Abstract
Deposition of amyloid around blood vessels, known as cerebral amyloid angiopathy (CAA), is a major pathological feature found in the majority of Alzheimer’s disease (AD) cases, and activated complement fragments have been detected on CAA deposits in AD brains. In this study, we demonstrate for the first time that human cerebrovascular smooth muscle cells (HCSMC) isolated from cortical vessels derived from postmortem brains can express mRNAs for complement genes C1qB, C1r, C1s, C2, C3, C4, C5, C6, C7, C8 and C9, the components of the classical complement pathway. Secretion of the corresponding complement proteins for these genes was also demonstrated, except for C1q and C5. Of particular significance was the observation that treatment of HCSMC with aggregated amyloid beta (Aβ)1–42 increased expression of complement C3 mRNA and increased release of C3 protein. Aβ treatment of HCSMC also increased expression of C6 mRNA. Interferon-γ induced expression and release of complement C1r, C1s, C2 and C4. As HCSMC are closely associated with Aβ deposits in vessels in the brain, their production of complement proteins could amplify the proinflammatory effects of amyloid in the perivascular environment, further compromising brain vascular integrity.
Keywords: Vasculature, Inflammation, Amyloid, Toxicity, Neurodegeneration, Cell culture, Gene expression
Introduction
There is now strong evidence that damage of the cerebrovasculature is involved in the etiology of Alzheimer’s disease (AD) (Luchsinger et al., 2005; Shi et al., 2000; Yip et al., 2005; Zlokovic et al., 2005). Cerebrovascular pathology is a common feature in AD brains and encompasses a variety of lesions including changes in endothelial and vascular smooth muscle cells, macroscopic and micro-infarction, hemorrhage, and white matter changes related to small vessel disease. Cerebral amyloid angiopathy (CAA) is found in approximately 90% of AD cases and consists of deposits of amyloid beta (Aβ) peptide in and around the perivascular spaces of vessels (Premkumar et al., 1996). This eventually leads to the death of smooth muscle cells and endothelial cells in the vicinity of amyloid deposits (Vonsattel et al., 1991). The presence of Aβ deposits in the vasculature can also lead to proinflammatory responses including microglia/macrophage activation (Giulian et al., 1995) and complement activation (McGeer et al., 1989). Although Aβ peptide can be directly toxic to HCSMC (Davis et al., 1999; Wilhelmus et al., 2005), the involvement of other mechanisms in mediating cerebrovascular damage, including inflammatory processes, has been indicated (Miao et al., 2005).
The complement system is one of the components of the innate immune system, and consists of a series of functionally-linked proteins, which, when activated, interact to amplify the immune response to targeted molecules (van Beek et al., 2003). The components of the complement system can mediate cytolysis if the complete pathway is activated. During the activation process, complement fragments are produced that can bind to invading microorganisms or cellular debris and target them for phagocytosis, or promote chemotaxis of inflammatory cells to sites of tissue damage through interaction with specific complement receptors (Aderem and Underhill, 1999; Gasque et al., 2002). Activation of the complement system in AD and in brains affected by other neurodegenerative diseases has been extensively characterized (Gasque et al., 2002; McGeer and McGeer, 2002; van Beek et al., 2003). Aβ plaques and other hallmark features of AD, including vascular amyloid deposits, are tagged with different complement fragments, indicating that the complement system is activated in the AD brain (McGeer et al., 1989; Rogers et al., 1992; Shen et al., 2001; Veerhuis et al., 1996). The presence of activated complement protein fragments C1q, C3c, C4d and C5b-9 on cerebrovascular-associated amyloid in AD brains has been observed (Verbeek et al., 1998), though the source of these proteins was not determined.
Aβ peptide can activate the classical complement pathway by binding C1q in the absence of specific antibodies (Rogers et al., 1992; Webster et al., 1997), as well as activating the alternative complement pathway in the absence of C1q (Bergamaschini et al., 1999; Bradt et al., 1998). Studies have shown localized expression of different complement mRNAs within the brain (Johnson et al., 1992; Walker and McGeer, 1992), with significant increases of complement mRNA expression in brain regions selectively affected by AD (Yasojima et al., 1999). It is now known that neurons, microglia, astrocytes, oligodendrocytes and microvascular endothelial cells have the capacity to express many of the genes and gene products of complement pathway proteins (Gasque et al., 1995; Hosokawa et al., 2003; Klegeris et al., 2000; Shen et al., 1997; Terai et al., 1997; Thomas et al., 2000; Walker et al., 1995, 1998).
In this study, we demonstrate for the first time that HCSMC derived from cerebral vessels from human postmortem brains have the potential to express the complete range of mRNAs of the classical complement pathway, and to secrete most of the corresponding proteins. This may be of significance as HCSMC are closely associated with deposited Aβ in the basement membrane around vessels; the production and subsequent activation of complement in this perivascular environment could contribute to vascular dysfunction.
