Abstract
Background
HOXB4 transcription factor plays an important role in embryonic and adult hematopoiesis. Over-expression of HOXB4 in murine and human embryonic stem cells (ESCs) has been used to generate hematopoietic stem cells (HSCs) via embryoid body formation method.
Methods
We used FuGENE-6 based transfection of YPL2-HOXB4 vector to generate HOXB4-expressing colonies from human ESC line H9 and investigated the differentiation potential of these cells into primitive CD34+ hematopoietic cells, via co-culture methodology with OP9 murine bone marrow stromal cells. Expression of HOXB4 in transfected human ESC colonies and their derivatives was verified using immunocytochemistry and RT-PCR.
Results
Utilizing OP9 stromal cell co-culture methodology, we generated CD34+ cells from HOXB4-expressing H9 human ESCs at a frequency similar to, and not higher than, non-transfected human ESCs. However, we observed that some colonies of HOXB4-expressing human ESCs, not co-cultured on OP9 cells, differentiated into mesenchymal stromal/stem cells (MSCs) while preserving their HOXB4 expression. These HOXB4-expressing MSCs expressed CD29, CD73, CD44, CD90, CD105, and HLA-class I; were negative for the expression of CD34, CD45, CD54, CD71, CD106 and HLA DR; and could be differentiated into adipocytes and osteocytes.
Discussion
In our specific experimental system we observed that over-expression of HOXB4 in human ESCs did not improve the generation of CD34+ hematopoietic cells via OP9 co-culture methodology. Furthermore, we could generate MSCs from human ESCs over-expressing HOXB4.
Keywords: Human embryonic stem cells, Mesenchymal stromal/stem cells, HOXB4
INTRODUCTION
Human embryonic stem cells (ESCs) have the potential to revolutionize medicine by providing cellular therapeutic options for a wide variety of human diseases such as Parkinson’s and Alzheimer’s disease, diabetes, and hematological disorders [1]. Generation of both hematopoietic stem cells (HSCs) and more lineage restricted progenitor cells from ESCs has been studied extensively from both mouse and human ESCs. ESCs grown in a suspension culture without feeder cells spontaneously form complex cell aggregates called embryoid bodies (EBs), which contain components of all three germ layers [2]. Doetschman et al. were the first to note that EBs which develop from murine ESCs contain hematopoietic cells [3]. This method has been widely used to generate primitive hematopoietic cells from murine [4–6] or human ESCs [7, 8]. Alternatively, ESCs can undergo hematopoietic differentiation if they are grown in the presence of some type of hematopoietic supportive stromal cells [9]. This method has also been investigated extensively for the generation of hematopoietic cells from murine [10] or human ESCs [11–13], utilizing a variety of supportive stromal cells.
Nevertheless, until recently it was difficult to demonstrate robust in vivo hematopoiesis after the transplantation of primitive hematopoietic cells derived from ESCs in vitro using either the EB or stromal cell co-culture methodology. However, when Kyba et al. transduced day 6 murine EB-derived cells with the HOXB4 gene and then co-cultured them with OP9 murine bone marrow stromal cells, primitive hematopoietic cells expressing cell surface markers such as c-kit were generated [14]. Importantly, transplantation of these cells into mice resulted in hematopoietic reconstitution in both primary and secondary recipients indicating their long-term engraftment potential and thus their identity as HSCs. Since this seminal report the potential role of over-expression of HOXB4 in promoting generation of HSCs form murine or human ESCs have been extensively investigated, using a wide variety of methodologies and experimental conditions. Most of these studies involve a component of EB formation step to generate hematopoietic cells [15–19]. We initiated our current study to investigate the differentiation potential of human ESCs stably expressing HOXB4 utilizing the co-culture methodology with OP murine stromal cells instead of EB methodology, as this specific approach has not been reported yet.
METHODS
Human ESC culture
The human ESC line H9 was obtained from WiCell (Madison, WI). The cells were originally maintained in the undifferentiated state by culturing on irradiated murine embryonic fibroblasts (MEF). Prior to transfection cells were maintained in MEF- conditioned media (CM) on matrigel coated plates (BD Biosciences, San Jose, CA), for few passages to make sure no MEF cells left in culture, as described previously [20].
Construction of plasmids
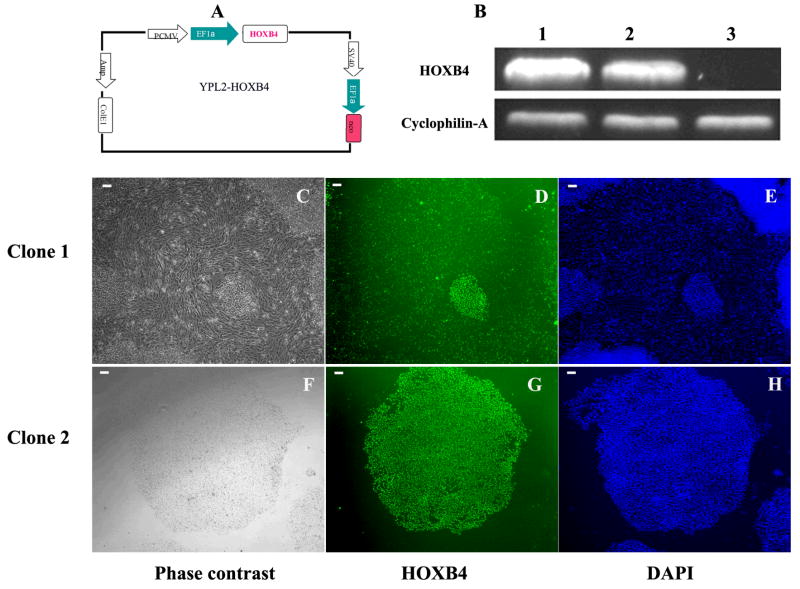
HOXB4 gene was amplified from pTAT-HA-HOXB4 [21], kindly provided by Dr. Guy Sauvageau [22], with EcoR I and Xba I sites, and its sequence was confirmed. HOXB4 cDNA was then inserted into YPL2 vector [20], with EcoRI and XbaI, and designated YPL2-HOXB4 (Figure-1A). In this vector, there are two EF1-alpha promoters, one drives HOXB4 and the other drives Neomycin resistance gene expression (Figure-1-A). For the control transfection we used YPL2-GFP.
