Abstract
Objectives. We assessed the health impact of a housing and case management program, the Chicago Housing for Health Partnership, for homeless people with HIV.
Methods. HIV-positive homeless inpatients at a public hospital (n = 105) were randomized to usual care or permanent housing with intensive case management. The primary outcome was survival with intact immunity, defined as CD4 count ≥ 200 and viral load < 100 000. Secondary outcomes were viral loads, undetectable viral loads, and CD4 counts.
Results. Outcomes were available for 94 of 105 enrollees (90%). Of 54 intervention participants, 35 (65%) reached permanent housing in program housing agencies. After 1 year, 55% of the intervention and 34% of the usual care groups were alive and had intact immunity (P = .04). Seventeen intervention (36%) and 9 usual care (19%) participants had undetectable viral loads (P = .051). Median viral loads were 0.89 log lower in the intervention group (P = .03). There were no statistical differences in CD4 counts.
Conclusions. Homelessness is a strong predictor of poor health outcomes and complicates the medical management of HIV. This housing intervention improved the health of HIV-positive homeless people.
Homelessness is a strong predictor of poor health outcomes.1–5 HIV infection among homeless people is common, with a prevalence ranging from 2% to 22%, depending on the location and sample selected.6–12 The combination of HIV and homelessness is particularly ominous, as homeless people are less likely to take the medications responsible for improved health among the general HIV-positive population.13 When antiretroviral therapy is prescribed, adherence can be challenging for homeless patients because of the necessary focus on meeting day-to-day needs for food, shelter, and clean clothing.14,15 In addition, a multitude of other daily challenges that are prevalent among the homeless—substance abuse, mental illness, physical disability, and poverty—makes it unlikely that any single intervention can entirely reverse these health outcomes.16
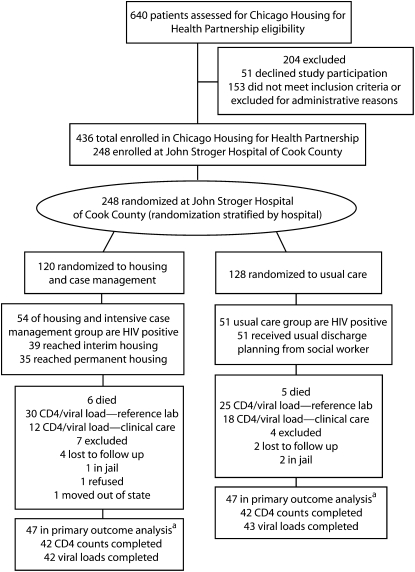
FIGURE 1.
Randomization, flow through the study, and follow-up of participants in the Chicago Housing for Health Partnership Program: Chicago, IL, October 2003–May 2006.
aSome participants who died also had CD4 counts and viral loads completed as part of their usual outpatient care after enrollment.
Despite this generally gloomy outlook, cohort studies have demonstrated that interim housing17 and supportive housing18 can reduce hospitalizations and that case management can improve medication adherence.19 As a next step, prospective, randomized controlled trials are needed to reliably estimate the true effect of supportive housing and case management. Although randomized trials of supportive housing have documented improved rates of abstinence among homeless drug users seeking treatment,20 there are no published randomized trials assessing health outcomes. Therefore, we conducted a randomized controlled trial to determine the health benefits of a broad-based intervention of permanent housing and intensive case management among homeless persons hospitalized with a chronic medical illness.
METHODS
The Chicago Housing for Health Partnership (CHHP) was developed to meet the challenge of providing housing to the most disadvantaged homeless people in the city: those with chronic illnesses who are being discharged from a hospital. The program model was community based and collaboratively created and monitored by an oversight committee composed of the leadership of all involved partner agencies. The CHHP was a group of 8 nonprofit agencies that provided supportive housing and 2 agencies that provided interim housing or respite care. Supportive housing was defined by the intervention as housing without time limits combined with services to help participants to live more stable, productive lives.
