Abstract
Anaphylaxis is a severe allergic reaction that can be rapidly progressing and occasionally fatal. In instances where the triggering allergen is not obvious, establishing the etiology of anaphylaxis is pivotal to long-term management. Assigning etiology is limited, however, by the number of known exposures associated with anaphylaxis. Therefore, identification of novel causative agents can provide an important step forward in facilitating new, allergen specific approaches to management. In contrast to the view that carbohydrate-directed IgE has minimal, if any, clinical significance, recent data suggests that IgE antibodies to carbohydrate epitopes can be an important factor in anaphylaxis that may otherwise appear to be idiopathic. Specifically, IgE antibodies to the carbohydrate galactose-α-1,3-galactose (alpha-gal) were found to be capable of eliciting serious, even fatal, reactions to the monoclonal antibody (ab) cetuximab.1 Moreover, alpha-gal has recently been identified as a novel food allergen.2 Patients who have IgE to alpha-gal report delayed anaphylaxis or urticaria occurring 3-6 hours after eating beef, pork or lamb. Here, we review the evidence relating to carbohydrates in food allergy and anaphylaxis and discuss the implications of a new mammalian cross-reactive carbohydrate determinant (CCD).
Keywords: anaphylaxis, cross-reactive carbohydrate determinant (CCD), alpha-gal, glycosylation
Introduction
In those cases of anaphylaxis where the etiology is known, this syndrome appears to be mediated by a specific IgE (sIgE) response to the causative allergen that leads to systemic release of histamine and other mediators from mast cells and basophils. This process may lead to shock with generalized urticaria, laryngeal edema, lower-airway obstruction and hypotension. Establishing the etiology of recurrent anaphylaxis is a critical aspect of treatment, as the identification of causal allergens allows the use of either avoidance or immunotherapy in the management. The most frequent allergens involved in anaphylactic reactions are proteins found in peanuts, tree nuts, fish, shellfish, bee and wasp venoms, as well as drug haptens and latex. Carbohydrates in the form of complex oligosaccharides are also present on many foods and can be the target of anti-glycan IgE responses. Since these carbohydrate moieties can be present on multiple different types of proteins, they are prone to extensive cross-reactivity. These epitopes are called cross-reactive carbohydrate determinants (CCD), however, until recently the clinical significance of IgE antibodies directed against them has been unclear.
Overview of CCD
Role in producing clinical symptoms
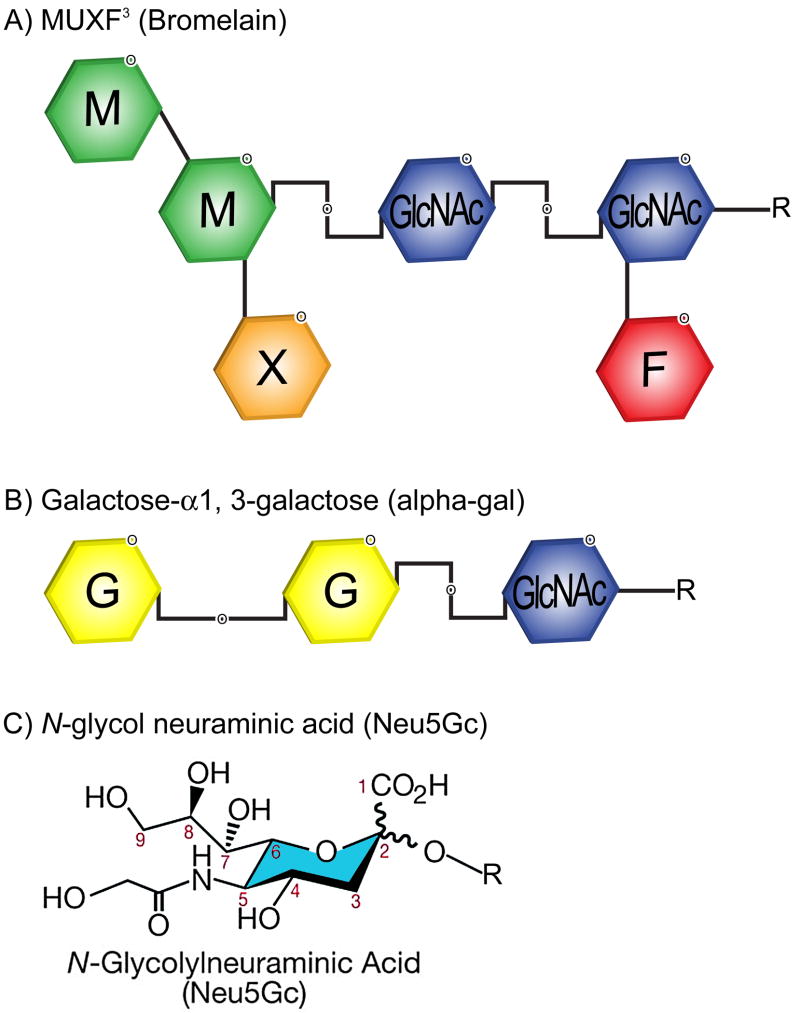
The study of glycosylation on proteins as a target for IgE in relation to food antigens began in the 1970s when a Japanese group reported the structure of a protease from pineapple stem.3 It was subsequently shown that this protease, bromelain, carried an oligosaccharide with two structural features that had not been found in mammalian glycoproteins: core α1,3-fucose and xylose (Fig 1). In fact, xylose and core-3-linked fucose may be the most common carbohydrate epitopes recognized by human IgE antibodies. Subsequently, the binding of IgE to Api m 1 (honeybee venom phospholipase A2) in sera of bee allergic patients was shown to be inhibited by glycopeptides from pineapple stem bromelain.4
Fig. 1.
