Abstract
Light treatment has been used as a non-pharmacological tool to help mitigate poor sleep quality frequently found in the older people. In order to increase compliance to non-pharmacological light treatments, new, more efficacious light-delivery systems need to be developed. A prototype personal light-treatment device equipped with low brightness blue light-emitting diodes (LEDs) (peak wavelength near 470 nm) was tested for its effectiveness in suppressing nocturnal melatonin, a measure of circadian stimulation. Two levels of corneal irradiance were set to deliver two prescribed doses of circadian light exposure. Eleven older subjects, between 51 and 80 yrs of age who met the selection criteria, were exposed to a high and to a low level of light for 90 min on separate nights from the personal light-treatment device. Blood and saliva samples were collected at prescribed times for subsequent melatonin assay. After 1 h of light exposure, the light-induced nocturnal melatonin suppression level was about 35% for the low light level and about 60% for the high light level. The higher level of blue light suppressed melatonin slightly faster, to a greater extent over the course of the 90 min exposure period, and was maintained after 60 min. The constant exposure of the low light level resulted in a decrease in nocturnal melatonin suppression for the last sampling time, whereas for the high light level, suppression continued throughout the entire exposure period. The present study performed with healthy adults suggests that the tested personal light-treatment device might be a practical, comfortable, and effective way to deliver light treatment to those suffering from circadian sleep disorders; however, the acceptance and effectiveness of personal light-treatment devices by older people and by other segments of the population suffering from sleep disorders in a real-life situation needs to be directly tested.
Keywords: melatonin, blue light, sleep disorders, elderly, circadian
Introduction
Sleep disturbances increase as we age. Surveys indicate that 40 to 70% of the oldest members of the population (>65 yrs old) suffer from chronic sleep disturbances. In general, older adults tend to go to bed earlier in the evening and wake earlier in the morning than young adults. Frequent nocturnal awakenings, difficulty falling asleep, and an increased number of naps during the day are also more common in the oldest adults (Buysse et al., 1991; Van Someren, 2000). Sleep disturbances are associated with decreased physical health, including increased cardiovascular problems, disruption of endocrine functions, and decline of immune functions in older people (Van Cauter et al., 1998; von Kanel et al., 2006). Sleep disturbances are particularly common in persons with Alzheimer’s disease (AD). It is estimated that in the late years of the disease, AD patients spend approximately 40% of their time at night awake and a significant amount of their daytime hours asleep (Ancoli-Israel et al., 1989; Vitiello et al., 1991; McCurry et al., 2000). An irregular sleep-activity pattern is a major source of difficulty for family-member caregivers and is one of the main reasons AD patients are institutionalized (Pollak & Perlick, 1991). Once institutionalized, nursing home residents who experience a high level of sleep disruption at night are more likely to exhibit aggressive behavior during the day (Cohen-Mansfield et al., 1995).
Although the homeostatic drive is important for sleep, the timing and consolidation of sleep is driven by the circadian system (Borbely, 1982; Dijk et al., 2000). The 24 h light-dark cycle is the most important exogenous stimulus for regulating the timing of the circadian system (Moore, 1997) and thereby is an important determinant of consolidated and predictable sleep. As people age, however, optical changes in the eye (senile miosis and increased crystalline lens opacity) reduce the amount of light reaching the retina (Weale, 1963). This age-dependent reduction in retinal illuminance is compounded by the static, low-level lighting conditions commonly found in senior housing (Espiritu et al., 1994). The threshold for activation of the circadian system is higher than for the visual system (Rea et al., 2002), so the much reduced regulatory power of the muted light-dark cycle associated with a sedentary lifestyle that limits access to daylight (Ancoli-Israel et al., 1997; Jean-Louis et al., 2000; Higgins et al., 2008) can go largely unnoticed by older people and their caregivers. Moreover, as one ages, retinopathy and apoptosis begin to compromise the mechanisms necessary for phototransduction and for processing neural signals generated by the retina (Weale, 1992). Degeneration of the suprachiasmatic nuclei (SCN), the site of the master circadian clock in the brain, is prevalent in persons with AD (Swaab et al., 2002; Wu & Swaab, 2007). Thus, the neural machinery necessary to process environmental lighting conditions is compromised in older adults, and given the muted light-dark patterns that older people often experience, it should not be surprising that older people, particularly those with AD, have irregular and episodic sleep-activity patterns (Van Someren et al., 1996; Yoon et al., 2003).
As might be expected, then, a marked increase in daytime lighting levels for older people can counteract the age-dependent losses in retinal light exposure and thereby provide a stronger modulated signal for processing by the SCN. Clinical research has shown that exposure to very bright light during the day and darkness at night can consolidate rest and activity patterns in people with AD (Van Someren et al., 1997). Bright light exposure (>2500 lux of a white light at the cornea) during the morning has been shown to improve nighttime sleep, increase daytime wakefulness, reduce evening agitation behavior, and consolidate rest/activity patterns of people with AD (Lovell et al., 1995; Van Someren et al., 1997; Mishima et al., 1998; Koyama et al., 1999; Lyketosos et al., 1999; Ancoli-Israel et al., 2002). More recently, Riemersma-van der Lek and colleagues (2008) demonstrated that continuous daytime exposure to 1000 lux at the eye of a white light (4100 K correlated color temperature [CCT]) improved sleep efficiency and cognition in patients with AD as well as reduced symptoms of depression. Evening light exposure has also been shown to be effective in consolidating rest/activity rhythms of AD patients and helping them to sleep better at night (Ancoli-Israel et al., 2003). Light applied in the evening has also been shown to be effective in phase delaying the circadian system, which allows older people to fall asleep later and wake up later (Campbell et al., 1995). Although there is strong evidence for the positive impact of light on sleep disorders in older adults, it must be noted that some studies failed to find any impact of light treatment on sleep quality of AD subjects. Dowling and colleagues (2005, 2008) reported that light treatment alone (1 h of 2,500 lux at the angle of gaze) did not improve nighttime sleep or daytime wake and rest activity in AD patients.
