Abstract
Emerging evidence illustrates the importance of the Positive Transcription Elongation Factor b (P-TEFb) in control of global RNA synthesis which constitutes a major feature of the compensatory response to diverse hypertrophic stimuli in cardiomyocytes. P-TEFb complex, composed of cyclin T and cdk9, is critical for elongation of nascent RNA chains via phosphorylation of the carboxyl terminal domain of RNA polymerase II (CTD RNA Pol II). We and others have shown that the activity of P-TEFb is inhibited by its association with CLP-1, the mouse homolog of human HEXIM1, in various physiological and pathological conditions. To investigate the mechanism of CLP-1’s control of the P-TEFb activity in cardiac hypertrophy, we used a transgenic mouse model of hypertrophy caused by over-expression of calcineurin in the heart. We observed that the level of CLP-1 associated with P-TEFb was reduced markedly in hypertrophic hearts. We also generated bigenic mice (MHC-cyclin T1/CLP-1+/−) by crossing MHC-cyclin T1 transgenic mice with CLP-1 heterozygote. The bigenic mice exhibit enhanced susceptibility to hypertrophy which is accompanied with an increase in cdk9 activity via an increase in serine 2 phosphorylation of CTD and an increase in GLUT1/GLUT4 ratio. These mice have compensated systolic function without evidence of fibrosis and reduced life span. These data suggest that the reduced level of CLP-1 introduced in the background of elevated levels of cyclin T1 elevates de-repression of P-TEFb activity and emphasizes the importance of CLP-1’s role in the mechanism governing compensatory hypertrophy in cardiomyocytes.
Keywords: myocardial hypertrophy, transcriptional elongation, CLP-1, HEXIM1
Introduction
Cardiac hypertrophy, a compensatory response to several clinical settings, such as, hypertension, myocardial infarction, arrhythmias, inherited cardiomyopathies, vascular abnormalities, and mechanical stress 1–3, is characterized by an increase in myocyte size to accommodate increased sarcomeric elaboration resulting from the recruitment of contractile and structural proteins. Although it is initially an adaptative response to normalize ventricular wall stress, prolonged application of deleterious stimuli leads to a pathological state of hypertrophy and eventually to heart failure 4. The transition to the hypertrophic phenotype is highlighted by a global increase in total RNA synthesis, a shift toward dependence upon glucose to meet metabolic demands with altered expression of glucose transporters, and by re-expression of genes that resembles the fetal gene expression program5, 6. There has been significant progress in understanding the mechanisms underlying fetal gene re-activation during hypertrophy 1, 2, 7–11. However, the mechanism that governs the activation of a wide-scale genetic response culminating in a global increase in RNA synthesis in hypertrophic cardiomyocytes remains elusive. Recent studies using microarray analysis identified a wide spectrum of genes that show increased expression levels in response to hypertrophic stimuli 12, 13. Such a wide-scale genomic response is likely to be achieved not through activation of a specific battery of genes via distinct regulatory controls, but rather through a generalized mechanism of transcriptional activation or through activation of a common part of the transcriptional apparatus critical to a wide spectrum of genes.
It is now believed that in addition to translation and transcription initiation steps, phosphorylation of RNA polymerase II (Pol II) in the CTD constitutes a critical step in control of the production of full-length mRNAs 14. Phosphorylation of Pol II by cyclin-dependent kinases (cdk) allows progression from transcription initiation to RNA chain elongation and pre-mRNA processing 15. Recent studies have implicated P-TEFb activity as pivotal to the hypertrophic response to pressure overload in the myocardium7, 16. It appears that P-TEFb is dynamically partitioned between inactive versus active states suggesting that its transcriptional control can be subject to regulatory control. Studies in HeLa cells have shown that a critical step in the response of cells to stress stimuli is dissociation of the inhibitory protein CLP-1/HEXIM1 from P-TEFb 17, 18. These observations provide additional insights into how P-TEFb activity might be regulated.
We have cloned the mouse homolog of human HEXIM1, called CLP-119, 20, and knocked out the CLP-1 gene in mice21 which resulted in expression of the genetic hallmarks of cardiac hypertrophy during the late stages of fetal development 20, 21. We have shown that CLP-1 is co-localized with cdk9 and cyclin T1 in heart during the period in which the CLP-1 knockout fetuses develop hypertrophy. Subsequently, we demonstrated the release of CLP-1 from the P-TEFb complex in cardiomyocytes in culture rendered hypertrophic by mechanical stretch or agonist 22. In the present study, we have extended our findings to demonstrate the regulatory function of CLP-1 in the control of cardiac growth in mouse models of hypertrophy. Transgenic mice that express an activated form of calcineurin show a dissociation of CLP-1 from the P-TEFb complex accompanied by increased serine 2 phosphorylation of Pol II during the compensatory phase of hypertrophy. To study the inhibitory effects of CLP-1 on P-TEFb activity, we crossed the heart-specific transgenic MHC-cyclin T1 mice with CLP-1+/− and show a marked increase in hypertrophic features in the cyclin T1 transgenic with CLP-1 heterozygous offspring (MHC-cyclin T1/CLP-1+/−). We evaluated the hearts of these bigenic mice using a variety of well-documented biochemical and physiological parameters and show that reduced levels of CLP-1 promotes appearance of features characteristic of compensatory hypertrophy in cardiomyocytes.
Materials and Methods
Transgenic lines
Transgenic mice with cardiac-restricted expression of calcineurin (MHC-CnA), a kind gift of Dr. Jeffery Molkentin, have been described previously23, 24. MHC-CnA mice were bred on a congenic C57/Bl6 background before performing the experiments. The CLP-1 heterozygote knockout mice generated in our Laboratory have been described earlier21. The CLP-1+/− mice were also bred on a congenic C57/Bl6 background. Transgenic Mice with cardiac restricted overexpression of cyclin T1 (Fvb background), a kind gift of Dr. Michael Scheneider, have been descrived previously 7, 25. The MHC-cyclin T1/CLP-1+/− mice were produced by crossing females CLP-1 heterozygote mice with males MHC-cyclin T1 mice. All animal experiments were conducted in accordance with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85–23, revised 1996).
Immunoprecipitation and Western blot analysis
The hearts were homogenated with a glass tissue grinder in ice-chilled buffer A (10 mM HEPES (pH 7.9), 1.5 mM MgCl2, 10 mM KCl, 200 mM NaCl, 0.2 mM EDTA), supplemented with 1 mM DTT, protease inhibitor cocktail (P-8340, Sigma), 40 U ml−1 of RNasin (promega), phosphatase Inhibitor cocktails (P2850, P5726, Sigma), β-glycerol phosphate (102893, MP Biochemicals) and 0.5% Nonidet P-40. The extracts were subjected to sequential centrifugations for 5 min each at 500 g and 9000 g at 4°C. The final supernatant was used in all experiments. For the immunoprecipitation experiments, the supernatant was incubated overnight with goat antibody against cyclin T1 (Santa Cruz Biotechnology) followed by 3 hr incubation with protein G agarose. Proteins were separated by polyacrylamide electrophoresis (10% tris-HCl) and electrotransferred onto nitrocellulose membrane. The blots were blocked in Tris-buffered saline, 0.1% Tween 20 (TBST) with 5% bovine serum albumin powder for 1 hr at room temperature. After incubation with the primary antibody, detection was done using secondary horseradish peroxide-coupled antibody and ECL-enhanced chemiluminescence according to the supplier's recommendations (Amersham Biosciences). The following antibodies were used: rabbit anti-CLP-1 (Proteintech Group Inc.), rabbit anti-GAPDH (Abcam), rabbit anti-cyclin T1 (Santa Cruz Biotechnology), mouse monoclonal anti-Ser2 Pol II (Covance), mouse monoclonal anti-Ser5 Pol II (Covance), rabbit anti-RNA Pol II (Santa Cruz Biotechnology), mouse monoclonal anti-cdk9 (Santa Cruz Biotechnology), rabbit anti-HEXIM2 (Santa Cruz Biotechnology), Rabbit anti-GLUT1 (Santa Cruz Biotechnology), and rabbit anti-GLUT4 (Santa Cruz Biotechnology).
