Abstract
Many lines of independent research have provided convergent evidence regarding oxidative stress, cerebrovascular disease, dementia, and Alzheimer’s disease (AD). Clinical studies spurred by these findings engage basic and clinical communities with tangible results regarding molecular targets and patient outcomes. Focusing on recent progress in characterizing age-related diseases specifically highlights oxidative stress and mechanisms for therapeutic action in AD. Oxidative stress has been investigated independently for its relationship with aging and cardiovascular and neurodegenerative diseases and provides evidence of shared pathophysiology across these conditions. The mechanisms by which oxidative stress impacts the cerebrovasculature and blood-brain barrier are of critical importance for evaluating antioxidant therapies. Clinical research has identified homocysteine as a relevant risk factor for AD and dementia; basic research into molecular mechanisms associated with homocysteine metabolism has revealed important findings. Oxidative stress has direct implications in the pathogenesis of age-related neurodegenerative diseases and careful scrutiny of oxidative stress in the CNS has therapeutic implications for future clinical trials. These mechanisms of dysfunction, acting independently or in concert, through oxidative stress may provide the research community with concise working concepts and promising new directions to yield new methods for evaluation and treatment of dementia and AD.
Keywords: Alzheimer disease, antioxidants, cerebrovascular, dementia, oxidative stress, treatment
Introduction
Management of oxidative stress is essential to cellular and tissue-specific homeostasis, and excess oxidative stress has been identified in the etiology and progression of numerous pathological conditions including cancer, age-related diseases, diabetes, and various mechanisms of toxicity. Oxidative stress is generally defined as an imbalance between production of reactive oxygen species (ROS) and capacity for removing ROS. While free radicals contributing to oxidative stress are generally unstable and decay spontaneously, accumulated effects of reactive species are particularly relevant to cell injury. While Alzheimer’s disease (AD) has been defined primarily through molecular mechanisms of neuronal dysfunction, a broad body of evidence indicates that ROS and oxidative stress contribute to associated dementias through multiple mechanisms. Importantly, there is a growing body of evidence that AD and oxidative stress are associated with profound cerebrovascular changes [26,36,45,64,114,115]. While cerebrovascular dysfunction and neurodegeneration exist independently, both conditions can impact cognition, and treatments with antioxidants have shown broad therapeutic effects on these conditions.
ROS production under normal conditions is tied directly to well characterized biochemical pathways [79]. Indeed, the production of ROS is an inescapable consequence of respiration and also occurs through other oxidative processes generating multiple signaling molecules and specific defensive oxidative species [65,111,112]. Importantly, the brain has a high rate of oxygen utilization and consumes approximately 20% of cardiac output and must continually compensate for the products of high oxygen metabolism and acute sensitivity to changes in blood flow. This ROS balance is dependent on a number of cellular and tissue specific antioxidant mechanisms such that impaired or suboptimal ability to remove ROS results in conditions of oxidative stress. If these normal mechanisms of compensation and regulation become ineffective in resolving cellular insults, dysregulated mechanisms of pathology characteristic for disease processes are initiated. Mechanisms of dysfunction can ultimately cause similar responses; notably age-related cardiovascular disease and age-related neurodegenerative diseases including AD show remarkable convergence in inflammatory mechanisms [20]. Pro-oxidant conditions are not limited to inflammation, ischemic conditions, and aging, and therefore therapeutic treatments with broad action on the vasculature and central nervous system (CNS) may well prevent these conditions from producing dementia. Indeed, research on antioxidant therapies for CNS and peripheral conditions are generating a foundation of information utilized by basic and clinical scientists. As the causes of AD have yet to be successfully resolved, evaluating recent clinical studies and progress across all these fields is exceedingly important and instructive.
Cerebrovascular Disease: Impact on Dementia
AD is the most prevalent form of age-related dementia, however, aging itself is more broadly considered the most prominent determinant of disease in Western societies [28]. Noteworthy, aging is a major risk factor for both vascular and cerebrovascular diseases. Cerebrovascular integrity is critical for proper metabolism and perfusion of the brain and oxidative stress has been widely characterized in vascular dysfunction. Considerations of the effects of oxidative stress on CNS and cerebrovascular components should be made collectively due to parallels in pathophysiology and associated mortality. Additionally, cerebrovascular dysfunction directly impacts dementia, a broad clinical determination for specific impairments, and it is unfortunate that cognitive testing does not have the sensitivity to always discriminate the underlying specific pathological condition. Importantly, global evaluation of vascular pathologies allows for greater attention to prevention of dementia and AD.
Characterizing the relationship between cerebrovascular and CNS disorders has been evaluated but additional research is clearly needed. Indeed, a report from The National Heart, Lung, and Blood Institute (NHLBI) issued in 2006 prioritized a set of recommendations for research in cerebrovascular biology and disease. The working group for NHLBI identified large gaps of understanding in the following areas: (a) molecular and cellular neurobiology of cerebral blood vessels focusing on genomics and proteomics; (b) resource development for new methodological approaches and collaborative research in cerebrovascular pathobiology; and (c) translational programs to address mechanisms of cerebrovascular disease. The report highlights which information has yet to be collected regarding neurovascular relationships and interactions with the brain [37].
While these efforts are broadly focused on improving understanding for all cerebrovascular disorders, specific cerebrovascular dysfunction is associated with dementia and occurs simultaneously with AD. Indeed, chronic or acute ischemia, hypoperfusion, atrophy, and hemorrhagic stroke can all independently induce indistinguishable cognitive deficits [3]. A large body of evidence has emerged reflecting that many cases of age-related dementia have cerebrovascular pathology affecting performance of the CNS [19]. Upon autopsy, substantial vascular pathology is observed in both demented and non-demented elderly, and while AD and vascular pathology are major pathological correlates with cognitive decline, there are no clear thresholds for pathological features predicting dementia [56]. Clinical diagnosis of vascular dementia and AD is distinguishable by mode of presentation, progression, and accompanying clinical findings (reviewed by [70]). Latent AD, which progresses through structures of the brain in a well-defined, hierarchical spatial sequence, may take years to impact executive functions while vascular dementia pathology manifests in cognitive impairment.
Observations regarding cerebrovascular disease have revealed that interruption of prefrontal subcortical areas by ischemic lesions increases the risk of clinical expression of dementia in AD more than 20 fold [92]. Lacunes and microinfarcts are lesions of vascular disease and reflect vascular dysfunction causing pathology in the brain. While 20 different lacunar lesions have been described, almost all of these lesions are associated with small vessel disease and hypertension [22], and often correlate with pathology and cognitive deficits [21]. While overlapping pathologies are difficult to interpret, significant relationships have been found providing evidence that microinfarcts and demyelination contribute to cognitive deficits in aging and AD [41]. Lacunes and white matter hyperintensities have been shown to be independently associated with cognitive function in the elderly as measured by magnetic resonance imaging and cognitive scoring through Alzheimer’s Disease Assessment Scale-cognitive subscale (ADAS-Cog) and mini-mental state examination scores [98]. These findings have led to suggestions that magnetic resonance imaging will play an increasingly important role in directing and evaluating treatments [99].
Relatedly, awareness and improvement for cardiovascular health has produced substantial improvement in statistically evaluated measures of risk factor prevalence, disease incidence, and mortality. Indeed, the 2008 Statistical Update from the American Heart Association reflects that while vascular disease burden remains high, death rates from vascular disorders, as well as heart disease, have declined recently. Specifically, the report describes that the death rate from stroke declined 24.2% between 1994 and 2004, whereas the actual number of stroke only declined 6.8% during that time [71]. This effect may be attributable to improved acute care, or rather the outcome of better detection and treatment for hypertension [47]. A recent study of hypertension in cognitively normal elderly has identified abnormal cerebral blood flow in this population [14], a risk factor predisposing them to AD. Population studies have revealed an elevated risk for AD in patients with hypertension [23]. Parallels in disease processes and risk factors offer insight into disease management and strategies, in fact, angiotension converting enzyme inhibitors improve daily living measures of AD patients [27]. Thus, strict characterization for these conditions is beginning to falter as more evidence has been brought to light that prevention of one condition can ultimately provide protection against other age-related conditions. Future therapeutic strategies need to be considered within this context – multiple factors act in coordination to produce ultimate disease states; broad therapeutic action has the greatest probability for success in a diverse population.
Cerebrovascular Disease: Mechanisms and Direct Relationships with Alzheimer’s Disease
Vascular dementia, stroke, and multi-infarct dementia are well-characterized age-related CNS disorders and reflect shared pathophysiology. Indeed, the term Vascular Cognitive Impairment has been used recently to describe a heterogeneous group of cognitive disorders sharing a presumed vascular cause [51]. Emerging evidence suggests that neurovascular dysfunction is a feature of cerebrovascular diseases and neurodegenerative conditions such as AD [36]. Further characterization of cerebrovascular and neurodegenerative conditions is needed.
