Abstract
Chlamydiae are obligate intracellular pathogens that must coordinate the acquisition of host cell-derived biosynthetic constituents essential for bacterial survival. Purified chlamydiae contain several lipids that are typically found in eukaryotes, implying the translocation of host cell lipids to the chlamydial vacuole. Acquisition and incorporation of sphingomyelin occurs subsequent to transport from Golgi-derived exocytic vesicles, with possible intermediate transport through endosomal multivesicular bodies. Eukaryotic host cell-derived sphingomyelin is essential for intracellular growth of Chlamydia trachomatis, but the precise role of this lipid in development has not been delineated. The present study identifies specific phenotypic effects on inclusion membrane biogenesis and stability consequent to conditions of sphingomyelin deficiency. Culturing infected cells in the presence of inhibitors of serine palmitoyltransferase, the first enzyme in the biosynthetic pathway of host cell sphingomyelin, resulted in loss of inclusion membrane integrity with subsequent disruption in normal chlamydial inclusion development. Surprisingly, this was accompanied by premature redifferentiation to and release of infectious elementary bodies. Homotypic fusion of inclusions was also disrupted under conditions of sphingolipid deficiency. In addition, host cell sphingomyelin synthesis was essential for inclusion membrane stability and expansion that is vital to reactivation of persistent chlamydial infection. The present study implicates both the Golgi apparatus and multivesicular bodies as key sources of host-derived lipids, with multivesicular bodies being essential for normal inclusion development and reactivation of persistent C. trachomatis infection.
Author Summary
The genus Chlamydia is composed of a group of obligate intracellular bacterial pathogens that cause several human diseases of medical significance. C. trachomatis is the most commonly encountered sexually transmitted pathogen, as well as the leading cause of preventable blindness worldwide. The prevalence of chlamydial infections, and the extraordinary morbidity and health care costs associated with chronic persisting disease, justifies the research efforts in this area of microbial pathogenesis. Despite their clinical importance, the mechanisms by which these intracellular bacteria obtain nutrients essential to their growth remain enigmatic. Acquisition of sphingolipids, from the cells that chlamydiae infect, is essential for bacterial propagation. This study identifies a requirement for the lipid sphingomyelin from the infected host cell for bacterial replication during infection, and for long-term subsistence in persistent chlamydial infection. Blockage of sphingomyelin acquisition results in premature release of bacteria, a reduced bacterial number, and failure of the bacteria to cause a persisting infection. In this study, we have identified and subsequently disrupted specific sphingomyelin transport pathways, providing important implications on therapeutic intervention targeting this successful microbial pathogen.
Introduction
The genus Chlamydia is composed of obligate intracellular prokaryotic pathogens that cause a range of clinical sequelae in humans encompassing ocular, genital, and respiratory tract infections. Consequences of subsequent chronic disease include blindness, infertility, arthritis, and possible coronary heart disease [1],[2]. Despite their notoriety clinically, the molecular interactions between Chlamydia and its host cell that allow for propagation, persistence, and subsequent pathology, remain elusive. The defining biological characteristic of these successful pathogens is a unique process of intracellular development, with an infectious elementary body (EB) initiating uptake into a target host cell. The chlamydial EB subsequently differentiates to the noninfectious, metabolically active reticulate body (RB) within the confines of a membrane-bound vacuole termed an inclusion. Successive growth and replication, giving rise to a large inclusion body containing a multitude of infectious EBs, is contingent upon the acquisition of biosynthetic constituents from the nutrient-rich host cell cytosol. In response to nutrient or immunological stress [3], Chlamydiae can also enter into a persistent phase of development characterized by morphologically altered RBs that can be maintained intracellularly for extended periods of time. Alternating infectious and persistent phases of chlamydial growth correlate with acute and chronic infections in vivo [4]. The cellular biosynthetic constituents that sustain persistent chlamydiae, and allow for emergence from a persistent state, are poorly understood.
The intricacies of this host-pathogen interaction, which allow for acquisition of biosynthetic precursors from the host cell, remain largely undefined. Vacuole-bound chlamydiae attain nucleotides, amino acids, and lipids from the host cell [5]. Eukaryotic-derived phospholipids, sphingomyelin, and cholesterol are found within purified chlamydiae, suggesting that these host-derived constituents traverse the inclusion membrane with subsequent incorporation into the bacterium [6],[7]. Translocation of lipid droplets to the chlamydial inclusion lumen represents one potential source of neutral lipids [8],[9]. Host cell sphingolipids are required for the intracellular growth of C. trachomatis [10], with sphingomyelin attained via the intersection of the chlamydial inclusion with Golgi-derived exocytic vesicles destined for the plasma membrane [11]–[13]. Multivesicular bodies (MVBs), late endocytic organelles abundant in sphingolipids and central to intracellular lipid segregation, also serve as a source for host-derived lipids and a potential intermediate in Golgi to inclusion transport [14],[15]. To further delineate lipid acquisition pathways pirated by the chlamydial inclusion, specific inhibitors of host cell lipid biosynthesis and/or trafficking were evaluated for their effects on chlamydial growth and inclusion development. The present study focuses on sphingomyelin biosynthesis, a host cell pathway validated as essential for growth and replication of chlamydiae by Engel and colleagues [10]. Our studies indicate that sphingomyelin biosynthesis is requisite to inclusion membrane biogenesis and stability, and demonstrate that MVBs are a major source for this essential lipid.
Results
Inhibition of host cell sphingomyelin biosynthesis results in loss of inclusion membrane integrity
Specific inhibitors of sphingomyelin biosynthesis and trafficking were evaluated for effects on chlamydial growth and inclusion development. Treatment of infected cells with 25 µM myriocin, a potent inhibitor of serine palmitoyltransferase (SPT), the initial enzyme in the biosynthesis of sphingomyelin (Figure 1) [16], revealed striking morphological alterations in inclusion maturation. Confocal analysis of untreated Chlamydia-infected cells revealed normal inclusion development with the vacuole expanding in size from 24 to 36 hr postinfection (pi) (Figure 2). Infected cells cultured in the presence of myriocin, revealed a marked loss of inclusion membrane integrity with disruption of the inclusion and release of intracellular bacteria, initially evident at 24 hr pi (22% of infected cells with disrupted inclusions) and most notable at 30 hr pi (61%) (Figure 2). At 36 hr pi, myriocin-treated cells contained small multiple inclusions of heterogeneous size, rather than the large single inclusion typical of untreated cells (Figure 2). The concentration of myriocin used in these studies had no effect on host cell viability.
