoparticles at a given distance from the edge of the synaptic specialization. The measurements show that GABAB1 is redistributed along the extrasynaptic plasma membrane of PC spines in the GIRK3 KO mice. Scale bars, 0.2 µm.
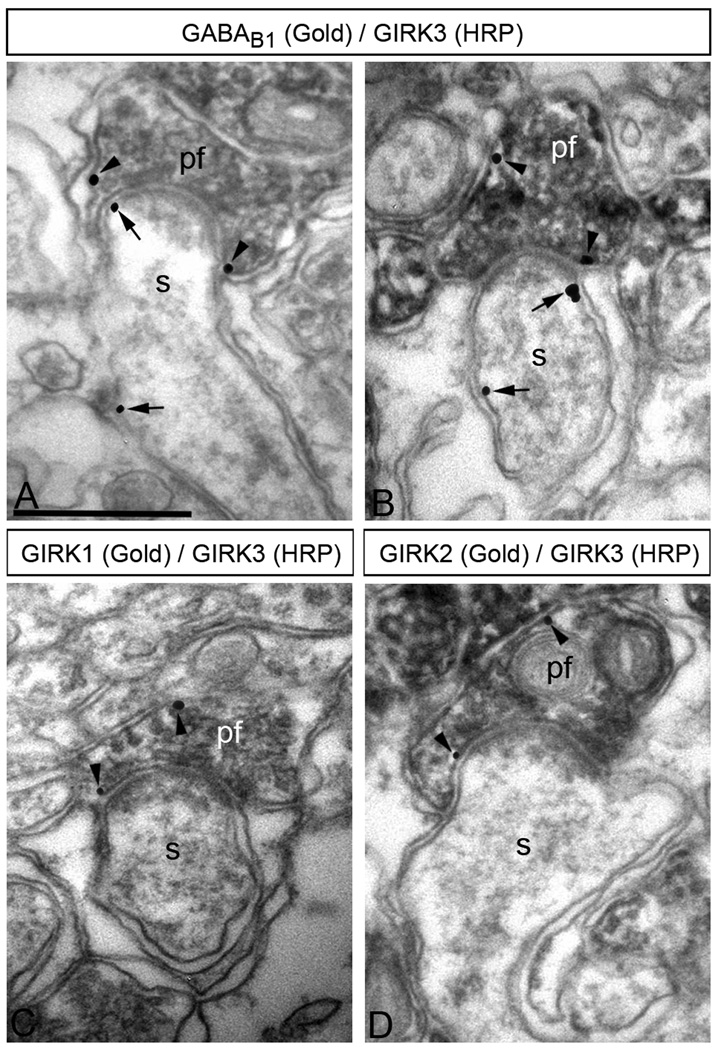
We next investigated the possibility that GIRK channel subunits and GABAB receptors are present in the same axon terminals by immunoelectron microscopy (Fig. 6). Using double labelling pre-embedding techniques we detected co-localization of GABAB1 with GIRK3 (Fig. 6A–B), as well as co-localization of GIRK1 with GIRK3, and of GIRK2 with GIRK3 (Fig. 6C–D) in the same parallel fibre terminals. Essentially all parallel fibre terminals that GIRK1 (61 out of 63) or GIRK2 (58 out of 59) also expressed GIRK3, and most axon terminals containing GABAB1 also expressed GIRK3 (104 out of 111).
Figure 6. GABAB receptors co-localize with GIRK subunits in parallel fibre terminals.
Electron micrographs show co-localization of GABAB1 and GIRK3 (A,B), as well as co-localization of GIRK1 or GIRK2 with GIRK3 (C, D), in the same parallel fibre terminal, as revealed using double-labelling pre-embedding methods. (A–B) The peroxidase reaction product (HRP) indicating GIRK3 immunoreactivity filled parallel fibre terminals (pf), whereas immunoparticles (GABAB1 immunoreactivity) were mainly located along the extrasynaptic plasma membrane, and occasionally in the presynaptic membrane specialization of parallel fibre terminals (pf) establishing excitatory synapses on PC spines (s) (arrows). (C,D) The peroxidase reaction product (GIRK3 immunoreactivity) filled parallel fibre terminals (pf), whereas immunoparticles (GIRK1 immunoreactivity or GIRK2 immunoreactivity) were mainly located along the extrasynaptic plasma membrane, and occasionally in the presynaptic membrane specialization of parallel fibre terminals (pf) establishing putative excitatory synapses on PC spines (s) (arrows). Scale bars, 0.5 µm.
Altogether, our ultrastructural analyses showing subcellular regulation of GIRK3 and GABAB1 in cerebellar PCs of GABAB1 KO and GIRK3 KO mice, respectively, is consistent with the idea that GABAB receptors may functionally interact with GIRK3-containing channels in PC spines and parallel fibre axon terminals.
