Abstract
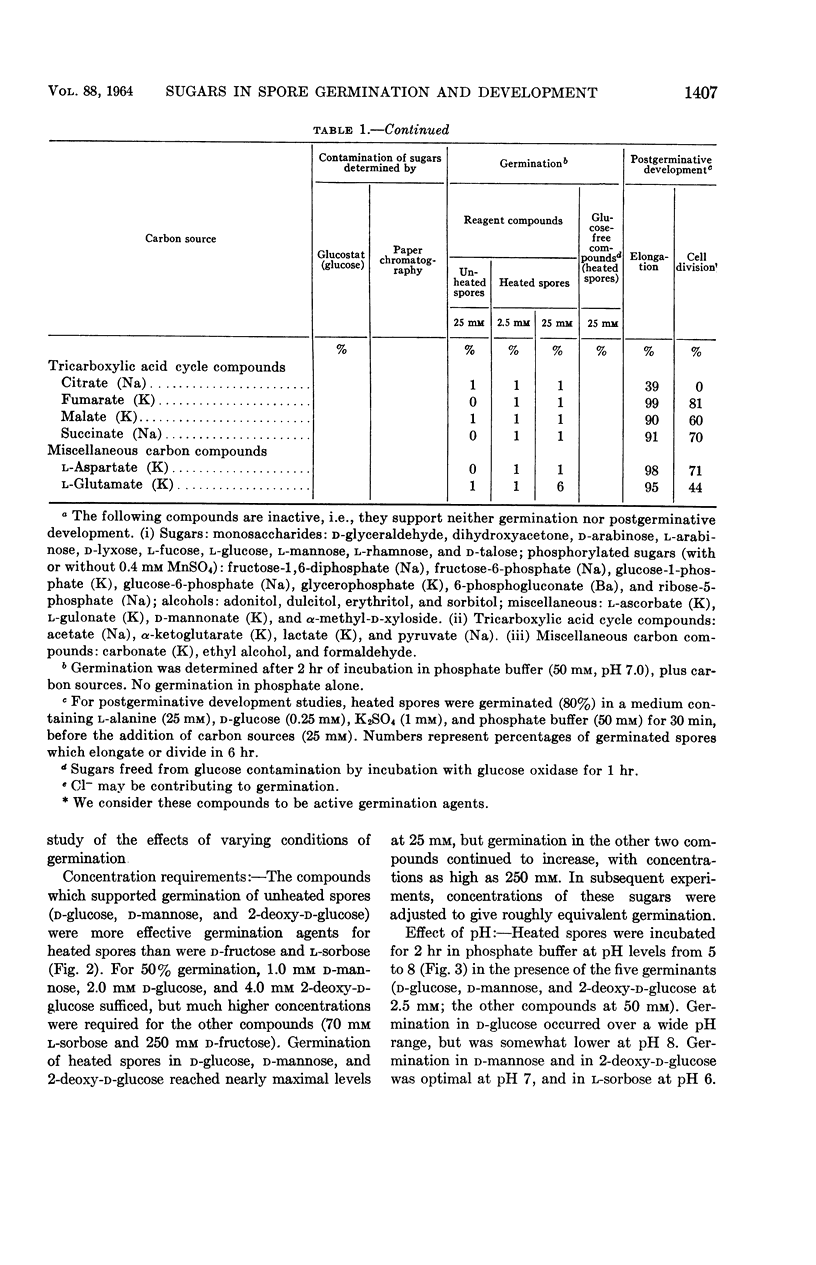
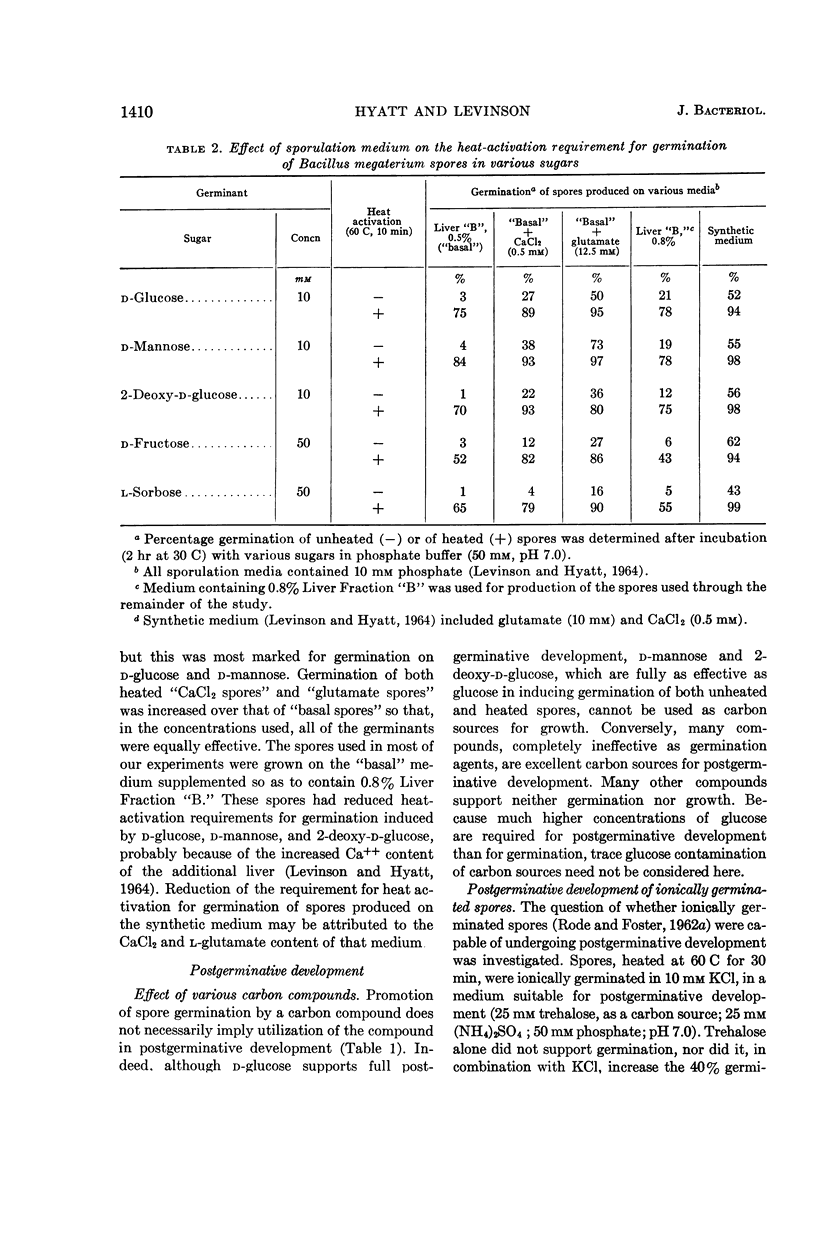
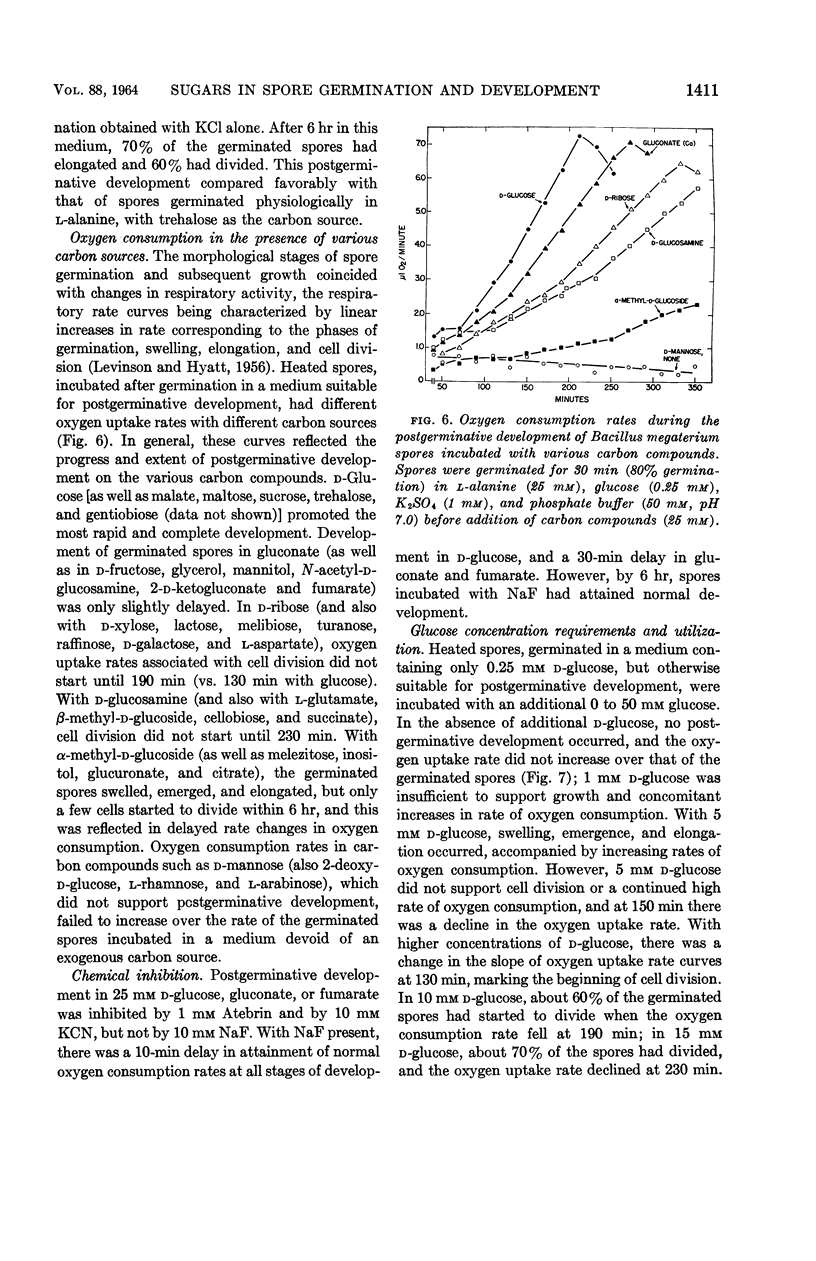
Hyatt, Mildred T. (Pioneering Research Division. U.S. Army Natick Laboratories, Natick, Mass.), and Hillel S. Levinson. Effect of sugars and other carbon compounds on germination and postgerminative development of Bacillus megaterium spores. J. Bacteriol. 88:1403–1415. 1964.—A total of 77 carbon-containing compounds were tested for their ability to support germination and postgerminative development of Bacillus megaterium spores. The only effective germination agents were certain of the hexose sugars and their derivatives. With unheated spores, only d-glucose, d-mannose, 2-deoxy-d-glucose, d-glucosamine, and N-acetyl-d-glucosamine (all at 25 mm) supported appreciable germination (ca. 25%). Heat-shock at 60 C for 10 min increased germination and decreased the concentration of sugar required for germination, so that these compounds, at 2.5 mm, supported 40 to 60% germination. Higher concentrations (25 mm) of other compounds, d-fructose, l-sorbose, d-allose, d-altrose, 2-hydroxyethyl-d-glucose, and β-methyl-d-glucoside, were required for appreciable germination of heated spores. Glucose or mannose contamination accounted for the germination apparently induced by certain other sugars. Ionic contamination did not appear to contribute to the germination induced by d-glucose, d-fructose, 2-deoxy-d-glucose, or l-sorbose. There was no clear-cut evidence for a multiplicity of metabolic pathways in the triggering of B. megaterium spore germination by various sugars. Postgerminative development of germinated spores was supported by a wider variety of carbon compounds, including some pentoses and hexoses, many