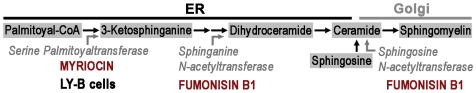
Figure 1. The sphingomyelin biosynthetic pathway.
The precursors of sphingomyelin are synthesized in the endoplasmic reticulum with subsequent transfer of ceramide to the Golgi apparatus, the site of the final step in sphingomyelin biosynthesis. The targets for the sphingomyelin inhibitors myriocin and fumonsin B1 are indicated, as well as, the site of enzymatic deficiency of LY-B cells.
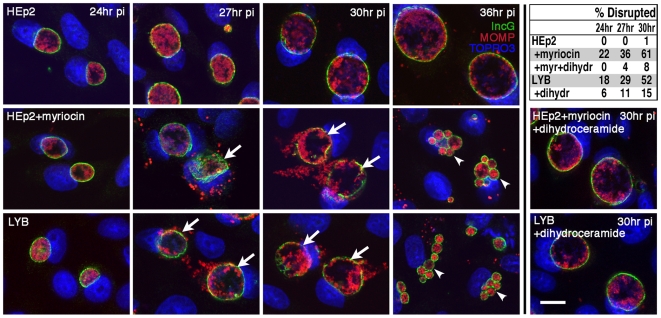
Figure 2. Inhibition in sphingomyelin biosynthesis results in the disruption of inclusion stability.
HEp-2 or LY-B cells were infected with C. trachomatis E (MOI 0.2) and treated with 25 µM myriocin at 1 hr pi where indicated. Infected cells were fixed at 24, 27, 30, and 36 hr pi and subsequently immunolabeled with anti-incG antibody (anti-rabbit Alexa Fluor 488) and anti-MOMP antibody (anti-mouse Alexa Fluor 568) to precisely identify the boundary of the chlamydial inclusion and the intrainclusion bacteria, respectively. TOPRO-3 labeling was used to identify both intracellular bacteria and the host cell nuclei. Analysis of 0.5 µm confocal optical sections of infected cells revealed disruption of inclusion integrity in HEp-2 cells treated with myriocin, or in SPT-deficient LY-B cells (indicated by white arrows). Disruption of inclusions resulted in early lysis of infected cells and reinfection evident at 36 hr pi (indicated by white arrowheads identifying multiple inclusions). Lower right panels: Supplementing the culture medium with 5 µM dihydroceramide for 48 hr prior to infection of HEp-2 or LY-B cells reversed the inhibitory effect of SPT inactivity. Scale bar = 20 µm. Upper right table: The percent of disrupted inclusions was quantitated for the cells and treatment conditions indicated.
The CHO-K1 mutant cell line, LY-B [17], which contains a mutation in the LCB1 gene and therefore does not express SPT, was used to independently test the role of sphingomyelin. C. trachomatis inclusions in LY-B cells showed a collapse of membrane integrity, similar to myriocin treatment (Figure 2). In addition, at 36 hr pi, LY-B-infected cells contained small multiple inclusions comparable to those observed in myriocin-treated HEp-2 cells. The complemented cell line, LY-B/LCB1, supported normal inclusion development comparable to that observed in both CHO-K1 and HEp-2 cells (data not shown), confirming that maintenance of inclusion membrane integrity was dependent on host cell SPT activity.
To confirm that the loss of inclusion membrane integrity was a consequence of a deficiency in host cell sphingomyelin rather than an indirect effect of depleted SPT activity, cells were cultured in the presence of 5 µM dihydroceramide or 5 µM sphingosine prior to infection. Dihydroceramide and sphingosine are precursors of sphingomyelin, positioned downstream of SPT, allowing for the restoration of sphingomyelin synthesis under conditions of SPT inactivity (Figure 1) [10],[18]. These sphingomyelin precursors reversed the detrimental effects of SPT-deficiency in LY-B cells or myriocin-treated HEp-2 cells, with growth and expansion of intact inclusions morphologically comparable to those present in untreated control cells at 24 to 36 hr pi (Figure 2) (data for sphingosine not shown).
Inhibition of host cell sphingomyelin biosynthesis results in early redifferentiation and premature release of infectious chlamydiae
The intracellular developmental cycle of C. trachomatis E requires approximately 72 hr to complete, with redifferentiation of RBs to infectious EBs occurring prior to release of infectious progeny. At 24 to 36 hr pi, the expanding inclusion contains predominantly noninfectious RBs that, if released indiscriminately from the infected cell, are incapable of initiating an infectious cycle. The presence of multiple small inclusions at 36 hr pi, under conditions of disrupted host cell sphingomyelin biosynthesis, suggested premature release of infectious progeny and subsequent reinfection. To analyze possible early emergence of infectious EBs, the expression of OmcB, an EB-specific protein detectable late in the developmental cycle, was analyzed. In untreated cells, low levels of OmcB were evident at 30 to 36 hr pi (Figure 3), with peak levels emerging at 48 to 72 hr as inclusions reached maximal size and approached lysis (not shown). Myriocin treatment resulted in expression of OmcB as early as 24 hr pi with EBs being dispersed upon premature loss of both inclusion and host cell membrane integrity (Figure 3). Infected SPT-deficient LY-B cells also displayed early emergence of OmcB-positive EBs, temporally similar to those observed under conditions of myriocin treatment (not shown). Western blot analysis confirmed the higher levels of OmcB at 27–36 hr pi in infected cells treated with myriocin as compared to control cells (Figure 3). In addition, higher levels of infectious progeny were released from myriocin-treated cells versus control cells at early times post infection (Figure 3). Collectively, these results indicate that the absence of sphingomyelin results in loss of inclusion membrane integrity, early redifferentiation, and premature release of infectious chlamydiae.
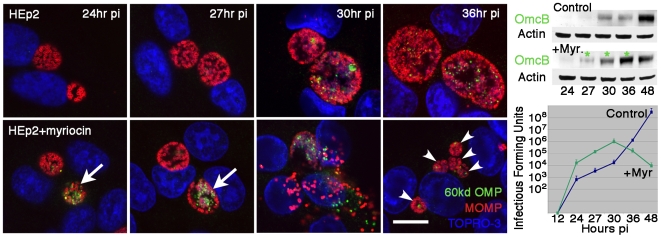
Figure 3. Inhibition in sphingomyelin biosynthesis results in the early redifferentiation of RBs to infectious EBs.
