Abstract
Data from the International Collaborative Gaucher Group Gaucher Registry were analysed to assess the relationship between enzyme replacement therapy with imiglucerase (ERT) and incidence of avascular necrosis (AVN) in type 1 Gaucher disease (GD1), and to determine whether the time interval between diagnosis and initiation of ERT influences the incidence rate of AVN. All patients with GD1 enrolled in the Gaucher Registry who received ERT and did not report AVN prior to starting therapy (n = 2700) were included. The incidence rate of AVN following initiation of ERT was determined. An incidence rate of AVN of 13·8 per 1000 person-years was observed in patients receiving ERT. Patients who initiated ERT within 2 years of diagnosis had an incidence rate of 8·1 per 1000 person-years; patients who started ERT ≥2 years after diagnosis had an incidence rate of 16·6 per 1000 person-years. The adjusted incidence rate ratio was 0·59 [95% confidence interval (CI) 0·36–0·96, P = 0·0343]. Splenectomy was an independent risk factor for AVN (adjusted incidence rate ratio 2·23, 95% CI 1·61–3·08, P < 0·0001). In conclusion, the risk of AVN was reduced among patients who initiated ERT within 2 years of diagnosis, compared to initiating treatment ≥2 years after diagnosis. A higher risk of AVN was observed among patients who had previously undergone splenectomy.
Keywords: Gaucher disease, enzyme replacement therapy, avascular necrosis, imiglucerase
Gaucher disease (GD), the most common lysosomal storage disorder, results from defective activity of acid β-glucosidase (EC 3.2.1.45; lysosomal glucocerebrosidase) due to mutations in the GBA gene. Enzyme deficiency leads to accumulation of glucocerebroside in the lysosomes of mononuclear phagocytes and a complex multisystemic phenotype (Grabowski et al, 2006). The glucocerebroside-engorged macrophages trigger a chronic inflammatory state, immune dysfunction and, occasionally, fibrosis (Cox & Schofield, 1997). The estimated prevalence of GD is 1/57 000 (Meikle et al, 1999) to 1/75 000 newborns (Grabowski, 2005) although its prevalence in people of Ashkenazi Jewish ancestry is higher, at c. 1 in 500 (Weinreb et al, 2008). Type 1 GD (GD1) comprises c. 94% of all cases, and it is differentiated from type 2 and type 3 GD by the absence of primary central nervous system involvement (Grabowski et al, 2006).
The most prominent sites of pathology in GD1 are the liver, spleen, bone marrow, skeleton and lungs (Charrow et al, 2000; Grabowski et al, 2006). Clinical and radiological evidence of diverse skeletal involvement occurs in the majority of patients (Charrow et al, 2000; Grabowski et al, 2006; Weinreb et al, 2007). However, skeletal involvement may occur in the absence of significant haematological and visceral abnormalities, underscoring the extreme heterogeneity of GD1 within the same genotype groups and even among affected siblings (Taddei et al, 2009).
Type 1 GD is a progressive condition in which irreversible disease sequelae can occur in untreated patients (Kaplan et al, 2006; Maaswinkel-Mooij et al, 2000; Taddei et al, 2009). A major irreversible complication of GD1 is avascular necrosis (AVN), which can lead to joint destruction, the need for joint replacement surgery, and chronic disability. AVN may rarely result in pseudotumors of the bone known as ‘Gaucheromas’ and pathological fractures (Elstein et al, 1997). In the natural course of GD1, the occurrence of AVN appears to be unpredictable and its risk factors are not understood.
The standard of care for GD1 is enzyme replacement therapy with imiglucerase (ERT). While enzyme therapy is highly effective in reversing visceral and haematological manifestations and some aspects of skeletal disease, its precise impact on the risk of bone disease, such as AVN, is not known (Andersson et al, 2005; Barton et al, 1991; Charrow et al, 2007; Cox et al, 2008; Grabowski et al, 2006, 1998; Sims et al, 2008; Weinreb et al, 2007, 2002; Wenstrup et al, 2007). A key unanswered question regarding the management of GD1 concerns the timing of treatment initiation, and whether early initiation of ERT after diagnosis reduces the incidence rate of clinically significant events, such as AVN. The present study used the International Collaborative Gaucher Group (ICGG) Gaucher Registry database to determine whether the rate of AVN following treatment initiation varied according to particular risk factors, including the interval between diagnosis and initiation of enzyme therapy. Our results provide evidence on which clinical decisions regarding the optimal timing to begin ERT can be based.
Methods
ICGG Gaucher Registry
The ICGG Gaucher Registry was launched in 1991 to track the clinical, demographic, genetic, biochemical and therapeutic characteristics of patients with GD throughout the world, irrespective of disease severity and treatment status (Charrow et al, 2000). The goals of the Registry are to define the clinical spectrum of GD, assess its natural history though longitudinal follow-up and assess the effect of treatment. An independent international group of physicians who are experts in GD provide scientific direction and governance of the Registry, with logistical support from Genzyme Corporation (Cambridge, MA, USA). Since 1991, with Institutional Review Board/Ethics Committee approvals, over 700 physicians from 60 countries have voluntarily submitted de-identified data on over 5000 patients to the Registry.
Study population
Data included in this study were recorded in the Registry as of July 2007. ERT refers to mannose-terminated glucocerebrosidase, whether human placenta-derived, alglucerase (Ceredase®; Genzyme Corporation) or human recombinant Chinese Hamster Ovary cell-generated, imiglucerase (Cerezyme®; Genzyme Corporation). Alglucerase and imiglucerase have been shown to be therapeutically equivalent in a randomized, two-arm clinical trial (Grabowski et al, 1995). Of the patients included in this analysis, 44% initiated therapy with alglucerase and 56% initiated therapy with imiglucerase. Within several years, most of the patients who initiated therapy with alglucerase eventually switched to imiglucerase; only 16% of patients were treated solely with alglucerase during follow-up.
The dataset for this analysis included all GD1 Registry patients who received imiglucerase with the following information: date of GD diagnosis; age at GD diagnosis; dates of first and last assessments in the Registry; and date of initiation of therapy. The diagnosis of GD1 was usually based on the assay of acid β-glucosidase activity and/or genotyping of GBA.
The objective of the analysis was to estimate the incidence rate of new onset AVN following treatment initiation. Therefore, patients with AVN reported before initiation of therapy were excluded from this analysis.
