Abstract
Person identification represents a unique category of semantic knowledge that is commonly impaired in Alzheimer's Disease (AD), but has received relatively little investigation in patients with Mild Cognitive Impairment (MCI). The current study examined the retrieval of semantic knowledge for famous names from three time epochs (recent, remote, and enduring) in two participant groups; 23 aMCI patients and 23 healthy elderly controls. The aMCI group was less accurate and produced less semantic knowledge than controls for famous names. Names from the enduring period were recognized faster than both recent and remote names in both groups, and remote names were recognized more quickly than recent names. Episodic memory performance was correlated with greater semantic knowledge particularly for recent names. We suggest that the anterograde memory deficits in the aMCI group interferes with learning of recent famous names and as a result produces difficulties with updating and integrating new semantic information with previously stored information. The implications of these findings for characterizing semantic memory deficits in MCI are discussed.
Keywords: semantic memory, aging, fame recognition, person identity, remote memory, temporal gradient
Introduction
Semantic memory impairment is evident in AD, even in the early phases of the disease (Albert et al., 2001; Hodges & Patterson, 1995; Jacobs et al., 2001; Martin & Fedio, 1983; Vogel et al., 2005). Although it remains unclear whether these difficulties reflect degraded memory stores or actual loss of knowledge (Giffard et al., 2001), it has been suggested that the deterioration of the semantic knowledge network may be a useful marker for tracking the rate of progression of cognitive changes in AD (Chan et al., 1995).
AD patients exhibit difficulty in several categories of semantic knowledge, including living (e.g., animals) and non-living objects (e.g., tools) (Hodges & Patterson, 1995). Person-specific identity knowledge is also impaired in mild AD. For example, AD patients show reduced recognition accuracy for both famous faces and famous names and also have difficulty providing names of famous faces (Becker et al., 1995; Delazer et al., 2003; Hodges et al., 1993). In addition, AD patients provide less semantic information about famous people, even when they are correctly recognized (Hodges et al., 1993). Indeed, there is evidence to suggest that person-identity semantic knowledge is disproportionately affected in AD. For example, AD patients have more difficulty naming famous faces than naming other categories of objects (Semenza et al., 2000; Thompson et al., 2002).
Mild cognitive impairment (MCI) is considered to be a transitional state of cognitive impairment associated with a high rate of conversion to AD (Petersen et al., 2001). Given the association of MCI with AD, it is not surprising that recent investigations have also revealed significant semantic memory processing deficits in MCI (Kramer et al., 2006; Lopez et al., 2006; Murphy et al., 2006; Nordlund et al., 2005; Ribeiro et al., 2006). Adlam et al. (2006) reported that both MCI subjects and mild AD patients showed diminished performance on measures of category fluency, object knowledge, and naming low frequency objects. The AD group also showed impairment on additional semantic memory tasks such as naming high frequency words, comprehension, and semantic association. Other studies have reported reduced levels of performance for MCI subjects on measures of semantic clustering during word-list learning tasks, and susceptibility to semantic proactive interference (Loewenstein et al., 2004; Murphy et al., 2006; Ribeiro et al., 2007). In fact, it has been suggested that amnestic MCI may not be an accurate concept unless semantic memory impairment is included as a core impairment (Hodges et al., 2006).
Impairment in person-specific semantic knowledge appears to be present in MCI patients (Dudas et al., 2005; Joubert et al., 2008). A recent study reported that MCI patients who converted to AD two years later performed significantly worse on a baseline measure of famous face recognition compared to MCI patients who did not convert over that time period (Estevez-Gonzalez et al., 2004). They hypothesized that early atrophy in the temporal lobe was responsible for the face recognition deficit, which is consistent with neuroimaging findings in MCI and findings concerning the impact of temporal lobe damage on face recognition (Seidenberg et al., 2002; Viskontas et al., 2002). However, this study did not directly examine retrieval of person-identity semantic knowledge or the time epoch from which the famous face stimuli were drawn. Thus, a more extensive investigation of person-specific knowledge could be useful in understanding the nature of the semantic memory deficits in MCI.
A unique feature of investigating famous person recognition is that it provides the opportunity to study the effect of memory age or the time interval since initial encoding on subsequent memory retrieval. For example, Harry Truman first came to popular exposure in the 1940's while Barack Obama has come to public attention much more recently. This contrast of memory age has been the basis for the study of the temporal gradient underlying remote memory in both healthy persons and a variety of clinical groups (Albert et al., 1979; Barr et al., 1990; Hodges & Graham, 1998; McCarthy & Warrington, 1990). It is also an important source of data for discussions concerning the role of the hippocampus and neocortex in the consolidation, storage, and retrieval of long term memory (Moscovitch & Nadel, 1998; Squire & Alvarez, 1995).
The nature of the temporal gradient is not similar across various patient groups. AD patients appear to show a (mild) temporal gradient in their recognition and identification of famous faces and famous names. That is, performance is better for stimuli from a more remote time period than from a more recent time period (Beatty et al., 1988; Greene & Hodges, 1996). Similar finding have been reported for patients with unilateral temporal lobe epilepsy (TLE), particularly when left TLE subjects were asked to name famous faces (Seidenberg et al., 2002). In contrast, semantic dementia patients show a reverse temporal gradient in which recent famous people are more accurately recognized than famous people from remote time periods (Hodges & Graham, 1998). Subcortical dementia groups (e.g., HIV dementia, Huntington's disease) typically produce a flat temporal gradient characterized by similar levels of impairment in famous face recognition across both recent and remote periods (Albert et al., 1981; Beatty et al., 1988; Sadek et al., 2004). To date, the temporal gradient for person-identity has not been examined in patients with MCI and a better understanding of this aspect of semantic memory could provide useful information in characterizing the nature of semantic memory functioning during this transitional phase.