Hypertension is the strongest risk factor for AD and vascular dementia when these conditions are considered together [82]. The penetrating arteries in the circle of Willis are particularly sensitive to the effects of hypertension and suffer early and selective damage during chronic hypertension [24,35]. Hypertension is closely associated with atherosclerosis and vascular function, and in the brain this results in hypoperfusion and ischemic conditions of the nucleus basalis Meynert. Targeting molecular mechanisms and using dietary methods and therapies are grounded in reducing free radicals and associated oxidative stress related damage initiating hypertension [96,100]. Animal models of induced hypertension include glutathione depletion whereby disruption of nitric oxide (NO) signaling, and therefore depressed NO availability and significant accumulations of nitrotyrosine result in severe hypertension [101]. Although the initiating factors for hypertensive disorders may vary, oxidative stress and inflammation have major roles in the pathogenesis of hypertension, and these three conditions participate in a self-perpetuating cycle that can lead to progressive cardiovascular disease [102]. Clinical evidence supports this hypothesis as biomarkers of systemic oxidative stress are observed in hypertension [67]. Polymorphonuclear leukocytes and platelets rich with ROS have also been identified in hypertensive patients, participating in the vascular oxidative stress [50,107]. Experimental evidence showing oxidative stress involvement in vascular damage has been of great interest for targeting ROS for the treatment of hypertension and other cardiovascular diseases (reviewed by [95]). Hypertension and resultant atherosclerosis reduce brain perfusion and may precipitate chronic ischemic conditions inducing AD. While documented in AD cases, it is speculative to indicate atherosclerosis is causal for AD, though these pathologies may have independent and convergent processes [10].
Neurovascular changes and hypoperfusion associated with aging and AD have are also being closely examined within the context of oxidative stress. Normal aging and neurodegenerative diseases are associated with structural and functional alterations of the cerebrovasculature and brain endothelial cells. This loss of endothelium function manifests as loss of responsiveness to vasodilators such as NO and increased formation of ROS [9]. Histological changes include loss of brain endothelial cell elongation and reduction of endothelial mitochondria. These deficit,s working in concert, produce hypoperfusion, aberrant angiogenesis and remodeling, potentially inducing neuronal injury and loss. It has been suggested that vascular hypoperfusion may be a causal effect in neuronal mitochondrial dysfunction [3] and oxidative stress leading to compensation mechanisms in the endothelium [61]. Recent findings with mouse models of AD support the role of dysfunctional NO signaling in the pathogenesis of the disease. Transgenic inducible nitric oxide synthase knockout mice develop pathology in the brain characteristic of AD (amyloid plaques, tau phosphorylation, and neuronal loss) indicating the NO has a protective role [13,104]. Additional considerations have identified that shared cholinergic deficits occurring in vascular dementia and AD are due to susceptibility of basal forebrain neurons to the effects of arterial hypertension, hypoperfusion, and ischemia [70]. The exact manner in which neurovascular changes contribute to cognitive decline have yet to be elucidated, however, present data suggest these mechanisms play a significant role in AD and related dementias.
The blood brain barrier (BBB) is essential for brain performance and considered substantially compromised in a subpopulation of AD patients [6,8]. Normal functioning of the BBB is critical for proper neuronal function including synaptic transmission, remodeling, angiogenesis, and neurogenesis (reviewed by [116]). The BBB is characterized by tight junctions between adjacent brain endothelial cells providing a unique boundary that is highly specialized with diverse transport systems. Integrity of the BBB is dependent on the health of the vasculature, and in AD, the total length of capillaries is reduced [5] and microvascular endothethial degeneration has been described [70]. Additional work has shown that a subset of genes is considerably altered in brain endothelial cells [105] directly implicating altered gene expression in this cell population in AD pathogenesis. Altered brain endothelial cells in AD reflect dysfunctional angiogenesis, reduced lipoprotein receptor-related protein 1, and resultant impairment of amyloid-β (Aβ) efflux from the brain, providing evidence that impaired BBB contributes to the disease. Dysfunction of the BBB also has substantial effects on Aβ influx. Receptor for advanced glycation endproducts (RAGE) is the major transporter of Aβ, affecting influx of the peptide across the BBB and mediating pathophysiological responses. RAGE is expressed in at least 3 major isoforms and the splice isoform soluble RAGE (sRAGE) lacks a transmembrane domain and is thought to compete with membrane bound RAGE for circulating ligands. Ligands include a variety of proinflammatory agents including advanced glycation end products, S100/calgranulins, and Aβ that are known to be elevated in AD [11,84,85]. Elevations in circulating sRAGE are associated with reduced risk of coronary artery disease, metabolic disorder, hypertension, arthritis, and AD (reviewed by [25]). Accumulation of RAGE ligands, such as advanced glycation endproducts and Aβ, cause increased cerebrovascular expression of RAGE resulting in the transcytosis of Aβ into the brain parenchyma where it binds to neurons.
A pro-inflammatory state may be further promoted through nuclear factor κB (NFκB), redox-sensitive transcription factors responding to loss of NO. Endothelium activation mediated through NFκB signaling results in induction of proinflammatory cytokines directly acting on local neurons. A more direct interaction has been shown for neurons expressing RAGE that are directly susceptible to oxidative damage mediated death by activated microglia secreting proinflammatory cytokines [106]. These mechanisms indicate that reductions of circulating RAGE ligands can reduce Aβ influx to the brain by mediating BBB transcytosis and reduce inflammatory cascades initiated through the endothelium and microglia impacting survival of RAGE expression neurons.
Further investigations into the vascular components of AD are warranted due to the high amount of overlap in risk factors with vascular dementia, atherosclerosis, stroke, homocysteine, hypertension, hyperlipidemia, diabetes, and apolipoprotein expression [15,36,115]. Causal relationships that have been identified with oxidative stress should be further investigated as targets of intervention which prohibit or diminish clinical manifestations of AD and dementia. Collectively this information indicates that oxidative stress and antioxidant therapies can substantially impact clinically diagnosed dementia and AD.
Homocysteine, Alzheimer’s Disease and Dementia: Oxidative Stress Mediates Broad Effects
Homocysteine has been identified as a peripheral marker directly relevant to oxidative stress and reflecting a nefarious relationship with AD, neuropathy, and vascular dysfunction. Epidemiogical evidence regarding the relationship between homocysteine and AD has provided substantial evidence that homocysteine is an independent risk factor for development of dementia and AD [80]. Plasma homocysteine concentrations above 14 μM increase the risk for developing AD two-fold. Despite well characterized enzymes and biochemical pathways, the pathophysiology of homocysteine remains largely unknown, however, hyperhomocysteinemia has been correlated in other neurological disorders besides AD [12], including Parkinson’s disease [54], and brain atrophy [74]. Homocysteine levels have also been correlated to white matter hyperintensities and increased risk of small and large vessel disease [73]. While generation of free radicals from oxidation of homocysteine has been evaluated as a mechanism of generalized oxidative stress, and these diseases involve multiple physiological insults, it is not clear which mechanism of action is responsible for homocysteine toxicity.
Homocysteine is a sulfur containing amino acid generated by hydrolysis of S-adenosyl homocysteine. In this well known biochemical pathway, S-adenosyl methionine provides methyl groups to various methyltransferases, directing normal methylation of proteins and DNA. Importantly, it has been estimated that hyperhomocysteinemia occurs in 20–30% of the elderly population [4], and this figure may be higher in the psychogeriatric population [57]. Oxidation to homocysteine thiols to disulfides occurs rapidly and is accompanied by the generation of free radicals. This creates mixed disulfides with homocysteine bonding with cysteine, homocysteine, and most predominantly albumin [68]. This pro-oxidant effect has been hypothesized to explain increased lipid peroxidation associated with hyperhomocysteinemia [18,103]. Information regarding intracellular homocysteine and mechanisms of BBB transport have not been addressed and could provide insight into the associated pathophysiology. Reports have been issued showing the homocysteine has a toxic effect on microvessels and disrupts the BBB in transgenic models of hyperhomocysteinemia [39]. These experimental observations regarding homocysteine have yielded insights into the observed clinical relationships that have been reported. homocysteine decreases NO synthesis and bioavailability in the endothelium. It has been suggested that NO plays an important role in the detoxification of homocysteine by formation of S-nitrohomocysteine, which functions as a vasodilator and inhibits platelet aggregation [94]. Indeed, primates with mild hyperhomocysteinemia exhibit endothethial dysfunction [44]. Oxidation of homocysteine has been shown to mediate toxic effects of the amino acid, however, other reports have shown wide-ranging effects that are implicated in AD pathophysiology.