HEp-2 were infected with C. trachomatis E (MOI 0.2) and treated with 25 µM myriocin at 1 hr pi where indicated. Infected cells were fixed at 24, 27, 30, and 36 hr pi and subsequently immunolabeled with anti-OmcB antibody (anti-rabbit Alexa Fluor 488) and anti-MOMP antibody (anti-mouse Alexa Fluor 568). TOPRO-3 labeling was used to identify both intracellular bacteria and the host cell nuclei. Analysis of 0.5 µm confocal optical sections of infected cells revealed early redifferentiation from RBs to EBs as indicated by the presence of OmcB specific for EBs in myriocin-treated cells (indicated by white arrows). Disruption of inclusions resulted in early lysis of infected cells and reinfection evident at 36 hr pi (indicated by white arrowheads identifying multiple inclusions). Scale bar = 20 µm. Upper right panel: Western blot analysis of OmcB levels in untreated control and myriocin-treated cells revealed the early emergence of OmcB in myriocin-treated cells (indicated by asterisks). Analysis of actin levels served as a loading control. Lower right panel: Recovery of infectious Chlamydia from untreated control and myriocin-treated cells. Data are presented as mean infectious forming units of triplicate cultures+/−s.e.m.
Host cell-derived sphingomyelin is required for homotypic fusion of inclusions
A distinguishing trait of prototypic C. trachomatis strains is homotypic fusion of inclusions [19]. Infecting a single cell with multiple EBs of a defined serovar, results in multiple bacterial-containing vacuoles that fuse early in the developmental cycle to form a single inclusion. The presence of multiple inclusions at 36 hr pi in sphingomyelin-depleted cells, suggests reinfection with subsequent disruption of homotypic fusion. To analyze the effect of sphingomyelin deficiency on homotypic fusion, cells were infected with a high MOI of five bacteria per cell and inclusion numbers were determined at 16 hr pi (Figure 4). HEp-2 and CHO-K1 cells generally contained a single inclusion per infected cell as shown in the histogram inserts. HEp-2 cells cultured in the presence of 25 µM myriocin or 5 µg/ml fumonisin B1 (a potent inhibitor of sphingonine and sphinosine N-acetyltransferase, Figure 1), or the SPT-deficient LY-B cells, revealed multiple inclusions per cell. Complementation of the LY-B cells with the LCB1 gene, resulted in the restoration of host cell sphingomyelin biosynthesis, and the recovery of the inclusion fusion phenotype as shown by a single inclusion per infected cell (Figure 4). To confirm that lack of inclusion fusion was a consequence of a deficiency in host cell sphingomyelin rather than an indirect effect of depleted SPT activity, cells were cultured in the presence of dihydroceramide and sphingosine prior to infection. These sphingomyelin precursors restored the fusion capability to infected cells cultured under conditions of SPT-deficiency with a majority of cells containing a single inclusion (Figure 4). Collectively, these findings indicate that host cell sphingomyelin biosynthesis is required for homotypic fusion of chlamydia inclusions within a single infected cell.
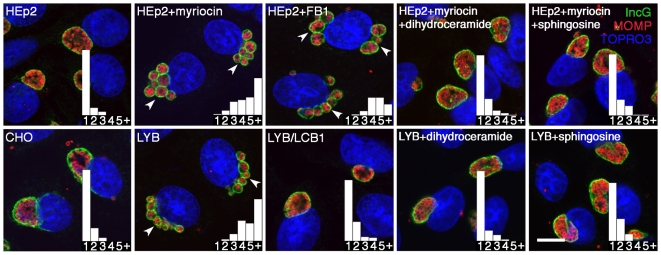
Figure 4. Host cell sphingomyelin-deficiency results in the inhibition of homotypic fusion of inclusions.
HEp-2, CHO-K1, LY-B, and LY-B/LCB1 cells were infected with C. trachomatis E (MOI 5) and treated with 25 µM myriocin or 5 µg/ml fumonisin B1 at 1 hr pi where indicated. Infected cells were fixed at 16 hr pi and subsequently immunolabeled with anti-incG antibody (anti-rabbit Alexa Fluor 488) and anti-MOMP antibody (anti-mouse Alexa Fluor 568) to precisely identify the boundary of the chlamydial inclusion and the intrainclusion bacteria, respectively. TOPRO-3 labeling was used to identify both intracellular bacteria and the host cell nuclei. Analysis of 0.5 µm confocal optical sections of infected cells revealed the inhibition of fusion of multiple inclusions to a single inclusion in HEp-2 cells treated with myriocin or fumonisin B1, or in SPT-deficient LY-B cells (indicated by white arrowheads). Right panels: Supplementing the culture medium with 5 µM dihydroceramide or 5 µM sphingosine for 48 hr prior to infection of HEp-2 or LY-B cells reversed the inhibitor effect of SPT inactivity. The relative number of inclusions per infected cell is shown in graph inserts. Scale bar = 20 µm.
Host cell-derived sphingomyelin is required for reactivation of persistent chlamydial infection
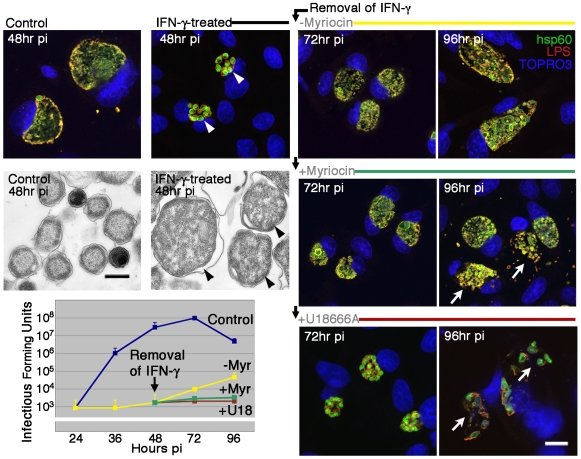
Persistence is a hallmark of natural chlamydial diseases, and is characterized by the retention of nonreplicating, aberrant reticulate bodies within the host cell for extended periods of time [3]. Host cell sphingomyelin biosynthesis is essential for maintenance of inclusion integrity during normal chlamydial development, and is likely essential during reactivation of persistent infection, a process concurrent with inclusion membrane expansion. The role of host cell sphingomyelin was tested in a model system of IFN-γ-induced persistence [20]. HEp-2 cells were infected with C. trachomatis B, a strain sensitive to IFN-γ-mediated alterations in intracellular growth [21]. Untreated Chlamydia-infected cells revealed normal inclusion development with large inclusions at 48 hr pi, while IFN-γ-treated cells harbored smaller inclusions containing enlarged RBs as confirmed by fluorescence and electron microscopy (Figure 5). The persistent state was reversible as shown by the expansion of the inclusion and reactivation of infectious EBs following removal of IFN at 48 hr pi and culturing in fresh medium for an additional 48 hr (Figure 5). In contrast, culturing in the presence of myriocin during the recovery phase resulted in disruption in inclusion membrane integrity and failure of persistent forms to completely reactivate to infectious EBs (Figure 5). These results were confirmed in an alternate in vitro model system of penicillin-induced persistence [22],[23]. In C. trachomatis serovar B- and servovar E-infected cells treated with penicillin to induce aberrant, persistent chlamydial development, the presence of myriocin during the recovery phase prevented the recovery of infectious EBs (not shown). These studies implicate host cell-derived sphingomyelin as an essential component for maintenance of inclusion membrane integrity during reactivation of persistent chlamydial infection.