In the current study, we examined semantic knowledge for names of famous people across three different time epochs; recent (1990-2003) and remote (1950-1965), in persons with MCI and healthy control participants. A third stimulus condition, enduring, was included and consisted of names of famous people who have remained in the public domain for several decades (enduring Names), such as Bob Hope. Previous research from our group has shown that accuracy was higher and reaction time was faster for enduring famous names compared to recent famous names in healthy older adults, a pattern that was not evident among younger adult controls (Nielson et al., 2006; Woodard et al., 2007). We expected MCI participants to provide less semantic knowledge for famous people, regardless of time epoch, compared to controls. Furthermore, given the presence of difficulties in the acquisition of new information, we expected that MCI participants would show poorer performance (recognition accuracy, reaction time, and semantic knowledge) when identifying recent famous names compared to names from the remote and enduring time periods. In addition, famous names from the enduring condition were also expected to be negatively affected in MCI because the anterograde memory impairment characterizing the group was expected to disrupt the potential effects of frequent and recent updating on name recognition and person-identity semantic knowledge. We also included ratings of emotional valence (positive, negative) and emotional arousal to ensure that differences in semantic knowledge between groups and time epochs could not be accounted for by these stimulus attributes.
Methods
The data reported in this manuscript were acquired in compliance with the regulations of our local institutions and with the review and approval of the Institutional Review Board
Participants
Twenty-three older adults with MCI were recruited from medical clinics in the metropolitan Milwaukee area. All subjects met Petersen (2001) criteria for amnestic MCI (aMCI): (1): memory complaint preferably corroborated by an informant, (2) objective memory impairment by neuropsychological testing (see below), (3) normal general cognitive functioning, (4) intact activities of daily living, and (5) not demented. Twenty-two of the aMCI subjects met criterion for aMCI single domain and one subject met criterion for aMCI multiple domains (Petersen, 2004).
To evaluate memory performance, local norms were collected from 91 healthy adult subjects to establish cutoff scores for the delayed recall and long term percentage recall (LTPR) indices from the Rey Auditory Verbal Learning Test (RAVLT; Rey, 1958). Separate cutoff scores were established for men and women as there were significant sex differences in the local group performance on the RAVLT. Using a criterion corresponding to a performance of 1.5 standard deviation below the mean, delayed recall of 5 words or lower for women and 4 words or lower for men, and percent retention scores below 60% were the established cutoffs scores used to identify the MCI group. All aMCI subjects scored below both these cutoff scores while all healthy controls (see below) scored above these cutoff scores.
To further characterize the cognitive status of the MCI and control groups, participants were administered the Mini Mental Status Exam (MMSE; Folstein et al., 1975) and the Dementia Rating Scale −2 (DRS-2; Jurica et al., 2001; Mattis, 1988). Age and educated corrected MOANS scaled scores of 5 or lower (1.5 standard deviation) on the DRS-2 subscales (other than memory) was used to differentiate subjects meeting criteria for aMCI multiple domain from those meeting criteria for aMCI single domain (Petersen, 2004).
All MCI subjects also obtained MMSE scores above 23. A score less than 20 on the GDS was also required to rule out moderate to severe depression. Finally, all MCI subjects scored in the normal range on the Lawton and Brody Personal Self-Maintenance and Instrumental Activities of Daily Living Scale (Lawton & Brody, 1969). It consists of specific questions about instrumental activities of daily living and personal self-maintenance activities to derive an overall ADL rating on an ordinal scale from 1-5. All MCI subjects scored in the 4-5 range (good to excellent).Whenever possible, a collateral reviewed subject responses.
Subjects were also seen by a neurologist with expertise in dementia to rule out other possible bases for the memory impairment. Prospective participants were excluded if there was a history or evidence of current or previous neurological illness, major psychiatric disturbance, or substance abuse. All MCI subjects obtained a modified Hachinski ischemia score below 4. Any participant with an abnormality seen on high resolution MRI was excluded. An extensive blood chemistry screen was also administered to ensure that levels of TSH, homoctsyteine, vitamin B12, folate, and creatinine were within normal limits.
Twenty-three control participants for the study were recruited from the metropolitan Milwaukee community from newspaper advertisements. All controls scored above the cutoff scores (identified above) on the RAVLT and scored 28 or above on the MMSE. In addition, controls also did not report any previous or current history of neurological disease, no major psychiatric disturbance meeting DSM-IV Axis I criteria, no substance abuse meeting DSM-IV Axis I criteria, and no current use of psychoactive medications.
Stimuli
Name stimuli were selected through a carefully standardized procedure used in an earlier study conducted with 24 young and 24 older individuals (Douville et al., 2005). From an initial corpus of 784 famous names selected from the internet, trivia books, magazines, and newspapers, along with unfamiliar names selected from a metropolitan telephone directory, a total pool of 60 names were selected for the current study. These 60 stimuli were composed of: (1) Recent stimuli: 10 names of people who achieved public prominence in the 1990-2003 period and were correctly identified by 90% of both older and younger participants, (2) Remote stimuli: 10 names of persons who achieved prominence in the 1950-1965 period, but who have been out of the public eye for some time and are not as likely to appear frequently in the news or entertainment media; these stimuli were correctly identified by 90% of older and only 10% of younger participants, (3) Enduring stimuli: 10 names of persons who achieved fame in the 50's and are still well-known; these stimuli were correctly identified by 90% of older and younger participants, and (4) Unfamiliar stimuli: 30 unfamiliar names correctly identified as unfamiliar by 90% of older and younger participants.
Procedure
The initial presentation of familiar names occurred during an event- related fMRI scanning session in which each name stimulus was presented for 4 seconds. Only the behavioral data from the scanning session (accuracy, reaction time, semantic knowledge) were considered for the present study. Participants were instructed to make a right index finger (i.e., dominant hand) key press if the name was famous and a right middle finger key press if the name was unfamiliar (all conditions). Response accuracy and reaction time to respond to each name was recorded.
The semantic knowledge data were collected immediately following the recognition phase. Subjects were asked to complete (in writing) information about the famous names which were correctly recognized. Semantic knowledge was determined by having subjects provide information in response to four distinct probes: (1) Reason this person is well known (e.g., occupation), (2) Known works/accomplishments of this individual, (3) Names of specific individuals or events associated with this individual, (4) History and background (e.g., family life, health status). Each of these four probes was scored on a 0-3 point scale. A total semantic knowledge score (range 0-12) was derived for each item by adding scores from the four probes. Scoring was conducted independently and blinded to group membership by two raters who demonstrated an intra-class correlation reliability of r = 0.93. Subjects also rated each famous name on a 7-point Likert scale of emotional arousal and emotional valence (positive/negative). Appendix 1 provides a sample item with responses and scores for the semantic knowledge measure.