Materials and methods
Human brain vessel isolation
HCSMC were cultured from human postmortem brains using the following protocol to firstly isolate viable human brain vessels. In this study, HCSMC isolated from 9 separate human postmortem cases were used in the described experiments. Except where noted, all isolates produced similar results. Human brain tissues were received by the Sun Health Research Institute Brain Donation program within 3h of death of the donor. Collection of human brain tissues in this program had received approval from the Institutional Review Board of the Sun Health Corporation. Brains used in this study were from 4 AD, 2 Parkinson’s disease with dementia and 3 non-demented (ND) donors (mean age 85yr ± 5.3 (std. dev.); postmortem delay 2.3h ± 0.4 (std. dev.)). For vessel isolation, gray matter (approximately 25g) was dissected from coronal slices of frontal cortex. Tissue was homogenized in Hanks balanced salt solution (HBSS) using a wide-bore Dounce homogenizer. Tissue homogenate was then centrifuged (250g, 10min), and resuspended in Dextran 70 (Sigma-Aldrich, St. Louis, MO) to a final concentration of 15%. Homogenates were centrifuged (5800g/15min) and the resulting vessel-enriched pellets were recovered. This fraction was digested for 60–90min in 0.1% collagenase type II (Worthington Biochemicals, Lakewood, NJ) at 37°C and then filtered sequentially through 100, 70 and 40μm mesh filters (BD, Bedford, MA). HCSMC were cultured from vessels retained on the 100 and 70μm mesh filters. These vessels were plated onto collagen type-I coated culture dishes (BD Biosciences, Bedford, MA). Earlier isolates were cultured in Dulbecco’s modified Eagles medium (DMEM) supplemented with 20% fetal bovine serum. Later isolates were grown in Smooth Muscle Cell Medium (Sciencell Research Laboratories, San Diego, CA), a proprietary media containing growth factors that supported more rapid cell growth.
Purification of cerebrovascular smooth muscle cells
After 14–21days of growth, the microvessel cultures from the 100μm and 70μm filter-retained fractions contained mainly HCSMC with some endothelial cells. To enrich for HCSMC, cells growing from the primary vessel isolates were treated with 0.25% trypsin in HBSS containing 1mM EDTA to release single cells. These were then mixed with magnetic beads (Dynal Biotech—Invitrogen, Carlsbad, CA) conjugated with biotinylated Ulex europaeus agglutinin-1 (Vector Laboratories, Burlingame, CA), which binds selectively to endothelial cells. After 30min incubation on ice, beads with bound cells were removed by absorption to a magnet, and the unbound fraction was cultured further as enriched HCSMC.
Characterization of cerebrovascular smooth muscle cells
Prior to use in experiments, cultures were stained by immunocytochemistry with antibodies to smooth muscle α-actin (SMA), CD31, glial fibrillary acidic protein (GFAP) and LN3 (Table 2) to confirm the purity of the cultures. As positive controls for immunocytochemistry, brain endothelial cells were stained with CD31, astrocytes were stained with GFAP, and microglia were stained with LN3. For reference purposes, a culture of HCSMC was obtained from a commercial source (Sciencell Research Laboratories). The method of isolation for these cells and age of donor is not available, but more than 98% of these cells were stained for SMA.
Table 2.
Details of antibodies used
| Antibodies | Supplier | Dilution | Application |
|---|---|---|---|
| C1Q | Dako (R) | 1:5000 | W |
| C1R | MP (G) | 1:5000 | W |
| C1S | MP (G) | 1:5000 | W |
| C2 | ART (G) | 1:5000 | W |
| C3 | ART (G) | 1:10,000 | W |
| C4 | ART (G) | 1:10,000 | W |
| C5 | ART (G) | 1:5000 | W |
| C6 | ART (G) | 1:5000 | W |
| C7 | ART (G) | 1:5000 | W |
| C8 | ART (G) | 1:10,000 | W |
| C9 | ART (G) | 1:5000 | W |
| SMA | Sigma (M) | 1:5000 | ICC |
| CD31 | Dako (M) | 1:1000 | ICC |
| LN3 | MP (M) | 1:500 | ICC |
| GFAP | Dako (R) | 1:2000 | ICC |
Abbreviations: Dako; Dako North America, Carpinteria, CA: MP; MP Biomedicals, Solon, OH: ART; Advanced Research Technologies, San Diego, CA; Sigma; Sigma Chemical Company, St. Louis,MO: (R), rabbit polyclonal: (G), goat polyclonal: (M), mouse monoclonal: W, western blots: ICC; immunocytochemistry: SMA, smooth muscle α-actin
Cell stimulation treatments
HCSMC at passage 3 or 4 were plated at 105 cells/well in 6-well plates in growth media and allowed to attach. The following day, cultures were rinsed and then refed with 1ml serum-free DMEM. To these cultures, either Aβ (1–42, 5μM), interferon-γ (IFN-γ, Peprotech, Princeton, NJ) (100ng/ml) or interleukin (IL)-1β (IL-1β) (Peprotech; 20ng/ml) was added as stimulating agents. Aβ peptide was obtained from rPeptide (Bogart, GA) and preaggregated according to standard procedures. In brief, peptide was dissolved in 5mM sodium hydroxide, to which was added 1/10 volume of 10× Phosphate Buffered Saline (PBS) for a final Aβ concentration of 500μM. This peptide solution was incubated at 37°C for 18h to promote aggregation. Characterization of peptide by atomic force microscopy and non-denaturing gel electrophoresis showed that the majority of the peptide was in an oligomeric form, with a small percentage as fibrils. Treated cells were harvested for RNA extraction and media for protein analysis after 24h treatment.
RNA isolation and reverse transcription-polymerase chain reaction (RT-PCR)
RNA was prepared using Trizol reagent from 8 different isolates of HCSMC cultured from postmortem brains and from one commercially-obtained HCSMC culture, according to the manufacturer’s instructions (Invitrogen, Carlsbad, CA). RNA samples (1μg) from these cultures were reverse transcribed according to our standard procedure (Walker et al., 2002). Our published RT-PCR methods were employed to measure gene expression (Walker et al., 1995, 2002). Initial studies were carried out to qualitatively determine the presence of complement-specific mRNA sequences. As expression of these genes was detectable in 5 different HCSMC isolates, subsequent studies employed a semi-quantitative method for assessing relative levels of gene expression in response to relevant stimuli. Due to the varying abundance in expression of these genes, primers were pre-tested through an increasing number of cycles to ensure PCR product formation was being measured while the amplifications were in the exponential range. The expression level of each of the complement genes was normalized for relative levels of β-actin in each sample. The complement and β-actin primers used and cycle numbers employed for each gene are listed in Table 1. The identities of the HCSMC cDNA-derived amplification fragments were confirmed by restriction digest mapping with 2–3 different restriction endonucleases. The sizes of fragments identified corresponded to those expected using published sequences for each of these genes. All primers were designed to span an intron sequence to ensure that detected PCR fragments were derived from reverse transcribed RNA, and not due to genomic DNA contamination. Real time PCR analysis for C3 expression was carried out to confirm the detected gene expression changes. Samples were amplified using the listed primers for C3 using SYBR green-containing master mix (Superarray Bioscience, Frederick, MD) with a Stratagene Mx3000p qPCR machine.