The spectral sensitivity of the circadian system peaks between 450 and 480 nm (Brainard et al., 2001; Thapan et al., 2001; Berson et al., 2002; Rea et al., 2005), whereas the achromatic visual channel used for reading and fine acuity, and which underlies all current lighting standards, peaks at 555 nm (Rea, 2000). Logically then, commercially available lighting systems, like those used to illuminate senior residences, assisted-living facilities, and nursing homes for the purpose of supporting vision, are not necessarily efficacious for generating light to stimulate the circadian system. In other words, since the spectral power distribution of these fabricated sources and the intensities at which they are employed are designed for visual effectiveness and minimal energy use rather than for circadian effectiveness, much higher levels of electric light used to illuminate architectural spaces would be required to be effective for the circadian system. An alternative approach is to deliver light directly to the patient using luminous panels located at close distances to the observer or head worn luminous visors. However, the high brightness of these luminous objects in an otherwise dim environment can cause glare and thereby reduce compliance of individuals seeking this treatment. Recently, Rea and colleagues (2005) developed a model of human circadian phototransduction that predicts the relative effectiveness of any spectral power distribution for stimulating the circadian system. By selecting a spectral power distribution that is optimized for the peak spectral sensitivity of the circadian system, it is possible to maximize source efficacy, minimize energy use, and avoid the very bright levels needed from “white” electric light sources that can cause glare and compromise compliance.
In the present experiment, we tested the effectiveness of a prototype personal light-treatment device equipped with low brightness blue light-emitting diodes (LEDs), with peak wavelengths near 470 nm for suppressing nocturnal melatonin in older subjects. Four LEDs were placed inside a plastic diffuser in the upper rim of a transparent pair of goggles so that direct and reflected light from the goggle lenses reached the subject’s eyes. The placement of the LEDs, which delivered light off the visual axis, along with the choice of LED emission wavelength, minimized glare while achieving the desired level of corneal irradiance. Two levels of corneal irradiance were set to deliver two prescribed doses of circadian light exposure. According to the model by Rea et al., both of the selected light levels (see Methods) should produce reliable levels of melatonin suppression after 60 min, but at suppression levels below response saturation (Rea et al. 2005; Bullough et al., 2008). If the personal light-treatment device can be shown to deliver effective levels of circadian stimulation (measured via nocturnal melatonin suppression in blood plasma and saliva), it should then be possible to subsequently determine if the device can increase sleep consolidation and efficiency in older subjects when worn for a prescribed duration and at a prescribed circadian time.
Methods
Subjects between the ages of 50 and 80 yrs (mean age ± standard deviation: 59 ± 10 yrs) were recruited for the study through email notices, posters, and word-of-mouth. This study was approved by the Rensselaer Polytechnic Institute Institutional Review Board and meets the international ethical standards of this journal (Portaluppi et al., 2008).
Individuals were considered eligible for participation if they reported that they were free from major health problems, including cardiovascular disease, diabetes, and high blood pressure. They were excluded from consideration if they reported taking over-the-counter melatonin, antidepressants, sleep medicine, beta blockers, or hormone replacement therapy. Subjects did not go through an ophthalmologic examination, but were asked to self-report any eye disorders, such as cataract or glaucoma. Any subject who reported having any known eye disorder was excluded from the experiment. Subjects were not tested for color blindness because a previous study reported that there is no significant difference in the magnitude of melatonin suppression between a normal control group and a group with the most common forms of color confusions (protans and deutans) (Ruberg et al., 1996). From the pool of eligible candidates, subjects were selected according to their responses to the Munich ChronoType questionnaire (Roenneberg et al., 2003); only “normal” or “early” sleep types (from slight to extreme) were selected, because the experiment was scheduled to start at 23:50 h, and we wanted to ensure that subjects would be synthesizing melatonin during the light exposure periods. Subjects were instructed to maintain their regular sleep schedules (bed times between 21:00 and 23:00 h) during the week prior to each experimental session. Twelve subjects participated in the study; one of these subjects had nocturnal melatonin levels that were not reliably different than baseline values, so his data had to be excluded from subsequent analyses. The results of the present study are based upon eleven subjects between the ages of 51 and 80 yrs who met all of the selection criteria, completed the experiment, and provided melatonin levels high enough for reliable assessment of light-induced nocturnal melatonin suppression.