A number of findings demonstrate that the CNS is acutely sensitive to homocysteine. Homocysteine is an agonist for N-methyl-D-aspartic acid receptors, stimulates calcium influx, and promotes glutamate excitotoxicity [42,46]. In vitro experiments have also demonstrated that homocysteine causes DNA damage, activation of poly(ADP-ribose)polymerase and p53 induction [42]. Attenuation of these neurotoxic mechanisms by superoxide dismutase, catalase, and N-acetyl-L-cysteine are consistent with the role of ROS-mediated insults [32,40]. The S-adenosyl methionine/S-adenosyl homocysteine ratio is of special consideration due to regulation of specific methyltransferases; hypomethylation of enzyme PP2A has been implicated in amyloid-β protein precursor pathophysiology [93] under conditions of hyperhomocysteinemia.
The evidence around homocysteine supports clinical efforts that have been pursued to reduce concentrations of plasma homocysteine. Indeed, given the broad effects that homocysteine may have on vascular and neuronal cell populations, targeting this molecule directly may have substantial benefits. However, because the evidence regarding the generation and toxicity of homocysteine is not completely understood, is will be beneficial to use homocysteine as a biomarker when evaluating various antioxidant therapies evaluated in the clinic.
Oxidative Stress, Neurodegeneration, and Alzheimer’s Disease
Thus, the elements addressed regarding aging and oxidative stress demonstrate many potential mechanisms exist by which these conditions can induce cellular dysfunction. Observed vascular dysfunctions influence CNS performance independent of changes in neuronal environment. Direct evidence has also been collected suggesting the oxidative stress plays a large role in neuronal susceptibility to cellular dysfunction and CNS lesions.
Oxidative stress, reduced glucose metabolism, and mitochondrial abnormalities are associated with AD [31,52,58,60,69]; mitochondria are considered the primary source of oxidative stress associated with normal respiration. Oxidative phosphorylation produces superoxide radicals subsequently transformed to hydrogen peroxide (H2O2) and highly reactive hydroxyl radicals (•OH). While most of these free radicals are sequestered in the mitochondria, oxidative insult is exacerbated by age, metabolic demand, and disease [83]. Heightened superoxide radical formation in AD also correlates with heightened superoxide dismutase levels that may allow the release of H2O2 from the mitochondria to the cytoplasm (H2O2 is diffusible through the mitochondrial and cell membranes). Mitochondrial abnormalities have been associated with deficiencies in enzymatic activities, specifically α-ketoglutarate dehydrogenase complex, pyruvate dehydrogenase complex, and cytochrome oxidase in AD neurons [48,53,72,81,108], which may increase either production of free radicals or alternatively alter cellular mechanisms for clearance. It has been shown that metals are dysregulated in AD [90], specifically that redox-active transition metals are aberrantly accumulated in the cytoplasm of AD susceptible neurons [78,88]. Increased cytoplasmic H2O2 may cause localized increases in concentrations of ROS in the presence of redox active metals [43]. The literature reflects that oxidative stress is not limited to mitochondrial dysfunction and Fenton chemistry. Indeed, Aβ peptide and oligomers have been widely characterized in the context of oxidative stress and neuronal toxicity. Aβ peptide is considered a strong redox active agent capable of reducing transition metals in the cytoplasm and allowing for conversion of molecular oxygen (O2) to H2O2 [7,30,34,55,78].
Increased intraneuronal ROS may have multiple effects on cellular metabolism, especially in the context of inflammation, metabolic loss, and endocrine signaling mechanisms. Oxidation of lipids and RNA has accurately been described by the field; 8-hydroxyguanosine is a marker of RNA oxidation that is increased in the cytoplasm of neurons in AD [33]. Evaluating these pathways in AD has identified multiple mechanisms by which oxidative stress may accumulate and create dysfunctional neuronal responses, suggesting various insults are required for the development of the AD phenotype [110,112,113].
Mechanisms for dealing with endogenous antioxidants may be compromised in AD and other diseases characterized by oxidative stress. Metal homeostasis and the associated Fenton chemistry that is associated with free metal ions induces pro-oxidant conditions in broad neurological conditions. Only trace amounts of metals are necessary for redox cycling to occur; these free metal ions or low-affinity complexes with amino acids mediate oxidative stress reactions [77,87]. Oxidation of protein side chains by ROS or reactive nitrogen species introduces hydroxyl groups or generates carbonyl groups detectable by 2,4,-dinitrophenylhydrazine [86,89]. Oxidation to polyunsaturated lipids and membrane lipoproteins, lipoxidation is of particular relevance in the brain which is rich in polyunsaturated lipid [76]. Oxidative damage to nucleic acids, particularly RNA, has been documented in AD [58–60].
Neurodegenerative diseases show conserved mechanisms of pathology and collectively demonstrate that components of oxidative stress are involved with neurodegenerative diseases. While Parkinson’s disease and amyotrophic lateral sclerosis have different genetic and environmental characteristics, they are characterized by neuroinflammation and chronic oxidative stress. Additionally, these conditions are characterized as protein conformational diseases; accumulations of misfolded proteins have genetic and environmental contributions, however, this conserved pathology demonstrates parallels in conserved cell biology. Dysregulated metabolism of metal ions has also been observed in all three conditions providing evidence of parallel but distinct mechanisms of compensation. Indeed, progress in identifying the role oxidative stress in AD etiology and progression can provide substantial insight into the multitude of neurodegenerative diseases.
Relevant Clinical Studies and Future Opportunities
Clinical studies conducted with antioxidant therapies in the areas of vascular disease and AD provide a wealth of new information due to the age-related nature of these conditions. Indeed, research methodologies and evidence collected can provide insight into treatment paradigms and the anticipated clinical outcomes; ultimately reviewing clinical studies utilizing antioxidants may provide insight such that dramatic decreases in mortality, such as those recently reported for stoke, can be observed.
The relationship between oxidative stress and vascular disease has been evaluated in at least seven large clinical studies evaluating antioxidants; only one study showing that antioxidant supplementation through vitamins E and C can reduce atherosclerosis [29]. These studies have utilized patients with significant cardiovascular disease and additionally other considerations should be made regarding failure to demonstrate beneficial effects. Accordingly, the American Heart Association has not adopted recommendations for population-wide antioxidant supplementation but have made recommendations that the general population consume a balanced diet with antioxidant-rich fruit, vegetables, and grains [97].
The Gingko Evaluation of Memory Study was initiated to evaluate the effects of Gingko biloba in prevention of dementia, targeting early intervention to impact clinical dementia [16]. Importantly, the Gingko Evaluation of Memory Study reflects that only recently has progress in dementia research allowed for prevention of dementia trials. Gingko Evaluation of Memory Study enrolled over 3000 participants and this large cohort will enable the investigators to evaluate the effects of combinations of cardiovascular and cerebrovasculature status on cognion and dementia. A preliminary report from the study was released in July 2007 [17].
A randomized, placebo controlled trial that involved traditionally identified antioxidants provided evidence that antioxidant therapies can significantly impact AD patient outcomes. When severity of dementia at baseline is used as a covariate for analysis, significant delays in time-to-death, placement in a nursing home, development of severe dementia, or a defined severity of impairment of activities of daily living were observed for patients in the selegiline, α-tocopherol, and combination-therapy groups [75].
Previous studies have found that antioxidants may ablate cognitive decline [38,62]. Randomized trials have shown that selegiline hydrochloride or vitamin E may slow progression of AD. A large epidemiological study has shown that use of vitamin E and C in combination was associated with reduced AD prevalence and incidence [109]. The authors of this report suggest that vitamin E and C may offer protection if taken together at high doses. This evidence suggests the antioxidant strategies are beneficial for reducing the risk of developing AD; however, once a disease state has been initiated, reversal through the use of antioxidants is mechanistically improbable. Indeed, therapy outcomes should be evaluated with the realization that multiple disease factors are acting in conjunction.