Figure 1.
A: HOXB4-expressing plasmid, B: RT-PCR using HOXB4 specific primers on RNA isolated from the 2 different colonies of HOXB4 transfected H9 ESC (column 1 from clone#1 and column 2 from clone#2), and from non-transfected H9 human ESCs (column 3). Cyclophilin-A was used as a house keeping control gene. Two colonies generated from HOXB4 plasmid transfection in H9 ESCs after 2 weeks of G-418 selection. Clone#1 showed mesenchymal cell morphology (C) but clone# 2 remained undifferentiated (F). Both clones showed HOXB4 expression in the cells (D, G). E and H are nuclei staining with DAPI. Scale bar = 100 μm.
Transfection
Plasmid transfection was performed as described before [20] with some modification. Briefly, 10 μg/well of YPL2-HOXB4 plasmid and 15 μl/well of FuGENE 6 (Roche Applied Science, Indianapolis, IN) were mixed in 100 μl OptiMEM medium, and incubated at room temperature for 30 minutes. Following incubation, the plasmid/FuGENE 6 mixture was added to human ESCs cultured in 6-well plates containing CM. Twenty-four hours after transfection, the media was changed to 2.5 mL of fresh CM containing 100 μg/mL of G418 (Invitrogen, Carlsbad, CA). After two weeks of G418 selection, the remaining colonies, undifferentiated or partially differentiated, were individually transferred to 4-well plates, each well containing one colony. One week later, cells from each well were distributed to 4 wells of a new 4 well-plate. From each of these secondary 4-well plates one well was immunostained with HOXB4 antibody (Developmental Studies Hybridoma Bank, Iowa City, IA) to identify HOXB4 positive colonies under microscope. Colonies that remained undifferentiated were used for co-culture experiments with OP9 cells but for colonies with mesenchymal looking cells further culturing was done using MSC media (α-MEM containing 20% FBS).
Co-culture with OP9 cell
HOXB4+ human ESCs were co-cultured with OP9 stromal cells by plating undifferentiated HOXB4-expressing human ESC colonies at a density of 1 × 105 cells per mL onto six-well plates containing a confluent monolayer of OP9 cells, previously irradiated with 80 Gy, and differentiation medium containing α-modified minimum essential medium (α-MEM) with 10% fetal bovine serum (Hyclone Laboratories, Logan, UT), 0.1 nonessential amino acids, 2 mM L-glutamine, and 0.1 mM β-mercaptoethanol. Co-cultured cells were then incubated at 37° centigrade (C)/5% CO2 with half medium changes on days 4, 6, 8, 10, 12 and 14.
Immunostaining
All of the immunocytochemistry analyses were performed after fixation in 4% paraformaldehyde for 30 minutes, rinsing twice with phosphate-buffered saline (PBS), and incubation with 20% goat blocking serum in 4% Triton/PBS for 2 hours at room temperature. All primary antibodies, at 4 μg/mL in 4% Triton/PBS, were applied overnight at 4°C. The primary antibodies used for immunostaining included: anti-Oct4 (Santa Cruz Biotechnology, Santa Cruz, CA), and anti-HOXB4 (Developmental Studies Hybridoma Bank). For negative controls, we used the same concentration of the primary antibodies of mouse IgG isotype (Caltag, Burlingame, CA). After washing the cells three times with PBS, they were incubated with fluorescence-conjugated secondary goat anti-mouse antibody (Santa Cruz Biotechnology) at a 1:50 dilution.
Flow cytometry
The cells were dissociated with trypsin (0.25%) plus EDTA (1 mM), washed twice in PBS containing 0.1% bovine serum albumin (BSA), and suspended at a concentration of 6 × 105 cells/mL. Five μL of Phycoerythrin (PE) or Allophycocyanin (APC) conjugated antibodies were added to each 0.1 mL volume of the cells, incubated for 30 minutes at 4 °C in dark, washed twice in PBS containing 0.1% BSA, and then immediately analyzed on a FACSCalibur flow cytometer (BD Biosciences, San Jose, Ca) with FlowJo acquisition and analysis software (Tree Star, Inc., San Carlos, CA). The antibodies used for detection of cell surface markers by FACS included: BD Biosciences antibodies: CD29 PE (#557332), CD34 PE (# 550619), CD44 PE (# 550989), CD73 PE (# 550257), CD90 APC (#559869), CD54 PE (#555511), CD106 PE (#555647), HLA-DR PE (#347363); eBiosciences (San Diego, Ca) antibodies: CD45 PE (clone HI30) and CD105 APC (Clone SN6); Santa Cruz antibody: CD71 PE (#SC7327); and Sigma antibody: HLA Class-I FITC (Clone W6/32). In each experiment control staining with the appropriate isotype monoclonal antibodies was included.
Reverse transcribed-polymerase chain reaction (RT-PCR)
The total RNA was extracted from 1 million cells with TRIZOL reagent using the manufacturer’s recommendations (Invitrogen). One μg of total RNA was reverse transcribed using M-MuLV Reverse Transcriptase (Roche, Indianapolis, IN). PCR mixtures were prepared as described (Promega protocol for Taq polymerase, Madison, WI), denatured at 94 °C for 2 minutes, followed by 30 cycles of 94 °C for 30 seconds, 55 °C for 30 seconds, and 72 °C for 60 seconds. A final extension at 72 °C for 10 minutes was performed after cycling. Primer sequences used included: HOXB4 sense: GAATTCAATGGCTATGAGTTCT, HOXB4 antisense TCTAGACTAGAGCGCGCGGGG; bone specific alkaline Phosphatase (ALP) sense TGGAGCTTCAGAAGCTCAACACAA, ALP antisense ATCTCGTTGTCTGAGTACCAGTCC; Bone Sialoprotein (BSP) sense AATGAAAACGAAGAAAGCGAAG, BSP antisense ATCATAGCCATCGTAGCCTTGT; Peroxisome Proliferator Activated Receptor-γ (PPAR-γ) sense CTCCTATTGACCCAGAAAGC, PPAR-γ antisense GTAGAGCTGAGTTCTTCTCAG; Cyclophilin-A sense: CCGAGGAAAACCGGTACTAT, Cyclophilin-A antisense AGATTCTAGGATACTGCGAGCA.