To evaluate this new service model, we designed a prospective randomized trial to examine the effect of supportive housing and intensive case management on health service utilization. For the study, we enrolled homeless patients with at least 1 of 15 chronic illnesses from 2 urban hospitals that were members of the partnership and known to have large numbers of unstably housed patients. The chronic illnesses were associated with increased mortality in the homeless21 and were verified through review of physician hospital notes. This trial is the health outcomes portion of CHHP. From these 15 chronic illnesses, we chose to focus our assessment of the health impact of CHHP on those patients who had HIV because it was the most common chronic illness that qualified patients for the study (35% of enrollees) and because HIV has objective biological markers of disease progression.
We collected blood specimens from the HIV-positive patients who were enrolled in the CHHP trial at John Stroger Hospital of Cook County from October 2003 through May 2006. We chose to prospectively focus the study on this enrollment site because pilot data indicated that the vast majority of HIV-positive enrollees would be recruited at this site and that this site includes the largest outpatient HIV care center in the Midwest that would capture CD4 counts and viral loads completed as part of usual clinical care. To contain costs, we did not obtain blood specimens from participants enrolled at the other site.
Patients
We recruited participants from the inpatient medical and surgical service of John Stroger Hospital of Cook County (formerly Cook County Hospital) in Chicago, Illinois. The hospital's social work staff recruited patients as part of their usual work activities. One of the hospital social workers was supported by the program and acted as each enrolled participant's initial case manager. We considered patients eligible for enrollment if they met the following criteria: they had no source of stable housing (i.e., housing for which there were no time limits and for which the person had adequate resources to maintain occupancy) during the 30 days before hospital admission and they were seropositive for HIV. We excluded patients if they were (1) unable to speak or understand English or Spanish, (2) unable to provide informed consent, (3) unable to care for themselves in an independent living environment, or (4) guardians of minors that required housing. To improve the generalizability of our results, we required no run-in period and made no attempts to identify participants who would be more likely to adhere to study visits.
Assessment at Enrollment
Research associates interviewed participants at the time of enrollment to obtain written informed consent and to collect demographic information. We collected information on participants' self-reported race/ethnicity because, based on the demographics of other similar homeless cohorts, we expected that a large proportion of participants would be African American, and therefore the results of the study would have implications for addressing racial/ethnic health disparities. We assessed baseline substance use with the Addiction Severity Index.22
Randomization and Intervention
We stratified the randomization for the CHHP study by the hospital of enrollment but not by diagnosis. We assigned patients to groups through the use of a computerized list generated by D. B. with group assignment sealed in an opaque numbered envelope. We completed the baseline interview before revealing the group assignment to the participant and interviewer.
Patients in the usual care group received the discharge planning usually provided to homeless individuals during a hospital stay. This planning included referrals to overnight shelters or to interim housing providers. All usual care participants were eligible to receive case management through an existing Ryan White program in the hospital-affiliated HIV/AIDS clinic. The Ryan White case managers were free to refer their clients to housing programs in the community. Peer substance abuse mentors interviewed all inpatients at this hospital as part of a separate project and provided referrals to substance abuse treatment facilities when appropriate.
A case manager who was part of the hospital's social work staff saw patients in the intervention group. This individual explained the intervention and provided referrals for interim housing after hospital discharge. Patients who could abstain from drug and alcohol use were referred to a respite care center described previously.17 Patients who could not abstain from drug or alcohol use were referred to a shelter where they could stay as long as they were not disruptive to the other guests. Although this was an overnight shelter, CHHP participants were able to stay there during the day to finish recovering from their hospitalization. Participants in the intervention group who were at the respite center or the overnight shelter were provided with a case manager who helped the participant apply for permanent supportive housing at 1 of the CHHP partner agencies.