Plant and mammalian glycans. A) Structure of MUXF3 (bromelain), which contains the structural features of xylose and core α1,3-fucose. Xylose and core α1,3-fucose are the main motifs for CCD-directed IgE and are the essential part of two independent epitopes. B) Structure of the galactose-α-1,3-galactose (alpha-gal) epitope found in lower mammals and depiction of the important α(1→3) linkage. C) Structure of N-glycol neuraminic acid that, like alpha-gal, is found in lower mammals but not humans.
Current estimates are that 15-30% of allergic patients generate specific anti-glycan IgE.5 This frequent occurrence of serum IgE to CCD contrasts with their modest effect on skin tests and their apparent inability to produce clinical symptoms. Historically, a study by van der Veen et al is often cited as evidence against a significant clinical impact of carbohydrate-directed IgE.6 In that study, basophils from patients who had IgE antibodies to a carbohydrate moiety on peanut but without clinical symptoms were found to release histamine only after stimulation with very high concentrations of a peanut extract. Those results contrasted with the much lower concentrations needed to produce histamine release from basophils of patients with sIgE to peanut protein and documented clinical peanut allergy. Based upon these differences, it was concluded that anti-glycan IgE detected by in vitro methods was clinically irrelevant or required high concentrations to produce degranulation, possibly owing to its monovalent nature. Mari and his colleagues reached a similar conclusion when testing over 4,500 patients with possible inhalant allergy for reactivity to bromelain revealed that 23% had positive in vitro results, while only 0.1% had positive reactions on skin testing.5
A clinically benign role for CCD is now being questioned, though, as several studies have shown the ability of anti-CCD IgE to trigger mediator release from basophils.7 Careful analysis of the van der Veen study 6 reveals that those patients were primarily sensitized to grass pollens and their anti-glycan IgE antibodies were more likely induced as part of this sensitization and only cross-reactive with peanut glycans (mannose). Thus, a high concentration of peanut extract may have been required to achieve basophil stimulation because the IgE antibodies bound poorly to, or were partially cross-reactive against, carbohydrates present on peanut. In the aforementioned study which examined the prevalence of bromelain sensitization, skin testing to bromelain revealed few positives. This may be expected, though, given that degranulation requires cross-linking on the surface of the mast cell and bromelain contains only one oligosaccharide chain per molecule. It has also been suggested that antibodies to relatively uncharged carbohydrate epitopes would have low affinity,8 thus rendering skin testing less reliable. However, the evidence that IgE antibodies to CCDs are of low affinity is poor, and recent work has indicated that these antibodies have affinities comparable to IgG antibodies.9 Our studies have shown that IgE antibodies specific for the carbohydrate galactose-α-1,3-galactose (alpha-gal) are capable of eliciting serious, even fatal, reactions to the monoclonal ab cetuximab.1 We subsequently extended that observation by demonstrating that IgE antibodies to alpha-gal are associated with an unusual form of delayed anaphylaxis which follows 3-6 hours after eating meat that carries alpha-gal (e.g., beef, pork or lamb).2 In contrast to previously described CCD motifs of xylose and core-3-linked fucose, which are common in plants and insects, the alpha-gal epitope is abundantly expressed on cells and tissues of non-primate mammals. This expression pattern makes alpha-gal potentially clinically relevant either as a food allergen (e.g., beef, pork, lamb) or as an inhaled allergen (e.g., cat, dog).