Demographic and clinical characteristics of patients
Patient demographics and clinical manifestations of GD1 were characterized at the time of therapy initiation. Clinical manifestations included whether prior therapeutic splenectomy had been performed, haemoglobin concentration, platelet count, spleen volume, liver volume, bone mineral density and reports of bone pain and/or bone crisis. Criteria for diagnosis of bone crises in the Registry include acute onset of bone pain requiring immobilisation of the affected area and narcotics for the relief of pain, accompanied by one or more of the following: periosteal elevation, elevated white blood cell count, elevated inflammatory markers, fever or debilitation >3 d (Charrow et al, 2007). This definition excluded fracture and osteomyelitis. Bone pain was defined as patient-reported pain experienced during the 30-d period preceding the report and attributable to Gaucher disease according to the clinical judgment of the reporting physician.
Ascertainment of avascular necrosis
Avascular necrosis was typically ascertained from X-ray or magnetic resonance imaging (MRI) results. The recommended radiological guidelines for patients with GD1 include assessment of the hip, femur, pelvis and lumbar spine. The date of onset and site of AVN was recorded on the skeletal assessment case report forms of the Registry. If a patient had AVN reported on more than one date, the patient’s earliest date of AVN onset was identified.
Person-time of follow-up
Follow-up began on each patient’s date of initiation of therapy in the Registry. Patients were followed until either the date of AVN onset, or for patients who did not develop AVN, the date of their last recorded assessment in the Registry. Each patient was classified according to the duration of time between diagnosis of GD1 and initiation of therapy. The categories were as follows: <1; 1–<2; 2–<5; 5–<10; 10–<20; 20–<30; and 30+ years.
Data analysis
Descriptive statistics were used to analyse data in this study according to demographics and clinical characteristics of GD1. Proportions were calculated for categorical variables (i.e. gender, genotype, ethnicity). Summary statistics (mean, standard deviation, percentiles) were calculated for continuous measures (i.e. age). Incidence rates were calculated as follows:
 |
Other candidate risk factors for new onset AVN were collectively examined through construction of a multivariate Poisson regression model. Variables in the model included interval between GD diagnosis and initiation of therapy (<2, 2 years or more), age at initiation of therapy, age at Gaucher diagnosis, gender, year of diagnosis (before 1991, 1991–1999, 2000 or later), splenectomy before initiation of therapy, calendar year of initiation of therapy (before 1995, 1995–1999, 2000 or later), and genotype category (N370S/N370S, N370S/Other, Other/Other). Results from the multivariate Poisson regression model were expressed as adjusted incidence rate ratios. The risk of AVN over time following initiation of therapy was depicted by construction of a Kaplan–Meier curve. An alpha-level of 0·05 was used to determine statistical significance.
All analyses were conducted in SAS 8.2 (SAS Institute Inc., Cary, NC, USA) in accordance with STROBE guidelines (http://www.strobe-statement.org, accessed 15 June 2009).
Results
As of July 2007 of a total of 4783 patients were enrolled in the ICGG Gaucher Registry; 3176 patients met the study inclusion criteria, i.e., received ERT, reported dates of therapy initiation and GD1 diagnosis, and reported age at diagnosis. The final analysis was based on 2700 patients; 476 of 3176 patients were excluded because they had a history of AVN before therapy initiation.
The incidence rate of new onset AVN among GD1 patients following initiation of therapy was 13·8 per 1000 person-years (Table I). Incidence rates then were stratified by interval in years between diagnosis and initiation of ERT. Among patients who initiated therapy within 2 years after diagnosis, the incidence rate of AVN following initiation of imiglucerase ranged from 8·0 to 8·2 per 1000 person-years. In contrast, the incidence rate of AVN among patients starting therapy more than 2 years after diagnosis (up to 30 years) rose progressively from 12·7 to 22·1 per 1000 person-years. Therefore, we consolidated our analysis of the rate of AVN on ERT into two groups: patients who started ERT within 2 years of diagnosis and those who started ERT 2 years or more after diagnosis.
Table I.
Incidence rates of avascular necrosis according to time interval between Gaucher disease diagnosis and initiation of therapy.
| AVN cases | Number of patients | Person-years of follow-up | Incidence rate* | |
|---|---|---|---|---|
| Total | 213 | 2700 | 15 468 | 13·8 |
| Years between Gaucher disease diagnosis and initiation of ERT with imiglucerase | ||||
| <1 | 24 | 639 | 3018 | 8·0 |
| 1–<2 | 17 | 408 | 2066 | 8·2 |
| 2–<5 | 30 | 394 | 2369 | 12·7 |
| 5–<10 | 35 | 375 | 2468 | 14·2 |
| 10–<20 | 48 | 440 | 2844 | 16·9 |
| 20–<30 | 33 | 244 | 1493 | 22·1 |
| 30+ | 26 | 200 | 1210 | 21·5 |
Incidence rate per 1000 person-years.
Ascertainment of AVN was by MRI in 54% of cases and plain radiology for 41% of cases. The sites of AVN were the hip (52%), femur (21%), shoulder (6%), humerus (3%), tibia (2%), knee (2%), spine (<1%), ankle (<1%), wrist (<1%) and jaw (<1%); in 11% of cases, AVN occurred at multiple sites.
The characteristics of patients included in this analysis are depicted in Table II, stratified according to whether therapy was initiated within 2 years or 2 or more years after diagnosis. Additional patient characteristics are provided in Table SI. There were similar proportions of men (47%) and women (53%). The majority of patients were from the US (40%), Americas including Canada (21%) and Europe (25%). The most common race/ethnicities were non-Jewish Caucasians (41%) and Jewish (35%). Genotypes were reported for c. 70% of the patients; the majority (87%) had at least one N370S allele (26% homozygous, 61% heteroallelic).
Table II.