All subjects participated in a two-night, within-subjects protocol (Table 1). The two experimental sessions were scheduled one or two weeks apart. Experiments were conducted between June and November 2008 (Higuchi et al., 2007). Blood and saliva samples were collected at prescribed times for subsequent melatonin assay. Every subject was exposed to a high and to a low level (Table 2) of light for 90 min on separate nights from the personal light-treatment device; the order of light level exposures was counterbalanced across subjects. Following previous studies (Brainard et al., 2001; Thapan et al., 2001; Rea et al., 2005), light levels were selected to provide corneal irradiances above threshold and below saturation for nocturnal melatonin suppression. During the entire experimental session, before and during light treatment, the subjects watched a movie projected on a screen approximately 4 m away in a large room dimly illuminated by red LEDs (peak wavelength = 630 nm). Illuminance levels at the subjects’ eyes from the dim LEDs and the screen were less than 5 lux. Subjects arrived at the laboratory at approximately 22:30 on the night of data collection.
Table 1.
Experimental protocol
| Time 1 | Time 2 | Time 3 | Time 4 | Time 5 | Time 6 | Time 7 | |
|---|---|---|---|---|---|---|---|
| Blood Collection | 23:50 | 00:00 | 00:10 | 00:20 | 00:30 | 01:00 | 01:30 |
| Dark | Dark | Blue light | Blue light | Blue light | Blue light | Blue light | |
| Saliva Collection | 23:50 | 00:00 | 00:05 | 00:15 | 00:25 | 00:45 | 01:15 |
| Dark | Dark | Blue light | Blue light | Blue light | Blue light | Blue light |
Table 2.
Mean and standard deviations of measurements taken from both eyes for each light-treatment device and mean pupil diameter measured using videography. Note: peak wavelengths for the two irradiances are different because two light goggles were calibrated to deliver the high light level and two light goggles were calibrated to deliver the low light level; so, different goggles were used for each lighting condition.
| Light condition | Irradiance (W/m2) | Photon Flux Density [phot/(cm2 s)] | Illuminance (lux) | Peak wavelength (nm) | FWHM* (nm) | Pupil diameter** (mm) |
|---|---|---|---|---|---|---|
| High | 0.65 ± 0.15 | 1.53 ± 0.35 × 1014 | 53 ± 15.5 | 468 ± 6.5 | 20.1 ± 1.5 | 3.2 ± 0.4 |
| Low | 0.11 ± 0.03 | 2.62 ± 0.71 × 1013 | 10.4 ± 2.6 | 474 ± 1.8 | 19.7 ± 0.6 | 3.7 ± 0.6 |
FWHM = Full width half maximum
Pupils were not dilated for the experiment, but at the end of the experiment, pupils were measured while subjects wore the energized goggles using infrared videography. Ten samples of pupil diameters were obtained and averaged for each subject.
Subjects were instructed to go to bed by 22:00 h the night before and to refrain from napping and consuming caffeine after 10:00 h on the day of their experimental session. Soon after arrival, a registered nurse inserted an indwelling catheter into an arm vein of each subject. Overhead room lights were turned off at 23:00 h. Light exposures began at 00:00 h and ended at 01:30 h. As shown in Table 1, a total of seven blood and seven saliva samples were taken over the 110 min sessions, both of which began at 23:50 h. Three, 3-ml blood samples were drawn at the prescribed times; the second and third samples were spun for 15 min at 1000× g for plasma extraction. Saliva samples were collected using the salivette system (ALPCO Diagnostics, Salem, NH, USA). Each subject moved a plain cotton cylinder around in the mouths until saturated after which it was placed in a test tube and spun for 5 min at 1000× g. To avoid contamination of the saliva samples, subjects were asked to brush their teeth prior to data collection and were not allowed to eat or drink (other than water) during data collection. The plasma and saliva samples were immediately frozen (−20°C) and later sent to a laboratory (Pharmasan Labs, Osceola, WI) for melatonin radioimmunoassay. The sensitivity of the saliva assay was 0.7 pg/ml, and the intra- and inter-assay coefficients of variability (CVs) were 12.1% and 13.2%, respectively. The sensitivity of the plasma assay was 3.5 pg/ml, and the intra- and inter-assay CVs were 8.1% 14.8%, respectively.
The personal light-treatment devices (Figure 1) were fabricated by Topbulb.com from commercially available safety goggles and blue LEDs (LUXEON Rebel). Two LEDs were mounted above each lens of the safety glasses on the upper rim. The irradiance produced by the LEDs mounted above each lens could be adjusted using a small, custom-built power supply. The spectral irradiances provided by the LEDs at the location where the subjects’ corneas would be when wearing the personal light-treatment devices were measured with a laboratory spectrometer (Acton Research, model 2300i double monochromator) using a 5 mm diameter cosine-corrected input optic on the end of an optical fiber and inserted in a mannequin head (Figure 1) to approximate the size and location of a person’s pupil. Both the left and right lenses of the goggles had to be measured independently because the spectral power distributions of the nominally equivalent LEDs varied. Table 2 shows the average irradiance, illuminance, photon flux density, peak wavelength, and full width half maximum values for every goggle lens, together with their associated standard deviations. Pupils were not dilated for the experiment, but at the end of the experiment, pupils were photographed using infrared videography while wearing the energized goggles and pupil diameters for each subject were calculated and averaged; results are presented in Table 2.
Figure 1.