Presence of CCD can complicate immunoassays
In most cases CCD are likely to have a marginal effect on in vitro test results. However, in certain subgroups of patients CCD reactivity may have clinical relevance and an awareness of possible CCD response may therefore be of great value when making diagnoses. For instance, if the patient was not originally sensitized to the allergen tested, but the carbohydrate epitopes recognized by the patient’s IgE are cross-reactive, the positive test result may not have the same clinical relevance. In particular, the glycan epitopes present in a latex extract can bind carbohydrate-directed IgE present in the serum of a pollen allergic patient not originally sensitized to latex, resulting in a positive in vitro assay for IgE to latex. At times, if a patient is sensitized to the allergen tested by immunoassay, the presence of carbohydrate-directed IgE in addition to anti-peptide IgE can result in a higher quantitative result, suggesting a more severe sensitization than is actually the case. Moreover, a recent study underscores the high occurrence of clinically irrelevant results for peanut sIgE in patients sensitized to grass pollen who have no symptoms related to peanuts.10 Some have suggested that tests for CCD sIgE should be carried out systematically to improve the in vitro diagnosis of certain allergies; at least, all in vitro tests should be evaluated together with the clinical history.
The carbohydrate epitope on cat IgA is alpha-gal
In 2006, Adedoyin et al. reported that IgE ab specific for cat IgA, present in the serum of cat-sensitized patients, bound to a glycan moiety localized on the α-chain.11 In addition, they reported that these carbohydrates are also present on IgM from cat, as well as on IgM from many different mammalian species, but not human immunoglobulins. Unexpectedly, IgE antibodies to cat IgM and cat IgA showed complete cross-reactivity, whereas cat IgG did not, suggesting an identical oligosaccharide on the 2 former immunoglobulin classes.12 As the first mammalian carbohydrate IgE epitope described, it was of major interest to identify the structure responsible for the broad cross-reactivity. Recent collaboration between our group and the Swedish group has established that the IgE-binding oligosaccharide on cat IgA is alpha-gal.12
Necessity for understanding glycosylation in the production of recombinant molecules
The cell type used for expression of a recombinant therapeutic glycoprotein has significant implications for the presence, number and diversity of protein-linked oligosaccharides attached during the synthesis and secretion of the molecule. From a pharmacological perspective, the potential for changes in glycosylation to influence the activity, serum half-life or immunogenicity of the recombinant protein is an obvious cause for concern. Studies have shown, for example, that variations exist in the glycosylation pattern of tissue plasminogen activator isolated from different cell lines.13 The most commonly used production cell lines for monoclonal antibodies are CHO, NS0 and Sp2/0 and each of these can add sugar residues that are not present in normal serum-derived IgG (reviewed by Jefferis14). As recent studies have shown,1 a particular concern is the addition of galactose in an α(1→3) linkage by NS0 and Sp2/0 cells such that galactose-α-1,3-galactose (alpha-gal) is formed. In humans and higher primates the gene encoding alpha-1,3-galactosyltransferase is not functional, so these species cannot produce alpha-gal; by contrast, the alpha-gal negative animals make IgG antibodies specific for this oligosaccharide.8 The implications of antibodies directed against the monoclonal ab are that the response to treatment may be influenced by accelerated clearance of the molecule or of sensitization potentially causing reactions upon re-exposure. In the case of cetuximab, which carries alpha-gal, the patients who reacted had sIgE prior to the exposure and anaphylaxis occurred during the first infusion.1
In addition to alpha-gal, the production cell lines can add an α(2→3) linked N-glycylneuraminic acid (Fig. 1C) that is not present in humans and may have immunogenic properties. CHO cells in particular can add N-acetylneuraminic acid in α(2→3) linkage rather than the α(2→6) linkage found in humans14. Moreover, there is new evidence that fucose residues (or the absence of such sugars) on IgG Fc may influence activation of FcγRIIa and FcγRIIIa. The FcγRs may have differential glycosylation patterns themselves. Thus, knowledge and awareness of the oligosaccharides present on all recombinant molecules (not only monoclonal antibodies) is critical to understanding the etiology of infusion reactions. While glycosylation of the Fc portion of the molecule (Asn 297) is known to play a significant role in Fc binding and the activation of antibody-dependent cellular cytotoxicity (ADCC), it is not clear that the same is true for glycosylation on the Fab side. Although current knowledge limits the ability to change glycosylation patterns, it is possible to engineer the molecules so that the glycosylation sites on the Fab are not present.