The CHHP housing partners were a group of 8 nonprofit agencies that provided supportive housing and 2 agencies that provided interim housing or respite care. Each agency also had a CHHP case manager on staff that facilitated housing referrals for intervention participants and provided intensive case management to participants housed by their agency. The particular housing program the individual and case manager selected depended on a range of clinical and demographic factors such as gender, presence of HIV infection, ability to abstain from drug and alcohol use, and the desire of the individual to live in a particular part of the city. Some of the housing was provided in buildings owned by the housing partners, but the majority was provided in scattered site apartments that were leased from traditional landlords. Intervention housing utilized tenant-based, federal grant–funded vouchers that allowed participants to change housing location if necessary.
Federal rules were enforced at all sites and rents varied from $550 to $800 a month, with participants contributing based on their income level. In addition to the housing referrals, the case managers provided a wide range of supports to clients, including assistance with applying for benefits, obtaining government identification, negotiating with landlords, learning life skills, and organizing medical appointments. No traditional health care services were provided to the intervention participants at the permanent housing sites.
Outcomes
Although the phlebotomists, laboratory technicians, and data analyst were blinded to group assignments, study participants and study personnel arranging transportation to the phlebotomy sites could not be blinded. Therefore, we focused on outcomes that subjective interpretations would not affect. We chose survival with intact immunity as the primary endpoint of our study after enrollment began but before data analysis. We selected it because we believed it to be the best overall objective measure of participants' health. We defined this endpoint as being alive 12 months after enrollment with a CD4 count of greater than or equal to 200 and a viral load of less than 100 000. These cutoffs were chosen based on epidemiological studies that indicated that patients with a CD4 count less than 200 or a viral load greater than 100 000 have increased mortality and more opportunistic infections.23 Secondary outcomes were CD4 counts, viral loads, and the fraction of patients with undetectable viral loads.
Patients were taken to a reference laboratory, Quest Diagnostics, for phlebotomy 1 year (±28 days) after enrollment and were given $40 in compensation. For patients who could not be located, were in jail or prison, had died, or had moved out of state, we reviewed the laboratory records from the enrolling public hospital system. We collected the results completed as part of participants' usual outpatient care during the 2 years after enrollment. The laboratory results that were closest to the 1-year postenrollment date were included in the analysis. When patients died but had CD4 counts and viral loads completed as part of their postenrollment clinical care, we included these results in the CD4 count and viral load analysis to not bias the results toward analyzing healthier participants.
The viral load measurements completed at the reference laboratory used the Bayer Versant BDNA assay 3.0 (Bayer Healthcare, Diagnostics Division, Tarrytown, NY),24 which detects viral loads within the range of 75 to 500 000 copies. Viral loads completed as part of participants' usual care after February 9, 2005, also used this assay in the public hospital's laboratory. Before this date, the public hospital's laboratory used the Roche Cobas Amplicor 1.5 assay (La Roche Ltd, Basel, Switzerland) for assessing viral loads, which detects viral loads within the range of 50 to 750 000 copies. We multiplied the results of the Roche assay by 0.317 to make them equivalent to the Bayer assay results. We obtained this conversion factor with the results of the American College of Pathologists survey from 2006.26 In this survey, 10 reference samples were sent to 71–80 labs that used the Bayer Versant assay and to 159–174 labs that used the Roche Cobas Amplicor 1.5 assay. We used the mean results for each sample in a linear regression that produced the conversion factor. The R2 for the relation was 0.92. We also analyzed the results with the data from the Roche assay excluded.
We collected information about deaths by 2 methods. First, our research staff became aware of deaths through tracking the participants to complete interviews at 1, 3, 6, 9, and 12 months. We verified the dates and locations of these deaths by reviewing death certificates or medical records. Second, for participants we could not verify to be alive, we reviewed the Social Security Death Index.26
Statistical Analysis
We considered participants to have remained part of their randomized groups regardless of whether they successfully found housing. Although the laboratory observations closest to the 12-month postenrollment target were judged to be best for accurately assessing the intervention's impact, a last observation forward analysis was also completed in which the last observation before the 12-month target was analyzed. A last observation forward analysis for the missing data is more conservative, as it tends to underestimate the effect of the intervention. We used the χ2 test for all comparisons of categorical variables in the study. We used the independent sample t test to compare continuous variables for the groups' baseline statistics. We used the Mann–Whitney U test to compare HIV viral loads and CD4 counts. We analyzed the data with SPSS for Windows version 14 (SPSS Inc, Chicago, IL).