Personal light-treatment device mounted on a mannequin head used for calibration (left) and seen from behind (right).
Results
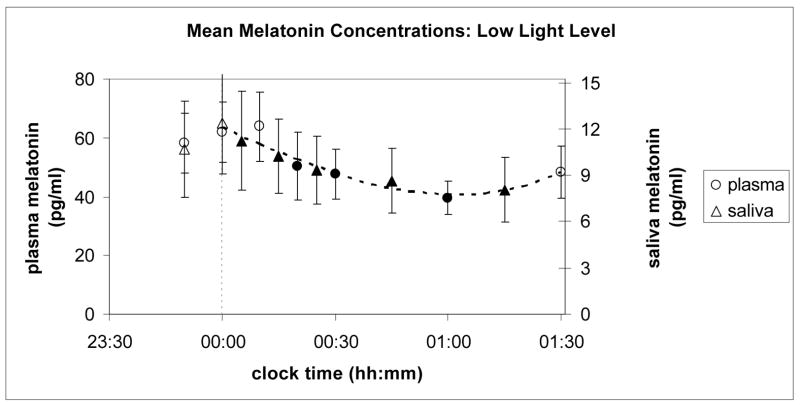
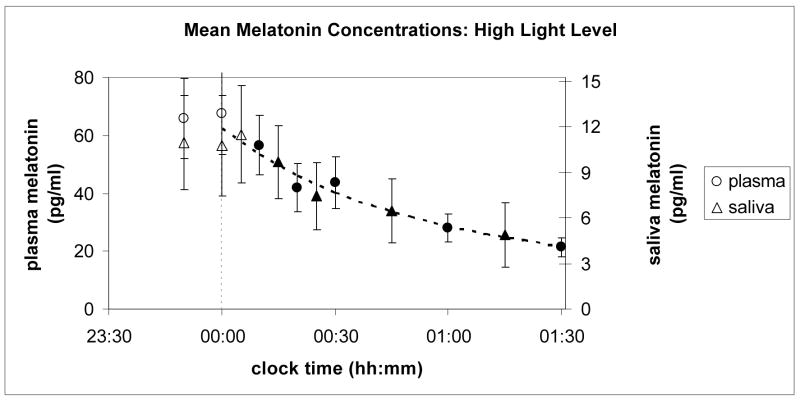
Figures 2 and 3 show the results of the experiment. Ordinates for plasma and saliva melatonin were related to one another for both light levels (high and low) by a coefficient equal to the ratio of their overall average concentrations, which was 5.25. Saliva melatonin concentrations are generally lower than those found in plasma for the same subject and circadian time. As seen in these two figures, relative changes in melatonin concentration levels for plasma and saliva are quite similar for both light levels over the entire course of data collection.
Figure 2.
Mean melatonin concentrations (pg/ml) from plasma (left ordinate) and from saliva (right ordinate) samples collected from subjects prior to and during continuous exposure to the low light level at the cornea from narrowband light sources peaking near 470 nm. Black shaded points are statistically significantly different (p < 0.05) from the last dark value prior to turning on the blue light (at 00:00 h, corresponding to the dashed vertical line).
Figure 3.
Mean melatonin concentrations (pg/ml) from plasma (left ordinate) and from saliva (right ordinate) samples collected from 11 subjects prior to and during continuous exposure to the high light level at the cornea from narrowband light sources peaking near 470 nm. One subject did not produce enough saliva for the assay process during exposure to this light level; so, data from only 10 subjects are included in the saliva melatonin data. The plasma melatonin concentrations are based upon data from all 11 subjects. Black shaded points are statistically significantly different (p < 0.05) from the last dark value prior to turning on the blue light (at 00:00 h, corresponding to the dashed vertical line).
In both Figures 2 and 3, the circles represent values from plasma samples and the triangles from saliva. For each set of data (plasma and saliva and low and high light levels), paired, one-tailed t-tests were conducted on the subjects’ melatonin concentration values at midnight and at each time following midnight to determine whether the mean melatonin concentrations at each time were reliably lower than they were at midnight. The filled symbols in Figures 2 and 3 represent the mean concentrations that were statistically significantly (p < 0.05) different from those at midnight. The curves in Figures 2 and 3 are cubic functions constrained to go through the combined average last dark value from both plasma and saliva samples, and fitted to both the plasma and the scaled saliva data collected between 00:00 and 01:30 h. Both the plasma and scaled saliva values in each of the figures lie along single cubic polynomial functions that depend solely on light level.
Melatonin suppression values (defined as the mean percentage reduction in melatonin concentrations from those at midnight) corresponding to light exposure durations of 30, 60 and 90 min (for plasma) and of 45 and 75 min (for saliva) were calculated and the data for each submitted to a repeated measures, two factor (light level and sample time) analysis of variance (ANOVA). For the saliva data at the high light level, only 10 subjects were included in the analysis, since one subject did not provide enough saliva during that experimental night. For all other situations, the data from all 11 subjects were included. Table 3 shows the mean percent melatonin suppression values and associated standard deviations for plasma and for saliva at the collection times. For plasma, there were significant main effects for both light level (F1,10 = 14.4, p < 0.01) and sample time (F2,10 = 5.2, p < 0.05), and there was a significant interaction between light level and sample time (F2,10 = 4.4, p < 0.05). For saliva, only the main effect of sample time (F1,9 = 7.1, p < 0.05) was statistically significant, reflecting a higher variance in melatonin concentrations from saliva.