IgE to a Mammalian CCD
Prior evidence
As discussed, although IgE ab to CCD is known to be common, until recently the clinical data was almost uniformly negative. Thus, although patients are exposed to plant products (either inhaled or oral) that carry a CCD to which they have IgE antibodies, they do not report symptoms. In addition, investigation of sera from patients in Europe with chronic urticaria did not identify a significant number of cases with IgE antibodies to plant derived CCD [Rob Aalberse, personal communication]. Other carbohydrate antigens that are recognized as immunogens in man include A and B blood group substances. Until the past few years, however, no evidence existed for a significant mammalian CCD. Recent work by Wong et al. using deglycosylation techniques hinted at the existence of another CCD in mammalian tissue.15 Our description of IgE to alpha-gal in a cohort of patients who reported delayed symptoms after eating mammalian meat fits the results of Wong et al. and alpha-gal may well be the first clinically relevant CCD that is specifically found in mammalian tissue.
IgE ab to alpha-gal
Over the last three years, it has become increasingly clear that there are many cases of “delayed anaphylaxis to red meat” occurring in an area of the United States that includes primarily VA, NC, TN, AR and MO, but also extends into the surrounding states.2 The basis for these reactions appears to be an IgE ab response to the oligosaccharide galactose-α-1,3-galactose, awareness of which was triggered by severe hypersensitivity reactions to the monoclonal antibody cetuximab.1 These reactions to beef, pork, or lamb present several features that are strikingly different from established teaching on food allergy. First, this form of allergy to meat develops in adult life. Second, the reactions do not start until several hours after eating meat. Third, the patients generally give negative or very weak wheal responses to prick tests with meat extracts.2,16 Because of these features, in many cases the patient’s histories had previously been dismissed by other physicians including allergists. Here we discuss the various syndromes and evidence in the literature that supports the unique relevance of IgE to alpha-gal as a significant factor in this anaphylactic syndrome.
The mammalian pattern: IgE to beef, pork, cat, dog and milk
In 2005, Mamikoglu reported the existence of 18 patients in a Southeastern state (Arkansas) whose sera were positive by ImmunoCAP testing for IgE to beef, pork, cat, dog and milk.17 This pattern of positive titers to mammalian allergens could well be explained by IgE to alpha-gal, and is mirrored in the 24 patients that we recently reported.2 It seems unlikely that cross-reactivity to a protein epitope on serum albumin would explain the reported mammalian pattern as no current reports exist to link dog dander allergy to meat allergy. Alternatively, alpha-gal is present on proteins from the epithelia and milk of all lower mammals, consistent with the reported pattern.
Similar anaphylaxis syndromes not due to a CCD
There are additional types of cross-reaction in patients suffering from meat allergy:
-
Cross-reactivity between meats from different animal species and animal dander
The probability of cross-reaction is increased the more closely related the animals are evolutionarily, such that patients allergic to beef may react to mutton or pork but not poultry or fish. In some cases this cross-reactivity has been shown to be due to IgE specific for common determinants on serum albumin and this has been referred to as the ‘pork-cat syndrome’.18 A recent publication specifically addressed whether IgE to alpha-gal was present in patients with pork-cat syndrome and found no evidence for sIgE to alpha-gal, supporting the central role of serum albumin in the pork-cat syndrome.16
-
Cross-reactivity between beef and cow’s milk
DBPCFC studies of children allergic to beef have shown immediate reactivity to cow’s milk.19 Again, sensitivity to BSA was the main predictive marker of this cross-reactivity and that of a subset of cow’s milk allergic patients who also react to beef.
Desensitization possibilities
Modulation of the anaphylactic responses should be possible if IgE to alpha-gal has similar properties to more classical, peptide-directed IgE. Indeed, preliminary experiments in our lab have found that basophils and mast cells activate and release histamine, respectively, in response to the carbohydrate antigen in appropriately sensitized patients. Additionally, desensitization to cetuximab has been performed in a patient who had a grade 3 hypersensitivity reaction during the initial infusion. The patients serum was tested and found to have IgE ab to alpha-gal but there was a clinical need for cetuximab therapy and desensitization was successfully performed.20
Evidence Related to Ticks
A recent report on 25 patients from Sydney, New South Wales documents an association between tick bite reactions and red meat allergy.21 These 25 patients reported clinical reactions ranging from urticaria to anaphylaxis, and 10 of the 25 patients (40%) reported a delayed onset of ≥4 hours after ingestion of mammalian meat.21 In this report from Australia by Van Nunen et al., nearly all of the patients described large local reactions to tick bites.21 Likewise, >90% of patients with IgE to alpha-gal report tick or chigger bites. Interestingly, screening of sera from a separate cohort of Australian patients also with tick bites and reactions to red meat showed the presence of IgE ab to alpha-gal in 9 of 13 meat allergic patients (Mullins, Commins, James, Platts-Mills; unpublished data).