Figure 5. Host cell sphingomyelin-deficiency results in inhibition of reactivation of persistent chlamydial infection.
HEp-2 cells were treated with 1 ng/ml IFN-γ where indicated, for 48 hr prior to and 1 hr after infecting with C. trachomatis B (MOI 0.2). At 48 hr pi, the IFN-γ was removed and replaced with fresh culture medium with or without myriocin (25 µM) or U18666A (10 µM), and cultured until 96 hr pi. At the indicated times pi, infected cells were immunolabeled with anti-hsp60 antibody (anti-rabbit Alexa Fluor 488) and anti-LPS antibody (anti-mouse Alexa Fluor 568). TOPRO-3 labeling was used to identify both intracellular bacteria and the host cell nuclei. Upper left panels: Analysis of 0.5 µm confocal optical sections and electron micrographs of infected cells revealed aberrant, persistent chlamydial development when cultured in the presence of IFN-γ (indicated by arrowheads). Upper right and center panels: Analysis by confocal microscopy revealed inclusion expansion with normal inclusion development when cultured in the absence of myriocin but disruption of inclusion integrity when cultured in the presence of myriocin (indicated by white arrows). Lower right panels: Culturing IFN-γ-induced persistent chlamydiae in the presence of U18666A revealed a lack of inclusion expansion and loss of inclusion membrane integrity (indicated by white arrows). Scale bar of fluorescent images = 20 µm. Scale bar of electron micrographs = 0.5 µm. Lower left panel: The effect of indicated culture conditions on the recovery of infectious organisms. Data are presented as mean infectious forming units of triplicate cultures+/−s.e.m.
Trafficking of host cell sphingomyelin from the Golgi or MVBs is required for homotypic fusion and normal inclusion development
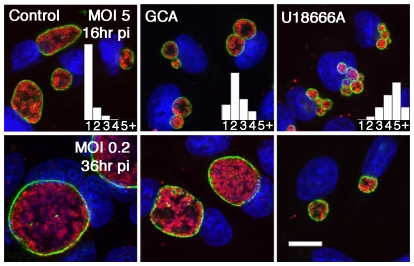
The precursors of sphingomyelin are synthesized in the endoplasmic reticulum with subsequent transfer of ceramide to the Golgi apparatus, the site of the final step in sphingomyelin biosynthesis (Figure 1). Hackstadt and colleagues have demonstrated the transport of sphingomyelin from the Golgi to the chlamydial inclusion, with incorporation of the sphingolipid into the inclusion membrane and the cell wall of chlamydiae [12],[13]. MVBs, late endocytic organelles abundant in sphingomyelin, have been proposed to provide essential lipids to the chlamydial inclusion and may be an intermediate in Golgi to inclusion transport [14],[15]. To decipher the source of Chlamydia-acquired sphingomyelin, the phenotypic effects of inhibitors of Golgi and MVB transport on inclusion maturation were compared to inclusion development under conditions of sphingomyelin deficiency. The inhibitors were used at concentrations that disrupt transport of ceramide-derived sphingomyelin from the Golgi apparatus to the chlamydial inclusion, but have no effect on host cell viability [13],[14]. HEp-2 cells were infected with a high MOI of five bacteria per cell and treated with the indicated inhibitors at 1 hr pi, then analyzed for homotypic fusion at 16 hr pi (Figure 6). Control cells generally contained a single inclusion per infected cell as shown in the histogram inserts. HEp-2 cells were cultured in the presence of golgicide A (GCA), a potent, highly specific inhibitor of GBR1 (Golgi BFA resistence factor 1) that disrupts both anterograde and retrograde transport through the Golgi [24]. GCA-treatment revealed a slight disruption in vacuole fusion with a mean of 2.6 inclusions per infected cell (Figure 6), with a similar result observed upon treatment with 1 µg/ml brefeldin A (BFA) another inhibitor of Golgi function [25] (not shown). HEp-2 cells cultured in the presence of 10 µM U18666A, a pharmacological agent that disrupts trafficking from MVBs [26]–[28], revealed multiple inclusions per infected cell (Figure 6), similar to the conditions of sphingomyelin deficiency (Figure 4). Therefore, interruption of sphingomyelin trafficking from the Golgi delayed inclusion fusion, while a block in MVB trafficking completely impeded fusion, implicating MVBs, an organelle abundant in sphingolipids, as a principle source of chlamydiae-acquired sphingomyelin.
Figure 6. Inhibition of Golgi or MVB transport interrupts homotypic fusion and normal inclusion development.
HEp-2 cells were infected with C. trachomatis E (MOI 0.2 or 5) and treated with GCA (10 µM) or U18666A (10 µM) at 1 hr pi where indicated. Infected cells were fixed at 16 or 36 hr pi and subsequently immunolabeled with anti-incG antibody (anti-rabbit Alexa Fluor 488) and anti-MOMP antibody (anti-mouse Alexa Fluor 568) to precisely identify the boundary of the chlamydial inclusion and the intrainclusion bacteria, respectively. TOPRO-3 labeling was used to identify both intracellular bacteria and the host cell nuclei. Analysis of 0.5 µm confocal optical sections of infected cells revealed the effects of inhibitors on homotypic inclusion fusion (upper panels) or normal inclusion development (lower panels). The relative number of inclusions per infected cell is shown in graph inserts (upper panels). Scale bar = 20 µm.