Table 3.
Mean melatonin suppression values (defined as the mean percentage reduction in melatonin concentrations relative to those at midnight) and associated standard deviations (mean percent suppression ± SD) corresponding to light exposure durations of 30 (T30), 60 (T60), and 90 (T90) min (for plasma) and of 45 (T45) and 75 (T75) min (for saliva; shaded cells)
| Melatonin Suppression (mean % ± SD) | |||||
|---|---|---|---|---|---|
| Light Condition | T30 | T45 | T60 | T75 | T90 |
| High | 32 ±19 | 25 ± 35 | 53 ± 25 | 40 ± 27 | 61 ± 22 |
| Low | 20 ± 29 | 19 ± 33 | 28 ± 36 | 25 ± 32 | 13 ± 51 |
As shown in Figure 2, there was an increase in the mean melatonin concentration under the low light level between 01:00 and 01:30 h. In fact, the mean concentration at 01:30 h is not reliably (p < 0.05) different from the mean concentration at midnight. The melatonin suppression value after 90 min of exposure to the low light level was lower than the suppression after 60 min. While not firmly decisive, these results and, in particular, the significant light level by sample time interaction revealed by the ANOVA, add support for the inference that the circadian system habituates to prolonged exposure to low light levels. A follow-up study is recommended to formally test the hypothesis that the circadian system might exhibit habituation to a prolonged, weak-light stimulus.
Discussion
Sleep is governed by both circadian and homeostatic processes (Borbely, 1982). Often circadian timing is misaligned with homeostatic drive, leading to poor or fragmented sleep. Light has been used to align circadian timing with the homeostatic process and thereby help regulate and consolidate sleep in both AD and older, non-demented individuals (Figueiro et al., 2008). A personal light-treatment device that could provide the proper dosage at the right time could, therefore, offer a non-pharmacological method of improving sleep quality in older individuals.
The key question raised here was “Would personal light-treatment devices be effective for reliably stimulating the circadian system?” Indeed, both light exposure levels delivered by the “light goggles” reliably suppressed nocturnal melatonin in plasma and saliva and exhibited simple dose-dependent (cubic) functions. The data and the functions relating exposure time to melatonin concentration indicate that, compared to the low light level, the higher level of blue light (λmax ≈ 470 nm): (a) suppresses melatonin slightly faster, (b) suppresses melatonin to a greater extent over the course of a 90 min exposure period, and (c) is able to maintain suppression after 60 min. Even though it is known that the aging lens will absorb slightly more irradiance at 470 nm (Weale, 1963) and, therefore, reduce the sensitivity of older subjects to blue light for nocturnal melatonin suppression (Herljevic et al., 2005), the corneal irradiances used in our study were clearly sufficient to suppress nocturnal melatonin in healthy adults age ≥50 yrs. From a practical perspective, subjects were able to watch a movie throughout the two light exposure periods without difficulty or complaint, an important finding considering that high levels of white light can contribute to glare and compromise compliance. These findings demonstrate both the efficacy of “blue light goggles” for affecting the circadian system and their acceptability among non-demented and otherwise healthy senior subjects. Although it will be important to next demonstrate circadian phase adjustment after wearing the personal light-treatment device, it seems reasonable to suppose, based upon the findings by Zeitzer et al. (2000), that circadian phase shifting leading to entrainment and nocturnal melatonin suppression are similarly affected, although perhaps not precisely identically, by light exposure. Consistently, Warman et al. (2003) showed that phase shifting of the human circadian system by light is also maximally sensitive to short-wavelength light, similar to acute nocturnal melatonin suppression. The results from both studies suggest that light-induced nocturnal melatonin suppression and light-induced circadian phase shifts utilize the same retinal neural apparatus and follow a similar stimulus-response, function. Therefore, the present study offers a positive first step in developing and demonstrating a practical method for efficaciously delivering light to individuals for circadian alignment to improve sleep quality in older individuals. Naturally, it would be useful to extend these techniques to other populations, taking into account, of course, different lens transmissions and pupil areas (Herljevic et al., 2005).
Finally, it is also worth noting that the present study demonstrates a useful methodology for collecting frequent samples of melatonin concentrations. Since it is practically very difficult to obtain multiple blood samples from subjects over the course of an experimental session, the present results indicate that interleafed samples from saliva and blood can be very informative. By combining relative melatonin concentrations from saliva and blood, more reliable estimates of the dynamics of light-induced nocturnal melatonin suppression than those obtained with point estimates can be obtained. This conclusion is also consistent with the findings from a number of earlier studies that have shown that the relative changes in melatonin concentrations from plasma and from saliva are well correlated (Benloucif et al., 2008).