As discussed, homocysteine has also been a central target in the association of oxidative stress with AD. Clinical trials have been conducted to evaluate the impact of high-dose vitamin supplementation on plasma homocysteine levels in patients with AD [1]. This study provides evidence that high-dose vitamins can lower homocysteine in AD patients. More recent evidence has been collected, reflecting that both elevated plasma homocysteine and low serum levels of folate are independent predictors for the development of dementia and AD [66]. These finding have spurred placebo controlled studies regarding the efficacy of folic acid supplementation on dementia and AD. The Alzheimer’s Disease Cooperative Study Homocysteine Trial was initiated to explore supplementation for AD patients over an 18 month period. Treatment groups demonstrated reduction of homocysteine levels by high-dose folic acid/B6/B12 supplementation in a multicenter, randomized, controlled clinical trial. Treatment resulted in 20–30% reduction in peripheral homocysteine, however, ADAS-Cog scores showed no change. In elevated homocysteine population (>13 uM homocysteine), no significant change in ADAS-Cog testing was observed. Post-hoc analysis on mildly demented patients (Clinical Dementia Rating = 0.5) demonstrated B vitamin supplementation provided clinically significant stabilization in ADAS-Cog scores from three months through 18 months. Depression was reported as an adverse event in the vitamin supplement group [2].
Alternatively, antioxidants B12 and N-acetyl-L-cysteine have been administered to patients with dementia. While the size of these studies in general clinical settings are quite small, these reports have found that antioxidants may indeed have an apparent clinical benefit. It is well noted that patients responses are quite varied as the circumstances under which these hyperhomocysteimic patients are enrolled in the trials [49].
Conclusions
AD is recognized as a chronic condition with a long asymptomatic period preceding recognizable clinical deterioration and dementia. Collected evidence has shown strong implications that oxidative stress plays an early and important role in the pathogenesis of the disease through a number of mechanisms. Collectively examining the role of oxidative stress on cerebrovasculature, hypertension, BBB, and neurons, provides substantial evidence that therapies impacting these conditions independently may ultimately influence onset, severity, or progression of clinical AD. These therapies should therefore be considered as disease modifying and continued to be pursued as more information regarding antecedent biomarkers becomes available [63,91].
Currently large collaborations are underway in the United States and Europe to improve clinical trials by characterizing mild cognitive impaired and AD patients. The Alzheimer Disease Neuroimaging Initiative is an unprecedented study in size and scope; this partnership between the National Institutes of Health and private industry is generating parallel data from magnetic resonance imaging, positron emission tomography, cognitive scores, and biomarkers in a longitudinal study, creating a wealth of data that will allow for improved treatment trials.
In June 2007, in concert with the Alzheimer’s Association, the Centers for Disease Control released the “Healthy Brain Initiative: A National Public Roadmap to Maintaining Cognitive Health”; the roadmap is intended to give the public and scientific communities insights into the progress that is being made in the areas of progress and prevention for AD and dementia. While the direct relationships between vascular and cognitive health are still not fully understood, sufficient evidence exists to support the association between vascular health and cognitive health, clinical trials are necessary to establish the effectiveness of interventions targeted to vascular risk factors.
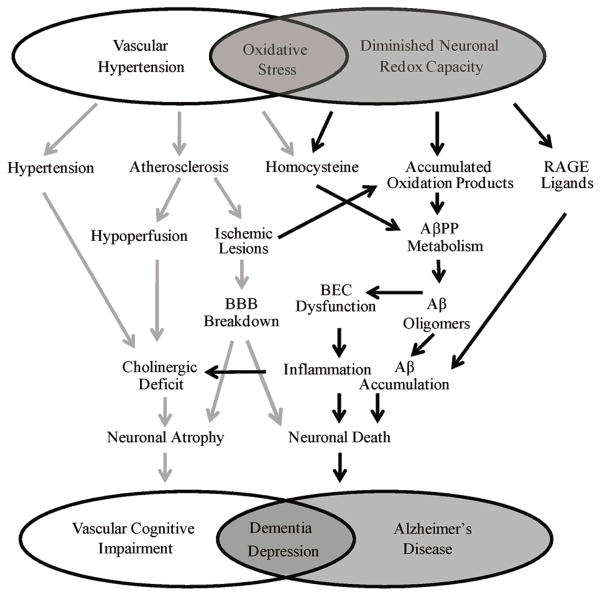
Figure 1.
Cerebrovascular oxidative stress and diminished neuronal redox capacity both independently and coordinately impact dementia and AD.
Acknowledgments
Work in the authors’ laboratories is supported by the National Institutes of Health (AG026151 to MAS; AG024028 to XWZ). Dr. Smith is, or has in the past been, a paid consultant for, owns equity or stock options in and/or receives grant funding from Neurotez, Neuropharm, Edenland, Panacea Pharmaceuticals, and Voyager Pharmaceuticals. Dr. Perry is a paid consultant for and/or owns equity or stock options in Takeda Pharmaceuticals, Voyager Pharmaceuticals, Panacea Pharmaceuticals and Neurotez Pharmaceuticals.
References
- 1.Aisen PS, Egelko S, Andrews H, Diaz-Arrastia R, Weiner M, DeCarli C, Jagust W, Miller JW, Green R, Bell K, Sano M. A pilot study of vitamins to lower plasma homocysteine levels in Alzheimer disease. Am J Geriatr Psychiatry. 2003;11:246–249. [PubMed] [Google Scholar]
- 2.Aisen PS, Shelia J, Ronald GT, Mary S, Ramon DA, Leon T. S3-02-01: ADCS homocysteine trial. Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association. 2007;3:S199. [Google Scholar]
- 3.Aliev G, Smith MA, Obrenovich ME, de la Torre JC, Perry G. Role of vascular hypoperfusion-induced oxidative stress and mitochondria failure in the pathogenesis of Azheimer disease. Neurotox Res. 2003;5:491–504. doi: 10.1007/BF03033159. [DOI] [PubMed] [Google Scholar]
- 4.Allen RH, Stabler SP, Lindenbaum J. Relevance of vitamins, homocysteine and other metabolites in neuropsychiatric disorders. Eur J Pediatr. 1998;157(Suppl 2):S122–126. doi: 10.1007/pl00014295. [DOI] [PubMed] [Google Scholar]
- 5.Bailey TL, Rivara CB, Rocher AB, Hof PR. The nature and effects of cortical microvascular pathology in aging and Alzheimer’s disease. Neurol Res. 2004;26:573–578. doi: 10.1179/016164104225016272. [DOI] [PubMed] [Google Scholar]
- 6.Bonda DJ, Webber KM, Siedlak SL, Perry G, Friedland RP, Smith MA. The pathology of Alzheimer disease elicits an in vivo immunological response. Am J Immunol. 2007;3:10–14. [Google Scholar]
- 7.Bondy SC, Guo-Ross SX, Truong AT. Promotion of transition metal-induced reactive oxygen species formation by beta-amyloid. Brain Res. 1998;799:91–96. doi: 10.1016/s0006-8993(98)00461-2. [DOI] [PubMed] [Google Scholar]
- 8.Bowman GL, Kaye JA, Moore M, Waichunas D, Carlson NE, Quinn JF. Blood-brain barrier impairment in Alzheimer disease: stability and functional significance. Neurology. 2007;68:1809–1814. doi: 10.1212/01.wnl.0000262031.18018.1a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brandes RP, Fleming I, Busse R. Endothelial aging. Cardiovasc Res. 2005;66:286–294. doi: 10.1016/j.cardiores.2004.12.027. [DOI] [PubMed] [Google Scholar]
- 10.Casserly I, Topol E. Convergence of atherosclerosis and Alzheimer’s disease: inflammation, cholesterol, and misfolded proteins. Lancet. 2004;363:1139–1146. doi: 10.1016/S0140-6736(04)15900-X. [DOI] [PubMed] [Google Scholar]