RESULTS
Patient demographic characteristics are summarized in Table 1. The typical profile for an enrolled participant was an African American man in his 40s who had completed a high school education and had never married. The majority used cocaine and alcohol, and many used heroin. The most common locations where patients had slept during the past 30 days were with family or friends, on the streets, or in shelters. There were no statistically significant differences in the baseline demographic characteristics for the 2 study groups. Seventy-five percent of HIV-positive participants in the overall CHHP study were enrolled at John Stroger Hospital, the location examined for this health outcomes assessment.
TABLE 1.
Baseline Characteristics of Participants in the Chicago Housing for Health Partnership Program: Chicago, IL, October 2003–May 2006
| All Randomized Participants |
Participants Analyzed |
|||
| Intervention (n = 54) | Usual Care (n = 51) | Intervention (n = 47) | Usual Care (n = 47) | |
| Age, y, mean (SD) | 45 (6.9) | 43 (7.7) | 45 (7.2) | 44 (7.7) |
| Female, no. (%) | 15 (28) | 8 (16) | 13 (28) | 7 (15) |
| Veterans, no. (%) | 3 (6) | 5 (10) | 2 (4) | 5 (11) |
| Marital status, no. (%) | ||||
| Never married | 36 (67) | 33 (65) | 32 (68) | 30 (64) |
| Divorced or separated | 14 (26) | 11 (21) | 11 (23) | 10 (21) |
| Married | 2 (4) | 2 (4) | 2 (4) | 2 (4) |
| Widow | 2 (4) | 2 (4) | 2 (4) | 2 (4) |
| Other | … | 2 (4) | … | 2 (4) |
| No response | … | 1 (2) | … | 1 (2) |
| Education, no. (%) | ||||
| Eighth grade or less | 7 (13) | 7 (14) | 6 (13) | 5 (11) |
| Some high school | 25 (46) | 14 (28) | 20 (43) | 14 (30) |
| Completed high school | 14 (26) | 19 (37) | 13 (28) | 17 (37) |
| Beyond high school | 8 (15) | 11 (22) | 8 (17) | 11 (23) |
| Race, no. (%) | ||||
| African American | 49 (91) | 44 (86) | 43 (91) | 40 (85) |
| Latino | 1 (2) | 3 (6) | 1 (2) | 3 (6) |
| White | 2 (4) | 1 (2) | 2 (4) | 1 (2) |
| Other | 2 (4) | 3 (6) | 1 (2) | 3 (6) |
| Residence 30 days before hospitalization, no. daysa | ||||
| With family | 12 | 10 | 12 | 11 |
| Street | 8 | 11 | 7 | 10 |
| Shelter | 4 | 5 | 5 | 5 |
| Hospital | 2 | 3 | 3 | 3 |
| Jail or prison | 1 | 2 | 1 | 1 |
| Other | 3 | … | 3 | … |
| Lifetime substance use | ||||
| Alcohol to intoxication | ||||
| Mean | 15 | 12 | 14 | 11 |
| 25th percentile | 0 | 1 | 0 | 0 |
| Median | 16 | 7 | 14 | 6 |
| 75th percentile | 23 | 23 | 22 | 23 |
| Heroin | ||||
| Mean | 9.3 | 8.9 | 9.8 | 9.4 |
| 25th percentile | 0 | 0 | 0 | 0 |
| Median | 1 | 0 | 1 | 0 |
| 75th percentile | 20 | 18 | 20 | 21 |
| Cocaine | ||||
| Mean | 12 | 10 | 12 | 10 |
| 25th percentile | 2 | 1 | 2 | 1 |
| Median | 11 | 10 | 11 | 10 |
| 75th percentile | 22 | 17 | 23 | 17 |
Note. There were no statistically significant differences in baseline characteristics between the randomized or analyzed groups. We assessed lifetime substance use with the Addiction Severity Index.22 Ellipses indicate the branch point when patients were randomly allocated into the 2 groups.