GABAB and GIRK-mediated inhibition of glutamate release dependent
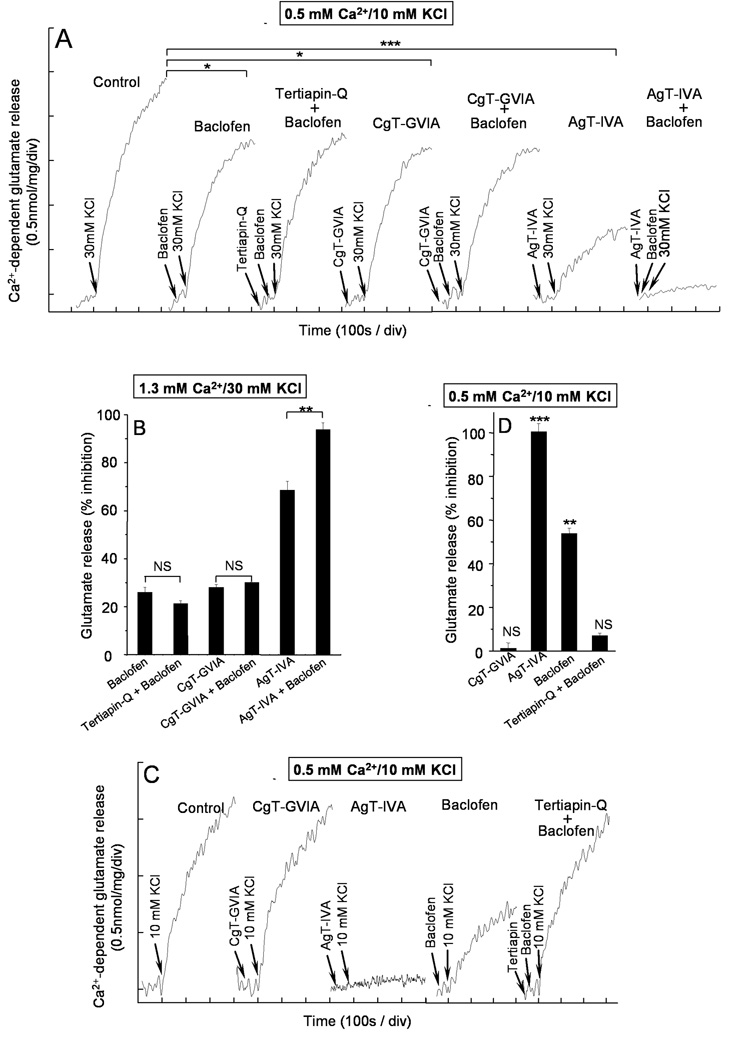
Next, we used synaptosome assays to probe the functional coupling between GABAB receptors and GIRK subunits at presynaptic sites. Depolarization of nerve terminals with 30 mM KCl opens voltage-dependent Ca2+ channels and initiates neurotransmitter release (Barrie et al. 1991). At cerebellar nerve terminals 30 mM KCl-induced release (2.3 ± 0.2 nmol glutamate/mg of protein (n=4) was reduced with the GABAB receptor agonist baclofen (26.0 ± 2.1%, p<0.05, n=4) (Fig 7A and B), while tertiapin-Q, which blocks GIRK channels, did not reverse the baclofen effect (21.3 ± 5.0%, p>0.05, n=4), indicating that GIRK channels do not mediate the inhibition of release by baclofen under 30 mM KCl stimulation conditions. As GABAB receptors reduce synaptic transmission by inhibiting presynaptic Ca2+ channels (Dittman and Regher, 1996; Isaacson and Hille, 1997; Takahashi et al. 1998) we sought to establish which type of Ca2+ channel was inhibited by GABAB receptors, by performing occlusion experiments with the Ca2+ channel antagonists ω-CgTx-GVIA and ω-Aga-IVA which selectively blocks N and P/Q channels, respectively (Mintz et al. 1995; Olivera et al. 1985). The blockage of N-type Ca2+ channels with 2µM ω-CTx-GVIA reduced glutamate release (28.1 ± 1.2%, p<0.05, n=3) and largely occluded a further inhibition of release by baclofen (30.1 ± 2.0%, p>0.05, n=3) (Fig. 7A and B). In contrast, blocking P/Q-type Ca2+ channels with 200 nM ω-Aga-IVA strongly reduced glutamate release (68.5 ± 3.7%, p<0.001, n=4) but still allowed further inhibition by baclofen (93.7 ± 2.9%, p<0.01, n=4) (Fig. 7A and C). Thus, GABAB receptor-dependent inhibition of release evoked by 30 mM KCl largely relies on N-type Ca2+ channels.
Figure 7. GIRK-channel dependent inhibition of release by GABAB receptors.
The Ca2+ dependent release was calculated by subtracting the release obtained during a 5 min period of depolarization at 200 nM free [Ca2+] from release at 1.33 mM CaCl2. The Ca2+-dependent release of glutamate was evoked by 30 mM KCl at 1.3 mM CaCl2 (A) or by 10 mM KCl at 0.5 mM CaCl2 (C) in the absence (control) and presence of agonists and antagonists added at the indicated times prior to depolarization (arrows). Baclofen (20µM, 40s); tertiapin-Q (100 nM, 60s); ω-CgTx-GVIA (2µM, 100s) and ω-Aga-IVA (200 nM, 100s). B and D histograms show the % inhibition of Ca2+-dependent release after a 5 min depolarization under the aforementioned conditions, both in the presence of 30 mM KCl plus 1.3 mM CaCl2 (B), or 10 mM KCl plus 0.5 mM CaCl2 (D). Data represent the mean ± SEM (n=3–5). NSp>0.05; *p<0.05; **p<0.01; ***p<0.001 (students t-test) when compared with control values, unless indicated otherwise.