To analyze the effect of inhibitors on inclusion maturation, HEp-2 cells were infected with a low MOI of C. trachomatis E, treated with the indicated inhibitors at 1 hr pi and analyzed at 36 hr pi. Confocal analysis of GCA-treated Chlamydia-infected cells revealed a slight delay in inclusion maturation with smaller inclusions compared to those in untreated control cells (Figure 6). There was no evidence of inclusion membrane instability as observed under conditions of sphingomyelin deficiency (Figure 2), indicating that sphingolipids may be acquired from an alternate source such as MVBs. Infected cells cultured in the presence of the MVB inhibitor U18666A, revealed a dramatic interruption in inclusion development with significantly smaller inclusions (Figure 6). There was no evidence of inclusion membrane instability as observed under conditions of sphingomyelin deficiency (Figure 2). However, the complete interruption in RB division and subsequent inclusion expansion, implicates additional MVB-derived constituents necessary for normal chlamydial inclusion expansion and development.
Trafficking of host cell sphingomyelin from MVBs is required for reactivation of persistent chlamydial infection
Host cell sphingomyelin biosynthesis is essential for maintenance of membrane integrity during expansion of the inclusion following reactivation of persistent infection (Figure 5). Because trafficking from MVBs was essential to sphingomyelin-dependent inclusion expansion, the potential significance of these sphingolipid-rich organelles in reactivation of persistent infection was analyzed. Following induction of the persistent state by IFN-γ treatment for 48 hr, removal of IFN and subsequent culturing in the presence of the MVB inhibitor U18666A for an additional 48 hr, resulted in a lack of inclusion expansion, disruption in inclusion membrane integrity, and complete failure of aberrant persistent forms to reactivate to infectious EBs (Figure 5). These studies implicate MVB-derived sphingomyelin, and potentially other MVB constituents, requisite to inclusion membrane integrity during reactivation of persistent chlamydial infection.
Discussion
The present studies were initiated to identify lipid biosynthetic and transport pathways essential to the intracellular propagation of chlamydiae. These studies revealed novel effects on the intracellular development of chlamydiae under conditions that inhibit sphingomyelin biosynthesis. As demonstrated in classic studies by Hackstadt and colleagues, sphingomyelin synthesized in the Golgi apparatus is transported from the trans-Golgi to the chlamydial inclusion with successive incorporation into the bacterial cell wall [12],[13]. In subsequent studies by Engel and colleagues, host cell-derived sphingomyelin was shown to be essential for intracellular development of C. trachomatis and optimal production of infectious progeny [10]. In the present study, we further explore this requirement and demonstrate that sphingomyelin biosynthesis is necessary for stability and expansion of the inclusion membrane during both normal intracellular development and reactivation of persistent infection. Blockage of this pathway results in premature egress, reduced bacterial output, and failure to emerge from a persistent state. Hence, disruption of lipid trafficking may provide a novel means to thwart intracellular pathogens.
Chlamydiae undergo their entire intracellular developmental cycle within an inclusion that is bound by a membrane, providing a protected intracellular environment for bacterial replication. Treatment of infected cells with myriocin interrupted inclusion membrane functionality, with complete disruption of membrane integrity resulting in premature dispersal of intracellular bacteria from their protected niche (Figure 2). Myriocin is a potent inhibitor of SPT, the initial enzyme in sphingomyelin biosynthesis (Figure 1) [16]. Analysis of inclusion development in SPT-deficient LY-B cells, and under conditions of concurrent pretreatment with precursors of sphingomyelin, revealed that the compromise in inclusion membrane integrity was a direct result of host cell sphingomyelin deficiency (Figure 2). Actin and intermediate filaments have been shown to stabilize the chlamydial inclusion, with disruption of these host cytoskeletal structures resulting in loss of inclusion membrane integrity and release of bacteria into the host cell cytosol [29]. In the present studies, immunofluorescence analyses of actin and intermediate filaments of both uninfected and chlamydiae-infected cells revealed no obvious morphological alterations in the cytoskeletal structure upon inhibition of sphingomyelin biosynthesis (data not shown).
The disruption of inclusion membrane integrity under conditions of sphingomyelin deficiency occurred concomitantly with the early redifferentiation of noninfectious RBs to infectious EBs (Figure 3). This implies that the procurement of host cell sphingomyelin may be required for inclusion membrane expansion and stability, and programmed conversion to infectious forms. The signals that trigger the replicative RBs to convert to infectious EBs remain elusive. However, it is clear that this developmental transformation coincides with a contact-dependent interaction of the type III secretion (TTS) system with the inclusion membrane. RBs amass at the periphery of the inclusion, with projections of the TTS system mediating intimate contact between the bacteria and the inner face of the inclusion membrane [30],[31]. The proposed chlamydial injectisome acts as a molecular syringe, translocating effector proteins directly from the intrainclusion chlamydiae to the host cell cytosol [32]. This association may be requisite to RB replication and potentially inclusion expansion allowing for nutrient acquisition from the host cell cytosol [33]. The physical detachment of RBs from the inclusion membrane, coupled to inactivation of TTS, signals the initation of late redifferentiation [32]. In the present studies, lipid deprivation may signal the loss of TTS intimate contact and RB detachment leading to premature conversion of RBs to infectious EBs. Host cell-derived sphingomyelin associates transiently with the chlamydial inclusion membrane and incorporates into the bacterial cell wall [12]. Failure of this sphingolipid to incorporate into the inclusion membrane may cause the normally contiguously intact membrane to become indiscriminately permeable to environmental changes that potentially signal RB to EB conversion. Alternately, incorporation of sphingomyelin into the chlamydial cell wall may be essential to RB division and proliferation, with lack of available sphingomyelin being a potential cue for premature redifferentiation.