Total days do not equal 30 in some groups because of rounding.
Of the 105 HIV-positive patients who were enrolled in the CHHP study at the public hospital site after October 2004, 11 died during the following 12 months. Of the remaining 94, 55 had blood tests completed at the reference laboratory and 28 had CD4 counts or viral loads completed as part of their usual outpatient care within 2 years of enrollment. Eleven patients (10%) were not known to have died and did not have any laboratory assessment completed. Of these, 3 were known to be in jail or prison, 1 had moved out of state, 1 refused the blood draw, and 6 could not be contacted.
Of the tests done as part of usual outpatient care, 9 had viral load measurements with the Roche assay (4 usual care, 5 intervention) and 21 had viral load measurements with the Bayer Versant assay. Of the 11 patients who had died, 4 had CD4 counts (3 intervention, 1 usual care) and 3 had viral loads (2 intervention, 1 usual care) completed as part of usual outpatient care after enrollment and before death. There were no statistical differences in baseline characteristics between those with and without laboratory data. Overall, 87 patients (83% of enrolled patients) had laboratory outcomes available for analysis.
Of the 54 patients randomly assigned to the intervention group, 39 (72%) reached interim housing and 35 (65%) reached permanent housing at 1 of the 8 housing agencies involved in the program.
Primary Outcome
For the primary outcome, 26 (55%) patients in the intervention group and 16 (34%) patients in the usual care group were alive after 12 months of follow-up and had intact immunity (CD4 ≥ 200 and viral load < 100 000 at the time of their laboratory assessment; P = .04). The difference in primary outcome was still significant when we excluded laboratory results from the Roche assay (P = .04), when we defined intact immunity as a CD4 count of greater than or equal to 200 and viral load of less than 10 000 (P = .03), or when we used survival with a CD4 count greater than or equal to 200 for the calculations (P = .05). When the last laboratory value preceding the 12 month follow-up was used instead of the laboratory value closest to the date, the difference in primary outcomes was still significant (P = .03).
Because of the presence of missing data, we performed 2 additional sensitivity analyses. In the first, we assumed that all missing data did not support our hypothesis that the intervention improved survival with intact immunity. In the second, we assumed that all missing data supported the hypothesis. Assuming the missing data did not support the hypothesis, the relative risk for the primary outcome among the intervention group was 1.2 (95% confidence interval [CI] = 0.8, 1.9; P = .36); assuming the data supported the hypothesis, the relative risk was 1.95 (95% CI = 1.2, 3.1; P = .002).
Secondary Outcomes
The difference between the CD4 counts for the 2 groups was not statistically significant. The median viral load was 1356 in the intervention group and 10 417 in the usual care group (P = .03), a difference in median log viral loads of 0.89. Seventeen patients in the intervention group (36%) and 9 in the usual care group (19%) had undetectable viral loads (P = .051). Six participants in the intervention group and 5 in the usual care group died within 12 months of enrollment (P = .75). Four of the intervention deaths and 3 of the usual care deaths appeared in the Social Security Death Index as of June 28, 2007.26 We confirmed the other deaths through medical records or family members. The primary and secondary outcomes are summarized in Table 2.
TABLE 2.