Conclusions
Light treatment has been used as a non-pharmacological tool to help mitigate poor sleep quality frequently found in the older population. Societal benefits associated with improving the sleep quality of older adults, including those with AD, can range from reducing the number of falls to increasing cognition and delaying transition of older adults and persons with AD to a more controlled living environment. In order to increase compliance to non-pharmacological light treatments, new, more efficacious light-delivery systems need to be developed. The current study suggests that “blue light goggles” might be a practical, comfortable, and efficacious way to deliver light treatment to those suffering from circadian sleep disorders. In particular, the use of blue light allowed us to limit discomfort glare without compromising the effectiveness of the light for stimulating the circadian system. Other light sources, e.g., white light, could be used in a personal light treatment device, but the irradiances would have to be carefully adjusted to maintain the same effectiveness for stimulating the circadian system (Rea et al., 2005: Revell et al., 2007), while limiting discomfort glare. Obviously, however, the acceptance and efficaciousness of personal light-treatment devices by older people with circadian sleep disorders and by other segments of the population also suffering from sleep disorders in a real-life situation needs to be directly tested.
Acknowledgments
Sources of Support: National Institute on Aging (1R41 AG029693) through a Small Business Technology Transfer Research (STTR) grant to Topbulb.com.
This study was supported by the National Institute on Aging (1R41 AG029693) through a Small Business Technology Transfer grant to Topbulb.com. The authors would like to acknowledge Phil Bonello of Topbulb.com for providing the goggles prototypes, Karen Kubarek, Chris Munson, Barbara Plitnick, and Bonnie Westlake of the Lighting Research Center for their assistance with data collection and goggle calibration. We would like to thank Jennifer Taylor for her assistance with the manuscript editing and formatting.
Footnotes
Declaration of Interest: None of the authors report conflict of interest.
References
- Ancoli-Israel S, Parker L, Sinaee R, Fell RL, Kripke DF. Sleep fragmentation in patients from a nursing home. J Gerontol. 1989;44:M18–21. doi: 10.1093/geronj/44.1.m18. [DOI] [PubMed] [Google Scholar]
- Ancoli-Israel S, Klauber MR, Jones DW, Kripke DF, Martin J, Mason W, Pat-Horenczyk R, Fell R. Variations in circadian rhythms of activity, sleep, and light exposure related to dementia in nursing-home patients. Sleep. 1997;20:18–23. [PubMed] [Google Scholar]
- Ancoli-Israel S, Martin J, Kripke D, Marler M, Klauber M. Effect of light treatment on sleep and circadian rhythms in demented nursing home patients. J Am Geriatr Soc. 2002;50:282–289. doi: 10.1046/j.1532-5415.2002.50060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ancoli-Israel S, Martin JL, Gehrman P, Shochat T, Corey-Bloom J, Marler M, Nolan S, Levi L. Effect of light on agitation in institutionalized patients with severe alzheimer disease. Am J Geriatr Psychiatry. 2003;11:194–203. [PubMed] [Google Scholar]
- Benloucif S, Burgess HJ, Klerman EB, Lewy AJ, Middleton B, Murphy PJ, Parry BL, Revell VL. Measuring melatonin in humans. J Clin Sleep Med. 2008;4:66–69. [PMC free article] [PubMed] [Google Scholar]
- Berson DM, Dunn FA, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 2002;295:1070–1073. doi: 10.1126/science.1067262. [DOI] [PubMed] [Google Scholar]
- Borbely AA. A two process model of sleep regulation. Human Neurobiology. 1982;1:195–204. [PubMed] [Google Scholar]
- Brainard GC, Hanifin JP, Greeson JM, Byrne B, Glickman G, Gerner E, Rollag MD. Action spectrum for melatonin regulation in humans: evidence for a novel circadian photoreceptor. J Neurosci. 2001;21:6405–6412. doi: 10.1523/JNEUROSCI.21-16-06405.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullough JD, Bierman A, Figueiro MG, Rae MS. On melatonin suppression from polychromatic and narrowband light. [Letter to Editor] Chronobiol Int. 2008;25:653–656. doi: 10.1080/07420520802247472. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, III, Monk TH, Hoch CC, Yeager AL, Kupfer DJ. Quantification of subjective sleep quality in healthy elderly men and women using the pittsburgh sleep quality index (psqi) Sleep. 1991;14:331–338. [PubMed] [Google Scholar]
- Campbell S, Terman M, Lewy A, Dijk D, Eastman C, Boulos Z. Light treatment for sleep disorders: Consensus report: V. Age-related disturbances. J Biol Rhythms. 1995;10:151–154. doi: 10.1177/074873049501000207. [DOI] [PubMed] [Google Scholar]