- 11.Castellani RJ, Harris PL, Sayre LM, Fujii J, Taniguchi N, Vitek MP, Founds H, Atwood CS, Perry G, Smith MA. Active glycation in neurofibrillary pathology of Alzheimer disease: N(epsilon)-(carboxymethyl) lysine and hexitol-lysine. Free Radic Biol Med. 2001;31:175–180. doi: 10.1016/s0891-5849(01)00570-6. [DOI] [PubMed] [Google Scholar]
- 12.Clarke R, Smith AD, Jobst KA, Refsum H, Sutton L, Ueland PM. Folate, vitamin B12, and serum total homocysteine levels in confirmed Alzheimer disease. Arch Neurol. 1998;55:1449–1455. doi: 10.1001/archneur.55.11.1449. [DOI] [PubMed] [Google Scholar]
- 13.Colton CA, Vitek MP, Wink DA, Xu Q, Cantillana V, Previti ML, Van Nostrand WE, Weinberg JB, Dawson H. NO synthase 2 (NOS2) deletion promotes multiple pathologies in a mouse model of Alzheimer’s disease. Proc Natl Acad Sci U S A. 2006;103:12867–12872. doi: 10.1073/pnas.0601075103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dai W, Lopez OL, Carmichael OT, Becker JT, Kuller LH, Gach HM. Abnormal regional cerebral blood flow in cognitively normal elderly subjects with hypertension. Stroke. 2008;39:349–354. doi: 10.1161/STROKEAHA.107.495457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de la Torre JC. Is Alzheimer’s disease a neurodegenerative or a vascular disorder? Data, dogma, and dialectics. The Lancet Neurology. 2004;3:184–190. doi: 10.1016/S1474-4422(04)00683-0. [DOI] [PubMed] [Google Scholar]
- 16.DeKosky ST, Fitzpatrick A, Ives DG, Saxton J, Williamson J, Lopez OL, Burke G, Fried L, Kuller LH, Robbins J, Tracy R, Woolard N, Dunn L, Kronmal R, Nahin R, Furberg C. The Ginkgo Evaluation of Memory (GEM) study: design and baseline data of a randomized trial of Ginkgo biloba extract in prevention of dementia. Contemp Clin Trials. 2006;27:238–253. doi: 10.1016/j.cct.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 17.DeKosky ST, Fitzpatrick A, Ives D, Saxton JA, Williamson J, Lopez OL, Burke G, Fried L, Kuller LH, Robbins J, Tracey R, Woolard N, Dunn LO, Kronmal R, Nahin R, Furberg C. The Ginkgo in evaluation of memory (GEM) study design, recruitment, and incident dementia rates: A preliminary report. Alzheimer’s and Dementia. 2007;3:S168. [Google Scholar]
- 18.Eberhardt RT, Forgione MA, Cap A, Leopold JA, Rudd MA, Trolliet M, Heydrick S, Stark R, Klings ES, Moldovan NI, Yaghoubi M, Goldschmidt-Clermont PJ, Farber HW, Cohen R, Loscalzo J. Endothelial dysfunction in a murine model of mild hyperhomocyst(e)inemia. J Clin Invest. 2000;106:483–491. doi: 10.1172/JCI8342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Etiene D, Kraft J, Ganju N, Gomez-Isla T, Gemelli B, Hyman BT, Hedley-Whyte ET, Wands JR, De La Monte SM. Cerebrovascular Pathology Contributes to the Heterogeneity of Alzheimer’s Disease. J Alzheimers Dis. 1998;1:119–134. doi: 10.3233/jad-1998-1205. [DOI] [PubMed] [Google Scholar]
- 20.Finch CE. Developmental origins of aging in brain and blood vessels: an overview. Neurobiol Aging. 2005;26:281–291. doi: 10.1016/j.neurobiolaging.2004.03.015. [DOI] [PubMed] [Google Scholar]
- 21.Fisher CM. Lacunes: Small, Deep Cerebral Infarcts. Neurology. 1965;15:774–784. doi: 10.1212/wnl.15.8.774. [DOI] [PubMed] [Google Scholar]
- 22.Fisher CM. Lacunar strokes and infarcts: a review. Neurology. 1982;32:871–876. doi: 10.1212/wnl.32.8.871. [DOI] [PubMed] [Google Scholar]
- 23.Foroughan M, Farahani ZG, Shariatpanahi M, Vaezinejad M, Kamerani AA, Sheikhvatan M. Risk factors of Alzheimer’s disease among Iranian population. Curr Alzheimer Res. 2008;5:70–72. doi: 10.2174/156720508783884594. [DOI] [PubMed] [Google Scholar]
- 24.Fox NC, Warrington EK, Seiffer AL, Agnew SK, Rossor MN. Presymptomatic cognitive deficits in individuals at risk of familial Alzheimer’s disease. A longitudinal prospective study. Brain. 1998;121(Pt 9):1631–1639. doi: 10.1093/brain/121.9.1631. [DOI] [PubMed] [Google Scholar]
- 25.Geroldi D, Falcone C, Emanuele E. Soluble receptor for advanced glycation end products: from disease marker to potential therapeutic target. Curr Med Chem. 2006;13:1971–1978. doi: 10.2174/092986706777585013. [DOI] [PubMed] [Google Scholar]
- 26.Girouard H, Iadecola C. Neurovascular coupling in the normal brain and in hypertension, stroke, and Alzheimer disease. J Appl Physiol. 2006;100:328–335. doi: 10.1152/japplphysiol.00966.2005. [DOI] [PubMed] [Google Scholar]
- 27.Hajjar IM, Keown M, Lewis P, Almor A. Angiotensin converting enzyme inhibitors and cognitive and functional decline in patients with Alzheimer’s disease: an observational study. Am J Alzheimers Dis Other Demen. 2008;23:77–83. doi: 10.1177/1533317507309803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harman D. Aging: Overview. Ann NY Acad Sci. 2001;928:1–21. doi: 10.1111/j.1749-6632.2001.tb05631.x. [DOI] [PubMed] [Google Scholar]
- 29.Hasnain BI, Mooradian AD. Recent trials of antioxidant therapy: what should we be telling our patients? Cleve Clin J Med. 2004;71:327–334. doi: 10.3949/ccjm.71.4.327. [DOI] [PubMed] [Google Scholar]
- 30.Hayashi T, Shishido N, Nakayama K, Nunomura A, Smith MA, Perry G, Nakamura M. Lipid peroxidation and 4-hydroxy-2-nonenal formation by copper ion bound to amyloid-beta peptide. Free Radic Biol Med. 2007;43:1552–1559. doi: 10.1016/j.freeradbiomed.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 31.Hirai K, Aliev G, Nunomura A, Fujioka H, Russell RL, Atwood CS, Johnson AB, Kress Y, Vinters HV, Tabaton M, Shimohama S, Cash AD, Siedlak SL, Harris PL, Jones PK, Petersen RB, Perry G, Smith MA. Mitochondrial abnormalities in Alzheimer’s disease. J Neurosci. 2001;21:3017–3023. doi: 10.1523/JNEUROSCI.21-09-03017.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ho PI, Collins SC, Dhitavat S, Ortiz D, Ashline D, Rogers E, Shea TB. Homocysteine potentiates beta-amyloid neurotoxicity: role of oxidative stress. J Neurochem. 2001;78:249–253. doi: 10.1046/j.1471-4159.2001.00384.x. [DOI] [PubMed] [Google Scholar]
- 33.Honda K, Smith MA, Zhu X, Baus D, Merrick WC, Tartakoff AM, Hattier T, Harris PL, Siedlak SL, Fujioka H, Liu Q, Moreira PI, Miller FP, Nunomura A, Shimohama S, Perry G. Ribosomal RNA in Alzheimer disease is oxidized by bound redox-active iron. J Biol Chem. 2005;280:20978–20986. doi: 10.1074/jbc.M500526200. [DOI] [PubMed] [Google Scholar]
- 34.Huang X, Atwood CS, Hartshorn MA, Multhaup G, Goldstein LE, Scarpa RC, Cuajungco MP, Gray DN, Lim J, Moir RD, Tanzi RE, Bush AI. The A beta peptide of Alzheimer’s disease directly produces hydrogen peroxide through metal ion reduction. Biochemistry. 1999;38:7609–7616. doi: 10.1021/bi990438f. [DOI] [PubMed] [Google Scholar]
- 35.Hughes A, Tonks RS. Platelets, Magnesium, and Myocardial Infarction. Lancet. 1965;1:1044–1046. doi: 10.1016/s0140-6736(65)91317-6. [DOI] [PubMed] [Google Scholar]
- 36.Iadecola C. Neurovascular regulation in the normal brain and in Alzheimer’s disease. Nat Rev Neurosci. 2004;5:347–360. doi: 10.1038/nrn1387. [DOI] [PubMed] [Google Scholar]