The opening of GIRK channels by GABAB receptors is expected to hyperpolarize nerve terminals and to reduce their excitability. The finding that stimulations with 30 mM KCl failed to uncover a role for GIRK channels suggest either that GIRK channels have no role in release modulation, or alternatively, that the extent of depolarization by 30 mM KCl is large enough to activate all nerve terminals, including those hyperpolarized by the GABAB receptor-GIRK signalling system. For this reason, we used a submaximal (10 mM KCl) concentration, a condition in which hyperpolarized nerve terminals are expected not to activate. In addition, we isolated the release component coupled to P/Q-type Ca2+ to avoid the effect of baclofen on release supported by N-type Ca2+ channels. Because P/Q channels exhibit improved coupling to exocytosis when compared to N-type channels (Millán and Sánchez-Prieto, 2002; Millán et al. 2003), decreasing [Ca2+]o from 1.3 to 0.5 mM selectively eliminated the release mediated by N-type Ca2+ channels (1.1 ± 2.7%, p>0.05, n=3) making the remaining release fully governed by P/Q-type Ca2+ channels (100.4 ± 3.9%, p<0.001, n=3) (Fig 7C and D). Low KCl- induced release (1.60 ± 0.15, nmol of glutamate/mg of protein, n=5) was reduced by the GABAB receptor agonist baclofen (53.8 ± 2.6%, p<0.01, n=5). Interestingly, the GIRK channels blocker tertiapin-Q, which have no effect on control release (101.0 ± 2%, p>0.05, n=5) in the absence of baclofen, largely prevented the inhibition of release by baclofen (7.0 ± 1.2%, p>0.05, n=5) (Fig. 7C and D) suggesting a role for GIRK channels in the actions of GABAB receptors at P/Q-type nerve terminals.
Discussion
The present study describes the anatomical and functional coupling of GIRK channel subunits with GABAB receptors and their spatial relationship on cerebellar PCs at postsynaptic and parallel fibre presynaptic sites. GIRK1, 2 and 3 are primarily found postsynaptically and are localized to dendritic spines of PCs, where they can form at least two channel complexes in a subcellular compartment-dependent manner. At extrasynaptic sites GIRK1, GIRK2 and GIRK3 are coexpressed suggesting that they form heterotetrameric complexes. This is supported by the subcellular regulation of the GIRK subunits in KO mice. In postsynaptic densities GIRK channels are likely formed by the combination of GIRK2 and GIRK3 subunits. Dendritic shafts of PCs express only GIRK1 and GIRK3, while Bergmann glia that ensheath parallel fibre-PC synapses contain only GIRK3. At presynaptic sites, the GIRK channel found in parallel fibre terminals is likely formed by GIRK1, 2, and 3 and co-localizes with GABAB receptors. Consistent with this morphological evidence, we demonstrate their functional interaction in a subpopulation of axon terminals in the cerebellum by showing that GIRK channels play a role in the inhibition of glutamate release by GABAB receptors. Thus, the observed close spatial relationship of the three GIRK channel subunits and GABAB receptors at pre- and postsynaptic sites reflects their functional interaction in PC dendritic spines contacted by parallel fibres (Fig. 8).
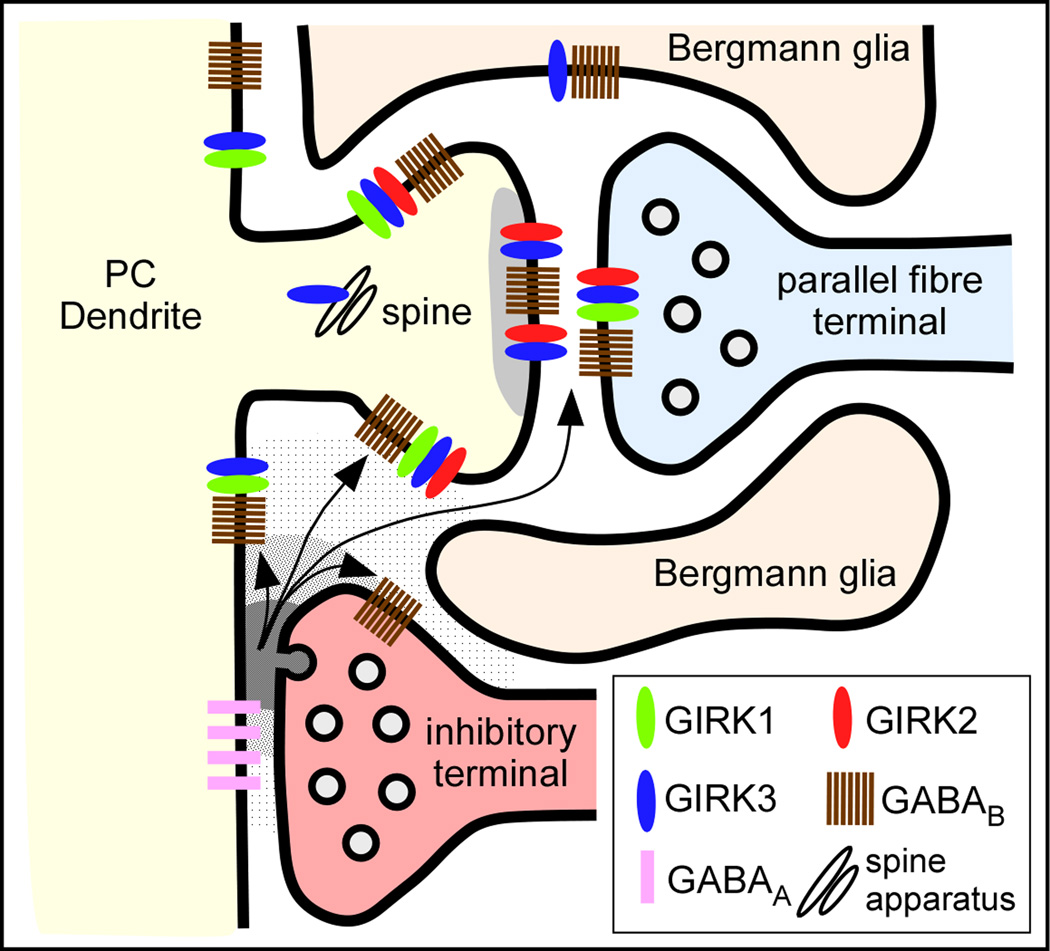
Figure 8.