Data Analyses
Recognition accuracy was determined by performance during scanning, and both reaction time and semantic knowledge analyses were conducted only for famous names that were accurately identified during scanning. A mixed design 2 (group) × 3 (time epoch) ANOVA was conducted to identify group differences in recognition accuracy, reaction time, and semantic knowledge for famous names from the different time periods. All follow-up pairwise contrasts were conducted using Bonferroni correction.
Results
Demographics and Cognitive Screening
There were no significant differences between the MCI and control groups for chronological age, t (44) = −0.20, p >.05, education level, t (44) = 0.13, p >.05, or gender distribution. Table 1 provides the findings for the cognitive screening battery. As expected given the diagnostic selection criteria, the MCI group scored significantly below controls on the RAVLT indices of delayed recall, t (44) = 9.90, p<.001, and long-term percent retention, t (44) = 8.63, p<.001. There were also significant group differences on the MMSE, t (44) = 3.06, p<.01, the total score of the DRS-2, t (44) = 5.94, p<.001, and all DRS-2 subtests (p's < .05), except for the construction scale (p = 0.39).
Table 1.
Demographic and Neuropsychological performance
| Measures |
Healthy Controls Mean (S.D.) (n=23) |
MCI Mean (S.D.) (n=23) |
p value |
|---|---|---|---|
| Age | 75.3 (4.7) | 75.6 (5.6) | .84 |
| Education | 14.7 (2.6) | 14.4 (3.2) | .90 |
| Gender | 20 women, 3 men | 20 women, 3 men 21 aMCI, 2 multidomain |
ns |
| MCI Type | |||
| MMSE | 29.26 (0.92) | 27.91 (1.90) | .004 |
| DRS-2 | |||
| Attention | 12.48 (1.04) | 11.13 (2.30) | .014 |
| Initiation/Perseveration | 11.26 (1.89) | 9.09 (2.86) | .004 |
| Construction | 9.74 (0.86) | 9.48 (1.16) | .39 |
| Concentration | 11.87 (1.32) | 9.61 (1.88) | <0.001 |
| Memory | 11.48 (1.53) | 7.48 (3.44) | <0.001 |
| Total | 13.00 (1.98) | 8.57 (2.98) | <0.001 |
| RAVLT Delayed Recall | 10.39 (2.76) | 3.17 (2.15) | <0.001 |
| RAVLT LTPR | 0.85 (0.11) | 0.39 (0.23) | <0.001 |
LTPR = Long Term Percent Retention
Famous Name Recognition Accuracy
When famous name accuracy was examined across the three time epochs, the main effect of group was not significant, F (1, 44) = 2.32, p > .05, eta2 = .05. The absence of an overall group difference in accuracy may have been due to ceiling effects (see Table 3). However, the main effect for time epoch was significant, F (2, 43) = 19.18, p > .001, eta2 = .47. Recognition accuracy for famous names from the recent epoch was lower than recognition accuracy for the remote time epoch and the enduring time epochs (p's < .001), which did not differ from each other (p > .05). The interaction effect of group × epoch only approached significance, F (2, 43) = 2.64 p = .08, eta2 = .11, but given our prediction, we examined group differences by time epoch with t-test contrasts. As predicted, the MCI group were significantly less accurate than controls for famous names in the recent epoch, t (44) = 2.44, p = .019, but were not significantly different in accuracy for the remote, t (44) = 1.57, p > .05 or enduring time period, t (44) = 1.30, p > .05.
Table 3.
Semantic Knowledge and RAVLT Memory Indices (n = 46)
| Recent Knowledge | Remote Knowledge | Enduring Knowledge | |
|---|---|---|---|
| Delayed Recall | .40** | .40** | .63** |
| LTPR | .34* | .30* | .49** |
p<0.01
p<0.05
LTPR = Long term percent retention
Famous Name Reaction Time
Analyses of reaction time for correct trials (Figure 1) yielded a non-significant main effect of group, F (1, 44) = .365, p > .05, eta2 = .008. The main effect of time epoch was significant, F (2, 43) = 90.75, p < .001, eta2 = .67. Both groups showed a steep temporal gradient with faster reaction time for remote names than recent names, and enduring famous names showed a faster reaction time than remote and recent names, (all p's < .001). The group × time period interaction was not significant, F (2, 43) = .13, p > .05, eta2 = .003.
Figure 1.
Reaction time (RT) for recognizing famous names. For both groups, RT is fastest for enduring famous names, followed by remote names, and slowest for recent famous names. No group differences on RT. The main effect of time epoch for RT was significant across groups. RT for enduring was faster than remote which in turn was faster than recent names.
* indicates a significant difference between time epochs.