Virological and Immunological Outcomes for the Intervention and Usual Care Groups at 12 months: Chicago Housing for Health Partnership Program, Chicago, IL, October 2003–May 2006
| Intervention | Usual Care | Relative Risk (95% CI) | P | |
| Primary endpoint (n = 47) | ||||
| Survival with intact immunity, no. (%) | 26 (55) | 16 (34) | 1.63 (1.01, 2.61) | .04a |
| Deaths, no. (%) | 6 (13) | 5 (11) | .75a | |
| CD4 count quartiles, no. (n = 42) | .23b | |||
| 25% | 90 | 33 | ||
| 50% | 236 | 187 | ||
| 75% | 386 | 303 | ||
| Mean | 271 | 246 | ||
| HIV viral load (n = 42c) | ||||
| Undetectable viral load, no. | 17 | 9 | 1.93 (0.97, 3.84) | .051a |
| Quartiles, copies per mL | .03b | |||
| 25% | < 75 | 217 | ||
| 50% | 1356 | 10 417 | ||
| 75% | 18 835 | 78 900 | ||
| Mean | 38 619 | 68 959 | ||
Note. CI = confidence interval. Survival with intact immunity was defined as being alive with a CD4 count ≥ 200 and a viral load < 100 000.
By χ2 test.
By Mann–Whitney U test.
For the usual care group, n = 43.
DISCUSSION
In this randomized trial, we found that housing hospitalized homeless HIV-positive individuals and providing them with intensive case management can increase the proportion surviving with intact immunity and decrease overall viral loads. The 63% relative increase and 21% absolute increase in survival with intact immunity is clinically meaningful. For every 5 patients offered this intervention and for every 3.25 patients provided housing in a program agency, 1 additional patient will be alive with intact immunity.
This study helps to confirm that a “Housing First”27–29 strategy can be effective in housing HIV-infected homeless people and that it can improve health. Housing First is a theory that homeless individuals are best stabilized through housing regardless of the personal challenges they may experience. This contrasts with the traditional housing readiness system that preferentially houses more stable and organized individuals by requiring repeated follow-up visits, stable contact information, and often sobriety. In the CHHP, there were no sobriety or adherence requirements, and there was no run-in period required to gain entry into the program. Although this study did not compare the Housing First and housing ready models, it confirms that a Housing First strategy can be successfully implemented for hospitalized patients. Because the study sample includes primarily African Americans, our findings also suggest that housing and case management may be effective strategies for addressing health disparities for vulnerable groups.
Homeless patients with HIV have been documented to have high hospital readmission rates30 even when compared with other medically ill homeless patients. Cohort studies have suggested that housing may reduce these admissions.17 If this reduction is confirmed in other studies, our findings would suggest that a portion of this reduction is likely because of an improvement in the individuals' disease status.
Because the CHHP intervention was complex, we could not determine the independent contributions of the various supports provided. Observational studies have found an association between improved adherence to antiretroviral medications and more intensive case management.19 Although we did not collect information on medication adherence and the intensity of case management as part of this study, case management was likely more intensive in the intervention group than in the usual care group through the Ryan White program. We also expect that the stability afforded to those living in permanent housing improved the ability of individuals to focus on their medical illness rather than on meeting this basic need for housing.14
Our study has several important limitations. First, the intervention was not blinded. Blinding participants is not possible when housing and case management are the interventions. Our use of objective biological markers of disease hopefully limits the effect of this on our results. Second, we did not measure baseline CD4 counts and viral loads. Although the demographic characteristics of the 2 groups appear to have been well distributed, we cannot state with certainty that the 2 groups did not differ at baseline in their virological or immunological status. Third, 6% of participants could not be located and had no laboratory tests completed after enrollment. This was because of the challenges of prospectively following chronically ill homeless participants, many of whom had substance abuse disorders or mental illness.
Some participants may have died during the study period without being indicated in the Social Security Death Index. Additionally, because of the same challenges following homeless participants noted in the last paragraph, one third of test results analyzed were from a routine outpatient clinical care rather than from specimens analyzed at the reference laboratory. Fourth, using the results of viral loads from 2 different assays may have added additional uncertainty about the accuracy of our outcomes. Fifth, we enrolled only participants who passed through a public hospital, so the applicability of these results to other settings may be questioned. Sixth, because of our small sample size, the presence of missing data, and the borderline statistical significance of some of our findings, these results should be confirmed by additional studies.