Characteristics of patients.
| Years between Gaucher diagnosis and initiation of ERT with imiglucerase |
|||
|---|---|---|---|
| <2 years (N = 1047) | ≥2 years (N = 1653) | Total (N = 2700) | |
| Gender, n (%) | n = 1047 | n = 1653 | n = 2700 |
| Male | 539 (51·5) | 719 (43·5) | 1258 (46·6) |
| Female | 508 (48·5) | 934 (56·5) | 1442 (53·4) |
| Age at Gaucher diagnosis (years), n (%) | n = 1047 | n = 1653 | n = 2700 |
| 0–9 | 471 (45·0) | 713 (43·1) | 1184 (43·9) |
| 10–19 | 171 (16·3) | 317 (19·2) | 488 (18·1) |
| 20–29 | 120 (11·5) | 278 (16·8) | 398 (14·7) |
| 30–39 | 111 (10·6) | 151 (9·1) | 262 (9·7) |
| 40–49 | 84 (8·0) | 107 (6·5) | 191 (7·1) |
| 50–59 | 46 (4·4) | 47 (2·8) | 93 (3·4) |
| ≥60 | 44 (4·2) | 40 (2·4) | 84 (3·1) |
| Age at initiation of ERT with imiglucerase (years), n (%) | n = 1047 | n = 1653 | n = 2700 |
| 0–9 | 444 (42·4) | 164 (9·9) | 608 (22·5) |
| 10–19 | 174 (16·6) | 326 (19·7) | 500 (18·5) |
| 20–29 | 116 (11·1) | 276 (16·7) | 392 (14·5) |
| 30–39 | 117 (11·2) | 307 (18·6) | 424 (15·7) |
| 40–49 | 95 (9·1) | 265 (16·0) | 360 (13·3) |
| 50–59 | 54 (5·2) | 161 (9·7) | 215 (8·0) |
| ≥60 | 47 (4·5) | 154 (9·3) | 201 (7·4) |
| Year of Gaucher diagnosis, n (%) | n = 1047 | n = 1653 | n = 2700 |
| Before 1991 | 21 (2·0) | 1175 (71·1) | 1196 (44·3) |
| 1991–1999 | 504 (48·1) | 387 (23·4) | 891 (33·0) |
| 2000 or later | 522 (49·9) | 91 (5·5) | 613 (22·7) |
| Year of initiation of ERT with imiglucerase, n (%) | n = 1047 | n = 1653 | n = 2700 |
| Before 1995 | 170 (16·2) | 682 (41·3) | 852 (31·6) |
| 1995–1999 | 329 (31·4) | 532 (32·2) | 861 (31·9) |
| 2000 or later | 548 (52·3) | 439 (26·6) | 987 (36·6) |
| Genotype, n (%) | n = 704 | n = 1166 | n = 1870 |
| N370S/N370S | 169 (24·0) | 317 (27·2) | 486 (26·0) |
| N370S/Other | 396 (56·3) | 739 (63·4) | 1135 (60·7) |
| Other/Other | 139 (19·7) | 110 (9·4) | 249 (13·3) |
In the group of patients who initiated therapy within 2 years following diagnosis, there were substantially more patients below age 10 years at therapy initiation (42%) and non-Jewish patients (76%) compared to the group of patients who initiated therapy 2 years or more following diagnosis (10% and 58%, respectively). However, the distributions of age at Gaucher diagnosis and genotypes were similar between the groups. As expected, 98% of patients who initiated therapy within 2 years following diagnosis were diagnosed after 1991 (when alglucerase first became available, i.e., during the ERT era), while the majority (71%) of patients in the group of patients who initiated therapy 2 years or more following diagnosis were diagnosed before 1991, i.e., during the pre-ERT era.
The haematological, visceral organ and skeletal characteristics at time of therapy initiation are shown in Table III. Overall, among patients with assessments reported, 43% had anaemia; 62% had platelet counts <120 × 109/l; 89% had spleen volumes >5 multiples of normal, and 71% had liver volumes >1·25 multiples of normal. Bone pain around the time of first infusion was reported in 46% of all patients and low bone mineral density in 26%. There were no substantial differences between the <2 and ≥2 years groups in haematological and visceral findings or with regards to bone pain or bone mineral density. However, patients who initiated therapy 2 years or more following diagnosis were more likely to have reported a bone crisis around the time of initiation of treatment (16%, n = 123 of 772) compared to those in the <2 years group (6%, n = 35 of 607). Furthermore, there was a striking difference in prevalence of splenectomy in the two treatment groups: 32% (n = 522 of 1653) of the patients in the ≥2 years group had undergone splenectomy compared to only 6% (n = 65 of 1047) of the patients in whom treatment was initiated <2 years from date of diagnosis.
Table III.
Clinical characteristics of patients.
| Years between Gaucher diagnosis and initiation of ERT with imiglucerase |
|||
|---|---|---|---|
| <2 years (N = 1047) | ≥2 years (N= 1653) | Total patients (N = 2700) | |
| Anaemia at first infusion*†, n (%) | N = 598 | n = 967 | n = 1565 |
| Yes | 269 (45·0) | 397 (41·1) | 666 (42·6) |
| No | 329 (55·0) | 570 (58·9) | 899 (57·4) |
| Platelet count (×109/l) at first infusion*, n (%) | N = 600 | n = 963 | n = 1563 |
| ≥120 | 219 (36·5) | 372 (38·6) | 591 (37·8) |
| 60–<120 | 290 (48·3) | 378 (39·3) | 668 (42·7) |
| <60 | 91 (15·2) | 213 (22·1) | 304 (19·4) |
| Spleen volume (multiples of normal) at first infusion‡, n (%) | n = 334 | n = 435 | n = 769 |
| ≤5 | 35 (10·5) | 53 (12·2) | 88 (11·4) |
| >5–≤15 | 161 (48·2) | 185 (42·5) | 346 (45·0) |
| >15 | 138 (41·3) | 197 (45·3) | 335 (43·6) |
| Liver volume (multiples of normal) at first infusion‡, n (%) | n = 317 | n = 543 | n = 860 |
| ≤1·25 | 90 (28·4) | 163 (30·0) | 253 (29·4) |
| >1·25–≤2·5 | 188 (59·3) | 295 (54·3) | 483 (56·2) |
| >2·5 | 39 (12·3) | 85 (15·7) | 124 (14·4) |
| Low bone mineral density at first infusion§¶, n (%) | n = 104 | n = 137 | n = 241 |
| Yes | 33 (31·7) | 30 (21·9) | 63 (26·1) |
| No | 71 (68·3) | 107 (78·1) | 178 (73·9) |
| Bone pain at first infusion§, n (%) | n = 214 | n = 487 | n = 701 |
| Yes | 106 (49·5) | 214 (43·9) | 320 (45·6) |
| No | 108 (50·5) | 273 (56·1) | 381 (54·4) |
| Bone crisis at first infusion§, n (%) | n = 607 | n = 772 | n = 1379 |
| Yes | 35 (5·8) | 123 (15·9) | 158 (11·5) |
| No | 572 (94·2) | 649 (84·1) | 1221 (88·5) |
| Partial or total splenectomy prior to initiation of ERT with imiglucerase, n (%) | n = 1047 | n = 1653 | n = 2700 |
| Yes | 65 (6·2) | 522 (31·6) | 587 (21·7) |
| No | 979 (93·5) | 1127 (68·2) | 2106 (78·0) |
| Unknown | 3 (0·3) | 4 (0·2) | 7 (0·3) |
Represents the data point closest to the first infusion date, no more than 8 weeks before through to 2 weeks following the date of first infusion.