Person-Identity Semantic Knowledge
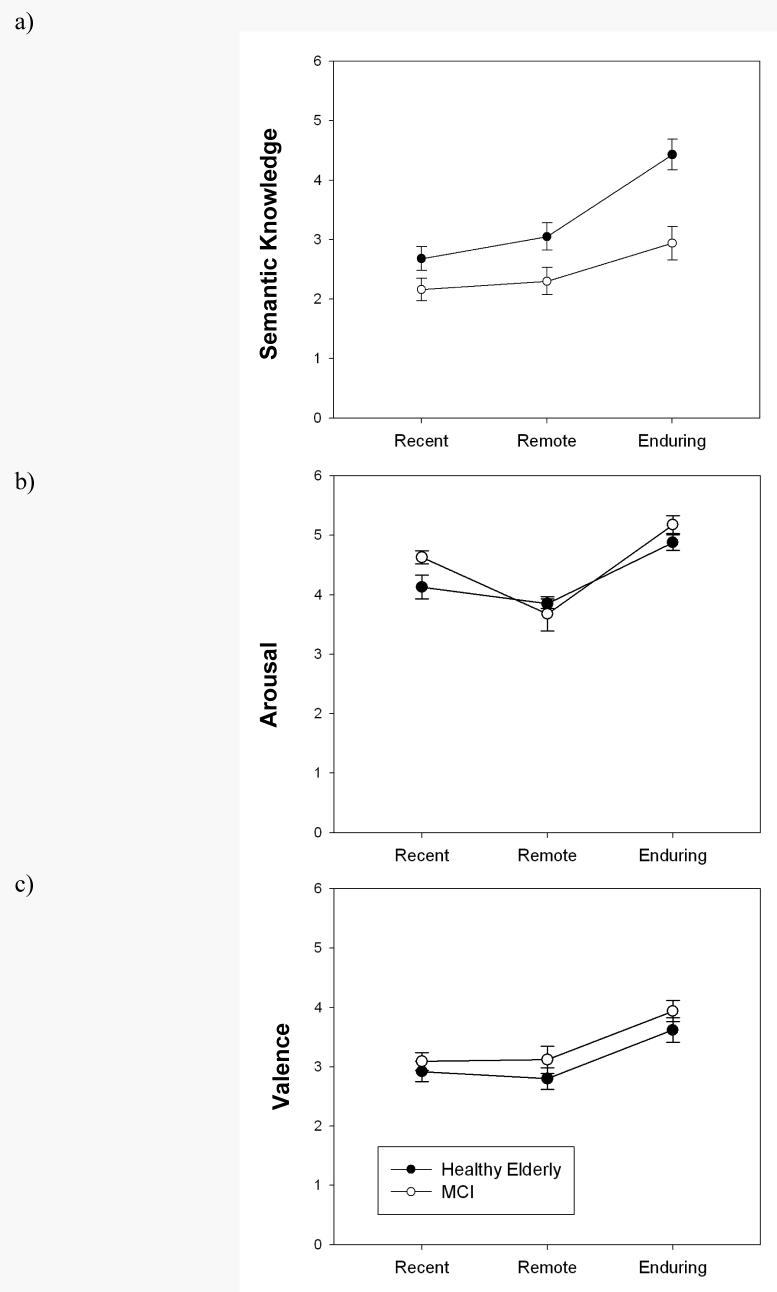
As indicated previously, only trials of famous names correctly recognized were analyzed in the post-scan assessment of semantic knowledge. The control group provided more semantic knowledge overall than the MCI group across all time epochs, F (1, 44) = 12.42, p<0.001, eta2 = .22, but there was also a significant group × time epoch interaction, F (2, 43) = 5.59, p < .001, eta2 = .11. The control group produced substantially more semantic information for the enduring time epoch than either the recent time period, t (22) = 5.98, p < .001, or the remote period, t (22) = 11.87, p < .001. In addition, the control group produced more semantic knowledge for the remote than the recent time epoch, t (22) = 2.13, p < .05. In contrast, the MCI group produced only slightly more semantic knowledge for the enduring stimuli than the recent stimuli, t (22) = 2.33, p = .03, and the remote stimuli t (22) = 3.33, p < .05.
Arousal and Valence
Ratings of arousal for famous names did not differ between the healthy controls and MCI group, F (1, 44) = .04, p > .05. However, arousal ratings did significantly differ by time epoch, F (2, 43) = 44.27, p < .001, eta2 = .67. For both groups, enduring famous names received significantly higher ratings than famous names from either the remote or recent time epochs (p's < .001). In addition, the recent time epoch produced higher arousal ratings than the remote time epoch in both groups (p's < .001). There was a non-significant group × time interaction effect, F (2, 43) = 1.62, p > .05, eta2 = .07.
Ratings for valence also did not yield a significant main effect of group, F (1, 44) = .12, p > .05, or a significant group × time epoch interaction, F (2, 43) = .31, p > .05. There was, however, a significant main effect of time epoch, F (2, 43) = 27.57, p < .001, eta 2 = .56. Enduring names received more positive ratings in both groups than either the recent or remote famous names in both groups (p < .001), but there was no difference in valence rating between the remote and recent time epochs.
In summary, there were no group differences in ratings of emotional arousal or valence, and enduring stimuli obtained higher arousal ratings and more positive ratings than names from either the recent or remote time periods. The same pattern was evident for both the MCI and control groups.
Categories of Semantic Knowledge
When the four categories of semantic knowledge were compared between the two groups, a significant main effect of group emerged, F (1, 44) = 27.50, p < .001, eta2 = .39; with the healthy controls providing more semantic information than the MCI group across all four categories (all p's < .001). A main effect of category was also significant, F (3, 42) = 222.62, p < .001, eta2 = .94; semantic knowledge for the reason for fame category was significantly greater than in the other three categories; famous works and acknowledgements, history and background, and names of associated events and people (all p's < .001). Among the three latter category types of knowledge history and background produced significantly more semantic knowledge than the other two categories (p's <.05). The interaction effect of group × category was also significant, F (3, 42) = 3.6, p < .05, eta2 = .21, reflecting the disproportionate amount of knowledge reported between the MCI group and controls for the more specific categories compared to the general fame category.
Episodic Memory and Person-identity Semantic Knowledge
The relationship between person-identity semantic knowledge and new learning episodic memory, as measured by the Rey Auditory Verbal Learning Test Delayed Recall and Long Term Percent Retention (LTPR) indices, is provided in Table 3. When the samples were separated, no significant relationships emerged, probably due to the restricted ranges within each group for memory scores. When both groups are combined, a significant correlation was obtained between the RAVLT indices and semantic knowledge in each of the three time epochs. In all instances, better general episodic memory performance was associated with greater semantic knowledge retrieval.
Discussion
Recognition accuracy and semantic knowledge retrieval for famous names were impaired in MCI subjects compared to controls, and time epoch influenced the degree of observed impairment. As predicted, the MCI group was not as accurate in recognizing famous names from the recent time epoch compared to the controls, a finding that is consistent with the anterograde memory impairment that characterizes the MCI group. Although the healthy control group provided more semantic information for famous names than the MCI group across all three stimulus time epochs, the difference between groups was substantially greater for the enduring time epoch. We hypothesize that this pattern reflects MCI patients' difficulties to acquire new information about person-identity and to “update” or “integrate” more recent information with previously acquired information about these people.
Consistent with expectations, the MCI group showed significantly poorer performance than controls on episodic memory and learning indices from the RAVLT. Furthermore, episodic memory performance was also significantly correlated with the amount of semantic knowledge reported, suggesting that the degree of impairment in new learning ability influenced the amount of semantic knowledge reported. By our operational definition, enduring famous names included people who have been “famous” or in the public domain for several decades and for whom information continues to be available in the public domain (e.g., John Kennedy). Therefore, the semantic knowledge base for enduring names can undergo continued updating of additional information and facts and re-exposure to already known information. In contrast, remote famous names were selected on the basis of their very low recognition rate among younger people and high recognition rates among older people (Douville et al., 2005). Thus, remote famous names includes people whose popularity was restricted to the past and were much less likely to have continued exposure into the more recent time interval. That is, there are fewer opportunities to update information about remote famous names than enduring famous names. Given their episodic memory impairment, the MCI group appears less able than controls to take advantage of the ongoing learning opportunities associated with continued exposure of the enduring famous names. This notion is consistent with current models of mesial temporal lobe involvement in acquiring new information, as well as its role in updating previously acquired information (Squire & Alvarez, 1995; Nadel & Moscovitch, 1997).