- 37.Iadecola C, Goldman SS, Harder DR, Heistad DD, Katusic ZS, Moskowitz MA, Simard JM, Sloan MA, Traystman RJ, Velletri PA. Recommendations of the National Heart, Lung, and Blood Institute working group on cerebrovascular biology and disease. Stroke. 2006;37:1578–1581. doi: 10.1161/01.STR.0000221297.57305.8a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jama JW, Launer LJ, Witteman JC, den Breeijen JH, Breteler MM, Grobbee DE, Hofman A. Dietary antioxidants and cognitive function in a population-based sample of older persons. The Rotterdam Study. Am J Epidemiol. 1996;144:275–280. doi: 10.1093/oxfordjournals.aje.a008922. [DOI] [PubMed] [Google Scholar]
- 39.Kamath AF, Chauhan AK, Kisucka J, Dole VS, Loscalzo J, Handy DE, Wagner DD. Elevated levels of homocysteine compromise blood-brain barrier integrity in mice. Blood. 2006;107:591–593. doi: 10.1182/blood-2005-06-2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim WK, Pae YS. Involvement of N-methyl-d-aspartate receptor and free radical in homocysteine-mediated toxicity on rat cerebellar granule cells in culture. Neurosci Lett. 1996;216:117–120. doi: 10.1016/0304-3940(96)13011-1. [DOI] [PubMed] [Google Scholar]
- 41.Kovari E, Gold G, Herrmann FR, Canuto A, Hof PR, Michel JP, Bouras C, Giannakopoulos P. Cortical microinfarcts and demyelination significantly affect cognition in brain aging. Stroke. 2004;35:410–414. doi: 10.1161/01.STR.0000110791.51378.4E. [DOI] [PubMed] [Google Scholar]
- 42.Kruman II, Culmsee C, Chan SL, Kruman Y, Guo Z, Penix L, Mattson MP. Homocysteine elicits a DNA damage response in neurons that promotes apoptosis and hypersensitivity to excitotoxicity. J Neurosci. 2000;20:6920–6926. doi: 10.1523/JNEUROSCI.20-18-06920.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee HG, Zhu X, O’Neill MJ, Webber K, Casadesus G, Marlatt M, Raina AK, Perry G, Smith MA. The role of metabotropic glutamate receptors in Alzheimer’s disease. Acta Neurobiol Exp (Wars) 2004;64:89–98. doi: 10.55782/ane-2004-1494. [DOI] [PubMed] [Google Scholar]
- 44.Lentz SR, Sobey CG, Piegors DJ, Bhopatkar MY, Faraci FM, Malinow MR, Heistad DD. Vascular dysfunction in monkeys with diet-induced hyperhomocyst(e)inemia. J Clin Invest. 1996;98:24–29. doi: 10.1172/JCI118771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lim A, Tsuang D, Kukull W, Nochlin D, Leverenz J, McCormick W, Bowen J, Teri L, Thompson J, Peskind ER, Raskind M, Larson EB. Clinico-neuropathological correlation of Alzheimer’s disease in a community-based case series. J Am Geriatr Soc. 1999;47:564–569. doi: 10.1111/j.1532-5415.1999.tb02571.x. [DOI] [PubMed] [Google Scholar]
- 46.Lipton SA, Kim WK, Choi YB, Kumar S, D’Emilia DM, Rayudu PV, Arnelle DR, Stamler JS. Neurotoxicity associated with dual actions of homocysteine at the N-methyl-D-aspartate receptor. Proc Natl Acad Sci U S A. 1997;94:5923–5928. doi: 10.1073/pnas.94.11.5923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Luepker RV, Arnett DK, Jacobs DR, Jr, Duval SJ, Folsom AR, Armstrong C, Blackburn H. Trends in blood pressure, hypertension control, and stroke mortality: the Minnesota Heart Survey. Am J Med. 2006;119:42–49. doi: 10.1016/j.amjmed.2005.08.051. [DOI] [PubMed] [Google Scholar]
- 48.Mastrogiacomo F, Bergeron C, Kish SJ. Brain alpha-ketoglutarate dehydrogenase complex activity in Alzheimer’s disease. J Neurochem. 1993;61:2007–2014. doi: 10.1111/j.1471-4159.1993.tb07436.x. [DOI] [PubMed] [Google Scholar]
- 49.McCaddon A. Homocysteine and cognitive impairment; a case series in a General Practice setting. Nutr J. 2006;5:6. doi: 10.1186/1475-2891-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Minuz P, Patrignani P, Gaino S, Seta F, Capone ML, Tacconelli S, Degan M, Faccini G, Fornasiero A, Talamini G, Tommasoli R, Arosio E, Santonastaso CL, Lechi A, Patrono C. Determinants of platelet activation in human essential hypertension. Hypertension. 2004;43:64–70. doi: 10.1161/01.HYP.0000105109.44620.1B. [DOI] [PubMed] [Google Scholar]
- 51.Moorhouse P, Rockwood K. Vascular cognitive impairment: current concepts and clinical developments. Lancet Neurol. 2008;7:246–255. doi: 10.1016/S1474-4422(08)70040-1. [DOI] [PubMed] [Google Scholar]
- 52.Moreira PI, Harris PL, Zhu X, Santos MS, Oliveira CR, Smith MA, Perry G. Lipoic acid and N-acetyl cysteine decrease mitochondrial-related oxidative stress in Alzheimer disease patient fibroblasts. J Alzheimers Dis. 2007;12:195–206. doi: 10.3233/jad-2007-12210. [DOI] [PubMed] [Google Scholar]
- 53.Moreira PI, Siedlak SL, Wang X, Santos MS, Oliveira CR, Tabaton M, Nunomura A, Szweda LI, Aliev G, Smith MA, Zhu X, Perry G. Autophagocytosis of mitochondria is prominent in Alzheimer disease. J Neuropathol Exp Neurol. 2007;66:525–532. doi: 10.1097/01.jnen.0000240476.73532.b0. [DOI] [PubMed] [Google Scholar]
- 54.Muller T, Werne B, Fowler B, Kuhn W. Nigral endothelial dysfunction, homocysteine, and Parkinson’s disease. Lancet. 1999;354:126–127. doi: 10.1016/s0140-6736(99)01660-8. [DOI] [PubMed] [Google Scholar]
- 55.Nakamura M, Shishido N, Nunomura A, Smith MA, Perry G, Hayashi Y, Nakayama K, Hayashi T. Three histidine residues of amyloid-beta peptide control the redox activity of copper and iron. Biochemistry (Mosc) 2007;46:12737–12743. doi: 10.1021/bi701079z. [DOI] [PubMed] [Google Scholar]
- 56.Neuropathology Group of the Medical Research Council Cognitive Function and Ageing Study. Pathological correlates of late-onset dementia in a multicentre, community-based population in England and Wales. Lancet. 2001;357:169–175. doi: 10.1016/s0140-6736(00)03589-3. [DOI] [PubMed] [Google Scholar]
- 57.Nilsson K, Gustafson L, Faldt R, Andersson A, Hultberg B. Plasma homocysteine in relation to serum cobalamin and blood folate in a psychogeriatric population. Eur J Clin Invest. 1994;24:600–606. doi: 10.1111/j.1365-2362.1994.tb01111.x. [DOI] [PubMed] [Google Scholar]
- 58.Nunomura A, Perry G, Pappolla MA, Wade R, Hirai K, Chiba S, Smith MA. RNA oxidation is a prominent feature of vulnerable neurons in Alzheimer’s disease. J Neurosci. 1999;19:1959–1964. doi: 10.1523/JNEUROSCI.19-06-01959.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nunomura A, Perry G, Pappolla MA, Friedland RP, Hirai K, Chiba S, Smith MA. Neuronal oxidative stress precedes amyloid-beta deposition in Down syndrome. J Neuropathol Exp Neurol. 2000;59:1011–1017. doi: 10.1093/jnen/59.11.1011. [DOI] [PubMed] [Google Scholar]
- 60.Nunomura A, Perry G, Aliev G, Hirai K, Takeda A, Balraj EK, Jones PK, Ghanbari H, Wataya T, Shimohama S, Chiba S, Atwood CS, Petersen RB, Smith MA. Oxidative damage is the earliest event in Alzheimer disease. J Neuropathol Exp Neurol. 2001;60:759–767. doi: 10.1093/jnen/60.8.759. [DOI] [PubMed] [Google Scholar]
- 61.Obrenovich ME, Smith MA, Siedlak SL, Chen SG, de la Torre JC, Perry G, Aliev G. Overexpression of GRK2 in Alzheimer disease and in a chronic hypoperfusion rat model is an early marker of brain mitochondrial lesions. Neurotox Res. 2006;10:43–56. doi: 10.1007/BF03033333. [DOI] [PubMed] [Google Scholar]
- 62.Perrig WJ, Perrig P, Stahelin HB. The relation between antioxidants and memory performance in the old and very old. J Am Geriatr Soc. 1997;45:718–724. doi: 10.1111/j.1532-5415.1997.tb01476.x. [DOI] [PubMed] [Google Scholar]