The normal pattern of allergic disease is a primary exposure that gives rise to an IgE antibody response, and subsequent exposure to the same antigen then gives rise to symptoms. However, there are some well documented situations where exposure to one foreign antigen gives rise to an IgE response which cross-reacts with an apparently different antigen that may be encountered by a different route. The obvious example is birch pollen exposure giving rise to IgE antibodies to Bet v1 which cross reacts with the closely related proteins of apple, hazelnut or the cherry derived allergen Pru a 1. These reactions are due to close structural similarity between the proteins. There are also examples where insect stings can sensitize to a CCD which is present on pollens. Thus, honey bee venom induces IgE responses to the oligosaccharide MUXF3 which is present on the pineapple protein bromelain and also on proteins derived from grass pollen.4 Therefore, there is an existing model of an arthropod bite or sting in the skin inducing IgE antibodies to a carbohydrate antigen.
Interestingly, immediate anaphylactic and large hypersensitivity reactions to tick bites have been reported with several different species of ticks.22 These IgE-mediated responses are due to tick salivary proteins22 and are distinct reactions from the delayed, food-induced anaphylactic and urticarial reactions that we have reported. Clinically, these hypersensitivity reactions are reported to occur within minutes of a tick bite and have been reported both with Ixodes holocyclus and with the ‘pigeon tick’ (Argas reflexus).22, 23 Thus, on current evidence it appears that tick bites can give rise to IgE responses to both carbohydrates and to tick-derived proteins. This should not be a surprise since ticks are well-recognized for their potent adjuvant and immunogenic effects.24
Commentary and Future Work
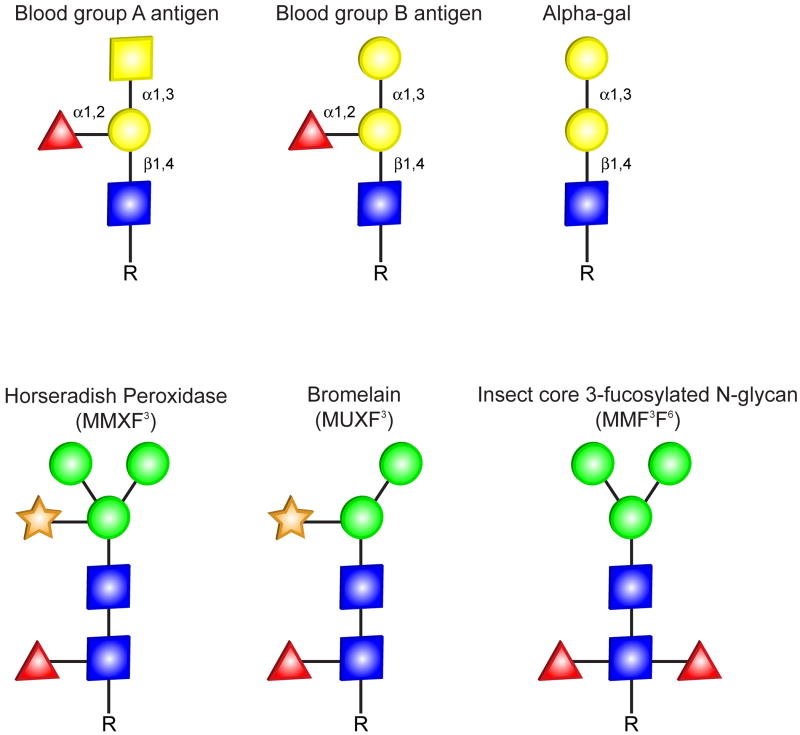
Although the oligosaccharide alpha-gal has only recently been recognized as a target for IgE antibodies, it has long been recognized as a significant factor in transplant immunology. In the 1930s, Karl Landsteiner recognized the existence of a blood group B-like substance on mammalian cells which was the target of agglutinating antibodies in human sera. This substance was probably alpha-gal, because blood group B antigen and the alpha-gal epitope differ only in that blood group B antigen has a fucose linked α(1→2) to the penultimate galactose (Fig 2). Other distinct but equally interesting work is unfolding from Dr. Varki and his colleagues showing that the carbohydrate N-glycol neuraminic acid (NeuGc) is found in high amounts in lamb, pork and beef, intermediate amounts in cow’s milk, and low/undetectable levels in poultry and fish. This group found anti-NeuGc antibodies of IgA, IgM and IgG isotypes in patient sera and that these antibodies represented up to 0.25% of total circulating IgG in some subjects.25 This falls into the ranges of IgG antibodies to alpha-gal but there was no direct correlation between the two antibody levels in a given individual.25 We have screened a large number of sera for IgE ab to NeuGc but the results were negative (Platts-Mills, James, Commins; unpublished data). Certainly, the finding of IgE to alpha-gal raises the issue that there may be other naturally-occurring non-protein epitopes such as other sialic acid-based sugars (for example, Lewis X), carbohydrates on horseradish peroxidase, and hydroxyl-proline-linked arabinogalactan that could be the target of IgE responses.