Table 1.
Polymerase chain reaction primer sequences
| Gene | Fragment size (bp) |
Cycle no. |
|---|---|---|
| C1QB-F CCCAGGGATAAAAGGAGAGAAA C1QB-R GGCGTGGTAGGTGAAGTAGTAGAG |
358 | 38 |
| C1R-F CCTCCCTGACAACGATACCTTCTA C1R-R CGTCCTGCTTTAGAGATGGGTGT |
215 | 26 |
| C1S-F AGAGAAGATTTTGATGTGGAAGCA C1S-R ACAGGTTGACATTTCAGTTTGGA |
444 | 26 |
| C2-F CTCTACCTGCTCCTGGACTGTT C2-R GTCGCATTTGGTTGTTCATCAT |
304 | 30 |
| C3-F CCTGGCTCCACAGTTCTCTATCG C3-R GTTCCCTCCACTTTCTTCCCGTA |
383 | 27 |
| C4-F TGGTTCCTATGCGGCTTGGTTGT C4-R GAGGCTTCCACTCTCTGCTTCAAT |
324 | 26 |
| C5-F TGGCATTAGCAGCAGTGGACAGT C5-R CAGGCTCCATCGTAACAACATTTC |
313 | 34 |
| C6-F GGCGTGTATGACCTTCTCTATC C6-R CACAGGGGATGTTTCTTACC A |
382 | 34 |
| C7-F ACTGTTGAGGGGACCCATT C7-R ACCGTAAATCTTCTCCACATCTG |
457 | 28 |
| C8-F ACTGCGACCCTCTTGACTCTG C8-R AGGACCCCTGTGTCTCCATAG |
311 | 37 |
| C9-F AATGAGCCCCTGGAGTGAATGGT C9-R ATTTCCGCAGTCATCCTCAGCAT |
180 | 38 |
| β-actin-F CCACGAAACTACCTTCAACTCC β-actin-R ACTCGTCATACTCCTGCTTGCT |
262 | 22 |
Western blot analysis of secreted complement proteins
To detect the secretion of complement proteins by HCSMC, samples of conditioned media from HCSMC cultures were incubated with Strataclean resin (20μl resin for 1ml of media) (Stratagene, LaJolla, CA) to bind secreted proteins. After 10min incubation with mixing, the resin was collected by centrifugation and washed; bound proteins were eluted by resuspension of resin in NuPAGE gel-sample buffer (Invitrogen) with heating at 70°C for 10min. Eluted proteins were separated under reducing conditions through 4–12% NuPAGE gels (Invitrogen) using MOPS electrophoresis buffer according to the manufacturer’s instructions. Separated proteins were transferred to nitrocellulose membranes and reacted with antibodies to complement proteins (Table 2) followed by reaction with the appropriate horseradish peroxidase-conjugated anti-immunoglobulin antibody (1:20,000: Pierce Chemical Company, Rockland, IL). Bound antibodies were detected by reaction of membranes with chemiluminescent peroxidase substrate (Perkin Elmer, Boston, MA), followed by exposure to film, or by direct imaging using a Fluorochem 8900 imaging system (Alpha Innotech, San Leandro, CA) (Walker et al., 2002). To confirm that there was quantitative recovery of protein using this technique, we performed experiments using media samples to which different concentrations of purified complement C1-inhibitor (Calbiochem, San Diego, CA) over the range 10pg/ml to 100ng/ml had been added. These proteins were detected and quantified using these same methods. As a loading control, replicate tubes (n = 8) containing 100ng/ml of purified GFAP (Calbiochem) were processed by Strataclean concentration and western blot detection. Data showed reproducible recovery of sample in these replicate samples (shown in Fig. 4).
Fig. 4.

Composite figure of western blots demonstrating secretion of different complement proteins into conditioned media by unstimulated (lane 1), and Aβ (lane 2), IFN-γ (lane 3) and IL-1β (lane 4) stimulated HCSMC. Diluted human serum (lane 5) was used as a positive control. Conditioned media samples from HCSMC cultures were concentrated with Strataclean resin; the bound proteins were eluted from the resin, separated through polyacrylamide gels and transferred to nitrocellulose membranes. Replicate membranes were probed with the indicated antibodies. Polypeptide(s) with the expected molecular weights were detected in each sample except for C1q and C5. The high molecular weight bands in C4 antibody probed HCSMC samples represent the unprocessed form of complement C4. As a surrogate loading control, in order to demonstrate the reliability and reproducibility of the Strataclean concentration method, replicate aliquots of samples containing the same amount of GFAP protein were processed and detected in parallel (GFAP loading).
Statistical analysis
Results for quantitative PCR are expressed as mean ± standard error of mean of duplicate samples from 3 separate HCSMC isolates. Statistical analysis was carried out by one-way ANOVA or by student’s t test, using GraphPad Prism 4 software (San Diego, CA). Significance in treatments is assumed if p < 0.05 is obtained.