Anaemia was defined according to age and gender norms for haemoglobin concentrations as follows: <120 g/l for males older than 12 years; <110 g/l for females older than 12 years; <105 g/l for children aged >2–12 years; <95 g/l for children aged 6 months–2 years; <101 g/l for children younger than 6 months of age.
Represents the data point closest to the first infusion date, no more than 6 months before through to 6 weeks following the date of first infusion.
Represents the data point closest to the first infusion date, no more than 2 years before through to 6 weeks following the date of first infusion.
Among patients less than age 50 years, lumbar spine z-score of −2 or lower; Among patients aged 50 years or older, lumbar spine t-score of −2·5 or lower.
The adjusted incidence rate ratio of AVN according to the time interval between GD diagnosis and initiation of therapy, which was analysed using a multivariate Poisson regression model, is shown in Table IV. Patients in whom ERT was initiated within 2 years of diagnosis of GD1 had approximately half the incidence rate of AVN (8·1 per 1000 person-years) compared to patients who started therapy 2 or more years after diagnosis (16·6 per 1000 person-years). The incidence rate difference between the two groups was 8·5 per 1000 person years [95% confidence interval (CI) 5·0–12·0 per 1000 person-years]. The multivariate Poisson regression analysis indicates that the adjusted incidence rate ratio of 0·59 (95% CI 0·36–0·96, P = 0·0343) represents a 41% decrease in the risk of AVN in patients who began ERT within 2 years following diagnosis. Splenectomy was an independent risk factor for AVN after adjusting for all other variables in the model. The adjusted incidence rate ratio for AVN among patients who were asplenic at the time of initiation of therapy was 2·23 (95% CI 1·61–3·08, P < 0·0001) compared to those who had an intact spleen. Women had a lower risk of AVN than men (adjusted incidence rate ratio 0·75, 95% CI 0·57–0·99, P = 0·0412). There were no other clear trends in the risk of AVN according to age at therapy initiation, age at Gaucher diagnosis, year of diagnosis, year of therapy initiation and genotype.
Table IV.
Multivariate analysis: incidence rates and adjusted incidence rate ratios of AVN.
| 95% confidence interval |
||||||||
|---|---|---|---|---|---|---|---|---|
| AVN cases | Number of patients | Person-years of follow-up | Incidence rate* | Adjusted incidence rate ratio† | Lower | Upper | P-value | |
| Total | 213 | 2700 | 15 468 | 13·8 | ||||
| Years between diagnosis and initiation of ERT with Imiglucerase | ||||||||
| <2 | 41 | 1047 | 5084 | 8·1 | 0·59 | 0·36 | 0·96 | 0·0343 |
| ≥2 | 172 | 1653 | 10 384 | 16·6 | Ref | |||
| Gender | ||||||||
| Female | 110 | 1442 | 8499 | 12·9 | 0·75 | 0·57 | 0·99 | 0·0412 |
| Male | 103 | 1258 | 6969 | 14·8 | Ref | |||
| Age at Gaucher diagnosis | ||||||||
| 0–9 | 97 | 1184 | 7421 | 13·1 | 1·23 | 0·66 | 2·3 | 0·5193 |
| 10–19 | 41 | 488 | 2563 | 16·0 | 1·30 | 0·71 | 2·36 | 0·3910 |
| 20–29 | 36 | 398 | 2223 | 16·2 | 1·19 | 0·65 | 2·19 | 0·5649 |
| 30–39 | 17 | 262 | 1415 | 12·0 | Ref | |||
| 40–49 | 11 | 191 | 998 | 11·0 | 0·96 | 0·44 | 2·09 | 0·9100 |
| 50–59 | 7 | 93 | 491 | 14·3 | 1·03 | 0·40 | 2·65 | 0·9448 |
| ≥60 | 4 | 84 | 357 | 11·2 | 0·93 | 0·27 | 3·28 | 0·9156 |
| Age at initiation of ERT with imiglucerase | ||||||||
| 0–9 | 28 | 608 | 3547 | 7·9 | 0·67 | 0·35 | 1·28 | 0·2226 |
| 10–19 | 36 | 500 | 3025 | 11·9 | 0·84 | 0·50 | 1·41 | 0·5102 |
| 20–29 | 46 | 392 | 2228 | 20·6 | 1·42 | 0·90 | 2·25 | 0·1345 |
| 30–39 | 34 | 424 | 2342 | 14·5 | Ref | |||
| 40–49 | 28 | 360 | 2123 | 13·2 | 0·93 | 0·55 | 1·58 | 0·8005 |
| 50–59 | 26 | 215 | 1218 | 21·3 | 1·63 | 0·93 | 2·88 | 0·0904 |
| ≥60 | 15 | 201 | 985 | 15·2 | 1·21 | 0·58 | 2·53 | 0·6049 |
| Year of Gaucher diagnosis | ||||||||
| Before 1991 | 141 | 1196 | 8295 | 17·0 | 0·63 | 0·27 | 1·48 | 0·2880 |
| 1991–1999 | 56 | 891 | 5614 | 10·0 | 0·87 | 0·42 | 1·79 | 0·7072 |
| 2000 or Later | 16 | 613 | 1559 | 10·3 | Ref | |||
| Year of initiation of ERT with imiglucerase | ||||||||
| Before 1995 | 117 | 852 | 6708 | 17·4 | 1·23 | 0·74 | 2·04 | 0·4242 |
| 1995–1999 | 60 | 861 | 5905 | 10·2 | 0·76 | 0·46 | 1·28 | 0·3059 |
| 2000 or Later | 36 | 987 | 2855 | 12·6 | Ref | |||
| Splenectomy before initiation of ERT with imiglucerase‡ | ||||||||
| Yes | 94 | 587 | 3500 | 26·9 | 2·23 | 1·61 | 3·08 | <0·0001 |
| No | 117 | 2106 | 11 943 | 9·8 | Ref | |||
| Genotype | ||||||||
| N370S/N370S | 36 | 486 | 3109 | 11·6 | Ref | |||
| N370S/Other | 103 | 1135 | 7415 | 13·9 | 1·11 | 0·74 | 1·67 | 0·6005 |
| Other/Other | 23 | 249 | 1612 | 14·3 | 1·21 | 0·68 | 2·13 | 0·5181 |
| Not reported | 51 | 830 | 3332 | 15·3 | 1·15 | 0·73 | 1·80 | 0·5402 |
Incidence rate per 1000 person-years.