Our study indicates that a community-based housing program with intensive case management can improve the health of hospitalized homeless patients with HIV/AIDS. Future planned work by us will examine the effect of this intervention on health service use and costs. Future studies should explore whether these findings extend to other chronic diseases and other housing and case management models.
Acknowledgments
This study was supported by grants from the Michael Reese Health Trust, Chicago Community Trust, the AIDS foundation of Chicago, the Fields Foundation, the Prince Charitable Trusts, the Siragusa Foundation, and Housing and Urban Development's Housing Opportunities for People with AIDS (HOPWA; grant #ILH020015).
Art Evans, MD, MPH, provided statistical support, and Brendan Reilly, MD, Margot Kushel, MD, and Robert Weinstein, MD, provided critical revisions to this article. Kathleen Beavis, MD, suggested the use of the information from the American College of Pathologists for analyzing the results of the HIV viral load assays.
Note. The funding foundations played no role in the concept or design of the study; the acquisition, analysis, and interpretation of the data; the drafting or revision of the article; or the technical support or supervision of the study.
Human Participant Protection
The institutional review board of the Cook County Bureau of Health Services approved all procedures. Written informed consent was obtained from each participant.
References
- 1.Breakey WR, Fischer PJ, Kramer M, et al. Health and mental health problems of homeless men and women in Baltimore. JAMA. 1989;262(10):1352–1357 [PubMed] [Google Scholar]
- 2.Hwang SW. Mortality among men using homeless shelters in Toronto, Ontario. JAMA. 2000;283(16):2152–2157 [DOI] [PubMed] [Google Scholar]
- 3.Hibbs JR, Benner L, Klugman L, et al. Mortality in a cohort of homeless adults in Philadelphia. N Engl J Med. 1994;331(5):304–309 [DOI] [PubMed] [Google Scholar]
- 4.Hwang SW, Orav EJ, O'Connell JJ, et al. Causes of death in homeless adults in Boston. Ann Intern Med. 1997;126(8):625–628 [DOI] [PubMed] [Google Scholar]
- 5.Barrow SM, Herman DB, Cordova P, et al. Mortality among homeless shelter residents in New York City. Am J Public Health. 1999;89(4):529–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Robertson MJ, Clark RA, Charlebois ED, et al. HIV seroprevalence among homeless and marginally housed adults in San Francisco. Am J Public Health. 2004;94(7):1207–1217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Herndon B, Asch SM, Kilbourne AM, et al. Prevalence and predictors of HIV testing among a probability sample of homeless women in Los Angeles County. Public Health Rep. 2003;118(3):261–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hahn JA, Bangsberg DR, McFarland W, et al. HIV seroconversion among the homeless and marginally housed in San Francisco: a ten-year study. J Acquir Immune Defic Syndr. 2004;37(5):1616–1619 [DOI] [PubMed] [Google Scholar]
- 9.Surratt HL, Inciardi JA. HIV risk, seropositivity and predictors of infection among homeless and nonhomeless women sex workers in Miami, Florida, USA. AIDS Care. 2004;16(5):594–604 [DOI] [PubMed] [Google Scholar]
- 10.Bucher JB, Thomas KM, Guzman D, et al. Community-based rapid HIV testing in homeless and marginally housed adults in San Francisco. HIV Med. 2007;8(1):28–31 [DOI] [PubMed] [Google Scholar]
- 11.Pfeifer RW, Oliver J. A study of HIV seroprevalence in a group of homeless youth in Hollywood, California. J Adolesc Health. 1997;20(5):339–342 [DOI] [PubMed] [Google Scholar]