Fig. 2.
Comparison of representative glycans referenced in the text. The oligosaccharide structures are shown in the symbolic depiction suggested by the Consortium of Functional Glycomics. Note that the lack of a core fucose residue separates the structure of blood group B antigen from alpha-gal.
The discovery of IgE antibodies to the oligosaccharide galactose alpha -1, 3-galactose has made it possible to investigate several novel aspects of allergic disease. One obvious issue is that the glycosylation of therapeutic recombinant molecules, particularly monoclonal antibodies, can create a risk for severe hypersensitivity reactions. The syndrome of ‘delayed anaphylaxis to beef’ has changed our approach to what would normally be regarded as ‘spontaneous’ or ‘idiopathic’ anaphylaxis in a large area of the southeastern US. In the future, an understanding of the factors that control the delay may provide real insight into the factors that control anaphylaxis. At this time, there remain several, major unexplained issues:
Is IgE to alpha-gal induced by ticks and, if so, is it related to a certain species?
Why are the reactions to red meat delayed?
Why is this IgE ab that binds alpha-gal on dog and cat proteins and is present in high titer in the serum not related to asthma or immediate nasal symptoms?
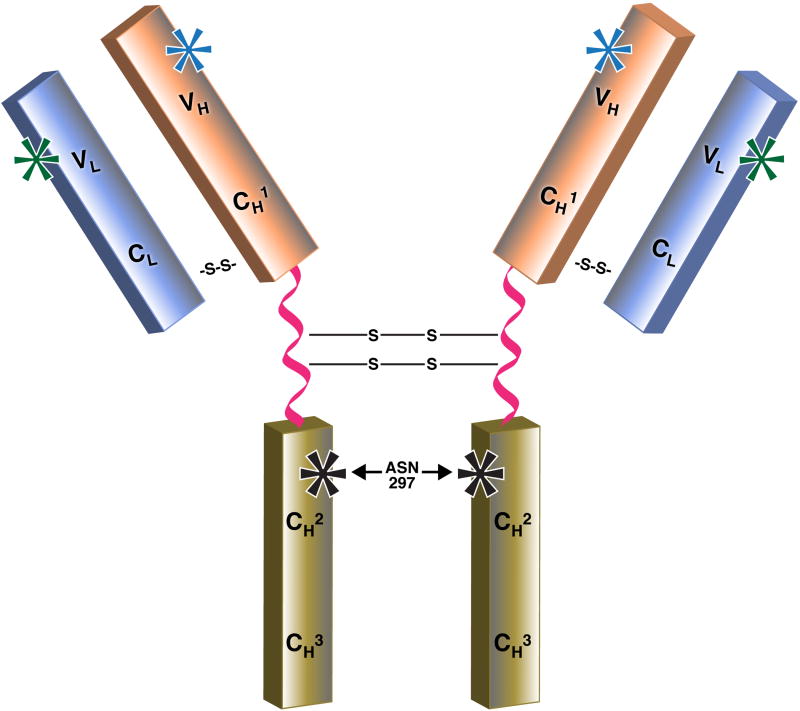
Fig. 3.