A secondary function of the inclusion membrane of C. trachomatis, distinct from inclusion membrane integrity, is homotypic fusion of multiple inclusions to a single vacuole in multiply-infected cells. The resulting multiple inclusions with greater surface area would require more lipid incorporation into the chlamydial inclusion membrane, indicating that early in infection other host cell lipids are available for incorporation into the expanding inclusion under conditions of sphingomyelin deficiency. Fusion of inclusions is a temperature-dependent process that requires export of the chlamydial incA protein to the inclusion membrane [34],[35]. Characteristic homotypic fusion of inclusions was interrupted when multiply-infected cells were cultured in the presence of myriocin (Figure 4). Analysis of the fusion of multiple inclusions in SPT-deficient LY-B cells, and under conditions of concurrent pretreatment with precursors of sphingomyelin, revealed that the disruption in homotypic fusion was a direct result of host cell sphingomyelin deficiency (Figure 4). These studies did not reveal an alteration in IncA incorporation into the inclusion membrane under conditions of sphingomyelin deficiency, implicating a role for host cell sphingolipids in homotypic fusion independent of incA. Culturing C. trachomatis-infected cells under conditions of sphingomyelin deficiency has two distinct phenotypic effects on chlamydial inclusion biogenesis. Interruption in homotypic fusion is observed early in chlamydial inclusion development, while a compromise in inclusion membrane integrity occurs later. These distinct anomalies may result from the failure of sphingomyelin incorporation into the inclusion membrane, implicating a direct role for host cell lipid in maintaining normal inclusion functionality. However, the effect of sphingomyelin deficiency on other lipid biosynthetic or signaling pathways that indirectly alter inclusion biogenesis cannot be disregarded.
Further studies determined the source of sphingomyelin essential to inclusion biogenesis, which includes membrane stability and the capacity for homotypic fusion. As described previously, inhibition of sphingomyelin transport from the Golgi apparatus using the inhibitor BFA, results in smaller, compact inclusions that retain a burst size comparable to untreated controls [12]. In the present studies, this observation was reproduced using both BFA and GCA. In addition, treatment of infected cells with concentrations of BFA or GCA that prevent the incorporation of newly synthesized Golgi-derived sphingomyelin into the chlamydial inclusion, failed to completely disrupt inclusion fusion or inclusion membrane integrity (Figure 6). This implicates another source of sphingomyelin available to the chlamydial inclusion under conditions of disrupted Golgi transport. These studies identify MVBs, late endocytic organelles abundant in sphingolipids and pivotal for intracellular distribution, as a potential source of sphingomyelin essential to homotypic fusion and maintenance of inclusion membrane integrity. U18666A treatment of infected cells, utilizing concentrations that block MVB transport and prevent the incorporation of newly synthesized Golgi-derived sphingomyelin into the chlamydial inclusion [14],[15], revealed complete inhibition of homotypic fusion of inclusions (Figure 6). These findings were identical to the disruption of inclusion fusion observed under conditions of sphingomyelin deficiency (Figure 4). However, inhibition of MVB transport had much more profound effects on RB division and normal inclusion development than what was observed under conditions of sphingomyelin deficiency. A deficit in host cell sphingomyelin resulted in RB division and the expansion of the chlamydial inclusion to a moderate size with subsequent loss of inclusion membrane integrity at 24 to 36 hr pi (Figure 2). In contrast, interruption in MVB transport impeded early RB division and inclusion membrane expansion at a stage in development prior to imposing stress on inclusion membrane integrity. Collectively these studies implicate sphingomyelin, and potentially additional constituents derived from MVBs, essential for inclusion expansion during normal development and the reactivation of persistent C. trachomatis infection. However, a pleiotropic effect of inhibitors of MVB transport, on cellular function or potential acquisition of sphingomyelin from alternate sources, cannot be disregarded.
Within the confines of a protected intracellular environment, chlamydiae coordinate the expansion of the inclusion and acquisition of biosynthetic constituents from the host cell cytosol. In the presence of eukaryotic protein synthesis inhibitors, intracellular development proceeds normally, indicating that inclusion expansion may be linked to host cell lipid biosynthesis. These studies identify host cell sphingomyelin biosynthesis as a requisite to C. trachomatis inclusion membrane biogenesis and functionality. This encompasses inclusion membrane expansion, homotypic fusion, and stability during normal inclusion development and reactivation of a persistent chlamydial infection. In addition, identification of potential sphingomyelin transport pathways may have important implications when deciphering this unique host-pathogen interaction.
Materials and Methods
Antibodies and reagents
Rabbit anti-incG was kindly provided by Dr. Ted Hackstadt (Rocky Mountain Laboratories, NIH, NIAID, Hamilton, MT). Rabbit anti-outer membrane complex protein B (OmcB) was generously provided by Dr. Thomas Hatch (University of Tennessee Health Science Center, Memphis, TN). Monoclonal antibody (mAb) L2I-10 to the major outer membrane protein (MOMP) of C. trachomatis, was kindly provided by Dr. Harlan Caldwell (Rocky Mountain Laboratories, NIH, NIAID, Hamilton, MT). MAb A57B9 against the chlamydial heat shock protein-60 (hsp60), was generously provided by Dr. Richard Morrison (University of Arkansas for Medical Sciences, Little Rock, AK). Antibodies to chlamydial LPS and eukaryotic actin (clone C4) were obtained from Chemicon International (Billerica, MA). TOPRO-3 (monomeric cyanine nucleic acid stain), and secondary antibodies conjugated to Alexa Fluor 488 and Alexa Fluor 568 were obtained from Invitrogen (Eugene, OR). Myriocin, fumonisin B1, dihydroceramide, sphingosine, 3-β-(2-diethylaminoethoxy)-androstenone HCl (U18666A), and brefeldin A were obtained from BioMol International (Plymouth Meeting, PA). Recombinant human IFN-γ was purchased from BD Biosciences (San Jose, CA). Golgicide A was kindly provided by Dr. David Haslam (Washington University School of Medicine, St. Louis, MO).
Cell culture and propagation of chlamydiae
C. trachomatis serovar E (provided by Dr. Harlan Caldwell) and C. trachomatis serovar B (provided by Dr. Ted Hackstadt) were propagated in HEp-2 cells (ATCC, Manassas, VA) and elementary bodies (EBs) were purified by Renografin gradient centrifugation as previously described [36]. HEp-2 cells were maintained in Iscove's DME medium supplemented with 12.5 mM HEPES, 10% (vol/vol) FBS, and 10 µg/ml gentamicin, and grown at 37°C with 5.5% CO2. CHO-K1, LY-B, and LY-B/LCB1 cells, obtained from Dr. Kentaro Hanada (National Institute of Infectious Disease, Tokyo, Japan), were maintained in Ham's F12 medium supplemented with 10% (vol/vol) FBS, and 10 µg/ml gentamicin at 37°C with 5.5% CO2. Cells were infected by incubating monolayers with Chlamydia EBs at a multiplicity of infection (MOI) of 0.2 or 5 for 1 hr at 37°C, washed and incubated in fresh culture medium for the times indicated.