Summary diagram illustrating the subcellular compartment-specific molecular diversity of GIRK channels in PC-parallel fibre synapses and their coupling with GABAB receptors as determined in this study. We propose that there are at least five different populations of GABAB-activated GIRK channels with specific subunit compositions: 1) In dendritic spines, GIRK channels are composed by GIRK2/GIRK3 at postsynaptic densities and 2) by GIRK1/GIRK2/GIRK3 at extrasynaptic sites; 3) In dendritic shafts, GIRK channels are composed by GIRK1/GIRK3 at extrasynaptic sites; 4) In parallel fibre terminals, GIRK channels are composed by GIRK1/GIRK2/GIRK3; and 4) In Bergmann glia, GIRK channels are composed by GIRK3. GABAB receptors may be activated by the GABA spilling over from neighbouring GABAergic synapses.
GIRK subunits are preferentially and differentially localized to dendritic spines in PCs
Although neuronal GIRK channels have been described to be heteromeric complexes of GIRK1 and GIRK2 (Duprat et al. 1995; Kofuji et al. 1995; Liao et al. 1996), by showing the co-immunoprecipitation of GIRK1, GIRK2 and GIRK3 in the cerebellum (Aguado et al. 2008) we suggested that neuronal GIRK channels are more diverse than previously thought. As shown by in situ hybridization (Kobayashi et al. 1995; Karschin et al. 1996; Liao et al. 1996) and immunohistochemical studies (Liao et al. 1996; Signorini et al. 1997; Inanobe et al. 1999; Aguado et al. 2008), the three neuronal GIRK channel subunits are abundantly expressed in the cerebellum, where they are expressed in a cell type-specific manner (Aguado et al. 2008). Among cerebellar cell types, using quantitative RT-PCR and immunohistochemistry, we demonstrated recently that PCs express GIRK1, GIRK2 and GIRK3, but not GIRK4 (Aguado et al. 2008). However, the subunit combinations of functional GIRK channels that interact in vivo in specific subcellular compartments remain unexplored. Our data showing that different compartments of PC dendrites contain unique combinations of GIRK channel subunits suggests that GIRK channels subtypes differ not only across cell types but also across subcellular compartment. For example, in PCs most putative GIRK1/GIRK2/GIRK3 heteromultimeric channels are located in the extrasynaptic plasma membrane of dendritic spines, as suggested by: 1) their strikingly similar distributions relative to neurotransmitter release sites, 2) the dramatic subcellular regulation of GIRK1 in the double GIRK2/GIRK3 KO mice, restricted to the rER of PCs, and 3) the down-regulation of GIRK3 in both the GIRK1 and GIRK2 KO mice. This is parallel to the regulation described in the hippocampus (Signorini et al. 1997; Koyrakh et al. 2005) and spinal cord (Marker et al. 2006).
In contrast to the presence of GIRK1, GIRK2 and GIRK3 at extrasynaptic sites of spines, only GIRK2 and GIRK3 were detected within the synaptic specialization of PC-parallel fibre synapses. The absence of GIRK1 in the postsynaptic specialization is an additional evidence for the molecular diversity of GIRK channels in the CNS and is consistent with recent observations made in the hippocampus and spinal cord dorsal horn (Koyrakh et al. 2005; Marker et al. 2005). Therefore, channels within the synaptic specialization are likely GIRK2/GIRK3 heterotetramers. This specific distribution of GIRK channels within the PSDs may reflect the presence of a PDZ interaction motif on the GIRK2c splice isoform and/or GIRK3 (Inanobe et al. 1999; Nehring et al. 2000; Kurachi and Ishii, 2004). At present, the functional significance of GIRK channels within the synaptic specialization is unknown, but these ion channels might be activated by GABAB receptors, which are known to be also present in the same PSD (Kulik et al. 2006).
The functional consequences arising from heteromeric channel formation in different subcellular compartments of PCs remain largely unknown. GIRK channel properties arise from the assembly of different subunits (Duprat et al. 1995; Kofuji et al. 1995; Krapivinsky et al. 1995). For example, expression of slow kinetics depends on the insertion of GIRK1 subunits into GIRK2-containing channels (Slesinger et al. 1996). Despite the broad distribution of GIRK3 in the CNS, its role is still uncertain. Our data on the high density and regulation of GIRK3 along the neuronal surface of PCs drives us to predict a relevant role for this GIRK subunit. Several studies point out that GIRK3 contributes to both channel formation (Jelacic et al. 2000; Torrecilla et al. 2002; Cruz et al. 2004; Koyrakh et al. 2005; Labouèbe et al. 2007) and channel trafficking (Ma et al. 2002; Lunn et al. 2007). For instance, GIRK2/GIRK3 heteromultimers exhibit a decreased sensitivity to receptor activation (Jelacic et al. 2000; Cruz et al. 2004). A role for GIRK3 in mediating interactions between GIRK channels and RGS proteins in VTA dopamine neurons was also recently suggested (Labouebe et al. 2007).