Morphological analysis
Hearts from three-month old male mice of wild type, CLP-1+/−, MHC-cyclin T1 and MHC-cyclin T1/CLP-1+/− genotypes were fixed in 4% paraformaldehyde, embedded in paraffin, and 10 micron coronal tissue sections were prepared. Masson Trichrome staining was done in accordance with the manufacturer’s instructions (HT15-KT, Sigma HT15 Trichrome Stain (Masson) Kit).
Echocardiography
The mice chests were shaved and allowed to rest by at least 1 hour before echocardiography. Echocardiography was performed on conscious mice to avoid any cardiodepression produced by anesthesia26. Mice were ascertained using the M mode short axis view, measuring systolic and diastolic cardiac dimensions. LV fractional shortening was calculated with the formula: (LVEDD-LVESD)/LVEDD). Echocardiography was performed using the SONOS 5500 with a linear probe (15 mhz) as reported earlier27.
Statistical analysis
The signal value of the Western blots was determinated with the imagen J program (NIH, Bethesda). Differences between means of the Western blots values and the heart/body ratios were compared by a one-way ANOVA and a paried T-test, using Microsoft Excel. The level of significance for rejection of the null hypothesis was set at P<0.05.
Results
CLP-1 expression in the calcineurin model of cardiac hypertrophy
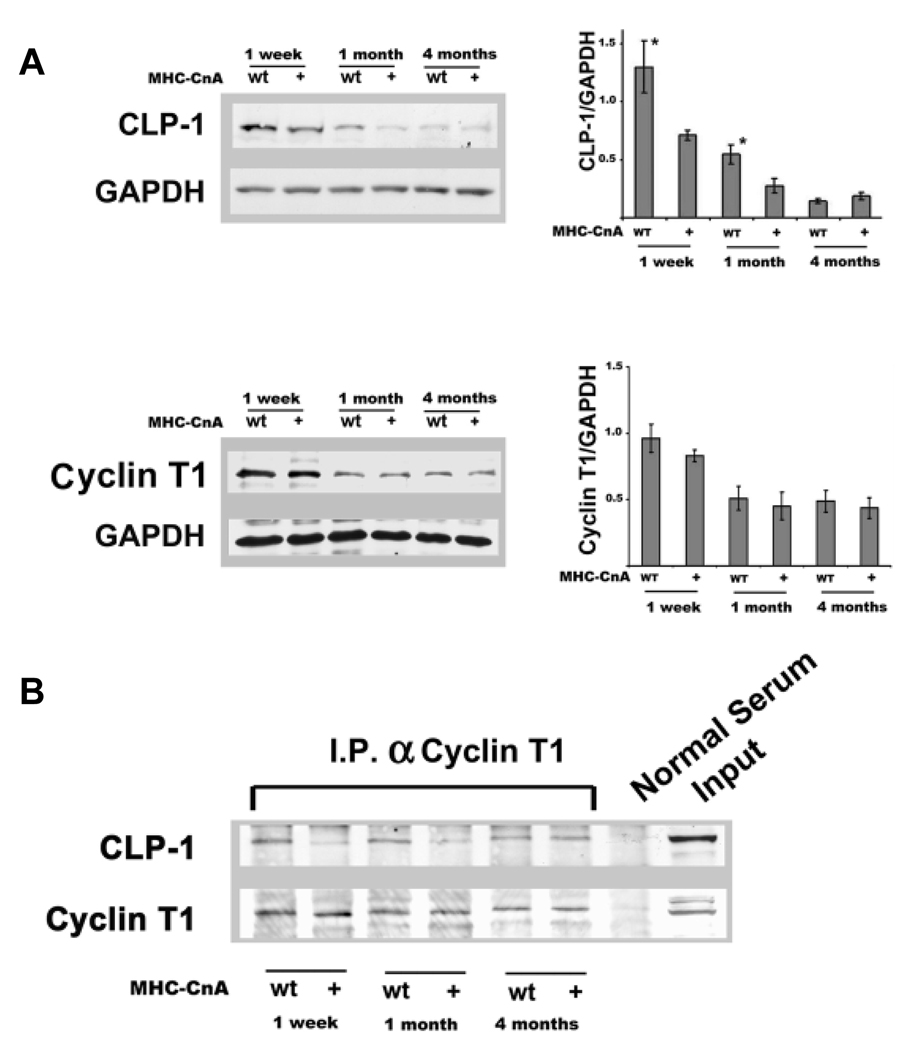
To determine whether the expression of CLP-1 is regulated differentially in the progression to cardiac hypertrophy, we examined total CLP-1 protein levels by Western blot and the levels of CLP-1 associated with P-TEFb in a hypertrophic hearts of MHC-CnA mice which over-expresses an activated form of calcineurin in the heart. Calcineurin is a calmodulin dependent phosphatase, which is activated by the elevation of intracellular calcium and promotes cardiac hypertrophy23. MHC-CnA mice exhibit two distinct and well defined phases in the progression of hypertrophy: during the first month, MHC-CnA mice have hypertrophic characteristics without diastolic dysfunction, however, after one month, the transgenic hearts show cardiac dysfunction associated with sudden death 24. We have previously shown that the levels of CLP-1 and cyclin T1 in the heart decrease after birth with the lowest level being in the adult heart 22. This is in agreement with another report that cyclin T1 and cdk9 levels decrease in heart after birth7. Figure 1A (upper panel) shows that CLP-1 levels in MHC-CnA decrease in comparison with wild type littermates. CLP-1 expression decreased in MHC-CnA mice in 1 week (P< 0.05, 45%), and 1 month (P<0.05, 49%). However, there was no difference between the four-month old transgenic and wild type mice, although both levels were low compared to 1 week and 1 month old mice, suggesting that CLP-1 is differentially regulated in the two stages of hypertrophy. There was no discernable difference in the 4 month transgenic mice in the maladaptive stage of heart failure compared to wild type hearts. Figure 1A (lower panel) shows that cyclin T1 levels did not change in MHC-CnA in comparison with wild type littermates in any of the different stages of hypertrophy (T-student, p>0.05).
Figure 1.
Postnatal changes in the P-TEFb complex in wild type and calcineurin (MHC-CnA) transgenic mice. A, immunoblot with anti-CLP-1 and anti-cyclin T1 antibodies of the heart lysates from wild-type (WT) and MHC-CnA (+) mice. GAPDH was used as loading control (n = 6). B, Association of CLP-1 with P-TEFb in postnatal wild type and MHC-CnA mice as a function of age. Heart lysates from wild type (WT) and MHC-CnA (+) of 1 week, 1 month and 4 month old mice were immunoprecipitated with Cyclin T1 antibody and probed with anti-CLP-1 and anti-Cyclin T1 antibodies. Input is non-immunoprecipitated cell lysates. The data is representative three of independent experiments. C, Phosphorylation of Ser2 RNA Pol II and total RNA Pol II in postnatal wild type and CnA transgenic mice. Immunoblot incubated with anti-phosphorylated Ser2 RNA Pol II and anti-total Pol II of cell lysates of hearts from wild-type (WT) and MHC-CnA (+) of 1 week, 1 month, and 4 months old mice (n =4). Data are expressed as mean ± SE . *P < 0.05 versus control.