It is generally acknowledged that the mesial temporal lobe (MTL) is the primary location of neuropathology observed in MCI (Twamley et al., 2006). Recent famous names or faces have been shown to produce greater fMRI activation than remote famous names in MTL regions such as the entorhinal cortex (Haist et al., 2001), the hippocampus (Douville et al., 2005), and interconnected regions, such as the posterior cingulate, which play an important role in memory retrieval (Woodard et al., 2007). In addition, activity in these regions, as well as in frontal cortex, is greater for older subjects than younger subjects, primarily for recent famous names (Nielson et al., 2006). Taken together, the current study findings are consistent with the notion that the functional disruption of the mesial temporal lobe in MCI plays a role in the different levels of performance observed for semantic knowledge across time epochs.
Both the MCI and control groups showed evidence for a steep temporal gradient for famous name recognition reaction time. As predicted, remote famous names showed an advantage over recent names. Thus, even when famous names were accurately recognized, remote famous names appeared to have faster accessibility in semantic memory than did recent famous names. The observed temporal gradient is consistent with the notion that well-consolidated semantic information (remote) has advantages in retrieval access compared to information that has less of an opportunity to be consolidated (recent). An advantage for “dated” stimuli over “contemporary” stimuli has also been observed in a name retrieval task. Of interest, this advantage was seen only for older subjects and not younger subjects (Small & Sandhu, 2007). A similar advantage for “dated” stimuli has also been reported for older adults on an episodic face recognition task (Backman & Herlitz, 1990; Wahlin et al., 1993) and recollection of media-mediated past events (Bizzozero et al., 2008). We found that reaction time for recognition of enduring names was significantly faster than the reaction time for recognition of remote and recent names in both the healthy and MCI groups, despite the differences in recognition levels. This finding suggests increased accessibility in semantic memory for enduring names overall.
Recently, Westmacott & Moscovitch (2003) suggested that the semantic knowledge base for famous people is composed of both a “semantic” component and an “episodic” component. They suggested that well-known public individuals have more autobiographical significance than others, and may therefore provide a more distinct episodic component to their long term memory representation. In a series of studies, they showed that famous names rated as autobiographically significant were read faster, were more quickly judged to be famous, and were better recalled and recognized than famous names without autobiographical significance. The current study solicited information from the “semantic” component of the subjects' conceptual knowledge base (i.e., reason for fame, known works, associated people, and background history), as we did not specifically probe for information about autobiographical significance. Nevertheless, it is certainly possible that retrieval of the famous name automatically elicits this information. Recent studies have suggested that personal episodic information for famous people is distinct from its semantic information (Denkova et al., 2006; Piolino et al., 2007). Thus, it would be of considerable interest to examine the relative impact and interaction of both of the semantic and autobiographical knowledge components for famous name recognition and identification across time epochs, across subject age, and in different clinical groups. For example, Westmacott et al. (2004) demonstrated that the performance of semantic dementia patients but not AD patients was affected by autobiographical significance, but they did not examine the potential influence of time epoch.
Our data indicated that the MCI group performed more poorly than the healthy controls across all four categories of semantic knowledge examined. However, both groups retrieved more semantic knowledge in the reason for fame (e.g., occupation) category than the remaining three knowledge categories summed together. Thus, reason for fame may be a primary attribute of the conceptual knowledge base for famous people. After all, this information is what distinguishes “famous” from a “non-famous” person. In support of this notion, there is considerable data attesting to a processing priority (i.e., memory) in person identification for occupation (essentially reason for fame) over other attributes such as nationality or whether the individual is currently dead or alive (Crutch & Warrington, 2004; Moran et al., 2005).
The MCI subjects studied here all met Petersen (1999) criteria for aMCI. However, several studies have suggested that some aMCI subjects also show impairment in other non-memory cognitive domains (Alladi et al., 2006; Frutos-Alegria et al., 2007; Lopez et al., 2006, Ribeiro et al., 2006). Petersen (2004) distinguishes aMCI single domain from aMCI multiple domains. In this study, we used a 1.5 standard deviation cutoff score on the DRS non-memory domains to identify subjects with impairment in domains other than memory. With this cutoff score, only 1/23 aMCI subjects met criteria for a multiple domain impairment. The remainder of the subjects met criteria for aMCI single domain.
Another issue to consider is the potential influence of stimulus dimensions such as arousal and valence on the current findings. Although enduring famous names received higher ratings of arousal and higher positive valence ratings than recent and remote names, there were no significant group differences in ratings of arousal or valence across the three time epochs. In addition, remote famous names showed lower arousal and valence rating than recent famous names in both groups, which does not correspond to the accuracy, reaction time, and semantic knowledge findings. It should be noted that both the MCI and healthy control groups were composed of substantially more women than men, and this imbalance is likely to reflect the influence of recruitment of a sample of convenience. Nevertheless, the sex bias should be kept in mind when considering the findings. Finally, it would be of interest to determine the predictive validity of person-identity semantic knowledge performance for progression to AD.
In summary, MCI patients appear to show substantial deficits on a semantic memory task involving famous name recognition and semantic knowledge. Furthermore, these differences are influenced by memory age and are consistent with expectations based on the disruption in the acquisition of new information and updating previously known information. We suggest that additional investigation of the person-identity network across time epochs may prove quite useful in characterizing the memory impairment observed in MCI, AD and other dementias.
Figure 2.
(top panel) Semantic knowledge for famous names. Semantic knowledge is greater for the healthy controls across all three time epochs. However, the healthy control group showed a more extensive increase in semantic knowledge compared to the other two time epochs compared to the MCI group. (middle panel) Arousal ratings for famous names. There was no difference in arousal ratings between groups for all time epochs. For both groups, arousal ratings for enduring stimuli were higher than names from the recent and remote time epochs.(bottom panel) Valence ratings for famous names. There was no difference in valence ratings between groups for all time epochs. For both groups, valence ratings for enduring stimuli were higher (more positive) than names from the recent and remote time epochs.