Results
Characterization of human postmortem brain-derived HCSMC
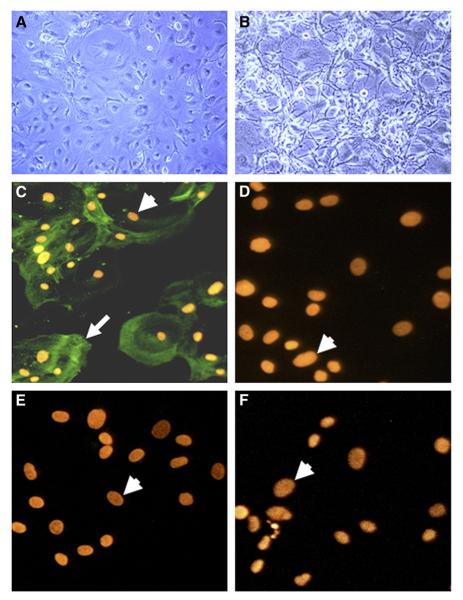
HCSMC isolated from postmortem cerebral blood vessels had a predominantly flat but heterogeneous morphology consistent with this cell type (Fig. 1A). When stimulated with IL-β, HCSMC appeared to contract, developing a fibrous reactive morphology (Fig. 1B). All HCSMC used in the experiments in this report were characterized by immunocytochemistry to establish the identity of the cultures. Cells grown on coverslips were reacted with antibodies to smooth muscle α-actin (SMA) (Fig. 1C). Positive immunoreactivity for SMA (indicated by arrow) can be observed surrounding the more brightly propidium iodide-stained nuclei (arrowheads). SMA positive cells represented at least 95% of cultures. These cells did not react with an antibody to CD31 (Fig. 1D), which identifies endothelial and microglial cells; an antibody to GFAP, which identifies astrocytes (Fig. 1E); or an antibody to HLA-DR (LN3), which identifies microglia (Fig. 1F).
Fig. 1.
Representative photomicrographs showing human postmortem brain-derived HCSMC. Phase contrast photomicrographs showing morphology of unstimulated HCSMC (A) and IL-1β stimulated HCSMC (B) (magnification 150×). Immunofluorescence micrographs of HCSMC stained with antibodies to smooth muscle α-actin (C—magnification 650×), and CD31 (D), GFAP (E) and LN3 (F) (magnification of D, E and F—800×), and detected by reaction with Alexa 488-labeled goat-anti-mouse or -anti-rabbit secondary antibodies. Positive immunoreactivity (arrow) was only observed in cells stained with SMA antibody (C); panels D, E and F showed no reactivity. Immunoreacted cells were counterstained with propidium iodide to identify nuclei (arrowheads).
Expression of complement pathway mRNAs by human postmortem brain-derived HCSMC
To demonstrate whether HCSMC could express all of the genes involved in the classical complement pathway, RT-PCR analyses for these individual genes were carried out using cDNAs derived from unstimulated HCSMC and HCSMC stimulated with Aβ, IFN-γ, or IL-1β. Gels demonstrating representative results of PCR-amplified DNA bands using primers specific for each gene are shown in Fig. 2 as a composite panel. Qualitative or quantitative analyses for complement gene expression were carried out with cDNAs from a total of 8 separate HCSMC isolates, and also the commercial isolate of HCSMC. With the exception of C1qB mRNA expression (see below), each set of cultures produced similar results for each of the genes analyzed. As positive controls, cDNAs from brain or human microglia RNA were used (data not shown). These data demonstrated that brain-derived HCSMC could express mRNAs for all of the components of the classical complement pathway. Quantitative measurements of complement gene expression were determined by RT-PCR using 3 of the HCSMC isolates (Fig. 3). The abundance of gene expression varied with C4 mRNA being the most highly expressed and C5, C6, C8 and C9 mRNAs being expressed at relatively low levels. C1qB mRNA expression was not consistent, being undetectable in 4 of the isolates, and at relatively low levels in the positively expressing cultures, though a significant stimulation of expression with IFN-γ could be observed (Figs. 2 and 3); amplification for 38 cycles was required to demonstrate C1qB expression in the gel shown in Fig. 2. C7 mRNA was considerably more highly expressed by HCSMC than the other components of the terminal complement complex (C5, C6, C8 and C9), requiring only 28 PCR cycles to reach optimum amplification intensity compared to 34 to 38 cycles for the other mRNAs (Table 1). Differential patterns of expression and regulation of the complement genes were apparent in HCSMC. Constitutive C3 mRNA expression was low, but was strongly induced by treatment of cells with aggregated Aβ (1–42) or IL-1β (Figs. 2 and 3). Quantitative measurement of changes in C3 gene expression by HCSMC was confirmed using real time polymerase chain reaction methodology. Both agents have been identified to have a significant proinflammatory effect in AD affected brains. Increased C6 gene expression was also detected in response to Aβ treatment (Figs. 2 and 3). C1r, C2 and C4 mRNA expression was noticeably increased when cells were treated with IFN-γ, but not with Aβ (Figs. 2 and 3). However, IFN-γ reduced expression of C5, C6, C7, C8 and C9 mRNA, an effect that reached statistical significance for C5, C8 and C9 (Fig. 3). IL-1β significantly reduced expression of C5, C8 and C9 (Fig. 3). Treatment of HCSMC with these agents did not induce apoptosis or necrosis in significant numbers of cells (data not shown).
Fig. 2.