- Cohen-Mansfield J, Werner P, Freedman L. Sleep and agitation in agitated nursing home residents: An observational study. Sleep. 1995;18:674–680. [PubMed] [Google Scholar]
- Dijk DJ, Duffy JF, Czeisler CA. Contribution of circadian physiology and sleep homeostasis to age-related changes in human sleep. Chronobiol Int. 2000;17:285–311. doi: 10.1081/cbi-100101049. [DOI] [PubMed] [Google Scholar]
- Dowling GA, Hubbard EM, Mastick J, Luxenberg JS, Burr RL, Van Someren EJ. Effect of morning bright light treatment for rest-activity disruption in institutionalized patients with severe Alzheimer’s disease. Int Psychogeriatr. 2005;17:221–36. doi: 10.1017/S1041610205001584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowling GA, Burr RL, Van Someren EJ, Hubbard EM, Luxenberg JS, Mastick J, Cooper BA. Melatonin and bright-light treatment for rest-activity disruption in institutionalized patients with Alzheimer’s disease. J Am Geriatr Soc. 2008;56:239–46. doi: 10.1111/j.1532-5415.2007.01543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espiritu RC, Kripke DF, Ancoli-Israel S, Mowen MA, Mason WJ, Fell RL, Klauber MR, Kaplan OJ. Low illumination experienced by San Diego adults: Association with atypical depressive symptoms. Biol Psychiatry. 1994;35:403–407. doi: 10.1016/0006-3223(94)90007-8. [DOI] [PubMed] [Google Scholar]
- Figueiro MG, Saldo E, Rea M, Kubarek K, Cunningham J, Rea MS. Developing architectural lighting designs to improve sleep in older adults. The Open Sleep Journal. 2008;12:40–51. [Google Scholar]
- Hattar S, Lucas RJ, Mrosovsky N, Thompson S, Douglas RH, Hankins MW, Lem J, Biel M, Hofmann F, Foster RG, Yau KW. Melanopsin and rod-cone photoreceptive systems account for all major accessory visual functions in mice. Nature. 2003;424:76–81. doi: 10.1038/nature01761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herljevic M, Middleton B, Thapan K, Skene DJ. Light-induced melatonin suppression: age-related reduction in response to short wavelength light. Exp Gerontol. 2005;40:237–242. doi: 10.1016/j.exger.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Higgins PA, Hornick T, Figueiro MG. Circadian rhythms and light exposure in caregivers and elders of persons with dementia: A case study. Presented at National State of the Science Congress on Nursing Research; October 2–4, 2008; Washington, DC. 2008. [Google Scholar]
- Higuchi S, Motohashi Y, Ishibashi K, Maeda T. Less exposure to daily ambient light in winter increases sensitivity of melatonin to light suppression. Chronobiol Int. 2007;24:31–43. doi: 10.1080/07420520601139805. [DOI] [PubMed] [Google Scholar]
- Jean-Louis G, Kripke DF, Ancoli-Israel S, Klauber MR, Sepulveda RS, Mowen MA, Assmus JD, Langer RD. Circadian sleep, illumination, and activity patterns in women: Influences of aging and time reference. Physiol Behav. 2000;68:347–352. doi: 10.1016/s0031-9384(99)00186-9. [DOI] [PubMed] [Google Scholar]
- Koyama E, Matsubara H, Nakano T. Bright light treatment for sleep-wake disturbances in aged individuals with dementia. Psychiatry Clin Neurosci. 1999;53:227–229. doi: 10.1046/j.1440-1819.1999.00483.x. [DOI] [PubMed] [Google Scholar]
- Lovell B, Ancoli-Israel S, Gevirtz R. Effect of bright light treatment on agitated behavior n institutionalized elderly subjects. Psychiatry Res. 1995;57:7–12. doi: 10.1016/0165-1781(95)02550-g. [DOI] [PubMed] [Google Scholar]
- Lyketosos C, Lindell Veiel L, Baker A, Steele C. A randomized, controlled trial of bright light therapy for agitated behaviors in dementia patients residing in long-term care. Int J Geriatr Psychiatry. 1999;14:520–525. [PubMed] [Google Scholar]
- Mawad K, Van Gelder N. Absence of long-wavelength photic potentiation of murine intrinsically photosensitive retinal ganglion cell firing in vitro. J Biol Rhythms. 2008;23:387–391. doi: 10.1177/0748730408323063. [DOI] [PubMed] [Google Scholar]
- McCurry S, Reynolds C, Ancoli-Israel S, Teri L, Vitiello M. Treatment of sleep disturbance in alzheimer’s disease. Sleep Med Rev. 2000;4:603–628. doi: 10.1053/smrv.2000.0127. [DOI] [PubMed] [Google Scholar]
- Mishima K, Hishikawa Y, Okawa M. Randomized, dim light controlled, crossover test of morning bright light therapy for rest-activity rhythm disorders in patients with vascular dementia and dementia of alzheimer’s type. Chronobiol Int. 1998;15:647–654. doi: 10.3109/07420529808993200. [DOI] [PubMed] [Google Scholar]
- Moore R. Circadian rhythms: Basic neurobiology and clinical applications. Annu Rev Med. 1997;48:253–266. doi: 10.1146/annurev.med.48.1.253. [DOI] [PubMed] [Google Scholar]
- Mure LS, Rieux C, Hattar S, Cooper HM. Melanopsin-Dependent Nonvisual Responses: Evidence for Photopigment Bistability In Vivo. J Biol Rhythms. 2007;22:411–424. doi: 10.1177/0748730407306043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollak CP, Perlick D. Sleep problems and institutionalization of the elderly. J Geriatr Psychiatry Neurol. 1991;4:204–210. doi: 10.1177/089198879100400405. [DOI] [PubMed] [Google Scholar]