Adipogenic Differentiation
Human ESC-derived MSCs were induced into adipocyte differentiation by exposure to 0.25 μM dexamethasone, 10 μg/mL insulin, and 0.5 mM isobutylxanthine (Sigma) in high glucose DMEM medium containing 10% FBS for 2–4 weeks [23]. Adipocyte-specific granules were detected via Red Oil O staining (Cambrex Bio Science, Walkersville, MD) according to the manufactory protocol. Cells were viewed with a phase contrast microscope; lipids appeared red and nuclei appeared blue. Differentiation of adipocytes was further confirmed using RT-PCR to detect expression of PPAR-γ.
Osteogenic Differentiation
Human ESC-derived MSCs were plated at low density (1–2.5 × 103 cells/cm2) on tissue-culture treated dishes in the presence of 10 mM β-glycerol phosphate (Sigma), 0.1 μM dexamethasone, and 200 μM ascorbic acid (Sigma) in alpha MEM medium containing 10% FBS for 3–4 weeks [23]. Osteogenesis was demonstrated by Von Kossa staining using silver stain and counterstaining with nuclear fast red, and then viewed on a phase contrast microscope. Osteogenesis was further confirmed by RT-PCR to detect bone specific ALP and BSP gene expression.
RESULTS
Generation of different colonies of HOXB4-expressing human ESCs
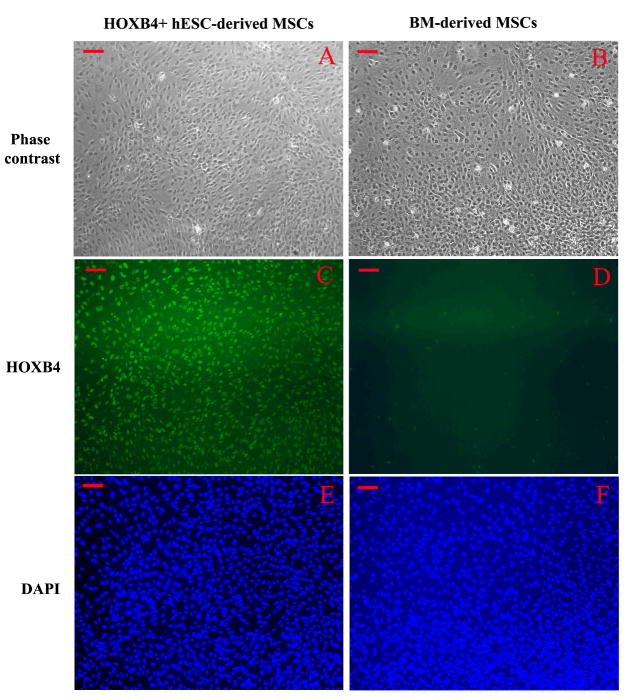
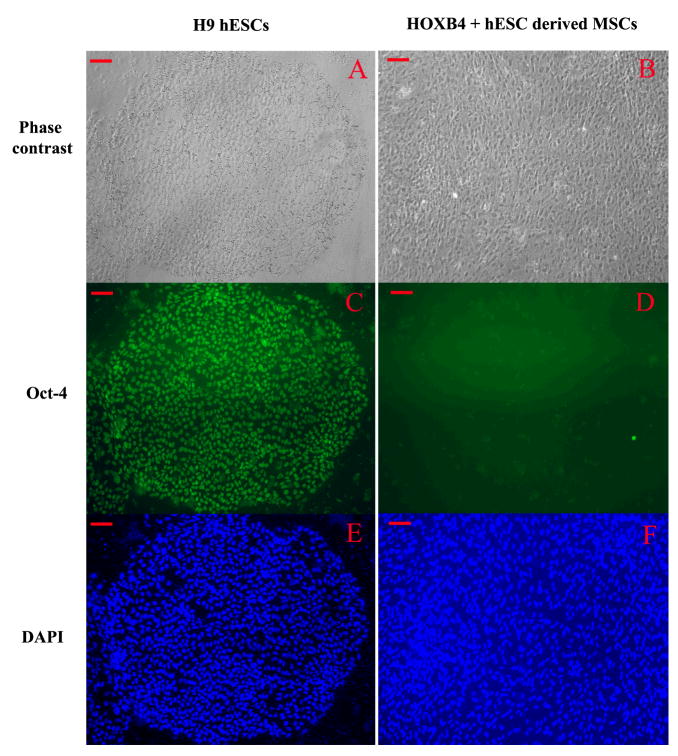
We used FuGENE 6-based transfection and our YPL2 vector to generate human ESC lines over-expressing human HOXB4 cDNA. Two different types of colonies appeared after YPL2-HOXB4 transfection and during G418 selection period. Colonies like colony#1 (Figure-1-C) showed differentiation towards mesenchymal looking cells, at the periphery of the colonies, similar to what we reported previously for non-transfected human ESC cultures [24, 25]. Colonies like Colony#2 maintained morphology of undifferentiated human ESC colonies (Figure-1-F). Both colonies expressed HOXB4 in RNA level as shown by RT-PCR (Figure-1-B) and protein level as shown by immunocytochemistry (Figure 1-D and 1-G). After the two-week selection period colonies like colony#1 were singly transferred into new 4-well plates and upon further passaging differentiation process continued, without any specific intervention, until all cells in the colony appeared to have mesenchymal looking morphology. All cells differentiated from this type of colony remained positive for HOXB4 as shown by immunocytochemistry (Figure-2-C). We did not find evidence of HOXB4 expression in BM-derived MSCs by immunocytochemistry (Figure-2-D). On the other hand, undifferentiated human ESCs (Figure-3-C) continue to express Oct-4, a marker of undifferentiated human ESCs [26]; however, in contrast, MSCs derived from HOXB4 expressing human ESCs were no longer expressing Oct-4 (Figure-3-D). These data provide evidence that MSCs derived from HOXB4-expressing human ESCs continue to express high levels of HOXB4 transcription factor but have lost the expression of markers of undifferentiated ESCs.