oligosaccharides, sugar derivatives, some alcohols, and some of the tricarboxylic acid cycle intermediates. Compounds effective for germination were not necessarily utilizable for growth, and vice versa. Oxygen consumption rates reflected the progress and extent of postgerminative development on the various carbon compounds. Utilization of glucose during postgerminative development was followed, and the concentration requirements were determined.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CHURCH B. D., HALVORSON H. Intermediate metabolism of aerobic spores. I. Activation of glucose oxidation in spores of Bacillus cereus var terminalis. J Bacteriol. 1957 Apr;73(4):470–476. doi: 10.1128/jb.73.4.470-476.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOLDMAN M., BLUMENTHAL H. J. PATHWAYS OF GLUCOSE CATABOLISM IN BACILLUS CEREUS. J Bacteriol. 1964 Feb;87:377–386. doi: 10.1128/jb.87.2.377-386.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HACHISUKA Y., ASANO N., KANEKO M., KANBE T. Evolution of respiratory enzyme system during germination of Bacillus subtilis. Science. 1956 Jul 27;124(3213):174–175. doi: 10.1126/science.124.3213.174. [DOI] [PubMed] [Google Scholar]
- HACHISUKA Y., ASANO N., SUGAI K. Studies on spore germination. III. Development of enzymes concerning glucose oxidation during spore germination of Bacillus subtilis. Jpn J Microbiol. 1958 Jan;2(1):79–88. [PubMed] [Google Scholar]
- HACHISUKA Y., KATO N., ASANO N., KUNO T. Studies on spore germination. II. Effect of caramels from sugars and other carbon sources on spore germination. J Bacteriol. 1955 Apr;69(4):407–411. doi: 10.1128/jb.69.4.407-412.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HACHISUKA Y., SUGAI K. Studies on spore germination. IV. Relationship between germination and appearance of glucose dehydrogenase activity in B. subtilis spore. Jpn J Microbiol. 1959 Apr;3:211–222. doi: 10.1111/j.1348-0421.1959.tb00117.x. [DOI] [PubMed] [Google Scholar]