- 12.Zolopa AR, Hahn JA, Gorter R, et al. HIV and tuberculosis infection in San Francisco's homeless adults. Prevalence and risk factors in a representative sample. JAMA. 1994;272(6):455–461 [PubMed] [Google Scholar]
- 13.Bangsberg D, Tulsky JP, Hecht FM, et al. Protease inhibitors in the homeless. JAMA. 1997;278(1):63–65 [PubMed] [Google Scholar]
- 14.Gelberg L, Gallagher TC, Andersen RM, et al. Competing priorities as a barrier to medical care among homeless adults in Los Angeles. Am J Public Health. 1997;87(2):217–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moss AR, Hahn JA, Perry S, et al. Adherence to highly active antiretroviral therapy in the homeless population in San Francisco: a prospective study. Clin Infect Dis. 2004;39(8):1190–1198 [DOI] [PubMed] [Google Scholar]
- 16.Hwang SW. Is homelessness hazardous to your health? Obstacles to the demonstration of a causal relationship. Can J Public Health. 2002;93(6):407–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buchanan D, Doblin B, Sai T, et al. The effects of respite care for homeless patients: a cohort study. Am J Public Health. 2006;96(7):1278–1281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kessell ER, Bhatia R, Bamberger JD, et al. Public health care utilization in a cohort of homeless adult applicants to a supportive housing program. J Urban Health. 2006;83(5):860–873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kushel MB, Colfax G, Ragland K, et al. Case management is associated with improved antiretroviral adherence and CD4+ cell counts in homeless and marginally housed individuals with HIV infection. Clin Infect Dis. 2006;43(2):234–242 [DOI] [PubMed] [Google Scholar]
- 20.Milby JB, Schumacher JE, Vuchinich RE, et al. Transitions during effective treatment for cocaine-abusing homeless persons: establishing abstinence, lapse, and relapse, and reestablishing abstinence. Psychol Addict Behav. 2004;18(3):250–256 [DOI] [PubMed] [Google Scholar]
- 21.Hwang SW, Lebow JM, Bierer MF, et al. Risk factors for death in homeless adults in Boston. Arch Intern Med. 1998;158:1454–1460 [DOI] [PubMed] [Google Scholar]
- 22.Zanis DA, McLellan AT, Cnaan RA, et al. Reliability and validity of the Addiction Severity Index with a homeless sample. J Subst Abuse Treat. 1994;11(6):541–548 [DOI] [PubMed] [Google Scholar]
- 23.Egger M, May M, Chene G, et al. Prognosis of HIV-1-infected patients starting highly active antiretroviral therapy: a collaborative analysis of prospective studies. Lancet. 2002;360(9327):119–129 [DOI] [PubMed] [Google Scholar]
- 24.Peter JB, Sevall JS. Molecular-based methods for quantifying HIV viral load. AIDS Patient Care STDS. 2004;18(2):75–79 [DOI] [PubMed] [Google Scholar]
- 25.American College of Pathologists American College of Pathologists Proficiency Testing Program; 2006 [Google Scholar]
- 26.Social Security Death Index Interactive Search. Available at: http://ssdi.rootsweb.ancestry.com. Accessed May 3, 2008.
- 27.Stefancic A, Tsemberis S. Housing First for long-term shelter dwellers with psychiatric disabilities in a suburban county: a four-year study of housing access and retention. J Prim Prev. 2007;28(3–4):265–279 [DOI] [PubMed] [Google Scholar]
- 28.Legander S. Housing first. A program to help people move off the streets and into treatment. Behav Healthc. 2006;26(5):38. [PubMed] [Google Scholar]
- 29.Tsemberis S, Gulcur L, Nakae M. Housing First, consumer choice, and harm reduction for homeless individuals with a dual diagnosis. Am J Public Health. 2004;94(4):651–656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Culhane DMS, Hadley T. Public service reductions associated with placement of homeless persons with severe mental illness in supportive housing. Health Policy Debate. 2002;13:107–163 [Google Scholar]