Figure 2.
Comparison of HOXB4 immunostaining in mesenchymal stromal cells (MSCs) differentiated from HOXB4+ human embryonic stem cells (ESCs) (A, C, E) and bone marrow (BM)-derived MSCs (B, D, F). BM-derived MSCs do not express HOXB4. A: Phase contrast view of human ESC-derived MSCs; B: Phase contrast view of BM-derived MSCs; C: HOXB4 antibody staining of MSCs derived from HOXB4+ human ESCs, and D: HOXB4 antibody staining of BM-derived MSCs; E and F: DAPI staining of cell nuclei. Scale bar = 100 μm.
Figure 3.
A: Phase contrast view of a colony of H9 human embryonic stem cells (ESCs) and B: a colony of mesenchymal stromal cells differentiating from HOXB4+ human ESCs; C and D: OCT4-specific antibody staining of H9 human ESCs and MSCs from HOXB4+ human ESCs, respectively; E and F: DAPI staining of nuclei for the same field. Scale bar = 100 μm.
Generation of CD34+ cell by co-culturing undifferentiated HOXB4-expressing human ESCs with OP9 cells
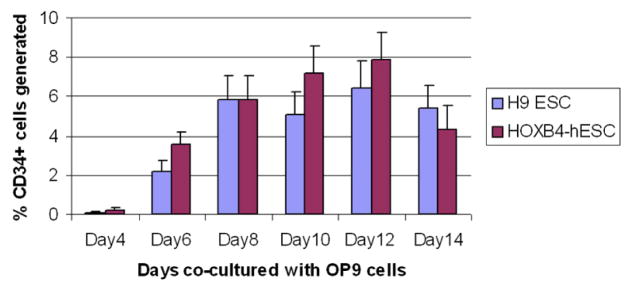
We co-cultured undifferentiated HOXB4-expressing human ESC colonies, similar to colony#2, or non-transfected H9 ESCs with OP9 cells for 2 weeks. Single-cell suspensions from day 4, 6, 8, 10, 12, and 14 of co-cultures were incubated with PE-CD34 antibodies and percentages of CD34+ were analyzed with flow cytometry. In three sets of experiments, simultaneously comparing HOXB4-expessing human ESCs and non-transfected human ESCs, we could not detect any statistically significant difference in the percentage of CD34+ cells generated at any time point during the co-culture period (Figure-4).
Figure 4.
Flow cytometric analysis of HOXB4+ human ESCs co-cultured with OP9 cells. Over-expression of HOXB4 in human ESCs did not cause any statistically significant differences in CD34+ cell generation.
Cells differentiated from HOXB4-expressing human ESCs without OP co-culture express cell surface markers of MSCs
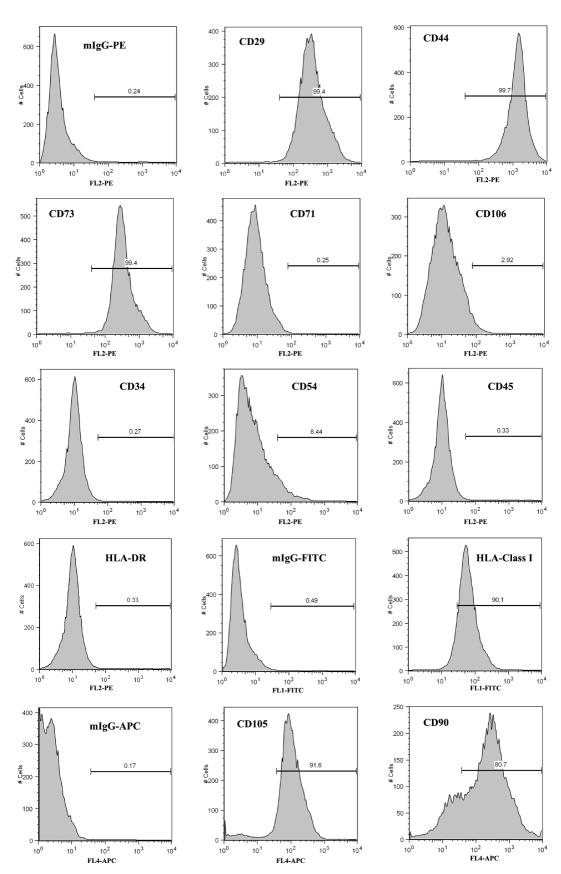
We used CD73+, a well known marker for MSCs [27], as a screening marker to follow the phenotype of mesenchymal looking cells in our differentiation cultures. Two weeks after initiation of transfection of human ESCs, and when they were maintained in CM with 100 μg/mL G418, 36% of the total cells expressed CD73 on their cell surfaces as detected by flow cytometry. Upon three further passages and culture of the differentiating colonies in MSC media (α-MEM containing 20% FBS) all cells assumed a homogenous morphology. At this time the cells were positive for CD29, CD44, CD73, CD90, and CD105 cell surface markers, consistent with a MSC phenotype, but did not express hematopoietic markers such as CD34, CD45, or CD71 (Figure-5). Furthermore, these cells were positive for HLA class I, but negative for HLA-DR. Interestingly, our cells did not express CD106 marker. As we and others have already reported [24, 25, 28, 29], our human ESC-derived MSCs showed slower proliferation after 12–15 passages; however, at these later passages they were still positive for MSC markers such as CD73 and CD105 (data not shown)
Figure 5.