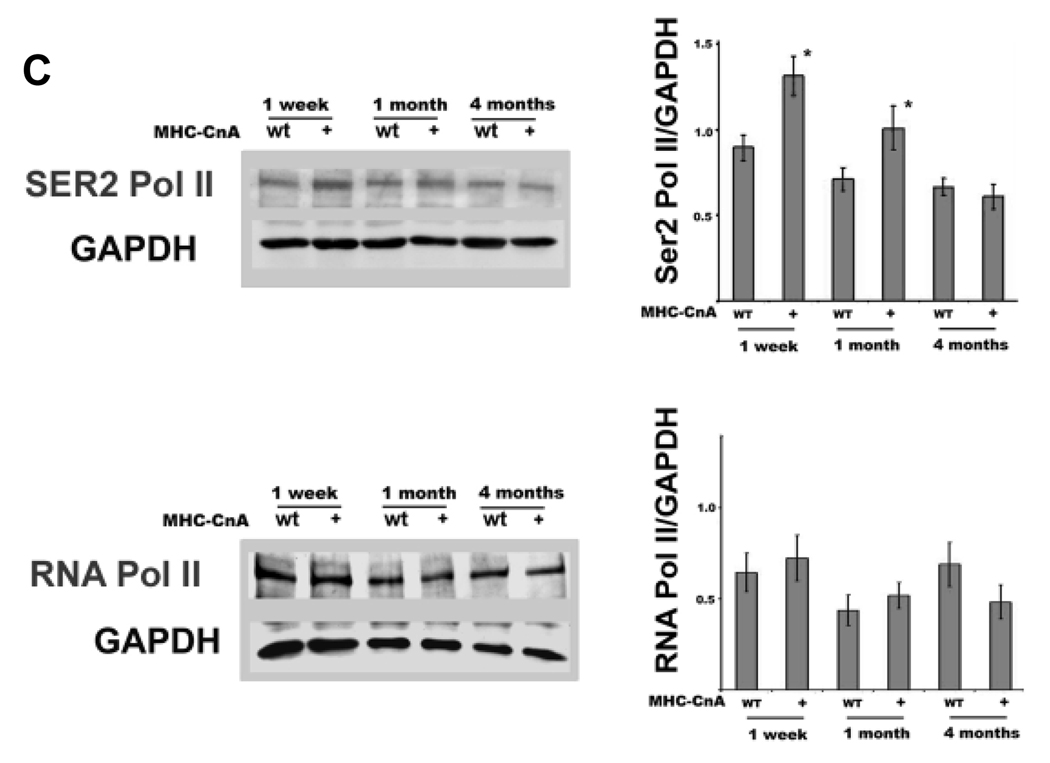
To examine the levels of CLP-1 associated with the P-TEFb complex in MHC-CnA hypertrophic hearts, we performed immunoprecipitations with the cyclin T1 antibody and western blotting with an antibody against CLP-1. Figure 1B shows that there was a decrease in binding of CLP-1 to P-TEFb in young hearts, but not in four months old hearts. The cyclin T1 levels, on the other hand, remained unchanged between littermates. The major substrate for phosphorylation by cdk9 activity is serine 2 of CTD terminal of Pol II (Ser2 Pol II) which allows Pol II to produce full-length RNA transcripts. We determined the phosphorylation status of Pol II by immunobloting protein extracts of MHC-CnA hearts with monoclonal antibody specific to Ser2 Pol II. We observed an increase in phosphorylation in the MHC-CnA heart at one-week (P<0.05, 47%) and one-month old mice (P<0.05, 42%), but not in four-month old mice (Figure 1C, upper panel). There was no significant change in the total RNA Pol II levels of MHC-CnA in comparison with wild type littermates (one way ANOVA, P>0.05) (lower panel).
CLP-1 mediates cardiac hypertrophy in the MHC-cyclin T1/CLP-1+/− mice
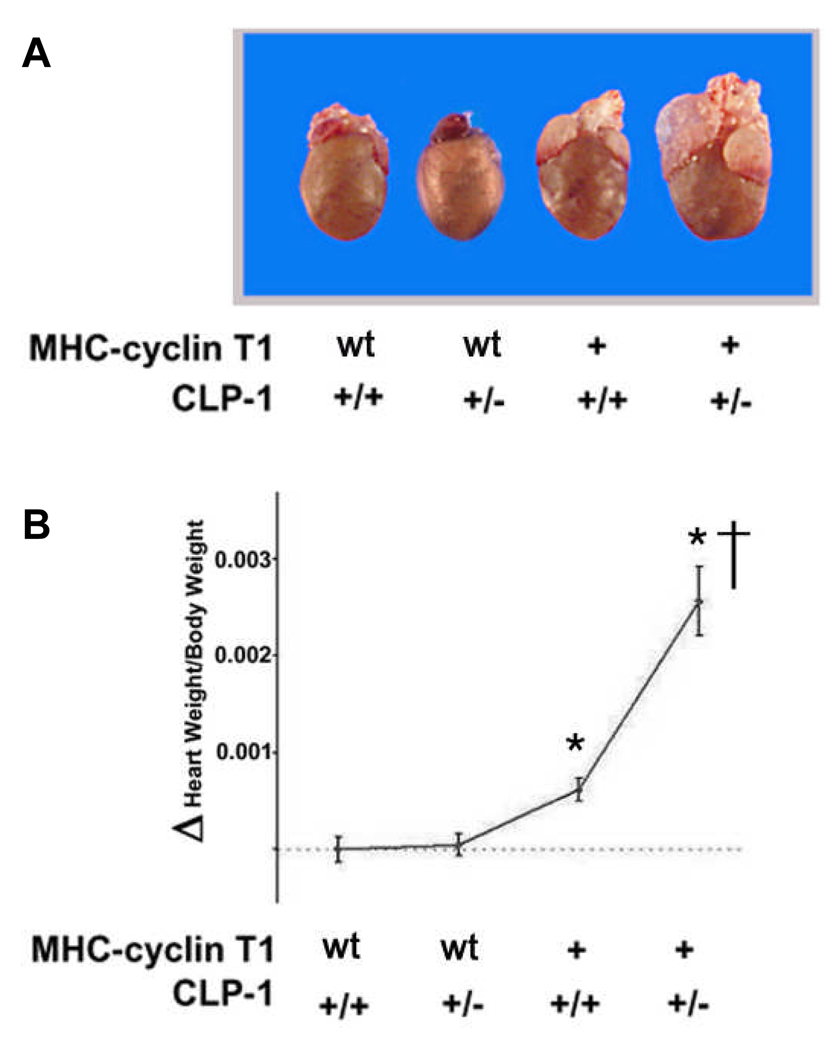
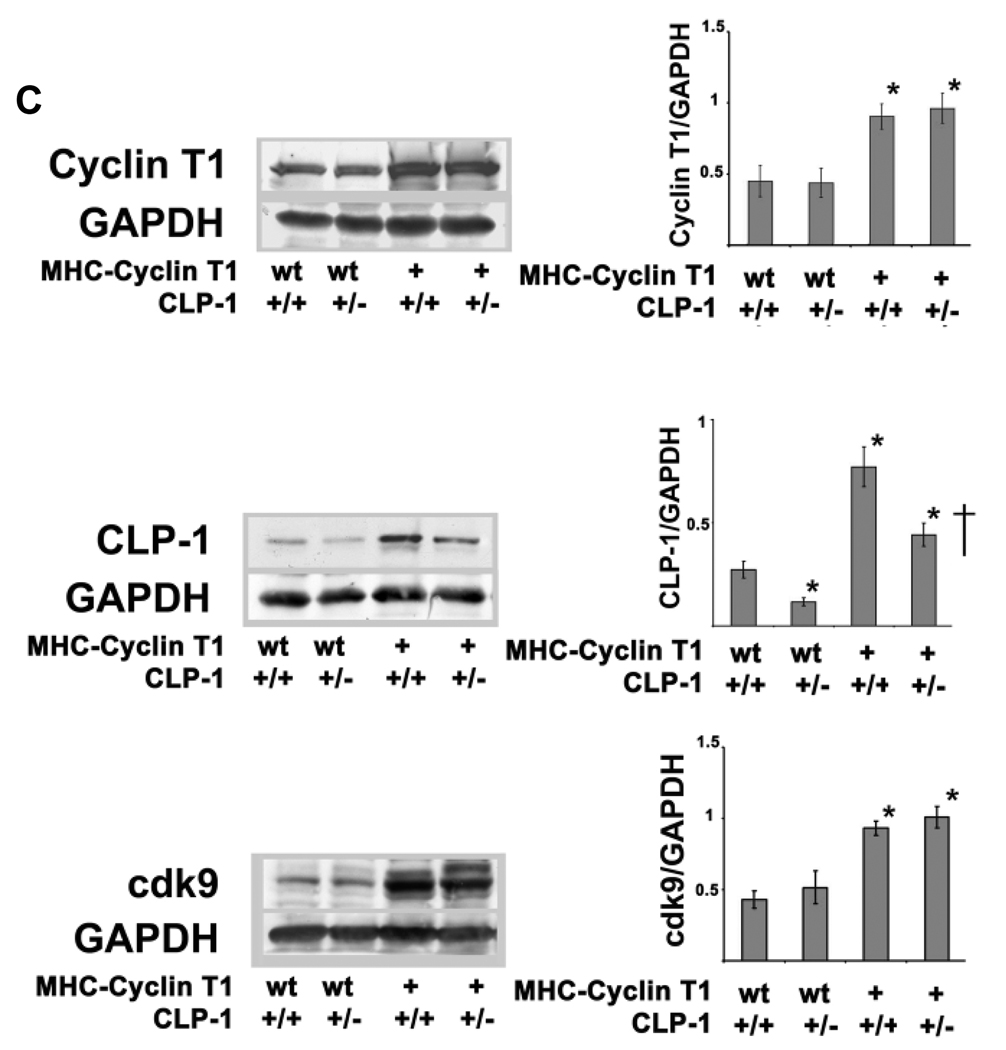
We have previously shown a correlation of de-repression of the P-TEFb activity with the onset of hypertrophy22. We have produced a CLP-1 knockout mouse which exhibits the physical and genetic hallmarks of cardiac hypertrophy and dies during late fetal development, Whereas CLP-1 heterozygote mice appear to be normal and survive without any phenotypic abnormalities 21. We did not observe a change in the interaction between CLP-1 and P-TEFb in wild type and CLP-1 heterozygote mice (see Online Figure I). Cdk9 exerts its kinase activity only when associated with its cyclin partner 14. The overexpression in the heart of cyclin T1 promotes hypertrophy and increase cdk9 activity; however, cdk9 overexpression does not increase cdk9 activity or produce cardiac growth 7, 25, suggesting that cyclin T1 is limiting for the activation of the P-TEFb complex. To establish a direct cause-effect relationship between P-TEFb activity and cardiac hypertrophy by further derepressing the P-TEFb, we used the previously described transgenic mice model, the MHC-cyclin T1 mouse (kindly provided by Dr. Michael Schneider).We used this model to test if decreased levels of CLP-1, a negative regulator of P-TEFb activity, could in combination with elevated levels of the cyclin T1 activator, further increases P-TEFb activity and lead to a more robust hypertrophy. We therefore crossed the MHC-cyclin T1 mice with CLP-1 heterozygotes to produce bigenic offspring (MHC-cyclin T1/CLP-1+/−). These offspring develop significant ventricular hypertrophy with enlargement of the atrium (Figure 2A) that resemble morphologically the MHC-cyclin T1 mice that were subjected to transaortic constriction25. Three month old MHC-cyclin T1/CLP-1+/− mice have increased heart/body ratio in comparison with