Incidence rate ratios adjusted for all variables shown in the table through a multivariate Poisson regression model. Model excludes seven patients who received a splenectomy with an unknown date of procedure.
Excludes seven patients who received a splenectomy but the date of the procedure was unknown.
Ref = reference category used to calculate adjusted incidence rate ratio for each variable.
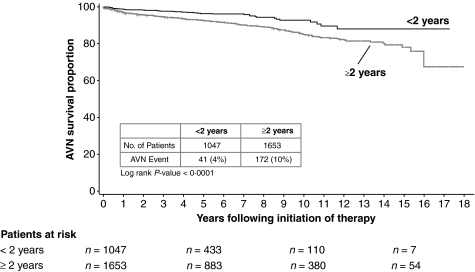
The Kaplan–Meier analysis revealed a reduction in risk of AVN after 5 years of ERT among patients who began treatment within 2 years of diagnosis and this decrease in risk became more striking with subsequent follow up (Fig 1).
Fig 1.
Kaplan–Meier curve.
Discussion
Avascular necrosis is a severe and irreversible complication of GD1 that frequently leads to functional disability (Wolfe & Taylor-Butler, 2000). Even after acute debilitating symptoms subside, patients suffer from chronic joint destruction, pain and progressive immobility. Joint replacement is frequently necessary, but, despite modern techniques, surgery is not always effective in restoring freedom from dependency on orthopaedic assistive devices or wheelchair confinement (Sims et al, 2008; Weinreb et al, 2007). Reports of patients on imiglucerase treatment, which is highly effective in reversing the haematological and splenic/hepatic manifestations of GD1, indicate decreased bone pain (El-Beshlawy et al, 2006) and bone crises (Charrow et al, 2007), improved marrow composition, bone mass (El-Beshlawy et al, 2006; Rosenthal et al, 1995), bone mineral density (Bembi et al, 2002; Wenstrup et al, 2007), growth rates in children (Bembi et al, 2002; El-Beshlawy et al, 2006) and decreased numbers of other skeletal complications (Sims et al, 2008).
Using data from the ICGG Gaucher Registry, this study reports the incidence rates of AVN in a large, international, diverse GD1 patient population after initiation of ERT. The overall risk of AVN following treatment initiation was 13·8 per 1000 person years of follow-up. The risk of AVN on ERT was lower among patients who began treatment within 2 years of diagnosis compared to those who started treatment after 2 years or more. The risk following treatment initiation was not associated with any GBA genotype category but was highly associated with splenectomy status.
The natural history and clinical manifestations of GD1 are markedly heterogeneous. In particular, the occurrence of AVN is unpredictable and its predisposing risk factors and mechanistic basis are not yet understood. There is no apparent correlation of risk of AVN with the severity of splenic, hepatic or haematological disease. However, we observed that patients who started treatment 2 years or more after GD diagnosis reported more bone crises around the time of treatment initiation (Table III). It is possible that bone crises may be an intermediate step in disease progression and indicator of future development of AVN. In addition, one report (Wolfe & Taylor-Butler, 2000) linked AVN and bone disease in GD1 with blood cell damage, which may be associated with activation of the coagulation cascade including the abnormal presence of thrombin-anti-thrombin complexes or increases in D-dimer (a particular fibrin degradation product) concentrations (Hollak et al, 1997). Abnormalities in red cell deformability may also be associated with GD (Bax et al, 2005) and AVN (Wolfe & Taylor-Butler, 2000). Persistent increases in plasma macrophage inflammatory proteins MIP-α and MIP-β as well as other biomarkers of chronic inflammation have also been reported in patients both with and without GD1-associated AVN (van Breemen et al, 2007). Perturbations in immunoregulatory anti-inflammatory cytokines such as interleukin-10 have also been reported in association with AVN (Mori et al, 2001) as well as in experimental GD models and GD1 patients (de Fost et al, 2008; Kacher & Futerman, 2009).
It is unclear as to how these abnormalities relate to the primary pathophysiology of GD1 or whether they directly contribute to the development of AVN. Patients who were diagnosed with GD1 before ERT was available often suffered from progressive disease complications. Currently, however, once diagnosed, symptomatic patients may be promptly treated. The incremental risk for AVN with increasing interval from diagnosis to initiation of ERT suggests that the probability of developing post-treatment AVN is substantially impacted by the total duration of the pre-treatment phase. Studies using animal models (Enquist et al, 2006) may provide insight into whether prolonged untreated GD1 leads to refractory changes in bone microvasculature due to chronic inflammation that may limit the effectiveness of ERT for prevention of AVN.
For patients without reported osteonecrosis who begin treatment 2 or more years after diagnosis of GD1, the Kaplan–Meier analysis suggests there is a 20% probability of developing AVN within 15–16 years (Fig 1). In children and young adults, this represents a significant lifetime risk. However, the multivariate Poisson regression model shows that, aside from timing of therapy initiation, prior splenectomy is also an independent risk factor for the development of AVN while on imiglucerase therapy. Because nearly one third of the patients who began treatment 2 or more years after diagnosis of GD1 had undergone splenectomy and as this procedure is performed much more rarely at present, our projection of lifetime risk of post-ERT AVN may be overstated for newly diagnosed patients.