- Portaluppi F, Touitou Y, Smolensky MH. Ethical and methodological standards for laboratory and medical biological rhythm research. Chronobiol Int. 2008;25:999–1016. doi: 10.1080/07420520802544530. [DOI] [PubMed] [Google Scholar]
- Rea M, editor. IESNA lighting handbook: Reference and application. 9. Illuminating Engineering Society of North America; New York: 2000. p. 1000. [Google Scholar]
- Rea M, Figueiro M, Bullough J. Circadian photobiology: An emerging framework for lighting practice and research. Light Res Technol. 2002;34:177–190. [Google Scholar]
- Rea M, Figueiro M, Bullough J, Bierman A. A model of phototransduction by the human circadian system. Brain Res Rev. 2005;50:213–228. doi: 10.1016/j.brainresrev.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Revell VL, Skene DJ. Light-induced melatonin suppression in humans with polychromatic and monochromatic light. Chronobiol Int. 2007;24:1125–37. doi: 10.1080/07420520701800652. [DOI] [PubMed] [Google Scholar]
- Riemersma-van der Lek RF, Swaab DF, Twisk J, Hol EM, Hoogendijk WJ, Van Someren EJ. Effect of bright light and melatonin on cognitive and noncognitive function in elderly residents of group care facilities: A randomized controlled trial. JAMA. 2008;299:2642–2655. doi: 10.1001/jama.299.22.2642. [DOI] [PubMed] [Google Scholar]
- Roenneberg T, Wirz-Justice A, Merrow M. Life between clocks: Daily temporal patterns of human chronotypes. J Biol Rhythms. 2003;18:80–90. doi: 10.1177/0748730402239679. [DOI] [PubMed] [Google Scholar]
- Ruberg FL, Skene DJ, Hanifin JP, Rollag MD, English J, Arendt J, Brainard GC. Melatonin regulation in humans with color vision deficiencies. J Clin Endocrinol Metab. 1996;81:2980–2985. doi: 10.1210/jcem.81.8.8768862. [DOI] [PubMed] [Google Scholar]
- Swaab DF, Dubelaar EJ, Hofman MA, Scherder EJ, van Someren EJ, Verwer RW. Brain aging and alzheimer’s disease; use it or lose it. Prog Brain Res. 2002;138:343–373. doi: 10.1016/S0079-6123(02)38086-5. [DOI] [PubMed] [Google Scholar]
- Thapan K, Arendt J, Skene DJ. An action spectrum for melatonin suppression: evidence for a novel non-rod, non-cone photoreceptor system in humans. J Physiol. 2008;535:261–267. doi: 10.1111/j.1469-7793.2001.t01-1-00261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Someren E, Hagebeuk E, Lijzenga C, Scheltens P, de Rooij S, Jonker C, Pot A, Mirmiran M, Swaab D. Circadian rest-activity rhythm disturbances in alzheimer’s disease. Biol Psychiatry. 1996;40:259–270. doi: 10.1016/0006-3223(95)00370-3. [DOI] [PubMed] [Google Scholar]
- Van Someren E, Kessler A, Mirmiran M, Swaab D. Indirect bright light improves circadian rest-activity rhythm disturbances in demented patients. Biol Psychiatry. 1997;41:955–963. doi: 10.1016/S0006-3223(97)89928-3. [DOI] [PubMed] [Google Scholar]
- Van Someren EJ. Circadian and sleep disturbances in the elderly. Exp Gerontol. 2000;35:1229–1237. doi: 10.1016/s0531-5565(00)00191-1. [DOI] [PubMed] [Google Scholar]
- Vitiello MV, Poceta JS, Prinz PN. Sleep in alzheimer’s disease and other dementing disorders. Can J Psychol. 1991;45:221–239. doi: 10.1037/h0084283. [DOI] [PubMed] [Google Scholar]
- von Kanel R, Dimsdale JE, Ancoli-Israel S, Mills PJ, Patterson TL, McKibbin CL, Archuleta C, Grant I. Poor sleep is associated with higher plasma proinflammatory cytokine interleukin-6 and procoagulant marker fibrin d-dimer in older caregivers of people with alzheimer’s disease. J Am Geriatr Soc. 2006;54:431–437. doi: 10.1111/j.1532-5415.2005.00642.x. [DOI] [PubMed] [Google Scholar]
- Warman V, Dijk D, Warman G. Phase advancing human circadian rhythms with short wavelength light. Neurosci Lett. 2003;342:37–40. doi: 10.1016/s0304-3940(03)00223-4. [DOI] [PubMed] [Google Scholar]
- Weale RA. The ageing eye. HK Lewis and Company; London: 1963. p. 200. [Google Scholar]
- Weale RA. The senescence of human vision. Oxford University Press; Oxford: 1992. p. 288. [Google Scholar]
- Wu YH, Swaab DF. Disturbance and strategies for reactivation of the circadian rhythm system in aging and alzheimer’s disease. Sleep Med. 2007;8:623–636. doi: 10.1016/j.sleep.2006.11.010. [DOI] [PubMed] [Google Scholar]
- Yoon IY, Kripke DF, Elliott JA, Youngstedt SD, Rex KM, Hauger RL. Age-related changes of circadian rhythms and sleep-wake cycles. J Am Geriatr Soc. 2003;51:1085–1091. doi: 10.1046/j.1532-5415.2003.51356.x. [DOI] [PubMed] [Google Scholar]
- Zeitzer J, Dijk D, Kronauer R, Brown E, Czeisler C. Sensitivity of the human circadian pacemaker to nocturnal light: Melatonin phase resetting and suppression. J Physiol. 2000;526:695–702. doi: 10.1111/j.1469-7793.2000.00695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]