the control littermates (Figure 2B). The statistical significance by one-way ANOVA was calculated on heart/body ratio and the analysis was found to be significant F (3,48) = 29.05, P<0.01. The heart/body ratios of MHC-cyclin T1/CLP-1+/− mice show a 56% increase in comparison to wild type (T-student, p<0.01), and a 37% increase in comparison with MHC-cyclin T1 mice (T-student, p<0.01). Moreover, there was a 14% increase in MHC-cyclin T1 in comparison with wild type (T-student, p<0.05). The Western blots in the upper panel of Figure 2C show a 102% increase in expression of cyclin T1 protein in the MHC-cyclin T1 transgenic and a 115% increase in the MHC-cyclin T1/CLP-1+/− in comparison with wild type mice (T-student, p<0.01). There was not a significant change between the wild type and CLP-1 heterozygote and between MHC-cyclin T1 and MHC-cyclin T1/CLP-1+/− mice in the cyclin T1 protein levels (T-student, p>0.05). The Western blots in the middle of panel Figure 2C show a 55% decrease expression of CLP-1 in the CLP-1 heterozygote in comparison with the control animals (T-student, p<0.05). There is a 47 % decreased expression of cyclin T1 in the MHC-cyclin T1/CLP-1+/− in comparison with the MHC-cyclin T1 (T-student, p<0.05). Moreover, we observed a secondary increase in the CLP-1 level in the MHC-cyclin T1 (P<0.05, 199%) and MHC-cyclin T1/CLP-1+/− (P<0.05, 89%) in comparison with the wild type possible because of the cyclin T1 dependent chaperon pathway that stabilize the complex 28. Western blots in the lower panel of Figure 2C show a 115% increased expression of cdk9 protein in the MHC-cyclin T1 transgenic and a 133% in the MHC-cyclin T1/CLP-1+/− in comparison with wild type mice (T-student, p<0.01). There was not a significant change between the wild type and CLP-1 heterozygote and between MHC-cyclin T1 and MHC-cyclin T1/CLP-1+/− mice in the cdk9 protein levels (T-student, p>0.05). Interestingly, we observed an increased reactivity of a small band over the cdk9 band from the MHC-cyclin T1/CLP-1+/− extracts which may be the autophosphorylated cdk929. Together, these data suggest a synergistic relationship between CLP-1 heterozygosity and the cyclin T1 overexpression that results in a more robust hypertrophy and strongly implicates CLP-1 in the regulation of hypertrophic growth. We did not observe a compensatory increase in the CLP-1 related protein HEXIM2 in response to the changes of levels of CLP-1 and P-TEFb (see Online Figure II; one way ANOVA, P>0.05).
Figure 2.
Exacerbated hypertrophy response in MHC-Cyclin T1/CLP-1+/− mice. A, photograph of adult mice hearts of the indicated genotypes. B, heart/body weight ratios in MHC-Cyclin T1 and MHC-Cyclin T1/CLP-1+/− mice. The data shown are means +/− SE of the Δheart/body ratio. *P < 0.05 versus wild type of three-month old mice (n =13). C, Western blots showing levels of CLP-1, Cyclin T1 and cdk9 hearts of the indicated genotypes of three-month old mice. GAPDH was used as loading control (n = 5). *P < 0.05 versus control; †P < 0.05 versus MHC-Cyclin T1.