The role of splenectomy in increasing the risk of AVN has been controversial with reports both in favour (Fleshner et al, 1991) as well as against such an association (Lee, 1982). Here, we found the risk of AVN in splenectomized patients after starting ERT is more than twofold higher than that of non-splenectomized patients; furthermore this increased risk persisted with enzyme therapy. Prior to the availability of ERT, splenectomy was commonly performed to control signs and symptoms of GD1 including severe cytopenias and abdominal discomfort (Cox et al, 2008). Splenectomy should only be considered in exceptional circumstances after assessment by a physician experienced in the management of GD (Cox et al, 2008).
This analysis has a number of limitations related to the Gaucher Registry design and study exclusion criteria. All Registry data are retrospective and unaudited. We have not attempted to ascertain the incidence of AVN in asymptomatic or mildly affected GD1 patients who are not considered to be candidates for ERT. Because criteria for initiation of ERT are not dictated by the Registry and vary considerably among physicians and geographic locales, the population of treated patients that analysed in this study was clinically heterogeneous. The treatment groups were comparable in terms of ERT dosing. However, we did not attempt to determine whether imiglucerase dose or schedule affects the overall incidence of AVN.
Furthermore, because this was not a randomized trial, there is always the concern of residual confounding, particularly according to variables not captured in the Registry. We were unable to assess the influence of known non-GD1 related risk factors for AVN, such as corticosteroids, alcohol, trauma and cancer (Wolfe & Taylor-Butler, 2000). However, we believe residual confounding according to these variables is unlikely, as bias would have been introduced into our study only if the distributions of such risk factors varied according to the time interval between Gaucher diagnosis and initiation of therapy.
A common concern in any observational study that relies on diagnostic screening for outcome ascertainment is the possibility of lead-time bias (Rothman, 2002). If this bias were present in our study, the differences in the incidence of AVN may be explained by systematically different levels of AVN screening in the study groups. It may be hypothesized that patients who initiated therapy sooner following Gaucher diagnosis may have received more screening and follow-up than others. This increased screening and follow-up would be expected to detect onset of AVN earlier and more frequently. However, our data indicate lower rates of AVN in patients who initiated therapy within 2 years following diagnosis, suggesting that lead-time bias could not have played a role in our study.
In this study we did not assess the incidence rates of AVN in untreated patients as such an analysis could invite assumptions about the effect of ERT on rates of AVN in Gaucher disease. The analytical methods used in this paper are not designed to assess the therapeutic effect of any particular treatment. A separate study of the factors that influence the rates of AVN in untreated Gaucher disease is in progress.
The Registry does not have standardized diagnostic criteria for reporting AVN. AVN may, at times, be asymptomatic and evolve slowly over years rendering the diagnosis difficult. The Registry has formulated a recommended schedule of skeletal assessments but compliance is voluntary. The requested anatomical sites for serial imaging are restricted to the hips, femora, pelvis, and, more recently, the lumbosacral spine. Asymptomatic AVN sites in other skeletal areas may therefore be missed, causing underestimation of the incidence in all groups that we studied. Moreover, we do not yet know the extent to which a radiological finding of AVN in an asymptomatic patient correlates with eventual functional disability and decreased health-related quality of life. Therefore, some caution should be exercised in concluding that the decreased incidence of de novo post-treatment AVN that we observed in GD1 patients in whom imiglucerase infusions were initiated within 2 years of diagnosis translates into an unequivocal clinical benefit. However, our findings clearly demonstrate that in some patients, later initiation of therapy following diagnosis can potentially result in skeletal pathology that may cause irreversible morbidity and disability. Pending a better understanding of the biological mechanisms that contribute to the development of AVN in patients with GD1 our findings support earlier rather than later therapeutic intervention in symptomatic patients.
Acknowledgments
We would like to thank the patients with type 1 (non-neuronopathic) Gaucher disease and their physicians and health care personnel who submit data to the Gaucher Registry; the Gaucher Registry support team at Genzyme Corporation (Cambridge, MA, USA); Andrea Gwosdow, PhD for assistance in preparing the manuscript and Robert Brown for assistance preparing the figure. Logistical support for this work was provided by Genzyme Corporation. The database for the ICGG Gaucher Registry is supported by Genzyme Corporation.
Author disclosures
Neal Weinreb receives educational grants from Genzyme Corporation; Pramod Mistry and Ashok Vellodi receive research grants from Genzyme Corporation; Patrick Deegan, Ashok Vellodi and Pramod Mistry have received speaking fees from Genzyme Corporation. Michael Yeh and Alexander Cole are employed by Genzyme Corporation.
Author contributions
Pramod Mistry, MD: Dr Mistry was responsible for the hypothesis, overall concept, analyses and data interpretation. He oversaw the writing of the manuscript.
J. Alexander Cole, DSc, MPH: Dr Cole was primarily responsible for the overall statistical analyses, including the overall concept and data interpretation.
Ashok Vellodi, FRCPCH: Dr Vellodi made contributions to the overall interpretation of the data and the content of the manuscript.
Patrick Deegan, MD: Dr Deegan assisted in the overall interpretation of the data, compiling the site of AVN data and the content of the manuscript.
Neal Weinreb, MD: Dr Weinreb assisted in the drafting and editing of the manuscript and interpretation of data.
Michael Yeh, MD: Dr Yeh assisted in the interpretation of data, the content of the manuscript and editing the manuscript.
Other contributors
Andrea Gwosdow, PhD: Dr Gwosdow is a professional medical writer supported by Genzyme Corporation. Dr Gwosdow was responsible for writing and managing the manuscript. This included writing the first draft of the manuscript, managing author reviews and synthesising the comments of each individual author into each draft of the manuscript.
Robert Brown: Robert Brown is a graphic artist employed by Genzyme Corporation who imported the figure into a graphics programme to produce the final figure submitted.
Funding sources
Biostatistical support for this study was provided by the Biostatistics Unit of Genzyme Corporation. Pramod Mistry is supported by NIH NIDDK K24DK066306 mid-career clinical investigator award.
Conflict of interests
Pramod Mistry, Patrick Deegan, Ashok Vellodi and Neal Weinreb have received travel reimbursements and/or honoraria and/or research support from Genzyme Corporation, Shire Pharmaceuticals, Amicus Therapeutics and Actelion. Alexander Cole and Michael Yeh were employed by Genzyme Corporation when the work was conducted.