Confocal microscopy
For immunofluorescence analyses, infected cells were fixed and permeabilized for 1 min with cold methanol. Cells were then incubated with the indicated primary and fluorophore-conjugated secondary antibodies, labeled with the nucleic acid stain TOPRO-3, and mounted in ProLong Anti-Fade (Invitrogen), as previously described [14]. Images were acquired using a Zeiss LSM510 Meta laser scanning confocal microscope (Carl Zeiss Inc., Thornwood, NY) equipped with a 63X, 1.4 numerical aperature Zeiss Plan Apochromat oil objective. Confocal Z slices of 0.5 µm were obtained using the Zeiss LSM510 software.
Analysis of inhibitors
One hour post infection (pi), infected HEp-2 cells were incubated with medium containing inhibitors and the effects on inclusion development were determined by immunofluorescence, Western blot analysis, and infectivity assays, when indicated. To quantify the disruption of inclusions, one hundred infected cells were scored by fluorescence microscopy as indicated. Data are presented as the mean percent of disrupted inclusions. To quantify the number of inclusions per cell, one hundred infected cells were scored by fluorescence microscopy at 16 hr pi and presented as the mean number of inclusions per infected cell.
Infectivity assays
Infected monolayers cultured in the presence of myriocin or IFN-γ were scraped from culture dishes, and supernatant and cells were analyzed to determine the number of infectious forming units (IFU) per ml (per 7.5×105 infected cells). Data are presented as the mean+/−standard error of mean (s.e.m.) from one of three representative experiments.
SDS-PAGE and immunoblotting
At the times indicated, infected monolayers were dissolved in Laemmli buffer and equivalent protein concentrations were analyzed by 10% SDS-PAGE. Western blots were probed with antibody to chlamydial OmcB, and antibody to host cell actin, which served as a loading control.
Induction of persistence
HEp-2 cells were pretreated with 1 ng/ml IFN-γ for 48 hr prior to infecting with C. trachomatis B. Infected cells were then cultured in the presence of 1 ng/ml IFN for 48 hr, IFN was subsequently removed, and cells were incubated for an additional 48 hr with fresh culture medium with or without 25 µg/ml myriocin or 10 µM U18666A. At the indicated time points, inclusion development and infectivity were analyzed by immunofluorescence analysis and infectivity assays, respectively.
Transmission electron microscopy
For ultrastructural analysis, infected HEp-2 cells were fixed in 2% paraformaldehyde/2.5% glutaraldehyde (Polysciences Inc., Warrington, PA) in 100 mM phosphate buffer, and processed as described previously [14].
Acknowledgments
Sincere thanks to L. David Sibley for insightful discussions and comments on this manuscript.
Footnotes
The authors have declared that no competing interests exist.
This work was supported by an award from the American Heart Association (0950022G) www.americanheart.org. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Grayston JT. Background and current knowledge of Chlamydia pneumoniae and atherosclerosis. J Infect Dis. 2000;181(Suppl 3):S402–410. doi: 10.1086/315596. [DOI] [PubMed] [Google Scholar]
- 2.Schachter J. Microbiology of Chlamydia. In: Barron AL, editor. CRC Press: Boca Raton; 1988. pp. 153–165. [Google Scholar]
- 3.Beatty WL, Morrison RP, Byrne GI. Persistent chlamydiae: from cell culture to a paradigm for chlamydial pathogenesis. Microbiol Rev. 1994;58:686–699. doi: 10.1128/mr.58.4.686-699.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beatty WL, Byrne GI, Morrison RP. Repeated and persistent infection with Chlamydia and the development of chronic inflammation and disease. Trends Microbiol. 1994;2:94–98. doi: 10.1016/0966-842x(94)90542-8. [DOI] [PubMed] [Google Scholar]
- 5.McClarty G. Chlamydiae and the biochemistry of intracellular parasitism. Trends in Microbiology. 1994;2:157–164. doi: 10.1016/0966-842x(94)90665-3. [DOI] [PubMed] [Google Scholar]
- 6.Newhall WJ. Microbiology of Chlamydia. In: Barron AL, editor. Boca Raton; 1988. pp. 47–70. [Google Scholar]
- 7.Wylie JL, Hatch GM, McClarty G. Host cell phospholipids are trafficked to and then modified by Chlamydia trachomatis. J Bacteriol. 1997;179:7233–7242. doi: 10.1128/jb.179.23.7233-7242.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cocchiaro JL, Kumar Y, Fischer ER, Hackstadt T, Valdivia RH. Cytoplasmic lipid droplets are translocated into the lumen of the Chlamydia trachomatis parasitophorous vacuole. Proc Natl Acad Sci U S A. 2008;105:9379–9384. doi: 10.1073/pnas.0712241105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kumar Y, Cocchiaro J, Valdivia RH. The obligate intracellular pathogen Chlamydia trachomatis targets host lipid droplets. Curr Biol. 2006;16:1646–1651. doi: 10.1016/j.cub.2006.06.060. [DOI] [PubMed] [Google Scholar]
- 10.van Ooij C, Kalman L, van Ijzendoorn S, Nishijima M, Hanada K, et al. Host cell-derived sphingolipids are required for the intracellular growth of Chlamydia trachomatis. Cell Microbiol. 2000;2:627–637. doi: 10.1046/j.1462-5822.2000.00077.x. [DOI] [PubMed] [Google Scholar]
- 11.Carabeo RA, Mead DJ, Hackstadt T. Golgi-dependent transport of cholesterol to the Chlamydia trachomatis inclusion. Proc Natl Acad Sci U S A. 2003;100:6771–6776. doi: 10.1073/pnas.1131289100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hackstadt T, Rockey DD, Heinzen RA, Scidmore MA. Chlamydia trachomatis interrupts an exocytic pathway to acquire endogenously synthesized sphingomyelin in transit from the Golgi apparatus to the plasma membrane. EMBO Journal. 1996;15:964–977. [PMC free article] [PubMed] [Google Scholar]