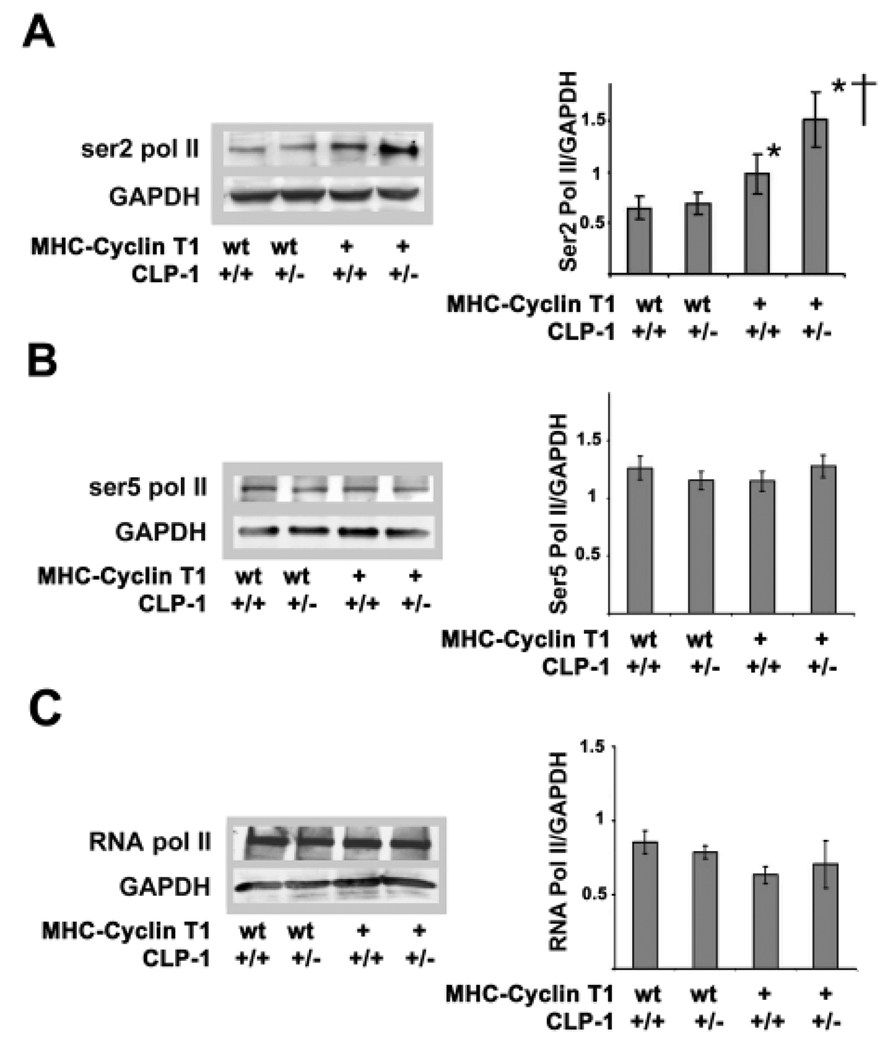
CLP-1 deficiency enhances cdk9-mediated phosphorylation of Pol II in MHC-cyclin T1/CLP-1+/− mice
Phosphorylation of Pol II at serine 2 and serine 5 sites by cdk9 and cdk7 respectively plays a fundamental role in RNA synthesis. It appears that there is a selectivity in utilizing either or both the kinases depending upon the nature of hypertrophic stimuli 7, 30. For example, activation of cdk9 alone was shown to be involved in hypertrophy triggered by acute aortic banding or in the agonist endothelin-induced hypertrophy in cardiomyocytes in culture, whereas both kinases were activated in most animal models with genetic signaling for cardiac growth 7. We, therefore, examined the phosphorylation state of Pol II in the bigenic MHC-cyclin T1/CLP-1+/− mice with monoclonal antibodies specific to each phosphorylation site of the CTD terminus of Pol II. The Western blot in Figure 3A shows an increased level of phosphorylation of Ser2 of the CTD terminal in the MHC-cyclin T1/CLP-1+/− mice in comparison with the wild type and MHC-cyclin T1 mice. The increase in Ser2 Pol II phosphorylation in MHC-cyclin T1/CLP-1+/− heart extracts compared to extracts from wild type mice was 133% (P<0.05) and 55% compared with MHC-cyclin T1 (P<0.05). There was a 51% increase in phosphorylation of Ser2 Pol II in MHC-cyclin T1 mice (P<0.05) compared with wild type mice. We did not notice a change in Ser2 Pol II phosphorylation in CLP-1+/− in comparison with the wild type mice. In contrast, the levels of ser5 Pol II phosphorylation and total RNA Pol II remained basically unchanged (Figure 3B & 3C; one way ANOVA, P>0.05). These data suggest that the decreased CLP-1 level and increased cyclin T1 level act synergistically to increase activity of Cdk9 to phosphorylate RNA Pol II and increase transcriptional elongation of RNA in myocardial hypertrophy.
Figure 3.
Phosphorylation levels of CTD RNA Pol II in MHC-Cyclin T1 and MHC-Cyclin T1/CLP-1+/− mice. A, anti-Ser2 Pol II immunoblot of heart lysates showing phosphorylation levels of RNA Pol II in MHC-Cyclin T1 and MHC-Cyclin T1/CLP-1+/− mice. B, anti-Ser5 Pol II immunoblot heart lysates showing phosphorylation levels of CTD RNA Pol II. C, anti-RNA Pol II immunoblot heart lysates showing total protein levels of RNA Pol II. Data are expressed as mean ± SE of five independent experiments. *P < 0.05 versus control. *P < 0.05 versus control; †P < 0.05 versus MHC-Cyclin T1.
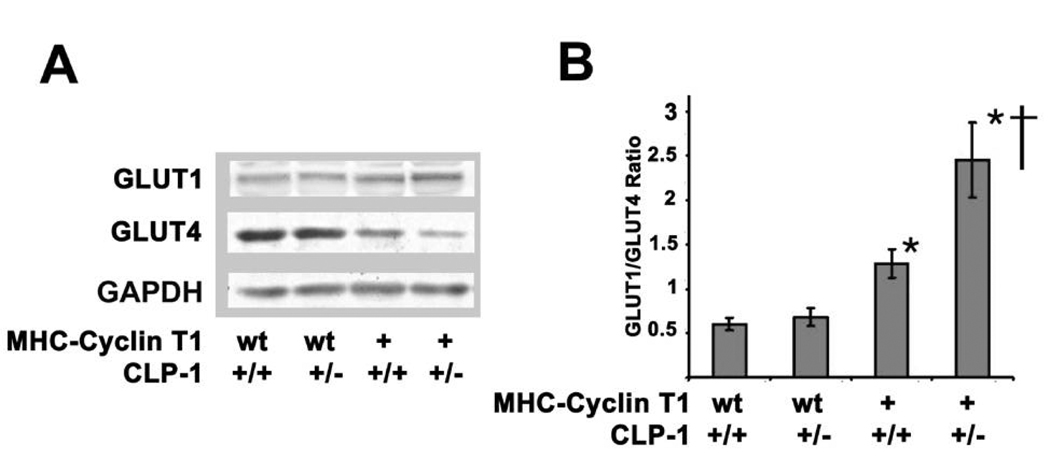
Modulation in GLUT transporter expression in cyclin T1/CLP-1+/− mice
The heart, under normal conditions, derives its energy from oxidation of lipids, but under pathological conditions, such as, myocardial hypertrophy, it becomes increasingly dependent upon glucose oxidation with an increase in GLUT1/GLUT4 ratio6. The global gene knockout of glucose transporter GLUT4 in mice produces cardiac hypertrophy and heart failure 31, but cardiac specific deletion of GLUT4 resulted in development of compensory hypertrophy with normal life span 32. To determine whether altered GLUT expression is also a feature of hypertrophy induced by the CLP-1 de-regulation of the P-TEFb complex in the bigenic mice, we examined GLUT1 and GLUT4 proteins by Western blotting of heart extracts as above (Figure 4). We observed a 4.1 fold increase in GLUT1/GLUT4 ratio in the MHC-cyclin T1/CLP-1+/− compared with wild type mice (P<0.01) and these bigenic mice have a 2.6 fold increase when compared with MHC-cyclin T1 mice (P<0.05). The MHC-cyclin T1 mice show about a two-fold increase in the GLUT1/GLUT4 ratio in comparison with wild type mice. No change was observed in the CLP-1+/− mice. Therefore, it appears that the changes in GLUT1/GLUT4 ratio in the MHC-cyclin T1/CLP-1+/− mice mimic the metabolic remodeling observed in cardiac hypertrophy.
Figure 4.
Glucose transporters GLUT1/GLUT4 ratio in MHC-Cyclin T1/CLP-1+/− mice. A, Western blot shows expression levels of GLUT1, GLUT4, and GAPDH in heart lysates of the indicated genotypes. B, quantification of GLUT1/GLUT4 ratio. Data are expressed as mean ± SE of five independent experiments of three-month old mice. *P < 0.05 versus control; †P < 0.05 versus MHC-Cyclin T1.