Expression of complement mRNAs by human brain HCSMC. Panel shows a composite of ethidium bromide-stained gels with results of PCR analyses of human HCSMC cDNAs amplified with the indicated gene specific complement primers. Panels demonstrate expression of complement genes C1qB through C9 and β-actin in cDNAs from HCSMC (lanes 1–4). Lanes: 1; unstimulated HCSMC: 2; HCSMC stimulated with IFN-γ (100 ng/ml): 3; HCSMC stimulated with 5 μM Aβ: 4; HCSMC stimulated with IL-β (20 ng/ml); M: DNA molecular size ladder with position of closest DNA marker (base pairs). Panel for each complement gene shows representative gels for one of the HCSMC isolates, but with the exception of C1qB, the patterns of expression were consistent between all the HCSMC isolates analyzed (see text).
Fig. 3.
Quantitative RT-PCR analyses showing effects of IFN-γ, Aβ and IL-1β on expression by stimulated HCSMC of early complement genes C1qB to C9. Results represent the mean values of gene expression measurements for duplicate determinations for 3 different HCSMC isolates (2 AD cases; 1 non-AD case). Relative abundances (expressed as relative units) were measured from ethidium bromide-stained gels of PCR-amplified complement fragments for each sample with values normalized for the relative intensity of β-actin in the corresponding sample.
HCSMC secrete complement proteins
The significance of complement gene expression by a particular cell type is only realized if these cells can secrete the proteins that might form the bioactive complement components. To assess the secretion of complement proteins by HCSMC, conditioned media from stimulated and unstimulated HCSMC cultures were concentrated by Strataclean resin. This silica resin can bind proteins from aqueous solutions, thus concentrating them. We have shown essentially quantitative recovery of spiked test protein samples over the range 10pg/ml to 100ng/ml following absorption to and elution from Strataclean resin (data not shown). Proteins eluted from the resin were separated by gel electrophoresis and analyzed by western blotting techniques using a range of monospecific complement antibodies (Fig. 4). As a form of loading control, to show reproducible recovery between identical samples, recovery of samples containing 100ng/ml of purified GFAP was carried out and measured (Fig. 4 — GFAP loading). These results (Fig. 4) are representative of those obtained using 4 different isolates of HCSMC. Diluted human serum was analyzed as a positive control for each complement protein. Consistent with the PCR results, we demonstrated that HCSMC secreted complement proteins C1r, C1s, C2, C3, C4, C6, C7, C8 and C9; however, C1q and C5 secretion by HCSMC could not be detected even though the mRNAwas detectable in the corresponding cell cultures, and the antibodies used readily detected C1q and C5 polypeptides in serum (Fig. 4, lane 5). The induction of secretion of C1r, C1s, C2 and C4 following treatment with IFN-γ (Fig. 4, lane 3) was consistent with the RT-PCR results. Particularly noticeable was the strong induction of C3 secretion following Aβ treatment (Fig. 4, lane 2; C3).
Discussion
The results from this study demonstrating expression of components of the classical complement pathway establish brain-derived HCSMC as possible participants in inflammatory responses that occur in neurodegenerative diseases such as AD. We present a potential mechanism whereby complement production, which could lead to its activation by deposited Aβ,could amplify the damaging consequences of Aβ deposition in vessels. Inflammatory stimulation appeared to increase production of the early complement pathway components, while expression of the terminal complement pathway proteins was unchanged or downregulated, particularly by IFN-γ and IL-1β.Aβ stimulation significantly increased expression of C3 and C6, while having no effect on C5, C7, C8 or C9. Although assembly of the terminal complement components into the membrane attack complex is necessary for the cytolytic action of complement activation, increased expression of the early complement component, particularly C3 and C4, results in the production of a number of subfragments (e.g. C3bi, C3b, C4b) that are chemoattractant to macrophage/microglia cells, thus potentially amplifying perivascular inflammation (Barnum, 2002).
HCSMC have multiple roles in supporting and maintaining the integrity of the vessels. In addition to their contractile role, HCSMC have been characterized as supportive cells for larger vessels, capable of secreting growth factors that aid in the maintenance of endothelial cells (Belgore et al., 2003; Motoyama et al., 2006). However, vascular smooth muscle cells (SMC) from different tissues can participate in inflammatory processes particularly those associated with atherosclerosis (Jaulmes et al., 2006; Peng et al., 2007; Rathcke and Vestergaard, 2006). Activation of aortic SMC with tumor necrosis factor-α (TNF-α) resulted in upregulated expression of mRNAs for granulocyte macrophage-colony stimulating factor, monocyte chemotactic protein-1 (MCP-1), IL-6 and CXCL5 (ENA-78) (Bandman et al., 2002). Low doses of lipopolysaccharide stimulated expression of MCP-1, IL-6 and IL-1α by aortic SMC (Rice et al., 2003). The neutrophil protein CAP-37 induced expression of intercellular adhesion molecule-1 by SMC (Gonzalez et al., 2004). IL-18 and angiotensin II, agents involved in atherosclerosis, activated MAP kinase and NF-κB signaling in vascular SMC, resulting in enhanced expression of IL-6, IL-8 and MCP-1 gene expression and increased functional IL-18 receptor activity (Gerdes et al., 2002; Sahar et al., 2005). Aβ induced secretion of IL-6 and induced matrix metalloproteinase-2 (MMP-2) activity in HCSMC; this proinflammatory effect could be inhibited by the anti-inflammatory agent dexamethasone (Previti et al., 2006). In light of these data on the expression of inflammatory mediators by SMC, the expression of complement pathway genes by HCSMC is consistent with the possible involvement of these cells in inflammatory responses.