- HERMIER J. [Germination of the Bacillus subtilis spore. I. Effect of sugars and amino acids on the initial phase of germination]. Ann Inst Pasteur (Paris) 1962 May;102:629–643. [PubMed] [Google Scholar]
- HILLS G. M. Chemical factors in the germination of spore-bearing aerobes: observations on the influence of species, strain and conditions of growth. J Gen Microbiol. 1950 Jan;4(1):38–47. doi: 10.1099/00221287-4-1-38. [DOI] [PubMed] [Google Scholar]
- HYATT M. T., LEVINSON H. S. Conditions affecting Bacillus megaterium spore germination in glucose or various nitrogenous compounds. J Bacteriol. 1962 Jun;83:1231–1237. doi: 10.1128/jb.83.6.1231-1237.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HYATT M. T., LEVINSON H. S. Correlation of respiratory activity with phases of spore germination and growth in Bacillus megaterium as influenced by manganese and L-alanine. J Bacteriol. 1956 Aug;72(2):176–183. doi: 10.1128/jb.72.2.176-183.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HYATT M. T., LEVINSON H. S. Sulfure requirement for postgerminative development of Bacillus megaterium spores. J Bacteriol. 1957 Jul;74(1):87–93. doi: 10.1128/jb.74.1.87-93.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HYATT M. T., LEVINSON H. S. Utilization of phosphates in the postgerminative development of spores of Bacillus megaterium. J Bacteriol. 1959 Apr;77(4):487–496. doi: 10.1128/jb.77.4.487-496.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEVINSON H. S., HYATT M. T. EFFECT OF SPORULATION MEDIUM ON HEAT RESISTANCE, CHEMICAL COMPOSITION, AND GERMINATION OF BACILLUS MEGATERIUM SPORES. J Bacteriol. 