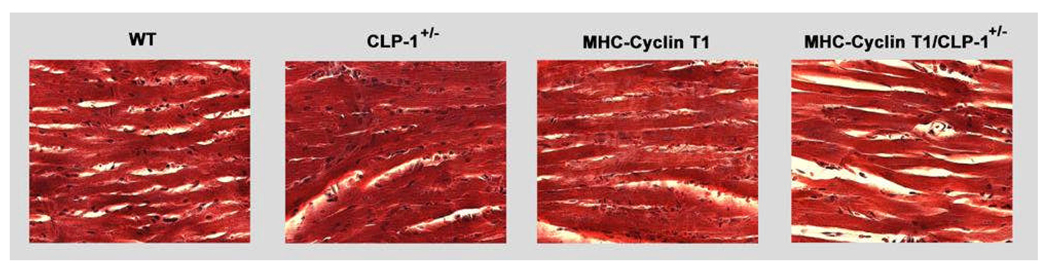
MHC-cyclin T1/CLP-1+/− mice show compensated hypertrophy
In failing hearts, there is disruption of the equilibrium between the synthesis and degradation of types I and III collagens resulting in excessive aggregation of collagen types I and III fibers within the myocardium33. To ascertain whether the MHC-cyclin T1/CLP-1+/− mice heart develop fibrotic foci, we did histological examination of ventricular tissue by Masson trichrome-staining and found no increase in interstitial deposition in MHC-cyclin T1/CLP-1+/− mice (Figure 5).
Figure 5.
Mason trichrome staining of representative ventricular transverse sections of the indicated genotypes (three-month old mice) illustrating the absence of any marked increase in interstitial collagen (which stains blue).
Echocardiography is considered an important tool in assessing the phenotype and in defining the physiological functionality of genetically altered mice that exhibit cardiomyopathies. We assessed the left ventricular dimensions and contractile function of MHC-cyclin T1/CLP-1+/− transgenic mice by using M-mode echocardiography in five-month MHC-cyclin T1/CLP-1+/− mice (Table 1). The bigenic mice show a significant increase in LV end diastolic diameter and LV mass compared to with the MHC-cyclin T1 and wild type littermates. Fractional shortening, an echocardiographic index of LV contractile function, of MHC-cyclin T1/CLP-1+/− mice was normal. No discernable apoptotic changes were noted in MHC-cyclin T1/CLP-1+/− mice based on Bcl-2 and Bax expression (unpublished observations). MHC-cyclin T1/CLP-1+/− mice were born with the expected Mendelian frequency, indicating lack of embryonic lethality. The bigenic mice (3 males and 3 females) survived longer than 17 months, implying that there was no reduction in the life span of these mice. Together, these data indicate that MHC-cyclin T1 mice when challenged with CLP-1 heterozygosity exhibit heightened physiological and metabolic features characteristic of the compensatory phase of hypertrophy, supporting the role of CLP-1 in regulation of myocardial hypertrophy.
TABLE 1.
Echocardiographic evaluation of MHC-Cyclin T1/CLP-1+/− mice.
| Parameter | Genotype | |||
|---|---|---|---|---|
| WT | CLP-1+/− | MHC-Cyclin T1 | MHC-Cyclin T1/CLP-1+/− | |
| Heart rate (Beats/min) |
628+/− 24 | 618+/− 35 | 614 +/− 24 | 616 +/− 36 |
| LVESD (mm) |
0.095+/− 0.0086 | 0.0092 +/− 0.009 | 0.104 +/− 0.0091 | 0.120 +/− 0.00124 |
| LVEDD (mm) |
0.22 +/− 0.012 | 0.218 +/− 0.008 | 0.234 +/− 0.01* | 0.262 +/− 0.001*† |
| LV mass (mg) |
0.070 +/−0.0035 | 0.067 +/− 0.0055 | 0.078 +/− 0.0058 | 0.094 +/− 0.0073* |
| FS (%) |
0.57 +/− 0.023 | 0.58 +/− 0.038 | 0.55 +/− 0.050 | 0.54 +/− 0.043 |
Values are mean ±SE. Only data for five-month mice are shown.
Abbreviations: LVESD—Left Ventricular end-systolic diameter; LVEDD—LV enddiastolic diameter; LV mass— LV mass; FS—fractional shortening
P < 0.05 versus control
P < 0.05 versus MHC-Cyclin T1
Discussion
Substantial progress has been made in understanding the mechanisms that control the selective and differential expression of genes associated with the fetal gene expression program in the hypertrophic heart. However, the control of global expression of RNA, the most distinctive feature of cardiac hypertrophic growth, still remains enigmatic. Emerging evidence supports the notion that RNA elongation serves as an important step for control of gene expression. Recent data underscore the regulatory function of CLP-1 as a dynamic component of the P-TEFb complex that serves in its capacity to inhibit cdk9 kinase activity as a critical regulatory switch controlling RNA Pol II function and RNA synthesis. In this study, we have shown the role of CLP-1 in regulation of the P-TEFb activity and thereby in modulation of growth in cardiac hypertrophy. We have demonstrated that de-repression of P-TEFb complex activity correlates with decreased expression of CLP-1 in MHC-CnA mice. Calcineurin plays a significant role in physiological and pathological hypertrophy 34, 35. Activation of P-TEFb via dissociation of CLP-1 occurs predominately during the initial compensatory hypertrophic stage and there appears to be no clear correlative connection of CLP-1 with the cardiac dysfunction phase. Four-month old MHC-CnA hearts, which exhibit pathological phase of hypertrophy, show no difference in CLP-1 levels when compared to age-matched mice, suggesting that the reduced association of CLP-1 with P-TEFb and increase in cdk9 activity may contribute to the initial genomic response to hypertrophic stimuli that occurs during compensatory hypertrophy rather than the transition to heart failure. While modulations in the levels of CLP-1 are linked to changes in P-TEFb activity, we can not exclude the possibility that other factors can also contribute to regulation of P-TEFb function. Our studies, nevertheless, suggest that CLP-1 acts like a molecular switch to promote or inhibit RNA synthesis by regulating cdk9 kinase activity and the phosphorylation state of RNA Pol II. Upon decreased binding of CLP-1 to P-TEFb, cdk9 phosphorylates serine 2 of the CTD of RNA Pol II, releasing it from the “abortive state” to a state that promotes full length RNA chain elongation 14, 17. An alternative way of increasing cdk9 activity is to increase the amount of cyclin T1 in relation to cdk97. Because, the CLP-1 conventional knockout is likely to have an affect on non-myocytes and other tissues, our approach was to see if a change in relative amounts of cyclin T1 and CLP-1 targeted to myocardium alone is sufficient to induce change in cardiac growth. Cyclin T1 is the limiting molecule for the P-TEFb activity in the heart and the cdk9/cyclin T1 heterodimer is degraded very slowly in comparison with the free cdk9 maybe due to a chaperon dependent pathway 28. As such, our data suggest that the levels of CLP-1 and P-TEFb are regulated by the cyclin T1 levels. Furthermore, a change in the CLP-1/Cyclin T1 equilibrium is sufficient to release from its inhibited state to activate RNA Pol II and increased RNA synthesis. Such alteration in CLP-1/cyclin T1 equilibrium is also likely to occur in response to hypertrophy stimuli via the dissociation of CLP-1 from the P-TEFb complex. We have previously shown this to be the case in cardiomyocytes rendered hypertrophic by mechanical stretch and application of hypertrophic agonist22. We have thus documented by two alternative approaches, one an stimulus-dependent generation of hypertrophy, in which CLP-1 dissociates from the P-TEFb, the other by genetic alteration of CLP-1 and cyclin T1 levels to produce increased cdk9 activity to show that CLP-1’s regulation of P-TEFb is a critical point in the response of cardiomyocytes to hypertrophic stimuli.