Flow cytometric analysis of MSCs differentiated from HOXB4+ human ESCs. MSCs differentiated from HOXB4+ human ESCs express CD29, CD44, CD73 CD90 and CD105, and are positive for HLA-Class I (ABC); they do not express CD34, CD45, CD71, and HLA-DR.
MSCs generated from HOXB4-expressing human ESCs differentiate into adipocytes and osteoblasts
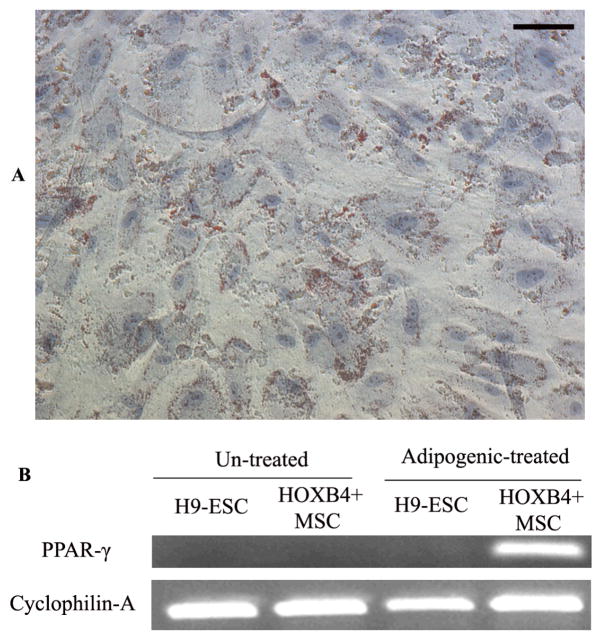
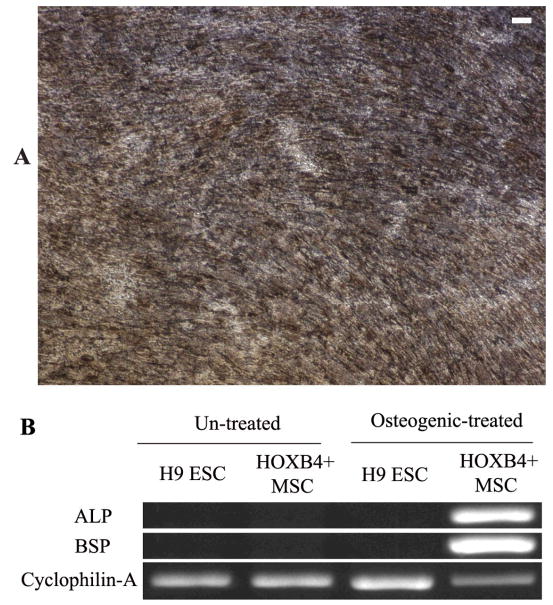
We then tested the differentiation potential of MSCs generated from HOXB4-expressing human ESCs into adipocytes when they were cultured in the adipogenic media for 4 weeks. The characteristic fat globules were first observed at 2 weeks after incubation, as visualized with Oil Red O staining, and after 4 weeks of induction about 30% of the cells were positive for the fat globules (Figure-6-A). In addition, the induced cells were positive for adipocyte marker PPAR-γ by RT-PCR (Figure-6-B). When MSCs generated from HOXB4-expressing human ESCs were cultured in osteogenic media for 4 weeks, at day 28, the majority of cells showed calcium crystal (Brown-black) deposits (Figure-7-A). These cells were also shown to be positive for ALP and BSP, osteogenic specific, genes by RT-PCR (Figure-7-B).
Figure 6.
Adipogenic differentiation of MSCs differentiated from HOXB4+ human ESCs when cultured in the adipogenic media. The adipocytes were characterized by accumulation of lipid (red) vacuoles inside the cells (Oil Red O staining) (A), and further verified by RT-PCR for Peroxisome Proliferator Activated Receptor-γ (PPAR-γ) gene (B).
Figure 7.
Osteogenic differentiation of mesenchymal stromal cells derived from HOXB4+ human embryonic stem cells (ESCs) in the osteogenic media. Osteoblast generation is indicated by presence of Ca+ crystals shown as dark brown spots (von Kossa staining) (A), and verified by RT-PCR for expression of bone-specific alkaline Phosphatase (ALP) and bone sialoprotein (BSP).
DISCUSSION
HOX genes are a highly conserved family of 39 genes, organized in 4 genomic clusters (A, B, C, and D), encoding transcription factor proteins that play an essential role in cell fate determination in lower and higher organisms [30]. Gene over-expression experiments have demonstrated the particular role of HOXB4 in enhancing the self-renewal of adult HSCs [31, 32], and its key role in embryonic hematopoiesis as well [33]. However, despite the prominent role of HOXB4 gene in hematopoiesis, generation of different types of blood cells is a very complex process involving many other HOX or non-HOX genes [34–36]. Brun et al. showed that a mouse model lacking the entire HOXB4 gene exhibits a significantly only a mild reduction in the numbers of primitive progenitors and stem cells in adult BM and fetal liver [37]. Furthermore, they showed that HOXB4 is not required for the generation of HSCs or maintenance of steady state hematopoiesis. More recently, Bijl et al. demonstrated HOXB4 (−/−) and HOXB1-B9 (−/−) fetal liver cells have full competitive repopulation potential and can regenerate all myeloid and lymphoid lineages [38].