Coupling between GIRK channels and GABAB receptors at postsynaptic sites
GIRK channels serve as postsynaptic effector for the major inhibitory transmitter GABA acting on GABAB receptors in heterologous expression systems as well as in several central neurons (Lüscher et al. 1997; Slesinger et al. 1997; Kaupmann et al. 1998; Chen and Johnston, 2005; Koyrakh et al. 2005; Tabata et al. 2005; Marker et al. 2005; Vigot et al. 2006; Labouèbe et al. 2007). In the cerebellum, functional GABAB receptors are heterodimers of GABAB1 and GABAB2 subunits (Kaupmann et al. 1998), and both are highly co-expressed in PCs and localize both pre- and post-synaptically (Kulik et al. 2003; Luján and Shigemoto, 2006). Based on the high level of expression of GIRK1, GIRK2, and GIRK3 in the cerebellum (Aguado et al. 2008), it seems reasonable to think that the three subunits interact functionally with GABAB receptors, at least in some cerebellar cell types. In this respect, we recently have shown that GIRK1, GIRK2, and GIRK3 subunits co-precipitated together in the cerebellum (Aguado et al. 2008), and in this study co-immunoprecipitation experiments further revealed that GIRK1, GIRK2, and GIRK3 antibodies each co-immunoprecipitated GABAB receptors in solubilized cerebellar membranes, likely reflecting their coupling at postsynaptic sites, where both are highly expressed (Luján & Shigemoto, 2006; Aguado et al. 2008). This coupling is supported by three observations: 1) the up-regulation of the GIRK3 subunit after genetic ablation of GABAB1; 2) the close spatial relationship of GIRK channel subunits and GABAB receptors, showing strikingly similar extrasynaptic distributions relative to the glutamate release site in PC spines; and 3) the subcellular regulation of extrasynaptic GABAB receptors in the PC spines after genetic ablation of GIRK3 KO. Consistent with these morphological data, electrophysiological studies show the presence of GABAB-activated GIRK currents in PCs (Tabata et al. 2005) and a reduction of these currents in the GIRK2 KO cerebellum (Slesinger et al. 1997).
Coupling between GIRK channels and GABAB receptors at presynaptic sites
Presynaptic GABAB receptors reduce neurotransmitter release by inhibiting Ca2+ channels (Takahashi et al. 1998). It has been reported that GIRK channels are localized at presynaptic sites in the brain (Morishige et al. 1996; Ponce et al. 1996; Kulik et al. 2006; Koyrakh et al. 2005; Marker et al. 2005; Aguado et al. 2008) and their proximity to the presynaptic active zone suggests an involvement in the regulation of neurotransmitter release. Although electrophysiological studies do not support presynaptic GIRK channel activation as a primary mechanism by which GABAB receptors modulate neurotransmitter release (Lüscher et al. 1997), using functional assays we recently found a GIRK channel-mediated inhibition of glutamate release through GABAB receptors at cerebrocortical nerve terminals (Ladera et al. 2008). The functional interaction between GABAB receptors and GIRK channels also occurs at presynaptic site of cerebellar synapses and this coupling is supported by the up-regulation of GIRK3 and GABAB receptors in parallel fibre terminals after genetic ablation of GABAB1 and GIRK3, respectively. We found that the presynaptic action of GABAB receptors involves two mechanisms in cerebellar nerve terminals. In one mechanism, the receptor reduces the release of glutamate supported by N-type Ca2+ channels. As this action is insensitive to the GIRK channel blocker tertiapin-Q we favour a direct inhibition of Ca2+ channels as responsible for this presynaptic mechanism (Dittman and Regher, 1996). However, the stimulation of nerve terminals with low KCl revealed another presynaptic action of GABAB receptors in down-regulating the glutamate release supported by P/Q-type Ca2+ channels. Since this inhibitory response is reversed by the GIRK channel blocker tertiapin-Q it is likely that the activation of GIRK channels accounts for this presynaptic action of GABAB receptors. That GIRK channels mediate the inhibition of release by GABAB receptors is consistent with data showing that GIRK channel localized to parallel fibre terminals are formed by GIRK1/GIRK2/GIRK3 and that these channels co-localize with GABAB receptors.
In summary, our combined biochemical, morphological, and functional analysis of WT and mutant mice clearly establishes that the three primary neuronal GIRK subunits assemble to form pools of functional channels that can differ in their subunit combinations across subcellular compartments in PCs. Furthermore, we present evidence that coupling between the GABAB receptor and neuronal GIRK channels of defined subunit compositions occurs at post- and presynaptic sites to fulfil distinct functions. The subcellular localization of GABAB receptors and GIRK channels and their close spatial relationship at excitatory synapses suggest that they may engage in the postsynaptic modulation of glutamate signalling in the cerebellum.
Supplementary Material
Acknowledgements
The authors would like to thank Drs. John P. Adelman and Juan Carlos Alvarado, members of the Wickman laboratory and members of the Luján laboratory for their comments on the manuscript. We also would like to thank to Diane Latawiec for the English revision of the manuscript and Mr. José Julio Cabanes and Laura Tortosa for the excellent technical assistance. This work was supported by grants from the Spanish Ministry of Education and Science (BFU-2006-01896) and CONSOLIDER (CSD2008-00005) to R.L., grants from the Spanish ‘Ministerio de Educación y Ciencia’ BFU2007-64154/BFI, the ‘Instituto de Salud Carlos III’ RD06/0026 and the ‘Comunidad de Madrid’ (S-BIO-0170/2006) to J. S-P., the Sociedad Española de Farmacología and Laboratorios Almirall to F.C., the NIH (MH061933 and DA011806) to K.W., and a grant of the Swiss Science Foundation (3100A0-117816) to B.B.