It is well established that the hypertrophic heart becomes dependent upon glucose for its energy. GLUT4, an insulin-responsive glucose transporter that allows the heart to derive its energy via the uptake of glucose, is down-regulated in various animal models of hypertrophy6. The cardiac specific GLUT4 knockout mice develop compensatory hypertrophy without fibrosis and have normal life spans32, while the cardiac specific overexpression of GLUT1 improves cardiac function and restrains heart failure in mice subjected to aortic constriction36. Moreover drugs, such as ranolazine and trimetazine, which stimulate the use of glucose, ameliorate cardiac function in heart failure models. It appears therefore that CLP-1 mediated derepression of P-TEFb complex promotes differential expression of GLUT transporters with an increase in GLUT1/GLUT4 ratio which is consistent with previously reported changes in GLUT transporters as an initial response to the hypertrophic stimulus.
Our data indicated that in agreement with a previous report7, an increase in cdk9 activity leads to compensatory hypertrophy with no discernable increase in collagen deposition in the MHC-Cyclin T1/CLP-1+/− mice in comparison with their control littermates. Also, we did not see a change in Bcl-2/Bax ratio (unpublished data). On the other hand, the MHC-cyclin T1/MHC-Gq bigenic mice, produced by the crossing MHC-Cyclin T1 and MHC-Gq mice, develop fibrosis and increased apoptosis which leads to heart failure25. This suggests that the increase in P-TEFb activity alone may not be sufficient to produce a transition to heart failure. Activation of specific signaling molecules and transcription factors may target specific genes such as mitochondrial energy-metabolizing genes whose altered expression may facilitate entry into descompensatory transition and failure. Therefore, although the dynamics of association/dissociation of CLP-1 from P-TEFb alone is sufficient to regulate the compensatory hypertrophic growth, other components of the transcriptional machinery are likely to be involved in its transition to heart failure. The activation of the Jak/STAT signaling pathway can induce P-TEFb activity by direct binding of STAT3 and P-TEFb37, 38. STAT3 is a well-documented signaling molecule in the cardiac hypertrophic response1. Our recent observation that CLP-1 does not dissociate from P-TEFb in the presence of Jak2 inhibitor, AG490, suggest that the association/dissociation of CLP-1 from the complex may be regulated by Jak2 signaling22. In summary, it appears that derepression of P-TEFb by dissociation and/or decreased levels of CLP-1 may be an important facet of the hypertrophic response and emphasizes the importance of CLP-1 as the regulator of RNA synthesis in development of cardiac hypertrophy.
Acknowledgments
Sources of funding
This work was supported by National Institutes of Health grant HL073399 to M.A.Q.S.
Footnotes
Subject codes: [15] Hypertrophy, [142] Gene expression, [143] Gene regulation, [145] Genetically altered mice, [155] Physiological and pathological control of gene expression
Disclosures
None.
Contributor Information
Jorge Espinoza-Derout, Department of Anatomy and Cell Biology, State University of New York Downstate Medical Center, 450 Clarkson Avenue, Brooklyn, NY 11203.
Michael Wagner, Department of Anatomy and Cell Biology, State University of New York Downstate Medical Center, 450 Clarkson Avenue, Brooklyn, NY 11203.
Louis Salciccioli, Center for Cardiovascular and Muscle Research and Division of Cardiology, State University of New York Downstate Medical Center, 450 Clarkson Avenue, Brooklyn, NY 11203.
Jason M. Lazar, Center for Cardiovascular and Muscle Research and Division of Cardiology, State University of New York Downstate Medical Center, 450 Clarkson Avenue, Brooklyn, NY 11203
Sikha Bhaduri, Department of Anatomy and Cell Biology, State University of New York Downstate Medical Center, 450 Clarkson Avenue, Brooklyn, NY 11203.
Eduardo Mascareno, Department of Anatomy and Cell Biology, State University of New York Downstate Medical Center, 450 Clarkson Avenue, Brooklyn, NY 11203.
Brahim Chaqour, Department of Anatomy and Cell Biology, State University of New York Downstate Medical Center, 450 Clarkson Avenue, Brooklyn, NY 11203.
M.A.Q. Siddiqui, Department of Anatomy and Cell Biology, State University of New York Downstate Medical Center, 450 Clarkson Avenue, Brooklyn, NY 11203
References
- 1.Wagner M, Mascareno E, Siddiqui MA. Cardiac hypertrophy: signal transduction, transcriptional adaptation, and altered growth control. Ann N Y Acad Sci. 1999;874:1–10. doi: 10.1111/j.1749-6632.1999.tb09219.x. [DOI] [PubMed] [Google Scholar]
- 2.MacLellan WR, Schneider MD. Genetic dissection of cardiac growth control pathways. Annu Rev Physiol. 2000;62:289–319. doi: 10.1146/annurev.physiol.62.1.289. [DOI] [PubMed] [Google Scholar]
- 3.Heineke J, Molkentin JD. Regulation of cardiac hypertrophy by intracellular signalling pathways. Nat Rev Mol Cell Biol. 2006;7:589–600. doi: 10.1038/nrm1983. [DOI] [PubMed] [Google Scholar]
- 4.van Empel VP, De Windt LJ. Myocyte hypertrophy and apoptosis: a balancing act. Cardiovasc Res. 2004;63:487–499. doi: 10.1016/j.cardiores.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 5.Olson EN, Schneider MD. Sizing up the heart: development redux in disease. Genes Dev. 2003;17:1937–1956. doi: 10.1101/gad.1110103. [DOI] [PubMed] [Google Scholar]
- 6.Abel ED. Glucose transport in the heart. Front Biosci. 2004;9:201–215. doi: 10.2741/1216. [DOI] [PubMed] [Google Scholar]
- 7.Sano M, Abdellatif M, Oh H, Xie M, Bagella L, Giordano A, Michael LH, DeMayo FJ, Schneider MD. Activation and function of cyclin T-Cdk9 (positive transcription elongation factor-b) in cardiac muscle-cell hypertrophy. Nat Med. 2002;8:1310–1317. doi: 10.1038/nm778. [DOI] [PubMed] [Google Scholar]
- 8.McKinsey TA, Olson EN. Cardiac hypertrophy: sorting out the circuitry. Curr Opin Genet Dev. 1999;9:267–274. doi: 10.1016/s0959-437x(99)80040-9. [DOI] [PubMed] [Google Scholar]
- 9.Lim HW, Molkentin JD. Calcineurin and human heart failure. Nat Med. 1999;5:246–247. doi: 10.1038/6430. [DOI] [PubMed] [Google Scholar]
- 10.Doud SK, Pan LX, Carleton S, Marmorstein S, Siddiqui MA. Adaptational response in transcription factors during development of myocardial hypertrophy. J Mol Cell Cardiol. 1995;27:2359–2372. doi: 10.1016/s0022-2828(95)92019-6. [DOI] [PubMed] [Google Scholar]
- 11.Mathew S, Mascareno E, Siddiqui MA. A ternary complex of transcription factors, Nished and NFATc4, and co-activator p300 bound to an intronic sequence, intronic regulatory element, is pivotal for the up-regulation of myosin light chain-2v gene in cardiac hypertrophy. J Biol Chem. 2004;279:41018–41027. doi: 10.1074/jbc.M403578200. [DOI] [PubMed] [Google Scholar]
- 12.Johnatty SE, Dyck JR, Michael LH, Olson EN, Abdellatif M. Identification of genes regulated during mechanical load-induced cardiac hypertrophy. J Mol Cell Cardiol. 2000;32:805–815. doi: 10.1006/jmcc.2000.1122. [DOI] [PubMed] [Google Scholar]