Although we originally assumed over-expression HOXB4 in human ESCs would increase the efficiency of human ESC differentiation to CD34+ hematopoietic cells, we did not see increased numbers of CD34+ cells using the methodology of co-culturing with OP9 stromal cells. The contrast between our studies and others which have shown improved generation of hematopoietic cells from HOXB4 over-expressing ESCs could have several explanations. Kyba et al. and Pilat et al. used murine, and not human, ESCs [14, 15] to show generation of long term engrafting HSCs from HOXB4-transduced ESC cells. Bowles et al. showed that over-expression of HOXB4 in human ESCs using lipofection method augments their in vitro differentiation of them into hematopoietic cells via EB method [16]. Chan et al. also used EB methodology to generate engraftable primitive hematopoietic cells from human ESCs [17]. To our knowledge there is no report on the generation of HSCs from HOXB4+ human ESCs through OP9 co-culture methodology without going through EB formation step. Thus, our results could be explained by unknown mechanisms in which EB formation is necessary to direct the generation of hematopoietic cells from HOXB-expressing human ESCs. However, we can not exclude the possibility that other factors such as differences in the level of expression of HOXB4, as suggested by Unger et al. [39], or other variables specific to our experimental methodology could have contributed to this observation.
Interestingly, we observed some of our human ESC colonies over-expressing HOXB4 during our selection process and before their transfer onto OP9 cells started to differentiate into cells with MSC morphology; and upon further passaging and using MSC specific culture conditions all differentiated cells expressed cell surface markers typical of a MSC phenotype [27]. However, in contrast to bone marrow derived MSCs our HOXB4+ human ESC-derived MSCs did not express CD106. Nevertheless, MSCs derived from human adipose tissue [40], umbilical vein [41], and amnion [42] have also been reported to lack expression of CD106. Since MSCs differentiated from our HOXB4+ human ESCs continues to express HOXB4, both at the RNA and protein levels, we believe generation of MSCs was not due to silencing of HOXB4 trans-gene. However, since the majority of HOXB+ human ESC colonies did not differentiate into MSCs it is quite possible that expression of HOXB4 had no direct role in generation of MSCs and was just an “innocent bystander”. Nevertheless, a recent study by Karner et al. showed that forced high level expression of HOXB4 by a lentiviral vector promoted osteogenesis and caused reduction of the number of CD34+ cells, while low level of HOXB4 expression caused a higher number of CD34+ cells generated from human ESCs [43]. Since osteogenic cells are progenies of MSCs, this latter study corroborates our assumption that different levels of expression of HOXB4 under different conditions could activate different cellular differentiation pathways.
Finally, generation of fibroblast/mesenchymal looking cells at the periphery of human ESC colonies has been reported as a frequent phenomenon in ESC cultures. Original investigators showed that such cells were able to support growth of human ESCs and possessed some characteristics of mesenchymal cells [44–46]. Recently, many investigators, including our group, have been able to generate pure populations of fibroblast/mesenchymal looking cells from human ESCs with or without co-culturing with murine stromal cells; and show these cells have characteristics very similar to those reported for MSCs derived from bone marrow and other sources [24, 25, 28, 29]. Thus, another working hypothesis of ours is that generation of mesenchymal cells could be the default pathway of differentiation of human ESCs. Such a differentiation process is reminiscent of the epithelial to mesenchymal transition, a ubiquitous process that plays a significant role, not only in the early stages of healthy embryonic development, but also in many disease processes including tissue reconstruction and carcinogenesis [47, 48]. We propose it is possible that the inherent propensity of ESCs to differentiate into MSCs might have played a role in preserving this differentiation potential even in the presence of over-expression of HOXB4.
Acknowledgments
This work was supported by grants from National Blood Foundation, Stem Cell Research Foundation, and NIH/NHLBI HL081076 K08 awards to Peiman Hematti.
References
- 1.Odorico JS, Kaufman DS, Thomson JA. Multilineage differentiation from human embryonic stem cell lines. Stem Cells. 2001;19(3):193–204. doi: 10.1634/stemcells.19-3-193. [DOI] [PubMed] [Google Scholar]
- 2.Smith AG. Embryo-derived stem cells: of mice and men. Annu Rev Cell Dev Biol. 2001;17:435–62. doi: 10.1146/annurev.cellbio.17.1.435. [DOI] [PubMed] [Google Scholar]
- 3.Doetschman TC, Eistetter H, Katz M, Schmidt W, Kemler R. The in vitro development of blastocyst-derived embryonic stem cell lines: formation of visceral yolk sac, blood islands and myocardium. Journal of embryology and experimental morphology. 1985 Jun;87:27–45. [PubMed] [Google Scholar]
- 4.Keller G, Kennedy M, Papayannopoulou T, Wiles MV. Hematopoietic commitment during embryonic stem cell differentiation in culture. Molecular and cellular biology. 1993 Jan;13(1):473–86. doi: 10.1128/mcb.13.1.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dang SM, Kyba M, Perlingeiro R, Daley GQ, Zandstra PW. Efficiency of embryoid body formation and hematopoietic development from embryonic stem cells in different culture systems. Biotechnology and bioengineering. 2002 May 20;78(4):442–53. doi: 10.1002/bit.10220. [DOI] [PubMed] [Google Scholar]