- 63.Perry G, Castellani RJ, Hirai K, Smith MA. Reactive oxygen species mediate cellular damage in Alzheimer disease. J Alzheimers Dis. 1998;1:45–55. doi: 10.3233/jad-1998-1103. [DOI] [PubMed] [Google Scholar]
- 64.Perry G, Smith MA, McCann CE, Siedlak SL, Jones PK, Friedland RP. Cerebrovascular muscle atrophy is a feature of Alzheimer’s disease. Brain Res. 1998;791:63–66. doi: 10.1016/s0006-8993(98)00006-7. [DOI] [PubMed] [Google Scholar]
- 65.Petersen RB, Nunomura A, Lee HG, Casadesus G, Perry G, Smith MA, Zhu X. Signal transduction cascades associated with oxidative stress in Alzheimer’s disease. J Alzheimers Dis. 2007;11:143–152. doi: 10.3233/jad-2007-11202. [DOI] [PubMed] [Google Scholar]
- 66.Ravaglia G, Forti P, Maioli F, Martelli M, Servadei L, Brunetti N, Porcellini E, Licastro F. Homocysteine and folate as risk factors for dementia and Alzheimer disease. Am J Clin Nutr. 2005;82:636–643. doi: 10.1093/ajcn.82.3.636. [DOI] [PubMed] [Google Scholar]
- 67.Redon J, Oliva MR, Tormos C, Giner V, Chaves J, Iradi A, Saez GT. Antioxidant activities and oxidative stress byproducts in human hypertension. Hypertension. 2003;41:1096–1101. doi: 10.1161/01.HYP.0000068370.21009.38. [DOI] [PubMed] [Google Scholar]
- 68.Refsum H, Smith AD, Ueland PM, Nexo E, Clarke R, McPartlin J, Johnston C, Engbaek F, Schneede J, McPartlin C, Scott JM. Facts and recommendations about total homocysteine determinations: an expert opinion. Clin Chem. 2004;50:3–32. doi: 10.1373/clinchem.2003.021634. [DOI] [PubMed] [Google Scholar]
- 69.Reiman EM, Caselli RJ, Yun LS, Chen K, Bandy D, Minoshima S, Thibodeau SN, Osborne D. Preclinical evidence of Alzheimer’s disease in persons homozygous for the epsilon 4 allele for apolipoprotein E. N Engl J Med. 1996;334:752–758. doi: 10.1056/NEJM199603213341202. [DOI] [PubMed] [Google Scholar]
- 70.Roman GC, Kalaria RN. Vascular determinants of cholinergic deficits in Alzheimer disease and vascular dementia. Neurobiol Aging. 2006;27:1769–1785. doi: 10.1016/j.neurobiolaging.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 71.Rosamond W, Flegal K, Furie K, Go A, Greenlund K, Haase N, Hailpern SM, Ho M, Howard V, Kissela B, Kittner S, Lloyd-Jones D, McDermott M, Meigs J, Moy C, Nichol G, O’Donnell C, Roger V, Sorlie P, Steinberger J, Thom T, Wilson M, Hong Y. Heart disease and stroke statistics--2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008;117:e25–146. doi: 10.1161/CIRCULATIONAHA.107.187998. [DOI] [PubMed] [Google Scholar]
- 72.Russell RL, Siedlak SL, Raina AK, Bautista JM, Smith MA, Perry G. Increased neuronal glucose-6-phosphate dehydrogenase and sulfhydryl levels indicate reductive compensation to oxidative stress in Alzheimer disease. Arch Biochem Biophys. 1999;370:236–239. doi: 10.1006/abbi.1999.1404. [DOI] [PubMed] [Google Scholar]
- 73.Sachdev P. Homocysteine, cerebrovascular disease and brain atrophy. J Neurol Sci. 2004;226:25–29. doi: 10.1016/j.jns.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 74.Sachdev PS, Valenzuela M, Wang XL, Looi JC, Brodaty H. Relationship between plasma homocysteine levels and brain atrophy in healthy elderly individuals. Neurology. 2002;58:1539–1541. doi: 10.1212/wnl.58.10.1539. [DOI] [PubMed] [Google Scholar]
- 75.Sano M, Ernesto C, Thomas RG, Klauber MR, Schafer K, Grundman M, Woodbury P, Growdon J, Cotman CW, Pfeiffer E, Schneider LS, Thal LJ. A controlled trial of selegiline, alpha-tocopherol, or both as treatment for Alzheimer’s disease. The Alzheimer’s Disease Cooperative Study. N Engl J Med. 1997;336:1216–1222. doi: 10.1056/NEJM199704243361704. [DOI] [PubMed] [Google Scholar]
- 76.Sayre LM, Zelasko DA, Harris PL, Perry G, Salomon RG, Smith MA. 4-Hydroxynonenal-derived advanced lipid peroxidation end products are increased in Alzheimer’s disease. J Neurochem. 1997;68:2092–2097. doi: 10.1046/j.1471-4159.1997.68052092.x. [DOI] [PubMed] [Google Scholar]
- 77.Sayre LM, Perry G, Smith MA. Redox metals and neurodegenerative disease. Curr Opin Chem Biol. 1999;3:220–225. doi: 10.1016/S1367-5931(99)80035-0. [DOI] [PubMed] [Google Scholar]
- 78.Sayre LM, Perry G, Harris PL, Liu Y, Schubert KA, Smith MA. In situ oxidative catalysis by neurofibrillary tangles and senile plaques in Alzheimer’s disease: a central role for bound transition metals. J Neurochem. 2000;74:270–279. doi: 10.1046/j.1471-4159.2000.0740270.x. [DOI] [PubMed] [Google Scholar]
- 79.Sayre LM, Perry G, Smith MA. Oxidative stress and neurotoxicity. Chem Res Toxicol. 2008;21:172–188. doi: 10.1021/tx700210j. [DOI] [PubMed] [Google Scholar]
- 80.Seshadri S, Beiser A, Selhub J, Jacques PF, Rosenberg IH, D’Agostino RB, Wilson PW, Wolf PA. Plasma homocysteine as a risk factor for dementia and Alzheimer’s disease. N Engl J Med. 2002;346:476–483. doi: 10.1056/NEJMoa011613. [DOI] [PubMed] [Google Scholar]
- 81.Simonian NA, Hyman BT. Functional alterations in Alzheimer’s disease: selective loss of mitochondrial-encoded cytochrome oxidase mRNA in the hippocampal formation. J Neuropathol Exp Neurol. 1994;53:508–512. doi: 10.1097/00005072-199409000-00010. [DOI] [PubMed] [Google Scholar]
- 82.Skoog I, Lernfelt B, Landahl S, Palmertz B, Andreasson LA, Nilsson L, Persson G, Oden A, Svanborg A. 15-year longitudinal study of blood pressure and dementia. Lancet. 1996;347:1141–1145. doi: 10.1016/s0140-6736(96)90608-x. [DOI] [PubMed] [Google Scholar]
- 83.Smith CD, Carney JM, Tatsumo T, Stadtman ER, Floyd RA, Markesbery WR. Protein oxidation in aging brain. Ann N Y Acad Sci. 1992;663:110–119. doi: 10.1111/j.1749-6632.1992.tb38654.x. [DOI] [PubMed] [Google Scholar]
- 84.Smith MA, Taneda S, Richey PL, Miyata S, Yan SD, Stern D, Sayre LM, Monnier VM, Perry G. Advanced Maillard reaction end products are associated with Alzheimer disease pathology. Proc Natl Acad Sci U S A. 1994;91:5710–5714. doi: 10.1073/pnas.91.12.5710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Smith MA, Sayre LM, Monnier VM, Perry G. Radical AGEing in Alzheimer’s disease. Trends Neurosci. 1995;18:172–176. doi: 10.1016/0166-2236(95)93897-7. [DOI] [PubMed] [Google Scholar]
- 86.Smith MA, Perry G, Richey PL, Sayre LM, Anderson VE, Beal MF, Kowall N. Oxidative damage in Alzheimer’s. Nature. 1996;382:120–121. doi: 10.1038/382120b0. [DOI] [PubMed] [Google Scholar]
- 87.Smith MA, Harris PL, Sayre LM, Perry G. Iron accumulation in Alzheimer disease is a source of redox-generated free radicals. Proc Natl Acad Sci U S A. 1997;94:9866–9868. doi: 10.1073/pnas.94.18.9866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Smith MA, Richey Harris PL, Sayre LM, Beckman JS, Perry G. Widespread peroxynitrite-mediated damage in Alzheimer’s disease. J Neurosci. 1997;17:2653–2657. doi: 10.1523/JNEUROSCI.17-08-02653.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Smith MA, Sayre LM, Anderson VE, Harris PL, Beal MF, Kowall N, Perry G. Cytochemical demonstration of oxidative damage in Alzheimer disease by immunochemical enhancement of the carbonyl reaction with 2,4-dinitrophenylhydrazine. J Histochem Cytochem. 1998;46:731–735. doi: 10.1177/002215549804600605. [DOI] [PubMed] [Google Scholar]