Although Aβ toxicity to HCSMC could be one mechanism of causing cerebrovascular disturbance (Paris et al., 2002), our results indicate that production of complement proteins by HCSMC also has the potential, in an Aβ-enriched or inflammatory environment, to contribute to cerebrovascular damage by amplifying proinflammatory mechanisms. The reason for the failure to consistently detect C1qB mRNA expression or to detect secretion C1q appears to be due to its low level of expression in this cell type. It is not clear why secretion of C5 could not be detected as its mRNA was expressed at similar levels to C6, which could be readily detected in media sample, but this result was replicated with 5 separate HCSMC isolates. The antibodies used could specifically detect C1q and C5 polypeptides in serum samples, similar to the others used (Fig. 4). Although secretion of C1q and C5 could not be demonstrated, since other cells in the microvascular environment, including macrophages/microglia, can secrete these proteins (Walker, 1998, unpublished data), there is the potential for all complement proteins to be produced in the localized microvascular environment. The strongly increased secretion of C3 by HCSMC following Aβ stimulation is of significance as this is a pivotal protein in both the classical and alternative activation pathways of the complement cascade (Bradt et al., 1998). Markers for complement activation have now been found in many neurodegenerative diseases or where brain injury has occurred (Barnum, 2002; van Beek et al., 2003). In addition, significantly increased expression of complement mRNAs in AD brains in regions of AD pathology indicate a role for local complement production in contributing to neurodegenerative processes (Yasojima et al., 1999). We have now demonstrated that cerebral HCSMC can express complement genes in vitro; these data indicate the potential for these cells to locally produce these proteins in regions affected by CAA pathology. Complement protein production by HCSMC in the perivascular environment associated with the abluminal surface of endothelial cells, where deposition of Aβ tends to occur in CAA, could contribute to cerebrovascular damage.
Acknowledgments
Supported in part by NIH grant R01 AG18345 (DGW) and a grant from Arizona Alzheimer’s Research Consortium (DGW, LF-L). The authors thank Ms. Emily Davenport for her technical assistance, and Dr. T.G. Beach and Ms. Lucia Sue for the operation of the Sun Health Research Institute Brain and Tissue Donation Program that provided brain tissue for culture.
References
- Aderem A, Underhill DM. Mechanisms of phagocytosis in macrophages. Annu. Rev. Immunol. 1999;17:593–623. doi: 10.1146/annurev.immunol.17.1.593. [DOI] [PubMed] [Google Scholar]
- Bandman O, Coleman RT, Loring JF, Seilhamer JJ, Cocks BG. Complexity of inflammatory responses in endothelial cells and vascular smooth muscle cells determined by microarray analysis. Ann. N. Y. Acad. Sci. 2002;975:77–90. doi: 10.1111/j.1749-6632.2002.tb05943.x. [DOI] [PubMed] [Google Scholar]
- Barnum SR. Complement in central nervous system inflammation. Immunol. Res. 2002;26:7–13. doi: 10.1385/IR:26:1-3:007. [DOI] [PubMed] [Google Scholar]
- Belgore F, Lip GY, Blann AD. Basic fibrobrast growth factor induces the secretion of vascular endothelial growth factor by human aortic smooth muscle cells but not by endothelial cells. Eur. J. Clin. Investig. 2003;33:833–839. doi: 10.1046/j.1365-2362.2003.01223.x. [DOI] [PubMed] [Google Scholar]
- Bergamaschini L, Canziani S, Bottasso B, Cugno M, Braidotti P, Agostoni A. Alzheimer’s beta-amyloid peptides can activate the early components of complement classical pathway in a C1q-independent manner. Clin. Exp. Immunol. 1999;115:526–533. doi: 10.1046/j.1365-2249.1999.00835.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradt BM, Kolb WP, Cooper NR. Complement-dependent proinflammatory properties of the Alzheimer’s disease beta-peptide. Cell Adhes. Commun. 1998;188:431–438. doi: 10.1084/jem.188.3.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis J, Cribbs DH, Cotman CW, Van Nostrand WE. Pathogenic amyloid beta-protein induces apoptosis in cultured human cerebrovascular smooth muscle cells. Amyloid. 1999;6:157–164. doi: 10.3109/13506129909007321. [DOI] [PubMed] [Google Scholar]
- Gasque P, Fontaine M, Morgan BP. Complement expression in human brain. Biosynthesis of terminal pathway components and regulators in human glial cells and cell lines. J. Immunol. 1995;154:4726–4733. [PubMed] [Google Scholar]
- Gasque P, Neal JW, Singhrao SK, McGreal EP, Dean YD, Van BJ, Morgan BP. Roles of the complement system in human neurodegenerative disorders: pro-inflammatory and tissue remodeling activities. Mol. Neurobiol. 2002;25:1–17. doi: 10.1385/mn:25:1:001. [DOI] [PubMed] [Google Scholar]