- 6.Choi K, Chung YS, Zhang WJ. Hematopoietic and endothelial development of mouse embryonic stem cells in culture. Methods in molecular medicine. 2005;105:359–68. doi: 10.1385/1-59259-826-9:359. [DOI] [PubMed] [Google Scholar]
- 7.Chadwick K, Wang L, Li L, Menendez P, Murdoch B, Rouleau A, et al. Cytokines and BMP-4 promote hematopoietic differentiation of human embryonic stem cells. Blood. 2003 Aug 1;102(3):906–15. doi: 10.1182/blood-2003-03-0832. [DOI] [PubMed] [Google Scholar]
- 8.Ng ES, Davis RP, Azzola L, Stanley EG, Elefanty AG. Forced aggregation of defined numbers of human embryonic stem cells into embryoid bodies fosters robust, reproducible hematopoietic differentiation. Blood. 2005;106(5):1601–3. doi: 10.1182/blood-2005-03-0987. [DOI] [PubMed] [Google Scholar]
- 9.Olsen AL, Stachura DL, Weiss MJ. Designer blood: creating hematopoietic lineages from embryonic stem cells. Blood. 2005 doi: 10.1182/blood-2005-09-3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nakano T, Kodama H, Honjo T. Generation of lymphohematopoietic cells from embryonic stem cells in culture. Science. 1994;265(5175):1098–101. doi: 10.1126/science.8066449. [DOI] [PubMed] [Google Scholar]
- 11.Kaufman DS, Hanson ET, Lewis RL, Auerbach R, Thomson JA. Hematopoietic colony-forming cells derived from human embryonic stem cells. ProcNatlAcadSciUSA. 2001;98(19):10716–21. doi: 10.1073/pnas.191362598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Slukvin II, Vodyanik MA, Thomson JA, Gumenyuk ME, Choi KD. Directed Differentiation of Human Embryonic Stem Cells into Functional Dendritic Cells through the Myeloid Pathway. JImmunol. 2006;176(5):2924–32. doi: 10.4049/jimmunol.176.5.2924. [DOI] [PubMed] [Google Scholar]
- 13.Qiu C, Hanson E, Olivier E, Inada M, Kaufman DS, Gupta S, et al. Differentiation of human embryonic stem cells into hematopoietic cells by coculture with human fetal liver cells recapitulates the globin switch that occurs early in development. Experimental hematology. 2005 Dec;33(12):1450–8. doi: 10.1016/j.exphem.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 14.Kyba M, Perlingeiro RC, Daley GQ. HoxB4 confers definitive lymphoid-myeloid engraftment potential on embryonic stem cell and yolk sac hematopoietic progenitors. Cell. 2002;109(1):29–37. doi: 10.1016/s0092-8674(02)00680-3. [DOI] [PubMed] [Google Scholar]
- 15.Pilat S, Carotta S, Schiedlmeier B, Kamino K, Mairhofer A, Will E, et al. HOXB4 enforces equivalent fates of ES-cell-derived and adult hematopoietic cells. Proceedings of the National Academy of Sciences of the United States of America. 2005 Aug 23;102(34):12101–6. doi: 10.1073/pnas.0505624102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bowles KM, Vallier L, Smith JR, Alexander MR, Pedersen RA. HOXB4 overexpression promotes hematopoietic development by human embryonic stem cells. Stem Cells. 2006 May;24(5):1359–69. doi: 10.1634/stemcells.2005-0210. [DOI] [PubMed] [Google Scholar]
- 17.Chan KM, Bonde S, Klump H, Zavazava N. Hematopoiesis and immunity of HOXB4-transduced embryonic stem cell-derived hematopoietic progenitor cells. Blood. 2008 Mar 15;111(6):2953–61. doi: 10.1182/blood-2007-10-117366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu SJ, Feng Q, Ivanova Y, Luo C, Li T, Li F, et al. Recombinant HoxB4 fusion proteins enhance hematopoietic differentiation of human embryonic stem cells. Stem cells and development. 2007 Aug;16(4):547–59. doi: 10.1089/scd.2007.0002. [DOI] [PubMed] [Google Scholar]
- 19.Ji J, Vijayaragavan K, Bosse M, Menendez P, Weisel K, Bhatia M. OP9 stroma augments survival of hematopoietic precursors and progenitors during hematopoietic differentiation from human embryonic stem cells. Stem Cells. 2008 Oct;26(10):2485–95. doi: 10.1634/stemcells.2008-0642. [DOI] [PubMed] [Google Scholar]
- 20.Liu YP, Dovzhenko OV, Garthwaite MA, Dambaeva SV, Durning M, Pollastrini LM, et al. Maintenance of pluripotency in human embryonic stem cells stably over-expressing enhanced green fluorescent protein. Stem cells and development. 2004 Dec;13(6):636–45. doi: 10.1089/scd.2004.13.636. [DOI] [PubMed] [Google Scholar]
- 21.Krosl J, Austin P, Beslu N, Kroon E, Humphries RK, Sauvageau G. In vitro expansion of hematopoietic stem cells by recombinant TAT-HOXB4 protein. NatMed. 2003;9(11):1428–32. doi: 10.1038/nm951. [DOI] [PubMed] [Google Scholar]
- 22.Kroon E, Krosl J, Thorsteinsdottir U, Baban S, Buchberg AM, Sauvageau G. Hoxa9 transforms primary bone marrow cells through specific collaboration with Meis1a but not Pbx1b. EMBO J. 1998;17(13):3714–25. doi: 10.1093/emboj/17.13.3714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143–7. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 24.Trivedi P, Hematti P. Simultaneous generation of CD34(+) primitive hematopoietic cells and CD73(+) mesenchymal stem cells from human embryonic stem cells cocultured with murine OP9 stromal cells. Experimental hematology. 2007 Jan;35(1):146–54. doi: 10.1016/j.exphem.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 25.Trivedi P, Hematti P. Derivation and immunological characterization of mesenchymal stromal cells from human embryonic stem cells. Experimental hematology. 2008 Mar;36(3):350–9. doi: 10.1016/j.exphem.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Adewumi O, Aflatoonian B, Ahrlund-Richter L, Amit M, Andrews PW, Beighton G, et al. Characterization of human embryonic stem cell lines by the International Stem Cell Initiative. Nature biotechnology. 2007 Jul;25(7):803–16. doi: 10.1038/nbt1318. [DOI] [PubMed] [Google Scholar]