Structure of IgG antibody molecule showing potential sites of glycosylation. The IgG-Fc region contains CH2 domains that are glycosylated through covalent attachment of oligosaccharide at asparagine 297 (Asn 297, indicated by black asterisks). The oligosacharide at Asn 297 is integral to the IgG-Fc structure, forms multiple noncovalent interactions with the protein surface of the CH2 domain and is important to the function of the molecule; therefore, the site cannot engineered out. In addition to the conserved glycosylation site at Asn 297, 15-20% of polyclonal human IgG molecules bear N-linked glycosylation within the IgG-Fab region. While there is no consensus sequence for N-linked oligosaccharide within the constant domain of the light chain or the CH1 domain of the heavy chain, glycosylation can be present in the variable regions of the kappa and lambda (VL, green asterisks) or heavy chains (VH, blue asterisks), and sometimes both. For example, the amino acid sequence of cetuximab has potential glycosylation sites at Asn43 of the light chain and at Asn88 and Asn299 of the heavy chain but the glycosylation site at Asn43 is not glycosylated. The biopharmaceutical industry and others have shown that differences in glycosylation products and manipulation of oligosaccharide placement (or deletion) can have significant effects on therapeutic efficacy for recombinant monoclonal antibodies. S–S denotes a disulfide bond.
Abbreviations
- ab
Antibody
- BSA
Bovine serum albumin
- CCD
Cross-reactive carbohydrate determinant
- alpha-gal
Galactose-α-1,3-galactose
- NeuGC
N-glycol neuraminic acid
- sIgE
Specific IgE
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chung CH, Mirakhur B, Chan E, Le QT, Berlin J, Morse M, et al. Cetuximab-induced anaphylaxis and IgE specific for galactose-alpha-1,3-galactose. N Engl J Med. 2008;358:1109–17. doi: 10.1056/NEJMoa074943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Commins SP, Satinover SM, Hosen J, Mozena J, Borish L, Lewis BD, et al. Delayed anaphylaxis, angioedema, or urticaria after consumption of red meat in patients with IgE antibodies specific for galactose-alpha-1,3-galactose. J Allergy Clin Immunol. 2009;123:426–33. doi: 10.1016/j.jaci.2008.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ishihara H, Takahashi N, Oguri S, Tejima S. Complete structure of the carbohydrate moiety of stem bromelain. An application of the almond glycopeptidase for structural studies of glycopeptides. J Biol Chem. 1979;254:10715–9. [PubMed] [Google Scholar]
- 4.Aalberse RC, Koshte V, Clemens JG. Immunoglobulin E antibodies that crossreact with vegetable foods, pollen, and Hymenoptera venom. J Allergy Clin Immunol. 1981;68:356–64. doi: 10.1016/0091-6749(81)90133-0. [DOI] [PubMed] [Google Scholar]
- 5.Mari A. IgE to cross-reactive carbohydrate determinants: analysis of the distribution and appraisal of the in vivo and in vitro reactivity. Int Arch Allergy Immunol. 2002;129:286–95. doi: 10.1159/000067591. [DOI] [PubMed] [Google Scholar]
- 6.van der Veen MJ, van Ree R, Aalberse RC, Akkerdaas J, Koppelman SJ, Jansen HM, et al. Poor biologic activity of cross-reactive IgE directed to carbohydrate determinants of glycoproteins. J Allergy Clin Immunol. 1997;100:327–34. doi: 10.1016/s0091-6749(97)70245-8. [DOI] [PubMed] [Google Scholar]
- 7.Foetisch K, Westphal S, Lauer I, Retzek M, Altmann F, Kolarich D, et al. Biological activity of IgE specific for cross-reactive carbohydrate determinants. J Allergy Clin Immunol. 2003;111:889–96. doi: 10.1067/mai.2003.173. [DOI] [PubMed] [Google Scholar]
- 8.Galili U. The alpha-gal epitope and the anti-Gal antibody in xenotransplantation and in cancer immunotherapy. Immunol Cell Biol. 2005;83:674–86. doi: 10.1111/j.1440-1711.2005.01366.x. [DOI] [PubMed] [Google Scholar]
- 9.Jin C, Hantusch B, Hemmer W, Stadlmann J, Altmann F. Affinity of IgE and IgG against cross-reactive carbohydrate determinants on plant and insect glycoproteins. J Allergy Clin Immunol. 2008;121:185–90 e2. doi: 10.1016/j.jaci.2007.07.047. [DOI] [PubMed] [Google Scholar]