The monoclonal antibody GABAB2 (Clone N81/37) was developed by and/or obtained from the UC Davis/NIH NeuroMab Facility, supported by NIH grant U24NS050606 and maintained by the Department of Neurobiology, Physiology and Behaviour, College of Biological Sciences, University of California, Davis, CA 95616.
References
- Aguado C, Colón J, Ciruela F, et al. Cell type-specific subunit composition of G protein-gated potassium channels in the cerebellum. J. Neurochem. 2008;105:497–511. doi: 10.1111/j.1471-4159.2007.05153.x. [DOI] [PubMed] [Google Scholar]
- Barrie AP, Nicholls DG, Sánchez-Prieto J, Shira TS. An ion channels locus for the protein kinase C potentiation of transmitter glutamate release from guinea pig cerebrocortical synaptosomes. J. Neurochem. 1991;57:1398–1404. doi: 10.1111/j.1471-4159.1991.tb08306.x. [DOI] [PubMed] [Google Scholar]
- Bettahi I, Marker CL, Roman MI, Wickman K. Contribution of the Kir3.1 subunit to the muscarinic-gated atrial potassium channel IKACh. J. Biol. Chem. 2002;277:48282–48288. doi: 10.1074/jbc.M209599200. [DOI] [PubMed] [Google Scholar]
- Burgueño J, Blake DJ, Benson MA, et al. The adenosine A2A receptor interacts with the actin-binding protein alpha-actinin. J. Biol. Chem. 2003;278:37545–37552. doi: 10.1074/jbc.M302809200. [DOI] [PubMed] [Google Scholar]
- Chen X, Johnston D. Constitutively active G-protein-gated inwardly rectifying K+ channels in dendrites of hippocampal CA1 pyramidal neurons. J. Neurosci. 2005;25:3787–3792. doi: 10.1523/JNEUROSCI.5312-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciruela F, Escriche M, Burgueno J, et al. Metabotropic glutamate 1alpha and adenosine A1 receptors assemble into functionally interacting complexes. J. Biol. Chem. 2001;276:18345–18351. doi: 10.1074/jbc.M006960200. [DOI] [PubMed] [Google Scholar]
- Cruz HG, Ivanova T, Lunn ML, et al. Bi-directional effects of GABA(B) receptor agonists on the mesolimbic dopamine system. Nat. Neurosci. 2004;7:153–159. doi: 10.1038/nn1181. [DOI] [PubMed] [Google Scholar]
- Dascal N. Signalling via the G protein-activated K+ channels. Cell Signal. 1997;9:551–573. doi: 10.1016/s0898-6568(97)00095-8. [DOI] [PubMed] [Google Scholar]
- Dittman J, Regher WG. Contributions of calcium-dependent and calcium-independent mechanisms to presynaptic inhibition at a cerebellar synapse. J. Neurosci. 1996;16:1623–1633. doi: 10.1523/JNEUROSCI.16-05-01623.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duprat F, Lesage F, Guillemare E, et al. Heterologous multimeric assembly is essential for K+ channel activity of neuronal and cardiac G-protein activated inward rectifiers. Biochem. Biophys. Res. Commun. 1995;212:657–663. doi: 10.1006/bbrc.1995.2019. [DOI] [PubMed] [Google Scholar]
- Inanobe A, Yoshimoto Y, Horio Y, et al. Characterization of G-protein-gated K+ channels composed of Kir3.2 subunits in dopaminergic neurons of the substantia nigra. J. Neurosci. 1999;19:1006–1017. doi: 10.1523/JNEUROSCI.19-03-01006.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacson JS, Hille B. GABAB-mediated presynaptic inhibition of excitatory transmission and synaptic vesicle dynamics in cultured hippocampal neurons. Neuron. 1997;18:143–152. doi: 10.1016/s0896-6273(01)80053-2. [DOI] [PubMed] [Google Scholar]
- Jelacic TM, Kennedy ME, Wickman K, Clapham DE. Functional and biochemical evidence for G-protein-gated inwardly rectifying K+ (GIRK) channels composed of GIRK2 and GIRK3. J. Biol. Chem. 2000;275:36211–36216. doi: 10.1074/jbc.M007087200. [DOI] [PubMed] [Google Scholar]
- Karschin C, Dissmann E, Stuhmer W, Karschin A. IRK(1–3) and GIRK(1–4) inwardly rectifying K+ channel mRNAs are differentially expressed in the adult rat brain. J. Neurosci. 1996;16:3559–3570. doi: 10.1523/JNEUROSCI.16-11-03559.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaupmann K, Schuler V, Mosbacher J, et al. Human gamma-aminobutyric acid type B receptors are differentially expressed and regulate inwardly rectifying K+ channels. Proc. Natl. Acad. Sci. USA. 1998;95:14991–1496. doi: 10.1073/pnas.95.25.14991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi T, Ikeda K, Ichikawa T, et al. Molecular cloning of a mouse G-protein-activated K+ channel (mGIRK1) and distinct distributions of three GIRK (GIRK1, 2 and 3) mRNAs in mouse brain. Biochem. Biophys. Res. Commun. 1995;208:1166–1173. doi: 10.1006/bbrc.1995.1456. [DOI] [PubMed] [Google Scholar]