Table 2.
Semantic knowledge variables between groups
| Measures |
Healthy Controls Mean (S.D.) (n=23) |
MCI Mean (S.D.) (n=23) |
p value | |
|---|---|---|---|---|
| Recognition Accuracy | ||||
| Recent | .89 (.13) | .75 (.23) | <.05 | |
| Remote | .97 (.06) | .92 (.13) | 0.12 | |
| Enduring | .98 (.05) | .95 (.08) | 0.20 | |
| Recognition Reaction Time | ||||
| Recent | 1497.82 (331.28) | 1603.9 (277.75) | 0.25 | |
| Remote | 1275.98 (263.38) | 1389.33 (258.87) | 0.15 | |
| Enduring | 1125.76 (259.41) | 1210.67 (218.45) | 0.24 | |
| Amount of Semantic Knowledge | ||||
| Recent | 2.68 (.97) | 2.16 (.93) | 0.07 | |
| Remote | 3.05 (1.12) | 2.3 (1.11) | <.05 | |
| Enduring | 4.44 (1.25) | 2.94 (1.34) | <.001 | |
| Arousal | ||||
| Recent | 4.13 (.94) | 4.63 (.53) | <.05 | |
| Remote | 3.85 (.39) | 3.68 (1.4) | 0.57 | |
| Enduring | 4.88 (.62) | 5.18 (.70) | 0.14 | |
| Valence | ||||
| Recent | 2.92 (.80) | 3.09 (.71) | 0.44 | |
| Remote | 2.80 (.88) | 3.12 (1.11) | 0.28 | |
| Enduring | 3.62 (.99) | 3.94 (.85) | 0.25 | |
Acknowledgements
This study was supported by grants from the National Institutes of Health (R01 AG022304), Medical College of Wisconsin General Clinical Research Center (M0 RR00058), the Medical College of Wisconsin Advancing a Healthier Wisconsin program, and the W.W. Keck Foundation.
Appendix 1
Stimulus item Example:
John F. Kennedy
1. Reason this person is well known
3 points: 35th President of the United States who was assassinated
2 points: President that was assassinated
1 point: President
2. Known works or accomplishments
Give 1 point for each specific work or accomplishment named Maximum of 3 points
Examples: Bay of Pigs Cuban Missile Crisis, involvement of the space program in the race to space
3. Names of specific individuals or events associated with the individual
Give 1 point for each individual or event named Maximum of 3 points
Examples: assassinated,Marilyn Monroe, Vietnam War
4. History and background
Give 1 point for each piece of information Maximum of 3 points
Examples: Roman Catholic, married to Jacqueline Onassis, brother is Robert Kennedy, 2 children (Caroline & John Jr.)
References
- Adlam A, Bozeat S, Arnold R, Watson P, Hodges J. Semantic knowledge in mild cognitive impairment and mild Alzheimer's disease. Cortex. 2006;42:675–84. doi: 10.1016/s0010-9452(08)70404-0. [DOI] [PubMed] [Google Scholar]
- Albert M, Moss M, Tanzi R, Jones K. Preclinical prediction of AD using neuropsychological tests. J Int Neuropsych Soc. 2001;7:631–39. doi: 10.1017/s1355617701755105. [DOI] [PubMed] [Google Scholar]
- Albert M, Butters N, Brandt J. Development of remote memory loss patients with Huntington's disease. J Clin Neuropsychol. 1981;3:1–12. doi: 10.1080/01688638108403109. [DOI] [PubMed] [Google Scholar]
- Albert M, Butters N, Levin J. Temporal gradients in the retrograde amnesia of patients with Korsakoff's disease. Arch Neurol. 1979;36:211–16. doi: 10.1001/archneur.1979.00500400065010. [DOI] [PubMed] [Google Scholar]
- Alladi S, Arnold R, Mitchell J, Nestor P, Hodges J. Mild cognitive impairment: applicability of research criteria in a memory clinic and characterization of cognitive profile. Psychol Med. 2006;36:507–15. doi: 10.1017/S0033291705006744. [DOI] [PubMed] [Google Scholar]
- Backman L, Herlitz A. The relationship between prior knowledge and face recognition in normal aging and Alzheimer's disease. J Gerontology. 1990;45:94–100. doi: 10.1093/geronj/45.3.p94. [DOI] [PubMed] [Google Scholar]
- Barr W, Goldberg E, Wasserstein J, Novelly R. Retrograde amnesia following unilateral temporal lobectomy. Neuropsychologia. 1990;28:243–55. doi: 10.1016/0028-3932(90)90018-j. [DOI] [PubMed] [Google Scholar]
- Beatty W, Salmon D, Butters N, Heindel W, Granholm E. Retrograde amnesia in patients with Alzheimer's disease or Huntington's disease. Neurobiology of Aging. 1988;9:181–86. doi: 10.1016/s0197-4580(88)80048-4. [DOI] [PubMed] [Google Scholar]
- Becker J, Lopez O, Boller F. Understanding impaired analysis of faces by patients with probable Alzheimer's disease. Cortex. 1995;31:129–37. doi: 10.1016/s0010-9452(13)80111-6. [DOI] [PubMed] [Google Scholar]
- Bizzozero I, Capitani E, Paglioni P, Luchelli F, Saetti M, Spinnler H. Recollection of public events in healthy people: A latent variable stochastic approach to disentangling retrieval and encoding. Cortex. 2008;44:150–160. doi: 10.1016/j.cortex.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Chan A, Salmon D, Butters N, Johnson S. Semantic network abnormatily predicts rate of cognitive decline in patients with probable Alzheimer's disease. J Int Neuropsychol Soc. 1995;1:297–303. doi: 10.1017/s1355617700000291. [DOI] [PubMed] [Google Scholar]
- Crutch S, Warrington E. The semantic organisation of proper nouns: the case of people and brand names. Neuropsychologia. 2004;42:584–96. doi: 10.1016/j.neuropsychologia.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Delazer M, Semenza C, Reiner M, Hofer R, Benke T. Anomia for people names in DAT – evidence for semantic and post-semantic impairments. Neuropsychologia. 2003;41:1593–98. doi: 10.1016/s0028-3932(03)00116-7. [DOI] [PubMed] [Google Scholar]
- Denkova E, Botzung A, Manning L. Neural correlates of remembering/knowing famous people: An event-related fMRI study. Neuropsychologia. 2006;44:2783–2791. doi: 10.1016/j.neuropsychologia.2006.05.029. [DOI] [PubMed] [Google Scholar]