- 13.Hackstadt T, Scidmore MA, Rockey DD. Lipid metabolism in Chlamydia trachomatis-infected cells: directed trafficking of Golgi-derived sphingolipids to the chlamydial inclusion. Proc Natl Acad Sci U S A. 1995;92:4877–4881. doi: 10.1073/pnas.92.11.4877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beatty WL. Trafficking from CD63-positive late endocytic multivesicular bodies is essential for intracellular development of Chlamydia trachomatis. J Cell Sci. 2006;119:350–359. doi: 10.1242/jcs.02733. [DOI] [PubMed] [Google Scholar]
- 15.Beatty WL. Late endocytic multivesicular bodies intersect the chlamydial inclusion in the absence of CD63. Infect Immun. 2008;76:2872–2881. doi: 10.1128/IAI.00129-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hanada K, Nishijima M, Fujita T, Kobayashi S. Specificity of inhibitors of serine palmitoyltransferase (SPT), a key enzyme in sphingolipid biosynthesis, in intact cells. A novel evaluation system using an SPT-defective mammalian cell mutant. Biochem Pharmacol. 2000;59:1211–1216. doi: 10.1016/s0006-2952(00)00251-3. [DOI] [PubMed] [Google Scholar]
- 17.Hanada K, Hara T, Fukasawa M, Yamaji A, Umeda M, et al. Mammalian cell mutants resistant to a sphingomyelin-directed cytolysin. Genetic and biochemical evidence for complex formation of the LCB1 protein with the LCB2 protein for serine palmitoyltransferase. J Biol Chem. 1998;273:33787–33794. doi: 10.1074/jbc.273.50.33787. [DOI] [PubMed] [Google Scholar]
- 18.Hanada K, Nishijima M, Kiso M, Hasegawa A, Fujita S, et al. Sphingolipids are essential for the growth of Chinese hamster ovary cells. Restoration of the growth of a mutant defective in sphingoid base biosynthesis by exogenous sphingolipids. J Biol Chem. 1992;267:23527–23533. [PubMed] [Google Scholar]
- 19.Ridderhof JC, Barnes RC. Fusion of inclusions following superinfection of HeLa cells by two serovars of Chlamydia trachomatis. Infect Immun. 1989;57:3189–3193. doi: 10.1128/iai.57.10.3189-3193.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beatty WL, Byrne GI, Morrison RP. Morphologic and antigenic characterization of interferon gamma-mediated persistent Chlamydia trachomatis infection in vitro. Proc Natl Acad Sci U S A. 1993;90:3998–4002. doi: 10.1073/pnas.90.9.3998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morrison RP. Differential sensitivities of Chlamydia trachomatis strains to inhibitory effects of gamma interferon. Infect Immun. 2000;68:6038–6040. doi: 10.1128/iai.68.10.6038-6040.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weiss E. The effect of antibiotics on agents of the psittacosis-lymphogranuloma group. I. The effect of penicillin. J Infect Dis. 1950;87:249–263. doi: 10.1093/infdis/87.3.249. [DOI] [PubMed] [Google Scholar]
- 23.Matsumoto A, Manire GP. Electron microscopic observations on the effects of penicillin on the morphology of Chlamydia psittaci. J Bacteriol. 1970;101:278–285. doi: 10.1128/jb.101.1.278-285.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saenz JB, Sun WJ, Chang JW, Li J, Bursulaya B, et al. Golgicide A reveals essential roles for GBF1 in Golgi assembly and function. Nat Chem Biol. 2009;5:157–165. doi: 10.1038/nchembio.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klausner RD, Donaldson JG, Lippincott-Schwartz J. Brefeldin A: insights into the control of membrane traffic and organelle structure. J Cell Biol. 1992;116:1071–1080. doi: 10.1083/jcb.116.5.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Higgins ME, Davies JP, Chen FW, Ioannou YA. Niemann-Pick C1 is a late endosome-resident protein that transiently associates with lysosomes and the trans-Golgi network. Mol Gen Metabol. 2001;68:1–13. doi: 10.1006/mgme.1999.2882. [DOI] [PubMed] [Google Scholar]
- 27.Kobayashi T, Beuchat MH, Lindsay M, Frias S, Palmiter RD, et al. Late endosomal membranes rich in lysobisphosphatidic acid regulate cholesterol transport. Nat Cell Biol. 1999;1:113–118. doi: 10.1038/10084. [DOI] [PubMed] [Google Scholar]
- 28.Kobayashi T, Vischer UM, Rosnoblet C, Lebrand C, Lindsay M, et al. The tetraspanin CD63/lamp3 cycles between endocytic and secretory compartments in human endothelial cells. Mol Biol Cell. 2000;11:1829–1843. doi: 10.1091/mbc.11.5.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kumar Y, Valdivia RH. Actin and intermediate filaments stabilize the Chlamydia trachomatis vacuole by forming dynamic structural scaffolds. Cell Host Microbe. 2008;4:159–169. doi: 10.1016/j.chom.2008.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moulder JW. Interaction of chlamydiae and host cells in vitro. Microbiol Rev. 1991;55:143–190. doi: 10.1128/mr.55.1.143-190.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bavoil PM, Hsia RC. Type III secretion in Chlamydia: a case of deja vu? Mol Microbiol. 1998;28:860–862. doi: 10.1046/j.1365-2958.1998.00861.x. [DOI] [PubMed] [Google Scholar]
- 32.Wilson DP, Timms P, McElwain DL, Bavoil PM. Type III secretion, contact-dependent model for the intracellular development of chlamydia. Bull Math Biol. 2006;68:161–178. doi: 10.1007/s11538-005-9024-1. [DOI] [PubMed] [Google Scholar]
- 33.Hackstadt T, Fischer ER, Scidmore MA, Rockey DD, Heinzen RA. Origins and functions of the chlamydial inclusion. Trends Microbiol. 1997;5:288–293. doi: 10.1016/S0966-842X(97)01061-5. [DOI] [PubMed] [Google Scholar]
- 34.Hackstadt T, Scidmore-Carlson MA, Shaw EI, Fischer ER. The Chlamydia trachomatis IncA protein is required for homotypic vesicle fusion. Cell Microbiol. 1999;1:119–130. doi: 10.1046/j.1462-5822.1999.00012.x. [DOI] [PubMed] [Google Scholar]
- 35.Fields KA, Fischer E, Hackstadt T. Inhibition of fusion of Chlamydia trachomatis inclusions at 32 degrees C correlates with restricted export of IncA. Infect Immun. 2002;70:3816–3823. doi: 10.1128/IAI.70.7.3816-3823.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Caldwell HD, Kromhout J, Schachter J. Purification and partial characterization of the major outer membrane protein of Chlamydia trachomatis. Infect Immun. 1981;31:1161–1176. doi: 10.1128/iai.31.3.1161-1176.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]