- Kofuji P, Davidson N, Lester HA. Evidence that neuronal G-protein-gated inwardly rectifying K+ channels are activated by G beta gamma subunits and function as heteromultimers. Proc. Natl. Acad. Sci. USA. 1995;92:6542–6546. doi: 10.1073/pnas.92.14.6542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyrakh L, Luján R, Colón J, et al. Molecular and cellular diversity of neuronal G-protein-gated potassium channels. J. Neurosci. 2005;25:11468–11478. doi: 10.1523/JNEUROSCI.3484-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krapivinsky G, Gordon EA, Wickman K, et al. The G-protein-gated atrial K+ channel IKACh is a heteromultimer of two inwardly rectifying K+-channel proteins. Nature. 1995;374:135–141. doi: 10.1038/374135a0. [DOI] [PubMed] [Google Scholar]
- Kulik A, Vida I, Lujan R, et al. Subcellular localization of metabotropic GABAB receptor subunits GABAB(1a/b) and GABAB(2) in the rat hippocampus. J. Neurosci. 2003;23:11026–11035. doi: 10.1523/JNEUROSCI.23-35-11026.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulik A, Vida I, Fukazawa Y, et al. Compartment dependent colocalization of Kir3.2-containing K+-channels and GABAB receptors in hippocampal pyramidal cells. J. Neurosci. 2006;26:4289–4297. doi: 10.1523/JNEUROSCI.4178-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurachi Y, Ishii M. Cell signal control of the G protein-gated potassium channel and its subcellular localization. J. Physiol. 2004;554:285–294. doi: 10.1113/jphysiol.2003.048439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labouèbe G, Lomazzi M, Cruz HG, et al. RGS2 modulates coupling between GABAB receptors and GIRK channels in dopamine neurons of the ventral tegmental area. Nat. Neurosci. 2007;10:1559–1568. doi: 10.1038/nn2006. [DOI] [PubMed] [Google Scholar]
- Ladera C, Godino MC, Cabañero MJ, et al. Presynaptic GABAB receptors inhibit glutamate release through GIRK channels. J. Neurochem. 2008;107:1506–1517. doi: 10.1111/j.1471-4159.2008.05712.x. [DOI] [PubMed] [Google Scholar]
- Liao YJ, Jan YN, Jan LY. Heteromultimerization of G-protein-gated inwardly rectifying K+ channel proteins GIRK1 and GIRK2 and their altered expression in weaver brain. J. Neurosci. 1996;16:7137–7150. doi: 10.1523/JNEUROSCI.16-22-07137.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luján R, Nusser Z, Roberts JD, et al. Perisynaptic location of metabotropic glutamate receptors mGluR1 and mGluR5 on dendrites and dendritic spines in the rat hippocampus. Eur. J. Neurosci. 1996;8:1488–1500. doi: 10.1111/j.1460-9568.1996.tb01611.x. [DOI] [PubMed] [Google Scholar]
- Luján R, Shigemoto R. Localization of metabotropic GABA receptor subunits GABAB1 and GABAB2 relative to synaptic sites in the rat developing cerebellum. Eur. J. Neurosci. 2006;23:1479–1490. doi: 10.1111/j.1460-9568.2006.04669.x. [DOI] [PubMed] [Google Scholar]
- Lunn ML, Nassirpour R, Arrabit C, et al. A unique sorting nexin regulates trafficking of potassium channels via a PDZ domain interaction. Nat. Neurosci. 2007;10:1249–1259. doi: 10.1038/nn1953. [DOI] [PubMed] [Google Scholar]
- Lüscher C, Jan LY, Stoffel M, et al. G protein-coupled inwardly rectifying K+ channels (GIRKs) mediate postsynaptic but not presynaptic transmitter actions in hippocampal neurons. Neuron. 1997;19:687–695. doi: 10.1016/s0896-6273(00)80381-5. [DOI] [PubMed] [Google Scholar]
- Ma D, Zerangue N, Raab-Graham K, et al. Diverse trafficking patterns due to multiple traffic motifs in G protein-activated inwardly rectifying potassium channels from brain and Herat. Neuron. 2002;33:715–729. doi: 10.1016/s0896-6273(02)00614-1. [DOI] [PubMed] [Google Scholar]
- Marker C, Luján R, Loh H, Wickman K. Spinal G protein-gated potassium channels contribute in a dose-dependent manner to the analgesic effect of mu and delta but not kappa opioids. J. Neurosci. 2005;25:3551–3559. doi: 10.1523/JNEUROSCI.4899-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marker C, Luján R, Colón J, Wickman K. Distinct populations of spinal cord lamina II interneurons expressing G-protein-gated potassium channels. J. Neurosci. 2006;26:12251–12259. doi: 10.1523/JNEUROSCI.3693-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millán C, Sánchez-Prieto J. Differential coupling of N and P/Q-type calcium channels to glutamate exocytosis in the rat cerebral cortex. Neurosci. Lett. 2002;330:29–32. doi: 10.1016/s0304-3940(02)00719-x. [DOI] [PubMed] [Google Scholar]
- Millán C, Luján R, Shigemoto R, Sánchez-Prieto J. The inhibition of glutamate release by metabotropic glutamate receptor7 affects both [Ca2+]c and cAMP: evidence for a strong reduction of Ca2+ entry in single nerve terminals. J. Biol. Chem. 2002;277:14092–14101. doi: 10.1074/jbc.M109044200. [DOI] [PubMed] [Google Scholar]