- Gerdes N, Sukhova GK, Libby P, Reynolds RS, Young JL, Schonbeck U. Expression of interleukin (IL)-18 and functional IL-18 receptor on human vascular endothelial cells, smooth muscle cells, and macrophages: implications for atherogenesis. Cell Adhes. Commun. 2002;195:245–257. doi: 10.1084/jem.20011022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giulian D, Haverkamp LJ, Li J, Karshin WL, Yu J, Tom D, Li X, Kirkpatrick JB. Senile plaques stimulate microglia to release a neurotoxin found in Alzheimer brain. Neurochem. Int. 1995;27:119–137. doi: 10.1016/0197-0186(95)00067-i. [DOI] [PubMed] [Google Scholar]
- Gonzalez ML, Ruan X, Kumar P, Grammas P, Pereira HA. Functional modulation of smooth muscle cells by the inflammatory mediator CAP37. Microvasc. Res. 2004;67:168–181. doi: 10.1016/j.mvr.2003.12.007. [DOI] [PubMed] [Google Scholar]
- Hosokawa M, Klegeris A, Maguire J, McGeer PL. Expression of complement messenger RNAs and proteins by human oligodendroglial cells. Glia. 2003;42:417–423. doi: 10.1002/glia.10234. [DOI] [PubMed] [Google Scholar]
- Jaulmes A, Thierry S, Janvier B, Raymondjean M, Marechal V. Activation of sPLA2-IIA and PGE2 production by high mobility group protein B1 in vascular smooth muscle cells sensitized by IL-1beta. FASEB J. 2006;20:1727–1729. doi: 10.1096/fj.05-5514fje. [DOI] [PubMed] [Google Scholar]
- Johnson SA, Lampert-Etchells M, Pasinetti GM, Rozovsky I, Finch CE. Complement mRNA in the mammalian brain: responses to Alzheimer’s disease and experimental brain lesioning. Neurobiol. Aging. 1992;13:641–648. doi: 10.1016/0197-4580(92)90086-d. [DOI] [PubMed] [Google Scholar]
- Klegeris A, Bissonnette CJ, Dorovini-Zis K, McGeer PL. Expression of complement messenger RNAs by human endothelial cells. Brain Res. 2000;871:1–6. doi: 10.1016/s0006-8993(00)02253-8. [DOI] [PubMed] [Google Scholar]
- Luchsinger JA, Reitz C, Honig LS, Tang MX, Shea S, Mayeux R. Aggregation of vascular risk factors and risk of incident Alzheimer disease. Neurology. 2005;65:545–551. doi: 10.1212/01.wnl.0000172914.08967.dc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeer PL, Akiyama H, Itagaki S, McGeer EG. Activation of the classical complement pathway in brain tissue of Alzheimer patients. Neurosci. Lett. 1989;107:341–346. doi: 10.1016/0304-3940(89)90843-4. [DOI] [PubMed] [Google Scholar]
- McGeer PL, McGeer EG. The possible role of complement activation in Alzheimer disease. Trends Mol. Med. 2002;8:519–523. doi: 10.1016/s1471-4914(02)02422-x. [DOI] [PubMed] [Google Scholar]
- Miao J, Xu F, Davis J, Otte-Holler I, Verbeek MM, Van Nostrand WE. Cerebral microvascular amyloid beta protein deposition induces vascular degeneration and neuroinflammation in transgenic mice expressing human vasculotropic mutant amyloid beta precursor protein. Am. J. Pathol. 2005;167:505–515. doi: 10.1016/s0002-9440(10)62993-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motoyama K, Fukumoto S, Koyama H, Emoto M, Shimano H, Maemura K, Nishizawa Y. SREBP inhibits VEGF expression in human smooth muscle cells. Biochem. Biophys. Res. Commun. 2006;342:354–360. doi: 10.1016/j.bbrc.2006.01.139. [DOI] [PubMed] [Google Scholar]
- Paris D, Townsend KP, Obregon DF, Humphrey J, Mullan M. Pro-inflammatory effect of freshly solubilized beta-amyloid peptides in the brain. Prostaglandins Other Lipid Mediat. 2002;70:1–12. doi: 10.1016/s0090-6980(02)00111-9. [DOI] [PubMed] [Google Scholar]
- Peng N, Liu JT, Gao DF, Lin R, Li R. Angiotensin II-induced C-reactive protein generation: inflammatory role of vascular smooth muscle cells in atherosclerosis. Atherosclerosis. 2007;193:292–298. doi: 10.1016/j.atherosclerosis.2006.09.007. [DOI] [PubMed] [Google Scholar]
- Premkumar DR, Cohen DL, Hedera P, Friedland RP, Kalaria RN. Apolipoprotein E-epsilon4 alleles in cerebral amyloid angiopathy and cerebrovascular pathology associated with Alzheimer’s disease. Am. J. Pathol. 1996;148:2083–2095. [PMC free article] [PubMed] [Google Scholar]
- Previti ML, Zhang W, Van Nostrand WE. Dexamethasone diminishes the pro-inflammatory and cytotoxic effects of amyloid beta-protein in cerebrovascular smooth muscle cells. J. Neuroinflammation. 2006;3:18. doi: 10.1186/1742-2094-3-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathcke CN, Vestergaard H. YKL-40, a new inflammatory marker with relation to insulin resistance and with a role in endothelial dysfunction and atherosclerosis. Inflamm. Res. 2006;55:221–227. doi: 10.1007/s00011-006-0076-y. [DOI] [PubMed] [Google Scholar]
- Rice JB, Stoll LL, Li WG, Denning GM, Weydert J, Charipar E, Richenbacher WE, Miller FJ, Jr., Weintraub NL. Low-level endotoxin induces potent inflammatory activation of human blood vessels: inhibition by statins. Arterioscler. Thromb. Vasc. Biol. 2003;23:1576–1582. doi: 10.1161/01.ATV.0000081741.38087.F9. [DOI] [PubMed] [Google Scholar]