- 27.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–7. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 28.Olivier EN, Rybicki AC, Bouhassira EE. Differentiation of human embryonic stem cells into bipotent mesenchymal stem cells. Stem Cells. 2006 Aug;24(8):1914–22. doi: 10.1634/stemcells.2005-0648. [DOI] [PubMed] [Google Scholar]
- 29.Lian Q, Lye E, Suan Yeo K, Khia Way Tan E, Salto-Tellez M, Liu TM, et al. Derivation of clinically compliant MSCs from CD105+, CD24- differentiated human ESCs. Stem Cells. 2007 Feb;25(2):425–36. doi: 10.1634/stemcells.2006-0420. [DOI] [PubMed] [Google Scholar]
- 30.McGinnis W, Krumlauf R. Homeobox genes and axial patterning. Cell. 1992 Jan 24;68(2):283–302. doi: 10.1016/0092-8674(92)90471-n. [DOI] [PubMed] [Google Scholar]
- 31.Sauvageau G, Thorsteinsdottir U, Eaves CJ, Lawrence HJ, Largman C, Lansdorp PM, et al. Overexpression of HOXB4 in hematopoietic cells causes the selective expansion of more primitive populations in vitro and in vivo. Genes & development. 1995 Jul 15;9(14):1753–65. doi: 10.1101/gad.9.14.1753. [DOI] [PubMed] [Google Scholar]
- 32.Thorsteinsdottir U, Sauvageau G, Humphries RK. Enhanced in vivo regenerative potential of HOXB4-transduced hematopoietic stem cells with regulation of their pool size. Blood. 1999 Oct 15;94(8):2605–12. [PubMed] [Google Scholar]
- 33.Helgason CD, Sauvageau G, Lawrence HJ, Largman C, Humphries RK. Overexpression of HOXB4 enhances the hematopoietic potential of embryonic stem cells differentiated in vitro. Blood. 1996 Apr 1;87(7):2740–9. [PubMed] [Google Scholar]
- 34.Magnusson M, Brun AC, Lawrence HJ, Karlsson S. Hoxa9/hoxb3/hoxb4 compound null mice display severe hematopoietic defects. Experimental hematology. 2007 Sep;35(9):1421–8. doi: 10.1016/j.exphem.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 35.Kubo A, Chen V, Kennedy M, Zahradka E, Daley GQ, Keller G. The homeobox gene HEX regulates proliferation and differentiation of hemangioblasts and endothelial cells during ES cell differentiation. Blood. 2005 Jun 15;105(12):4590–7. doi: 10.1182/blood-2004-10-4137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang Y, Yabuuchi A, McKinney-Freeman S, Ducharme DM, Ray MK, Chawengsaksophak K, et al. Cdx gene deficiency compromises embryonic hematopoiesis in the mouse. Proceedings of the National Academy of Sciences of the United States of America. 2008 Jun 3;105(22):7756–61. doi: 10.1073/pnas.0708951105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brun AC, Bjornsson JM, Magnusson M, Larsson N, Leveen P, Ehinger M, et al. Hoxb4-deficient mice undergo normal hematopoietic development but exhibit a mild proliferation defect in hematopoietic stem cells. Blood. 2004 Jun 1;103(11):4126–33. doi: 10.1182/blood-2003-10-3557. [DOI] [PubMed] [Google Scholar]
- 38.Bijl J, Thompson A, Ramirez-Solis R, Krosl J, Grier DG, Lawrence HJ, et al. Analysis of HSC activity and compensatory Hox gene expression profile in Hoxb cluster mutant fetal liver cells. Blood. 2006 Jul 1;108(1):116–22. doi: 10.1182/blood-2005-06-2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Unger C, Karner E, Treschow A, Stellan B, Felldin U, Concha H, et al. Lentiviral-mediated HoxB4 expression in human embryonic stem cells initiates early hematopoiesis in a dose-dependent manner but does not promote myeloid differentiation. Stem Cells. 2008 Oct;26(10):2455–66. doi: 10.1634/stemcells.2007-0876. [DOI] [PubMed] [Google Scholar]
- 40.Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001 Apr;7(2):211–28. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 41.Covas DT, Siufi JL, Silva AR, Orellana MD. Isolation and culture of umbilical vein mesenchymal stem cells. Brazilian journal of medical and biological research = Revista brasileira de pesquisas medicas e biologicas/Sociedade Brasileira de Biofisica [et al. 2003 Sep;36(9):1179–83. doi: 10.1590/s0100-879x2003000900006. [DOI] [PubMed] [Google Scholar]
- 42.Moon JH, Lee JR, Jee BC, Suh CS, Kim SH, Lim HJ, et al. Successful vitrification of human amnion-derived mesenchymal stem cells. Human reproduction (Oxford, England) 2008 Aug;23(8):1760–70. doi: 10.1093/humrep/den202. [DOI] [PubMed] [Google Scholar]
- 43.Karner E, Unger C, Cerny R, Ahrlund-Richter L, Ganss B, Dilber MS, et al. Differentiation of human embryonic stem cells into osteogenic or hematopoietic lineages: a dose-dependent effect of osterix over-expression. J Cell Physiol. 2009 Feb;218(2):323–33. doi: 10.1002/jcp.21605. [DOI] [PubMed] [Google Scholar]
- 44.Xu C, Jiang J, Sottile V, McWhir J, Lebkowski J, Carpenter MK. Immortalized fibroblast-like cells derived from human embryonic stem cells support undifferentiated cell growth. Stem Cells. 2004;22(6):972–80. doi: 10.1634/stemcells.22-6-972. [DOI] [PubMed] [Google Scholar]
- 45.Stojkovic P, Lako M, Stewart R, Przyborski S, Armstrong L, Evans J, et al. An autogeneic feeder cell system that efficiently supports growth of undifferentiated human embryonic stem cells. Stem Cells. 2005 Mar;23(3):306–14. doi: 10.1634/stemcells.2004-0137. [DOI] [PubMed] [Google Scholar]
- 46.Wang Q, Fang ZF, Jin F, Lu Y, Gai H, Sheng HZ. Derivation and growing human embryonic stem cells on feeders derived from themselves. Stem Cells. 2005 Oct;23(9):1221–7. doi: 10.1634/stemcells.2004-0347. [DOI] [PubMed] [Google Scholar]
- 47.Thiery JP, Sleeman JP. Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol. 2006 Feb;7(2):131–42. doi: 10.1038/nrm1835. [DOI] [PubMed] [Google Scholar]
- 48.Chaffer CL, Thompson EW, Williams ED. Mesenchymal to epithelial transition in development and disease. Cells, tissues, organs. 2007;185(1–3):7–19. doi: 10.1159/000101298. [DOI] [PubMed] [Google Scholar]