1964 Apr;87:876–886. doi: 10.1128/jb.87.4.876-886.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEVINSON H. S., HYATT M. T. Nitrogenous compounds in germination and postgerminative development of Bacillus megaterium spores. J Bacteriol. 1962 Jun;83:1224–1230. doi: 10.1128/jb.83.6.1224-1230.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEVINSON H. S., SEVAG M. G. Stimulation of germination and respiration of the spores of Bacillus megatherium by manganese and monovalent anions. J Gen Physiol. 1953 May;36(5):617–629. doi: 10.1085/jgp.36.5.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POWELL J. F. The sporulation and germination of a strain of Bacillus megatherium. J Gen Microbiol. 1951 Nov;5(5 Suppl):993–1000. doi: 10.1099/00221287-5-5-993. [DOI] [PubMed] [Google Scholar]
- RODE L. J., FOSTER J. W. Ionic and non-ionic compounds in the germination of spores of Bacillus megaterium Texas. Arch Mikrobiol. 1962;43:201–212. doi: 10.1007/BF00406436. [DOI] [PubMed] [Google Scholar]
- RODE L. J., FOSTER J. W. Ionic germination of spores of Bacillus megaterium QM B 1551. Arch Mikrobiol. 1962;43:183–200. doi: 10.1007/BF00406435. [DOI] [PubMed] [Google Scholar]
- STEDMAN R. L. Biochemical aspects of bacterial endospore formation and germination. II. Chemical changes during sporulation and germination. Am J Pharm Sci Support Public Health. 1956 Apr;128(4):114–130. [PubMed] [Google Scholar]
- WOESE C. R., MOROWITZ H. J., HUTCHISON C. A., 3rd Analysis of action of L-alanine analogues in spore germination. J Bacteriol. 1958 Dec;76(6):578–588. doi: 10.1128/jb.76.6.578-588.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]