- 90.Smith MA, Wehr K, Harris PL, Siedlak SL, Connor JR, Perry G. Abnormal localization of iron regulatory protein in Alzheimer’s disease. Brain Res. 1998;788:232–236. doi: 10.1016/s0006-8993(98)00002-x. [DOI] [PubMed] [Google Scholar]
- 91.Smith MA, Petot GJ, Perry G. Diet and oxidative stress: a novel synthesis of epidemiological data on Alzheimer’s disease. J Alzheimers Dis. 1999;1:203–206. doi: 10.3233/jad-1999-14-502. [DOI] [PubMed] [Google Scholar]
- 92.Snowdon DA, Greiner LH, Mortimer JA, Riley KP, Greiner PA, Markesbery WR. Brain infarction and the clinical expression of Alzheimer disease. The Nun Study. Jama. 1997;277:813–817. [PubMed] [Google Scholar]
- 93.Sontag E, Nunbhakdi-Craig V, Sontag JM, Diaz-Arrastia R, Ogris E, Dayal S, Lentz SR, Arning E, Bottiglieri T. Protein phosphatase 2A methyltransferase links homocysteine metabolism with tau and amyloid precursor protein regulation. J Neurosci. 2007;27:2751–2759. doi: 10.1523/JNEUROSCI.3316-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Stamler JS, Osborne JA, Jaraki O, Rabbani LE, Mullins M, Singel D, Loscalzo J. Adverse vascular effects of homocysteine are modulated by endothelium-derived relaxing factor and related oxides of nitrogen. J Clin Invest. 1993;91:308–318. doi: 10.1172/JCI116187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Touyz RM. Reactive oxygen species, vascular oxidative stress, and redox signaling in hypertension: what is the clinical significance? Hypertension. 2004;44:248–252. doi: 10.1161/01.HYP.0000138070.47616.9d. [DOI] [PubMed] [Google Scholar]
- 96.Touyz RM, Schiffrin EL. Reactive oxygen species in vascular biology: implications in hypertension. Histochem Cell Biol. 2004;122:339–352. doi: 10.1007/s00418-004-0696-7. [DOI] [PubMed] [Google Scholar]
- 97.Tribble DL. AHA Science Advisory. Antioxidant consumption and risk of coronary heart disease: emphasison vitamin C, vitamin E, and beta-carotene: A statement for healthcare professionals from the American Heart Association. Circulation. 1999;99:591–595. doi: 10.1161/01.cir.99.4.591. [DOI] [PubMed] [Google Scholar]
- 98.van der Flier WM, van Straaten EC, Barkhof F, Verdelho A, Madureira S, Pantoni L, Inzitari D, Erkinjuntti T, Crisby M, Waldemar G, Schmidt R, Fazekas F, Scheltens P. Small vessel disease and general cognitive function in nondisabled elderly: the LADIS study. Stroke. 2005;36:2116–2120. doi: 10.1161/01.STR.0000179092.59909.42. [DOI] [PubMed] [Google Scholar]
- 99.van der Flier WM, Barkhof F, Scheltens P. Shifting paradigms in dementia: toward stratification of diagnosis and treatment using MRI. Ann N Y Acad Sci. 2007;1097:215–224. doi: 10.1196/annals.1379.013. [DOI] [PubMed] [Google Scholar]
- 100.Vasdev S, Gill V, Singal P. Role of advanced glycation end products in hypertension and atherosclerosis: therapeutic implications. Cell Biochem Biophys. 2007;49:48–63. doi: 10.1007/s12013-007-0039-0. [DOI] [PubMed] [Google Scholar]
- 101.Vaziri ND, Wang XQ, Oveisi F, Rad B. Induction of oxidative stress by glutathione depletion causes severe hypertension in normal rats. Hypertension. 2000;36:142–146. doi: 10.1161/01.hyp.36.1.142. [DOI] [PubMed] [Google Scholar]
- 102.Vaziri ND, Rodriguez-Iturbe B. Mechanisms of disease: oxidative stress and inflammation in the pathogenesis of hypertension. Nat Clin Pract Nephrol. 2006;2:582–593. doi: 10.1038/ncpneph0283. [DOI] [PubMed] [Google Scholar]
- 103.Voutilainen S, Morrow JD, Roberts LJ, 2nd, Alfthan G, Alho H, Nyyssonen K, Salonen JT. Enhanced in vivo lipid peroxidation at elevated plasma total homocysteine levels. Arterioscler Thromb Vasc Biol. 1999;19:1263–1266. doi: 10.1161/01.atv.19.5.1263. [DOI] [PubMed] [Google Scholar]
- 104.Wilcock DM, Lewis MR, Van Nostrand WE, Davis J, Previti ML, Gharkholonarehe N, Vitek MP, Colton CA. Progression of amyloid pathology to Alzheimer’s disease pathology in an amyloid precursor protein transgenic mouse model by removal of nitric oxide synthase 2. J Neurosci. 2008;28:1537–1545. doi: 10.1523/JNEUROSCI.5066-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wu Z, Guo H, Chow N, Sallstrom J, Bell RD, Deane R, Brooks AI, Kanagala S, Rubio A, Sagare A, Liu D, Li F, Armstrong D, Gasiewicz T, Zidovetzki R, Song X, Hofman F, Zlokovic BV. Role of the MEOX2 homeobox gene in neurovascular dysfunction in Alzheimer disease. Nat Med. 2005;11:959–965. doi: 10.1038/nm1287. [DOI] [PubMed] [Google Scholar]
- 106.Yan SD, Chen X, Fu J, Chen M, Zhu H, Roher A, Slattery T, Zhao L, Nagashima M, Morser J, Migheli A, Nawroth P, Stern D, Schmidt AM. RAGE and amyloid-beta peptide neurotoxicity in Alzheimer’s disease. Nature. 1996;382:685–691. doi: 10.1038/382685a0. [DOI] [PubMed] [Google Scholar]
- 107.Yasunari K, Maeda K, Nakamura M, Yoshikawa J. Oxidative stress in leukocytes is a possible link between blood pressure, blood glucose, and C-reacting protein. Hypertension. 2002;39:777–780. doi: 10.1161/hy0302.104670. [DOI] [PubMed] [Google Scholar]
- 108.Yates CM, Butterworth J, Tennant MC, Gordon A. Enzyme activities in relation to pH and lactate in postmortem brain in Alzheimer-type and other dementias. J Neurochem. 1990;55:1624–1630. doi: 10.1111/j.1471-4159.1990.tb04948.x. [DOI] [PubMed] [Google Scholar]
- 109.Zandi PP, Anthony JC, Khachaturian AS, Stone SV, Gustafson D, Tschanz JT, Norton MC, Welsh-Bohmer KA, Breitner JC. Reduced risk of Alzheimer disease in users of antioxidant vitamin supplements: the Cache County Study. Arch Neurol. 2004;61:82–88. doi: 10.1001/archneur.61.1.82. [DOI] [PubMed] [Google Scholar]
- 110.Zhu X, Castellani RJ, Takeda A, Nunomura A, Atwood CS, Perry G, Smith MA. Differential activation of neuronal ERK, JNK/SAPK and p38 in Alzheimer disease: the ‘two hit’ hypothesis. Mech Ageing Dev. 2001;123:39–46. doi: 10.1016/s0047-6374(01)00342-6. [DOI] [PubMed] [Google Scholar]
- 111.Zhu X, Raina AK, Lee HG, Casadesus G, Smith MA, Perry G. Oxidative stress signalling in Alzheimer’s disease. Brain Res. 2004;1000:32–39. doi: 10.1016/j.brainres.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 112.Zhu X, Raina AK, Perry G, Smith MA. Alzheimer’s disease: the two-hit hypothesis. Lancet Neurol. 2004;3:219–226. doi: 10.1016/S1474-4422(04)00707-0. [DOI] [PubMed] [Google Scholar]
- 113.Zhu X, Lee HG, Perry G, Smith MA. Alzheimer disease, the two-hit hypothesis: an update. Biochim Biophys Acta. 2007;1772:494–502. doi: 10.1016/j.bbadis.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 114.Zhu X, Smith MA, Honda K, Aliev G, Moreira PI, Nunomura A, Casadesus G, Harris PL, Siedlak SL, Perry G. Vascular oxidative stress in Alzheimer disease. J Neurol Sci. 2007;257:240–246. doi: 10.1016/j.jns.2007.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zlokovic BV. Neurovascular mechanisms of Alzheimer’s neurodegeneration. Trends Neurosci. 2005;28:202–208. doi: 10.1016/j.tins.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 116.Zlokovic BV. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57:178–201. doi: 10.1016/j.neuron.2008.01.003. [DOI] [PubMed] [Google Scholar]