Supporting information
Additional Supporting Information may be found in the online version of this article:
Table SI. Additional characteristics of patients.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Andersson HC, Charrow J, Kaplan P, Mistry P, Pastores GM, Prakash-Cheng A, Rosenbloom BE, Scott CR, Wappner RS, Weinreb NJ. Individualization of long-term enzyme replacement therapy for Gaucher disease. Genetics in Medicine. 2005;7:105–110. doi: 10.1097/01.gim.0000153660.88672.3c. [DOI] [PubMed] [Google Scholar]
- Barton NW, Brady RO, Dambrosia JM, Di Bisceglie AM, Doppelt SH, Hill SC, Mankin HJ, Murray GJ, Parker RI, Argoff CE. Replacement therapy for inherited enzyme deficiency – macrophage-targeted glucocerebrosidase for Gaucher’s disease. New England Journal of Medicine. 1991;324:1464–1470. doi: 10.1056/NEJM199105233242104. [DOI] [PubMed] [Google Scholar]
- Bax BE, Richfield L, Bain MD, Mehta AB, Chalmers RA, Rampling MW. Haemorheology in Gaucher disease. European Journal of Haematology. 2005;75:252–258. doi: 10.1111/j.1600-0609.2005.00496.x. [DOI] [PubMed] [Google Scholar]
- Bembi B, Ciana G, Mengel E, Terk MR, Martini C, Wenstrup RJ. Bone complications in children with Gaucher disease. British Journal of Radiology. 2002;75(Suppl. 1):A37–A44. doi: 10.1259/bjr.75.suppl_1.750037. [DOI] [PubMed] [Google Scholar]
- van Breemen MJ, de Fost M, Voerman JS, Laman JD, Boot RG, Maas M, Hollak CE, Aerts JM, Rezaee F. Increased plasma macrophage inflammatory protein (MIP)-1alpha and MIP-1beta levels in type 1 Gaucher disease. Biochimica et Biophysica Acta. 2007;1772:788–796. doi: 10.1016/j.bbadis.2007.04.002. [DOI] [PubMed] [Google Scholar]
- Charrow J, Andersson HC, Kaplan P, Kolodny EH, Mistry P, Pastores G, Rosenbloom BE, Scott CR, Wappner RS, Weinreb NJ, Zimran A. The Gaucher registry: demographics and disease characteristics of 1698 patients with Gaucher disease. Archives of Internal Medicine. 2000;160:2835–2843. doi: 10.1001/archinte.160.18.2835. [DOI] [PubMed] [Google Scholar]
- Charrow J, Dulisse B, Grabowski GA, Weinreb NJ. The effect of enzyme replacement therapy on bone crisis and bone pain in patients with type 1 Gaucher disease. Clinical Genetics. 2007;71:205–211. doi: 10.1111/j.1399-0004.2007.00769.x. [DOI] [PubMed] [Google Scholar]
- Cox TM, Schofield JP. Gaucher’s disease: clinical features and natural history. Baillieres Clinical Haematology. 1997;10:657–689. doi: 10.1016/s0950-3536(97)80033-9. [DOI] [PubMed] [Google Scholar]
- Cox TM, Aerts JM, Belmatoug N, Cappellini MD, Vom Dahl S, Goldblatt J, Grabowski GA, Hollak CE, Hwu P, Maas M, Martins AM, Mistry PK, Pastores GM, Tylki-Szymanska A, Yee J, Weinreb N. Management of non-neuronopathic Gaucher disease with special reference to pregnancy, splenectomy, bisphosphonate therapy, use of biomarkers and bone disease monitoring. Journal of Inherited Metabolic Disease. 2008;31:319–336. doi: 10.1007/s10545-008-0779-z. [DOI] [PubMed] [Google Scholar]
- El-Beshlawy A, Ragab L, Youssry I, Yakout K, El-Kiki H, Eid K, Mansour IM, Abd El-Hamid S, Yang M, Mistry PK. Enzyme replacement therapy and bony changes in Egyptian paediatric Gaucher disease patients. Journal of Inherited Metabolic Disease. 2006;29:92–98. doi: 10.1007/s10545-006-0121-6. [DOI] [PubMed] [Google Scholar]
- Elstein D, Itzchaki M, Mankin HJ. Skeletal involvement in Gaucher’s disease. Baillieres Clinical Haematology. 1997;10:793–816. doi: 10.1016/s0950-3536(97)80041-8. [DOI] [PubMed] [Google Scholar]
- Enquist IB, Nilsson E, Ooka A, Mansson JE, Olsson K, Ehinger M, Brady RO, Richter J, Karlsson S. Effective cell and gene therapy in a murine model of Gaucher disease. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:13819–13824. doi: 10.1073/pnas.0606016103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleshner PR, Aufses AH, Jr, Grabowski GA, Elias R. A 27-year experience with splenectomy for Gaucher’s disease. American Journal of Surgery. 1991;161:69–75. doi: 10.1016/0002-9610(91)90363-i. [DOI] [PubMed] [Google Scholar]
- de Fost M, Out TA, de Wilde FA, Tjin EP, Pals ST, van Oers MH, Boot RG, Aerts JF, Maas M, Vom Dahl S, Hollak CE. Immunoglobulin and free light chain abnormalities in Gaucher disease type I: data from an adult cohort of 63 patients and review of the literature. Annals of Hematology. 2008;87:439–449. doi: 10.1007/s00277-008-0441-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabowski GA. Recent clinical progress in Gaucher disease. Current Opinion in Pediatrics. 2005;17:519–524. doi: 10.1097/01.mop.0000172702.33128.19. [DOI] [PubMed] [Google Scholar]
- Grabowski GA, Barton NW, Pastores G, Dambrosia JM, Banerjee TK, McKee MA, Parker C, Schiffmann R, Hill SC, Brady RO. Enzyme therapy in type 1 Gaucher disease: comparative efficacy of mannose-terminated glucocerebrosidase from natural and recombinant sources. Annals of Internal Medicine. 1995;122:33–39. doi: 10.7326/0003-4819-122-1-199501010-00005. [DOI] [PubMed] [Google Scholar]
- Grabowski GA, Leslie N, Wenstrup R. Enzyme therapy for Gaucher disease: the first 5 years. Blood Reviews. 1998;12:115–133. doi: 10.1016/s0268-960x(98)90023-6. [DOI] [PubMed] [Google Scholar]