- Rogers J, Cooper NR, Webster S, Schultz J, McGeer PL, Styren SD, Civin WH, Brachova L, Bradt B, Ward P, Lieberburg I. Complement activation by beta-amyloid in Alzheimer disease. Proc. Natl. Acad. Sci. U. S. A. 1992;89:10016–10020. doi: 10.1073/pnas.89.21.10016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahar S, Dwarakanath RS, Reddy MA, Lanting L, Todorov I, Natarajan R. Angiotensin II enhances interleukin-18 mediated inflammatory gene expression in vascular smooth muscle cells: a novel cross-talk in the pathogenesis of atherosclerosis. Circ. Res. 2005;96:1064–1071. doi: 10.1161/01.RES.0000168210.10358.f4. [DOI] [PubMed] [Google Scholar]
- Shen Y, Li R, McGeer EG, McGeer PL. Neuronal expression of mRNAs for complement proteins of the classical pathway in Alzheimer brain. Brain Res. 1997;769:391–395. doi: 10.1016/s0006-8993(97)00850-0. [DOI] [PubMed] [Google Scholar]
- Shen Y, Lue L, Yang L, Roher A, Kuo Y, Strohmeyer R, Goux WJ, Lee V, Johnson GV, Webster SD, Cooper NR, Bradt B, Rogers J. Complement activation by neurofibrillary tangles in Alzheimer’s disease. Neurosci. Lett. 2001;305:165–168. doi: 10.1016/s0304-3940(01)01842-0. [DOI] [PubMed] [Google Scholar]
- Shi J, Perry G, Smith MA, Friedland RP. Vascular abnormalities: the insidious pathogenesis of Alzheimer’s disease. Neurobiol. Aging. 2000;21:357–361. doi: 10.1016/s0197-4580(00)00119-6. [DOI] [PubMed] [Google Scholar]
- Terai K, Walker DG, McGeer EG, McGeer PL. Neurons express proteins of the classical complement pathway in Alzheimer disease. Brain Res. 1997;769:385–390. doi: 10.1016/s0006-8993(97)00849-4. [DOI] [PubMed] [Google Scholar]
- Thomas A, Gasque P, Vaudry D, Gonzalez B, Fontaine M. Expression of a complete and functional complement system by human neuronal cells in vitro. Int. Immunol. 2000;12:1015–1023. doi: 10.1093/intimm/12.7.1015. [DOI] [PubMed] [Google Scholar]
- van Beek J, Elward K, Gasque P. Activation of complement in the central nervous system: roles in neurodegeneration and neuroprotection. Ann. N. Y. Acad. Sci. 2003;992:56–71. doi: 10.1111/j.1749-6632.2003.tb03138.x. [DOI] [PubMed] [Google Scholar]
- Veerhuis R, Janssen I, Hack CE, Eikelenboom P. Early complement components in Alzheimer’s disease brains. Acta Neuropathol. (Berl.) 1996;91:53–60. doi: 10.1007/s004019570001. [DOI] [PubMed] [Google Scholar]
- Verbeek MM, Otte-Holler I, Veerhuis R, Ruiter DJ, De Waal RM. Distribution of A beta-associated proteins in cerebrovascular amyloid of Alzheimer’s disease. Acta Neuropathol. (Berl.) 1998;96:628–636. doi: 10.1007/s004010050944. [DOI] [PubMed] [Google Scholar]
- Vonsattel JP, Myers RH, Hedley-Whyte ET, Ropper AH, Bird ED, Richardson EP., Jr. Cerebral amyloid angiopathy without and with cerebral hemorrhages: a comparative histological study. Ann. Neurol. 1991;30:637–649. doi: 10.1002/ana.410300503. [DOI] [PubMed] [Google Scholar]
- Walker DG. Expression and regulation of complement C1q by human THP-1-derived macrophages. Mol. Chem. Neuropathol. 1998;34:197–218. doi: 10.1007/BF02815080. [DOI] [PubMed] [Google Scholar]
- Walker DG, McGeer PL. Complement gene expression in human brains: comparison between normal and Alzheimer disease cases. Mol. Brain Res. 1992;14:109–116. doi: 10.1016/0169-328x(92)90017-6. [DOI] [PubMed] [Google Scholar]
- Walker DG, Kim SU, McGeer PL. Expression of complement C4 and C9 genes by human astrocytes. Brain Res. 1998;809:31–38. doi: 10.1016/s0006-8993(98)00811-7. [DOI] [PubMed] [Google Scholar]
- Walker DG, Kim SU, McGeer PL. Complement and cytokine gene expression in cultured microglia derived from postmortem human brains. J. Neurosci. Res. 1995;40:478–493. doi: 10.1002/jnr.490400407. [DOI] [PubMed] [Google Scholar]
- Walker DG, Lue LF, Beach TG. Increased expression of the urokinase plasminogen-activator receptor in amyloid beta peptide-treated human brain microglia and in AD brains. Brain Res. 2002;926:69–79. doi: 10.1016/s0006-8993(01)03298-x. [DOI] [PubMed] [Google Scholar]
- Webster S, Bradt B, Rogers J, Cooper N. Aggregation state-dependent activation of the classical complement pathway by the amyloid beta peptide. J. Neurochem. 1997;69:388–398. doi: 10.1046/j.1471-4159.1997.69010388.x. [DOI] [PubMed] [Google Scholar]
- Wilhelmus MM, Otte-Holler I, Davis J, Van Nostrand WE, De Waal RM, Verbeek MM. Apolipoprotein E genotype regulates amyloid-beta cytotoxicity. J. Neurosci. 2005;25:3621–3627. doi: 10.1523/JNEUROSCI.4213-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasojima K, Schwab C, McGeer EG, McGeer PL. Up-regulated production and activation of the complement system in Alzheimer’s disease brain. Am. J. Pathol. 1999;154:927–936. doi: 10.1016/S0002-9440(10)65340-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip AG, McKee AC, Green RC, Wells J, Young H, Cupples LA, Farrer LA. APOE, vascular pathology, and the AD brain. Neurology. 2005;65:259–265. doi: 10.1212/01.wnl.0000168863.49053.4d. [DOI] [PubMed] [Google Scholar]
- Zlokovic BV, Deane R, Sallstrom J, Chow N, Miano JM. Neurovascular pathways and Alzheimer amyloid beta-peptide. Brain Pathol. 2005;15:78–83. doi: 10.1111/j.1750-3639.2005.tb00103.x. [DOI] [PMC free article] [PubMed] [Google Scholar]