- Douville K, Woodard J, Seidenberg M, Leveroni C, Nielson K, Franczak M, Antuono P, Rao S. Medial temporal lobe activity for recognition of recent and remote famous names: an event-related fMRI study. Neruopsychologia. 2005;43:693–703. doi: 10.1016/j.neuropsychologia.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Dudas R, Clague F, Thompson S, Graham K, Hodges J. Episodic and semantic memory in mild cognitive impairment. Neuropsychologia. 2005;43:1266–1276. doi: 10.1016/j.neuropsychologia.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Estevez-Gonzalez A, Garcia-Sanchez C, Boltes A, Otermin P, Pascual-Sedano B, Gironell A, Kulisevsky J. Semantic knowledge of famous people in mild cognitive impairment and progression to Alzheimer's disease. Dement Geriatr Cogn Disord. 2004;17:188–95. doi: 10.1159/000076355. [DOI] [PubMed] [Google Scholar]
- Folstein M, Folstein S, McHugh P. “Mini-mental state”. A practical method for grading the cognitive status of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Frutos-Alegria M, Molto-Jorda J, Morera-Guitart J, Sanchez-Perez A, Ferrer-Navajas M. The neuropsychological profile of mild cognitive impairment with involvement of multiple cognitive areas. The importance of amnesia in distinguishing two subtypes of patients. Rev Neurol. 2007;44:455–9. [PubMed] [Google Scholar]
- Giffard B, Desgranges B, Nore-Mary F, Lalevee C, de la Sayette V, Pasquier F, Eustache F. The nature of semantic memory deficits in Alzheimer's disease: new insights from hyperpriming effects. Brain. 2001;124:1522–32. doi: 10.1093/brain/124.8.1522. [DOI] [PubMed] [Google Scholar]
- Greene J, Hodges J. Identification of famous faces and famous names in early Alzheimer's disease. Relationship to anterograde episodic and general semantic memory. Brain. 1996;119:111–28. doi: 10.1093/brain/119.1.111. [DOI] [PubMed] [Google Scholar]
- Haist F, Bowden G, Mao H. Consolidation of human memory over decades revealed by functional magnetic resonance imaging. Nat Neurosci. 2001;4:1139–45. doi: 10.1038/nn739. [DOI] [PubMed] [Google Scholar]
- Hodges J, Erzinclioglu S, Patterson K. Evolution of cognitive deficits and conversion to dementia in patients with mild cognitive impairment: a very-long-term follow-up study. Dement Geriatr Cogn Disord. 2006;21:380–91. doi: 10.1159/000092534. [DOI] [PubMed] [Google Scholar]
- Hodges J, Graham K. A reversal of the temporal gradient for famous person knowledge in semantic dementia: implications for the neural organization of long-term memory. Neuropsychologia. 1998;36:803–25. doi: 10.1016/s0028-3932(97)00126-7. [DOI] [PubMed] [Google Scholar]
- Hodges J, Patterson K. Is semantic memory consistently impaired early in the course of Alzheimer's disease? Neuroanatomical and diagnostic implications. Neruopsychologia. 1995;33:441–59. doi: 10.1016/0028-3932(94)00127-b. [DOI] [PubMed] [Google Scholar]
- Hodges J, Salmon D, Butters N. Recognition and naming of famous faces in Alzheimer's disease: A cognitive analysis. Neuropsychologia. 1993;31:775–88. doi: 10.1016/0028-3932(93)90128-m. [DOI] [PubMed] [Google Scholar]
- Jacobs D, Sano M, Dooneief G, Marder K, Bell K, Stern Y. Neuropsychological detection and characterization of preclinical Alzheimer's disease. Neurology. 2001;45:957–62. doi: 10.1212/wnl.45.5.957. [DOI] [PubMed] [Google Scholar]
- Joubert S, Felician O, Barbeau EJ, Didic M, Poncet M, Ceccaldi M. Patterns of semantic memory impairment in mild cognitive impairment. Behavioural Neurology. 2008;19:35–40. doi: 10.1155/2008/859657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurica P, Leitten C, Mattis S. Dementia Rating Scale-2 (DRS-2) Psychological Assessment Resources; Odessa, FL: 2001. [Google Scholar]
- Kramer J, Nelson A, Johnson J, Yaffe K, Glenn S, Rosen H, Miller B. Multiple cognitive deficits in amnestic mild cognitive impairment. Dement Geriatr Cogn Disord. 2006;22(4):306–11. doi: 10.1159/000095303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawton M, Brody M. Assessment of older people: Self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9:179–86. [PubMed] [Google Scholar]
- Loewenstein D, Acevedo A, Luis C, Crum T, Barker W, Duara R. Semantic interference deficits and the detection of mild Alzheimer's disease and mild cognitive impairment without dementia. J Int Neuropsychol Soc. 2004;10:91–100. doi: 10.1017/S1355617704101112. [DOI] [PubMed] [Google Scholar]
- Lopez O, Becker J, Jagust W, Fitzpatrick A, Carlson M, DeKosky S, Breitner J, Lyketsos C, Jones B, Kawas C, Kuller L. Neuropsychological characteristics of mild cognitive impairment subgroups. J Neurol Neurosurg Psychiatry. 2006;77:159–65. doi: 10.1136/jnnp.2004.045567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin A, Fedio P. Word production and comprehension in Alzheimer's disease: the breakdown of semantic knowledge. Brain Lang. 1983;19:124–41. doi: 10.1016/0093-934x(83)90059-7. [DOI] [PubMed] [Google Scholar]
- Mattis S. Dementia Rating Scale (DRS) Psychological Assessment Resources; Odessa, FL: 1988. [Google Scholar]
- McCarthy R, Warrington E. Cognitive Neuropsychology: A Clinical Introduction. Academic Press; San Diego: 1990. [Google Scholar]
- Moran M, Seidenberg M, Sabsevitz D, Swanson S, Hermann B. The acquisition of face and person identity information following anterior temporal lobectomy. J Int Neuropsychol Soc. 2005;11:237–48. doi: 10.1017/S1355617705050290. [DOI] [PubMed] [Google Scholar]