- Grabowski G, Kolodny EH, Weinreb N, Rosenbloom BE, Prakash-Cheng A, Kaplan P, Charrow J, Pastores GM, Mistry PK. Gaucher disease: phenotypic and genetic variation. In: Scriver CR, Beaudet AL, Valle D, Vogelstein WS, Kinzler KW, Childs B, editors. The Online Metabolic and Molecular Basis of Inherited Metabolic Disease. New York, NY: McGraw-Hill Companies; 2006. Available at: http://genetics.accessmedicine.com/mmbid/public/co_contents/toc_part16.html (accessed 11 June 2009. [Google Scholar]
- Hollak CE, Levi M, Berends F, Aerts JM, van Oers MH. Coagulation abnormalities in type 1 Gaucher disease are due to low-grade activation and can be partly restored by enzyme supplementation therapy. British Journal of Haematology. 1997;96:470–476. doi: 10.1046/j.1365-2141.1997.d01-2076.x. [DOI] [PubMed] [Google Scholar]
- Kacher Y, Futerman AH. Impaired IL-10 transcription and release in animal models of Gaucher disease macrophages. Blood Cells and Molecules & Diseases. 2009;43:134–137. doi: 10.1016/j.bcmd.2009.03.006. [DOI] [PubMed] [Google Scholar]
- Kaplan P, Andersson HC, Kacena KA, Yee JD. The clinical and demographic characteristics of nonneuronopathic Gaucher disease in 887 children at diagnosis. Archives of Pediatric and Adolescent Medicine. 2006;160:603–608. doi: 10.1001/archpedi.160.6.603. [DOI] [PubMed] [Google Scholar]
- Lee RE. The pathology of Gaucher disease. In: Desnick RJ, Grabowski GA, editors. Gaucher Disease: A Century of Delineation and Research. New York, NY: Liss; 1982. pp. 177–217. [Google Scholar]
- Maaswinkel-Mooij P, Hollak C, van Eysden-Plaisier M, Prins M, Aerts H, Poll R. The natural course of Gaucher disease in The Netherlands: implications for monitoring of disease manifestations. Journal of Inherited Metabolic Disease. 2000;23:77–82. doi: 10.1023/a:1005655031239. [DOI] [PubMed] [Google Scholar]
- Meikle PJ, Hopwood JJ, Clague AE, Carey WF. Prevalence of lysosomal storage disorders. Journal of the American Medical Association. 1999;281:249–254. doi: 10.1001/jama.281.3.249. [DOI] [PubMed] [Google Scholar]
- Mori A, Hashino S, Kobayashi S, Tanaka J, Yamamoto Y, Asaka M, Imamura M. Avascular necrosis in the femoral head secondary to bone marrow infarction in a patient with graft-versus-host disease after unrelated bone marrow transplantation. Annals of Hematology. 2001;80:238–242. doi: 10.1007/s002770000253. [DOI] [PubMed] [Google Scholar]
- Rosenthal DI, Doppelt SH, Mankin HJ, Dambrosia JM, Xavier RJ, McKusick KA, Rosen BR, Baker J, Niklason LT, Hill SC. Enzyme replacement therapy for Gaucher disease: skeletal responses to macrophage-targeted glucocerebrosidase. Pediatrics. 1995;96:629–637. [PubMed] [Google Scholar]
- Rothman K. Epidemiology: An Introduction. Oxford; New York: Oxford University Press; 2002. [Google Scholar]
- Sims KB, Pastores GM, Weinreb NJ, Barranger J, Rosenbloom BE, Packman S, Kaplan P, Mankin H, Xavier R, Angell J, Fitzpatrick MA, Rosenthal D. Improvement of bone disease by imiglucerase (Cerezyme) therapy in patients with skeletal manifestations of type 1 Gaucher disease: results of a 48-month longitudinal cohort study. Clinical Genetics. 2008;73:430–440. doi: 10.1111/j.1399-0004.2008.00978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taddei TH, Kacena KA, Yang M, Yang R, Malhotra A, Boxer M, Aleck KA, Rennert G, Pastores GM, Mistry PK. The underrecognized progressive nature of N370S Gaucher disease and assessment of cancer risk in 403 patients. American Journal of Hematology. 2009;84:208–214. doi: 10.1002/ajh.21362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinreb NJ, Charrow J, Andersson HC, Kaplan P, Kolodny EH, Mistry P, Pastores G, Rosenbloom BE, Scott CR, Wappner RS, Zimran A. Effectiveness of enzyme replacement therapy in 1028 patients with type 1 Gaucher disease after 2 to 5 years of treatment: a report from the Gaucher Registry. American Journal of Medicine. 2002;113:112–119. doi: 10.1016/s0002-9343(02)01150-6. [DOI] [PubMed] [Google Scholar]
- Weinreb N, Barranger J, Packman S, Prakash-Cheng A, Rosenbloom B, Sims K, Angell J, Skrinar A, Pastores G. Imiglucerase (Cerezyme®) improves quality of life in patients with skeletal manifestations of Gaucher disease. Clinical Genetics. 2007;71:576–588. doi: 10.1111/j.1399-0004.2007.00811.x. [DOI] [PubMed] [Google Scholar]
- Weinreb NJ, Andersson HC, Banikazemi M, Barranger J, Beutler E, Charrow J, Grabowski GA, Hollak CE, Kaplan P, Mankin H, Mistry PK, Rosenbloom BE, Vom Dahl S, Zimran A. Prevalence of type 1 Gaucher disease in the United States. Archives of Internal Medicine. 2008;168:326–327. doi: 10.1001/archinternmed.2007.128. author reply 327–328. [DOI] [PubMed] [Google Scholar]
- Wenstrup RJ, Kacena KA, Kaplan P, Pastores GM, Prakash-Cheng A, Zimran A, Hangartner TN. Effect of enzyme replacement therapy with imiglucerase on BMD in type 1 Gaucher disease. Journal of Bone Mineral Research. 2007;22:119–126. doi: 10.1359/jbmr.061004. [DOI] [PubMed] [Google Scholar]
- Wolfe CJ, Taylor-Butler KL. Avascular necrosis. A case history and literature review. Archives of Family Medicine. 2000;9:291–294. doi: 10.1001/archfami.9.3.291. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.