- 13.Aronow BJ, Toyokawa T, Canning A, Haghighi K, Delling U, Kranias E, Molkentin JD, Dorn GW., 2nd Divergent transcriptional responses to independent genetic causes of cardiac hypertrophy. Physiol Genomics. 2001;6:19–28. doi: 10.1152/physiolgenomics.2001.6.1.19. [DOI] [PubMed] [Google Scholar]
- 14.Zhou Q, Yik JH. The Yin and Yang of P-TEFb regulation: implications for human immunodeficiency virus gene expression and global control of cell growth and differentiation. Microbiol Mol Biol Rev. 2006;70:646–659. doi: 10.1128/MMBR.00011-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Orphanides G, Reinberg D. A unified theory of gene expression. Cell. 2002;108:439–451. doi: 10.1016/s0092-8674(02)00655-4. [DOI] [PubMed] [Google Scholar]
- 16.Sano M, Schneider MD. Cyclins that don't cycle--cyclin T/cyclin-dependent kinase-9 determines cardiac muscle cell size. Cell Cycle. 2003;2:99–104. [PubMed] [Google Scholar]
- 17.Yik JH, Chen R, Nishimura R, Jennings JL, Link AJ, Zhou Q. Inhibition of P-TEFb (CDK9/Cyclin T) kinase and RNA polymerase II transcription by the coordinated actions of HEXIM1 and 7SK snRNA. Mol Cell. 2003;12:971–982. doi: 10.1016/s1097-2765(03)00388-5. [DOI] [PubMed] [Google Scholar]
- 18.Michels AA, Nguyen VT, Fraldi A, Labas V, Edwards M, Bonnet F, Lania L, Bensaude O. MAQ1 and 7SK RNA interact with CDK9/cyclin T complexes in a transcription-dependent manner. Mol Cell Biol. 2003;23:4859–4869. doi: 10.1128/MCB.23.14.4859-4869.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ghatpande S, Goswami S, Mascareno E, Siddiqui MA. Signal transduction and transcriptional adaptation in embryonic heart development and during myocardial hypertrophy. Mol Cell Biochem. 1999;196:93–97. [PubMed] [Google Scholar]
- 20.Huang F, Wagner M, Siddiqui MA. Structure, expression, and functional characterization of the mouse CLP-1 gene. Gene. 2002;292:245–259. doi: 10.1016/s0378-1119(02)00596-6. [DOI] [PubMed] [Google Scholar]
- 21.Huang F, Wagner M, Siddiqui MA. Ablation of the CLP-1 gene leads to down-regulation of the HAND1 gene and abnormality of the left ventricle of the heart and fetal death. Mech Dev. 2004;121:559–572. doi: 10.1016/j.mod.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 22.Espinoza-Derout J, Wagner M, Shahmiri K, Mascareno E, Chaqour B, Siddiqui MA. Pivotal role of cardiac lineage protein-1 (CLP-1) in transcriptional elongation factor P-TEFb complex formation in cardiac hypertrophy. Cardiovasc Res. 2007;75:129–138. doi: 10.1016/j.cardiores.2007.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Molkentin JD, Lu JR, Antos CL, Markham B, Richardson J, Robbins J, Grant SR, Olson EN. A calcineurin-dependent transcriptional pathway for cardiac hypertrophy. Cell. 1998;93:215–228. doi: 10.1016/s0092-8674(00)81573-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Semeniuk LM, Severson DL, Kryski AJ, Swirp SL, Molkentin JD, Duff HJ. Time-dependent systolic and diastolic function in mice overexpressing calcineurin. Am J Physiol Heart Circ Physiol. 2003;284:H425–H430. doi: 10.1152/ajpheart.00546.2002. [DOI] [PubMed] [Google Scholar]
- 25.Sano M, Wang SC, Shirai M, Scaglia F, Xie M, Sakai S, Tanaka T, Kulkarni PA, Barger PM, Youker KA, et al. Activation of cardiac Cdk9 represses PGC-1 and confers a predisposition to heart failure. Embo J. 2004;23:3559–3569. doi: 10.1038/sj.emboj.7600351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takuma S, Suehiro K, Cardinale C, Hozumi T, Yano H, Shimizu J, Mullis-Jansson S, Sciacca R, Wang J, Burkhoff D, et al. Anesthetic inhibition in ischemic and nonischemic murine heart: comparison with conscious echocardiographic approach. Am J Physiol Heart Circ Physiol. 2001;280:H2364–H2370. doi: 10.1152/ajpheart.2001.280.5.H2364. [DOI] [PubMed] [Google Scholar]
- 27.Beckles DL, Mascareno E, Siddiqui MA. Inhibition of Jak2 phosphorylation attenuates pressure overload cardiac hypertrophy. Vascul Pharmacol. 2006;45:350–357. doi: 10.1016/j.vph.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 28.O'Keeffe B, Fong Y, Chen D, Zhou S, Zhou Q. Requirement for a kinase-specific chaperone pathway in the production of a Cdk9/cyclin T1 heterodimer responsible for P-TEFb-mediated tat stimulation of HIV-1 transcription. J Biol Chem. 2000;275:279–287. doi: 10.1074/jbc.275.1.279. [DOI] [PubMed] [Google Scholar]
- 29.Garber ME, Mayall TP, Suess EM, Meisenhelder J, Thompson NE, Jones KA. CDK9 autophosphorylation regulates high-affinity binding of the human immunodeficiency virus type 1 tat-P-TEFb complex to TAR RNA. Mol Cell Biol. 2000;20:6958–6969. doi: 10.1128/mcb.20.18.6958-6969.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abdellatif M, Packer SE, Michael LH, Zhang D, Charng MJ, Schneider MD. A Ras-dependent pathway regulates RNA polymerase II phosphorylation in cardiac myocytes: implications for cardiac hypertrophy. Mol Cell Biol. 1998;18:6729–6736. doi: 10.1128/mcb.18.11.6729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Katz EB, Stenbit AE, Hatton K, DePinho R, Charron MJ. Cardiac and adipose tissue abnormalities but not diabetes in mice deficient in GLUT4. Nature. 1995;377:151–155. doi: 10.1038/377151a0. [DOI] [PubMed] [Google Scholar]
- 32.Abel ED, Kaulbach HC, Tian R, Hopkins JC, Duffy J, Doetschman T, Minnemann T, Boers ME, Hadro E, Oberste-Berghaus C, et al. Cardiac hypertrophy with preserved contractile function after selective deletion of GLUT4 from the heart. J Clin Invest. 1999;104:1703–1714. doi: 10.1172/JCI7605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ritter O, Neyses L. The molecular basis of myocardial hypertrophy and heart failure. Trends Mol Med. 2003;9:313–321. doi: 10.1016/s1471-4914(03)00114-x. [DOI] [PubMed] [Google Scholar]
- 34.De Windt LJ, Lim HW, Bueno OF, Liang Q, Delling U, Braz JC, Glascock BJ, Kimball TF, del Monte F, Hajjar RJ, Molkentin JD. Targeted inhibition of calcineurin attenuates cardiac hypertrophy in vivo. Proc Natl Acad Sci U S A. 2001;98:3322–3327. doi: 10.1073/pnas.031371998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rothermel BA, McKinsey TA, Vega RB, Nicol RL, Mammen P, Yang J, Antos CL, Shelton JM, Bassel-Duby R, Olson EN, Williams RS, et al. Myocyte-enriched calcineurin-interacting protein, MCIP1, inhibits cardiac hypertrophy in vivo. Proc Natl Acad Sci U S A. 2001;98:3328–3333. doi: 10.1073/pnas.041614798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liao R, Jain M, Cui L, D'Agostino J, Aiello F, Luptak I, Ngoy S, Mortensen RM, Tian R. Cardiac-specific overexpression of GLUT1 prevents the development of heart failure attributable to pressure overload in mice. Circulation. 2002;106:2125–2131. doi: 10.1161/01.cir.0000034049.61181.f3. [DOI] [PubMed] [Google Scholar]
- 37.Hou T, Ray S, Brasier AR. The functional role of an interleukin 6-inducible CDK9.STAT3 complex in human gamma-fibrinogen gene expression. J Biol Chem. 2007;282:37091–37102. doi: 10.1074/jbc.M706458200. [DOI] [PubMed] [Google Scholar]
- 38.Giraud S, Hurlstone A, Avril S, Coqueret O. Implication of BRG1 and cdk9 in the STAT3-mediated activation of the p21waf1 gene. Oncogene. 2004;23:7391–7398. doi: 10.1038/sj.onc.1207972. [DOI] [PubMed] [Google Scholar]