- Moscovitch M, Nadel L. Consolidation and the hippocampal complex revisited: In defense of the multiple-trace model. Curr Opin Neurobio. 1998;8:297–300. doi: 10.1016/s0959-4388(98)80155-4. [DOI] [PubMed] [Google Scholar]
- Murphy K, Rich J, Troyer A. Verbal fluency patterns in amnestic mild cognitive impairment are characteristic of Alzheimer's type dementia. J Int Neuropsychol Soc. 2006;12:570–4. doi: 10.1017/s1355617706060590. [DOI] [PubMed] [Google Scholar]
- Nadel L, Moscovitch M. Memory consolidation, retrograde amnesia, and the hippocampal complex. Curr Opin Neurobiol. 1997;7:217–227. doi: 10.1016/s0959-4388(97)80010-4. [DOI] [PubMed] [Google Scholar]
- Nielson K, Douville K, Seidenberg M, Woodard J, Miller S, Franczak M, Antuono P, Rao S. Age-related functional recruitment for famous name recognition: An event-related fMRI study. Neurobiology of Aging. 2006;27:1494–1504. doi: 10.1016/j.neurobiolaging.2005.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordlund A, Rolstad S, Hellstrom P, Sjogren M, Hansen S, Wallin A. The Goteborg MCI study: mild cognitive impairment is a heterogeneous condition. J Neurol Neurosurg Psychiatry. 2005;76:1485–90. doi: 10.1136/jnnp.2004.050385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen R, Doody R, Kurz A, Mohs R, Morris J, Rabins P, Ritchie K, Rossor M, Thal L, Winblad B. Current concepts in mild cognitive impairment. Arch Neurol. 2001;58:1985–92. doi: 10.1001/archneur.58.12.1985. [DOI] [PubMed] [Google Scholar]
- Petersen R. Mild cognitive impairment as a diagnostic entity. J Int Med. 2004;256:183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- Piolino P, Lamidey V, Desgranges B, Eustache F. The semantic and episodic subcomponents of famous person knowledge: dissociation in healthy subjects. Neuropsychology. 2007;21:122–35. doi: 10.1037/0894-4105.21.1.122. [DOI] [PubMed] [Google Scholar]
- Ribeiro F, DeMendonca A, Guerrerio M. Mild cognitive impairment: deficits in cognitive domains other than memory. Dement Geriatr Cogn Disord. 2006;21:284–90. doi: 10.1159/000091435. [DOI] [PubMed] [Google Scholar]
- Ribeiro F, Guerreiro M, DeMendonca A. Verbal learning and memory deficits in Mild Cognitive Impairment. J Clin Exp Neuropsychol. 2007;29:187–97. doi: 10.1080/13803390600629775. [DOI] [PubMed] [Google Scholar]
- Sadek J, Johnson S, White D, Salmon D, Taylor K, Delapena J, Paulsen J, Heaton R, Grant I. Retrograde amnesia in dementia: comparison of HIV-associated dementia, Alzheimer's disease, and Huntington's disease. Neuropsychology. 2004;18:692–9. doi: 10.1037/0894-4105.18.4.692. [DOI] [PubMed] [Google Scholar]
- Seidenberg M, Griffith R, Sabsevitz D, Moran M, Haltiner A, Bell B, Swanson S, Hammeke T, Hermann B. Recognition and identification of famous faces in patients with unilateral temporal lobe epilepsy. Neuropsychologia. 2002;40:446–56. doi: 10.1016/s0028-3932(01)00096-3. [DOI] [PubMed] [Google Scholar]
- Semenza C, Borgo F, Mondini S, Pasini M, Sgaramella T. Proper names in the early stages of Alzheimer's Disease. Brain Cognition. 2000;43:384–7. [PubMed] [Google Scholar]
- Small J, Sandhu N. Episodic and semantic memory influences on picture naming in Alzheimer's disease. Brain Lang. 2007 Jan 12; doi: 10.1016/j.bandl.2006.12.002. [DOI] [PubMed] [Google Scholar]
- Squire L, Alvarez P. Retrograde amnesia and memory consolidation: A neurobiological perspective. Curr Opin Neurobio. 1995;5:169–77. doi: 10.1016/0959-4388(95)80023-9. [DOI] [PubMed] [Google Scholar]
- Thompson S, Graham K, Patterson K, Sahakian B, Hodges J. Is knowledge of famous people disproportionately impaired in patients with early and questionable Alzheimer's disease? Neuropsychology. 2002;16:344–58. doi: 10.1037//0894-4105.16.3.344. [DOI] [PubMed] [Google Scholar]
- Twamley E, Ropacki S, Bondi M. Neuropsychological and neuroimaging changes in preclinical Alzheimer's disease. J Int Neuropsych Society. 2006;12:707–35. doi: 10.1017/S1355617706060863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viskontas I, McAndrews M, Moscovitch M. Memory for famous people in patients with unilateral temporal lobe epilepsy and excisions. Neuropsycholgy. 2002;16:472–80. [PubMed] [Google Scholar]
- Vogel A, Gade A, Stokholm J, Waldemar G. Semantic memory impairment in the earliest phase of Alzheimer's disease. Dement Geriatr Cogn Disord. 2005;19:75–81. doi: 10.1159/000082352. [DOI] [PubMed] [Google Scholar]
- Wahlin A, Backman L, Mantyla T, Herlitz A, Viitanen M, Winblad B. Prior knowledge and face recognition in a community-based sample of healthy, very old adults. J Gerontology. 1993;48:54–61. doi: 10.1093/geronj/48.2.p54. [DOI] [PubMed] [Google Scholar]
- Westmacott R, Black S, Freedman M, Moscovitch M. The contribution of autobiographical significance to semantic memory: evidence from Alzheimer's disease, semantic disease, and amnesia. Neuropsychologia. 2004;42:25–48. doi: 10.1016/s0028-3932(03)00147-7. [DOI] [PubMed] [Google Scholar]
- Westmacott R, Moscovitch M. The contribution of autobiographical significance to semantic memory. Mem & Cog. 2003;31:761–74. doi: 10.3758/bf03196114. [DOI] [PubMed] [Google Scholar]
- Woodard J, Seidenberg M, Nielson K, Miller S, Franczak M, Antuono P, Douville K, Rao S. Temporally graded activation of neocortical regions in response to memories of different ages. J Cog Neurosci. 2007;19:1–12. doi: 10.1162/jocn.2007.19.7.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]