- Millán C, Castro E, Torres M, et al. Co-expression of mGluR7 and N-type Ca2+ channels in single cerebrocortical nerve terminals from adult rats. J. Biol. Chem. 2003;278:23955–23962. doi: 10.1074/jbc.M211471200. [DOI] [PubMed] [Google Scholar]
- Mintz IM, Sabatini BL, Regehr WG. Calcium control of transmitter release at a cerebellar synapse. Neuron. 1995;15:675–688. doi: 10.1016/0896-6273(95)90155-8. [DOI] [PubMed] [Google Scholar]
- Morishige KI, Inanobe A, Takahashi N, et al. G protein gated K+ channel (GIRK 1) protein is expressed presynaptically in the paraventricular nucleus of the hypothalamus. Biochem. Biophys. Res. Commun. 1996;220:300–305. doi: 10.1006/bbrc.1996.0400. [DOI] [PubMed] [Google Scholar]
- Nehring RB, Wischmeyer E, Döring F, et al. Neuronal inwardly rectifying K(+) channels differentially couple to PDZ proteins of the PSD-95/SAP90 family. J. Neurosci. 2000;20:156–162. doi: 10.1523/JNEUROSCI.20-01-00156.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North A. Drug receptors and the inhibition of nerve cells. Br. J. Pharmacol. 1989;98:13–28. doi: 10.1111/j.1476-5381.1989.tb16855.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivera BM, Gray WR, Zeikus R, et al. Peptides neurotoxins from fish-hunting cone snails. Science. 1985;230:1338–1343. doi: 10.1126/science.4071055. [DOI] [PubMed] [Google Scholar]
- Perry CA, Pravetoni M, Teske JA, et al. Predisposition to late-onset obesity in GIRK4 knockout mice. Proc. Natl. Acad. Sci. USA. 2008;105:8148–8153. doi: 10.1073/pnas.0803261105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponce A, Bueno E, Kentros C, et al. G-protein-gated inward rectifier K+ channel proteins (GIRK1) are present in the soma and dendrites as well as in nerve terminals of specific neurons in the brain. J. Neurosci. 1996;16:1990–2001. doi: 10.1523/JNEUROSCI.16-06-01990.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuler V, Lüscher C, Blanchet C, et al. Epilepsy, hyperalgesia, impaired memory, and loss of pre- and postsynaptic GABA(B) responses in mice lacking GABA(B(1)) Neuron. 2001;31:47–58. doi: 10.1016/s0896-6273(01)00345-2. [DOI] [PubMed] [Google Scholar]
- Signorini S, Liao YJ, Duncan SA, et al. Normal cerebellar development but susceptibility to seizures in mice lacking G protein-coupled, inwardly rectifying K+ channel GIRK2. Proc. Natl. Acad. Sci. USA. 1997;94:923–927. doi: 10.1073/pnas.94.3.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slesinger PA, Patil N, Liao J, et al. Functional effects of the mouse weaver mutation on G protein-gated inwardly rectifying K+ channels. Neuron. 1996;16:321–331. doi: 10.1016/s0896-6273(00)80050-1. [DOI] [PubMed] [Google Scholar]
- Slesinger P, Stoffel M, Jan Y, Jan L. Defective g-aminobutyric acid type B receptor-activated inwardly rectifying K+ currents in cerebellar granule cells isolated from weaver and Girk2 null mutant mice. Proc. Natl. Acad. Sci. USA. 1997;94:12210–12217. doi: 10.1073/pnas.94.22.12210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabata T, Haruki S, Nakayama H, Kano M. GABAergic activation of an inwardly rectifying K+ current in mouse cerebellar Purkinje cells. J. Physiol. 2005;563:443–457. doi: 10.1113/jphysiol.2004.081000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T, Kajikawa Y, Tsujimoto T. G-protein-coupled modulation of presynaptic calcium currents and transmitter release by GABAB receptor. J. Neurosci. 1998;18:3138–3146. doi: 10.1523/JNEUROSCI.18-09-03138.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrecilla M, Marker CL, Cintora SC, et al. G-protein-gated potassium channels containing Kir3.2 and Kir3.3 subunits mediate the acute inhibitory effects of opioids on locus ceruleus neurons. J. Neurosci. 2002;22:4328–4334. doi: 10.1523/JNEUROSCI.22-11-04328.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigot R, Barbieri S, Bräuner-Osborne H, et al. Differential compartmentalization and distinct functions of GABAB receptor variants. Neuron. 2006;50:589–601. doi: 10.1016/j.neuron.2006.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickman K, Nemec J, Gendler SJ, Clapham DE. Abnormal heart rate regulation in GIRK4 knockout mice. Neuron. 1998;20:103–114. doi: 10.1016/s0896-6273(00)80438-9. [DOI] [PubMed] [Google Scholar]
- Wickman K, Pu WT, Clapham DE. Structural characterization of the mouse Girk genes. Gene. 2002;284:241–250. doi: 10.1016/s0378-1119(01)00884-8. [DOI] [PubMed] [Google Scholar]
- Yum DS, Cho JH, Choi IS, et al. Adenosine A1 receptors inhibit GABAergic transmission in rat tuberomammillary nucleus neurons. J. Neurochem. 2008;106:361–371. doi: 10.1111/j.1471-4159.2008.05400.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.