- 10.Guilloux L, Morisset M, Codreanu F, Parisot L, Moneret-Vautrin DA. Peanut allergy diagnosis in the context of grass pollen sensitization for 125 patients: roles of peanut and cross-reactive carbohydrate determinants specific IgE. Int Arch Allergy Immunol. 2009;149:91–7. doi: 10.1159/000189190. [DOI] [PubMed] [Google Scholar]
- 11.Adedoyin J, Gronlund H, Oman H, Johansson SG, van Hage M. Cat IgA, representative of new carbohydrate cross-reactive allergens. J Allergy Clin Immunol. 2007;119:640–5. doi: 10.1016/j.jaci.2006.11.637. [DOI] [PubMed] [Google Scholar]
- 12.Gronlund H, Adedoyin J, Commins SP, Platts-Mills TA, van Hage M. The carbohydrate galactose-alpha-1,3-galactose is a major IgE-binding epitope on cat IgA. J Allergy Clin Immunol. 2009;123:1189–91. doi: 10.1016/j.jaci.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buelens K, Hillmayer K, Compernolle G, Declerck PJ, Gils A. Biochemical importance of glycosylation in thrombin activatable fibrinolysis inhibitor. Circ Res. 2008;102:295–301. doi: 10.1161/CIRCRESAHA.107.157099. [DOI] [PubMed] [Google Scholar]
- 14.Jefferis R. Glycosylation as a strategy to improve antibody-based therapeutics. Nat Rev Drug Discov. 2009;8:226–34. doi: 10.1038/nrd2804. [DOI] [PubMed] [Google Scholar]
- 15.Wong KN, O T, Wang X, Lee WSe, Lim PA, Wang De Y, Chew FT. Deglycosylation of meat extracts reduced the binding of cross-reactive antibodies to meat. XVth Annual Congress of the European Academy of Allergology and Clinical Immunology; Vienna. June 10-14, 2006. [Google Scholar]
- 16.Jacquenet S, M-V D, Bihain BE. Mammal meat anaphylaxis: clinical relevance of anti-galactose-alpha-1,3-galactose IgE confirmed by skin tests to cetuximab. J Allergy Clin Immunol. 2009 doi: 10.1016/j.jaci.2009.06.014. in press. [DOI] [PubMed] [Google Scholar]
- 17.Mamikoglu B. Beef, pork, and milk allergy (cross reactivity with each other and pet allergies) Otolaryngol Head Neck Surg. 2005;133:534–7. doi: 10.1016/j.otohns.2005.07.016. [DOI] [PubMed] [Google Scholar]
- 18.Fuentes Aparicio V, Sanchez Marcen I, Perez Montero A, Baeza ML, de Barrio Fernandez M. Allergy to mammal’s meat in adult life: immunologic and follow-up study. J Investig Allergol Clin Immunol. 2005;15:228–31. [PubMed] [Google Scholar]
- 19.Werfel SJ, Cooke SK, Sampson HA. Clinical reactivity to beef in children allergic to cow’s milk. J Allergy Clin Immunol. 1997;99:293–300. doi: 10.1016/s0091-6749(97)70045-9. [DOI] [PubMed] [Google Scholar]
- 20.Jerath MR, Kwan M, Kannarkat M, Mirakhur B, Carey L, Valgus J, et al. A desensitization protocol for the mAb cetuximab. J Allergy Clin Immunol. 2009;123:260–2. doi: 10.1016/j.jaci.2008.09.046. [DOI] [PubMed] [Google Scholar]
- 21.Van Nunen SA, O’Connor KS, Clarke LR, Boyle RX, Fernando SL. An association between tick bite reactions and red meat allergy in humans. Med J Aust. 2009;190:510–1. doi: 10.5694/j.1326-5377.2009.tb02533.x. [DOI] [PubMed] [Google Scholar]
- 22.Gauci M, Loh RK, Stone BF, Thong YH. Allergic reactions to the Australian paralysis tick, Ixodes holocyclus: diagnostic evaluation by skin test and radioimmunoassay. Clin Exp Allergy. 1989;19:279–83. doi: 10.1111/j.1365-2222.1989.tb02384.x. [DOI] [PubMed] [Google Scholar]
- 23.Hilger C, Bessot JC, Hutt N, Grigioni F, De Blay F, Pauli G, et al. IgE-mediated anaphylaxis caused by bites of the pigeon tick Argas reflexus: cloning and expression of the major allergen Arg r 1. J Allergy Clin Immunol. 2005;115:617–22. doi: 10.1016/j.jaci.2004.11.052. [DOI] [PubMed] [Google Scholar]
- 24.Francischetti IM, Sa-Nunes A, Mans BJ, Santos IM, Ribeiro JM. The role of saliva in tick feeding. Front Biosci. 2009;14:2051–88. doi: 10.2741/3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tangvoranuntakul P, Gagneux P, Diaz S, Bardor M, Varki N, Varki A, et al. Human uptake and incorporation of an immunogenic nonhuman dietary sialic acid. Proc Natl Acad Sci U S A. 2003;100:12045–50. doi: 10.1073/pnas.2131556100. [DOI] [PMC free article] [PubMed] [Google Scholar]